Abstract
Food waste is generated in large amounts locally and globally, and requires expenditure for disposal. However, it has high nutritional value and almost no toxic components. Therefore, it can be returned to mushroom mediums for further use, leading to food waste circulation. Though disposing of spent mushroom substrate (SMS) after harvesting is an additional problem, there have been increased efforts to compost it and apply it to the soil for growing vegetables. This study, therefore, aimed to optimize (1) mushroom spawn production with rice hull, (2) mushroom substrates using food waste to accelerate food waste recycling, and (3) the utilization of SMS as an organic fertilizer. An optimal substrate composition and high yield were obtained at 120–140 g of food waste per bag among substrates from Pleorutus ostreatus and Pleorutus citrinopileatus; therefore, using a high ratio of food waste in the mushroom mediums was achieved. On the other hand, the SMS of P. citrinopileatus demonstrated higher plant biomass growth, at 36 g, than that of P. ostreatus, at 21.2 g, in a treatment using SMS + okara. The present discovery is that people may be encouraged to be mindful of food loss by the delivery of mushrooms and plants grown from agro/food waste to the dining table, and this circular system may therefore be used as a key resource in mushroom and plant cultivation and to achieve a zero-emission cycle.
1. Introduction
The changing global climate and weather patterns might result in the current agricultural systems being less stable, thus threatening a food-secure future [1]. The current world population is around 8.1 billion, according to the most recent United Nations estimates, and this instability adds to the existing challenges of feeding the expected world population by 2050 of around 10 billion [2]. It has been estimated that food production will need to grow by 70% to meet this demand [3]. Nevertheless, simultaneous increases in the generation of domestic food waste, leftovers, and/or trade refuse per capita due to population increases, affluence, and urbanization pose significant problems for disposal [4]. Food is lost or wasted throughout the supply chain, from initial agricultural production down to final household consumption [2]. In Japan, food waste recycling for family-based waste accounts for only 6.5% of waste when compared with that of industrial waste, which stands at 78% [5]. In general, the great advantage of recycling food waste is that, in addition to using the waste as a resource for new production processes, it also eliminates environmental pollution through the food chain. Although there are several different types of organic waste, food waste in particular has been investigated and used directly and indirectly in crop production [6]. The growing amount of food and trade waste can, therefore, be considered as a resource rather than waste. This may be utilized in several ways, such as in crop nutrient management. The most extensively used agro wastes for the production of edible mushrooms are currently wheat or rice straw, sawdust or wood chip, sugarcane bagasse, cotton waste and cotton seed hull, corn cob, rice or wheat bran, chicken or horse manure, and other green materials, such as cotton stalk and soybean straw [7], coffee pulp [8], etc. The effect of food waste compost on the antler-type fruiting body yield of Ganoderma lucidum [9] has also been studied.
In addition, the mushroom industry produces abundant spent mushroom substrate (SMS) after mushroom harvesting which still has sufficient mineral nutrients, lignocellulolytic enzymes, and proteins. The agricultural use of SMS is, therefore, not only as an excellent organic resource for plant nutrients but also as a resource that helps to maintain soil health [10]. However, in most countries, this SMS is disposed of as waste, with negative environmental impacts [11], because SMS is not suitable for composting by itself due to the presence of lignocellulose [12]. According to estimates, approximately 55 million tons of SMS are generated annually worldwide. It is estimated that 1 kg of fresh mushrooms produces approximately 5 kg of SMS [8,13]. Sendi et al. [12] attempted to replace the expensive peat moss with SMS under pot culture cultivation of Brassica oleracea var. Alboglobata. SMS and peat moss in the ratio of 1:1 along with NPK and peat moss alone performed equally in terms of yield and quality of B. oleraceae crop. Additionally, Ahlawat and Sagar [14] reported the reuse of SMS as a good growing medium for vegetables like cucumber, tomato, broccoli, tulip, cauliflower, pepper, spinach, etc. The SMSs of oyster mushrooms have a positive effect on growth of chili Capsicum. Despite this, the SMS of the grown Pleorutus spp., using food waste in combination with okara, has not been fully investigated for its agricultural use. Okara is a soybean by-product generated in large quantities (annual production of 14 million tones globally) from the soybean milk and bean curd industries [15,16]. Being high in moisture content (70–80%), it is highly perishable and is mostly discarded [17]. The increased interest in waste valorization has led to many looking into reusing okara, since okara contains about 40–60% fibril components, which are composed of cellulose, hemicellulose, and lignin, and about 30% proteins on a dry matter basis, which may help for providing plant nutrients [15,18,19,20]. This study, therefore, aimed to optimize the medium composition in mushroom cultivation using agro/food waste and the use of the SMSs of the Pleorutus ostreatus and Pleorutus citrinopileatus mushrooms as an organic fertilizer for Brassica rapa cultivation. The possibility of building a food production system and a zero-emission cycle was demonstrated based on the results.
