An Investigation into Sustainable Solutions: Utilizing Hydrated Lime Derived from Oyster Shells as an Eco-Friendly Alternative for Semiconductor Wastewater Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Compositions of Semiconductor Wastewater
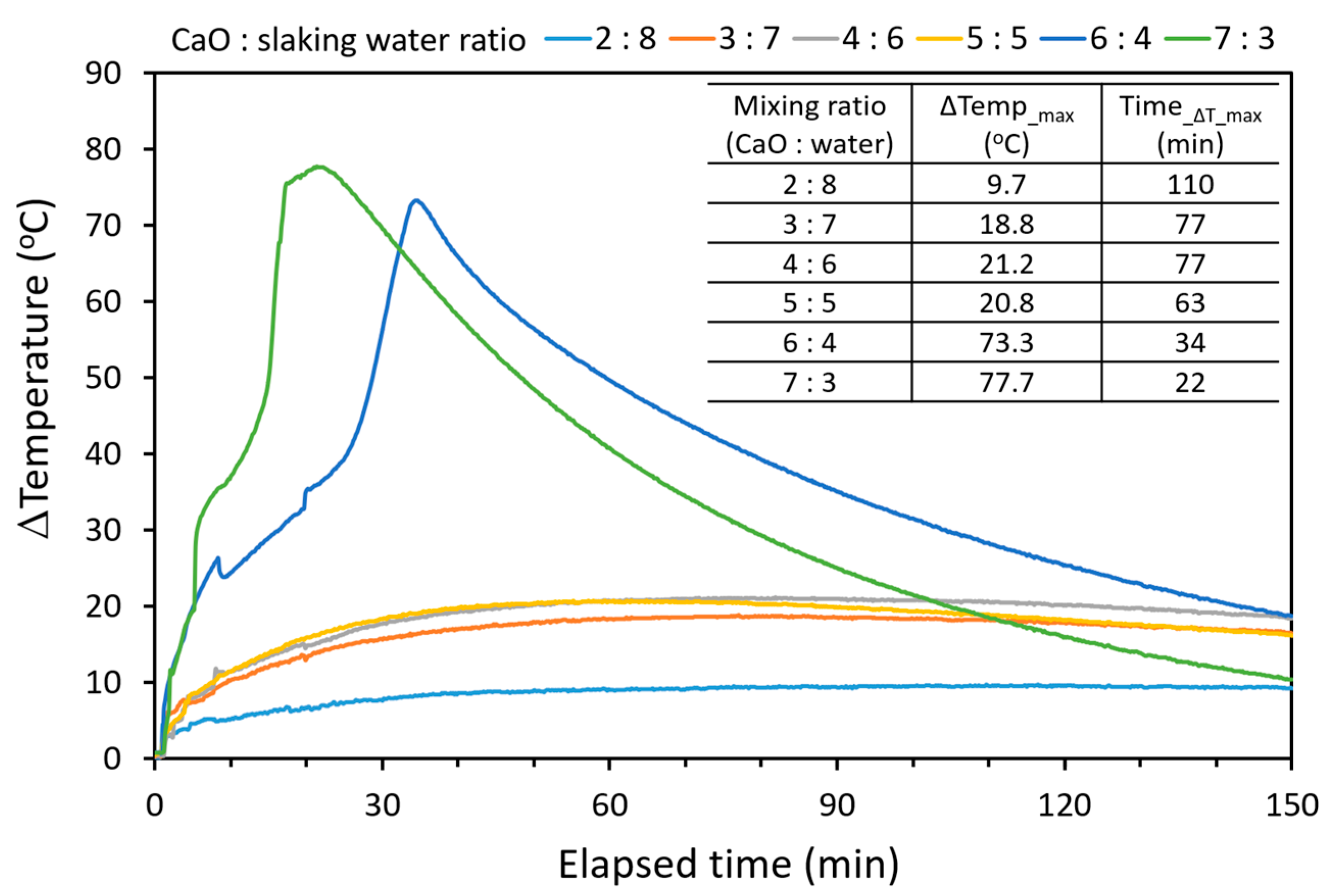
