1. Introduction
Hypertrabeculation, characterized by excessive trabecular meshwork within the left ventricular (LV) cavity, may be present in up to 20% of the general population, and current recommendations regard asymptomatic individuals without a history of cardiac disease as a physiological variant [
1,
2].
This morphology may arise in response to chronic volume overload, such as pregnancy, high-level sports activity, or hyperthyroidism, but a distinct subgroup also fulfills the diagnostic criteria of primary left ventricular noncompaction (LVNC) with preserved LV ejection fraction (LV_EF) [
3,
4,
5].
These patients with excessive trabeculation often show increased volumetric and decreased functional parameters, and in some cases, disease progression may lead to impaired LV function, heart failure, arrhythmias, or thromboembolic events [
6,
7,
8].
However, less is known about the role of right ventricular (RV) involvement in this population. While isolated RV noncompaction has been previously described in case reports, recent studies using echocardiography (TTE) and cardiac magnetic resonance imaging (CMR) have investigated the potential biventricular nature of LVNC. These studies revealed RV characteristics resembling those of the LV, namely, increased trabecular mass values, larger volumes, and reduced functional parameters [
6,
8]. This aspect is particularly studied in subjects with preserved LV_EF, as it allows the assessment of RV involvement without the confounding influence of impaired LV function. Despite these findings, evidence remains heterogeneous and often limited to conventional parameters, underscoring the need for more advanced imaging techniques to better characterize RV mechanics in LVNC.
Three-dimensional transthoracic echocardiography (3D TTE) has recently gained attention as a valuable imaging modality that enables comprehensive cardiac assessment by providing spatially detailed views of cardiac structures and motion [
9,
10]. Although it is less optimal for depicting fine trabecular details, 3D TTE offers high accuracy in quantifying functional and volumetric parameters (
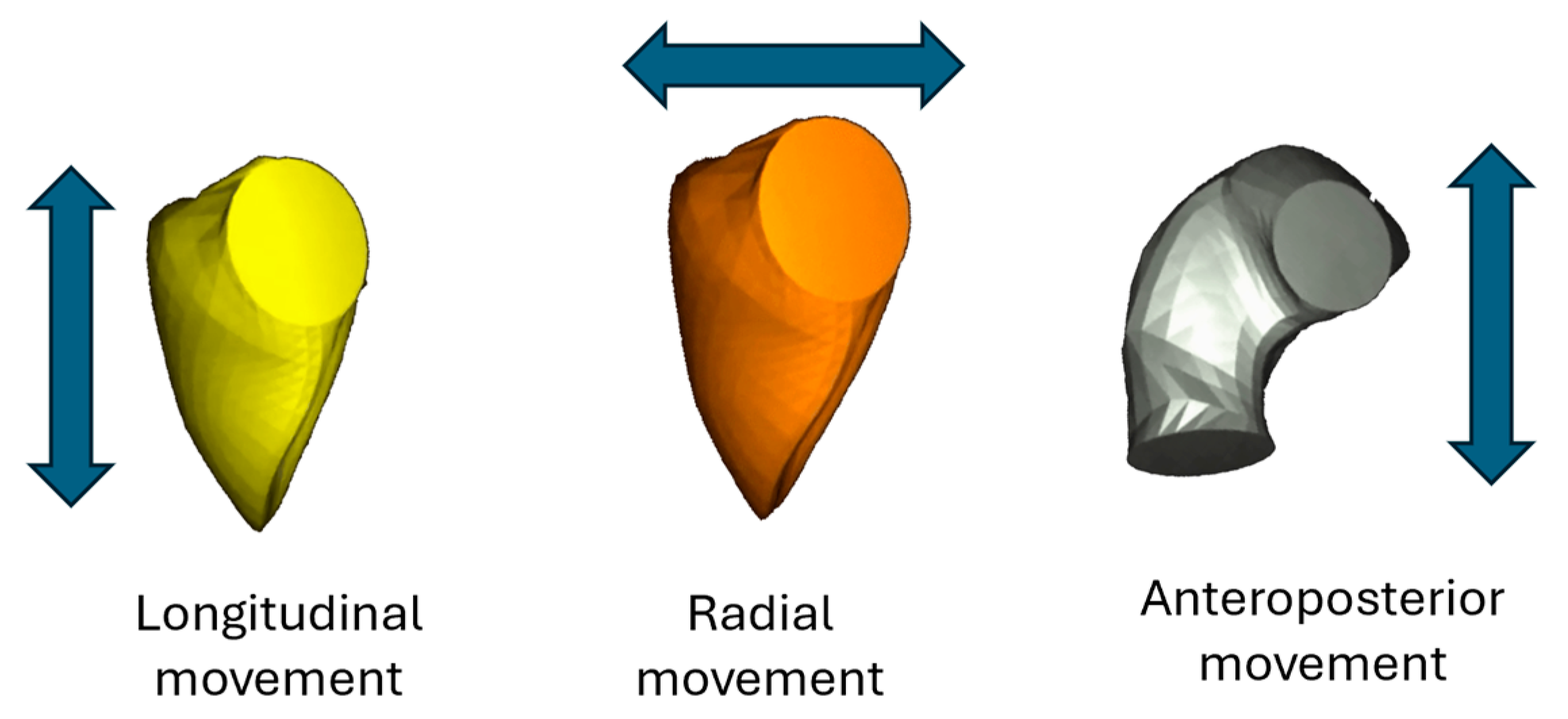
Figure 1), as also emphasized in recent guideline recommendations. A novel analytical approach based on 3D TTE facilitates the evaluation of right ventricular (RV) volumes, global function, and directional motion along three orthogonal planes that correspond to the anatomical fiber architecture of the RV [
11]. Prior studies across various cardiac conditions have indicated that alterations in RV motion patterns may hold prognostic relevance. Nevertheless, the specific features of RV mechanics, including directional contraction behavior, have not yet been systematically characterized in individuals with LVNC morphology.