2. Materials and Methods
2.1. Agar Culture for Spawn
P. ostreatus and P. citrinopileatus were maintained on potato dextrose agar (PDA) (Oxoid, CMO 139, Basingstoke, UK) plates for spawn production. The sterilized PDA medium was poured into sterilized Petri dishes, 15 mL in each, and allowed to solidify. Inoculation was conducted by transferring a 5 mL disc of the mycelial mat from the periphery of a 10-day incubation culture. The plates were then incubated at 25 ± 1 °C. Observations on colony diameter were taken when the maximum growth was attained on the medium selected to transfer to the mother spawn substrates.
2.2. Preparation of Spawn Substrates
The method of spawn substrate production used rice hull with rice bran and starch, for which the material percentage was calculated on a dry–wet basis based on our previous data. The rice hull, rice bran, starch, and calcium carbonate were mixed well to adjust the pH to 5.0–5.5, and the moisture content was maintained at 59–61%. Then, 400–450 g samples of the substrates were placed into 1000 cc polypropylene bottles. The bottles were sterilized at 121 °C for 30 min in the autoclave. After cooling at the clean bench, the bottles were inoculated by a piece of mycelia mat from the PDA plate medium culture over the bed surfaces and then incubated for 20–30 days at 25 °C in the incubation room. The spawn mycelia were colonized on the inside and surfaces of the bottles and then stored at 5 °C ± 1 °C for further inoculation into mushroom cultivation bags.
2.3. Preparation of Mushroom Cultivation Substrate
In order to determine suitable substrates and suitable ratios for the cultivation of the two oyster mushrooms, the chosen method followed those of several research reports which had documented the utilization of supplements in the production of edible mushrooms; these included wheat or rice straw, sawdust or wood chip, sugarcane bagasse, cotton waste and cotton seed hull, corn cob, rice or wheat bran, chicken or horse manure, and other green materials, such as cotton stalk and soybean straw [7] and coffee pulp [8]. A total of eight different substrates (T1–T8) were used, including barley tea residue, coffee residue, rice hull, food waste including vegetable and fruit peels and leftover food from diverse meat, egg, fish, and vegetable dishes, rice bran, and okara (the soybean residue that remains after the manufacture of soymilk or soybean curd) [7,16] (Table 1), and calcium carbonate was mixed in to adjust the pH to 5.5–6.0. These substrates varied in composition, water content, and pH value. The substrates’ ratios followed a completely randomized design with three replication bags each. Agro waste, comprising barley tea residue for swine which is discarded as waste after manufacturing a barley tea drink, coffee residue, rice hull, rice bran, and okara, was collected from Mitsuinorin Co., Yamanashi, Japan. In addition, food waste including vegetable and fruit peels and the leftover food from diverse meat and vegetable menus was collected and dried to approximately 9% moisture using a Hitachi Household Garbage Dryer and Processor ECO-B25 (Hitachi, Ltd., Tokyo, Japan). All the materials were mixed well and put in a polyethylene bag (38 cm × 15 cm). Each bag contained 500 g of media with 60–63% humidity and was autoclaved at 121 °C for 60 min. After being cooled at the clean bench, the bags were inoculated over substrate surfaces using 5–7 g of the mother spawn and then incubated for approximately 20–30 days at 25 °C in the incubation room until the formation of mushroom primordia.

