Abstract
This review article gathers the most recent recycling technologies for thermoset and thermoplastic polymers. Results about existing experimental procedures and their effectiveness are presented. For thermoset polymers, the review focuses mainly on fibre-reinforced polymer composites, with an emphasis on epoxy-based systems and carbon/glass fibres as reinforcement, due to the environmental concerns of their end-of-life management. Thermal processes (fluidised bed, pyrolysis) and chemical processes (different types of solvolysis) are discussed. The most recent combined processes (microwave, steam, and ultrasonic assisted techniques) and extraordinary recycling attempts (electrochemical, biological, and with ionic liquids) are analysed. Mechanical recycling that leads to the downgrading of materials is excluded. Insights are also given for the upcycling methodologies that have been implemented until now for the reuse of fibres. As for thermoplastic polymers, the most state-of-the-art recycling approach for the most common polymer matrices is presented, together with the appropriate additivation for matrix upcycling. Mechanical, chemical, and enzymatic recycling processes are described, among others. The use of fibre-reinforced thermoplastic composites is quite new, and thus, the most recent achievements are presented. With all of the above information, this extensive review can serve as a guide for educational purposes, targeting students and technicians in polymers recycling.
1. Introduction
The transition from a fossil- to renewable-based society and recycling are vital for the next generations, as the fundamental steps toward a circular economy regenerative system able to minimize resource input and waste, emission, and energy consumption [1]. According to Plastics Europe, worldwide plastic production in 2022 was 400.3 Mt, with a CAGR of 5–6% [2], with thermosets counting for around 11% of plastic production volume (42 Mt) [3]. Recent trends indicate significantly increased circular plastics production, reaching nearly 10% of global production (35.5 Mt) and showing a 16-fold increase compared to fossil-based plastics, with circular plastics production increasing by 29.2% since 2018 in Europe, reaching a share of 19.7% of overall European plastics production by 2022.
Thermoset polymers are one of the most produced and used material worldwide, due to their numerous advanced characteristics such as toughness and resistance in harsh environmental conditions. They are used mainly as matrices in composite materials, along with several other engineering applications, such as adhesives, coatings, insulating foams, etc., that find applications in naval, aerospace, aviation, building, and wind energy. Among the most common thermoset polymers are acrylic resins, polyesters and vinyl esters, epoxies, polyurethanes, phenolic, amino, and furan resins, as well as polybenzoxazines.
Thermoset polymers are mainly used for the manufacturing of fibre-reinforced polymers (FRPs) that offer significant advantages over conventional isotropic materials such as their steel and aluminium counterparts. According to the R-strategies [4], the recycling of FRP has a great advantage in that it can be applied to all structural parts made of composite materials. Furthermore, this approach can be an advantageous combination for repetitive applications, such as the production of non-structural components from end-of-life composite parts [5], as the waste stream of residual composites generated during the production process can be reduced.
Due to the high Young’s modulus of carbon fibres, carbon fibre-reinforced polymers (CFRPs) are used in a wide number of sectors in which high strength-to-weight ratio and stiffness are required, including the transport sector (i.e., automotive, aeronautic, shipbuilding) and energy sector (i.e., wind energy). CFRPs are expected to reach a global demand of 290 kt by the end of 2024 [6,7], and from renewable energy sectors alone, it has been estimated that by 2030, composite waste will reach 500 kt [8].
Glass fibre-reinforced plastics (GFRPs) composites remain the dominant material in the composites market, with a market share of over 95% [9] thanks to significant industrial interest (e.g., lightweight for automotive, unlimited design possibilities coupled with high performance for home appliances, and much lower cost for a railway).
Thermoplastic polymers are one of the most widely used materials in packaging, home appliances, and the automotive industry, among others. Specifically, the rapidly increasing amount of electronic waste (WEEE) reached ~52.2 Mt in 2021 [10], with estimations to exceed 74 Mt in 2030 [11], while formal documented collection and recycling only accounted for 9.3 Mt, a mere 17.4%, in 2019, illustrating that recycling activities are not at all keeping pace with the global growth of WEEE. Likewise, toys made from polymeric materials are identified to be the most plastic-intensive industry in the world, with 40 t of plastic used for every $1 M in revenue [12]. Considering that children grow quickly, and their abilities and interests shift even faster, toys are often thrown away after a short lifespan, with 80% [13] of them ending up in landfills or being incinerated. Textiles are another consumer sector of plastic products with high global revenue annually, having only 13% of the fibre input for clothing recycled [14] and less than 1% of closed-loop recycling, i.e., fibres recycled back into clothing rather than into lower value uses, such as cleaning cloths and insulation. The circular fibre concludes the crisis caused by the global COVID-19 pandemic and its economic consequences further highlight and exacerbate the vulnerabilities along the textile value chain, intensifying pressure on this already out-of-control problem and creating a significant increase in waste production across textile types, highlighting the urgency of transitioning from the industry’s current model to a more sustainable, socially inclusive, and circular model [15]. More than 8 Mt of pandemic-associated plastic waste have been generated globally [16], with more than 25 t entering the global ocean [17].
The European Green Deal and Circular Economy Action Plan 2.0, following the European Plastics Strategy [18], aim to increase the recycling of plastics, boost the recycled content in everyday products, and reduce the use of virgin materials. EU rules on single-use plastic products [19] aim to prevent and reduce the impact of certain plastic products on the environment and on human health, focusing on promoting the transition to a circular economy with innovative and sustainable business models, products, and materials, therefore also contributing to the efficient functioning of the internal market. Specific targets include a 77% separate collection target for plastic bottles by 2025, increasing to 90% by 2029, and incorporating 25% of recycled plastic in PET beverage bottles from 2025, and 30% in all plastic beverage bottles from 2030. The targets set by the environmental legislation for the recycling of EoL components and structures will mean that 33,000 tons of CFRP will be recycled every year in Europe by 2025 [20].
To reach all these goals, the recycling of thermoplastic waste industry must overcome a number of challenges. One of the most demanding tasks to be addressed is the rise in microplastic burden from recycling processes. The degradation of plastic and the subsequent release of microplastics into the environment have attracted significant public attention. Microplastic generation during plastic recycling, particularly during the size reduction phase, is a concerning unintended consequence. Despite being detected in recycling facility wastewater and sludge, pathways, factors, and minimization strategies for microplastic generation remain understudied. Material type and environmental weathering significantly influence microplastic generation rates, with material hardness strongly correlated to microplastic generation [21]. This suggests that plastic recycling facilities may inadvertently contribute to microplastic pollution. Mitigating microplastic release from recycling processes has become a key focus in the literature recently, highlighting the need for research into capturing methods and understanding the proportion of generated microplastics released into the environment [22]. With mechanical recycling expected to increase, urgent efforts are needed to comprehend the scale of microplastic generation and release, especially considering their presence in ecosystems worldwide [23].
Furthermore, thermoplastic manufacturing is highly dependent on the utilisation of fillers during the industrial production, for the improvement in properties as well as the reduction in costs. Most thermoplastics in their original form are impact resistant, often brittle, hard, combustible, etc.; fillers are incorporated during the processing to improve these properties, such as strength, stiffness, thermal conductivity, etc. Some common filler materials used are glass fibres, talc, calcium carbonate, silica, carbon black, flame retardants, etc. [24]. Although filler incorporation is vital for the industry and production, it causes several issues afterwards, during the recycling of those waste materials. Waste streams from the electrical and electronic equipment, and automotive and construction sector suffer from contamination, which in many cases is with substances of high concern. As a result, highly contaminated waste streams pose environmental issues and have lower market value, sometimes decreased properties, and increased complexity of processing [25]. Specifically, the mechanical recycling of polymers from WEEE and automotive waste suffer from the presence of brominated flame retardants, which have been used in products for years and have recently been evaluated as substances of very high concern (SVHC) or even persistent organic pollutants (POP). The presence of those substances poses threats to humans, animals, and the environment, and therefore, their removal from streams has to be dealt with. The detection of those substances is possible through spectroscopic techniques and their quantification using chromatographic techniques [26]. Regarding their purification, extraction in supercritical conditions has been proven to be effective [27,28], as well as their purification through solvent-based recycling processes, such as the CreaSolv® Process [29].
Nowadays, due to their strategic properties, the integration of thermoplastics and thermosets as matrices in composite materials is rising, with their market penetration growing in established sectors and their adoption in new sectors, although their deployment is currently limited due to a lack of recyclability and re-usability, which is hindered by their complexity [30]. Inevitably, this results in more waste from manufacturing, and an increasing challenge to develop economically sustainable recycling routes for end-of-life (EoL), taking also into account that, according to their chemical composition, from a technological point of view, thermoplastics are easily recycled and reprocessed by conventional methods, while thermosets have long been considered thermally unprocessable as a result of the presence of covalent intermolecular cross-links. However, each recycling technology has its challenges and limitations for either polymer recycling, reinforcement reclamation, upcycling, and re-use.
Thus, based on the needs resulting from the use of polymer materials, all recent trends, challenges, and outcomes in polymer and polymer composites recycling are analysed in this review.
2. Recycling Technologies of Thermoset Polymers and Their Composites
Thermosets are polymers with high molecular weight, offering alternative properties and applications compared to thermoplastics. This is due to their cross-linked molecular structure, which provides mechanical strength, thermal stability, endurance, and chemical resistance [31]. However, their crosslinked nature is what makes their recycling challenging. Thermosets can be found in coatings and insulation systems, but mostly as matrices in composite materials, reinforced by various fibres such as glass and carbon fibres [32]. Τhe recycling of thermoset materials mainly addresses their composites, as the reinforcement is the one to be reclaimed, mostly due to its high value [33]. The methods analysed below can also be applied to the neat thermoset resins.
2.1. Thermal Processes
Thermal processes are usually applied to the recycling of thermoset FRPs, as these materials lack processability aspects and the capabilities to separate fillers and reinforcement. After thermal recycling, the reinforcing fibres can be reclaimed and can be upcycled or re-used in composite applications. Recycling thermal processes are using heat as an energy source in order to break the polymer chain and crosslinks of the polymeric matrix, resulting in various by-products, such as gas, liquid molecules, and solids.
Thermal processes for the recycling of polymers are being used at an industrial level, targeting energy recovery (with suitable apparatus) compared to the incineration process, where the energy is lost during combustion and the collection of liquid by-products to be further used as fuels [34]. In the case of thermoset FRPs, thermal processes are being used to also recover the reinforcement. Two main processes are considered: (i) fluidised bed and (ii) pyrolysis. In the following paragraphs, the thermal processes and their recycling capabilities are summarised.
2.1.1. Fluidised Bed
Main Principle and Process Description: Fluidised bed is a thermal recycling method used for reclaiming size-down reinforcement and/or fillers from composite scraps. Fluidised bed has industrial applications, as it is very efficient in heat transfer and its parameters are easy to control. Usually, the composites are previously grinded to smaller granules (approx. 25 mm) to be fed into the fluidised bed reactor, consisting of a sand bed. Within reactor preheated air is circulated [35]. After the material insertion to the reactor, a hot gas stream (450–550 °C), e.g., oxygen, breaks down the polymer and separates the fibres and inorganic fillers. After this step and through a cyclone, separation is achieved depending on the weight of the produced products. In this method, high fibre fraction collection is feasible; however, the polymer matrix cannot be recycled since it totally decomposes to solid and gas by-products. Thus, fluidised bed is a suitable recycling method to reclaim fillers and clean short fibres for further valorisation in other applications.
Reclamation of GFs from polyesters: Kennerley et al. [36] studied the reclamation of glass fibres (GFs) from polyester moulding compound (SMC) scraps, with a total formulation (by weight) of 25% polyester resin, 22% chopped GFs, 35% hydroxide carbonate filler, 15% aluminium hydroxide filler, and 3% additives and processing aids. Recycling conditions were set to 450–550 °C with cyclone velocities of 1.3 m/s to 1.7 m/s. After a purification step (washing), the reclaimed fibres turned out to be up to 40% of the total fillers collected in the bin. It was identified that at 450 °C, the fibres had half of the virgin GF’s mechanical strength. To evaluate the reusability, the fibres were functionalised with polyester-compatible silane solution and used to manufacture a dough moulding compound in various concentrations. From tensile and flexural testing, it was concluded that in a material manufactured by Dough Moulding Compounding (DMC), the addition of up to 50% of reclaimed GFs can be performed without affecting the physical properties. Another study performed by Pickering et al. [37] evaluated the reclamation of GFs from three different post-industrial scraps; SMC, a filament wound pipe and an E-glass/polyester sandwich panel. The characteristics and properties of the obtained GFs were characterised by measuring the length distribution, as well as mechanical testing, and through comparison to virgin fibre properties. By image analysis, the average length was measured to be 5.6 mm. From mechanical testing, the results showed a 50% reduction at a 450 °C processing temperature, while at 650 °C, the reduction was more than 80%. A general conclusion obtained from the parametric study is that the degradation of properties depends on both temperature and time conditions. The authors highlighted that due to the high operating temperatures of the bed, composites need to be grinded in granules of 10 mm or smaller for the polymer to be combusted completely. For larger fragments, the residence times had to increase to receive clean fibres, and this will probably lead to a reduction in mechanical properties.
Reclamation of CFs from epoxies: Jiang et al. [38] studied the reclamation of carbon fibres (CFs) from epoxy-based thermoset composite using fluidised bed, and the effect of this thermal treatment was assessed. The temperature was set at 550 °C and the hot air was inserted with a velocity of 1.0 m s−1. The results showed that after 10 min, the epoxy was fully removed. To ensure that fibres were clean from any debris after the process, they dispersed them in acetone and cleaned in an ultrasonic bath for 30 min.
2.1.2. Pyrolysis
Main principle: For the reclamation of larger quantities and lengths of reinforcement from GFRPs/CFRPs, pyrolysis has been studied as a thermal treatment. Pyrolysis operates with the same principles as fluidised bed, as high temperatures that are applied; however, in pyrolysis, inert atmosphere is used in the process [39]. On principle, when polymers are being pyrolysed, depending on the process parameters, the polymeric main chains degrade or rapture, resulting in the creation of side groups. For the second case, typically, volatile products and char residues are formed. In the case of thermoset polymers, less volatiles and a higher amount of char by-products are formed, due to the crosslinked nature [40]. Resulting volatile compounds can be divided into gases (non-condensable gases) and liquids (condensable gases) [41]. The composition of those by-products, as well as the temperatures applied to the process, are highly dependent on the chemical nature of the thermoset matrix. To identify those compounds, usually the pyrolysis is coupled with gas chromatography/mass spectroscopy (Py-GC/MS) [42]. Evans et al. [43] have studied the thermal degradation of 23 types of polyesters, at 600 °C, and evaluated the decomposition by-products through GC. It was identified that styrene was the predominant degradation product from the degraded polyester and phthalic anhydride was the main product from the styrene-free polyesters.
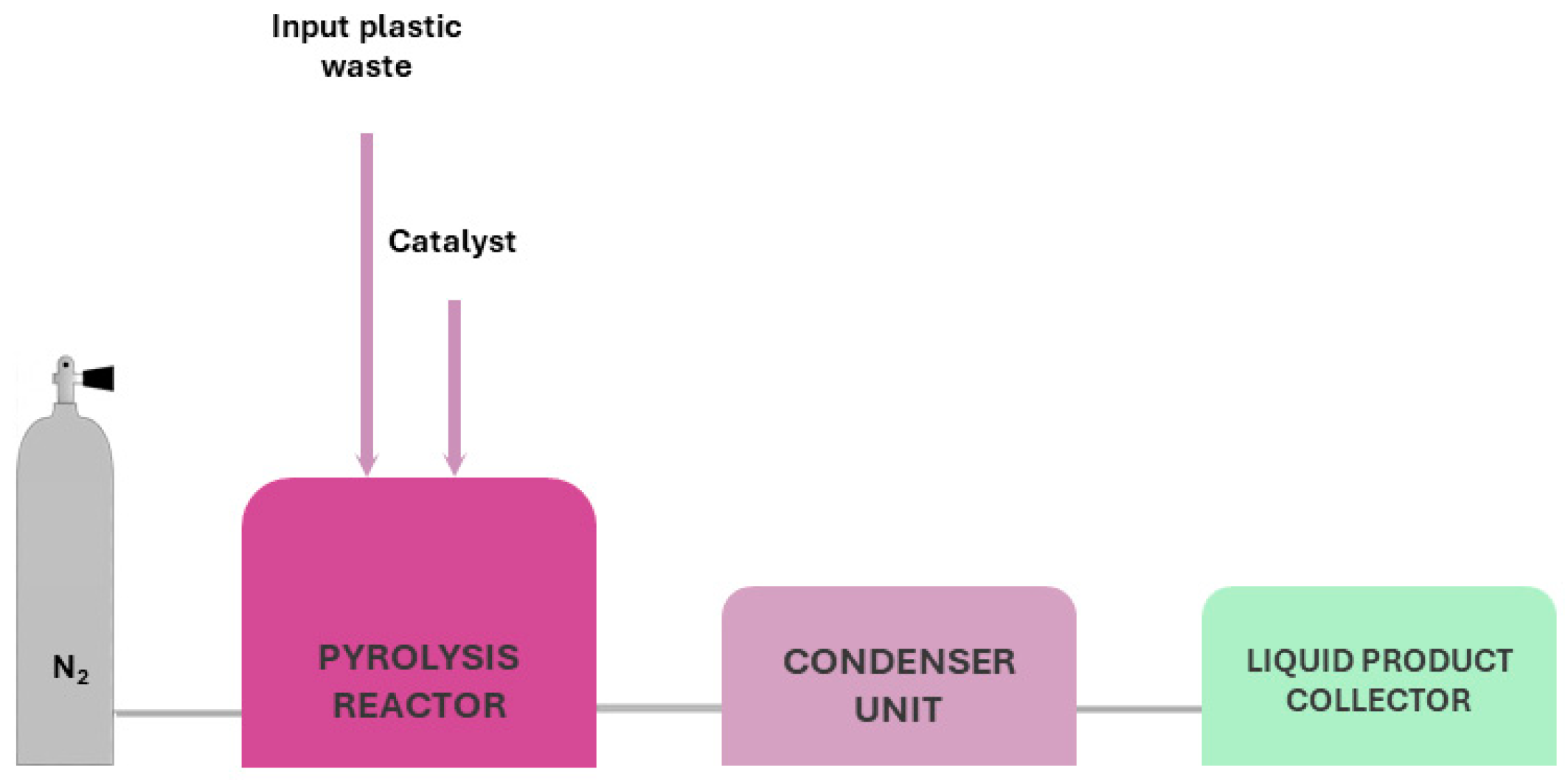
Process Description and Equipment: A typical pyrolysis system is the fixed bed reactor. The apparatus is horizontally or vertically mounted and in cylindrical shape. For a horizontal pyrolysis furnace, the main parts, as depicted in Figure 1, are:
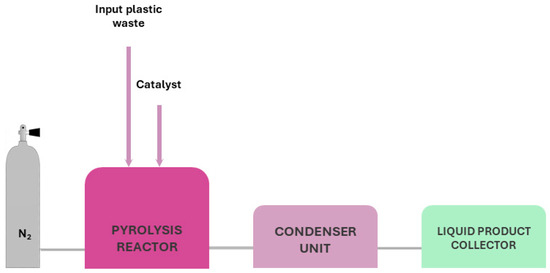
Figure 1.
Typical set up for pyrolysis of thermoset and thermoplastic waste.
- Pyrolysis chamber; the material is inserted to be pyrolysed;
- Gas inlet, located at beginning of the chamber; it is connected with the inert gas to ensure proper conditions;
- Gas outlet located at the end of the chamber to ensure the proper removal of gas and liquid by-products;
- Condenser chamber for condensable gases;
- Scrubber unit;
- Gas filtration system.
The process usually carried out is the following: The material is inserted in the centre of the chamber, where isothermal conditions are met, before the initiation of the process. Afterwards, the inert gas is inserted to remove oxygen and reach inert conditions (purging). Once purging is complete, the furnace is heated to the desired pyrolysis temperature with steady heating rate. During pyrolysis, the gas and liquid by-products are driven by gas flow, out of the chamber and into the condensers, to collect liquids and oils. Then, the rest of the gases are passed through scrubbers to entrap any solids. Afterwards, the rest of the gases are filtered before being exhausted. When pyrolysis is completed, the heating is stopped and the system is left to cool at room temperature, under inert conditions, before retrieving the solids [44].
Mechanism: The pyrolysis process needs to be adapted based on the composite material’s chemical nature, making it a challenge when it comes to composite reinforcement reclamation. A study from Cunliffe et al. [45] was performed to evaluate the correlation between applied temperature and polymeric matrix. Composites with polyester, polyester-styrene, polybenzoxazine, and epoxy resins as matrices were evaluated. The solid, liquid, and gas by-products were characterised depending on the pyrolysis temperature profiles for each type of matrices. Furthermore, the definition of the pyrolysis parameters was supported by thermogravimetric analysis. They concluded on several aspects, such as that the chemical nature of the polymeric matrix strongly influenced the produced solid and gas compositions. At higher temperatures, fillers such as calcium carbonate, influenced the yields of the process, and for phenolic and epoxy resin matrices, it was observed that the mass loss was continuous compared to the other resins, which could be attributed to carbonisation phenomena.
Epoxies Case: An example of a pyrolysis mechanism for thermosets is given for epoxy resin composed of DGEBA. It has been identified that the thermal degradation follows two main mechanisms: (1) the the cleavage of cross-linked and ether bonds, and (2) polycondensation stages. The thermal decomposition takes place between 330 °C and 390 °C, and a residue of 10% of the total mass is left behind. However, for bisphenol A compounds, higher temperatures that can reach up to 420 °C are needed [46].
The cleavage stage consists of the removal of side groups from the main polymer chain, producing a high number of free radicals and low molecular weight groups (gas by-products). From these groups, OH, C-C, and CH3 are the intermediate for the formation of H2O, CO, and CH4. From the main chain breakage, liquid by-products are being produced, such as BPA, phenol, benzene, etc. Dehydroxylation reaction can take place for phenol, forming aromatic hydrocarbons. Ring-opening reactions can also occur, resulting in chain-like hydrocarbons. After the complete cleaving stage, H/C mass ratio gaseous products, as well as low H/C mass ratio liquids and solids, are generated.