2.2. Hydration of CaO from Calcined Oyster Shell and Limestone
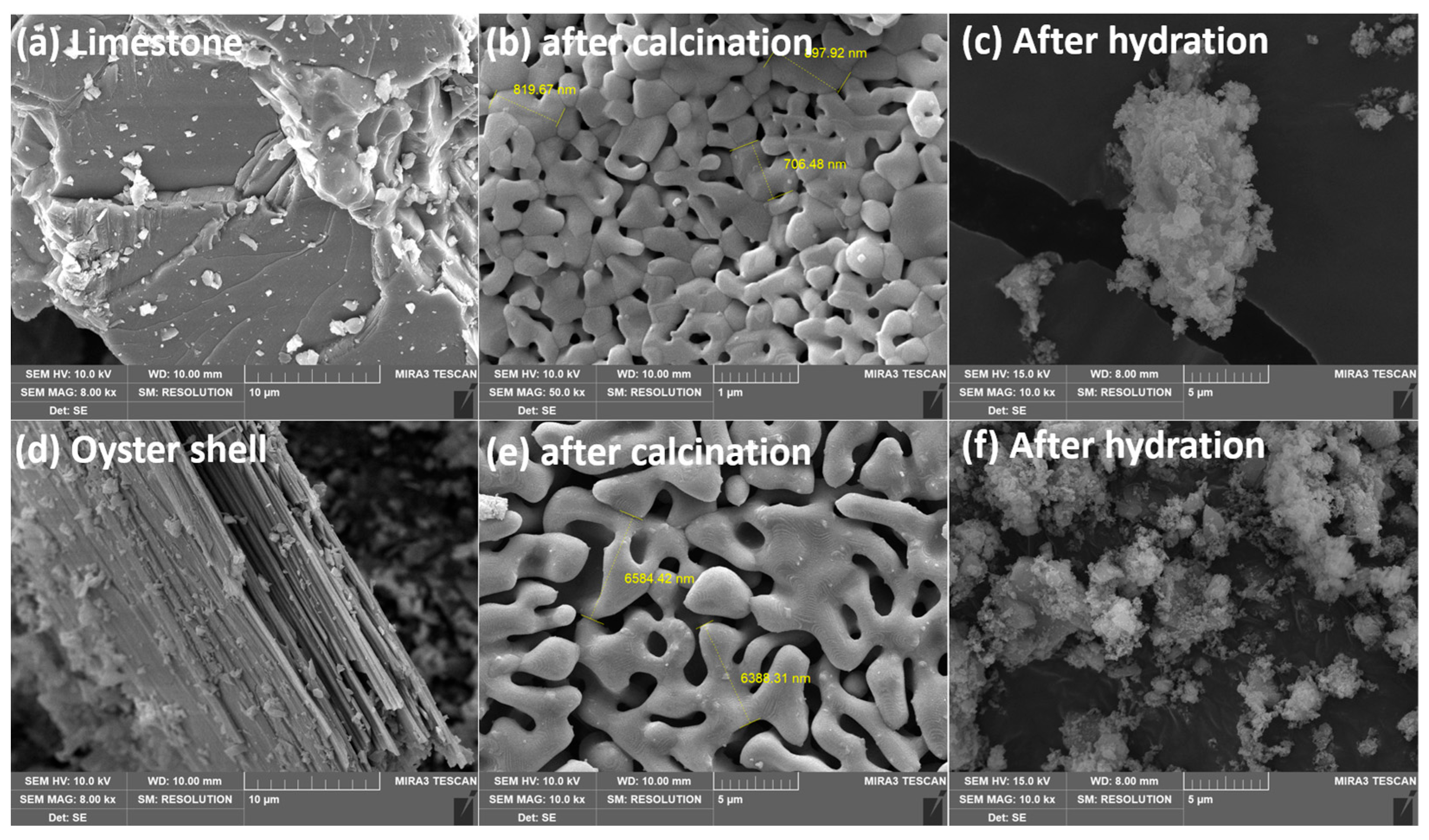
2.3. SEM Images for Quick and Hydrated Lime
2.4. Particle Distribution of Hydrated Lime
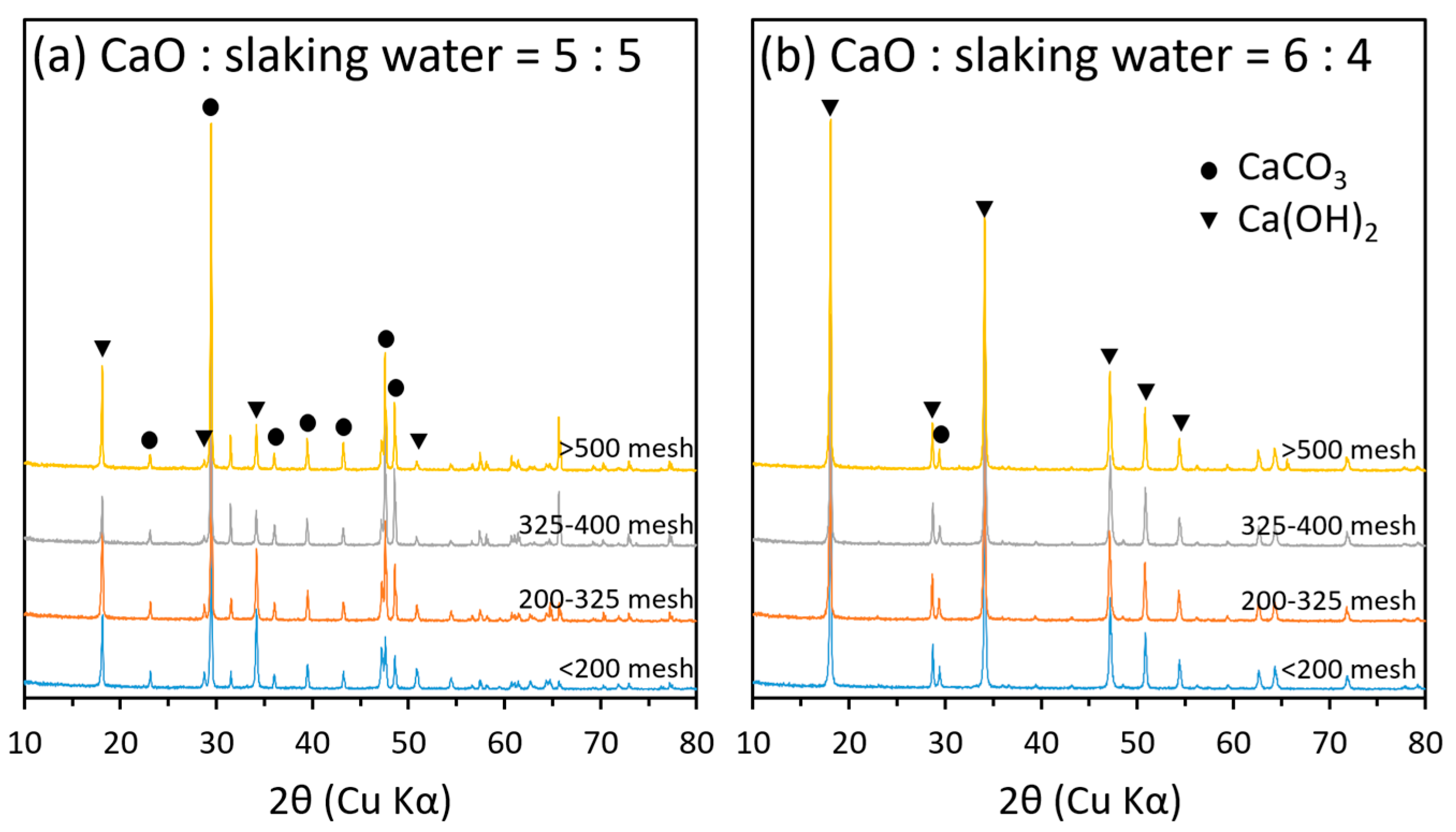
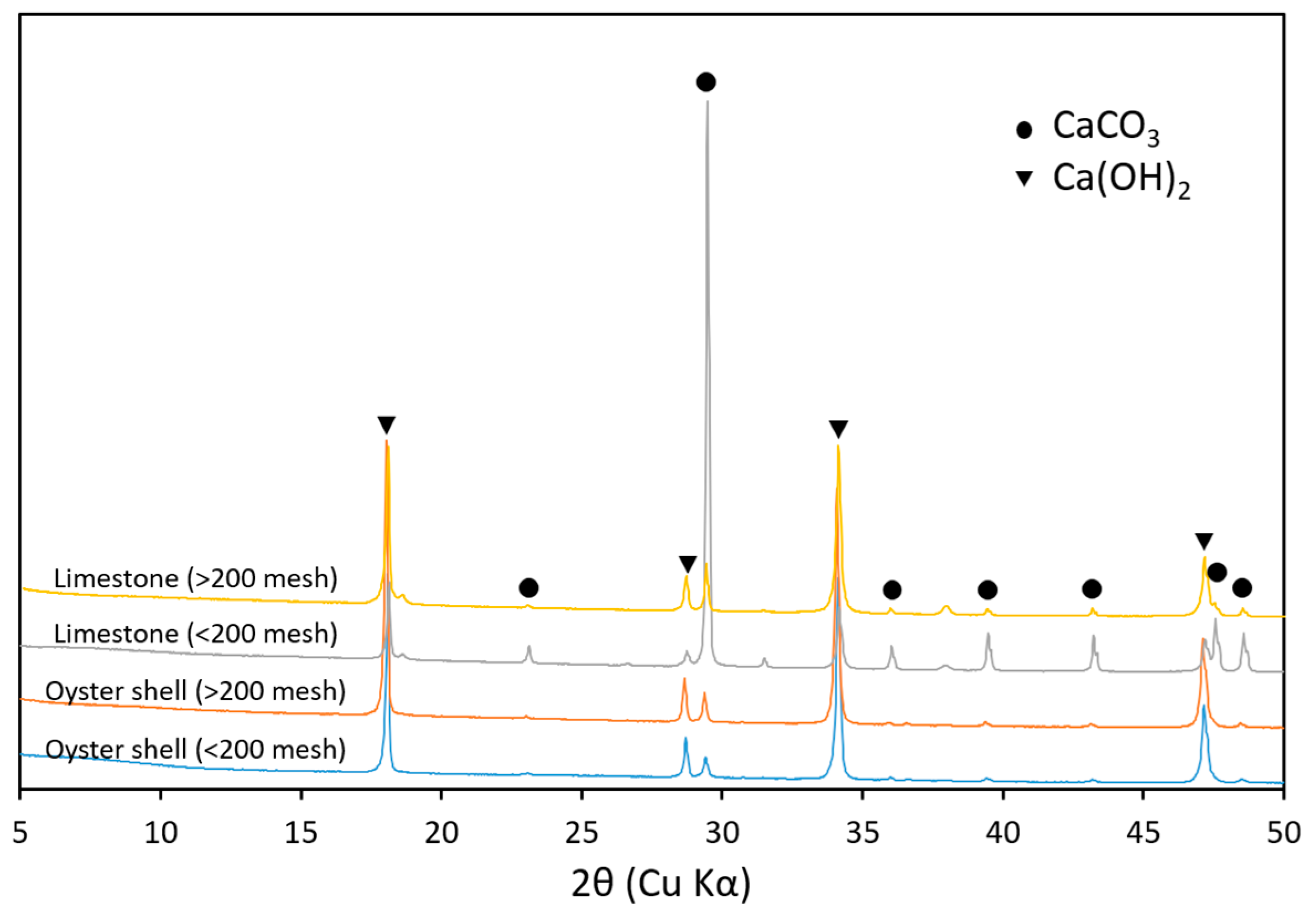
