Differentiation of Benign and Malignant Neck Neoplastic Lesions Using Diffusion-Weighted Magnetic Resonance Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Conventional MRI
2.2. DWI MRI
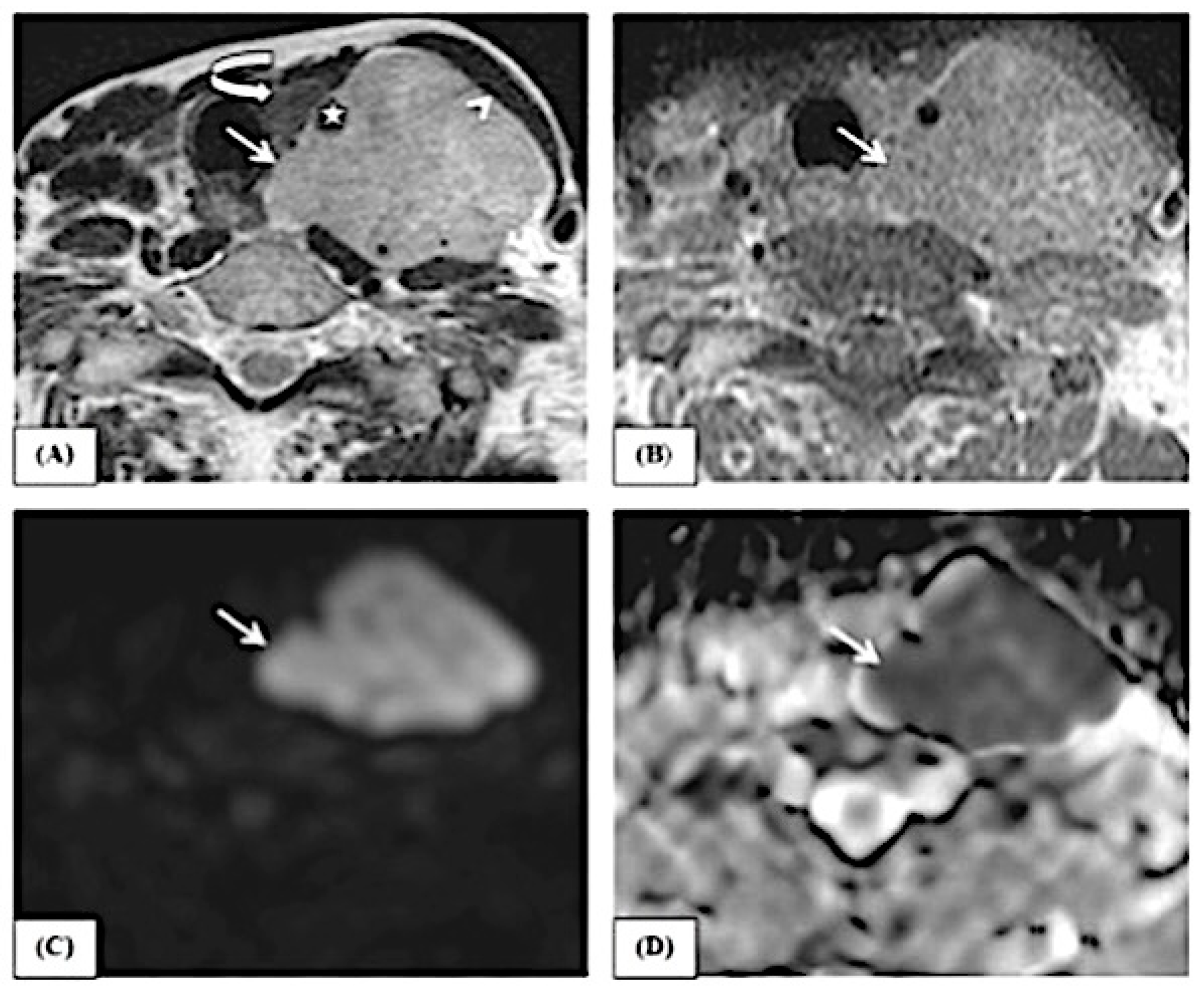
2.2.1. Image Analysis
2.2.2. Statistical Analysis
3. Results
Overall ADCs
4. Discussion
5. Conclusions
- The qualitative evaluation of diffusion-weighted imaging (DWI) and the results obtained from the apparent diffusion coefficient (ADC) map can effectively distinguish between benign and malignant neck tumors by using a threshold value of 1.1 mm2/s.