In the polycondensation stage, the produced liquid and solids are dehydrogenated to form pyrolysis char, while the gases generate H2, H2O, and CO. By increasing the pyrolysis temperature, the rates of the by-products are affected [47].
The presence of the reinforcing fibres in the pyrolysis process in thermoset composites and their influence on the pyrolysis process was evaluated by Ge et al. TGA characterisation was performed on pure epoxy and epoxy CFRPs, and the degradation rate of the two was studied [48]. Different ratios of carbon fibre/epoxy were characterised, and they identified that for ratios 2:7, 3:6, and 4:5 epoxy/carbon fibre, the final weight loss was smaller, when applying high heating rates. Compared to pure epoxy resin, they identified that the maximum weight loss was much larger than the weight loss of the composites at the higher heating rate. This phenomenon was attributed to the presence of carbon fibre, which made pyrolysis rate of epoxy resin lower. Finally, after TG-FTIR analysis and pyrolysis produced gas detection, they proposed a possible mechanism for the pyrolysis reaction in a CFRP system. The model indicates that in the first stage of pyrolysis, the long polymeric chains break down to monomers, e.g., bisphenol A and 2-methyloxirane. With the increase in temperature, the C-C bond dissociated to form phenol, methanol, and alkanes.
Another study performed by Adler et al. evaluated the degradation characteristics of epoxy CFRP composites and epoxy CFRPs composites manufactured with pyrolysed CFs, through TGA analysis [49]. The results showed that the curves between the two materials were not identical, and this deference was attributed to the influence of the fibres sizing on the epoxy resin’s network during curing. The phenomenon was explained based on the compatibility of the sizing on the fibres with epoxy matrix, compared to fibres with no sizing. The fibres with no sizing resulted to different cross-linked epoxy network, compared to the sized ones, presenting different degradation behaviour, with greater mass loss in the pyrolysed fibre-CFRP.
Table 1 summarises the literature results on pyrolysis studies conducted on reinforcing composites (FRPs).

Table 1.
Literature review on the pyrolysis of FRPs.
As can be observed from Table 1, for the separation and reclamation of fibre reinforcement, the applied pyrolysis temperature and residence time are important factors, especially when considering the yield of the by-products. The pyrolysis products, i.e., solids (reinforcing fibres, char) oils, and gases, produced under suitable conditions, can allow the valorisation of these products for enhancing the material circularity [53]. The gases can be valorised for energy recovery, the liquids when post-processed can be used as fuels, and solids, in case of fibres, can be used as reinforcement for composite applications.
When pyrolysis is applied as a recycling approach, apart from the thermal degradation of the polymer matrix, the structural and mechanical integrity of the reinforcing fibres should also be considered. Nahil et al. [50] have investigated the pyrolysis of woven CF/Polybenzoxazine resin composites as well as their produced by-products. The produced gases were analysed by GC and the produced liquids by coupled GC/MS. The investigation was performed at 350, 400, 450, 500, and 550 °C for 1 h in nitrogen atmosphere. They identified that by increasing the temperature from 350 °C to 700 °C, the solids residue yield was decreased while the liquids yield was increased. For the produced gases, the yield was similar to those of the liquids. With regard to the fibre reinforcement, solid residue was left on the fibres in the form of char from the resin carbonisation. Oxidation was performed, under different temperatures, to remove the formed char and then the fibres were evaluated for their mechanical performance through tensile testing. They concluded that the best conditions for CF reclamation with 93% retained tensile properties were pyrolysis and oxidation at 500 °C.
Post-pyrolysis treatment: As evident from Nahil et al.’s [50] study, in FRPs, the solid residue product from pyrolysis consists of the remaining fibres and char residue (pyrolytic carbon). In order for fibres to be re-used, further steps are needed to remove the char from the surface, as already mentioned. Post-pyrolysis or post-treatment in oxidative medium at a high temperature burns the char and results in clean fibres. Giorgini et al. [52] have investigated the correlation between residue char amount (thickness) formed on the fibre’s surface and the applied pyrolysis temperature. They identified that with the increase in the pyrolysis temperature from 500 °C to 600 °C, the amount formed on the fibre’s surface was reduced. Termine et al. [44] investigated the correlation between the removal of the formed char of the CFs with temperature and residence time. CF woven fabrics were post-pyrolysed for 10, 20, 30, 40, and 50 min of residence time at 400 °C and 500 °C in air. They concluded, through SEM analysis, that at 500 °C for 50 min, the char was removed from the fibres and the fibre structure was intact, as evaluated by Raman spectroscopy. The structure integrity is an important factor for the capability of CFs to be reused, as after the pyrolysis process, the fibres should result in their mechanical properties being close to those of a virgin CF. Termine et al. reclaimed CF fabrics from epoxy-based CFRPs and reused the fabrics with the most promising results as reinforcement to manufacture epoxy-based CFRPs. The composites were evaluated under tensile and flexural testing; however, the results showed a decrease of 30% and 12% in tensile and flexural strength, respectively. Thus, for the use of recycled CFs from pyrolysis, non-structural CFRP applications were proposed.
Last but not least, pyrolysis is also applied for the reclamation of GFs. However, in their case, the parameters should be further tailored, as the mechanical properties of reclaimed GFs can be greatly degraded [37].
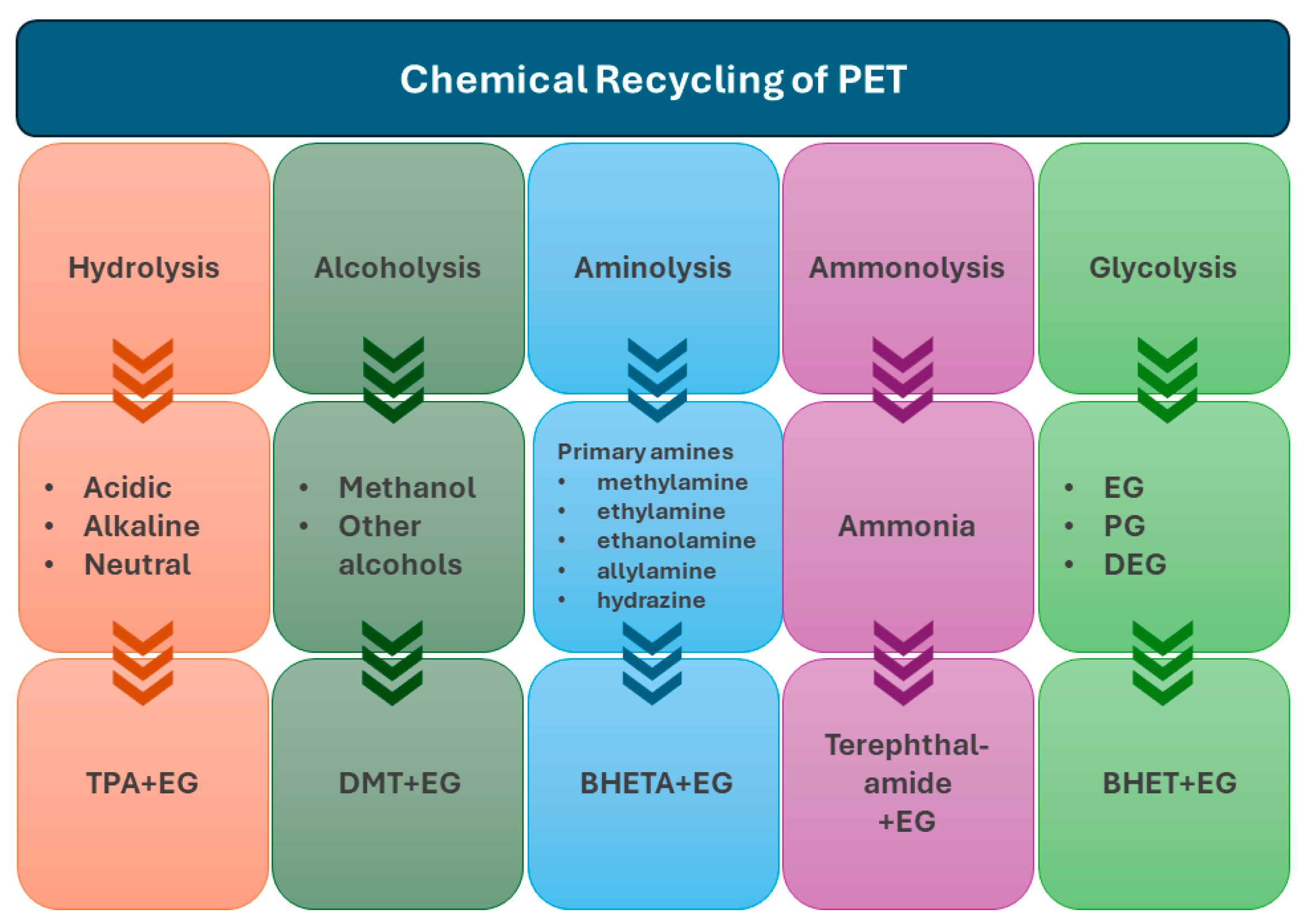
2.2. Chemical Processes—Solvolysis
Chemical recycling refers to the chemical decomposition of polymers into mono-oligomers and the recovery of fibres in the case of composite materials. However, the recovery of the mono-oligomers, due to the solvents and catalysts used, is a hazardous process; therefore, they are typically discarded as waste and only the reinforcement is recovered [33].
The main processes are solvolysis at low temperatures and ambient pressure, and solvolysis at near- or super-critical conditions. Solvolysis uses solvents such as water, alcohols, acetone, glycol, or acids in order to break down the chemical bond of the polymer matrix (either epoxy, polyester, or phenolic). The interest is that fibres can be recovered and can be reused. Solvolysis offers a large number of possibilities due to its wide range of solvents, catalysts, temperature, and pressure used, and can be applied successfully for both CFRPs and GFRPs, as shown in Figure 2.

Figure 2.
SEM images of (a) CFRP, (b) chemical recycled CFs, (c) GFRPs, (d) chemical recycled GFs.
2.2.1. Low Temperature and Pressure Solvolysis
Solvolysis at low temperatures and pressure is generally carried out below 200 °C and at atmospheric pressure. For that reason, in order to degrade the resin in mild conditions, the use of acids or bases and catalysts is necessary. Extensive research has been carried out for epoxy-based composites through this process. Epoxy-based CFRPs treated with bases: Regarding the treatment with bases, Peng et al. [54] conducted recycling of fibre/epoxy resin composite with a poly(ethylene glycol)/NaOH system at a temperature of 200 °C for 4 h. The experiment occurred with 200 g PEG200, 1 g NaOH, and 10 g of FRC with a high decomposition efficiency of 84.1–93.0%.
Epoxy-based CFRPs treated with acids: As for the use of acids, Feraboli et al. [55] developed a solvolysis process for FRCs using sulfuric acid with hydrogen peroxide to accelerate the reaction and oxidize the matrix. The experiment relies on 110 °C in ordinary pressure with mechanical stirring throughout the 30 min duration of the entire process. According to SEM images of recycled CFs, they appear to have a clean and smooth surface, with very limited traces of resin or coating residue. Mechanical properties were also tested through tension, compression, flexure, and short beam shear tests, which showed traits similar to those of virgin fibres. For the nitric acid solution, Liu et al. recycled carbon fibre with epoxy matrix. At a temperature of 90 °C in a glass vessel, the experiment took place with a nitric acid solution at a concentration of 8 M with a ratio of 40 g/L of the composite being the best. Under these parameters, the resin liquefied with a conversion of more than 99 wt.% and the undamaged CFs were recovered with tension strength loss of 1.1% [56]. A novel recycling method, using formic acid as a solvent, was proposed by Ballout et al. [57]. The recycling process transpires with CFs reinforced with epoxy resin at room temperature and ambient pressure. SEM and TGA results showed 10 wt.% of residual epoxy resin, indicating that the applied condition does not lead to the full dissolution of the cured epoxy resin.
Epoxy-based GFRPs treated with acids: Dang et al. [58] also investigated recycling using a nitric acid solution for GFRPs. They recycled GFs (E-glass and T-glass fibre type) by decomposing epoxy resin with a solution of nitric acid of 4 M at a temperature of 80 °C. Tensile strength and stiffness tests were not performed on the fibres; however, it was observed that T-glass fibre possessed more excellent corrosion resistance to the acid solution.
2.2.2. Solvolysis at Near- or Super-Critical Condition
Solvolysis at near- or super-critical conditions is a relatively new process that is more environmentally friendly and sustainable. Using high pressure as a processing tool can exempt the use of catalysts and toxic solvents. Sub- and super-critical fluids such as water, acetone, and alcohol are excellent reaction media for the depolymerization or decomposition of thermoplastics and thermosets. Composite materials are decomposed into smaller molecular components and fibres rapidly. The process is considered a green process, since there are no toxic products released into the environment, and the solvents can be recovered and reused. Epoxy-based CFRPs treated with supercritical acetone: Okajima et al. [59] studied the decomposition epoxy resin in CFRPs using super-critical acetone testing of different parameters of the experiment. The decomposition was at a maximum of 95.6% efficiency at 350 °C at 140 bar pressure for 60 min with a ratio of 4.35 mol of acetone per litre of the reactor volume. The reactor was a stainless-steel tube with an inner volume of 8.9 cm3, while the CFRP plate was a rectangular piece 1 mm thick, 50 mm × 5 mm. The supercritical mixture of acetone/water was also investigated by Keith et al. [60], with regard to decomposing carbon fibre-reinforced epoxy resin. The recycling process transpires in a volume ratio of 8:2 of the acetone/water mixture with temperatures and pressures at a range of 300–380 °C and 16–30 MPa, respectively, at a reaction time of up to 150 min. For this process, a stainless steel 100 mL reactor without stirring was used with 50 mL of solvent mixture. The CFRP contained a 35 wt.% of resin; therefore, the ratios of 30 g, 60 g, and 90 g of resin per litre of solvent were investigated. After processing 20 MPa and 30 g/L of resin for 120 min at 320 °C, the minimum conditions necessary for effective fibre recovery, up to 95 wt.% of the resin was decomposed and the original weave architecture of the fibre was retained.
Epoxy-based CFRPs treated with supercritical water: The use of solvent mixtures was also researched by Henry et al. [61] to separate the CFs from epoxy resin matrices. The experiment relies on super critical water (SCW) and (50/50 vol%) water/ethanol mixtures in supercritical conditions at a temperature of approximately 375 °C and 25 MPa pressure for a range of 15 to 120 min. SEM images coupled with TGA analysis of the reclaimed fibres showed that up to 98 wt.% of the resin was decomposed. The CFs that were treated by SCW proved to have a higher tensile strength; on the other hand, for the ones that were treated with the water/ethanol mixtures, their surface underwent a gentle in situ oxidation. Knight et al. [62] recycled epoxy CFRPs using only supercritical water but with 0.5 M KOH as catalyst. The process occurred at approximately 28.5 MPa, 410 °C and a reaction time of 15 to 120 min. The higher resin elimination was 99.2 wt.% at a reaction time of 120 min. Phenolic-based CFRPs treated with ethylene glycol: In a different approach to solvent mixtures, Yildirir et al. [63] recycled CFRPs with a phenolic matrix. The solvents used were ethylene glycol (EG) and an EG/water mixture, and they were placed in a 500 mL stainless steel reactor in a volume of 60 mL, along with a 2.5 g CFRP, under a N2 purge. Ethylene glycol alone decomposed 92.1% at 400 °C and 4.2 MPa; however, the addition of water in a volume ratio of EG/water 5:1 and 3:1 decomposed 97.6% and 95.2% at 400 °C in zero residence, respectively. The mechanical properties between virgin fibres and recovered fibres had minimal differences. Polyester-based GFRPs treated with supercritical water: With supercritical water, Oliveux et al. [64] performed chemical recycling on GFRPs. The composites contained 37.5 wt.% unsaturated polyester resin. The experiment took place in a 587 mL batch system testing different temperatures, reaction times, water-to-resin ratio, pressure, and NaOH catalyst concentration. Out of 19 experiments, the best conditions were carried out at 300 °C, 85 MPa, without NaOH catalyst for 30 min with a ratio mass of resin per distilled water volume at 0.01 g/mL. A second hydrolysis at 250 °C for 10 min with a ratio mass of contaminated fibres per distilled water volume of 0.05 g/mL, was performed on the GFs after the first hydrolysis. Then, the fibres were washed with dichloromethane (DCM), in order to clear the coated formation of an organic gluing substance of the fibres. Particularly, after two washes with DCM, the yield of eliminated resin reached 100%. An investigation of the mechanical properties of the fibres showed that a 35% to 65% reduction in strength was observed in a range of different temperatures and reaction times.
2.3. Extraordinary and Combined Recycling Processes
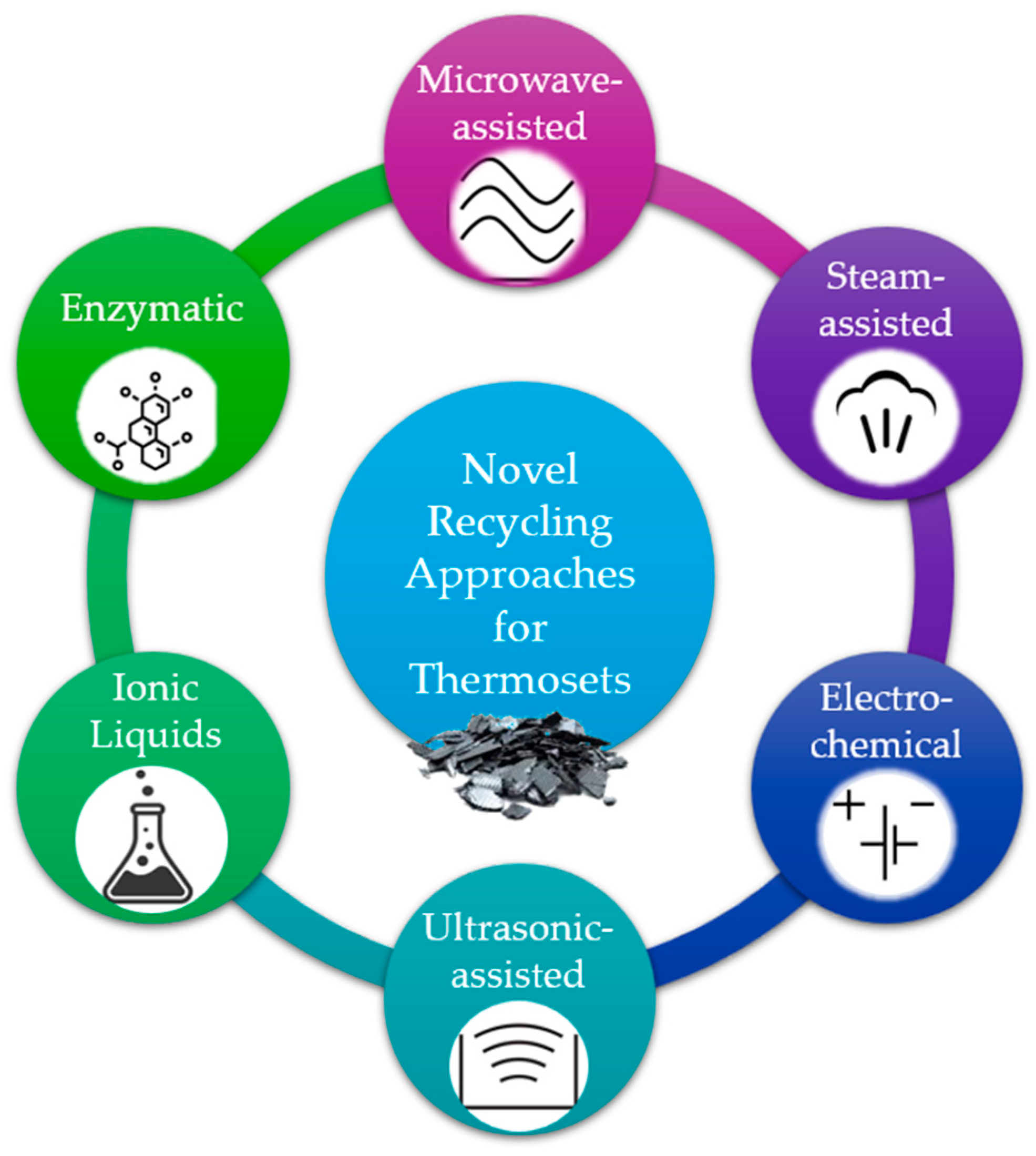
Several extraordinary techniques have been developed for the recycling of thermoset composite materials, either by combining steps of the aforementioned procedures or by exploiting other means of energy for the breakage of the chemical bonds of the polymers. Combining multiple recycling techniques, such as chemical and mechanical methods, in a hybrid approach can synergistically improve the overall efficiency and effectiveness of thermoset composite recycling processes. The most unique techniques are presented in Figure 3, and they are analysed in the following paragraphs.
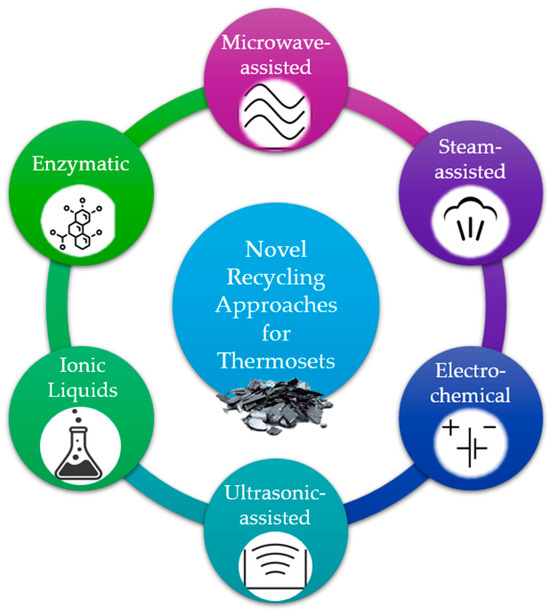
Figure 3.
Energy sources, triggers, and media of new recycling approaches.
