Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques
Abstract
1. Introduction
2. Imaging Techniques
2.1. Micro-Computed Tomography
2.2. Optical Microscopy
2.3. Confocal Laser Scanning Microscopy
2.4. Scanning Electron Microscopy
3. Case Studies
3.1. Biological
3.1.1. Effects of Climate Change on Gastropod Shells and Egg Capsules
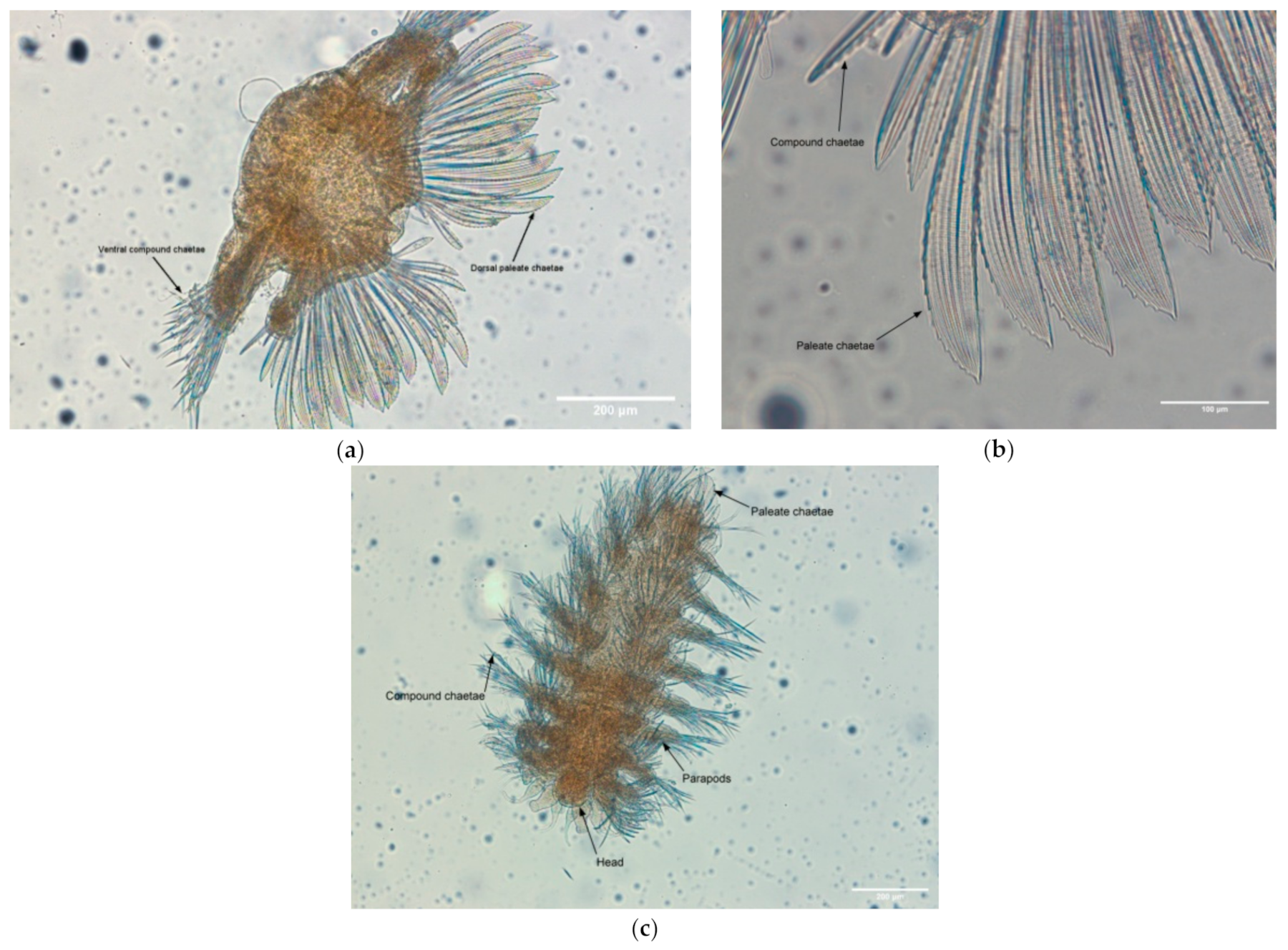
3.1.2. Taxonomic Identification of Polychaeta
3.2. Biomedical
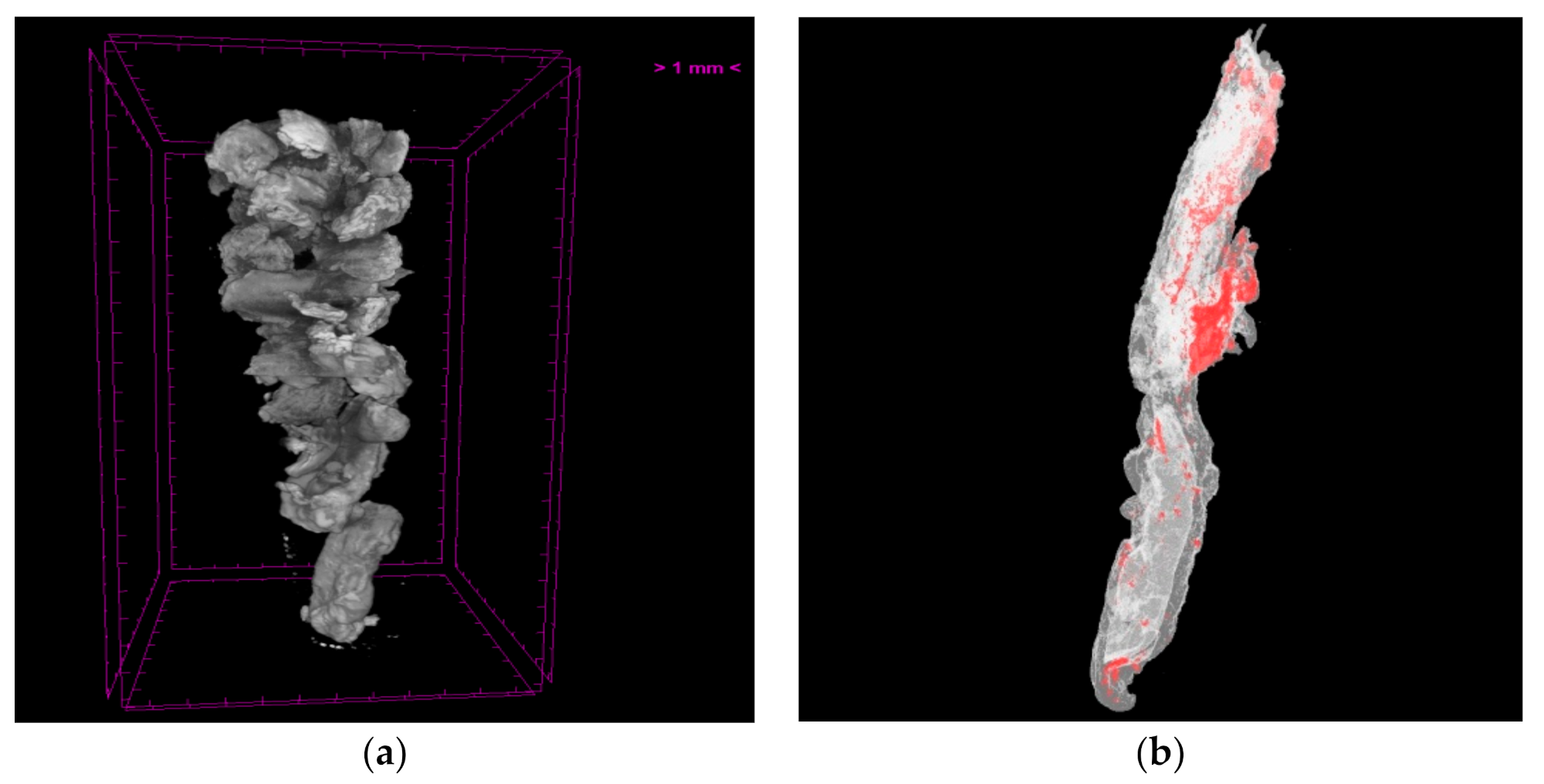
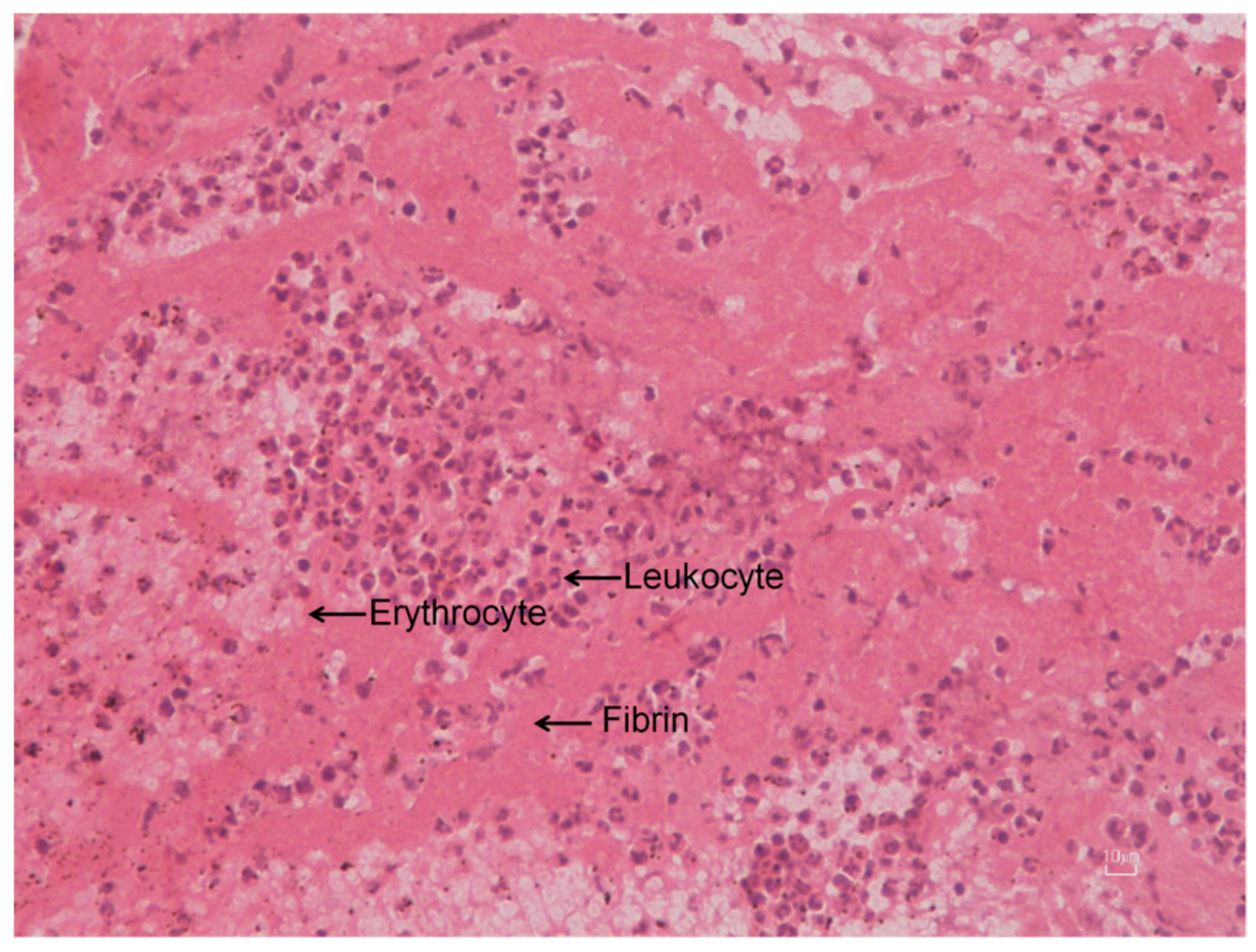
3.2.1. Evaluation of Extracted Thrombotic Material Characteristics
3.2.2. Cardiotoxicity Due to Proteasome Dysfunction
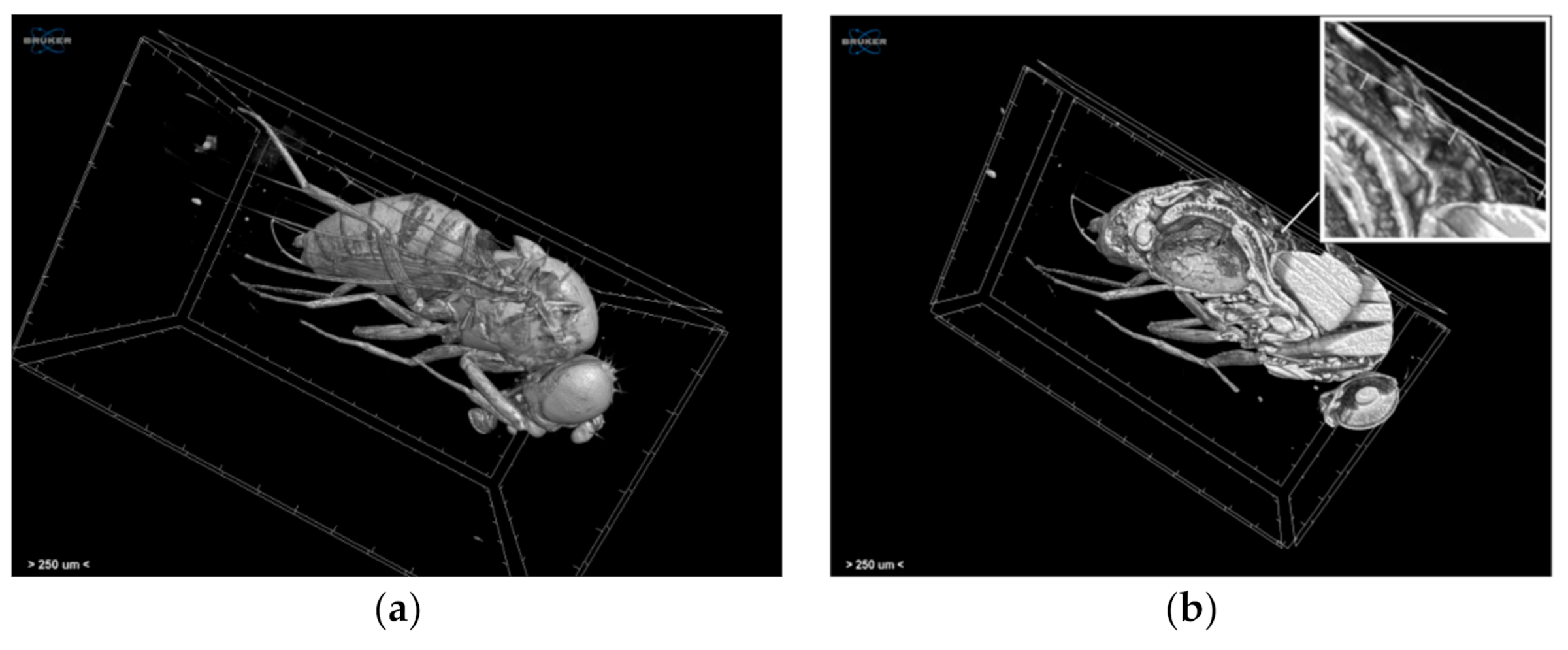
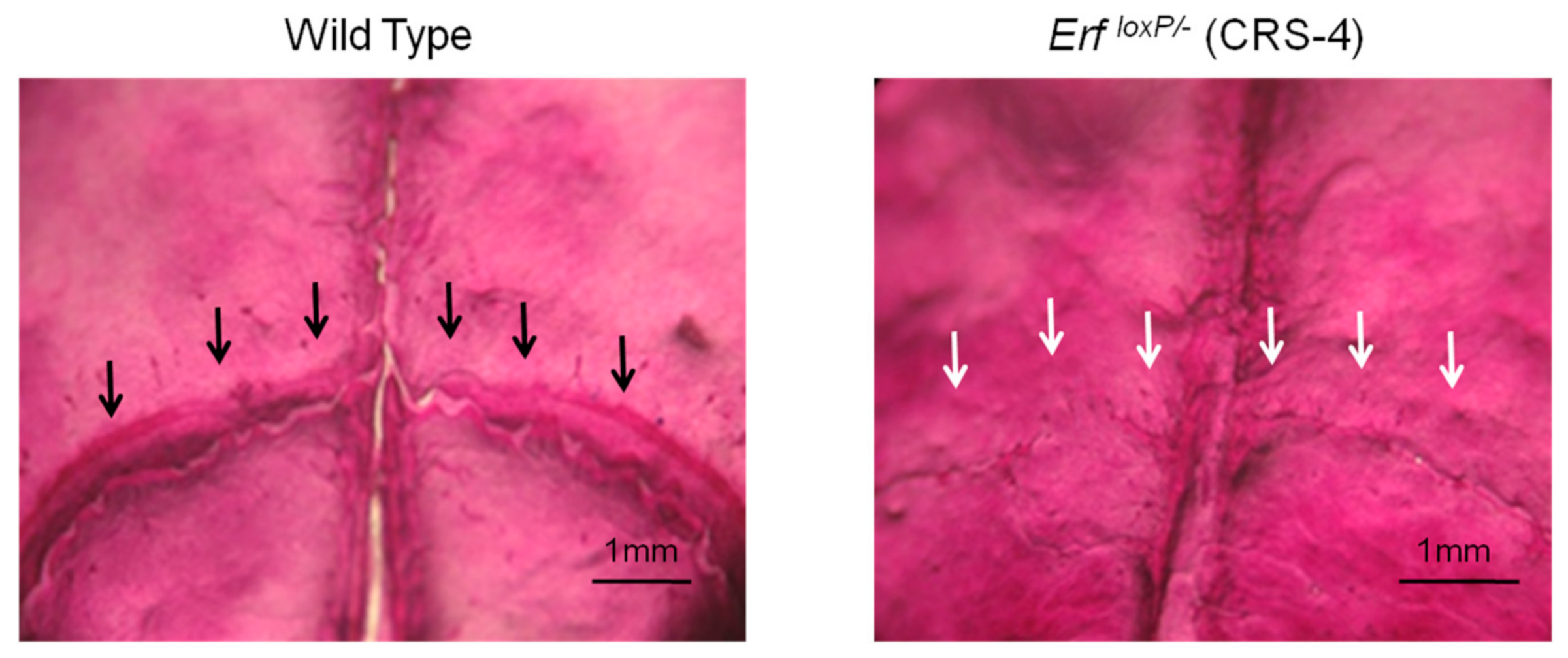
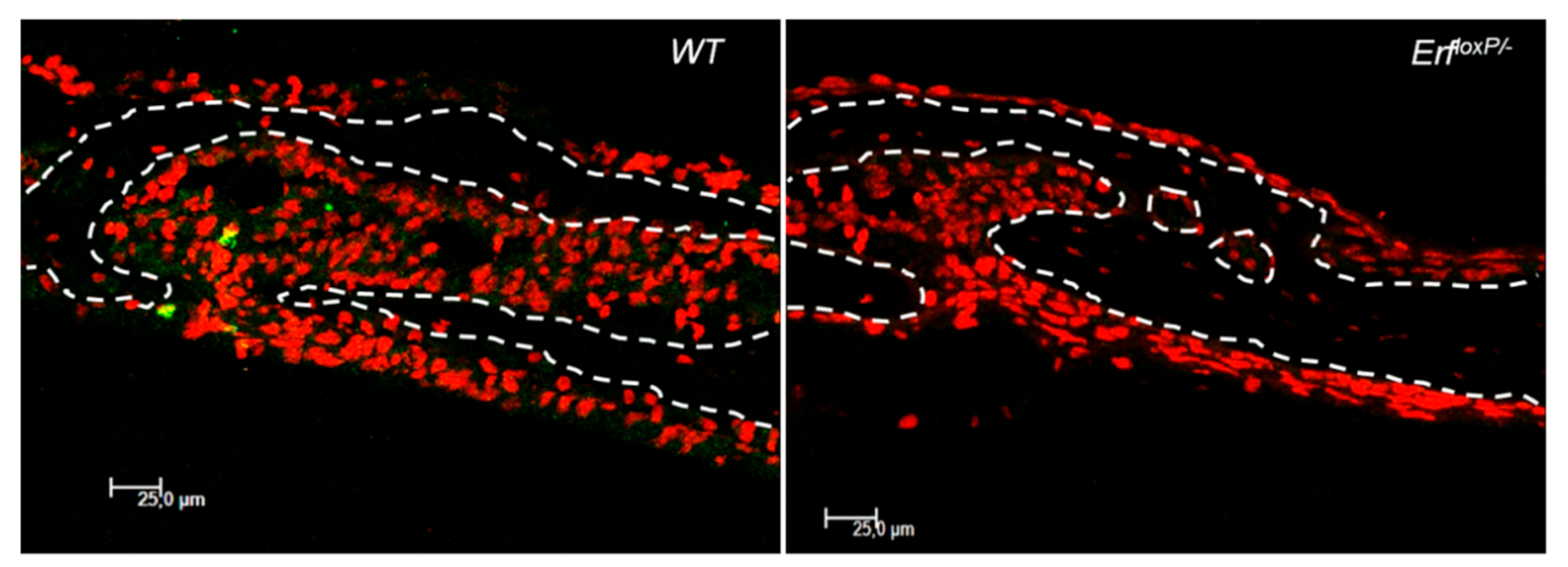
3.2.3. Craniosynostosis in Mice Due to Erf Insufficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weissleder, R.; Nahrendorf, M. Advancing biomedical imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 1–5. [Google Scholar] [CrossRef]
- Orhan, K. Introduction to Micro-CT Imaging. In Micro-Co Mputed Tomography (Micro-CT) in Medicine and Engineering; Orhan, K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; p. 312. ISBN 978-3-030-16640-3. [Google Scholar]
- Descamps, E.; Sochacka, A.; de Kegel, B.; Loo, D.V.; Hoorebeke, L.; Adriaens, D. Soft tissue discrimination with contrast agents using micro-ct scanning. Belgian J. Zool. 2014, 144, 20–40. [Google Scholar] [CrossRef]
- Faillace, M.E.; Rudolph, R.A.; Brunke, O. Micro and nano-CT as a valuable and complimentary tool for life science research. Microsc. Microanal. 2013, 19, 636–637. [Google Scholar] [CrossRef][Green Version]
- Handschuh, S.; Baeumler, N.; Schwaha, T.; Ruthensteiner, B. A correlative approach for combining microCT, light and transmission electron microscopy in a single 3D scenario. Front. Zool. 2013, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Herdina, A.N.; Plenk, H.; Benda, P.; Lina, P.H.C.; Herzig-Straschil, B.; Hilgers, H.; Metscher, B.D. Correlative 3D-imaging of Pipistrellus penis micromorphology: Validating quantitative microCT images with undecalcified serial ground section histomorphology. J. Morphol. 2015, 276, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): Part 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Fenster, A. A TRENDS Guide to Imaging Technologies. Trends Biotechnol. 2002, 20, S1–S2. [Google Scholar] [CrossRef]
- Keklikoglou, K.; Faulwetter, S.; Chatzinikolaou, E.; Wils, P.; Brecko, J.; Kvaček, J.; Metscher, B.; Arvanitidis, C. Micro-computed tomography for natural history specimens: A handbook of best practice protocols. Eur. J. Taxon. 2019, 522, 1–55. [Google Scholar] [CrossRef]
- Steiner, L.; Synek, A.; Pahr, D.H. Comparison of different microCT-based morphology assessment tools using human trabecular bone. Bone Rep. 2020, 12, 100261. [Google Scholar] [CrossRef]
- Schaad, L.; Hlushchuk, R.; Barré, S.; Gianni-Barrera, R.; Haberthür, D.; Banfi, A.; Djonov, V. Correlative Imaging of the Murine Hind Limb Vasculature and Muscle Tissue by MicroCT and Light Microscopy. Sci. Rep. 2017, 7, 41842. [Google Scholar] [CrossRef]