Table 1.
Mushroom cultivation substrates with different agro wastes.
2.4. Mushrooms Cultivation Process
When the mycelia covered the inside and surfaces of the mushroom cultivation bags, as well as when some mushroom pinheads were observed, the old spawn on top of the substrates were removed using a screening procedure and washed using cool clean water. The bags were then transferred to the cultivation chamber for three to ten days, depending on the mushroom species, where the temperature was controlled to approximately 20 °C ± 2 °C and the air humidity was set at 70–85% [21]. The fruiting body was then harvested and the data were recorded.
2.5. Plant Material and Experimental Setup
One portion of the SMSs of P. ostreatus and P. citrinopileatus was used for plant growth. B. rapa was selected as the plant since it is commonly cultivated in Japan, where the seeds can be obtained commercially. Eight treatments with different combinations of additive materials—chemical fertilizer (CK), 12 g of SMS, 24 g of SMS, 48 g of SMS, 12 g of SMS + 10 g of urea, 12 g of SMS + 20 g of urea, 12 g of SMS + 12 g of okara, 12 g of SMS + 24 g of okara—were used for the experiment using only soil as a control. Here, 300 g samples of air-dried soil with different combinations of SMS and water were mixed well and transferred into Neubauer pots (100 cm2 volume; Fujiwara Seisakusho, Ltd., Tokyo, Japan) and kept at 25 °C under dark conditions for two weeks. B. rapa was then grown in the pots for 37 days in a growth chamber at 25 °C, and three replicated pots were established for each treatment. B. rapa was then harvested after 37 days and the dry weight of above- and below-ground biomass was determined. The cultivation test was conducted twice; the second time, 24 g of SMS and 12 g of SMS + 12 g of okara were selected due to their good results in the first cultivation test. The dry weight of the plants was measured after harvest even in the second cultivation, and the soil chemical properties and bacterial and fungal biomass were investigated after harvest.
2.6. Statistical Analysis
The statistical analyses were performed using the one-way analysis of variance (ANOVA) in the SPSS program version 16.0 via the statistical software package SPSS 16.0 (https://spss.software.informer.com/16.0, accessed on 3 May 2023). The experiment followed a completely randomized design with three replications. The Tukey–Kramer multiple range test (MRT) was used to determine significance, where the different alphabetic letters indicate significance at p < 0.05.
3. Results
3.1. Effect of Rice Hull Spawn on P. ostreatus and P. citrinopileatus
Both mushrooms did not demonstrate differential mycelial colonization after inoculation in any of the spawn substrates during the incubation periods. A thicker mycelial density was observed in the spawn substrate containing rice hull, rice bran, and starch in both mushroom species. Additionally, faster mycelial colonization was observed in the spawn substrate containing rice hull, rice bran, and starch in both mushroom species in comparison with the spawn substrate containing with sawdust and rice bran. Among the cultivation substrates, the highest mushroom yield was obtained through the spawn inoculation of the medium comprising rice bran with starch. It is clear that by adding starch to rice hull, it is possible to create a spawn culture that is equivalent to or better than the conventional culture medium made from sawdust (Figure 1).

Figure 1.
Effects of rice hull spawn on different agro/food waste substrates in P. ostreatus: (A) 12 days of incubation using a substrate with sawdust + rice bran and with rice hull + rice bran + starch; (B) 21 days of incubation using a substrate with sawdust + rice bran and with rice hull + rice bran + starch.