2.3.1. Microwave-Assisted Recycling
Microwave-assisted recycling utilizes microwave energy to selectively heat and break down thermoset composites. This process can be more energy-efficient and selective compared to traditional thermal methods, allowing for the targeted decomposition of the polymer matrix while preserving the reinforcing fibres.
Epoxy-based CFRPs: One of the first successful trials of extracting CFs from waste CFRPs by microwave irradiation was that of Obunai et al. in 2015 [65]. In this study, two types of epoxy-based CFRPs were used as wastes, and a simplified microwave irradiation apparatus with three different atmospheres (Ar, N2, air) was utilized. For all experiments carried out, the power and frequency of microwaves was set to 700 W and 2.45 GHz. It was proven that the most effective extraction of CFs was that under an Ar atmosphere, since spark glow plasma was generated, which decomposed the gasified resin. On the other hand, the study of Jiang et al. [66] during the same year investigated the effect of the temperature during a similar procedure, in N2 atmosphere for epoxy-based CFRPs, for 30 min exposure in a microwave oven. From the chosen temperatures, the 500 °C was selected as the optimum one since it offered an acceptable level of fibres cleanliness and without severe defects. The most recent study on the recycling of CFRRs, with a low cost and green microwave-assisted chemical method, is that of Zabihi et al. (2020) [67]. This one-step process is fast (only 1 min exposure in microwaves), catalyst-free, and uses a mixture of hydrogen peroxide and tartaric acid. Different mixing ratios of the hydrogen peroxide and the acid were tested, while the microwave power was constant at 800 W, for different time durations. Compared to other chemical approaches, is seems that is the fastest until now in terms of process duration (25 min in total, including the cooling down), the lowest in temperature from those executed in elevated temperatures, and the most promising regarding tensile strength retention. Also, compared to the electrochemical techniques that will be presented in the next section, the microwave-assisted chemical method of Zabihi et al. excels in time but not in temperature.
Epoxy-based GFRPs: A recent study (Rani et al., 2022) [68] describes the recovery of GFs from epoxy-based waste composites through a microwave-based approach. In this procedure, a combination of solvolysis and microwave treatment was tried out. A chemical solution with acetic acid and hydrogen peroxide with different mixing ratios was prepared with an increment of volume ratio of hydrogen peroxide from 20% to 50% in the acetic acid. As a process parameter, the exposure time was investigated, while the power was constant at 700 W. Both the decomposition rate of the resin and the mechanical properties of the reclaimed GFs were studied. An exposure of 180 s in microwaves, in a stepwise process, was proven to be the most effective since the reclaimed GFs had similar strain-to-failure responses as the virgin ones. GFs were also reclaimed from scraps through microwave pyrolysis and reused in new composites, in combination with virgin GFs, according to the study of Akesson and Skrifvars [69]. The power was set at 1 kW and the samples were pyrolysed at 450 °C for 1 h, but the reclaimed GFs were covered with char that had not been degraded after the pyrolysis process.
2.3.2. Superheated Steam Recycling
Superheated steam treatment involves subjecting thermoset composites to high-pressure steam at elevated temperatures. This process can cause the polymer matrix to degrade, facilitating the separation of fibres from the resin. The resulting materials can then be reused in new composite applications.
Epoxy-based CFRPs: Extensive research of the superheated stream recycling of CFRPs has been executed by Cai et al. [70,71,72] for thermoplastic PA6 composites with CFs. They used an induction heating superheated stream generating system (5 kg/h steam flow rate) with a temperature range from 500 °C to 700 °C. The procedure included the following steps: (a) 1 h treatment at 400 °C with pure superheated stream, and (b) 1 h superheated steam treatment at 500 °C in the presence of 4 vol% O2. A similar apparatus and procedure were employed by Wada et al. [72] to study the effect of the superheated stream to the interfacial adhesion of CFs on epoxy resin and as a surface treatment method of CFs that can be used after their reclamation from composite wastes. The fastest and most promising route using steam for CFs recovery from CFRPs is described in the work of Joeng et al. (2019) [73]. In this work, steam was used as an oxidant to remove the resin from the CFRP, at a medium temperature range (600–800 °C) in a pyrolysis furnace. The heating rate and holding time at the target oxidation temperature were determined to be 10 °C/min and 60 min, respectively. Surface-clean CFs can be collected from waste CFRPs at 700–800 °C in 140 min of total process time, excluding cooling time with this methodology, which can also be transferred to a continuous process. Polyesters: Superheated steam recycling has also been implemented on terephthalic and ortho phthalic acid-based co-polyesters crosslinked with styrene to recover oligomers [74]. Since the process was promising, it can be reproduced for co-polyester thermoset composites, to valorise both the reinforcement and the matrix material.
2.3.3. Electrochemical Recycling
Electrochemical methods involve applying an electric current to thermoset composites immersed in an electrolyte solution. This process can induce chemical reactions at the polymer-electrolyte interface, leading to the breakdown of the polymer chains. Electrochemical recycling holds promise for selectively degrading thermoset materials while preserving valuable components like reinforcing fibres.
Epoxy-based CFRPs: In 2015, a study on the recycling of CFs from CFRPs through an electrochemical method was published by Sun et al. [75]. The electrochemical recycling system consisted of a direct current (4, 10, 20, and 25 mA) power supply, a stainless-steel cathode connected to the negative terminal of the power supply, and, as the anode, the CFRP specimen, connected to the positive terminal of the power source. As the electrolyte, a NaCl solution was used, with concentrations of 3%, 10%, and 20%. The recycling procedure lasted for 21 days and samples were taken on regular intervals to quantify the recycling depth (surface degradation). The cleanest fibres were obtained at applied current of 4 mA and NaCl solution concentration of 3%; the measured tensile strength of the reclaimed fibres on these conditions was 80% of the virgin ones. After 5 years, the study of Oshima et al. [76] presented a rapid removal of epoxy resin from a UD CFRP by high-voltage electrical treatment, causing water electrolysis, by using a similar setup of the electrochemical cell of Sun et al. This treatment was performed under a constant voltage of 2.5–15.0 V and different electrolytes were tested (aqueous solutions of NaCl, KCl, NaOH, KOH, and Na2CO3), with concentrations between 0.01 and 1.0 mol L−1. It was proven that the weight loss in neutral electrolytes such as the NaCl or KCl solution was higher than that in basic electrolytes. The weight loss and current density increased with an increase in the electrolyte concentration. This suggests that high electrolyte concentrations improve the effectiveness of the electrical treatment. In this process, the maximum electrical treatment time was 20 h. Weight loss was measured as an indicator of the process effectiveness and a removal rate of approximately 200 μm/day was achieved. However, the high voltage application in the long-time induced damage of the CFs made it difficult to operate as a main recycling method, but it worked more as a supporting one, in combination with other techniques.
Polyester-based GFRPs: GFRPs have been successfully recycled through high-voltage fragmentation, a technique developed by Mativenga et al. [77], which was first reported in 2016. The high-voltage fragmentation process uses repetitive pulse electrical discharges between two electrodes within a dielectric liquid environment (water in this case), to disintegrate the composite material. The discharge creates a spark channel that travels between material internal boundaries and the latter generates an intense shockwave with pressure up to 1010 Pa and temperature greater than 104 K. For the current study, the tests were conducted at 160 kV applied voltage, 10 mm electrode gap, and 1 Hz pulse frequency. The number of pulses (500, 1000, 1500, 2000) was selected as the process parameter and it was proven that residual resin content depends on the number of electrical pulses applied inversely. The process offers cleaner fibres, longer fibre length distribution of the reclaimed fibres, higher percentage of fibres at mean fibre length, and lower retained resin content than composites recycled mechanically. However, it is highly energy consuming.
Reclamation of continuous CFs from CFRPs: Recently, the recycling of continuous CFs from CFRPs was achieved by Sarmah et al. [78] by exploiting the Joule heating effect, using a simple electrical connection DC setup. The target was the composite specimen to reach 400 °C through Joule heating (recorded through an IR camera), which was proven to be an effective decomposition temperature for the resin, according to the TGA results. The reclaimed fibres showed a 10–15% decrease in tensile strength. The methodology can also be applied to continuous composite rolls, with a potential upscaling to industrial level. It is worth noting that current research through the European Project “EuReComp” of Horizon Europe investigates the use of plasma during solvolysis for the reclamation of continuous CFs from thermoset composites. In this case, the use of nitrogen plasma inside concentrated nitric acid solution is proposed, since a plethora of reactive species are produced (NO2+, H3O+, OH•, H+, H•, NO2•, H2O2, O•+, HO•−, H2, O2, NO3−, OH−, NO2−) that can enhance the resin degradation. It has been proven that the time needed for the matrix dissolution and complete detachment of the fibres is significantly shorter than the time reported for conventional HNO3 solvolysis [79].
2.3.4. Ultrasonic Recycling
Ultrasonic waves can be utilized to mechanically disrupt the molecular structure of thermoset composites. By subjecting the material to high-frequency vibrations, ultrasonic recycling aims to break down the polymer matrix and facilitate the separation of fibres. This technique offers potential advantages in terms of energy efficiency and scalability.
A sonochemical approach for the enhanced recovery of CFs from CFRP waste using a mild acid (diluted nitric acid) and peroxide mixture was presented by Das and Varughese in 2016 [80]. A two-stage process comprising a pretreatment stage followed by a sonochemical stage was carried out. In the first stage, composite samples were immersed for pretreatment in an aqueous mixture of 2 M HNO3 and 9 M H2O2 of various concentrations. In the second stage, sonication was carried out in an ultrasonication tank where solid-to-fluid ratio was maintained at 1:60 for optimum cavitation efficiency. The required pretreatment time was determined by measuring the mass uptake of the solution by the sample with immersion time. When the composite samples were fully swollen, the samples were placed in a 470 kHz ultrasonication tank maintained at 65 °C. It was proven that the sonochemical reaction increased the resin decomposition ratio to almost 3 times that of the process without ultrasound, and the recovered fibres had comparable tensile strength to that of virgin fibres. Increasing the H2O2 amount reduced the resin decomposition ratio and oxidized the fibre surface (as the H2O2 content increased in the solutions, the brittleness of the fibres increased and the resin remaining on the fibre surface appeared charred); thus, the optimum ratio of dilute HNO3 and H2O2 was 98–2% vol, since it gave better resin decomposition in the presence of ultrasound without damaging the fibre’s surface.
It is worth noting that recently [81], power ultrasonics have been used for the interlaminar pre-cracking of thermoplastic CF composites, in order to separate the layers and enable their reuse. The ultrasonic assisted pre-cracking proved to be a fast and robust method to initiate controlled artificial pre-cracks in thermoplastic composite materials, avoiding any fibre damage that could affect the mechanical properties.
2.3.5. Recycling with Ionic Liquids
Epoxy-based resins and CFRPs: The decomposition behaviour of epoxy-based CFRPs in a molten KOH was investigated by Nie et al. in 2015 [82]. The reaction was conducted in a stainless-steel reactor which heated in a salt bath. The KOH was put into the reactor in solid form and heated to a molten state and the CFRPs were fed into the molten KOH. CFRPs were decomposed under atmospheric pressure at temperatures from 285 to 330 °C, under N2 flow. Time and temperature are inversely proportional in this process, and it was proved that thermolysis plays an important role in breaking the chemical bond of epoxy resin in molten KOH. The results showed that more than 95% tensile strength compared to virgin CFs was retained. Also, the decomposition product was extracted selectively with water, dichloromethane, ethanol, and acetone. With this process, most of the contaminants in waste composites (thermoplastics, paints, sealants, and GFs) can be decomposed, which is promising for treatment of CFRP waste with many contaminates.
An ecologically friendly recycling method for both epoxy resins and epoxy-based CFRPs (with milled CFs) in the presence of ionic liquids and alcohols under mild working conditions (150 °C and atmospheric pressure) was reported by Perez et al. in 2021 [83]. From a variety of ionic liquids that have been studied, it was proven that the most effective mixture was that of 1-butyl-3-methyl imidazolium acetate with ethylene glycol, which worked in 150 min. The recovered monomer from the depolymerised resin was obtained through precipitation and was employed for the preparation of a recycled epoxy. Additionally, CFs were recovered and reused to manufacture new CFPRs. The mechanical properties of the recycled materials (both epoxy and CFRPs) were similar to the virgin ones.
Recently, a combination of solvolysis with degradation of the polymeric matrix through ionic liquids was presented by Perli et al. [84]. In this work, a cleavable building block for tailoring the degradation of thermoset networks was introduced, based on ionic liquids affording the programable deconstruction of composites. Specifically, a solvent-assisted transesterification reaction was carried out, using ethylene glycol and an acid ionic liquid. The introduction of 10% tetra-epoxidised ionic liquid comonomer in the network with 10% of acid ionic liquid lead to a degradation in activity of 83.7%. When the concentration reached 50% into the networks, it resulted in 95% degradation of the resin, while maintaining excellent thermal and mechanical properties. A significant disassembly of the composites was observed at 190 °C for 4.5 h by using 10% of acid ionic liquid.
Epoxy-based GFRPs: It is worth noting that in 2023, polyionic liquids were produced in situ during the recycling of GFs thermoset epoxy composites during their treatment with sulfuric acid in ambient conditions, which enabled the cleaning of the GFs and the maintenance of their mechanical and surface properties. H2SO4 liquefied the polymer matrix via the formation of oxonium poly ionic liquid and benzenesulfonic acid. This process may last up to two weeks; however, the by-product of this process (the dissolved epoxy) can be used in cement mixtures, increasing their compressive strength, since it can be transformed to a superplasticizer [85].
2.3.6. Biological Recycling
Recent research is exploring the potential for biological methods, such as enzymatic degradation or microbial treatment, to break down thermoset composites; however, the relevant studies are scarce. Enzymes or microorganisms capable of breaking down specific polymers within the composite matrix could offer a sustainable and environmentally friendly recycling solution. Biotechnology can provide solutions for the biodegradation of complex plastics and composites, by using oxidative enzymes. With a model compound simulating the epoxy resin RTM6, a colorimetric high-throughput screening assay was developed by Dolz et al. [86], used to screen unspecific peroxygenases mutant libraries of S. cerevisiae. It was proven that organic solvents help to solubilize the recalcitrant and enhance availability for enzymatic attack. Moreover, the use of chemical and mechanical pretreatment of resins is necessary to enhance the enzymatic recycling of composites and managing their disposal.
From the conventional thermoset composites, trials of enzymatic recycling have been carried out in polyurethane matrix, which contains ester and amide bonds that must be cleaved for efficient recycling. Enzymes target the ester bonds, and many high-value products can be obtained, including alcohols, acids, and aromatic precursors, that can be used as recycled raw materials in chemical industry [87].
The most recent research on thermoset recycling through engineering of oxidoreductases and hydrolytic enzymes is currently carried out by the EU project Bizente, funded by the European Commission. Through protein engineering, enzymes are molecularly modified (pre-engineered through directed evolution to adapt them to the degradation process) to enable the thermoset resin recovery from composites and give them a second life. The project develops a biodegradation technology using enzymes that will enable the recyclability of thermoset composites and recover resins and other products at their end of life, with the aim of making them reusable [88]. This research is still ongoing; thus, there are not yet available results.
2.4. Upcycling of Thermoset Composites to Vitrimers
In recent years, intrinsically recyclable epoxy resins based on covalent adaptable networks (CANs) have been employed for obtaining unconventional polymer networks with exchangeable bonds, rearrangeable under heat, pH, and UV light [89] or another stimulus, while keeping the network integrity, giving the potential to be recycled, reused, and reprocessed [90]. Based on the mechanism of bond exchange, CANs are classified as (1) dissociative CANs, in which the cross-linking bonds break upon heating and reform at lower temperature resulting in a decrease in network connectivity and modification of the cross-linking degree during network rearrangement and (2) associative CANs, which do not depolymerize and exhibit constant cross-link density when changing temperature. In both cases, the bond breaks only when a new bond is formed conducting to a permanent degree of connectivity [91]. Vitrimers are a class of associative dynamic covalent networks, a versatile class of materials that could potentially overcome some of the above-mentioned limitations of thermoset polymers and composites [90,92], as they do not lose their network integrity due to their stable cross-linking density of associative bonds during the plastic rearrangement, allowing for in situ reshaping and repairing by applying heat and pressure [93]. Different approaches have been reported [94,95], patented (e.g., 3R technology based on epoxy type matrix with reversible aromatic disulfide linkages [96], and explored in national and EU projects research projects (e.g., AIRPOXY, Carbo4Power, Ecoxy etc.), but EoL opportunities vs. traditional thermoset materials have not been proven at pilot level yet nor commercialized (e.g., available various reversible building-blocks of various thermoset materials e.g., acrylate, or vinylester) [91]. Current results show that materials present thermoplastic-like behaviour due to the reshuffling capacity of dynamic crosslinks and the polymer network can be regenerated by applying heat and pressure for repair of matrix microcracks and CFRP delamination’s; thermo-conformability; mechanical recyclability via grinding/compression-moulding and chemical recyclability has been proven at a lab scale showing easier fibre separation under mild conditions without causing surface damage on the fibres, but further research is required for upscale and valorisation [97].
Versatile dynamic covalent chemistries were reported, such as transesterification [98,99,100], imine amine exchange [101], vinylogous transamination [102], disulfide exchange [103], acetal bonds [104], and DA/retro-DA chemistry [105]. In contrast to some of the traditional exchange reactions, these associative mechanisms do not require high temperature treatments, petrol-based additional monomer, special processing (e.g., sonication), and/or metal catalysts to allow for the recycling process. Moreover, recent attention has been paid to exploring new recycling strategies, e.g., to convert the rigid networks into viscoelastic liquids; e.g., imine dynamic exchanges can combine both associative (transimination) and dissociative mechanisms (imine hydrolysis and reformation), where resulting vitrimers can be recycled through acidic hydrolysis [104,106].
More specifically, Liang Yue et al. introduced a new approach for the inversion of existing thermoset waste to vitrimers, allowing unrecyclable thermoset materials to be reprocessed and valorised in several applications. The methodology introduced relies on swelling–drying methods, milling, and hot pressing. More specifically, a solution of a catalyst, in this case, (tin(II) 2hexanoate (Sn(Oct)2)), is used for the swelling of the thermoset network (PU or epoxy), which causes transesterification reactions that enable the forming of dynamic bonds between ester and hydroxyl groups. Afterwards, the material can be milled using a ball milling set up and then formed using hot pressing technologies [107]. In the same frame, EVA thermoset waste was treated to be converted to vitrimer with the use of a zinc acetate catalyst and similar methodology, as described above, for the formation of dynamic covalent bonds between ester and hydroxyl groups via transesterification. The resulting material was able to be processed with extrusion and compression moulding methods [108]. The ability of vitrimer materials to undergo shape reconfigurability as the transesterification reaction is the underlying mechanism providing another superior possibility, which is the self-healing capabilities, as demonstrated by Hubbard et al.; furthermore, the ability of self-healing and processability are dependent on the catalyst and its sufficient concentration to enable, while the vitrimer’s shape memory capabilities are catalyst-independent [98].
Electroactive vitrimers—epoxy resins nanocomposites with carbon nanotubes (CNT)—have been mechanically recycled by milling and hot-pressing, conserving partial CNT integrity and dispersion, maintaining the mechanical strength of the pristine nanocomposites, and having the ability to be electrically welded through heating by the Joule effect [109]. Moreover, chemical recycling of polycyanurate thermosets has been demonstrated by activating ‘dormant’ covalent bonds, and they can be recycled into the original monomers, which can be circularly reused for their original purpose [110]. Moreover, vinylogous urethane vitrimers synthesized from bis-polyethylene glycol acetoacetates (aPEG) and tris(2-aminoethyl)amine can be degraded by water at a moderate temperature with almost quantitative recovery (>98%) of aPEG [102].
Epoxy vitrimers with outstanding mechanical properties and water insensitivity have been synthesized by crosslinking diglycidyl ether of bisphenol F (DGEBF) with a novel curing agent bearing imine backbones and amino terminals [111]. The dynamic imine bonds of the curing agent enable malleability, shape reconfiguration, and programming of the epoxy vitrimer; the aromatic structure of the curing agent imparts the epoxy vitrimer with high tensile strength, Young’s modulus, and thermal stability, while high water resistance was obtained due to the hydrophobicity and high crosslinking density of the network.
2.5. Upcycling of Thermoset Composites
Many recovery strategies for fibres have been discussed in the previous sections. In the case of the thermoset composite, upcycling refers mainly to the upgrade of the reclaimed fibres and specifically for CFs, which have a high cost and added value. An upcycling method of CFRPs was proposed by Zhang et al. in 2023 [112], where a combination of plasma under microwave irradiation in a nitrogen atmosphere pyrolysed the thermoset polymer matrix of a CFRP and in parallel improved the graphitization degree of the valorised CFs, in comparison to the virgin ones. In our recent research studies [113,114], nano-enabled solutions were investigated to evaluate the impact of nanomaterials on the surface morphology and mechanical properties of CFs. In the first one, recycled CFs were decorated with magnetic nanoparticles during a supercritical CO2-acetone process. The direct synthesis of magnetic nanoparticles in hydrothermal conditions and the deposition of already synthesized particles was investigated. In this process, no CF surface activation was needed thanks to the presence of functional groups due to the remaining matrix after the treatment in supercritical conditions. The resulting nano-enabled CFs showed a strong magnetic behaviour and no degradation. In the second one, a novel sizing approach was developed that can be applied as an upcycling route for reclaimed CFs, since it resulted in enhanced affinity between fibres and the polymeric epoxy matrix. The incorporation of nanomaterials, specifically N2-plasma-functionalized CNTs and few-layer graphene, demonstrated notable improvements in the interfacial shear properties (90% increase), verified by mechanical and push-out tests.
Also, the valorisation of the polymer matrix is of high importance, and the current research is seeking new ways of exploiting and upcycling the decomposed thermoset polymers. The study of Liu et al. [115] reported the direct conversion of waste epoxy resins into multiple-responsive (temperature, water, and pH) supramolecular materials via acid-catalysed oxidation. A strategy of upcycling an anhydride cured epoxy the reutilization of decomposed dual monomers into several applications was suggested by Shao et al. [116] recently. An aminolysis reaction took place in aminoethanol without using any catalysts. The epoxy resin was fully decomposed at 160 °C in 4 h, resulting in two distinct high purity monomers (HHPA-OH and BPA-OH). The latter was used to synthesize a polyurethane coating with superior properties (Tg of 88.9 °C, scratch hardness of 8 H, gouge hardness of 6 H, adhesive strength of 5B, and strong solvent resistance). The HHPA-OH reacted with methacrylic anhydride to form a dimethacrylate monomer, which was then used as a viable crosslinker for photo-curable 3D printing thermosetting polymer. This work demonstrates a feasible pathway to convert anhydride-cured epoxy waste to new monomeric recyclates.