Accordingly, our objective was to assess the volumetric, functional, and strain characteristics of both the left and right ventricles, as well as the detailed three-dimensional motion pattern of the RV in individuals with primary LVNC with preserved left ventricular ejection fraction (LV_EF > 50%) using 3D TTE based methods. The findings were subsequently compared to those obtained from an age- and sex-matched control (C) group.
2. Materials and Methods
From our registry of LVNC population, we enrolled 37 individuals (males n = 22, average age: 40.2 ± 15 years) with preserved left ventricular function who had undergone 3D TTE examinations. To create a control group (C), we also included 37 age- and sex-matched healthy volunteers without known cardiac or systemic disorders (males n = 22, average age: 40.3 ± 15 years).
Inclusion criteria for patients encompassed a diagnosis of LVNC confirmed by CMR, meeting both Petersen (ratio of noncompacted to compacted myocardial layer exceeding 2.3 at end-diastole) and Jacquier criteria (trabecular mass greater than 20% of total myocardial mass at end-diastole).
We excluded participants with reduced LV_EF (<50%), coronary artery disease, congenital heart disease, other forms of cardiomyopathies, or significant comorbidities (such as diabetes and untreated hypertension). Additionally, individuals engaged in physical training exceeding 6 h per week and those with images that could not be reliably processed for technical reasons were also excluded. The baseline characteristics of the population are shown in
Table 1.
This study involved human participants, all of whom provided written informed consent prior to enrollment. The study protocol adhered to the principles outlined in the Declaration of Helsinki and its subsequent amendments. Ethical approval was granted by the Central Ethics Committee of Hungary.
Three- and two-dimensional transthoracic echocardiographic (3D TTE) studies were performed using a GE Vivid E95 ultrasound system equipped with a 4Vc-D matrix-array transducer (GE Vingmed Ultrasound, Horten, Norway). Full-volume datasets of the left ventricle (LV) and right ventricle (RV) were acquired from an apical four-chamber view using ECG-gated, multibeat acquisition over four cardiac cycles.
All post-processing analyses were conducted by a single experienced operator using commercially available software (4D LV-Analysis (v. 3.1) and 4D RV-Function 2 (v. 2.2.4); TOMTEC Imaging Systems GmbH, Unterschleissheim, Germany). The LV datasets were evaluated first. The software automatically delineated the LV endocardial surface, which was subsequently adjusted manually as needed across long- and short-axis views throughout the cardiac cycle. Speckle tracking was used for the deformation analysis.