3.2. Effects of Different Agro/Food Waste Substrates on the Yield of P. ostreatus and P. citrinopileatus
First, in the cultivation using the conventional substrate, the yield was compared with the substrates with rice bran (T5) and okara (T6) as an additive material (Figure 2). On the other hand, T1 to T3 used rice bran as an additive material, and T4 to T6 used okara. All the treatments significantly increased the yield by using food waste instead of sawdust. Rice bran demonstrated a better yield than okara when food waste was used as the main material. In P. ostreatus, the mushrooms grew well in any substrates with inoculation with rice hull spawn with rice bran and starch, and were not significantly different in mushroom yield, even in substrates containing rice bran or okara, excluding T7–T8 which contained wood sawdust. The highest yield of 104–133.3 g was obtained in T2–T3, containing food waste with rice bran, and then the next-highest yield was 86 g in T1, which did not contain food waste but did contain rice bran. However, higher yields were obtained in T2–T3 than in the T5–T6 substrates, containing okara, and T7–T8, containing only wood sawdust with rice bran or okara. The lowest yield of 55–75 g was obtained in T7–T8, which contained wood sawdust with rice bran or okara (Figure 2A). The mushroom yield did not demonstrate any significant difference in substrates T4–T6, containing food waste with okara. However, the substrates T5 and T6 demonstrated relatively higher yields than those of substrate T4 as well as substrates T7 and T8. On the other hand, for P. citrinopileatus, the best yield of the compared treatment sections was observed in T2, which contained 25% food waste and rice bran, and T3, comprising 20% food waste, 10% coffee grounds, and 13.5% barley tea residue. The highest yield was determined to be 115 g in the cultivation substrate T2, and the lowest yield was determined to be 70–73.3 g in T4–T6, containing food waste with okara, and T7–T8 on wood sawdust, even with rice bran and okara (Figure 2B). Among the cultivation substrates comprising okara, the yield of substrates T5–T6 was relatively higher than substrate T4, which did not contain food waste but did contain okara.
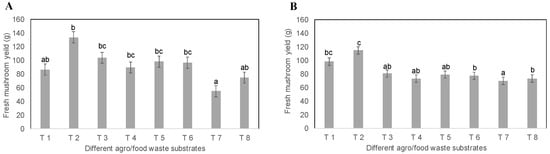
Figure 2.
Effects of rice hull spawn on different agro/food waste substrates: (A) P. ostreatus; (B) P. citrinopileatus; values are given on three replicates; different letters show significant differences among all treatments (p < 0.05). Statistical analysis was performed using the Tukey–Kramer test.
3.3. Effects of SMS of P. ostreatus and P. citrinopileatus on the Growth of B. rapa
For the SMS of P. ostreatus, different additive material combinations demonstrated significantly different biomass yields. The biomass of B. rapa in the chemical fertilizer plot presented a dry weight of 1.52 g, but when 12 g of okara (soybean residue) was added to the SMS, the plant growth was greatly improved and the plant dry weight increased to 2 g (Figure 3A). In terms of the SMS of P. citrinopileatus, the growth of B. rapa was observed to be the best in the plot where 12 g of okara was added to 12 g of SMS. The treatment with the next best growth was found to be the treatment in which urea was added to 12 g of SMS (Figure 3B). A similar result was obtained in the second cultivation test, revealing that SMS + okara is a good combination (Figure 4, Table 2). In terms of the soil chemical properties after cultivation, nitrate nitrogen accumulated in the treatment of SMS + okara in the pots incorporated with the SMS of P. ostreatus (Table 3). Other factors presented almost the same value as the control and chemical fertilizer. SMS + okara, therefore, was revealed to be the most suitable material for the growth of B. rapa. A photo of the plant growth is shown in Figure S1.
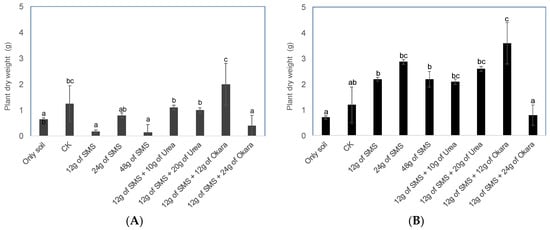
Figure 3.