- The qualitative assessment of DWI and the values of the ADC map can differentiate between squamous cell carcinoma and non-Hodgkin lymphoma with a cutoff value of 0.7 mm2/s.
- The quantitative assessment of the ADC value is more important than the qualitative assessment of the DWI in the characterization of the lesion.
- Adding a DWI sequence to conventional MRI in examining clinically detected neck lesions adds little to the examination time but adds much to the diagnostic performance; thus, we recommend using DWI MRI whenever there are unsolved or confusing cases by conventional imaging techniques.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razek, A.A. Diffusion-weighted magnetic resonance imaging of head and neck. J. Comput. Assist. Tomogr. 2010, 34, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Kim, S.; Wang, S.; Poptani, H. Diffusion-weighted imaging in head and neck cancers. Future Oncol. 2009, 5, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Dvorak, R.; Perni, K.; Rohrer, S.; Mukherji, S.K. Differentiation of benign and malignant pathology in the head and neck using 3T apparent diffusion coefficient values: Early experience. AJNR Am. J. Neuroradiol. 2008, 29, 40–44. [Google Scholar] [CrossRef]
- Imaizumi, A.; Yoshino, N.; Yamada, I.; Nagumo, K.; Amagasa, T.; Omura, K.; Okada, N.; Kurabayashi, T. A potential pitfall of MR imaging for assessing mandibular invasion of squamous cell carcinoma in the oral cavity. AJNR Am. J. Neuroradiol. 2006, 27, 114–122. [Google Scholar] [PubMed]
- Ahmad, A.; Branstetter, B.F. CT versus MR: Still a tough decision. Otolaryngol. Clin. N. Am. 2008, 41, 1–22. [Google Scholar] [CrossRef]
- Star-Lack, J.M.; Adalsteinsson, E.; Adam, M.F.; Terris, D.J.; Pinto, H.A.; Brown, J.M.; Spielman, D.M. In Vivo 1H MR spectroscopy of human head and neck lymph node metastasis and comparison with oxygen tension measurements. AJNR Am. J. Neuroradiol. 2000, 21, 183–193. [Google Scholar]
- Shukla-Dave, A.; Poptani, H.; Loevner, L.A.; Mancuso, A.; Serrai, H.; Rosenthal, D.I.; Kilger, A.M.; Nelson, D.S.; Zakian, K.L.; Arias-Mendoza, F.; et al. Prediction of treatment response of head and neck cancers with P-31 MR spectroscopy from pretreatment relative phosphomonoester levels. Acad. Radiol. 2002, 9, 688–694. [Google Scholar] [CrossRef]
- Wang, J.; Takashima, S.; Takayama, F.; Kawakami, S.; Saito, A.; Matsushita, T.; Momose, M.; Ishiyama, T. Head and neck lesions: Characterization with diffusion-weighted echo-planar MR imaging. Radiology 2001, 220, 621–630. [Google Scholar] [CrossRef]
- Vandecaveye, V.; De Keyzer, F.; Nuyts, S.; Deraedt, K.; Dirix, P.; Hamaekers, P.; Poorten, V.V.; Delaere, P.; Hermans, R. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: Correlation between radiologic and histopathologic findings. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 960–971. [Google Scholar] [CrossRef]
- Hermans, R.; Vandecaveye, V. Diffusion-weighted MRI in head and neck cancer. JBR-BTR 2007, 90, 264–267. [Google Scholar] [CrossRef][Green Version]