3. Recycling Technologies of Thermoplastic Polymers
3.1. Overview
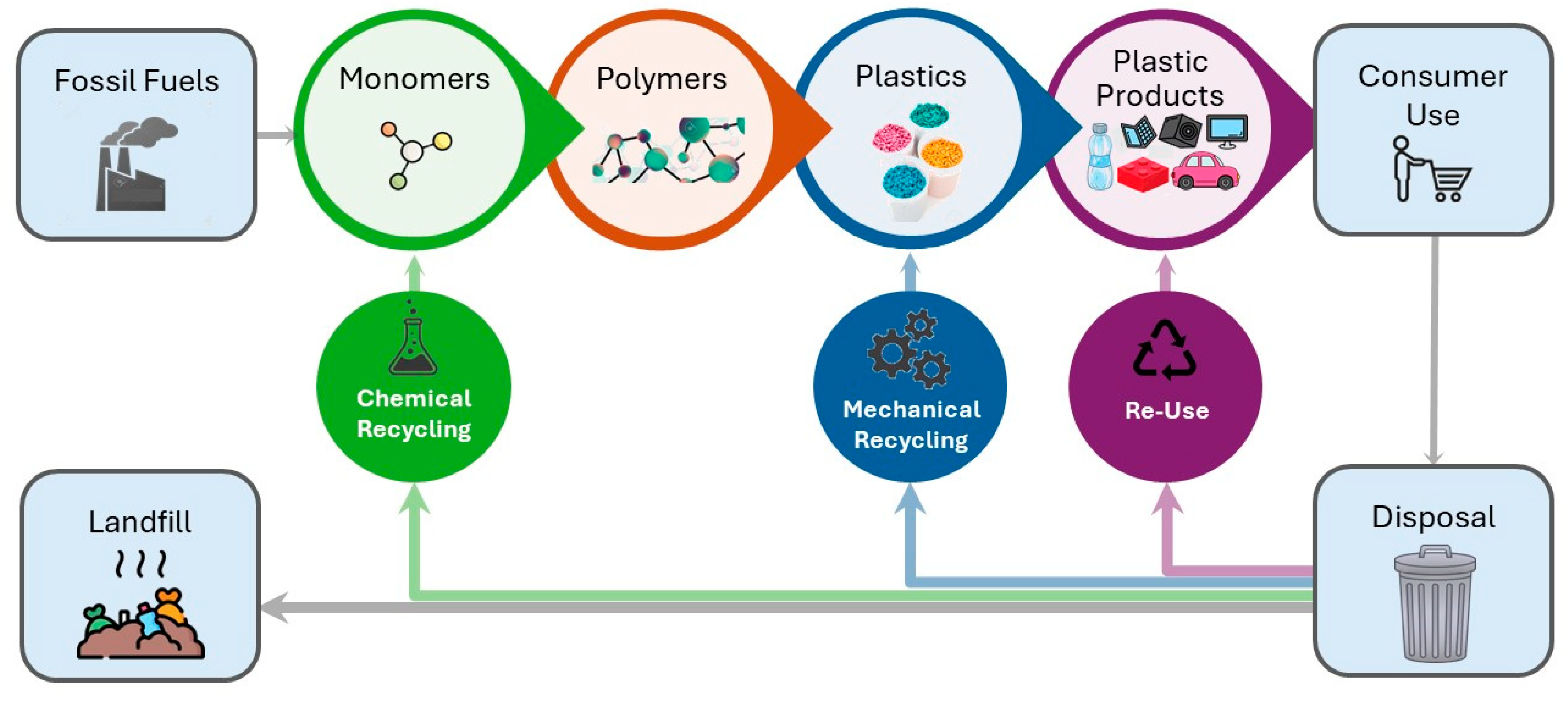
Thermoplastics have made significant contributions to both our daily lives and industrial growth. They have become ubiquitous and irreplaceable due to their adaptability and superior material qualities such as strength and durability, as well as their low-cost and automated manufacturing. Also, thermoplastics can be used for the manufacturing of high-performance composites in high-demanding applications, such as in the energy and marine sectors [117]. However, thermoplastics production relies on petroleum resources that are depleting, imposing limitations on their production. Also, plastics constitute an environmental threat when they are improperly discarded in the environment. Thermoplastics’ superior characteristic is their ability to remelt and dissolve, allowing reprocessing and reshaping, as well as chemical modifications, and chemical depolymerisation for monomer and feedstock reclamation. There are four approaches for polymer recycling, termed primary, secondary, tertiary, and quaternary recycling, which are presented in Table 2 and Figure 4.

Table 2.
Approaches for recycling of thermoplastics.
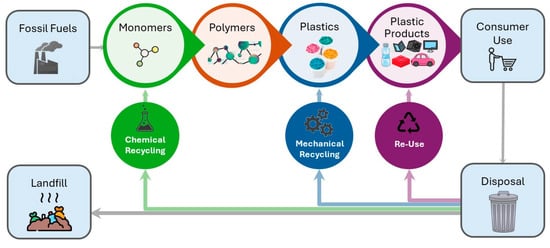
Figure 4.
Conventional process flow of plastics life cycle.
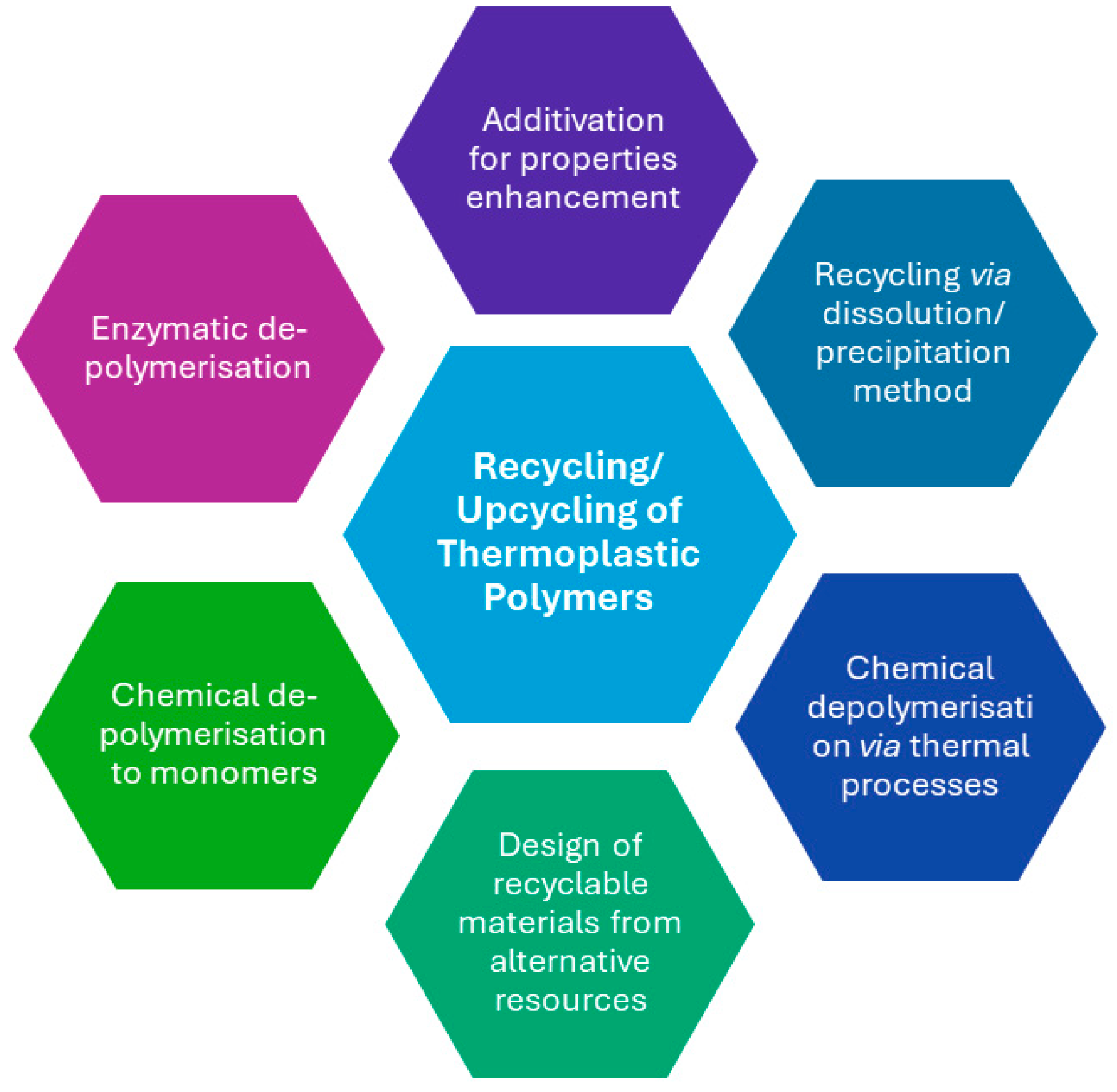
In general, the traditional recycling processes end up producing downgraded materials. The most promising technologies for the future are those attempting to end the materials life cycle by producing value-added materials. Upcycling refers to a process that converts by-products, undesirable, unwanted, or waste materials into new, higher-value materials. The concept of upcycling procedures can be defined as the use of post-industrial or post-consumer plastic waste as a feedstock for the synthesis of value-added products such as polymers, molecules, or materials, and is considered complementary to chemical and mechanical recycling [122,123,124]. Approaches for the upcycling of thermoplastic polymers that are discussed in this review are presented in Figure 5.
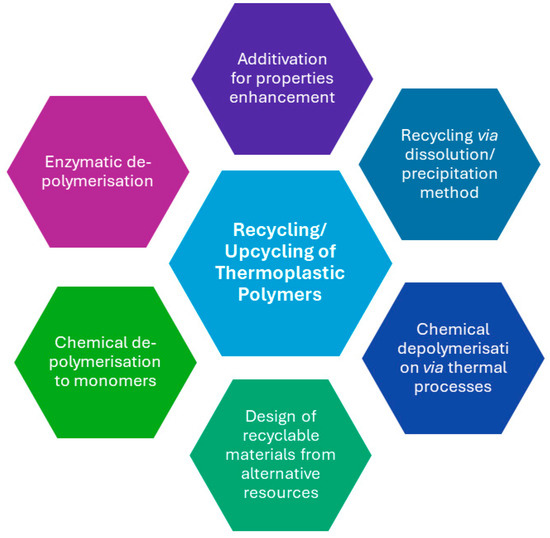
Figure 5.
Different approaches for upcycling of thermoplastic polymers.
3.2. Mechanical Recycling and Upcycling
The heterogeneity and low purity of post-consumer polymer waste streams, along with thermal degradation during the remelting and reshaping processes, lead to lower value products (downgrading). More specifically, the polymers degrade on a first level during their lifetime and afterwards during the thermal reprocessing, either due to chain scission reactions that happen due to presence of water molecules or acidic impurities [119,125]. This thermomechanical degradation results in decreased molecular weight and hence, the material does not retain its properties and the products that can be manufactured are downgraded [123]. To overcome this obstacle and achieve a higher number of polymer life cycles, recycling industries use several additives, such as stabilizers, chain extenders, flow enhancers, etc. [120].
The first step to recover polymers from various post-consumer waste streams before the recycling process is the identification and separation of plastic waste streams. There are several methods for automated sorting of polymers in one polymer rich stream, as shown in Table 3, based on the physical properties of the targeted polymer, such as electrostatic and density separation, as well as sensing-based methods, such as separation with near-infrared (NIR) spectroscopy. Sorting is insufficiently discussed so far, despite its potential to significantly increase the efficiency and yield of the mechanical recycling.
The reduction in the mechanical properties of reprocessed acrylonitrile butadiene styrene (ABS) has been extensively studied as one of the main industrial and most consumed polymers in the world. The assessment conducted by Scaffaro et al. [126] involved examining the impact of post-consumer ABS content in blends containing both virgin and post-consumer ABS, as well as analysing the influence of reprocessing cycles on the physical characteristics of these blends. The mechanical and thermo-mechanical properties of the blends deteriorated with an increase in the proportion of post-consumer ABS and with each subsequent reprocessing operation. In another study, Rahimi et al. [127] concluded that reprocessing leads to ABS degradation, primarily due to the breakage of polybutadiene bonds. They determined that the optimal blend of virgin and recycled material to minimize shrinkage was 50% w/w, while the most favourable mechanical properties were attained with blends containing up to 20% w/w recycled material. Polycarbonate (PC)/ABS blends were subjected to examination for up to 20 reprocessing cycles [128]. Despite PC/ABS demonstrating considerable thermal stability during reprocessing, its mechanical properties deteriorated with an escalation in reprocessing cycles. This decline was attributed to increased stiffness resulting from polymer chain scission. Recovery of the 20-time reprocessed blend was achieved by adding virgin material in combination with chain extender and styrene maleic anhydride at 30%, 1.5%, and 2% w/w, respectively.

Table 3.
Sorting methods for thermoplastics.
Table 3.
Sorting methods for thermoplastics.
| Sorting Method | Main Principle | Ref. |
|---|---|---|
| Magnetic density separation | A magnetic mixture is used to separate plastics by their differences in densities. | [129] |
| Electrostatic separation | Electrostatic field is used to separate different plastics according due to their differences in electrical properties. Usually, friction is used to electrostatically charge the different plastics and then the fractions are separated due to their negative or positive charges. | [129] |
| Froth flotation separation | In this method, different plastics are separated by hydrophobic and hydrophilic properties. | [130] |
| NIR separation | Separation based in the different spectrums of different polymers. | [131] |
| LIBS separation | Based on the different atomic emission spectrums of different polymers. | [132] |
Secondary or mechanical recycling has been used broadly and it has been a very successful recycling method, especially in the case of polyethylene terephthalate (PET) bottles [120,123,133]. It is a valuable method as it decreases the overall energy demand for processing, considering that the production of virgin materials require significantly more energy than materials derived from recycling process. Hence, it lacks two crucial objectives of circular economy planning, which are the elimination of waste and the development of value-added products in a circular way [125].
Mechanical processing of polyolefins induces degradation at the molecular level and the formation of aldehydes, ketones, and short-chain hydrocarbons [134]. Post-consumer high-density polyethylene (HDPE) was blended with virgin polymer at ratios of 0, 20, 40, 60, 80, and 100% by weight via extrusion by Curtzwiler et al. [135] in 2018 and the properties of the different blends were measured. A significant increase was observed in UVA absorption, in carbonyl and terminal vinyl functional groups, and in the degradation temperature. The fluorescence properties and the polymer crystal quality were deteriorated. In another study by Curtzwiler et al. [136] performed in 2019, the optical, thermal, mechanical, morphological, and gas barrier properties of post-consumer recycled polyolefin materials (87.5% post-consumer recycled polypropylene and 12.5% post-consumer recycled polyethylene) melt blended with virgin polypropylene at different blend ratios have been investigated. The presence of phase separated domains and increased compatibility from oxygenated functional groups resulted in increase in the yield stress by 74%, the strain at yield by 49% and the UV blocking by 160%, whereas the gas transmission was reduced by 30–40%. The effect of accelerated thermal and photo-aging and subsequent reprocessing on homopolymers of low-density polyethylene (LDPE), HDPE, polypropylene (PP) and high-impact polystyrene (HIPS) was monitored by Luzuriaga et al. [137]. LDPE and HIPS were more sensitive to thermo-oxidation than HDPE and PP, whereas HDPE and PP were affected more by UV exposure. It was concluded that the application of stabilizers prior to reprocessing is mandatory to prevent the accelerating effect of thermal and photo-aging caused by the structural inhomogeneities built up in the materials. The use of natural materials such as ground rice hull (RH) of 17 μm diameter as a modifier to a PE/PP recycled resin from waste fishing ropes, resulting in the improvement in the mechanical properties, has been reported by Sato and Shishido [138].
To deal with the challenges of mechanical recycling such as polymer incompatibility, phase separation, and polymer degradation, and to overcome the downgraded of thermoplastics during reprocessing and achieve a higher number of polymer life cycles, the recycling industries use a number of additives, such as compatibilizers, coupling agents, impact modifiers, stabilizers, chain extenders, flow enhancers, and many others [120,139]. Recycling of multi-layer barrier packaging [140] lays in this category and additives such as compatibilizers and impact modifiers are needed in these cases to recycle or upcycle waste polymers into new materials with desired properties.
To avoid low-quality recycled materials with inferior mechanical properties and to deal with constraints of mechanical recycling arising from the heterogeneous nature of polymers and the contaminants present in the plastic waste streams [141], the use of specialty additives [142] and techniques that improve the melt processability and mechanical performance of final products is necessary [143].
3.2.1. Compatibilizers
When multiple thermoplastics are blended without being chemically compatible, it is important to apply certain performance modifiers during their reprocessing to prevent phase separation and inferior-quality materials with poor mechanical characteristics. Differences in chemical structures, melting points, and degradation profile during high shear melt-extrusion need to be considered. Compatibilizers are used in order to reduce the interphase tension that can create phase dispersion and to stabilize the morphology in order to withstand high stress and strain application. Furthermore, compatibilizers can enhance the adhesion between the phases in the solid-state, improving the mechanical properties of the products. Compatibilization in polymer blends is achieved using additives such as low-molecular-weight coupling agents, traditional polymeric compatibilizers, impact modifiers, and reactive nano fillers. There are two different approaches for the compatibilization of polymer blends. The first one is the addition of compatibilising agents, usually block or core-shell copolymers, or components miscible with all phases that reduce the interfacial tension of the blend. The second approach is the in situ reactive compatibilization where trans-reactions such as transesterification, graft, or block copolymers’ in situ formation, ionically bonded structures formation and mechanochemical blending that generates copolymers by breaking and recombining polymer chains can be used [144]. The use of compatibilizers such as reactive low-molecular-weight coupling agents or crosslinkers [145], dialkyl peroxides [146], coupling agents based on silane, phosphite, isocyanate, bis-caprolactam, and bisoxazoline [144,147,148] and epoxy-based coupling agents [149] has been reported in the literature.
Polylactic acid (PLA) blends with three different thermoplastic elastomers were compatibilized by a low amount (2 wt.%) of 4,4-methylene diphenyl diisocyanate. Mechanical, thermomechanical, and impact strength measurements proved the favoured interfacial adhesion of PLA and thermoplastic elastomer (TPE). Compatibilized blends of high toughness were demonstrated by Vuillaume et al. [148]. Incompatible and immiscible blends of polyamide-6 (PA6) and polybutylene terephthalate (PBT) were effectively compatibilized by a multifunctional epoxy resin. The in situ-formed copolymer enhanced the mechanical properties of the PA6/PBT blends, as reported by Chiou at al. [149]. The in situ compatibilization of recycled PA6 with PP blends has been also reported by grafting maleic anhydride onto PP using benzoyl peroxide catalyst. Improved mechanical properties were achieved in comparison to the uncompatibilised blends [146].
Furthermore, polymeric compatibilizers such as functionalized polyolefins [150], styrenic block polymers [151], epoxy resins [152], anhydride based compatibilizers [153], and isocyanate polymers [154] have been widely used. Ha et al. [155] reported the use of chlorinated polyethylene (CPE), ethylene-propylene rubber (EPR), and their 1/1 mixture as compatibilizers for HDPE/PP/polyvinyl chloride (PVC) blends, while the CPE, styrene-ethylene-propylene block copolymer (SEP), or their 1/1 mixture were used as compatibilizers of the HDPE/PS/PVC blends. EPR and SEP exhibited the best behaviour as impact modifiers on the HDPE/PP/PVC and HDPE/PS/PVC blends, respectively. In another study, styrene ethylene butylene styrene (SEBS) elastomer functionalized by maleic anhydride (SEBS-g-MA) compatibilized recycled PP/PET blends improving blend homogeneity and properties [156]. A positive effect on the mechanical properties and thermal stability was observed through the addition of 5% PP-g-MA and epoxy resin as a compatibilizer to recycled ABS and recycled PC-rich blends [157].
3.2.2. Impact Modifiers
Impact modifiers are used to strengthen the impact resistance and mechanical properties of recycled thermoplastics that have undergone degradation due to exposure to heat, light, and several reprocessing cycles. The presence of additives usually used in plastic formulations such as flame retardants, fillers, or pigments can further downgrade the mechanical properties and impact resistance of the materials. The addition of impact modifiers can alter the material’s behaviour from stiff and brittle to flexible and tough [158,159]. Thermoplastic elastomers or rubbers are commonly introduced to thermoplastic materials to enhance their toughness. Factors such as the molecular structure, the molecular weight, and the ratio of soft and rigid blocks of the impact modifier affect the performance of the final products [160]. Impact modifiers have, in general, a lower young modulus compared to the polymer matrix and a low glass transition temperature (Tg). Phases compatibility with the polymer matrix and a fine particle size distribution of the rubbery phase are important factors [160]. Butadiene-based modifiers, acrylic modifiers, and elastomers are the most representative classes of impact modifiers used in the market [161,162].
Wang et al. [158] studied the influence of ethylene octene copolymer (EOC) as a soft rubber toughening agent and talc as a rigid reinforcing agent in PP before and after several recycling cycles. It was concluded that the PP/EOC/talc composites were not influenced by the reprocessing cycles. The addition of EOC improved the impact resistance of both PP and PP/talc and the reprocessing cycles had a positive effect on the PP/talc due to a fragmentation of the talc particles. Chain scission was observed on the PP/EOC composite after reprocessing. The impact strength of recycled PP/PE blends was also increased with the addition of EOC due to its elastomeric nature, as reported by Kazemi at al. [163]. Furthermore, styrene-ethylene-butylene-styrene elastomer (SEBS) has been successfully used as an impact modifier in blends of PET/PP [164] or PET/LDPE [165] or PP/HIPS [166]. SEBS copolymer has also been used in WEEE blends, where it significantly increased the impact strength [167], whereas styrene acrylonitrile resin (SAN) has been used for the enhancement of impact strength of ABS/HIPS [168]. The best performance in HIPS/ABS blend of 80/20 ratio obtained from waste from electrical and electronic equipment (WEEE) waste was achieved by using 2% w/w SAN.