The dry weight of B. rapa in the SMS of P. ostreatus (A) and P. citrinopileatus (B) SMS combinations. Values are given from three replicates; the error bars indicate standard deviation and different letters indicate significant differences determined using the Tukey–Kramer test (p < 0.05).
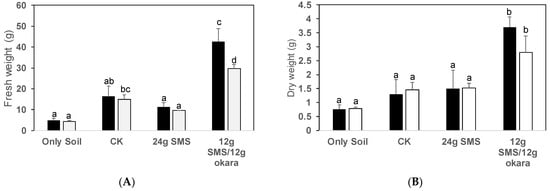
Figure 4.
A second pot experiment was conducted using the two treatments in which better growth was confirmed in the first pot experiment. The black bars indicate P. citrinopileatus and the white bars indicate P. ostreatus. Sub-figure (A) shows fresh weight after harvesting, and (B) shows dry weight. Values are given on three replicates; the error bars indicate standard deviation and different letters indicate significant differences determined using the Tukey–Kramer test (p < 0.05).

Table 2.
Effect of spent mushroom substrate (SMS) and SMS + okara mixtures on the growth traits of Brassica rapa plants grown under climate control chamber conditions. The values are given on three replicates; a, b, c, d notation shows the significance between all substrates (p < 0.05). Statistical analyses were performed using the Tukey–Kramer test.

Table 3.
Soil chemical properties after harvesting.
4. Discussion
It is said that food waste comprises approximately 80% water [5]. If the waste to be incinerated contains a lot of moisture, heat is used to evaporate the moisture, which reduces the efficiency of waste combustion. In particular, the heat utilization efficiency of waste incineration facilities that make effective use of the heat generated by incinerating waste decreases [5]. Rather than simply throwing away large quantities of food waste as garbage, it may be effectively utilized as a valuable unused resource, eliminating the burden on the environment and the costs of processing it. The accumulation of organic waste primarily originates from different food industry processes [18]. This waste varies in composition, water content, and pH value, carrying the risk of bacterial contamination and negative environmental influences [22,23]. The valorization of food waste and the application of new technologies to eliminate waste by producing value-added products through bioconversion processes is a unique way to manage these prevalent issues. Various technologies have been used to solve this problem of waste generation, like the extraction of natural pigments [24], extraction of omega-3 fatty acids from waste fish oil [25], and transforming apple peel waste into ethanol and acetic acid through the enzymatic hydrolysis of cellulose [22]. Food waste with a high cellulose and lignin content may be converted to renewable energy [3]. Food waste may additionally be bio-converted into other various products, such as biofuels, industrial enzymes, nutraceuticals, or even biodegradable plastics [18].