- Befera, N.T.; Badea, C.T.; Johnson, G.A. Comparison of 4D-MicroSPECT and MicroCT for Murine Cardiac Function. Mol. Imaging Biol. 2014, 16, 235–245. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Papazoglou, A.S.; Sofidis, G.; Chatzinikolaou, E.; Keklikoglou, K.; Panteris, E.; Kartas, A.; Stalikas, N.; Zegkos, T.; Girtovitis, F.; et al. Micro-CT-Based Quantification of Extracted Thrombus Burden Characteristics and Association With Angiographic Outcomes in Patients With ST-Elevation Myocardial Infarction: The QUEST-STEMI Study. Front. Cardiovasc. Med. 2021, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.C.; Nguyen, T.; Nowell, C.J.; Graham, B.; Boyd, B.J. Examining the gastrointestinal transit of lipid-based liquid crystalline systems using whole-animal imaging. Drug Deliv. Transl. Res. 2015, 5, 566–574. [Google Scholar] [CrossRef]
- Qiu, S.; Dorrius, M.D.; de Jongh, S.J.; Jansen, L.; de Vries, J.; Schröder, C.P.; Zhang, G.; de Vries, E.G.E.; van der Vegt, B.; van Dam, G.M. Micro-computed tomography (micro-CT) for intraoperative surgical margin assessment of breast cancer: A feasibility study in breast conserving surgery. Eur. J. Surg. Oncol. 2018, 44, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.K.; Ford, N.L. Investigating the effect of longitudinal micro-CT imaging on tumour growth in mice. Phys. Med. Biol. 2011, 56, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Wright, A.C.; Horng, D.; Padmanabhan, A.; Epstein, J.A. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ. Cardiovasc. Imaging 2010, 3, 314–322. [Google Scholar] [CrossRef]
- Faulwetter, S.; Vasileiadou, A.; Kouratoras, M.; Dailianis, T.; Arvanitidis, C. Micro-computed tomography: Introducing new dimensions to taxonomy. Zookeys 2013, 263, 1–45. [Google Scholar] [CrossRef]
- Chatzinikolaou, E.; Keklikoglou, K.; Grigoriou, P. Morphological Properties of Gastropod Shells in a Warmer and More Acidic Future Ocean Using 3D Micro-Computed Tomography. Front. Mar. Sci. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Metscher, B.D. MicroCT for developmental biology: A versatile tool for high-contrast 3D imaging at histological resolutions. Dev. Dyn. 2009, 238, 632–640. [Google Scholar] [CrossRef]
- Waarsing, J.; Day, J.; van der Linden, J.; Ederveen, A.; Spanjers, C.; De Clerck, N.; Sasov, A.; Verhaar, J.A.; Weinans, H. Detecting and tracking local changes in the tibiae of individual rats: A novel method to analyse longitudinal in vivo micro-CT data. Bone 2004, 34, 163–169. [Google Scholar] [CrossRef]
- Bilecenoğlu, B.; Ocak, M. Analysis of Fracture Callus Mechanical Properties Using Micro-CT. In Micro-Computed Tomography (Micro-CT) in Medicine and Engineering; Orhan, K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; p. 312. ISBN 978-3-030-16640-3. [Google Scholar]
- Metscher, B.D. MicroCT for comparative morphology: Simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, S.; Felix, M.C.; Hartmann, L.; Bierbaum, M.; Maros, M.E.; Kerl, H.U.; Wenz, F.; Glatting, G.; Kramer, M.; Giordano, F.A.; et al. In vivo micro-CT imaging of untreated and irradiated orthotopic glioblastoma xenografts in mice: Capabilities, limitations and a comparison with bioluminescence imaging. J. Neurooncol. 2015, 122, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Van Loo, D.; Cornillie, P.; Brabant, L.; Van Hoorebeke, L. An exploratory study of contrast agents for soft tissue visualization by means of high resolution X-ray computed tomography imaging. J. Microsc. 2013, 250, 21–31. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Keklikoglou, K.; Metscher, B.D.; Faulwetter, S. Exploring methods to remove iodine and phosphotungstic acid stains from zoological specimens. In Proceedings of the Micro-CT User Meeting, Bruges, Belgium, 4–7 May 2015; Bruker: Bruges, Belgium, 2015; pp. 116–123. [Google Scholar]