For the LV, volumetric indices—including end-diastolic volume (LV_EDV), end-systolic volume (LV_ESV), and stroke volume (LV_SV)—were calculated. Functional metrics such as ejection fraction (LV_EF), global longitudinal strain (LV_GLS), and global circumferential strain (LV_GCS) were also derived. All volumetric data were indexed to body surface area.
Following LV evaluation, the right ventricular (RV) 3D surface model was exported frame by frame throughout the cardiac cycle for further analysis using a dedicated post-processing tool (ReVISION—Right Ventricular Separate Wall Motion Quantification). This custom-designed software performs a vertex-based motion decomposition of the RV mesh model. Among the 2D parameters, the TAPSE and PAP were also calculated.
At each time point, directional volume changes were computed using the signed tetrahedron method, allowing motion to be separated along three anatomically defined orthogonal axes. This approach enabled the quantification of the individual contributions from longitudinal, radial, and anteroposterior wall displacement to overall RV volume change (
Figure 2).
Standard RV volumetric indices—including end-diastolic volume (RV_EDV), end-systolic volume (RV_ESV), and stroke volume (RV_SV)—were determined, along with global functional parameters such as ejection fraction (RV_EF), global longitudinal strain (RV_GLS), global circumferential strain (RV_GCS), and global area strain (RV_GAS). In addition, the longitudinal (LEF), radial (REF), and anteroposterior (AEF) components of ejection fraction were calculated separately. These were also expressed as ratios relative to RV_EF (LEF/RV_EF, REF/RV_EF, AEF/RV_EF), representing the directional contribution of each motion component to the overall RV function. Normative reference values for these parameters were adopted from Cotella et al. [
11].
The normality of data distribution was assessed using the Shapiro–Wilk test. For continuous variables with normal distribution, group comparisons were performed using the independent samples t-test; for non-normally distributed data, the Mann–Whitney U test was applied.
Relationships between continuous variables were explored using Pearson’s correlation analysis, with correlation strength interpreted as weak (<0.3), moderate (0.3–0.6), or strong (>0.6).
Interobserver agreement for key 3D echocardiographic parameters (e.g., RV EF, LEF, REF, AEF) was assessed using the two-way random-effects intraclass correlation coefficient (ICC), average measures, with corresponding 95% confidence intervals.
All tests were two-tailed, and a p-value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 28.0.1.0 (IBM Corp., Armonk, NY, USA) and Microsoft Excel for Microsoft 365 (Microsoft Corp., Redmond, WA, USA).
3. Results
The interobserver agreement of the LV and RV, as assessed by the ICC, was analyzed in ten randomly selected patients and ten healthy subjects. The results of the interobserver variability test are in
Table 2.
First, we analyzed the LV and RV volumetric and functional parameters. The LV volumes were significantly elevated, and the LV_EF and strain parameters were significantly decreased compared to the control group. It is worth noting that, except for the LV strain parameters, which were below the normal cutoff value, all the LV parameters remained within the normal range (
Table 2).
Examining the RV, no significant differences were found in volumetric parameters between the two groups, except the RV_EF, RV_GLS, and RV_GAS values of the LVNC group, which were significantly reduced compared to the controls (
Table 3).