3.2.3. Plasticizers
The addition of organic molecules as plasticizers in thermoplastics promotes the flexibility, extensibility and processability of the materials. The mechanism of plasticization involves the formation of secondary bonds between the plasticizer and the polymer chains, increasing the distance of ‘neighbour’ chains, preventing the interaction among them, and increasing their mobility. Plasticizers are categorized based on their chemical composition in two classes: phthalates and non phthalates. Bio-based plasticizers are increasingly used based on natural materials such as polysaccharides (starch, cellulose, chitosan, etc.), microbial polyhydroxyalkanoates (PHAs) and polyhydroxybutyrates (PHBs), and PLA [169,170,171,172,173]. Glycerol, polyols (such as sorbitol or xylitol), and vegetable oils are widely used plasticizers [160].
3.2.4. Antioxidants and UV Stabilizers
Weathering can extensively downgrade thermoplastics exposed to outdoor environment. Thermal- or UV light-induced oxidative phenomena mainly occurred and result in material discoloration as well as reduction in mechanical properties. The degradation process through oxidation involves the oxygen-induced formation of reactive oxygen species (ROS), which eventually provokes the chain scission or crosslinking of the macromolecular structure. Antioxidants are able to either scavenge free radicals or decompose hydroperoxides. Photooxidation can similarly occur through the absorption of photons from sunlight radiation and consequently leads to the oxidative degradation of photosensitive molecules [160,174,175,176].
Three different categories of additives were tested in post-consumer PET for stabilization. The commercial antioxidants Irganox 1010® and Irganox B561® were selected together with a metal deactivator and an anti-hydrolysis agent by Silva Freitas et al. [177] and presented a positive effect. Another commercial antioxidant, Irganox HP2921, was added to lab-synthesized thermoplastic polyurethane/silicone thermoplastic vulcanizate (TPV) in combination with a crosslinking agent, compatibilizers, and processing aid, and successfully enhanced the mechanical properties of the material [178]. The excellent anti-aging performance of a synthesized nano-TiO2-loaded antioxidant (TiO2-KH570-MB) in TPV matrix against UV/O3 was demonstrated by Yang et al. [179]. This nano antioxidant exhibits a high efficiency in anti-aging performance and achieves a significant increase in the service life of polymer products due to the better compatibility and the lower level of migration and volatility of the chemically bonded 2-mercaptobenzimidazole (MB). The increase in tensile strength (28.23%) and Young’s modulus (29.16%) of thermoplastic elastomer in the presence of antioxidant fillers was reported by Utracki et al. [180] Natural materials such as rosemary essential oil have been studied for their antioxidant activity by Azevedo et al. [181]. The mechanical properties of thermoplastic starch/whey protein (TPS/WPI) nanocomposites obtained by extrusion with rosemary essential oil revealed an antioxidant effect together with a less rigid, weaker behaviour.
3.3. Recycling of Multilayer Plastic Materials
Multilayer plastic packaging materials are constituted by distinct layers of polymers such as polyolefins and polyesters. The polymers of each layer of the packaging are selected in such way in order to provide individual properties to the packaging, always depending on the final application. Polyethylene, ethylene vinyl alcohol, and polyethylene terephthalate are only a few examples of thermoplastics present in multilayer plastic packaging selected for their mechanical and barrier properties [182,183].
The recycling of multilayer packaging materials [184,185,186] is challenging as they cannot be mechanically recycled due to the chemical incompatibility of their layers. No industrial technologies exist for the partial or complete deconstruction of multilayer materials into their constituent polymers. Multilayer plastic materials currently follow solvent extraction techniques which selectively dissolve the targeted polymers in a solvent system [187,188]. Two relevant technologies, the Newcycling® process by APK AG and the CreaSolv® Process by Unilever and the Fraunhofer Institute, are being currently commercialized. Both processes are based on the selective dissolution of polyolefins from multilayer plastics with the Newcycling® using a solvent system consisting of alkanes, isooctane, or cycloalkanes and the CreaSolv® using aliphatic hydrocarbons.
Georgiopoulou et al. [189] studied a selective dissolution-precipitation process for the recycling of post-consumer beverage cartons Tetra Pak® and the separation and recovery of LDPE and aluminium following paper recovery by hydro pulping. LDPE from the inner layers was recovered as white powder of high purity with thermal properties like those of pure LDPE, whereas the recovered LDPE from the outer layer remained contaminated with impurities, such as printing inks. In another study by Walker et al. [190], a solvent-targeted recovery and precipitation (STRAP) method to deconstruct multilayer films into their constituent resins through consequent solvent washes was demonstrated. STRAP method was supported by computational tools to guide the solvents selection. The research team managed to separate three representative polymers (PE, PVA, and PET from a commercially available multilayer film with nearly 100% material efficiency. Computational tools such as finite element analysis have been used by Mulakkal et al. [191] to investigate the recycling of multilayer packaging plastics and melt-blending based mechanical recycling solutions using compatibilizers. The influence of compatibilizers is explored and the finite element micromechanical modelling technique is used to estimate the mechanical properties of recycled blends. The model output proved to be in good agreement with the experimental data available in the literature and its predictive ability was demonstrated.
3.4. Chemical Recycling and Upcycling
The chemical recycling concept aims to benefit the environment in terms of resources and long-term retention of material value by promoting circular economy planning and designing. More specifically, it aims to reduce material downcycling and at the same time open possibilities for the upcycling of waste resulting in value-added feedstock for economic enhanced applications with the same or amplified product quality [192]. There have been several approaches for chemical recycling of thermoplastics including thermal, chemical, and biological degradation. Thermal degradation (pyrolysis, gasification) results in liquid or gas fuels, while chemical and biological degradation results in the breaking down of the polymer to building blocks for repolymerisation or production of fine chemicals which can be achieved through catalysed or not chemical mechanisms [123]. Another key advantage of chemical recycling is that through the proper design of reagents, catalysts, and reaction parameters, we can deal with highly contaminated plastics, as well as complex polymer mixtures, which, in the case of secondary recycling, requires multiple process steps and higher energy consumption [140]. The categories of polymers that are very promising candidates for chemical recycling include the following:
- Polyolefins (such as PE, PP) that can be chemically recycled via thermal degradation;
- Polycondensation polymers such as PET, PA, PLA, and PC that can be recycled by chemical degradation [121,193];
- Polyaddition polymers with charge de-localizing side groups (i.e., alkenyl, phenyl, ether groups, halogens) such as poly (methyl methacrylate) (PMMA) and poly (tetra fluoroethylene) (PTFE) can also be recycled via chemical degradation [120,193].
The polymer that has been investigated thoroughly so far for chemical recycling and seems to be the most promising one for mass monomer production is PET.
3.4.1. Pyrolysis
The selective degradation of unstable polyaddition polymers, such as polyolefins (PP, PE), is very challenging due to their degradation mechanism. These polymers degrade via random C-C bond scission mechanism and hence, it is very difficult to recover their monomer. Thermolysis (e.g., pyrolysis, gasification) is a more effective approach in these cases, as it provides crude liquid (oil, wax), gas, or solid feedstock [120]. The reaction mechanisms of pyrolysis include end-chain scission, random-chain scission, chain-stripping, and cross-linking, depending on the polymer being treated. More specifically, pyrolysis occurs by a radical mechanism that includes several stages, as follows:
- Initiation: scission of the initial bonds in the chain to produce two radicals (may arise at either random or end-chain positions);
- Hydrogen transfer reactions: intermolecular or intramolecular reactions that result in the formation of olefinic species and polymeric fragments and also the generation of secondary radicals through hydrogen abstraction between a primary radical and a polymeric fragment;
- Β-cleavage of secondary radicals: resulting in an end-chain olefinic group and a primary radical;
- Termination: coupling of two primary radicals or the disproportionation of primary macroradicals.
The recycling method of pyrolysis involves the use of heat, in an inert environment (N2), to break down polymer chains and convert them into small molecules. It is the most common method for chemical recycling of polyolefins such as PE and PP. Similar pyrolysis apparatus with those used for thermosets recycling are employed. The products stained from the process are a mixture of gaseous, liquid, and solid components that can be used as a fuel source [194]. The resulting products depend on several parameters such as process temperature, heat rate, residence time, the presence or not of catalysts, as well as the reactor design, the waste composition, and presence of impurities [195].
The processing temperature can be in the range of 350 to 800 °C, and along with residence time and heating rate, are crucial for the type of products that will be obtained. Slow pyrolysis (10 °C/s) is the slow, non-isothermal heating of plastics in absence of oxygen, which aims to the production of mainly solid fuel, char. In this method, the volatile compounds evaporate partly, instead of combusting, resulting in the production of coke, tar, and char along with oil fractions and gas. That process is also called carbonisation [196], although the most common pyrolysis method is performed using higher heating rates (100 °C/s) and isothermally. In fast pyrolysis, the plastics heat rapidly and quickly (a few seconds) to produce mostly liquid fuels, oils. Furthermore, pyrolysis can be even faster (flash pyrolysis), using heating rates from 100–10,000 °C/s to produce gases and bio-oils [197,198]. Singh et al. [199] investigated slow and fast pyrolysis impact on the degradation of mixed plastic waste and showed that slow pyrolysis promotes the formation of aromatics and aliphatic with higher molecular weight, thus, increasing the higher density oil fractions, while fast pyrolysis increases the number of gaseous hydrocarbons.
Pyrolysis can be performed in the presence of catalysts or not. Catalytic pyrolysis uses catalysts to enhance the speed of the reaction by lowering the activation energy, thus saving energy consumption of the process. Several catalysts have been utilized, such as Al-MSU-F, Z-503, FCC [200,201,202]. Lee et al. investigated the catalytic pyrolysis of PE and PP with two types of mesoporous catalysts, desilicated Beta and Al-MSU-F. Catalytic TGA studies revealed that both PE and PP had lower decomposition temperatures when the catalysts were used than non-catalytic process. The Al-MSU-F catalyst has been also investigated and proven to be efficient for the catalytic pyrolysis of PS and PET [203]. Oh et al. [204] showed that catalytic pyrolysis with an Al-MSU-F catalyst not only shifted the decomposition temperature much lower, but also revealed selectivity to the products obtained. Other studies use modified natural zeolite (NZ) catalysts [205,206]. Khazaal et al. [207] conducted catalytic pressurized pyrolysis process utilizing a zeolite catalyst loaded with nickel (Ni), molybdenum (Mo), and tungstate (W), referred to as Z-503, for the thermal recycling of municipal plastic waste, and showed that the chemical composition of the yield fractions varied after the use of the catalyst to higher amounts of light hydrocarbon gases and diesel fuel fractions, presenting a promising “waste to energy” technology. Wang et al. [208] used an FCC (fluid catalytic cracking) catalyst to treat muti-layer thermoplastic waste (PET/LDPE and PA6/LDPE). Results show that the FCC catalyst promotes the formation of gases rather than waxes and oils, more alkane, and aromatics than aliphatic compounds and stronger deoxygenation ability at fast pyrolysis condition.
Another approach for pyrolysis of plastic waste includes plasma treatment. In fact, all kinds of waste can be pyrolysed with plasma due to its extreme heating intensity. The process generates gases as H2, CO and hydrocarbons that are very toxic such as dioxins and furans [209]. Hence, plasma is only suitable for medical waste [210].
Thermoplastic waste contains an average of 7% of additives, including plasticizers, fillers, and flame retardants [211]. In the case of engineering polymers used in high-value applications such as electrical/electronic equipment, automotive, and construction industry, the flame-retardant content is quite high. Commonly used flame retardants consist of organophosphorus, brominated, and chlorinated compounds which raise substantial environmental concerns, as well as PVC wastes. Common additives and fillers contained in the PVC and PS streams, may affect the thermal degradation efficiency of the plastics. This impact the process as they require greater energy input, resulting to higher temperatures or/and residence times to achieve complete degradation [212].
Several advancements have been achieved regarding the pyrolysis of halogen-plastics for resource recovery and potential routes for upcycling of halogens [213]. Microwave heating, plasma or electron beam irradiation, and supercritical pyrolysis are newly emerging industrially relevant technologies available for the dechlorination or breakdown of chlorinated plastics. The supercritical H2O pyrolysis utilizes sc-H2O as a heat and solvent carrier to transform plastics into light or heavy oil and wax [214].
Another approach of pyrolysis utilization for upcycling applications is the conversion of plastic waste to carbon-based nanomaterials. Plastic waste pyrolysis can be converted to several kinds of carbon materials such as porous carbon materials graphene nanosheets, carbon dots and spherules, nanofiber web, and multi-walled carbon nanotubes [215,216,217].
3.4.2. Depolymerisation to Monomers
Chemical recycling via depolymerisation to monomers is mostly investigated in PET polymers due to its chemical structure which allows for the performance of chemical depolymerisation with several chemical mechanisms. This section will mostly focus on the PET case and all the recent advancements regarding its recycling. The main chemical recycling methods for PET and the products of each one are summarised in Figure 6. Other common thermoplastics that can be chemically recycled to their monomers are polycarbonate and PMMA [218].
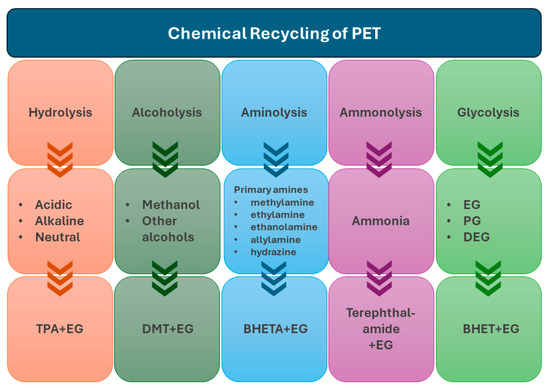
Figure 6.
Chemical recycling methods for PET and the products obtained from each one.
PET is a semicrystalline, thermoplastic polyester that is widely used and recycled many years now as one of the largest constituents in single use plastic waste. Mechanically recycled PET presents a significant decrease in its mechanical properties [219]. María del Mar Castro López et al. [220] showed that mechanically reprocessed PET specimens break before the elastic limit and the strain at break point undergoes from 35% to 0.7% after five extrusion cycles. Due to that, the research interest has shifted to chemical recycling routes. PET can be depolymerised with several chemical reagents and chemical mechanisms, such as hydrolysis, alcoholysis, aminolysis, and glycolysis, each of these define the end products obtained [193].
Hydrolysis of PET to its monomeric units can be achieved in acidic, alkaline, or neutral conditions, with or without high pressure and/or catalysts.
Acidic hydrolysis procedure utilizes high concentrated acidic aquatic solutions in which the PET waste is digested to its monomers, which are terephthalic acid (TPA) or terephthalic salt and ethylene glycol (EG). The acids that can be used are phosphoric, nitric, and sulfuric acid (which seems to be the most efficient). The concentrations must be very high to achieve high yields; for sulfuric and phosphoric acids, they should be 87% and higher, while for nitric acid, they should be 7–13 M [221]. Processing with sulfuric acid can achieve high yields of monomer recovery in relatively low temperatures and short reaction times, although the set-up of an industrial scale unit creates serious problems regarding the large quantities of highly acidic solutions that pose environmental concerns, as well as limitations and difficulties regarding the processing handling and the economic viability [222].
Alkaline hydrolysis of PET usually is carried out using aqueous alkaline solutions of NaOH or KOH at a concentration of 4–20 wt.%. Alkaline conditions can achieve very high yields when performed in high temperatures, such as 200 °C, and long reaction times (3–5 h) [223]. To reduce the reaction times and apply mild conditions of temperature and pressure, there has been research conducted on the utilisation of catalysts. The most promising investigation about catalysts, process development, and mechanistic understanding has focused on quaternary ammonium salts, such as TOMAB and HDTMAB [223], acting as phase transfer catalysts for PET ester bond cleavage under alkaline conditions. Another catalyst, [CTA] 3PW, is synthesised by combination of alkyl quaternary ammonium units with hetero-polyacid anion, resulting in a pH-responsive catalyst that can be easily separated after the end of the reaction by adjusting of pH value [224]. Catalysis can be further increased in a microwave assisted environment [225]. Another approach shows that mild conditions can be achieved by the introduction of ethanol in the reaction system; Ügdüler et al. [226] studied a two-step aqueous alkaline hydrolysis of PET waste under mild conditions (≤80 °C and atmospheric pressure) by optimising the parameters affecting the PET degradation rate, which are the temperature, ethanol to water ratio, NaOH concentration and stirring rate. They achieved approximately 95% product recovery in less than 20 min. Alkaline hydrolysis can also be performed to PET textiles or even polymer blends containing PET [227].
Neutral hydrolysis of PET without any catalyst can take place in high temperatures such as 200–300 °C and high pressure of 1–4 MPa. The use of catalysts can reduce the temperature and pressure requirements. Also, this reaction produces smaller amounts of inorganic salts, which makes it to be considered more environmentally friendly. The main disadvantage of this method is that the impurities that are present in the polymer, such as metal catalysts, dyes, pigments, other glycols, and dicarboxylic acids, cannot be easily removed from the recovered TPA monomer. As a result, TPA has considerably lower purity than the products of acid or alkaline hydrolysis. To obtain highly pure TPA, the product must undergo a second stage process of separation and purification, which makes the entire process much more time consuming and increases its cost [219,221,222].
The most investigated catalysts are alkali-metal salts such as zinc acetate [228,229], sodium acetate, zinc sulphate [230], and zinc chloride [231], along with imidazolium-based catalysts [232], which enhance the depolymerisation rate and allow mild reaction conditions but do not resolve the low purity problem of this process. The development of simply recoverable and reusable catalysts for PET hydrolysis in neutral pH media is a significant step for increasing the product purity, reducing the energy requirements, and setting the basis for upscaled application of the process. Kang et al. [233] developed ZSM-5 based zeolites with Brønsted and Lewis acidic sites, used herein as an acidic catalyst for the PET hydrolysis reaction, which can be easily recovered and regenerated catalysts. (Table 4)

Table 4.
Comparative table of chemical recycling methods for thermoplastic materials.
PET alcoholysis involves the breaking of backbone ester linkage via an alcohol attack on the carbonyl group, known as a transesterification reaction, which results in the formation of dimethyl terephthalate (DMT) and EG as main products. Even though DMT and EG are raw materials for PET synthesis, this method has a major drawback, which is the requirement of an additional process for purification of the products to remove impurities such as glycols, phthalate derivatives and alcohols [223]. Many alcohols have been investigated, such as ethanol, butanol, isooctyl alcohol, and trimethylolpropane, although the most attention has been drawn to methanol (methanolysis). The uncatalyzed reaction requires severe conditions of temperature and pressure [219]. Alcoholysis suffers from contamination of the products which adds economic barriers to industrialization. Tang et al. [246] introduced a method for producing gasoline and jet fuel range cycloalkanes and aromatics from PET wastes through methanolysis, which also might be considered as an upcycling route for PET waste. DMT product from PET degradation undergoes hydrogenation to dimethyl cyclohexane-1,4-dicarboxylate, catalysed by a noble metal, such as Pt/C. The dimethyl cyclohexane-1,4-dicarboxylate was further hydrodeoxygenated to C7-C8 cycloalkanes and aromatics that can be used as gasoline or density improvement additives of current bio-jet fuels. Jin-Tao Du et al. [247] proposed methanolysis of PET using a pseudohomogeneous catalytic system of ZnO nanodispersion; the results showed 97% PET degradation and 95% DMT recovery under temperature of 170 °C and a 15 min reaction time.
Another aspect of alcoholysis is that with the appropriate reaction parameters modification, such as temperature, pressure, alcohol, catalysts, and reagents ratios, it allows the precipitation of products with different molar weights and physical properties. In this framework, there is the possibility of upcycling applications. Scremin et al. [248] synthesized a polyol from post-consumer PET by the alcoholysis reaction using diethylene glycol (DEG) and sodium metasilicate (NaMs) as catalyst. The polyol obtained was used for polyurethane (PU) adhesives production, resulting in a product with enhanced properties. Another promising capability is to obtain dioctyl terephthalate (DOTP) from PET waste. DOPT is a is a new kind of green and non-toxic plasticizer that its traditional synthesis process is quite complicated and costly. Chen et al. [237] investigated a way to obtain DOPT through PET alcoholysis. They used 2-ethylhexanol alcohol, imidazole [Bmin]Cl ionic liquid as cosolvent and zinc acetate as catalyst, for the precipitation of DOPT, showing that 100% PET degradation and 93.1% DOTP recovery can be achieved under atmospheric pressure, at lower temperature (reflux temperature) and about 5 h reaction time. Another study by Zhou et al. [249] investigated the preparation of DOPT by alcoholysis of PET using 2-ethylhexanol alcohol as well, choline chloride-based deep eutectic solvent and zinc acetate as catalyst; results showed completely degradation of PET and 84.7% DOPT recovery under atmospheric pressure, temperature of 180 °C and 1 h reaction time.
In the latest studies, Fang et al. [250] utilized PET’s swelling capability under high temperature; PET undergoes swelling right before its melting point, which increases the specific surface area and hence, the degradation rate. Along with polyoxometalate (POM) catalysts and EG, the system formed is able of rapid degradation reactions [251]. The same team also investigated the controlled degradation of PET to oligomers for the synthesis of PET-PLA copolymers. The utilization of oligomer products for the development of a new material is highly environmentally proactive as it reduces the process and energy consumption needed for the complete recycle of PET to monomeric products.
Another agent that can be used for chemical depolymerisation of PET is ammonia or amines; the lone electron pair of nitrogen in amines/ammonia attacks on the electrophilic carbon of the ester bond resulting to bond cleavage. It is known that the more substituted structure is the more reactive, and thus, ammonia has not been investigated as much as amines have [252]. The product obtained by ammonolysis is terephthalic acid diamide (TPADA), which can be used as an additive to PVC [253]; otherwise, through via pyrolytic dehydration, we can obtain aromatic nitrile compounds that can be hydrogenated to aminomethyl derivatives, i.e., p-xylene, diamine, 4-(aminomethyl) benzoic acid, 1,4 benzene dicarboxamide, etc., that are valuable chemicals and can be used for many applications such as monomers for polyamide synthesis [254,255].