- Gignac, P.M.; Kley, N.J.; Clarke, J.A.; Colbert, M.W.; Morhardt, A.C.; Cerio, D.; Cost, I.N.; Cox, P.G.; Daza, J.D.; Early, C.M.; et al. Diffusible iodine-based contrast-enhanced computed tomography (diceCT): An emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. J. Anat. 2016, 228, 889–909. [Google Scholar] [CrossRef]
- Sharir, A.; Ramniceanu, G.; Brumfeld, V. High Resolution 3D Imaging of Ex-Vivo Biological Samples by Micro CT. J. Vis. Exp. 2011, 52, e2688. [Google Scholar] [CrossRef]
- Schambach, S.J.; Bag, S.; Schilling, L.; Groden, C.; Brockmann, M.A. Application of micro-CT in small animal imaging. Methods 2010, 50, 2–13. [Google Scholar] [CrossRef]
- Hall, A.C.; Sherlock, E.; Sykes, D. Does Micro-CT scanning damage DNA in museum specimens? J. Nat. Sci. Collect 2015, 2, 22–28. [Google Scholar]
- Paredes, U.M.; Prys-Jones, R.; Adams, M.; Groombridge, J.; Kundu, S.; Agapow, P.M.; Abel, R.L. Micro-CT X-rays do not fragment DNA in preserved bird skins. J. Zool. Syst. Evol. Res. 2012, 50, 247–250. [Google Scholar] [CrossRef]
- Haberthür, D.; Hintermüller, C.; Marone, F.; Schittny, J.C.; Stampanoni, M. Radiation dose optimized lateral expansion of the field of view in synchrotron radiation X-ray tomographic microscopy. J. Synchrotron Radiat. 2010, 17, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.T. Molluscan shell colour. Biol. Rev. 2017, 92, 1039–1058. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Otaki, J.M. Color-Pattern Evolution in Response to Environmental Stress in Butterflies. Front. Genet. 2012, 3, 15. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.-X. Depuration of metals by the green-colored oyster Crassostrea sikamea. Environ. Toxicol. Chem. 2014, 33, 2379–2385. [Google Scholar] [CrossRef]
- Ijiri, T.; Todo, H.; Hirabayashi, A.; Kohiyama, K.; Dobashi, Y. Digitization of natural objects with micro CT and photographs. PLoS ONE 2018, 13, e0195852. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Ebnesajjad, C. Surface Treatment of Materials for Adhesive Bonding, 2nd ed.; Elsevier: Oxford, UK, 2014; ISBN 9780323264358. [Google Scholar]
- Di Gianfrancesco, A. Technologies for chemical analyses, microstructural and inspection investigations. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Di Gianfrancesco, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 197–245. [Google Scholar]
- Ager, J.W. Overview of optical microscopy and optical microspectroscopy. In Proceedings of the 1998 International Conference on Characterization and Metrology for ULSI Technology, Gaithersburg, MD, USA, 23–27 March 1998; ASCE: Reston, VA, USA, 1998; pp. 641–652. [Google Scholar]
- Hopen, T.J.; Davis, M. Microscopy: Light Microscopes. In Wiley Encyclopedia of Forensic Science; Jamieson, A., Moenssens, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; p. 3104. [Google Scholar]
- Booth, M.J. Adaptive optical microscopy: The ongoing quest for a perfect image. Light Sci. Appl. 2014, 3, e165. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, B.; Liu, H. Optical and digital microscopic imaging techniques and applications in pathology. Anal. Cell. Pathol. 2011, 34, 5–18. [Google Scholar] [CrossRef]
- Kehlmaier, C.; Dierick, M.; Skevington, J.H. Micro-CT studies of amber inclusions reveal internal genitalic features of big-headed flies, enabling a systematic placement of Metanephrocerus Aczél, 1948 (Insecta: Diptera: Pipunculidae). Arthropod Syst. Phylogeny 2014, 72, 23–36. [Google Scholar]