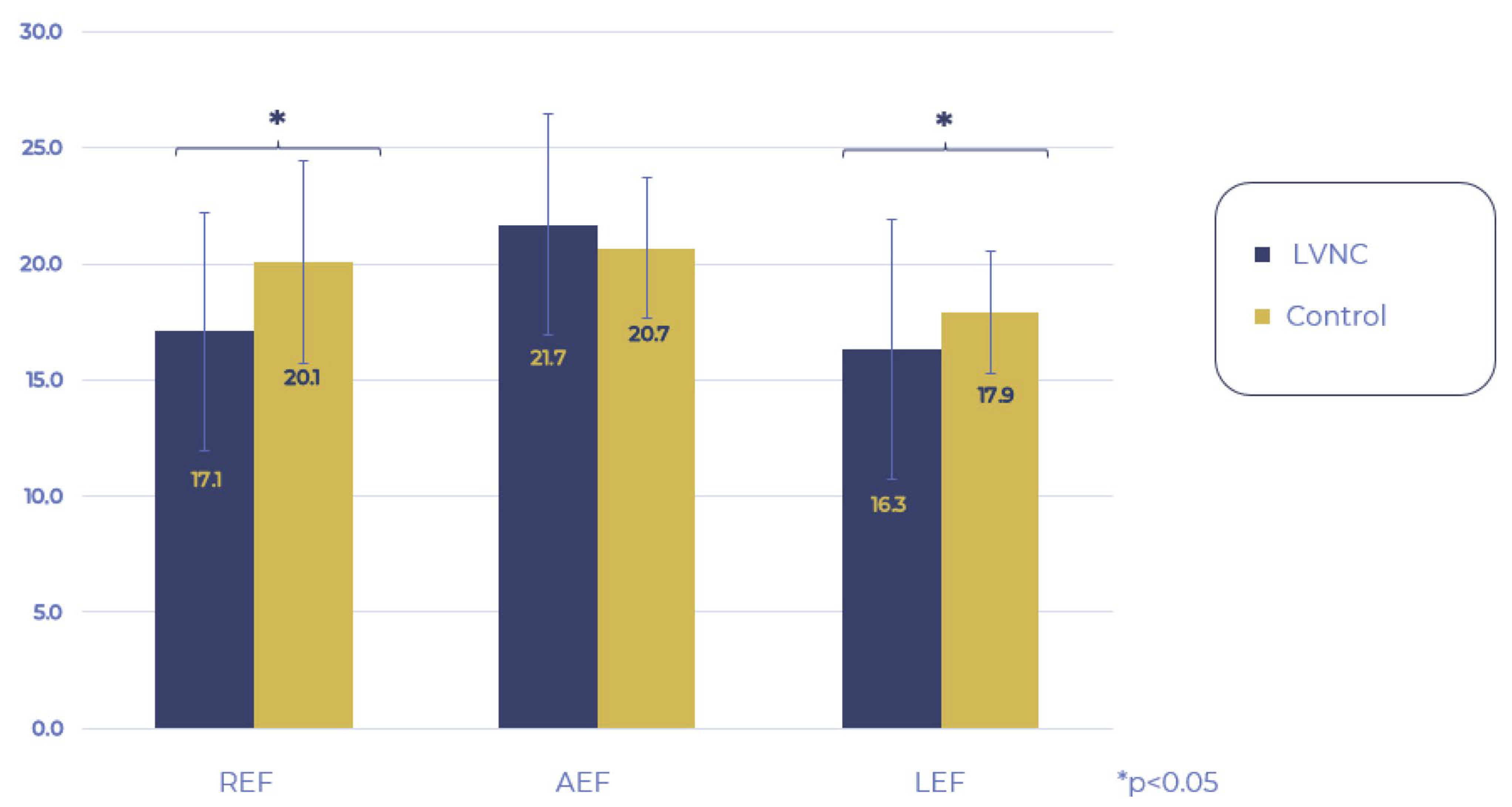
Then, we examined the movement pattern of the RV. When comparing the LEF, REF, and AEF of the LVNC and control groups, the REF and LEF components remained within the normal ranges but were significantly decreased compared to the control group, while AEF contraction remained unchanged. Analyzing the proportion of the three directions regarding the global RV_EF, the AEF had a significantly higher share in the LVNC group compared to the control group, whereas the contribution of REF and LEF contractions did not differ between the two groups (
Figure 3 and
Figure 4).
Finally, when studying the correlation of volumetric and functional parameters between the LV and RV, we found a moderately strong correlation in the case of EDV and SV, and it is noteworthy that almost all functional parameters showed a strong positive correlation between the two ventricles (
Table 4). Then, we examined the relationship of the RV’s three-directional motion components with left and right ventricular volumetric and functional parameters. While among the LV parameters, only the EF correlated with AP contraction, the RV parameters mainly correlated with the directions of contraction, most notably also with the AEF (
Table 5 and
Table 6).
As a supplementary observation, both the TAPSE and PAP values of LVNC patients were within the normal range (TAPSE: 26 ± 8 mm, PAP: 23 ± 8 Hgmm).
4. Discussion
This study used three-dimensional echocardiography to assess the characteristics of the left- and right ventricle, especially the 3D movement pattern of the RV in LVNC subjects with preserved LV_EF compared to healthy controls.