- Maeda, M.; Maier, S.E. Usefulness of diffusion-weighted imaging and the apparent diffusion coefficient in the assessment of head and neck tumors. J. Neuroradiol. 2008, 35, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.M.; Matzek, W.; Gentzsch, S.; Sulzbacher, I.; Czerny, C.; Herneth, A.M. Diffusion-weighted magnetic resonance imaging of head and neck squamous cell carcinomas. Eur. J. Radiol. 2008, 68, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, D.; Pace, A.; Iannella, G.; Rossetti, V.; Colizza, A.; Visconti, I.C.; Gulotta, G.; Messineo, D.; de Vincentiis, M.; Greco, A.; et al. Chronic obstructive Eustachian tube dysfunction: CT assessment with Valsalva maneuver and ETS-7 score. PLoS ONE 2021, 16, e0247708. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Galban, C.J.; Johnson, T.D.; Chenevert, T.L.; Ross, B.D.; Mukherji, S.K. Utility of the k-means clustering algorithm in differentiating apparent diffusion coefficient values of benign and malignant neck pathologies. AJNR Am. J. Neuroradiol. 2010, 31, 736–740. [Google Scholar] [CrossRef][Green Version]
- Iannella, G.; Stasolla, A.; Pasquariello, B.; Re, M.; Magliulo, G. Tympanomastoid cholesterol granuloma: radiological and intraoperative findings of blood source connection. Eur. Arch. Otorhinolaryngol. 2016, 273, 2395–2401. [Google Scholar] [CrossRef]
- ElSaid, N.A.; Nada, O.M.; Habib, Y.S.; Semeisem, A.R.; Khalifa, N.M. Diagnostic accuracy of diffusion weighted MRI in cervical lymphadenopathy cases correlated with pathology results. Egypt. J. Radiol. Nuclear Med. 2014, 45, 1115–1125. [Google Scholar] [CrossRef][Green Version]
- Choi, K.D.; Jo, J.W.; Park, K.P.; Kim, J.S.; Lee, T.H.; Kim, H.J.; Jung, D.S. Diffusion-weighted imaging of intramural hematoma in vertebral artery dissection. J. Neurol. Sci. 2007, 253, 81–84. [Google Scholar] [CrossRef]
- Perrone, A.; Guerrisi, P.; Izzo, L.; D’Angeli, I.; Sassi, S.; Mele, L.L.; Marini, M.; Mazza, D. Diffusion-weighted MRI in cervical lymph nodes: Differentiation between benign and malignant lesions. Eur. J. Radiol. 2011, 77, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, K.; Duetsch, S.; Fauser, C.; Eiber, M.; Rummeny, E.J.; Gaa, J. Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur. J. Radiol. 2009, 72, 381–387. [Google Scholar] [CrossRef]
- Ali, T.F.T. Neck lymph nodes: Characterization with diffusion-weighted MRI. Egypt. J. Radiol. Nuclear Med. 2012, 43, 173–181. [Google Scholar]
- de Bondt, R.B.; Hoeberigs, M.C.; Nelemans, P.J.; Deserno, W.M.; Peutz-Kootstra, C.; Kremer, B.; Beets-Tan, R.G.H. Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology 2009, 51, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Pace, A.; Mucchino, A.; Greco, A.; De Virgilio, A.; Lechien, J.R.; Maniaci, A.; Cocuzza, S.; Perrone, T.; Messineo, D.; Magliulo, G. A new 3D-printed temporal bone: ’the SAPIENS’-specific anatomical printed-3D-model in education and new surgical simulations. Eur Arch Otorhinolaryngol. 2024, 281, 4617–4626. [Google Scholar] [CrossRef]
- Ai, S.; Zhu, W.; Liu, Y.; Wang, P.; Yu, Q.; Dai, K. Combined DCE- and DW-MRI in diagnosis of benign and malignant tumors of the tongue. Front. Biosci. (Landmark Ed.) 2013, 18, 1098–1111. [Google Scholar] [PubMed]
- Li, S.; Cheng, J.; Zhang, Y.; Zhang, Z. Differentiation of benign and malignant lesions of the tongue by using diffusion-weighted MRI at 3.0 T. Dentomaxillofac. Radiol. 2015, 44, 20140325. [Google Scholar] [CrossRef] [PubMed]
- Habermann, C.R.; Gossrau, P.; Graessner, J.; Arndt, C.; Cramer, M.C.; Reitmeier, F.; Jaehne, M.; Adam, G. Diffusion-weighted echo-planar MRI: A valuable tool for differentiating primary parotid gland tumors? Rofo 2005, 177, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.; Sakihama, N.; Sumi, T.; Morikawa, M.; Uetani, M.; Kabasawa, H.; Shigeno, K.; Hayashi, K.; Takahashi, H.; Nakamura, T. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am. J. Neuroradiol. 2003, 24, 1627–1634. [Google Scholar]
- Abdel Razek, A.A.; Soliman, N.Y.; Elkhamary, S.; Alsharaway, M.K.; Tawfik, A. Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur. Radiol. 2006, 16, 1468–1477. [Google Scholar] [CrossRef]