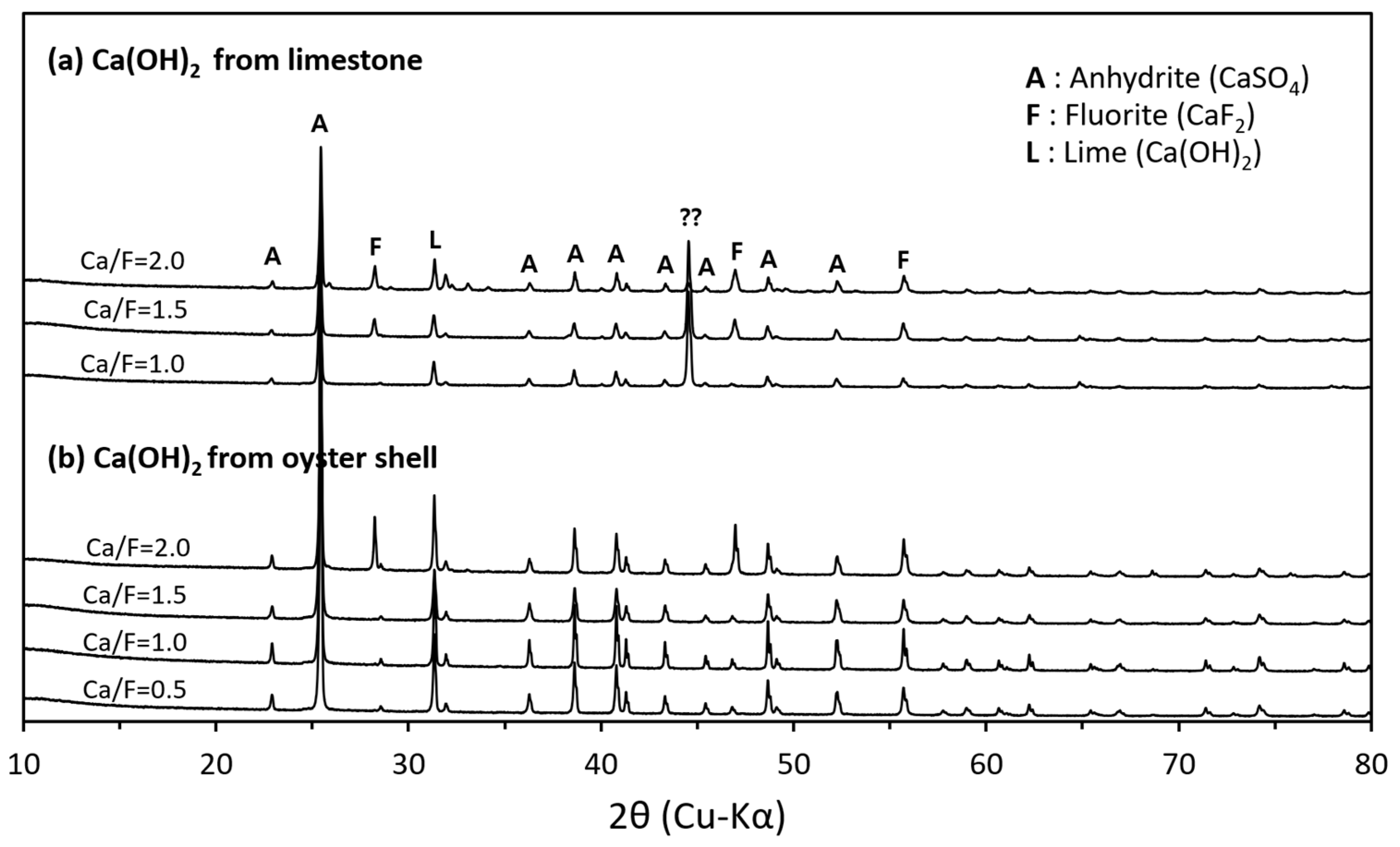
2.5. Mineralogical Properties of Hydrated Lime Derived from Oyster Shells
2.6. Fluoride Removal of Hydrated Lime
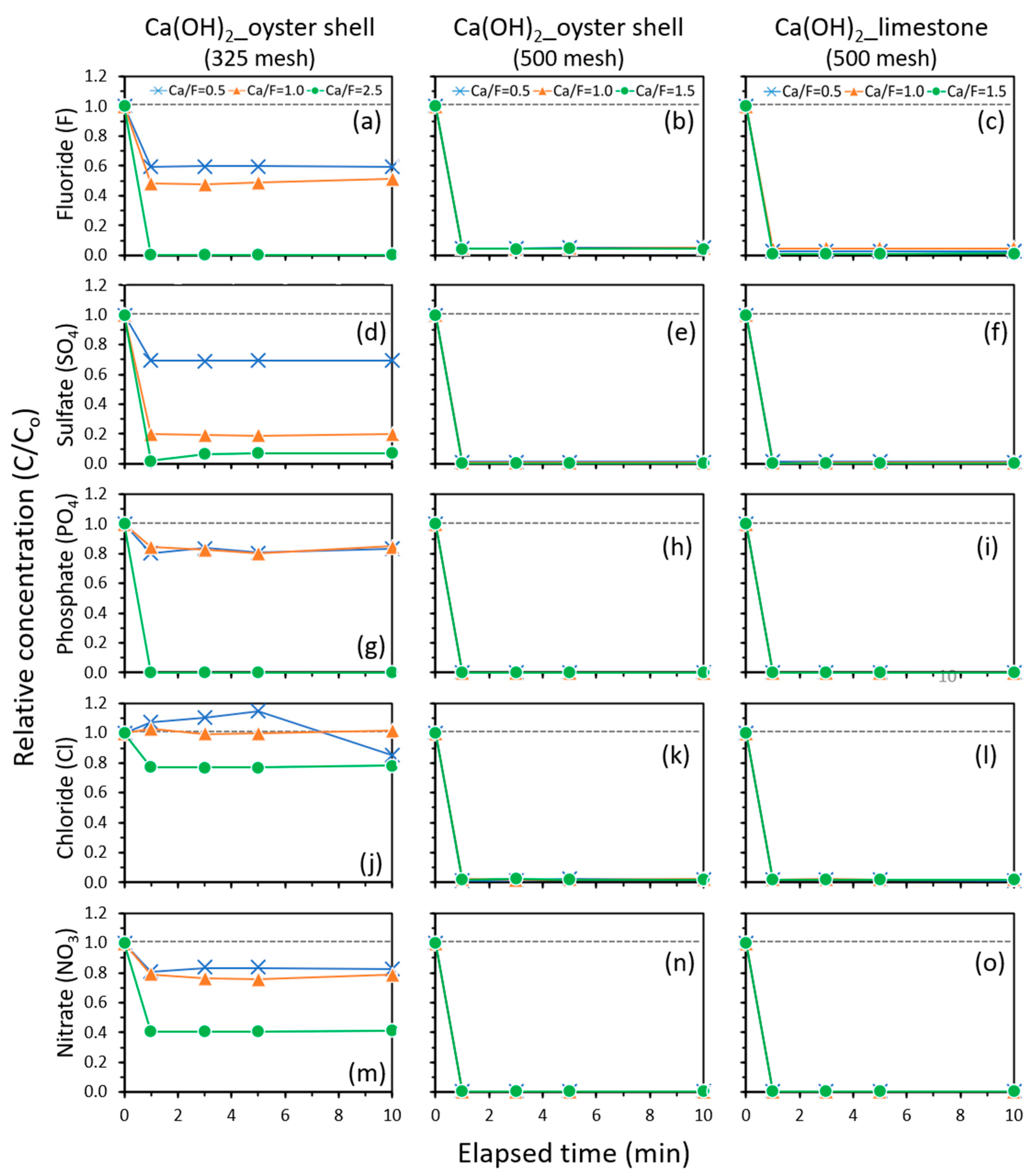
2.6.1. Comparative Results for Oyster Shell and Limestone
2.6.2. pH Variations of Hydrated Lime Produced from Oyster Shells and Limestones
2.6.3. Effects of the Ca/F Molar Ratio
2.7. Mineralogical Characteristics of the Recovered Solid
3. Materials and Methods
3.1. Preparation of Hydrated Lime
3.2. Experiment for Fluoride Removal
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Magara, Y. Fluoride in Drinking-Water; IWA Publishing: London, UK, 2006. [Google Scholar]
- Everett, E.T. Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J. Den. Res. 2011, 90, 552–560. [Google Scholar] [CrossRef] [PubMed]
- McClean, B. Semiconductor Units Forecast to Exceed 1 Trillion Devices Again in 2021—A 13% Increase Is Expected to Lift Total Semiconductor Shipments to a New Record High; IC Insights: Scottsdale, AR, USA, 2021; Available online: https://www.icinsights.com/files/data/articles/documents/1363.pdf (accessed on 16 February 2024).