Coffee pulp, either as the sole substrate or mixed with other organic materials, has been shown to be a suitable substrate for the cultivation of the edible mushrooms Pleurotus, Lentinula, and Auricularia [8]. The presence of caffeine inhibited mycelial growth on agar and in liquid culture in the laboratory [26]. However, despite growth inhibition, partial degradation of caffeine to xanthine by P. ostreatus mycelium was observed in all spent coffee grounds-containing substrate mixtures [11]. A study by [13] reported that the addition of appropriate ratios of tea residue could be used as an effective and economic substrate for oyster mushroom cultivation, resulting in high fruiting body yield, high biological efficiency, and a relatively short planting time. Approximately 700 tons of coffee and 150 tons of barley tea are generated annually in one industrial plant in Yamanashi prefecture in Japan. Since coffee and barley tea, which are familiar to Japanese people, are produced in large quantities, it is estimated that they generate a correspondingly large amount of waste, with coffee producing approximately 850,000 tons of waste and barley tea producing 120,000 tons of waste. Some of these wastes are recycled as fertilizer or feed, but most of them are treated as industrial waste. In this study, the yield of P. citrinopileatus was 115 g ± 2.8 g in T2 compared to 81 g ± 4.6 g in T3. The yield of P. ostreatus was 133.7 g ± 4.1 g in T2 compared to 104 g ± 0.8 g in T3. Substrates T2–T3 contained a higher proportion of food waste and lower proportions of barley tea and coffee residues, demonstrating that higher yields were obtained from P. ostreatus and P. citrinopileatus (Figure 2). However, although the effect of the high ratios of tea or coffee waste in cultivation substrates was reported as good for mycelial growth [19,21,27], the results of the present study demonstrated that they are deficient in terms of nutrients and energy, thereby limiting the generation of fruiting bodies (Figure 2). Moreover, excessive nitrogen-including antifungal substances such as catechin in the substrates comprising high ratios of tea waste may lead to a relatively lower mushroom yield [28]. On the contrary, the results indicate that mushroom mycelia utilize food waste as proper nitrogen sources for their mycelial growth and formation of fruit body. According to the results of this study, it is estimated that 1 ton of mushroom production per month can recycle 1.3 tons of agricultural/food waste, and 12 tons of mushroom production per year can recycle 16 tons of agricultural/food waste. However, the validation of these results on a larger scale is required.
SMS normally has a high C/N ratio. Mixing with soil, therefore, causes the nitrogen to be immobilized in the soil. The C/N ratio, which is the estimated compost, presented a higher value of more than C/N 50 after four months of composting. However, this dropped to approximately C/N 25 after six months. The same period was required to reduce the concentration of substances that inhibit the germination of plants. Therefore, Araki and Michigami [29] reported that a composting period of approximately six months is required to apply SMS to farmland with confidence. However, this study demonstrated that mixing urea and okara with SMS indicates a material that reduces the CN ratio and enables vegetable cultivation even if it is mixed with soil without passing through the composting process. First, considering the addition of SMS alone, if the amount of SMS was increased from 12 g to 24 g, the growth of B. rapa increased, but the addition of 48 g of SMS demonstrated a decrease in plant growth. This phenomenon demonstrated a similar trend for the SMS of both P. ostreatus and P. citrinopileatus. When urea or okara was added to 12 g of SMS, the plant growth was equivalent to that in the 24 g of SMS. In particular, 12 g of okara demonstrated the best growth and presented a high affinity with SMS (Figure 3 and Figure 4). When using the SMS from Pleorutus citrinopileatus, the growth of B. rapa was 5 g in only soil, but 42.6 g in 12 g SMS + 12 g okara, which is about eight times the growth. Compared to chemical fertilizer, the growth was 2.6 times greater, from 16.3 g to 42.6 g. The same result was seen for P. ostreatus, where the growth was 6.7 times greater, from 4.4 g in only soil to 29.6 g in 12 g SMS + 12 g okara. In other words, though the materials were mixed with the soil without going through the composting process, it has been shown that adding 12 g of okara to 12 g of SMS enabled the growth of vegetables. Moreover, interestingly, higher biomass was obtained through the application of P. citrinopileatus SMS than P. ostreatus SMS. These results also suggest that the SMS of P. citrinopileatus has a high ability to utilize SMS and has potential effects on the promotion of plant growth in B. rapa (Figure 4). The microbial population detected by the culture method also additionally increased. Okara is a soybean by-product generated in large quantities (annual production of 14 million tones globally) from the soybean milk and bean curd industries [15,16,20]. The increased interest in waste valorization has led to several studies looking into reusing okara, since okara contains approximately 40–60% fibril components composed of cellulose, hemicellulose, and lignin, and approximately 30% proteins on a dry matter basis, which may help provide plant nutrients [20,30]. It has, therefore, been reported that soil microorganisms, especially Bacillus spp., demonstrated vigorous growth and produced antibacterial substances [7].