- Steens, S.C.; Admiraal-Behloul, F.; Schaap, J.A.; Hoogenraad, F.G.; Wheeler-Kingshott, C.A.; Le Cessie, S.; Tofts, P.S.; Van Buchem, M.A. Reproducibility of brain ADC histograms. Eur. Radiol. 2004, 14, 425–430. [Google Scholar] [CrossRef]




| Complaint | No. | % |
|---|---|---|
| Neck swelling | 16 | 30.2 |
| Lingual mass | 9 | 17.0 |
| Hoarseness of voice | 9 | 17.0 |
| Retromolar ulcer | 4 | 7.5 |
| Floor of mouth mass | 2 | 3.8 |
| Headache | 2 | 3.8 |
| Parotid mass | 2 | 3.8 |
| Bleeding per nose | 1 | 1.9 |
| Multiple neck swellings | 1 | 1.9 |
| Nasal obstruction | 1 | 1.9 |
| Cheek swelling | 1 | 1.9 |
| Upper lip mass | 1 | 1.9 |
| Immobile tongue | 1 | 1.9 |
| Mandibular mass | 1 | 1.9 |
| Thyroid mass | 1 | 1.9 |
| Submandibular mass | 1 | 1.9 |
| Complaint | No. | % |
|---|---|---|
| Squamous cell carcinoma | 19 | 35.8 |
| NHL | 9 | 17.0 |
| Schwannoma | 5 | 9.4 |
| Inflammatory tongue ulcer | 4 | 7.5 |
| Thyroid cancer | 3 | 5.7 |
| Venolymphatic malformation | 2 | 3.8 |
| Glomus tumor | 2 | 3.8 |
| Lymphoid hyperplasia | 2 | 3.8 |
| Neurofibromas | 1 | 1.9 |
| Whartin | 1 | 1.9 |
| Inflammatory lymph node | 1 | 1.9 |
| Metastatic Nodal SCC | 2 | 3.8 |
| Parotid cancer | 1 | 1.9 |
| Acinic cell carcinoma of the left sublingual gland | 1 | 1.9 |
| ADC Value (Average) | Total (n = 53) | Diagnosis | U | p | |
|---|---|---|---|---|---|
| Benign (n = 18) | Malignant (n = 35) | ||||
| Min.–Max. | 0.40–2.53 | 0.57–2.53 | 0.40–2.0 | 86.0 * | 0.001 * |
| Mean ± SD. | 1.02 ± 0.45 | 1.43 ± 0.57 | 0.86 ± 0.28 | ||
| Median | 0.93 | 1.43 | 0.87 | ||
| AUC | P | 95% CI | Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | |||||||||
| Average ADC value | 0.811 * | 0.001 * | 0.628 | 0.994 | ≤1.1 | 97.14 | 77.78 | 91.9 | 90.9 | 86.2 |
| AUC | P | 95% CI | Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | |||||||||
| Average ADC value | 0.965 * | <0.001 * | 0.898 | 1.032 | ≤0.7 | 100.0 | 89.47 | 81.8 | 100.0 | 92.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamaleldin, O.; Iannella, G.; Cavalcanti, L.; Desouky, S.; Shama, S.; Gamaleldin, A.; Elwany, Y.; Magliulo, G.; Greco, A.; Pace, A.; et al. Differentiation of Benign and Malignant Neck Neoplastic Lesions Using Diffusion-Weighted Magnetic Resonance Imaging. J. Imaging 2024, 10, 257. https://doi.org/10.3390/jimaging10100257
Gamaleldin O, Iannella G, Cavalcanti L, Desouky S, Shama S, Gamaleldin A, Elwany Y, Magliulo G, Greco A, Pace A, et al. Differentiation of Benign and Malignant Neck Neoplastic Lesions Using Diffusion-Weighted Magnetic Resonance Imaging. Journal of Imaging. 2024; 10(10):257. https://doi.org/10.3390/jimaging10100257
Chicago/Turabian StyleGamaleldin, Omneya, Giannicola Iannella, Luca Cavalcanti, Salaheldin Desouky, Sherif Shama, Amel Gamaleldin, Yasmine Elwany, Giuseppe Magliulo, Antonio Greco, Annalisa Pace, and et al. 2024. "Differentiation of Benign and Malignant Neck Neoplastic Lesions Using Diffusion-Weighted Magnetic Resonance Imaging" Journal of Imaging 10, no. 10: 257. https://doi.org/10.3390/jimaging10100257
APA StyleGamaleldin, O., Iannella, G., Cavalcanti, L., Desouky, S., Shama, S., Gamaleldin, A., Elwany, Y., Magliulo, G., Greco, A., Pace, A., Virgilio, A. D., Maniaci, A., Lavalle, S., Messineo, D., & Bahgat, A. (2024). Differentiation of Benign and Malignant Neck Neoplastic Lesions Using Diffusion-Weighted Magnetic Resonance Imaging. Journal of Imaging, 10(10), 257. https://doi.org/10.3390/jimaging10100257