- Ngo, J.P.; Le, B.; Khan, Z.; Kett, M.M.; Gardiner, B.S.; Smith, D.W.; Melhem, M.M.; Maksimenko, A.; Pearson, J.T.; Evans, R.G. Micro-computed tomographic analysis of the radial geometry of intrarenal artery-vein pairs in rats and rabbits: Comparison with light microscopy. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Le Breton, A.; Jegoux, F.; Pilet, P.; Godey, B. Micro-CT scan, electron microscopy and optical microscopy study of insertional traumas of cochlear implants. Surg. Radiol. Anat. 2015, 37, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kind, M.; Stock, N.; Coindre, J.M. Histology and imaging of soft tissue sarcomas. Eur. J. Radiol. 2009, 72, 6–15. [Google Scholar] [CrossRef]
- Musumeci, G. Past, present and future: Overview on histology and histopathology. J. Histol. Histopathol. 2014, 1, 5. [Google Scholar] [CrossRef]
- Cotter, M.B.; Loda, M. Introduction to Histology. In Pathology and Epidemiology of Cancer; Loda, M., Mucc, L., Mittelstadt, M., Van Hemelrijck, M., Cotter, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 11–26. [Google Scholar]
- Titford, M. A Short History of Histopathology Technique. J. Histotechnol. 2006, 29, 99–110. [Google Scholar] [CrossRef]
- Macenko, M.; Niethammer, M.; Marron, J.S.; Borland, D.; Woosley, J.T.; Guan, X.; Schmitt, C.; Thomas, N.E. A method for normalizing histology slides for quantitative analysis. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, 28 June–1 July 2009; pp. 1107–1110. [Google Scholar]
- Cozma, K.; Henwood, A. An assessment of decoverslipping methods on histology slides. J. Histotechnol. 2019, 42, 64–67. [Google Scholar] [CrossRef]
- Taqi, S.; Sami, S.; Sami, L.; Zaki, S. A review of artifacts in histopathology. J. Oral Maxillofac. Pathol. 2018, 22, 279. [Google Scholar] [CrossRef]
- Minsky, M. Memoir on Inventing the Confocal Scanning Microscope. Scanning 1988, 10, 128–138. [Google Scholar] [CrossRef]
- Canette, A.; Briandet, R. Microscopy chapter: Confocal Laser Scanning Microscopy. In Encyclopedia of Food Microbiology; Batt, C., Tortorello, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 676–683. [Google Scholar]
- Vyplelová, P.; Ovečka, M.; Komis, G.; Šamaj, J. Advanced microscopy methods for bioimaging of mitotic microtubules in plants. Methods Cell Biol. 2018, 145, 129–158. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, O.; Harris, J.; Egan, C. Advanced Microscopy: Laser Scanning Confocal Microscopy. In Gene Expression Profiling. Methods and Protocols; O’Driscoll, L., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 169–180. [Google Scholar]
- Jonkman, J.; Brown, C.M. Any Way You Slice It—A Comparison of Confocal Microscopy Techniques. J. Biomol. Tech. 2015, 26, 54–65. [Google Scholar] [CrossRef]
- Bayguinov, P.O.; Oakley, D.M.; Shih, C.-C.; Geanon, D.J.; Joens, M.S.; Fitzpatrick, J.A.J. Modern Laser Scanning Confocal Microscopy. Curr. Protoc. Cytom. 2018, 85, e39. [Google Scholar] [CrossRef] [PubMed]
- Paddock, S.W.; Eliceiri, K.W. Laser Scanning Confocal Microscopy: History, Applications, and Related Optical Sectioning Techniques. In Confocal Microscopy. Methods in Molecular Biology (Methods and Protocols); Paddock, S., Ed.; Humana Press: New York, NY, USA, 2014; pp. 9–47. [Google Scholar]
- Choi, M.; Kwok, S.J.J.; Yun, S.H. In Vivo Fluorescence Microscopy: Lessons From Observing Cell Behavior in Their Native Environment. Physiology 2015, 30, 40–49. [Google Scholar] [CrossRef]
- Simionato, G.; Hinkelmann, K.; Chachanidze, R.; Bianchi, P.; Fermo, E.; van Wijk, R.; Leonetti, M.; Wagner, C.; Kaestner, L.; Quint, S. Red blood cell phenotyping from 3D confocal images using artificial neural networks. PLoS Comput. Biol. 2021, 17, e1008934. [Google Scholar] [CrossRef]