Regarding the LV, our results are in line with previous studies using CMR and cardiac ultrasound; namely, the volumes were increased, and the functional parameters were decreased compared to the controls [
6,
8]. Studying RV involvement in the LVNC population, our findings align with our previous investigation using 3D_TTE, as we also identified moderately decreased RV function [
6]. Previous CMR studies have also revealed subclinical changes, such as elevated RV volumetric parameters and decreased EF and strain values in LVNC individuals with both preserved and decreased LV_EF [
8]. This underscores the diagnostic complexity of LVNC, particularly in distinguishing pathologic forms from physiological remodeling [
12]. Interestingly, Stämpfli et al. concluded that quantifying RV trabeculation does not facilitate the differentiation between LVNC and healthy subjects, emphasizing the importance of focusing on the ventricle’s functional characteristics [
13].
The prognostic value of decreased RV function has been described in several studies in different pathologies [
14,
15,
16]. A study by Wang et al. involving 117 LVNC participants discovered that RV dysfunction was a strong predictor of all-cause mortality, independent of LV function. Moreover, impaired RV_GLS was another independent predictor of mortality as the authors concluded that RV dysfunction is a common and prognostic factor in patients with LVNC and suggested a regular and quantitative assessment of RV function in this population.
A new innovative technique with growing literature has the advantage of specifically characterizing RV function as it provides a complex 3D movement pattern of the RV. In this study, we found AEF-dominated compensation in the LVNC group compared to the controls.
In contrast to our investigation, Surkova and Kovács et al. found that in the case of decreasing LV_EF caused by various etiology, the LEF and AEF components of the global RV contraction showed notable decreases with a concurrent increase in the REF component to maintain the RV_EF. This mechanism could be in the background, as suggested by previous CMR studies reporting slightly decreased but still normal RV_EF in LVNC patients with reduced LV_EF.
In a study with a healthy population, Lakatos et al. found the proportion of the three contraction directions balanced [
17]. This publication also revealed that in patients with reduced LV function caused by various etiology, the REF compensation dominated with the early reduction in LEF and AEF, and these findings were also confirmed by other large case–control studies [
18,
19]. On the contrary, in pathologies associated with elevated RV strain, e.g., pulmonary embolism and atrial septal defect, longitudinal compensation was particularly pronounced independently from the LV function [
17]. Tokodi et al. also examined patients’ RV movement patterns before and after mitral valve replacement (MVR), and they found LEF dominated before MVR, which was turned into a radial compensation in the immediate postoperative period. Interestingly, after three months, the REF/RV_EF ratio was similar to that of healthy subjects. This study also suggests that preoperative 3D_TTE parameters may be prognostic for postoperative RV dysfunction [
6].
Among individuals with normal RV ejection fraction, the AEF emerged as a significant and independent predictor of adverse outcomes [
18]. Since AEF was normal in our study population, it may indicate a good prognosis in LVNC subjects with preserved ejection fraction. Moreover, Gregor et al. found the global RV function of LVNC patients to be preserved regardless of the LVEF being reduced or preserved, which might suggest a longer RV compensation in LVNC compared to other heart diseases.
In summary, this AEF-dominated movement has not been documented previously in the LVNC population; moreover, it differs from the RV movement patterns described in other diseases; thus, this might be a disease-specific pattern. Thus, our findings highlight the potential clinical relevance of 3D echocardiographic analysis. The identification of this subclinical RV dysfunction would serve as an early marker of the progression of this condition. Detecting these compensatory patterns may help to identify patients who could benefit from closer follow-up or additional diagnostic imaging, even in the absence of overt clinical deterioration. This directional analysis, extending beyond conventional 2D metrics, could support individualized risk stratification in line with contemporary ESC recommendations. Larger studies are warranted to confirm these observations and clarify the prognostic value of AEF in this population.
Limitations
The sample size was relatively small, reflecting the rarity of primary LVNC and the strict inclusion criteria. Nevertheless, the subgroup with preserved EF was derived from a well-established single-center registry, and future analyses will include patients with reduced LV function to investigate the impact of impaired EF on RV motion patterns.
The cross-sectional design limits conclusions regarding the temporal evolution or prognostic significance of the observed RV motion characteristics. However, longitudinal follow-up of this cohort is ongoing and will allow for serial 3D echocardiographic assessments.
While 3D echocardiography is increasingly adopted in clinical practice, its accuracy remains dependent on image quality and acoustic windows. In this study, only datasets with sufficient image quality were included to ensure reliable quantification. Additionally, the lack of laboratory data limits our ability to correlate functional findings with systemic biomarkers.