5. Conclusions
This study created an excellent spawn culture using rice hull with rice bran and starch as the main substrate, and it showed high productibility, using agricultural waste and food waste to optimize the growing media for P. ostreatus and P. citrinopileatus mushroom production and minimize incubation time. It was found that SMS could be used for farmland without the composting process by using okara as an additive material and that this does not cause nitrogen starvation in the soil. These results can be adapted to local recycling with food production systems that can produce new valuable agricultural products by regenerating local food loss, food waste, and agricultural waste; this will increase ethical consumption, reducing food loss and waste production in the future. Essentially, this circular process promotes recycling by separating and collecting food waste from households and production processes and using it as the raw material for new production processes. However, further investigation will be needed regarding the chemical composition of organic materials from different origins, especially for food wastes.
6. Patents
We are currently applying for a patent (patent application no. 2023-204585).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/recycling9040058/s1, Figure S1: Photos of plant harvesting in the pot experiment.
Author Contributions
Conceptualization, B.S.B. and R.K.; methodology, B.S.B. and R.K.; software, B.S.B.; validation, B.S.B., A.N. and R.K.; formal analysis, R.K. and B.S.B.; investigation, R.K.; resources, R.K.; data curation, B.S.B. and A.N.; writing—original draft preparation, B.S.B. and R.K.; writing—review and editing, R.K.; visualization, R.K.; supervision, R.K.; project administration, R.K.; funding acquisition, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Yamanashi.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank Makoto Higuchi in Mitsuinourin Co. Ltd., Yohei Mukouyama in Kurofuji farm A.P.C. for the provision of coffee residues, barley tea residues, and rice hulls.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Brien, P.; Kral-O’Brien, K.; Hatfield, J.L. Agronomic approach to understanding climate change and food security. Agron. J. 2021, 113, 4616–4626. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, How to Feed the World in 2050. 2009. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 9 July 2024).
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Bogner, J.; Pipatti, R.; Hashimoto, S.; Diaz, C.; Mareckova, K.; Diaz, L.; Kjeldsen, P.; Monni, S.; Faaij, A.; Gao, Q.; et al. Mitigation of global greenhouse gas emissions from waste: Conclusions and strategies from the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report. Working Group III (Mitigation). Waste Manag. Res. J. A Sustain. Circ. Econ. 2008, 26, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Consumer Affairs Agency (CAA), Government of Japan. 2016. Available online: https://www.caa.go.jp/policies/policy/consumer_policy/information/food_loss/conference/pdf/adjustments_index_10_161012_0007.pdf (accessed on 9 July 2024). (In Japanese).
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food waste to energy: An overview of sustainable approaches for food waste management and nutrient recycling. BioMed. Res. Int. 2017, 19, 2370927. [Google Scholar] [CrossRef] [PubMed]
- Ohno, A.; Ano, T.; Shoda, M. Use of soybean curd residue, okara, for the solid state substrate in the production of a lipopeptide antibiotic, iturin A, by Bacillus subtilis NB22. Process Biochem. 1996, 31, 801–806. [Google Scholar] [CrossRef]
- Martínez-Carrera, D.; Aguilar, A.; Martínez, W.; Bonilla, M.; Morales, P. Commercial production and marketing of edible mushrooms cultivated on coffee pulp in Mexico. In Coffee Biotechnology and Quality; Sera, T., Soccol, C., Pandey, A., Eds.; Kluwer Academic Publishers: Dordrecht, The Neterhlands, 2000; Chapter 45; pp. 471–488. ISBN 0-7923-6582-8. [Google Scholar]