- Peng, X.-H.; Cao, Z.-H.; Xia, J.-T.; Carlson, G.W.; Lewis, M.M.; Wood, W.C.; Yang, L. Real-time Detection of Gene Expression in Cancer Cells Using Molecular Beacon Imaging: New Strategies for Cancer Research. Cancer Res. 2005, 65, 1909–1917. [Google Scholar] [CrossRef]
- Bystricky, K.; Van Attikum, H.; Montiel, M.-D.; Dion, V.; Gehlen, L.; Gasser, S.M. Regulation of Nuclear Positioning and Dynamics of the Silent Mating Type Loci by the Yeast Ku70/Ku80 Complex. Mol. Cell. Biol. 2009, 29, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, O.; Webb, H.; O’Byrne, R.; Brabazon, E.; Treumann, A.; Sunter, J.D.; Carrington, M.; Voorheis, H.P. The Glycosylphosphatidylinositol-PLC in Trypanosoma brucei Forms a Linear Array on the Exterior of the Flagellar Membrane Before and After Activation. PLoS Pathog. 2009, 5, e1000468. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; Doyle, S.L.; McGettrick, A.F.; Hardy, M.; Husebye, H.; Banahan, K.; Gong, M.; Golenbock, D.; Espevik, T.; O’Neill, L.A.J. TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88–independent TLR4 pathway. Nat. Immunol. 2009, 10, 579–586. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of scanning electron microscopy (SEM). In Scanning Microscopy for Nanotechnology; Springer: New York, NY, USA, 2006; pp. 1–40. [Google Scholar]
- Inkson, B.J. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.-G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–43. [Google Scholar]
- Vernon-Parry, K.D. Scanning electron microscopy: An introduction. III-Vs Rev. 2000, 13, 40–44. [Google Scholar] [CrossRef]
- Knoll, M. Aufladepotentiel und Sekundäremission elektronenbestrahlterKörper. Z. Tech. Phys. 1935, 16, 467–475. [Google Scholar]
- Von Ardenne, M. Das Elektronen-Rastermikroskop. Theoretische Grund-lagen. Z. Phys. 1938, 109, 553–572. [Google Scholar] [CrossRef]
- Von Ardenne, M. Das Elektronen-Rastermikroskop. Praktische Aus-führung. Z. Phys. 1938, 19, 407–416. [Google Scholar]
- Zworykin, V.A.; Hillier, J.; Snyder, R.L. A scanning electron microscope. ASTM Bull. 1942, 117, 15–23. [Google Scholar] [CrossRef]
- McMullan, D. Scanning electron microscopy 1928–1965. Scanning 1995, 17, 175–185. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; pp. 7–9. [Google Scholar]
- Kashi, A.M.; Tahermanesh, K.; Chaichian, S.; Joghataei, M.T.; Moradi, F.; Tavangar, S.M.; Najafabadi, A.S.M.; Lotfibakhshaiesh, N.; Beyranvand, S.P.; Anvari-Yazdi, A.F.; et al. How to Prepare Biological Samples and Live Tissues for Scanning Electron Microscopy (SEM). Galen Med. J. 2014, 3, 63–80. [Google Scholar]
- Paterson, S.M.; Casadio, Y.S.; Brown, D.H.; Shaw, J.A.; Chirila, T.V.; Baker, M.V. Laser scanning confocal microscopy versus scanning electron microscopy for characterization of polymer morphology: Sample preparation drastically distorts morphologies of poly (2-hydroxyethyl methacrylate)-based hydrogels. J. Appl. Polym. Sci. 2013, 127, 4296–4304. [Google Scholar] [CrossRef]
- Ostrowski, S.G.; Paxon, T.L.; Denault, L.; McEvoy, K.; Smentkowski, V.S. Preparing Biological Samples for Analysis by High Vacuum Techniques. Micros. Today 2009, 17, 48–53. [Google Scholar] [CrossRef][Green Version]
- Danilatos, G.D. The examination of fresh or living plant material in an environmental scanning electron microscope. J. Microsc. 1981, 121, 235–238. [Google Scholar] [CrossRef]
- Echlin, P. Handbook of Sample Preparation for Scanning Electron Microscopy and X-Ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Gizzi, F.; Caccia, M.G.; Simoncini, G.A.; Mancuso, A.; Reggi, M.; Fermani, S.; Brizi, L.; Fantazzini, P.; Stagioni, M.; Falini, G.; et al. Shell properties of commercial clam Chamelea gallina are influenced by temperature and solar radiation along a wide latitudinal gradient. Sci. Rep. 2016, 6, 36420. [Google Scholar] [CrossRef]