On the other hand, aminolysis degradation of PET is much more investigated because amines are much more reactive; the reaction can take place in milder conditions (atmospheric pressure, room temperature) and without advanced apparatus or anhydrous environment requirements. The reaction results in terephthalamide derivatives (BHETA) and EG. For the acceleration of the reaction, several catalysts have been introduced: metal salts such as zinc acetate [256,257] and MgCl/NiCl [258], acetic acid for the swelling of PET [259], microwave-assisted conditions [256], etc. The BHETA derivatives can be the precursors for many added-value products [259]. An investigated application for PET upcycling via aminolysis is using the products as hardeners for epoxy resins such as DGEBA [260]. Kárpáti et al. [261] investigated the direct utilization of aminolysis products as cross-linking agents for epoxy resins without any purification process to the products. The authors proposed the accelerative nature of the EG side product, and the terephthalic-amide-diamine main products during the epoxy curing reaction. Recently, Alikin et al. [262] also showed that ED-20 epoxy-diane resin cured by amine derivatives from PET and PC waste presented enhanced mechanical and physical properties. In another upcycling attempt, Chan et al. [263] fabricated hydrogels through a two-step green process: aminolysis of PET waste and cross-linking of water-soluble poly(amidoamines) derivatives and ethylene glycol diglycidyl ether (EGDE). While the conversion of petrochemical derived polymers to hydrophilic and environmentally friendly materials is novel, they also showed a potential application for those hydrogels as absorbents for the removal of industrial dye, and how they may be addressed to other water-based fields too. Another novel study for upcycling PET waste though aminolysis is about the transformation of amine derivatives to N-doped carbon dots. Chan et al. [264] produced N-doped carbon dots with excellent fluorescent characteristics by treating PET aminolysis products with H2O2. The obtained upcycled material can be used for the sensitive detection of Fe3+ and Cu2+ ions in aqueous solutions at ppb and ppm concentrations, respectively.
Glycolysis of PET is the transesterification reaction in excess of a glycol such as EG, propylene glycol (PG), diethylene glycol (DEG), etc., and a catalyst. The product obtained is BHET and oligomers. The catalysts that can be used for glycolysis are metal acetates, metal oxides, ionic liquids, and deep eutectic solvents. Catalysts can control the yield of reaction and products [219,265]. The glycolysis approach is the most promising for the industrial scale depolymerisation of PET due to its more environmentally friendly reaction conditions, relatively milder temperature (<190 °C), and atmospheric pressure; the BHET product is less volatile and not toxic, and there are no toxic byproducts such as acid or alkali waste. Also, it can be performed in a continuous process and the separation and purification of products is easy. In contrast to those benefits, there are still challenges to be addressed for the success of industrialization, such as the relatively high reaction times and the low BHET yield. BHET can be precursor for PET synthesis, while oligomers can be further processed to produce polyurethanes, unsaturated polyesters, vinyl esters, epoxy resins, and polymeric concrete [219,265].
Depolymerisation via glycolysis can follow several catalytic routes such as metal oxides, anionic liquids, inorganic salts, organic bases, zeolites, clay catalysts, nanoparticles, and microwave-assisted catalysis [266,267]. In one of the latest research approaches, Shirazimoghaddam et al. [241] utilized a sulphated niobia-based catalyst treated at 573 K, and managed to obtain 100% conversion of PET and 85% yield toward BHET at a relatively low temperature of 195 °C in 220 min. This approach has great potential in the recycling of PET in mild conditions using nontoxic and inexpensive material as a catalyst. Other recent research approaches include the utilization of metal-doped SBA-15 (M/SBA-15) as heterogeneous Lewis acid catalysts [242] and biocompatible catalysts such as betaine [261].
3.4.3. Other Upcycling Approaches for Thermoplastics
Chemical upcycling holds promise for reducing the environmental impact of waste and enabling its transformation to valuable, added-value components. However, challenges remain in terms of scalability, cost-effectiveness, and the development of efficient processes for upcycling on an industrial scale. Ongoing research and technological advancements are essential for realizing the full potential of chemical upcycling in the management of polymer waste.
The chemical recycling of thermoplastic polymers is a field that has not yet been thoroughly investigated and, in fact, is still not completely applicable to some petroleum derived polymers. To tackle this, researchers have been focusing more on the designing of new, modified monomers for the synthesis of common thermoplastics, that have the ability also to depolymerise through chemolytic processes. The strategy to establish a circular plastic economy can be developed by designing chemically recyclable polymers [268].
Several studies propose ways to synthesize recyclable polyesters from bio-based and renewable feedstock; Tu et al. [269] successfully synthesized a series of biobased aromatic-aliphatic monomers that can build seven-membered-ring esters containing both the aromatic and aliphatic moieties. More importantly, the polymers are capable of depolymerizing into their monomers in solution or bulk with high efficiency, paving the way toward next-generation sustainable materials. Li et al. [270] presented a chemo-selective controlled ring-opening polymerisation (ROP) of bio-renewable bifunctional α-methylene-δ-valerolactone (MVL) that results in polyester with high molar mass that also exhibits tensile strength comparable to that of some commodity plastics. Also, the obtained polyester can be depolymerised to monomers with 96% yield by thermolysis, establishing a closed-loop life cycle. Li et al. [271] present a facile upcycling approach of bio-based polyester poly(3-hydroxybutyrate) (P3HB) into value-added polymerizable monomers, as well as the subsequent polymerisation of them via ROP toward degradable and recyclable polymers. The same team brought their research a step forward by investigating polymerisation parameters to achieve the synthesis of stereoregular polyesters with controlled molecular weights and well-defined chain ends [272].
The chemical recycling of polyolefins is approached mainly by thermolysis processes such as pyrolysis, gasification, hydrogenolysis etc. Lately, a lot of effort has been put into the investigation of synthesis of new polyolefin materials, polymers, and copolymers, from alternative monomers and synthetic routes, designed in a way that they are recyclable by chemical depolymerisation. Shi et al. [273] achieved the polymerisation and depolymerisation of two low-ceiling-temperature monomer structures, notoriously hard to polymerise, γ-butyrolactone and cyclohexene. This hybrid monomer can undergo orthogonal polymerisation either via ROP or ring opening metathesis polymerisation (ROMP), depending on the catalyst employed, resulting to two different polymer structures beginning from the same hybrid monomer; a polyester (ROP) and a poly (cyclic olefin) (ROMP). One of the latest achievements in this direction is the development of polyolefin-like multi-block polymers by Zhao et al. [274]. This new material consists of a soft and a hard kind of oligomeric building block, which can form via Ru-catalysed ROMP mechanisms, which are final materials with diverse mechanical characteristics varying from thermoplastics (polymer based on the hard building block) to elastomers (polymer based on the soft building block). Most importantly, all different multi-block polymers from this material after use can be combined and efficiently decomposed back to the initial hard and soft building blocks for separation and repolymerisation establishing a closed-loop recycling process.
The polycarbonate (PC) building block is quite reactive, since there are three available functionalization sites: methyl groups, carbonate groups, and aromatic rings. Chemical modification upcycling of waste polycarbonate materials can be achieved exploiting several functionalization methods such as halogenation, sulfonation, silanisation, hyper crosslinking via Friedel–Crafts alkylation and others [275]; the chemical recycling approach can be addressed through their synthesis. Saxon et al. [276] introduced the synthesis of polycarbonates derived from renewable alcohols (i.e., glucose tetraacetate, acetyl isosorbide, lauryl alcohol, and ethanol) and a cyclic carbonate bearing an imidazole-carboxylate, providing a completely function PC product that can also be chemically recycled using 1,8-Diazabicyclo(5.4.0) undec-7-ene (DBU) amidine. In the same direction, Zhang et al. [277] designed a seven-membered ring carbonate containing trans-cyclohexyl fused rings as monomer for the synthesis of PC, achieving a polymer that can be easily depolymerised to its monomer. Also, another approach for upcycling of polycarbonate waste is chemical conversion to vitrimers; Reddy et al. [278] developed polycarbonate imine vitrimers from waste polycarbonate with high thermal performance and hydrolytic stability that can be utilized for CFRP manufacturing. Composites can be chemically recycled via aminolysis in a catalyst-free, mild-conditions environment, providing a sustainable alternative for PC wastes.
Polyamides is another polymer family that is being widely used in high-performance applications, such as the automotive sector, as well as in commodity use products, thus its sustainable recycling and upcycling is vital. Cywar et al. [279] introduced a hybrid polyamide polymer, nylon 4/6, based on a bicyclic lactam composed of both HCT ε-caprolactam and LCT pyrrolidone motifs in a hybridized offspring structure. This hybrid nylon 4/6 product shows performance properties of the parent nylons, as well as facile depolymerisation characteristics. PMMA is also a polymer that is widely used in construction and other engineering applications. Wang et al. [280] introduced a catalyst-free depolymerisation strategy that can be applied to linear, bulky, cross-linked, and functional polymethacrylic materials synthesized by reversible addition-fragmentation chain-transfer (RAFT) polymerisation yielding high depolymerisation conversions (up to 92%). The method exploits the high end-group fidelity of RAFT polymers; specifically, the temperature of the polymethacrylate solution, chain-end radicals is being produced and triggers a rapid depolymerisation.
Lately, ‘one-pot’ depolymerisation approaches have attracted attention, showing emerging potential in dual-capable biodegradable and recyclable polyesters recycling, aiming to develop an energy-efficient process that can deconstruct mixed plastics with labile ester bonds into valuable, chemically defined feedstock to make virgin-grade new materials [281]. A “one-pot” depolymerisation is used to realize the simultaneous depolymerisation of BPA-PC/PET, PLA/PBS, and PLA/PBAT mixed plastics under the same conditions to their initial monomers or value-added chemicals, followed by the separation of products [282]. Taking advantage of the differences in depolymerisation reactivity of commercial plastics and versatile catalytic degradation ability of zinc bis[bis(trimethylsilyl)amide] [Zn(HMDS) 2], the selective depolymerisation process was smoothly achieved under mild conditions. These strategies provide ideas for promoting the sustainable development of plastics that are currently being used on a large scale.
3.5. Enzymatic Recycling and Upcycling
Another chemical approach that is very promising for the recycling and upcycling of thermoplastic waste is enzymatic recycling. Specialized enzymes are used to breakdown polymers into monomers; those enzymes are capable of hydrolysing plastics that are not easily biodegradable [283]. Enzymes like cutinase work as a catalyst for the depolymerisation process. One of the most used polymers in the plastic industry is polyethylene terephthalate (PET), which has a wide variety of uses. More than 50% is used for fabricating textiles and fibres, while the other half is used for plastic single-use bottles for liquids, packaging, and other applications. Therefore, recycling is essential to reduce the pollution crisis regarding the accumulation of these products in landfills and the environment [284].
Several studies have been conducted regarding the enzymatic activity of several fungus and microbial species. The main goal has been to degrade the polymer to monomeric building blocks. The main challenge of this enzyme breakdown of polymers, such as PP, PE, and PET, is their derivative nature (hydrophobicity inertness and stability), which makes them recalcitrant to biological degradation. The first step is the adhesion of the microorganism to the surface of the plastic, while the second step is the growth of the microorganisms followed by colonization with carbon-rich biofilm formation via metabolization, and the third step is the breakdown of the longer molecular chains of the polymer to smaller ones by means of enzymatic hydrolysis, and the fourth is the final degradation into oligomers as well as products such as H2O, CO2, and CH4 [285].
The enzyme cutinase from Humilica insolens, Pseudomonas mendocina, and Fusarium solani were tested regarding the degradation to PET films with low crystallinity and biaxial orientation. The results were promising when Humilica insolens were used on low crystallinity PET substrates at 70 °C, which catalysed the hydrolysis of the film at 97% ± 3% weight loss in 96 h, which corresponds to a loss of film thickness of 30 μm per day. Due to the preferential degradation of amorphous regions, the crystallinity was also increased to 27%. In contrast, both of the other two cutinases resulted in only 5% weight loss at 50 and 40 °C for 96 h [244].
As a semi-crystalline polymer, PET consists of crystalline and amorphous regions. When the temperature reaches the glass transition temperature, the embrittled amorphous regions become flexible. Studies have shown that at this stage, the enzymes can be more efficient regarding biodegradability because they can easily reach the polymer chains. Then et al. [286] used thermophilic actinomycete Thermobifida fusca KW3 to produce esterases TfH, BTA2, Tfu_0882, TfCut1 and TfCut2 to exhibit PET-hydrolysing activity. However, these enzymes have no thermal stability at temperatures close to PET Tg. Regarding this, the Ca2+ and Mg2+ cations were added to the catalysts and increased the Tm of the enzymes at 10.8 and 14.1, respectively. The addition of the cations was able to increase the thermostability at a temperature range of 55–65 °C for a 48 h duration. In particular, the effect of the cations on the degradation of low crystalline PET films by the hydrolases depended on the reaction temperature. At 55 °C and 48 h duration, all hydrolases caused similar weight loss (with or without cations) apart from TfCut1 and TfCut2 which caused a 2.5-fold decrease in PET hydrolysing in presence of Ca2+. At 60 °C Tfu_0882 caused no weight loss without cations, TfCut1 and TfCut2 have shown a decrease of 1.8–2.6-fold. At 65 °C no activity was detected without dications. TfH and TfCut2 have been the most effective, they caused a 12% weight loss. Cations were also replaced by arginine which caused an increase in thermal stability. TfCut2 developed versions, are stabilized catalysts that can cause the hydrolysability of PET just with positively charged arginine and without any dications.
In 2012, a bacterium named “Ideonella sakaiensis” was discovered that slowly “chews” the very common plastic: polyethylene terephthalate (PET), using it as carbon-based fuel for its growth. These bacteria do so by making an enzyme called PETase, which breaks PET into mono(2-hydroxyethyl) terephthalic acid (MHET), then another enzyme called MHETase breaks down MHET into terephthalic acid (TA) and ethylene glycol (EG), the basic building blocks of PET. These enzymes were found to be able to operate efficiently at temperatures close to 30 °C. Another enzyme was also discovered that evolved to break down the waxy protective coating on plant leaves. Its name is Leaf-branch compost cutinase (LCC) and it has an advantage over PETase, its operating temperature. LCC operates at 65 °C, a temperature close to PET glass transition temperature (Tg), which means that the polymer starts to melt, and the enzyme can have easier access to the polymer chains and break them down. Engineered E. coli was used as a host bacterium to catalyse the conversion of terephthalic acid to vanillin, a product widely used to give vanilla flavour to ice creams, chocolates, and other products in the food industry or to be used as fragrances in perfumes and other cosmetics. Using TA as a starting point a 79% conversion was achieved. No hazardous wastes were produced, and the reaction can be performed under ambient conditions in aqueous media [287].
Some more research has been conducted on thermoplastics such as polypropylene (PP), polyethylene (PE), polystyrene (PS), and polylactic acid (PLA). Oliveira et al. [288] have researched the impact of multiple extrusion cycles on the inoculated fungi Aspergillus sp. and Penicillium sp. PP/PBAT/thermoplastic starch blend. They have studied mass loss, chemical structure, hydrophilicity, and morphology of the blend before and after their inoculation with the fungi. They have found that the multiple extrusion cycles favour the deposition of fungi in the samples, enabling hydrophilicity and morphological changes. Multiple extrusions did not influence inoculated PP biodegradation; a slight increase in the biodegradation was observed with the addition of PBAT/TPS. In general, the degradation was very low; in fact, in the first extrusion cycle of the blend, the mass loss was only 1.04% and in the seventh cycle, it slightly increased to 2.32%. The action of the microorganism is affected by both the extrusion cycles and the addition of the PBAT/TPS.
Polylactic acid could be also hydrolysed using enzymes like lipase, esterase, and alcalase. The key factors that affect biodegradability in the environment are temperature and PH; material crystallinity and chain stereochemistry are also key factors. The process for the biodegradation of PLA follows some steps that eventually degrade not only the surface of the polymers but even the bulk. Thermoplastic starch (TPS) was added at 0–5 wt.% and 10 wt.% at the PLA film’s surface and after 627 h cracks, it became noticeable at the PLA film surface, which may be correlated to surface hydrolysis. Hydrolysis can also occur within the bulk polymer via the diffusion of water, which is then followed by bacterial growth and the secretion of enzymes at the polymer surface [289].
3.6. Recycling Technologies for Thermoplastic Composites
Thermoplastics can be used in various applications; however, in the last years, the investigation has focused on the use of thermoplastic matrices in manufacturing composite materials. This phenomenon can also be attributed to the increase in automated and additive manufacturing processes. These can be either general polymers, such as ABS, PP, and PE, or high-performance polymers, such as polyether ether ketone (PEEK), polyphenylene sulphide (PPS), PC), polyether sulfone (PES) and PA, etc. [290,291].
Thermoplastic composites can be recycled by the abovementioned recycling technologies (both thermoplastic and thermoset) such as mechanical, thermal, and chemical processes. However, due to the thermoplastic nature of the matrix, other processes can be applied. Roux et al. [292] have investigated the recycling of the PEEK composite reinforced with long CFs through electrodynamical fragmentation. In this process, the solid material under investigation is placed in an isolating liquid and then, through high voltage discharge applications, separation occurs. Lightweight rotorcraft door hinges were placed in the vessel and 6 cycles of 100 pulses/cycle, with 180 kV voltage of 5 Hz applied. Between each cycle, the contents were sieved through a metallic grid (4 mm mesh size). It was noticed that after each cycle, the amount of composite material was reduced, and the process yield was exponentially increased. Afterwards, fragments with targeted dimensions were used for remanufacturing hinges using compression moulding. The remanufactured hinges showed an 17% decrease compared to chopped tape hinges, and 18% increase in mechanical performance compared to hinges manufactured by injection moulding.
Another investigated process is the dissolution. This process allows the solvent to swell the polymer without breaking the bonds, allowing for the separation of reinforcements from the matrix. Cousins et al. [293] used an Elium® thermoplastic resin (methacrylates family) by Arkema and GFs to manufacture a spar cap component of a wind turbine blade. Part of the spar cap was then immersed in chloroform and soaked for 48 h. After the 48 h, the part was still adhered to the inner plies; thus, further immersion time was required, and finally, after another 24 h, the plies of the composite part were successfully separated. After dissolution, the composite part was precipitated into methanol. Finally, the part was dried for 24 h and then placed in vacuum at 60 °C for 12 h. Afterwards, it was observed that the GFs and polymer were completely separated, with fibre and polymer reclamation of total 91%. The authors also indicated that the economic feasibility of this process will be increased by 3–6 times when applied to CFRPs.
Apart for fibre reclamation, the thermoplastic composites can be recycled by exploiting the capabilities to be reprocessed, such as re-melting and re-moulding properties. Kiss et al. [294] shredded PP/PA6 laminates and separated the material to coarse and fine fractions. Then, granules of the material were used to manufacture a new thermoplastic composite through hot compression. Then, the compressed part was used for the manufacturing of sandwich laminated panel, by a reverse thermoforming technique.
4. Conclusions and Future Directions
In this review, the different state-of-the-art recycling technologies for both thermoset and thermoplastic polymers were assessed. With the increasing demand for composite structures, it is indispensable to address both their manufacturing and decommissioning. The identification of the suitable recycling technology is a key enabler of a circular concept for polymer and composites and their end-of-life management.
In the case of thermoset composites, the crosslinked structure of the polymeric matrix makes their recycling quite challenging, and conventional recycling techniques developed for thermoplastics cannot be applied. Three main recycling approaches were reviewed—thermal, chemical, and combined processes—that can successfully recycle and reclaim composite reinforcement while offering opportunities for upcycling and reuse. However, each type of composite requires its tailored recycling process, in order to be successful and exploitable in new applications. Thus, each process should be aligned with the chemical nature of the polymeric matrix, taking into account possible limitations and challenges. Pyrolysis, depending on the applied temperature, requires a post-processing oxidation step to remove solid residue left on the reinforcing fibres. The oxidation step may damage the fibres, making difficult to determine the optimum pyrolysis and post pyrolysis parameters for reclaiming high-quality fibres. Furthermore, the valorisation of by-products should be further investigated in order to enable circularity for the whole value chain. For the solvolysis process, the combination of solvent, temperature, pressure, and type of catalyst is critical for successful fibre reclamation. The chemical nature of the solvent should be chosen considering not only the polymeric matrix but also the type of reinforcement, e.g., GFs or CFs. In the case of supercritical solvolysis, less invasive solvents are being used (e.g., acetone, water, etc.) reducing the environmental impact. However, the handling of the solutions after the process should be evaluated, as well as the by-products valorisation together with the energy needs. The focus of the research should primarily aim at achieving pure fibres and minimising the processes’ impact on the fibres’ mechanical properties. Another challenge associated with solvolysis is its upscaling, which would allow larger quantities of material reclamation and industrial potential. In order to tackle limitations of the conventional thermal and chemical recycling, such as fibre degradation, extraordinary procedures as combination between processes or new approaches (e.g., electrochemical, ultrasonic, biological, etc.) are suggested. However, those technologies are still under investigation and further research is required to achieve exploitable results, focusing on efficient material separation, depolymerisation, and reclamation of valuable components. Furthermore, implementing disassembly techniques during the design phase of thermoset composites can enhance their recyclability at end-of-life, thereby facilitating circularity within the material lifecycle.