- Tsai, W.T.; Chen, H.P.; Hsien, W.Y. A review of uses, environmental hazards and recovery/recycle technologies of perfluorocarbons (PFCs) emissions from the semiconductor manufacturing processes. J. Loss Prev. Process Ind. 2002, 15, 65–75. [Google Scholar] [CrossRef]
- Wald, P.H.; Jones, J.R. Semiconductor manufacturing: An introduction to processes and hazards. Am. J. Ind. Med. 1987, 11, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Lee, J.; Rho, H.; Park, K.D.; Choi, Y.; Kim, D.; Kim, H.; Woo, Y.C. A review of semiconductor wastewater treatment processes: Current status, challenges, and future trends. J. Clean. Prod. 2023, 429, 139570. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Paudyal, H.; Inoue, K.; Kawakita, H.; Ohto, K.; Kamata, H.; Alam, S. Removal of fluoride by effectively using spent cation exchange resin. J. Mater. Cycl. Waste Manag. 2018, 20, 975–984. [Google Scholar] [CrossRef]
- Won, C.H.; Choi, J.; Chung, J. Evaluation of optimal reuse system for hydrofluoric acid wastewater. J. Hazard. Mater. 2012, 239, 110–117. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Lacson, C.F.Z.; Lu, M.C.; Huang, Y.H. Chemical precipitation at extreme fluoride concentration and potential recovery of CaF2 particles by fluidized-bed homogenous crystallization process. Chem. Eng. J. 2021, 415, 128917. [Google Scholar] [CrossRef]
- Ezzeddine, A.; Meftah, N.; Hannachi, A. Removal of fluoride from a industrial wastewater by a hybrid process combining precipitation and reverse osimosis. Desalin. Water Treat. 2015, 55, 2618–2625. [Google Scholar] [CrossRef]
- Katarzyna, M.N.; Martyna, G.; Malgorzata, K.K. Removal of fluoride ions by batch electrodialysis. Environ. Prot. Eng. 2015, 41, 67–81. [Google Scholar]
- Huang, C.J.; Liu, J.C. Precipitate flotation of fluoride-containing wastewater from a semiconductor manufacturer. Water Res. 1999, 33, 3403–3412. [Google Scholar] [CrossRef]
- Lu, N.C.; Liu, J.C. Removal of phosphate and fluoride from wastewater by a hybrid precipitation-microfiltration process. Sep. Purif. Technol. 2010, 74, 329–335. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, B.U.; Lee, J.W.; Cha, W.S.; Kim, K.; Moon, B.K. Evaluation of SO2 absorption efficiency for calcined oyster shell slurry using a simulated spray type-flue gas desulfurization (FGD) system: A comparative study with limestone slurry. Econ. Environ. Geol. 2019, 52, 119–128. [Google Scholar]
- Lim, J.; Cho, H.; Kim, J. Optimization of wet flue gas desulfurization system using recycled waste oyster shell as high-grade limestone substitutes. J. Clean. Prod. 2021, 318, 128492. [Google Scholar] [CrossRef]
- Kim, W.; Sigh, R.; Smith, J.A. Modified crushed oyster shells for fluoride removal from water. Sci. Rep. 2020, 10, 5759. [Google Scholar] [CrossRef]
- Terasaka, S.; Kamitakahara, M.; Yokoi, T.; Matsubara, H. Ability of hydroxyapatite synthesized from waste oyster shells to remove fluoride ions. Mater. Trans. 2015, 56, 1509–1512. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.H.; Kim, S.H.; Cha, W.S.; Kim, K.; Moon, B. Mineralogical changes of oyster shells by calcination: A comparative study with limestone. Econ. Environ. Geol. 2018, 51, 484–492. [Google Scholar]
- Ha, S.; Lee, J.W.; Choi, S.H.; Kim, S.H.; Kim, K.; Kim, Y. Calcination characteristics of oyster shells and their comparison with limestone from the perspective of waste recycling. J. Mater. Cycles Waste Manag. 2019, 21, 1075–1084. [Google Scholar] [CrossRef]