- Barclay, K.; Gaylord, B.; Jellison, B.; Shukla, P.; Sanford, E.; Leighton, L. Variation in the effects of ocean acidification on shell growth and strength in two intertidal gastropods. Mar. Ecol. Prog. Ser. 2019, 626, 109–121. [Google Scholar] [CrossRef]
- Andersson, A.J.; Mackenzie, F.T.; Lerman, A. Coastal ocean CO2 -carbonic acid-carbonate sediment system of the Anthropocene. Glob. Biogeochem. Cycles 2006, 20, GB1S92. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Arvanitidis, C.; Bellan, G.; Drakopoulos, P.; Valavanis, V.; Dounas, C.; Koukouras, A.; Eleftheriou, A. Seascape biodiversity patterns along the Mediterranean and the Black Sea: Lessons from the biogeography of benthic polychaetes. Mar. Ecol. Prog. Ser. 2002, 244, 139–152. [Google Scholar] [CrossRef]
- San Martín, G.; Aguado, M.T. Contribution of Scanning Electron Microscope to the Study of Morphology, Biology, Reproduction, and Phylogeny of the Family Syllidae (Polychaeta). In Scanning Electron Microscopy; Kazmiruk, V., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Lovell, L.L.; Fitzhugh, K. Taking a closer look: An SEM review of Levinsenia species (Polychaeta: Paraonidae) reported from California. Zootaxa 2020, 4751, 256–275. [Google Scholar] [CrossRef]
- Bhindi, R.; Kajander, O.A.; Jolly, S.S.; Kassam, S.; Lavi, S.; Niemelä, K.; Fung, A.; Cheema, A.N.; Meeks, B.; Alexopoulos, D.; et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: The optical coherence tomography sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur. Heart J. 2015, 36, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, E.; Konstantinidis, N.V.; Sofidis, G.; Chatzinikolaou, E.; Sianos, G. Rationale and design of a prospective, observational study for the QUantitative EStimation of Thrombus burden in patients with ST-Elevation Myocardial Infarction using micro-computed tomography: The QUEST-STEMI trial. BMC Cardiovasc. Disord. 2020, 20, 125. [Google Scholar] [CrossRef]
- Kuntz, S.H.; Jinnouchi, H.; Kutyna, M.; Torii, S.; Cornelissen, A.; Sakamoto, A.; Sato, Y.; Fuller, D.T.; Schwein, A.; Ohana, M.; et al. Co-Registration of Peripheral Atherosclerotic Plaques Assessed by Conventional CT Angiography, MicroCT and Histology in Patients with Chronic Limb Threatening Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 146–154. [Google Scholar] [CrossRef]
- Buytaert, J.; Goyens, J.; De Greef, D.; Aerts, P.; Dirckx, J. Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro-CT and light sheet fluorescence microscopy (LSFM). Microsc. Microanal. 2014, 20, 1208–1217. [Google Scholar] [CrossRef]
- Papazoglou, A.S.; Karagiannidis, E.; Moysidis, D.V.; Sofidis, G.; Bompoti, A.; Stalikas, N.; Panteris, E.; Arvanitidis, C.; Herrmann, M.D.; Michaelson, J.S.; et al. Current clinical applications and potential perspective of micro-computed tomography in cardiovascular imaging: A systematic scoping review. Hell. J. Cardiol. 2021, in press. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Papazoglou, A.S.; Stalikas, N.; Deda, O.; Panteris, E.; Begou, O.; Sofidis, G.; Moysidis, D.V.; Kartas, A.; Chatzinikolaou, E.; et al. Serum ceramides as prognostic biomarkers of large thrombus burden in patients with stemi: A micro-computed tomography study. J. Pers. Med. 2021, 11, 89. [Google Scholar] [CrossRef]