In the case of thermoplastic polymers, each recycling method offers unique advantages and challenges, and the choice of method depends on factors such as the type of thermoplastic, desired quality of recycled material, available infrastructure, and economic considerations. Mechanical recycling is a relatively simple and cost-effective process compared to other recycling methods, preserving the mechanical properties of the recycled material to some extent, though it is limited to thermoplastics with similar chemical compositions and may lead to the degradation of polymer properties due to thermal reprocessing; thus, it requires improved sorting and pre-treatment. Selective dissolution is effective for separation of mixed or contaminated plastics, offering higher purity levels compared to mechanical recycling; nevertheless, it may have higher energy consumption and operating costs, and limited scalability for certain techniques. Chemical recycling offers the highest purity and quality of recycled materials, regenerating monomers for virgin polymer production. It requires more complex and energy-intensive processes and may require high temperatures, pressures, and suitable catalysts. Although the most environmentally friendly process is enzymatic recycling, it is limited by enzyme stability and activity, slower reaction rates compared to chemical methods, and may require pre-treatment steps to enhance efficiency. Future research and development efforts should focus on improving existing recycling technologies and developing new methods to increase efficiency, reduce costs and environmental footprint, and improve the recyclate quality. Additional investments in recycling infrastructure are essential to support improvement in collection schemes and the implementing of advanced automated sorting and polymer processing technologies. Designing products with recyclability in mind should be prioritized, using materials that are easily separable and recyclable at the end of their life. Wide adoption of extended producer responsibility (EPR) schemes would encourage manufacturers to design for recyclability, use recycled materials, and invest in recycling infrastructure. Raising public awareness regarding the significance of recycling, adopting proper waste management practices, and understanding the advantages of utilizing recycled materials can facilitate the demand for recycled products and strengthen recycling initiatives.
Finally, all the recycling processes, in order to be industrially viable and exploitable, should provide product consistency and verified quality. The resulting properties of the recycled materials should be provided in technical and safety data sheets, as digital passports. The research activities should be orientated to waste transformation in secondary raw materials, to enable materials circularity.
Author Contributions
Conceptualization, C.P., S.T., D.S., A.-F.T. and T.K.M.; resources, C.C.; writing—original draft preparation, C.P. (Upcycling of Thermoset Composites to Vitrimers, Recycling Technologies of Thermoplastic Polymers, Overview, Chemical Recycling and Upcycling), S.T. (Thermal Processes for Thermosets, Thermoplastic Composites Recycling), M.M. (Chemical Processes for Thermosets), D.S. (Chemical Processes for Thermosets), C.T. (Recycling Technologies of Thermoplastic Polymers, Enzymatic Recycling and Upcycling), M.K. (Recycling Technologies of Thermoplastic Polymers, Mechanical Recycling and Upcycling), A.-F.T. (Extraordinary and Combined Recycling Processes for Thermosets), T.K.M. (Introduction, Thermoplastics and Thermosets, Upcycling of Thermoset Composites to Vitrimers); writing—review and editing, C.P., S.T. and A.-F.T.; visualization, C.P., M.M., D.S. and A.-F.T.; supervision, C.C.; project administration, C.C.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work is co-funded under the Horizon 2020 and Horizon Europe research and innovation funding programmes, entitled: “Plastics Recycling from and for home appliances, toys and textile” (PRecycling), under Horizon Europe with GA No. 101058670; “European recycling and circularity in large composite components” (EuReComp), under Horizon Europe with GA No. 101058089; “Sustainable structural sandwiches and hollow composites parts for automotive, boat and aerospace markets” (Suspens), under Horizon Europe with GA No. 101091906; “Recycle, Repurpose and Reuse End-Of-Life Wind Blade Composites—A Coupled Pre- and Co-Processing Demonstration Plant” (Blades2Build), under Horizon Europe with GA No. 101096437; “New generation of offshore turbine blades with intelligent architectures of hybrid, nano-enabled multi-materials via advanced manufacturing” (Carbo4Power), under H2020 with GA No. 953192; “Recycling and Repurposing of Plastic Waste for Advanced 3D Printing Applications” (Repair3D), under H2020 with GA No. 814588.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A New Sustainability Paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Walker-Franklin, I.; Jambeck, J. Plastics; Essential Knowledge series, eBook; The MIT Press: Cambridge, MA, USA, 2023; pp. 11–20. [Google Scholar]
- Recycling of Thermoset Materials: The Potential Impact on Reducing Fossil-Based Plastic Waste. Available online: https://www.prescouter.com/inquiry/recycling-of-thermoset-materials/ (accessed on 14 March 2024).
- Johst, P.; Kucher, M.; Bühl, M.; Schulz, P.; Kupfer, R.; Schilling, L.; Santos, R.M.; Carneiro, C.; Voigt, P.; Modler, N.; et al. Identification and Environmental Assessments for Different Scenarios of Repurposed Decommissioned Wind Turbine Blades. Mater. Circ. Econ. 2023, 5, 13. [Google Scholar] [CrossRef]
- Johst, P.; Chatzipanagiotou, K.-R.; Kucher, M.; Zschiebsch, W.; Voigt, P.; Breinl, D.; Koumoulos, E.P.; Böhm, R. Concept and Life Cycle Assessment of a Tiny House Made from Root Section Structures of a Decommissioned Large-Scale Wind Turbine Blade as a Repurposed Application. Mater. Circ. Econ. 2024, 6, 5. [Google Scholar] [CrossRef]
- Carbon Fibre Composites Market—By End-Use (Aerospace, Automotive, Wind Turbines, Sport & Leisure, Civil Engineering, Marine), By Matrix Material (Polymer [Thermosetting, Thermoplastics], Carbon, Ceramic, Metal, Hybrid) & Forecast, 2022–2030; For Insights Consultancy: New York, NY, USA, 2023.
- Composites Germany—Results of the 20th Composites Market Survey Now Available, PRESS RELEASE February 2023, No. 1/2023. Available online: https://www.composites-germany.org/index.php/en/news-en/market-information/737-20th-composites-market-survey (accessed on 14 March 2024).
- University of Sydney. The Looming 840,000 ton Waste Problem that Isn’t Single-Use Plastics. ScienceDaily. ScienceDaily, 3 July 2023. Available online: https://www.sydney.edu.au/news-opinion/news/2023/07/03/-the-looming-840-000-tonne-waste-problem-that-isn-t-single-use-p.html (accessed on 14 March 2024).
- Schmid, M.; Gonzalez Ramon, N.; Dierckx, A.; Wegman, T. Accelerating Wind Turbine Blade Circularity; WindEurope; Cefic; EuCIA: Brussels, Belgium, 2020; Available online: https://windeurope.org/intelligence-platform/product/accelerating-wind-turbine-blade-circularity/ (accessed on 14 March 2024).
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-waste Monitor—2017, United Nations University (UNU), International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Vienna. Available online: https://www.itu.int/en/ITU-D/Environment/Pages/Toolbox/Global-E-waste-Monitor-2017.aspx (accessed on 14 March 2024).
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)—Co-Hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Rotterdam. Available online: https://collections.unu.edu/eserv/UNU:7737/GEM_2020_def_july1.pdf (accessed on 14 March 2024).
- UNEP (2014) Valuing Plastics: The Business Case for Measuring, Managing and Disclosing Plastic Use in the Consumer Goods Industry. Available online: https://wedocs.unep.org/handle/20.500.11822/9238;jsessionid=5FE97F45C8AC6A7514ACBF870A6D35F6 (accessed on 14 March 2024).
- Ellen MacArthur Foundation. The Global Commitment 2021; Ellen MacArthur Foundation: Isle of Wight, UK, 2021. [Google Scholar]
- Ellen MacArthur Foundation. A New Textiles Economy: Redesigning Fashion’s Future; Ellen MacArthur Foundation: Isle of Wight, UK, 2017. [Google Scholar]
- Teresa Haukkala, K.N.; Turunen, L.L.M. Fashion in Turmoil: Impact of the COVID-19 Pandemic on Finland’s Textile and Fashion Industry. Sustain. Sci. Pract. Policy 2023, 19, 2173424. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic Waste Release Caused by COVID-19 and Its Fate in the Global Ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic Pollution during COVID-19: Plastic Waste Directives and Its Long-Term Impact on the Environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef]
- European Commission. Circular Economy Policy. 2020. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 14 March 2024).
- Directive (EU). 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment (Text with EEA Relevance). Off. J. Eur. Union 2019, L155/I, 1–19. [Google Scholar]
- Directorate-General for Environment Proposal for a Regulation on Circularity Requirements for Vehicle Design and on Management of End-of-Life Vehicles, 13 July 2023. Available online: https://environment.ec.europa.eu/publications/proposal-regulation-circularity-requirements-vehicle-design-and-management-end-life-vehicles_en (accessed on 14 March 2024).
- Stapleton, M.J.; Ansari, A.J.; Ahmed, A.; Hai, F.I. Evaluating the Generation of Microplastics from an Unlikely Source: The Unintentional Consequence of the Current Plastic Recycling Process. Sci. Total Environ. 2023, 902, 166090. [Google Scholar] [CrossRef]
- Guo, Y.; Xia, X.; Ruan, J.; Wang, Y.; Zhang, J.; LeBlanc, G.A.; An, L. Ignored Microplastic Sources from Plastic Bottle Recycling. Sci. Total Environ. 2022, 838, 156038. [Google Scholar] [CrossRef]
- Suzuki, G.; Uchida, N.; Tanaka, K.; Higashi, O.; Takahashi, Y.; Kuramochi, H.; Yamaguchi, N.; Osako, M. Global Discharge of Microplastics from Mechanical Recycling of Plastic Waste. Environ. Pollut. 2024, 348, 123855. [Google Scholar] [CrossRef]
- Jacob, J.; Linson, N.; Binoj, J.S.; Mansingh, B.B.; Kuriakose, S.; Thomas, S. Chapter 2—Polymers and Fillers Used in the Packaging Industry. In Nanostructured Materials for Food Packaging Applications; Jacob, J., Cacciotti, I., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2024; pp. 31–48. ISBN 978-0-323-99525-2. [Google Scholar]
- Eriksen, M.K.; Pivnenko, K.; Olsson, M.E.; Astrup, T.F. Contamination in Plastic Recycling: Influence of Metals on the Quality of Reprocessed Plastic. Waste Manag. 2018, 79, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Pöhlein, M.; Llopis, A.S.; Wolf, M.; van Eldik, R. Rapid Identification of RoHS-Relevant Flame Retardants from Polymer Housings by Ultrasonic Extraction and RP-HPLC/UV. J. Chromatogr. A 2005, 1066, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gripon, L.; Belyamani, I.; Legros, B.; Seaudeau-Pirouley, K.; Lafranche, E.; Cauret, L. Brominated Flame Retardants Extraction from Waste Electrical and Electronic Equipment-Derived ABS Using Supercritical Carbon Dioxide. Waste Manag. 2021, 131, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Preetam, A.; Jadhao, P.R.; Naik, S.N.; Pant, K.K.; Kumar, V. Supercritical Fluid Technology—An Eco-Friendly Approach for Resource Recovery from e-Waste and Plastic Waste: A Review. Sep. Purif. Technol. 2023, 304, 122314. [Google Scholar] [CrossRef]
- Strobl, L.; Diefenhardt, T.; Schlummer, M.; Leege, T.; Wagner, S. Recycling Potential for Non-Valorized Plastic Fractions from Electrical and Electronic Waste. Recycling 2021, 6, 33. [Google Scholar] [CrossRef]
- Devic, A.C.; Ierides, M.; Fernandez, V.; Verbenkov, M.; Bax, L. Polymer Composites Circularity, Suschem Material Working Group. 2018. Available online: https://suschem.org/publications/ (accessed on 14 March 2024).
- Ratna, D. Recent Advances and Applications of Thermoset Resins. Chapter 2—Properties and Processing of Thermoset Resin, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Guo, Q. (Ed.) Thermosets: Structure, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081010211. [Google Scholar]
- Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Legarreta, J.A.; Cabrero, M.A.; González, A.; Chomón, M.J.; Gondra, K. Recycling by Pyrolysis of Thermoset Composites: Characteristics of the Liquid and Gaseous Fuels Obtained. Fuel 2000, 79, 897–902. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling Technologies for Thermoset Composite Materials-Current Status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Kennerley, J.R.; Kelly, R.M.; Fenwick, N.J.; Pickering, S.J.; Rudd, C.D. The Characterisation and Reuse of Glass Fibres Recycled from Scrap Composites by the Action of a Fluidised Bed Process. Compos. Part A Appl. Sci. Manuf. 1998, 29, 839–845. [Google Scholar] [CrossRef]
- Pickering, S.J.; Kelly, R.M.; Kennerley, J.R.; Rudd, C.D.; Fenwick, N.J. A Fluidised-Bed Process for the Recovery of Glass Fibres from Scrap Thermoset Composites. Compos. Sci. Technol. 2000, 60, 509–523. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J.; Walker, G.S.; Wong, K.H.; Rudd, C.D. Surface Characterisation of Carbon Fibre Recycled Using Fluidised Bed. Appl. Surf. Sci. 2008, 254, 2588–2593. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Acha, E.; Caballero, B.M.; Orue, A.; Jiménez-Suárez, A.; Prolongo, S.G.; de Marco, I. Reclamation of Carbon Fibers and Added-Value Gases in a Pyrolysis-Based Composites Recycling Process. J. Clean. Prod. 2020, 273, 123173. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Majka, T.M. 1—Introduction to Thermal Degradation of Polymeric Materials. In Thermal Degradation of Polymeric Materials, 2nd ed.; Pielichowski, K., Njuguna, J., Majka, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–8. ISBN 978-0-12-823023-7. [Google Scholar]
- Meyer, L.O.; Schulte, K.; Grove-Nielsen, E. CFRP-Recycling Following a Pyrolysis Route: Process Optimization and Potentials. J. Compos. Mater. 2009, 43, 1121–1132. [Google Scholar] [CrossRef]
- Bradna, P.; Zima, J. Compositional Analysis of Epoxy Matrices of Carbon-Fibre Composites by Pyrolysis—Gas Chromatography/Mass Spectrometry. J. Anal. Appl. Pyrolysis 1992, 24, 75–85. [Google Scholar] [CrossRef]
- Evans, S.J.; Haines, P.J.; Skinner, G.A. Pyrolysis–Gas-Chromatographic Study of a Series of Polyester Thermosets. J. Anal. Appl. Pyrolysis 2000, 55, 13–28. [Google Scholar] [CrossRef]
- Termine, S.; Naxaki, V.; Semitekolos, D.; Trompeta, A.-F.; Rovere, M.; Tagliaferro, A.; Charitidis, C. Investigation of Carbon Fibres Reclamation by Pyrolysis Process for Their Reuse Potential. Polymers 2023, 15, 768. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Jones, N.; Williams, P.T. Recycling of Fibre-Reinforced Polymeric Waste by Pyrolysis: Thermo-Gravimetric and Bench-Scale Investigations. J. Anal. Appl. Pyrolysis 2003, 70, 315–338. [Google Scholar] [CrossRef]
- Blazsó, M. Composition of Liquid Fuels Derived from the Pyrolysis of Plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 315–344. ISBN 9780470021545. [Google Scholar]
- Xu, M.; Di, J.; Wu, Y.; Meng, X.; Ji, H.; Jiang, H.; Li, J.; Lu, Q. Insights into the Pyrolysis Mechanisms of Epoxy Resin Polymers Based on the Combination of Experiments and ReaxFF-MD Simulation. Chem. Eng. J. 2023, 473, 145404. [Google Scholar] [CrossRef]
- Ge, L.; Li, X.; Feng, H.; Xu, C.; Lu, Y.; Chen, B.; Li, D.; Xu, C. Analysis of the Pyrolysis Process, Kinetics and Products of the Base Components of Waste Wind Turbine Blades (Epoxy Resin and Carbon Fiber). J. Anal. Appl. Pyrolysis 2023, 170, 105919. [Google Scholar] [CrossRef]
- Adler, S.E.; Güttler, B.E.; Bendler, L.; Friedrich, K. Evaluation of Recycled Carbon Fibre/Epoxy Composites: Thermal Degradation Behaviour of Pyrolysed and Virgin Carbon Fibres Using Thermogravimetric Analysis. Adv. Ind. Eng. Polym. Res. 2021, 4, 82–92. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Recycling of Carbon Fibre Reinforced Polymeric Waste for the Production of Activated Carbon Fibres. J. Anal. Appl. Pyrolysis 2011, 91, 67–75. [Google Scholar] [CrossRef]
- Greco, A.; Maffezzoli, A.; Buccoliero, G.; Caretto, F.; Cornacchia, G. Thermal and Chemical Treatments of Recycled Carbon Fibres for Improved Adhesion to Polymeric Matrix. J. Compos. Mater. 2012, 47, 369–377. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Mazzocchetti, L.; Leonardi, C.; Zattini, G.; Minak, G.; Dolcini, E.; Cavazzoni, M.; Montanari, I.; Tosi, C. Recovery of Carbon Fibers from Cured and Uncured Carbon Fiber Reinforced Composites Wastes and Their Use as Feedstock for a New Composite Production. Polym. Compos. 2015, 36, 1084–1095. [Google Scholar] [CrossRef]
- Hadigheh, S.A.; Wei, Y.; Kashi, S. Optimisation of CFRP Composite Recycling Process Based on Energy Consumption, Kinetic Behaviour and Thermal Degradation Mechanism of Recycled Carbon Fibre. J. Clean. Prod. 2021, 292, 125994. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Q.; Li, X.-Y.; Yang, K.-K.; Wang, Y.-Z. Chemical Recycling of Fiber-Reinforced Epoxy Resin Using a Polyethylene Glycol/NaOH System. J. Reinf. Plast. Compos. 2014, 33, 2106–2114. [Google Scholar] [CrossRef]
- Feraboli, P.; Kawakami, H.; Wade, B.; Gasco, F.; DeOto, L.; Masini, A. Recyclability and Reutilization of Carbon Fiber Fabric/Epoxy Composites. J. Compos. Mater. 2012, 46, 1459–1473. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, L.; Huang, Y.; Du, J. Recycling of Carbon/Epoxy Composites. J. Appl. Polym. Sci. 2004, 94, 1912–1916. [Google Scholar] [CrossRef]
- Ballout, W.; Sallem-Idrissi, N.; Sclavons, M.; Doneux, C.; Bailly, C.; Pardoen, T.; Van Velthem, P. High Performance Recycled CFRP Composites Based on Reused Carbon Fabrics through Sustainable Mild Solvolysis Route. Sci. Rep. 2022, 12, 5928. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical Recycling of Glass Fiber Reinforced Epoxy Resin Cured with Amine Using Nitric Acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Okajima, I.; Sako, T. Recycling Fiber-Reinforced Plastic Using Supercritical Acetone. Polym. Degrad. Stab 2019, 163, 1–6. [Google Scholar] [CrossRef]
- Keith, M.J.; Román-Ramírez, L.A.; Leeke, G.; Ingram, A. Recycling a Carbon Fibre Reinforced Polymer with a Supercritical Acetone/Water Solvent Mixture: Comprehensive Analysis of Reaction Kinetics. Polym. Degrad. Stab. 2019, 161, 225–234. [Google Scholar] [CrossRef]
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W.M.; Aymonier, C.; Horn, S. Semi-Continuous Flow Recycling Method for Carbon Fibre Reinforced Thermoset Polymers by near- and Supercritical Solvolysis. Polym. Degrad. Stab. 2016, 133, 264–274. [Google Scholar] [CrossRef]
- Knight, C.C.; Zeng, C.; Zhang, C.; Wang, B. Recycling of Woven Carbon-Fibre-Reinforced Polymer Composites Using Supercritical Water. Environ. Technol. 2012, 33, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Yildirir, E.; Onwudili, J.A.; Williams, P.T. Recovery of Carbon Fibres and Production of High Quality Fuel Gas from the Chemical Recycling of Carbon Fibre Reinforced Plastic Wastes. J. Supercrit. Fluids 2014, 92, 107–114. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.-L.; Salle, E.L.G. La Chemical Recycling of Glass Fibre Reinforced Composites Using Subcritical Water. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1809–1818. [Google Scholar] [CrossRef]
- Obunai, K.; Fukuta, T.; Ozaki, K. Carbon Fiber Extraction from Waste CFRP by Microwave Irradiation. Compos. Part A Appl. Sci. Manuf. 2015, 78, 160–165. [Google Scholar] [CrossRef]
- Jiang, L.; Ulven, C.A.; Gutschmidt, D.; Anderson, M.; Balo, S.; Lee, M.; Vigness, J. Recycling Carbon Fiber Composites Using Microwave Irradiation: Reinforcement Study of the Recycled Fiber in New Composites. J. Appl. Polym. Sci. 2015, 132, 42658. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Liu, C.; Mahmoodi, R.; Li, Q.; Naebe, M. Development of a Low Cost and Green Microwave Assisted Approach towards the Circular Carbon Fibre Composites. Compos. B Eng. 2020, 184, 107750. [Google Scholar] [CrossRef]
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. Development of Sustainable Microwave-Based Approach to Recover Glass Fibers for Wind Turbine Blades Composite Waste. Resour. Conserv. Recycl. 2022, 179, 106107. [Google Scholar] [CrossRef]
- Åkesson, D.; Skrifvars, M. Recycling of Thermoset Composites by Microwave Pyrolysis. In Proceedings of the ICCM International Conferences on Composite Materials (ICCM 18), Jeju Island, Republic of Korea, 21–26 August 2011. [Google Scholar]
- Cai, G.; Wada, M.; Ohsawa, I.; Kitaoka, S.; Takahashi, J. Influence of Treatment with Superheated Steam on Tensile Properties of Carbon Fiber. Compos. Part A Appl. Sci. Manuf. 2018, 107, 555–560. [Google Scholar] [CrossRef]
- Cai, G.; Wada, M.; Ohsawa, I.; Kitaoka, S.; Takahashi, J. Interfacial Adhesion of Recycled Carbon Fibers to Polypropylene Resin: Effect of Superheated Steam on the Surface Chemical State of Carbon Fiber. Compos. Part A Appl. Sci. Manuf. 2019, 120, 33–40. [Google Scholar] [CrossRef]
- Wada, M.; Kawai, K.; Suzuki, T.; Hira, H.; Kitaoka, S. Effect of Superheated Steam Treatment of Carbon Fiber on Interfacial Adhesion to Epoxy Resin. Compos. Part A Appl. Sci. Manuf. 2016, 85, 156–162. [Google Scholar] [CrossRef]
- Jeong, J.-S.; Kim, K.-W.; An, K.-H.; Kim, B.-J. Fast Recovery Process of Carbon Fibers from Waste Carbon Fibers-Reinforced Thermoset Plastics. J. Environ. Manag. 2019, 247, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Wakisaka, M.; Nishida, H. Specific Oligomer Recovery Behavior from Cured Unsaturated Polyester by Superheated Steam Degradation. Polym. Degrad. Stab. 2019, 161, 1–6. [Google Scholar] [CrossRef]
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.-H.; Xing, F. Recycling of Carbon Fibers from Carbon Fiber Reinforced Polymer Using Electrochemical Method. Compos. Part A Appl. Sci. Manuf. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Oshima, K.; Matsuda, S.; Hosaka, M.; Satokawa, S. Rapid Removal of Resin from a Unidirectional Carbon Fiber Reinforced Plastic Laminate by a High-Voltage Electrical Treatment. Sep. Purif. Technol. 2020, 231, 115885. [Google Scholar] [CrossRef]
- Mativenga, P.T.; Shuaib, N.A.; Howarth, J.; Pestalozzi, F.; Woidasky, J. High Voltage Fragmentation and Mechanical Recycling of Glass Fibre Thermoset Composite. CIRP Ann. 2016, 65, 45–48. [Google Scholar] [CrossRef]
- Sarmah, A.; Sarikaya, S.; Thiem, J.; Upama, S.T.; Khalfaoui, A.N.; Dasari, S.S.; Arole, K.; Hawkins, S.A.; Naraghi, M.; Vashisth, A.; et al. Recycle and Reuse of Continuous Carbon Fibers from Thermoset Composites Using Joule Heating. ChemSusChem 2022, 15, e202200989. [Google Scholar] [CrossRef]
- Marinis, D.; Farsari, E.; Alexandridou, C.; Amanatides, E.; Mataras, D. Chemical Recovery of Carbon Fibers from Composites via Plasma Assisted Solvolysis. J. Phys. Conf. Ser. 2024, 2692, 012017. [Google Scholar] [CrossRef]
- Das, M.; Varughese, S. A Novel Sonochemical Approach for Enhanced Recovery of Carbon Fiber from CFRP Waste Using Mild Acid–Peroxide Mixture. ACS Sustain. Chem. Eng. 2016, 4, 2080–2087. [Google Scholar] [CrossRef]
- Ragupathi, B.; Bacher, M.F.; Balle, F. First Efforts on Recovery of Thermoplastic Composites at Low Temperatures by Power Ultrasonics. Clean. Mater. 2023, 8, 100186. [Google Scholar] [CrossRef]
- Nie, W.; Liu, J.; Liu, W.; Wang, J.; Tang, T. Decomposition of Waste Carbon Fiber Reinforced Epoxy Resin Composites in Molten Potassium Hydroxide. Polym. Degrad. Stab. 2015, 111, 247–256. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Opiri, M.M.; Ezzir, A.; Li, G.; Warner, I.M. Recycling Thermoset Epoxy Resin Using Alkyl-Methyl-Imidazolium Ionic Liquids as Green Solvents. ACS Appl. Polym. Mater. 2021, 3, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Perli, G.; Okada, C.Y.; Michelin, C.; El Omari, Y.; Gérard, J.-F.; Duchet-Rumeau, J.; Livi, S. Design for Disassembly of Composites and Thermoset by Using Cleavable Ionic Liquid Monomers as Molecular Building Blocks. Compos. B Eng. 2023, 264, 110899. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Yue, J.; Huan, H.; Bi, G.; Zhang, L. Recycling Glass Fibers from Thermoset Epoxy Composites by in Situ Oxonium-Type Polyionic Liquid Formation and Naphthalene-Containing Superplasticizer Synthesis with the Degradation Solution of the Epoxy Resin. Compos. B Eng. 2023, 254, 110435. [Google Scholar] [CrossRef]
- Dolz, M.; Mateljak, I.; Méndez-Sánchez, D.; Sánchez-Moreno, I.; de Santos, P.; Viña-Gonzalez, J.; Alcalde, M. Colorimetric High-Throughput Screening Assay to Engineer Fungal Peroxygenases for the Degradation of Thermoset Composite Epoxy Resins. Front. Catal. 2022, 2, 883263. [Google Scholar] [CrossRef]
- Pathak, G.S.; Hinge, M.; Otzen, D.E. Transdisciplinary pragmatic melioration for the plastic life cycle: Why the social, natural, and technical sciences should prioritize reducing harm. Sci. Total Environ. 2023, 895, 165154. [Google Scholar] [CrossRef] [PubMed]
- Nehls, G. European R&D Project Bizente Researches Enzymatic Technologies to Recover Thermosets, CompositesWorld, Published 19 December 2022. Available online: https://www.compositesworld.com/news/european-rd-project-bizente-researches-enzymatic-technologies-to-recover-thermoset-resins (accessed on 14 March 2024).