- Giorgi, R.; Dei, L.; Ceccato, M.; Schettino, C.; Baglioni, P. Nanotechnologies for conservation of cultural heritage: Paper and canvas deacidification. Langmuir 2002, 18, 8198–8203. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Imperfect oriented attachment: Dislocation generation in defect-free nanocrystals. Science 1998, 281, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.L.; Banfield, J.F. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Geochim. Cosmochim. Acta 1999, 63, 1549–1557. [Google Scholar] [CrossRef]
- Penn, R.L.; Oskam, G.; Strathamann, T.J.; Searson, P.C.; Stone, A.T.; Veblen, D.R. Epitaxial assembly in aged colloids. J. Phys. Chem. B 2001, 105, 2177–2182. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Ortega-Huertas, M.; Hansen, E. Nanostructure and irreversible colloidal behavior of Ca(OH)2: Implications in cultural heritage conservation. Langmuir 2005, 21, 10948–10957. [Google Scholar] [CrossRef]
- Labtaini, I.; El-Hami, K. X-ray diffraction characterization of the untreated calcium phosphate from two Moroccan mining zones. Mater. Sci. Eng. Int. J. 2019, 3, 67–70. [Google Scholar]



| pH | Ca2+ | Mg2+ | Na+ | K+ | F− | Cl− | NO3− | PO43− | SO42− |
|---|---|---|---|---|---|---|---|---|---|
| (−) | (mg/L) | ||||||||
| 1.841 | 100.7 | 3.79 | 608.6 | 3.97 | 3286 | 145 | 3994 | 3942 | 15,835 |
| Anionic Species | Sample ID | Ca/F Molar Ratio | ||||
|---|---|---|---|---|---|---|
| Ca/F = 0.5 | Ca/F = 1.0 | Ca/F = 1.5 | Ca/F = 2.0 | Ca/F = 2.5 | ||
| F− | Oyster_325 | 40.3 | 51.3 | N.A | N.A | 99.8 |
| Oyster_500 | 95.1 | 95.4 | 95.7 | 99.3 | N.A | |
| Limestone_500 | 97.3 | 95.3 | 98.9 | 100 | N.A | |
| Cl− | Oyster_325 | −14.8 | 0.0 | N.A | N.A | 23.1 |
| Oyster_500 | 97.8 | 98.0 | 97.9 | 98.3 | N.A | |
| Limestone_500 | 98.2 | 97.9 | 98.2 | 97.4 | N.A | |
| PO43− | Oyster_325 | 19.2 | 19.8 | N.A | N.A | 100 |
| Oyster_500 | 99.9 | 99.9 | 99.9 | 99.9 | N.A | |
| Limestone_500 | 99.9 | 99.9 | 99.9 | 100 | N.A | |
| NO3− | Oyster_325 | 16.6 | 24.5 | N.A | N.A | 59.2 |
| Oyster_500 | 99.4 | 99.5 | 99.5 | 99.4 | N.A | |
| Limestone_500 | 99.5 | 99.5 | 99.4 | 97.7 | N.A | |
| SO42− | Oyster_325 | 30.6 | 81.0 | N.A | N.A | 93.2 |
| Oyster_500 | 98.6 | 99.2 | 99.7 | 99.6 | N.A | |
| Limestone_500 | 98.5 | 99.4 | 99.5 | 98.3 | N.A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Lee, S.-E.; Kim, S. An Investigation into Sustainable Solutions: Utilizing Hydrated Lime Derived from Oyster Shells as an Eco-Friendly Alternative for Semiconductor Wastewater Treatment. Recycling 2024, 9, 61. https://doi.org/10.3390/recycling9040061
Lee H-J, Lee S-E, Kim S. An Investigation into Sustainable Solutions: Utilizing Hydrated Lime Derived from Oyster Shells as an Eco-Friendly Alternative for Semiconductor Wastewater Treatment. Recycling. 2024; 9(4):61. https://doi.org/10.3390/recycling9040061
Chicago/Turabian StyleLee, Hye-Jin, Sang-Eun Lee, and Seokhwi Kim. 2024. "An Investigation into Sustainable Solutions: Utilizing Hydrated Lime Derived from Oyster Shells as an Eco-Friendly Alternative for Semiconductor Wastewater Treatment" Recycling 9, no. 4: 61. https://doi.org/10.3390/recycling9040061
APA StyleLee, H.-J., Lee, S.-E., & Kim, S. (2024). An Investigation into Sustainable Solutions: Utilizing Hydrated Lime Derived from Oyster Shells as an Eco-Friendly Alternative for Semiconductor Wastewater Treatment. Recycling, 9(4), 61. https://doi.org/10.3390/recycling9040061