- Wu, P.; Oren, O.; Gertz, M.A.; Yang, E.H. Proteasome Inhibitor-Related Cardiotoxicity: Mechanisms, Diagnosis, and Management. Curr. Oncol. Rep. 2020, 22, 66. [Google Scholar] [CrossRef]
- Brigle, K.; Rogers, B. Pathobiology and Diagnosis of Multiple Myeloma. Semin. Oncol. Nurs. 2017, 33, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Tsakiri, E.N.; Terpos, E.; Papanagnou, E.-D.; Kastritis, E.; Brieudes, V.; Halabalaki, M.; Bagratuni, T.; Florea, B.I.; Overkleeft, H.S.; Scorrano, L.; et al. Milder degenerative effects of Carfilzomib vs. Bortezomib in the Drosophila model: A link to clinical adverse events. Sci. Rep. 2017, 7, 17802. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Catalgol, B.; Grune, T. The proteasomal system. Mol. Asp. Med. 2009, 30, 191–296. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Richardson, P.G.; Moreau, P.; Anderson, K.C. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat. Rev. Clin. Oncol. 2015, 12, 42–54. [Google Scholar] [CrossRef]
- Kastritis, E.; Laina, A.; Georgiopoulos, G.; Gavriatopoulou, M.; Papanagnou, E.-D.; Eleutherakis-Papaiakovou, E.; Fotiou, D.; Kanellias, N.; Dialoupi, I.; Makris, N.; et al. Carfilzomib-induced endothelial dysfunction, recovery of proteasome activity, and prediction of cardiovascular complications: A prospective study. Leukemia 2021, 35, 1418–1427. [Google Scholar] [CrossRef]
- Morriss-Kay, G.M.; Wilkie, A.O.M. Growth of the normal skull vault and its alteration in craniosynostosis: Insights from human genetics and experimental studies. J. Anat. 2005, 207, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Twigg, S.R.F.; Vorgia, E.; Mcgowan, S.J.; Peraki, I.; Fenwick, A.L.; Sharma, V.P.; Allegra, M.; Zaragkoulias, A.; Akha, E.S.; Knight, S.J.L.; et al. Reduced dosage of ERF causes complex craniosynostosis in humans and mice and links ERK1/2 signaling to regulation of osteogenesis. Nat. Genet. 2013, 45, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Le Gallic, L.; Virgilio, L.; Cohen, P.; Biteau, B.; Mavrothalassitis, G. ERF Nuclear Shuttling, A Continuous Monitor of Erk Activity That Links It to Cell Cycle Progression. Mol. Cell. Biol. 2004, 24, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
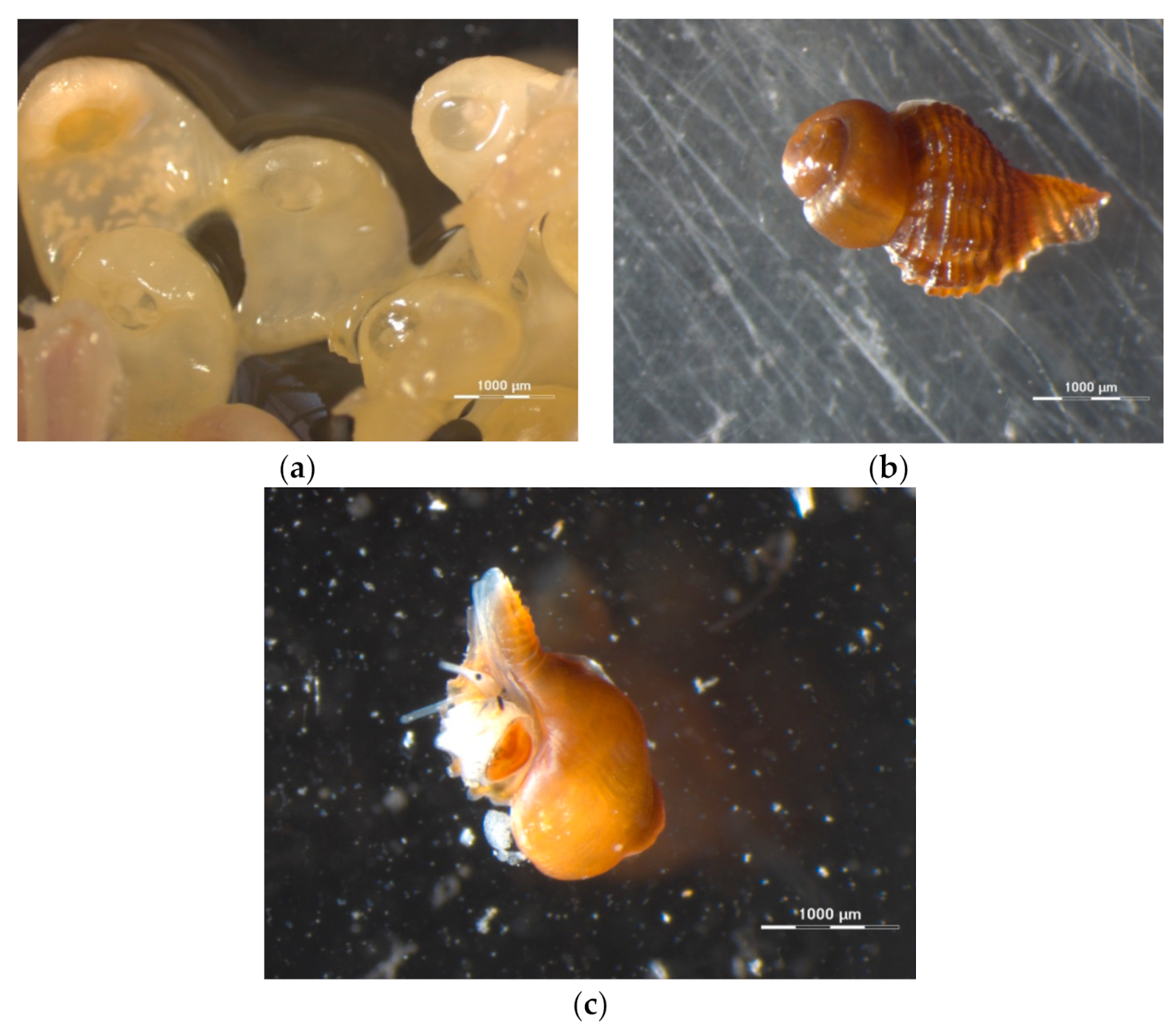
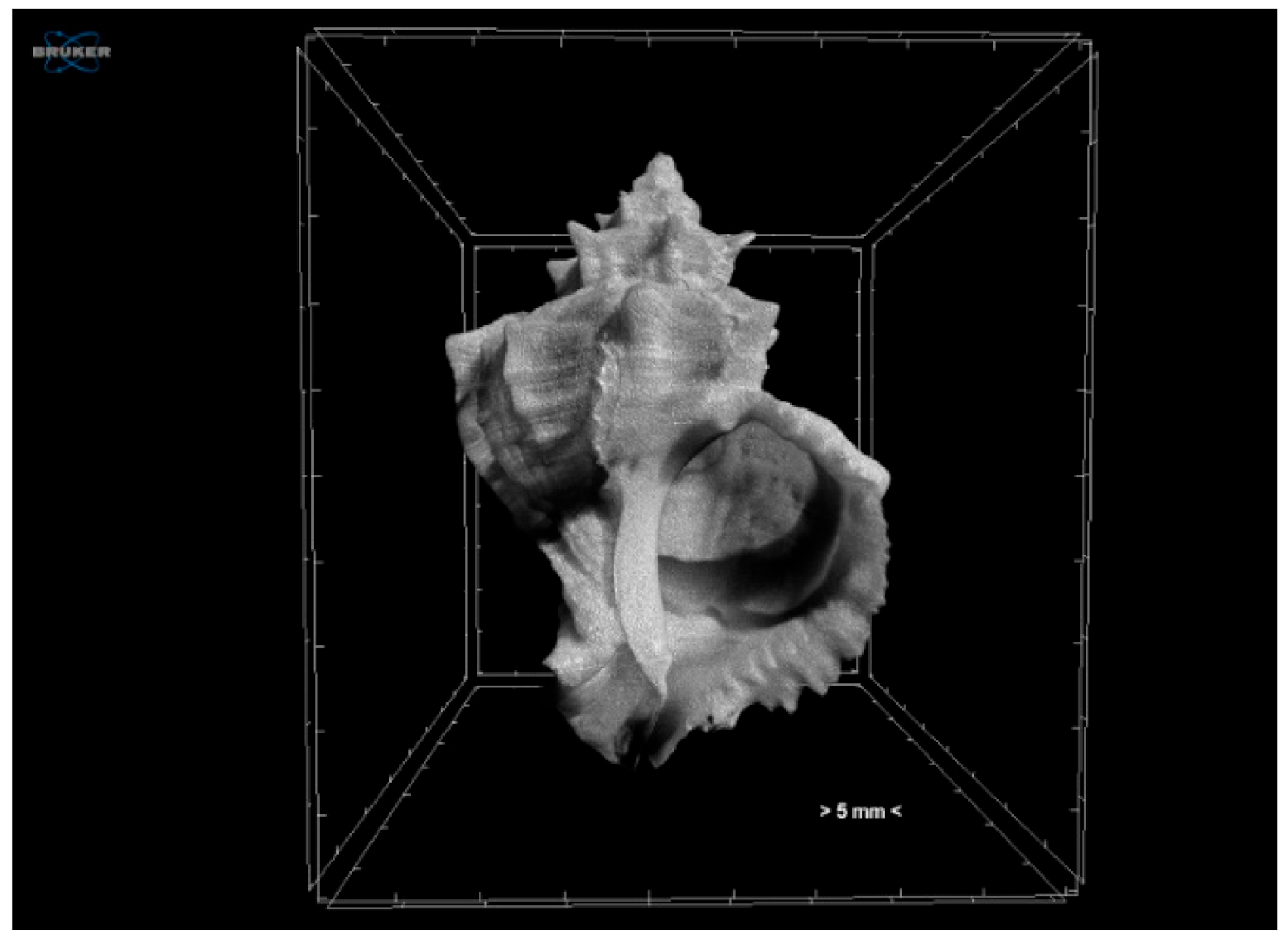
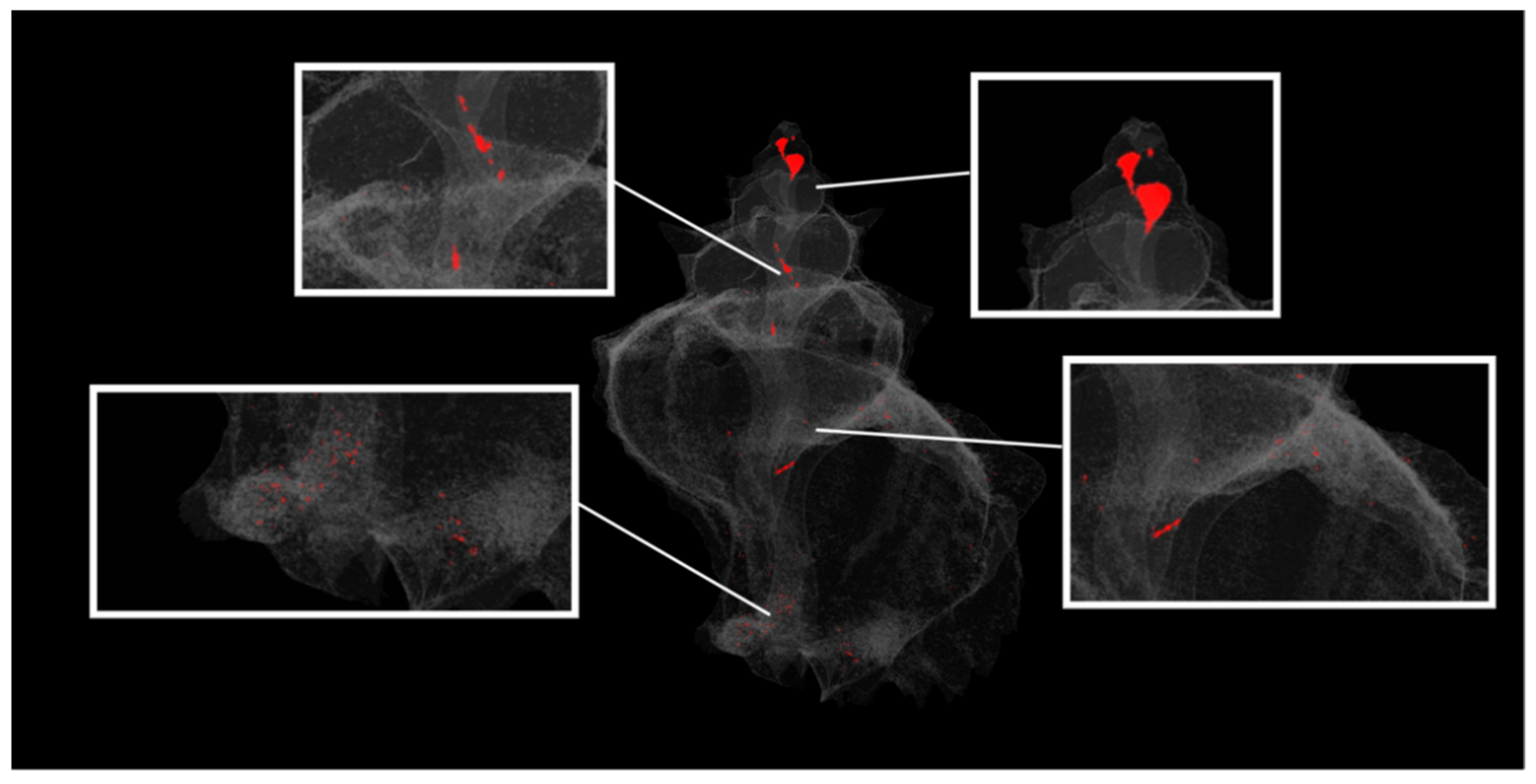
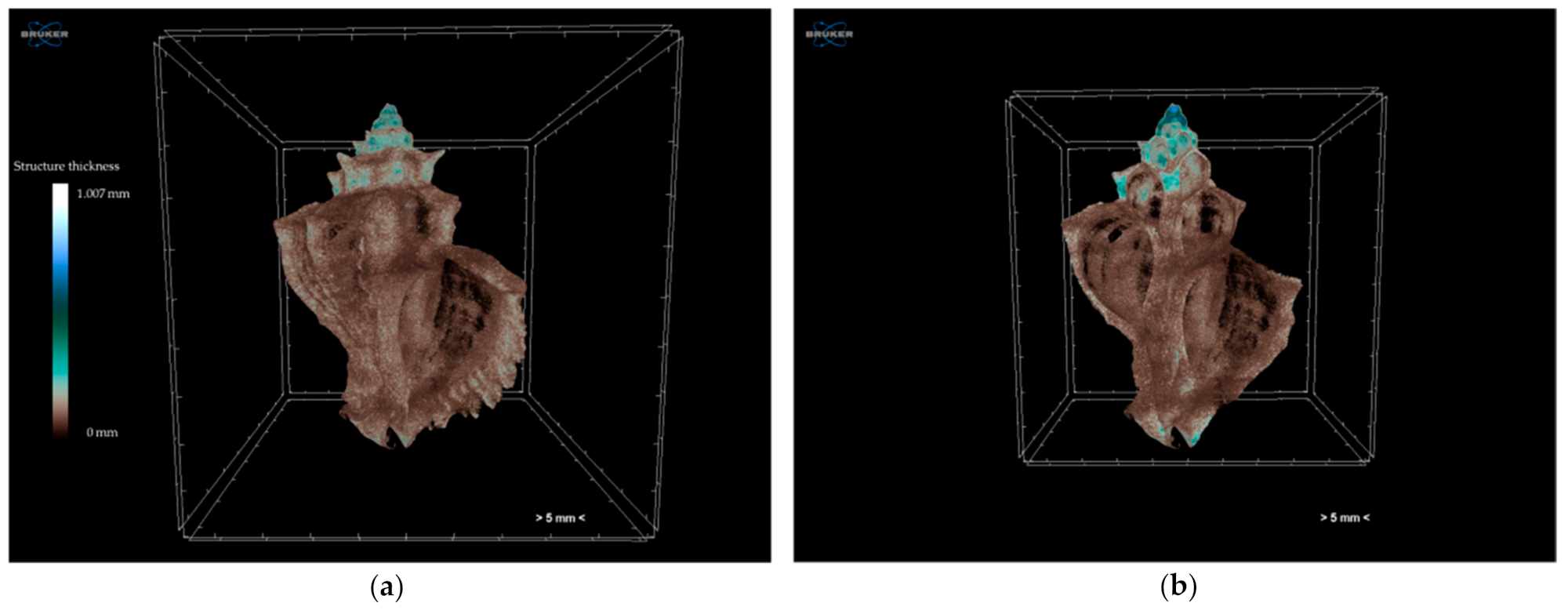




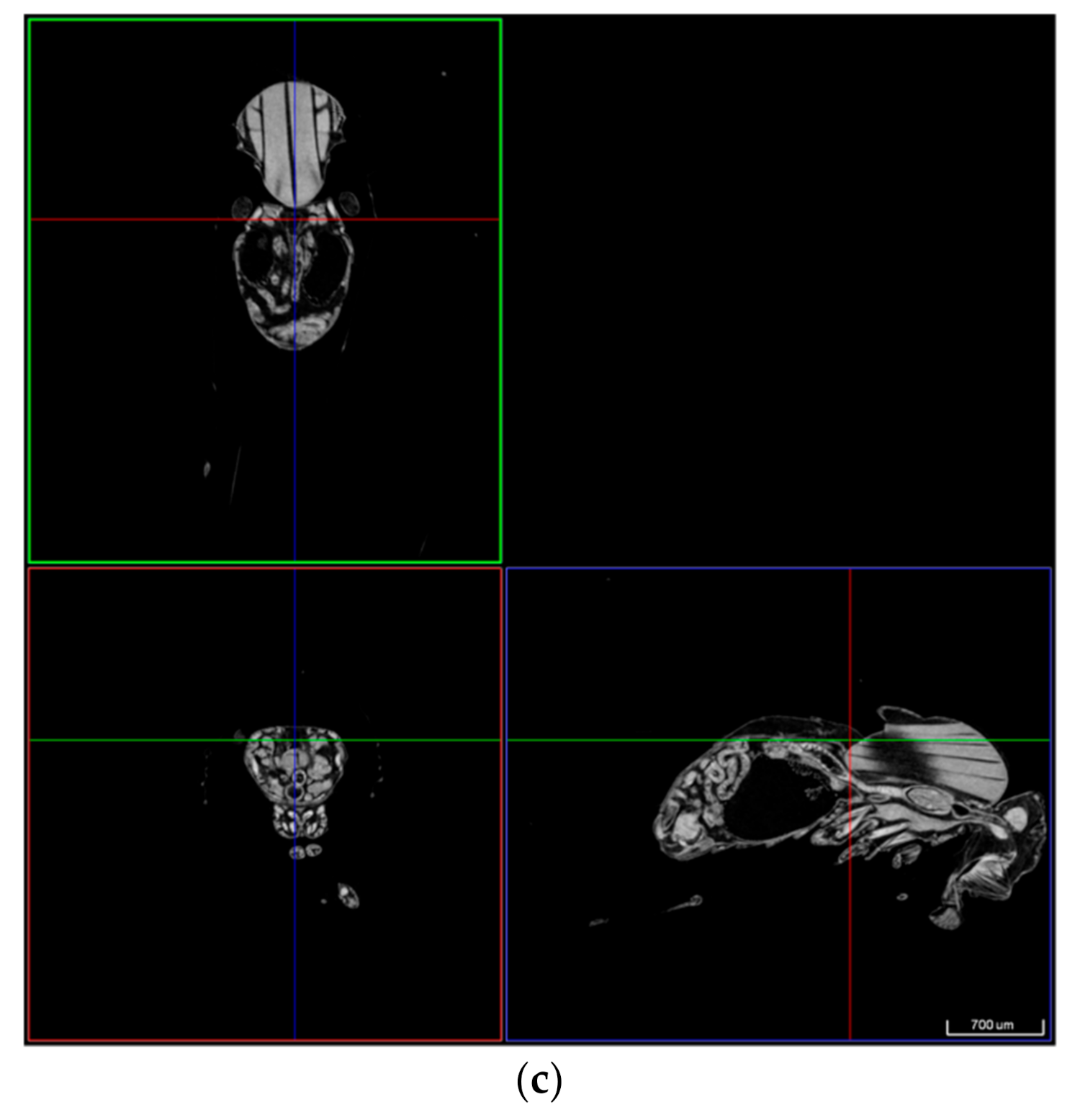
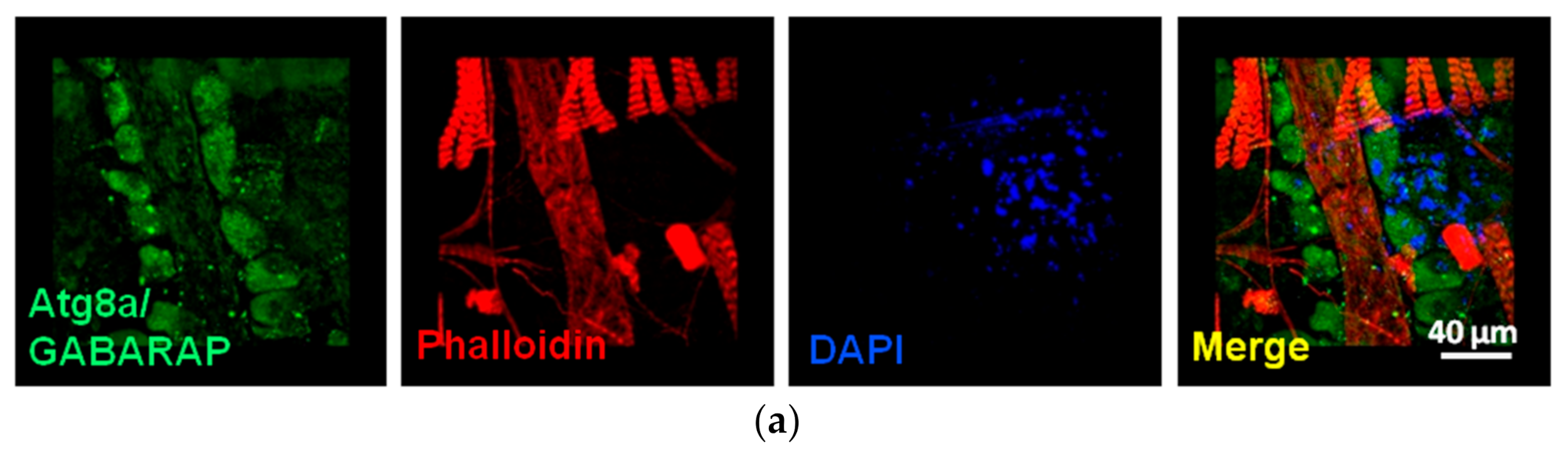
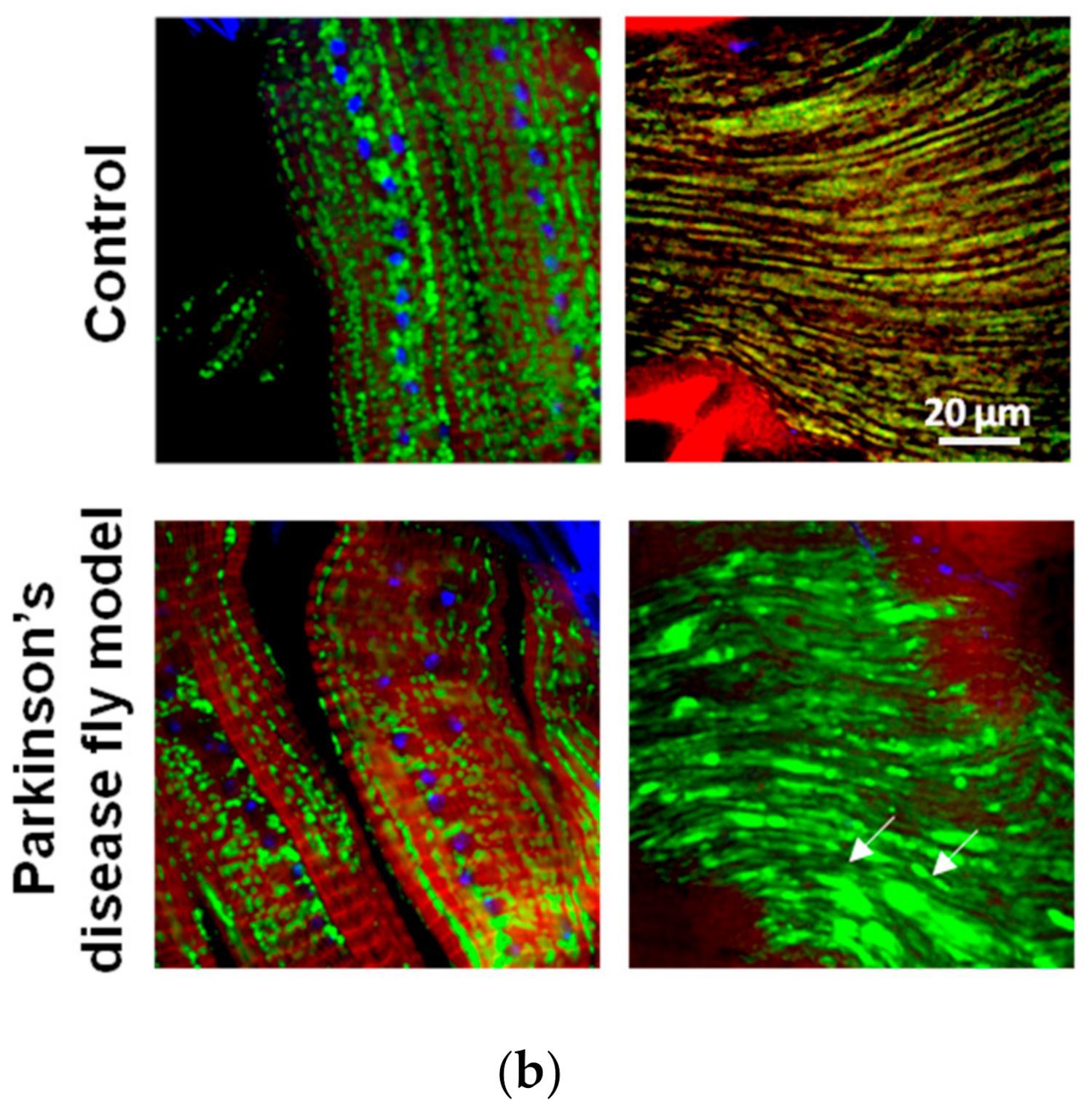
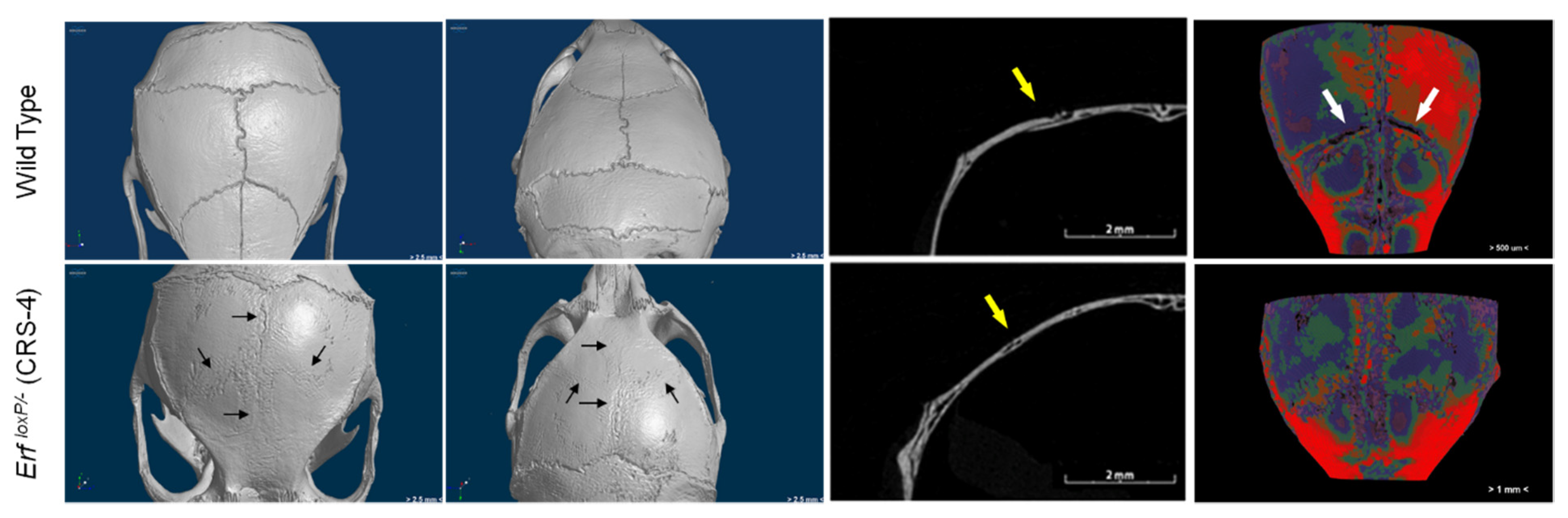
| Biological | Biomedical | Advantages | Disadvantages | |||||
|---|---|---|---|---|---|---|---|---|
| Case Study 1 (Section 3.1.1) | Case Study 2 (Section 3.1.2) | Case Study 3 (Section 3.2.1) | Case Study 4 (Section 3.2.2) | Case Study 5 (Section 3.2.3) | ||||
| Micro-CT | pixel size (μm) | 2–13.79 μm | 0.98 μm | 4 μm | 2.9 μm | 13.8 μm | ||
| sample preparation | only for egg capsules (fixation and staining—4 days) | no | yes (fixation and staining—2 days) | yes (fixation and staining—2 days) | no | internal specimen microstructure, measurements of specimen characteristics (volume, surface, density, thickness, porosity), reliable, reproducible, non-destructive | time consuming for high-resolution datasets, staining might alter specimen characteristics, large dataset size | |
| visualisation of features | external and internal shell and egg structures | external and internal structure | thrombotic characteristics | heart | facial deformities and fused sutures on the skull | |||