- Jo, E.-Y.; Cheon, J.-L.; Ahn, J.-H. Effect of food waste compost on the antler-type fruiting body yield of Ganoderma lucidum. Mycobiology 2013, 41, 42–46. [Google Scholar] [CrossRef]
- Mohd Hanafi, F.H.; Rezania, S.; Mat Taib, S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles. Waste 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Chiu, S.; Ching, M.; Fong, K.; Moore, D. Spent oyster mushroom substrate performs better than many mushroom mycelia in removing the biocide pentachlorophenol. Mycol. Res. 1998, 102, 1553–1562. [Google Scholar] [CrossRef]
- Sendi, H.; Mohamed, M.T.M.; Anwar, M.P.; Saud, H.M. Spent mushroom waste as a media replacement for peat moss in Kai-Lan (Brassica oleraceae var. Alboglabra) production. Sci. World J. 2013, 2013, 258562. [Google Scholar] [CrossRef]
- Williams, B.; McMullan, J.; McCahey, S. An initial assessment of spent mushroom compost as a potential energy feedstock. Bioresour. Technol. 2001, 79, 227–230. [Google Scholar] [CrossRef]
- Ahlawat, O.P.; Manikandan, K.; Sagar, M.P.; Rai, D.; Vijai, B. Effect of composted button mushroom spent substrate on yield, quality and disease incidence of Pea (Pisum sativum). Mushroom Res. 2011, 20, 87–94. [Google Scholar]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Gómez-Zavaglia, A. Okara: A nutritionally valuable by-product able to stabilize Lactobacillus plantarum during freeze-drying, spray-drying, and storage. Front. Microbiol. 2017, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Vong, W.C.; Lim, X.Y.; Liu, S.-Q. Biotransformation with cellulase, hemicellulase and Yarrowia lipolytica boosts health benefits of okara. Appl. Microbiol. Biotechnol. 2017, 101, 7129–7140. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Liu, W.-S.; Kwok, K.; Kwok, F. Isolation and characterization of proteins from soymilk residue (okara). Food Res. Int. 1996, 29, 799–805. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Food Losses and Food Waste–Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Parmar, I.; Rupasinghe, H.V. Bio-conversion of apple pomace into ethanol and acetic acid: Enzymatic hydrolysis and fermentation. Bioresour. Technol. 2013, 130, 613–620. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De Bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Cazón, P.; Silva, A.S. Natural pigments from food wastes: New approaches for the extraction and encapsulation. Curr. Opin. Green Sustain. Chem. 2024, 47, 100929. [Google Scholar] [CrossRef]
- Monsiváis-Alonso, R.; Mansouri, S.S.; Román-Martínez, A. Life cycle assessment of intensified processes towards circular economy: Omega-3 production from waste fish oil. Chem. Eng. Process. Process. Intensif. 2020, 158, 108171. [Google Scholar] [CrossRef]
- Carrasco-Cabrera, C.P.; Bell, T.L.; Kertesz, M.A. Caffeine metabolism during cultivation of oyster mushroom (Pleurotus ostreatus) with spent coffee grounds. Appl. Microbiol. Biotechnol. 2019, 103, 5831–5841. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liang, J.; Wang, Y.; Sun, F.; Tao, H.; Xu, Q.; Zhang, L.; Zhang, Z.; Ho, C.; Wan, X. Tea waste: An effective and economic substrate for oyster mushroom cultivation. J. Sci. Food Agric. 2016, 96, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Michigami, N. Effect of Spent Mushroom Substrates to Vegetable Fields (In Japanese), Shimane Prefecture Agricultural Experiment Station 2003; p. 104. Available online: https://www.pref.shimane.lg.jp/industry/norin/gijutsu/nougyo_tech/kenyui/kenkyu_seika/tayori/104-4.data/104-4.pdf (accessed on 9 July 2024).
- Mane, V.P.; Patil, S.S.; Syed, A.A.; Baig, M.M.V. Bioconversion of low quality lignocellulosic agricultural waste into edible protein by Pleurotus sajor-caju (Fr.) singer. J. Zhejiang Univ. B 2007, 8, 745–751. [Google Scholar] [CrossRef]
- Zang, T.T.; Hu, X.J.; Gu, H.D.; Yu, J.; Qu, J.J. Biosorption of Cu2+ by Auricularia auricula spent substrate and its mechanism. Acta Sci. Circumst. 2014, 34, 1421–1428. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).