| destructive | potential alterations in egg capsules due to staining procedure | no | potential alterations in thrombotic measurements due to staining procedure | potential alterations in heart structure due to staining procedure | no | |||
| imaging duration | 2.5–6 h | 3 h | 1.5 h | 2 h | 2 h | |||
| OM | magnification | 8–25× | 40–60× | 200× | 1× | |||
| sample preparation | no | no | yes (fixation and staining—1 day) | yes (fixation, staining and removal of remaining tissues—35–40 days) | no pre-treatment, original colours of the specimen, fast if no sample preparation is needed, no limitations for shape or geometries, application to in vivo trials, non-invasive | very thin focus area, destruction of the sample in case that the internal view is needed, time consuming for histological examination | ||
| visualisation of features | only external shell and egg structures | external structure | presence of different cell types | sutures | ||||
| destructive | no | yes (cross-sections from specific parts) | yes | yes | ||||
| imaging duration | immediate | immediate | immediate | immediate | ||||
| CLSM | magnification | 60× | 63× | availability of antibodies, photo-bleaching by laser power, destructive method | ||||
| sample preparation | yes (fixation, incubation with antibodies—2.5 h) | yes (fixation, incubation with antibodies—6–7 days) | study of organelles, visualisation at the cellular level, studying cellular and molecular events, can be applied to in vivo studies | |||||
| visualisation of features | organelles in selected tissues | proliferation, apoptosis, and cell type | ||||||
| destructive | yes (young flies were dissected, cardiac tubes or muscle tissues were isolated) | yes | ||||||
| imaging duration | immediate | 4–6 h | ||||||
| SEM | magnification | 85–600× | ||||||
| sample preparation | yes (dehydration and sputter coating with 20 nm thick gold—1 day) | High-resolution images with great focus depth | only surface images, destructive method | |||||
| visualisation of features | ||||||||
| destructive | yes (fixation, dehydration, sputtering) | |||||||
| imaging duration | immediate | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keklikoglou, K.; Arvanitidis, C.; Chatzigeorgiou, G.; Chatzinikolaou, E.; Karagiannidis, E.; Koletsa, T.; Magoulas, A.; Makris, K.; Mavrothalassitis, G.; Papanagnou, E.-D.; et al. Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques. J. Imaging 2021, 7, 172. https://doi.org/10.3390/jimaging7090172
Keklikoglou K, Arvanitidis C, Chatzigeorgiou G, Chatzinikolaou E, Karagiannidis E, Koletsa T, Magoulas A, Makris K, Mavrothalassitis G, Papanagnou E-D, et al. Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques. Journal of Imaging. 2021; 7(9):172. https://doi.org/10.3390/jimaging7090172
Chicago/Turabian StyleKeklikoglou, Kleoniki, Christos Arvanitidis, Georgios Chatzigeorgiou, Eva Chatzinikolaou, Efstratios Karagiannidis, Triantafyllia Koletsa, Antonios Magoulas, Konstantinos Makris, George Mavrothalassitis, Eleni-Dimitra Papanagnou, and et al. 2021. "Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques" Journal of Imaging 7, no. 9: 172. https://doi.org/10.3390/jimaging7090172
APA StyleKeklikoglou, K., Arvanitidis, C., Chatzigeorgiou, G., Chatzinikolaou, E., Karagiannidis, E., Koletsa, T., Magoulas, A., Makris, K., Mavrothalassitis, G., Papanagnou, E.-D., Papazoglou, A. S., Pavloudi, C., Trougakos, I. P., Vasileiadou, K., & Vogiatzi, A. (2021). Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques. Journal of Imaging, 7(9), 172. https://doi.org/10.3390/jimaging7090172