- You, Y.; Fu, M.R.; Rong, M.Z.; Zhang, M.Q. Improving Creep Resistance While Maintaining Reversibility of Covalent Adaptive Networks via Constructing Reversibly Interlocked Polymer Networks. Mater. Today Chem. 2022, 23, 100687. [Google Scholar] [CrossRef]
- Bowman, C.; Du Prez, F.; Kalow, J. Introduction to Chemistry for Covalent Adaptable Networks. Polym. Chem. 2020, 11, 5295–5296. [Google Scholar] [CrossRef]
- Guggari, S. Recyclability Purpose-Vitrimers Strategy Covalent Adaptable Networks; Specific Polymers: Castries, France, 2022. [Google Scholar]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent Adaptable Networks: Smart, Reconfigurable and Responsive Network Systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Long, Y.; Xu, S.; Liu, X.; Chen, L.; Wang, Y.-Z. Recovery of Epoxy Thermosets and Their Composites. Mater. Today 2023, 64, 72–97. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Du, S.; Zhu, J.; Ma, S. Upcycling of Thermosetting Polymers into High-Value Materials. Mater. Horiz. 2022, 10, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Odriozola, I.; De Luzuriaga, A.R.; Rekondo, A.; Martín, R.; Markaide, N.; Cabañero, G.; Grande, H.-J. Thermomechanically Reprocessable Epoxy Composites and Processes for Their Manufacturing. WO2015181054A1, 21 May 2015. [Google Scholar]
- Ruiz De Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy Resin with Exchangeable Disulfide Crosslinks to Obtain Reprocessable, Repairable and Recyclable Fiber-Reinforced Thermoset Composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Hubbard, A.M.; Ren, Y.; Sarvestani, A.; Konkolewicz, D.; Picu, C.R.; Roy, A.K.; Varshney, V.; Nepal, D. Recyclability of Vitrimer Materials: Impact of Catalyst and Processing Conditions. ACS Omega 2022, 7, 29125–29134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Z.; Wang, B.; Xu, X.; Feng, H.; Li, P.; Zhu, J.; Ma, S. High-Performance Epoxy Covalent Adaptable Networks Enabled by Alicyclic Anhydride Monoesters. Eur. Polym. J. 2022, 173, 111272. [Google Scholar] [CrossRef]
- Zhang, B.; Yuan, C.; Zhang, W.; Dunn, M.L.; Qi, H.J.; Liu, Z.; Yu, K.; Ge, Q. Recycling of Vitrimer Blends with Tunable Thermomechanical Properties. RSC Adv. 2019, 9, 5431–5437. [Google Scholar] [CrossRef] [PubMed]
- Memon, H.; Liu, H.; Rashid, M.A.; Chen, L.; Jiang, Q.; Zhang, L.; Wei, Y.; Liu, W.; Qiu, Y. Vanillin-Based Epoxy Vitrimer with High Performance and Closed-Loop Recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, X.; Shi, Z.; Berrocal, J.A.; Weder, C. Closed-Loop Recycling of Vinylogous Urethane Vitrimers. Angew. Chem. Int. Ed. 2023, 62, e202306188. [Google Scholar] [CrossRef]
- Zhou, Z.; Kim, S.; Bowland, C.C.; Li, B.; Ghezawi, N.; Lara-Curzio, E.; Hassen, A.; Naskar, A.K.; Rahman, M.A.; Saito, T. Unraveling a Path for Multi-Cycle Recycling of Tailored Fiber-Reinforced Vitrimer Composites. Cell Rep. Phys. Sci. 2022, 3, 101036. [Google Scholar] [CrossRef]
- Tran, T.N.; Mai, B.T.; Setti, C.; Athanassiou, A. Transparent Bioplastic Derived from CO2-Based Polymer Functionalized with Oregano Waste Extract toward Active Food Packaging. ACS Appl. Mater. Interfaces 2020, 12, 46667–46677. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, K.; Zhang, K.; Jiang, B.; Huang, Y. Facile Preparation of Reprocessable and Degradable Phenolic Resin Based on Dynamic Acetal Motifs. Polym. Degrad. Stab. 2022, 196, 109818. [Google Scholar] [CrossRef]
- Fukuda, K.; Shimoda, M.; Sukegawa, M.; Nobori, T.; Lehn, J.M. Doubly Degradable Dynamers: Dynamic Covalent Polymers Based on Reversible Imine Connections and Biodegradable Polyester Units. Green Chem. 2012, 14, 2907–2911. [Google Scholar] [CrossRef]
- Yue, L.; Bonab, V.S.; Yuan, D.; Patel, A.; Karimkhani, V.; Manas-Zloczowe, I. Vitrimerization: A Novel Concept to Reprocess and Recycle Thermoset Waste via Dynamic Chemistry. Glob. Chall. 2019, 3, 1800076. [Google Scholar] [CrossRef] [PubMed]
- Bandegi, A.; Gray, T.G.; Mitchell, S.; Jamei Oskouei, A.; Sing, M.K.; Kennedy, J.; Miller McLoughlin, K.; Manas-Zloczower, I. Vitrimerization of Crosslinked Elastomers: A Mechanochemical Approach for Recycling Thermoset Polymers. Mater. Adv. 2023, 4, 2648–2658. [Google Scholar] [CrossRef]
- Lorero, I.; Mujica, A.; Campo, M.; Prolongo, S.G. Mechanical Recycling and Electro-Thermal Welding of Epoxy Vitrimer Nanocomposites. Polym. Compos. 2024, 45, 6041–6058. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, H.; Luo, C.; Rong, Y.; Hu, Y.; Jin, Y.; Long, R.; Yu, K.; Zhang, W. Recyclable and Malleable Thermosets Enabled by Activating Dormant Dynamic Linkages. Nat. Chem. 2022, 14, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Wang, H.; Huang, X.; Huang, G.; Wu, J. Weldable, Malleable and Programmable Epoxy Vitrimers with High Mechanical Properties and Water Insensitivity. Chem. Eng. J. 2019, 368, 61–70. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Jiang, H.; Zhang, X.; Shang, Y.; Jiang, C.; Wang, X.; Qi, G.; Li, B.; Xu, P.; et al. Upcycling of Carbon Fiber-Reinforced Polymer Composites. Compos. Sci. Technol. 2023, 231, 109824. [Google Scholar] [CrossRef]
- Martin, S.; Milickovic, T.K.; Charitidis, C.A.; Moisan, S. Ready-to-Use Recycled Carbon Fibres Decorated with Magnetic Nanoparticles: Functionalization after Recycling Process Using Supercritical Fluid Chemistry. J. Compos. Sci. 2023, 7, 236. [Google Scholar] [CrossRef]
- Semitekolos, D.; Papadopoulos, I.; Anagnou, S.; Dashtbozorg, B.; Li, X.; Dong, H.; Charitidis, C.A. Nanomaterial-Enhanced Sizings: Design and Optimisation of a Pilot-Scale Fibre Sizing Line. Fibers 2024, 12, 16. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; An, W.; Zhou, X.; Zhang, S.; Xu, S.; Wang, Y.-Z. Upcycling of Waste Thermosets into Multiple-Responsive Supramolecular Materials via Acid-Catalyzed Oxidation. Polymer 2023, 270, 125774. [Google Scholar] [CrossRef]
- Shao, L.; Chang, Y.-C.; Zhao, B.; Yan, X.; Bliss, B.J.; Fei, M.; Yu, C.; Zhang, J. Bona Fide Upcycling Strategy of Anhydride Cured Epoxy and Reutilization of Decomposed Dual Monomers into Multipurpose Applications. Chem. Eng. J. 2023, 464, 142735. [Google Scholar] [CrossRef]
- Reis, J.P.; de Moura, M.; Samborski, S. Thermoplastic Composites and Their Promising Applications in Joining and Repair Composites Structures: A Review. Materials 2020, 13, 5832. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.J.; Wiebeck, H. Current Options for Characterizing, Sorting, and Recycling Polymeric Waste. Prog. Rubber Plast. Recycl. Technol. 2020, 36, 284–303. [Google Scholar] [CrossRef]
- Ayre, D. Technology Advancing Polymers and Polymer Composites towards Sustainability: A Review. Curr. Opin. Green Sustain. Chem. 2018, 13, 108–112. [Google Scholar] [CrossRef]
- Valerio, O.; Muthuraj, R.; Codou, A. Strategies for Polymer to Polymer Recycling from Waste: Current Trends and Opportunities for Improving the Circular Economy of Polymers in South America. Curr. Opin. Green Sustain. Chem. 2020, 25, 100381. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.A.; Jo, S.Y.; Song, H.M.; Park, S.Y.; Park, S.K.; Pyo, J.; Cha, H.G.; Kim, H.; et al. Recent Advances in Sustainable Plastic Upcycling and Biopolymers. Biotechnol. J. 2020, 15, 1900489. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; van Geem, K. Mechanical and Chemical Recycling of Solid Plastic Waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Morici, E.; Carroccio, S.C.; Bruno, E.; Scarfato, P.; Filippone, G.; Dintcheva, N.T. Recycled (Bio)Plastics and (Bio)Plastic Composites: A Trade Opportunity in a Green Future. Polymers 2022, 14, 2038. [Google Scholar] [CrossRef] [PubMed]
- Abu-Thabit, N.Y.; Pérez-Rivero, C.; Uwaezuoke, O.J.; Ngwuluka, N.C. From Waste to Wealth: Upcycling of Plastic and Lignocellulosic Wastes to PHAs. J. Chem. Technol. Biotechnol. 2021, 97, 3217–3240. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Di Benedetto, G. Physical Properties of Virgin-Recycled ABS Blends: Effect of Post-Consumer Content and of Reprocessing Cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Rahimi, M.; Esfahanian, M.; Moradi, M. Effect of Reprocessing on Shrinkage and Mechanical Properties of ABS and Investigating the Proper Blend of Virgin and Recycled ABS in Injection Molding. J. Mater. Process. Technol. 2014, 214, 2359–2365. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Huang, J.-K.; Kuo, M.-T.; Huang, J.-H. Characterisation of PC/ABS Blend during 20 Reprocessing Cycles and Subsequent Functionality Recovery by Virgin Additives. J. Polym. Res. 2018, 25, 124. [Google Scholar] [CrossRef]
- Serranti, S.; Bonifazi, G. 2—Techniques for Separation of Plastic Wastes. In Use of Recycled Plastics in Eco-Efficient Concrete; Pacheco-Torgal, F., Khatib, J., Colangelo, F., Tuladhar, R., Eds.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Cambridge, UK, 2019; pp. 9–37. ISBN 978-0-08-102676-2. [Google Scholar]
- Fraunholcz, N. Separation of Waste Plastics by Froth Flotation—A Review, Part I. Miner. Eng. 2004, 17, 261–268. [Google Scholar] [CrossRef]
- Near Infrared (NIR) Sorting in the Plastics Recycling Process; Document number Res-Sort-2. Association of Plastic Recyclers: Washington, DC, USA, 2018; Available online: https://plasticsrecycling.org/images/Design-Guidance-Tests/APR-RES-SORT-2-NIR-sorting-resource.pdf (accessed on 14 March 2024).
- Gondal, M.A.; Siddiqui, M.N. Identification of Different Kinds of Plastics Using Laser-Induced Breakdown Spectroscopy for Waste Management. J. Environ. Sci. Health Part A 2007, 42, 1989–1997. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Militky, J. LCA (Life Cycle Assessment) on Recycled Polyester. In Environmental Footprints of Recycled Polyester; Textile Science and Clothing Technology; Springer Nature Singapore Pte Ltd.: Singapore, 2020; ISBN 978-981-13-9578-9. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding Plastics Recycling Technologies: Chemical Aspects, Technology Status and Challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Curtzwiler, G.; Williams, E.; Hurban, E.; Greene, J.; Vorst, K. Certification Markers for Empirical Quantification of Post-Consumer Recycled Content in Extruded Polyethylene Film. Polym. Test. 2018, 65, 103–110. [Google Scholar] [CrossRef]
- Curtzwiler, G.; Schweitzer, M.; Li, Y.; Jiang, S.; Vorst, K. Mixed Post-Consumer Recycled Polyolefins as a Property Tuning Material for Virgin Polypropylene. J. Clean. Prod. 2019, 239, 117978. [Google Scholar] [CrossRef]
- Luzuriaga, S.; Kovářová, J.; Fortelný, I. Degradation of Pre-Aged Polymers Exposed to Simulated Recycling: Properties and Thermal Stability. Polym. Degrad. Stab. 2006, 91, 1226–1232. [Google Scholar] [CrossRef]
- Sato, T.; Shishido, M. Mechanical and Structural Properties for Recycled Thermoplastics from Waste Fishing Ropes. J. Mater. Cycles Waste Manag. 2020, 22, 1682–1689. [Google Scholar] [CrossRef]
- Ghosh, A. Performance Modifying Techniques for Recycled Thermoplastics. Resour. Conserv. Recycl. 2021, 175, 105887. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.M.; Ragaert, K.; De Meester, S. Detailed Analysis of the Composition of Selected Plastic Packaging Waste Products and Its Implications for Mechanical and Thermochemical Recycling. Environ. Sci. Technol. 2020, 54, 13282–13293. [Google Scholar] [CrossRef]
- Sankar, V.; Kumar, S. Common Additives Used in Recycling of Polymers: Methods, Characterization and Applications; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 11–53. ISBN 9783527338481. [Google Scholar]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical Recycling: Compatibilization of Mixed Thermoplastic Wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Toughening of Biodegradable Polylactide/Poly(Butylene Succinate-Co-Adipate) Blends via in Situ Reactive Compatibilization. ACS Appl. Mater. Interfaces 2013, 5, 4266–4276. [Google Scholar] [CrossRef]
- Pionteck, J.; Sadhu, V.; Jakisch, L.; Pötschke, P.; Häuβler, L.; Janke, A. Crosslinkable Coupling Agents: Synthesis and Use for Modification of Interfaces in Polymer Blends. Polymer 2005, 46, 6563–6574. [Google Scholar] [CrossRef]
- Soundararajan, S.R.; Palanivelu, K. Studies on Mechanical, Thermal & Electrical Properties and Insitu Compatibilization of Recycled Nylon 6-Poly Propylene Blends. IOSR J. Appl. Chem. 2012, 2, 48–51. [Google Scholar]
- Demir, T.; Tinçer, T. Preparation and Characterization of Poly(Ethylene Terephthalate) Powder-Filled High-Density Polyethylene in the Presence of Silane Coupling Agents. J. Appl. Polym. Sci. 2001, 79, 827–835. [Google Scholar] [CrossRef]
- Vuillaume, P.Y.; Vachon, A.; Grey, W.; Pépin, K.; Zhang, C.J. Compatibilisation of Various PLA/Thermoplastic Elastomer Blends with Diisocyanate Coupling Agent. Plast. Rubber Compos. 2018, 47, 95–105. [Google Scholar] [CrossRef]
- Chiou, K.-C.; Chang, F.-C. Reactive Compatibilization of Polyamide-6 (PA 6)/Polybutylene Terephthalate (PBT) Blends by a Multifunctional Epoxy Resin. J. Polym. Sci. B Polym. Phys. 2000, 38, 23–33. [Google Scholar] [CrossRef]
- Oner, B.; Gokkurt, T.; AYTAC, A. Studies On Compatibilization Of Recycled Polyethylene/Thermoplastic Starch Blends By Using Different Compatibilizer. Open Chem. 2019, 17, 557–563. [Google Scholar] [CrossRef]
- Zander, N.E.; Gillan, M.; Burckhard, Z.; Gardea, F. Recycled Polypropylene Blends as Novel 3D Printing Materials. Addit. Manuf. 2019, 25, 122–130. [Google Scholar] [CrossRef]
- Zhou, J.; Min, B.G. Compatibilizing Effect of Epoxy Resin on the Phase Separation and Properties of Polyamide 6/Poly(Ethylene Terephthalate) Blends. IEEE Trans. Softw. Eng. 2014, 51, 21–26. [Google Scholar]
- Raffaella, A.; Antonio, A. Liquid Crystalline Polymers Compatibilization and Adhesion Enhancement by Reactive Blending in Post-Consumers PET’s. Am. J. Eng. Appl. Sci. 2016, 9, 530–539. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, Q.; Clemons, C.; Guo, W. Phase Structure and Properties of Poly(Ethylene Terephthalate)/High-Density Polyethylene Based on Recycled Materials. J. Appl. Polym. Sci. 2009, 113, 1710–1719. [Google Scholar] [CrossRef]
- Ha, C.-S.; Park, H.; Cho, W. Compatibilizers for Recycling of the Plastic Mixture Wastes. II. the Effect of a Compatibilizer for Binary Blends on the Properties of Ternary Blends. J. Appl. Polym. Sci. 2000, 76, 1048–1053. [Google Scholar] [CrossRef]
- Araujo, L.; Morales, A. Compatibilization of Recycled Polypropylene and Recycled Poly (Ethylene Terephthalate) Blends with SEBS-g-MA. Polímeros 2018, 28, 84–91. [Google Scholar] [CrossRef]
- Mahanta, D.; Dayanidhi, S.; Mohanty, S.; Nayak, S. Mechanical, Thermal, and Morphological Properties of Recycled Polycarbonate/Recycled Poly(Acrylonitrile-Butadiene-Styrene) Blend Nanocomposites. Polym. Compos. 2012, 33, 2114. [Google Scholar] [CrossRef]
- Wang, K.; Addiego, F.; Bahlouli, N.; Ahzi, S.; Rémond, Y.; Toniazzo, V. Impact Response of Recycled Polypropylene-Based Composites under a Wide Range of Temperature: Effect of Filler Content and Recycling. Compos. Sci. Technol. 2014, 95, 89–99. [Google Scholar] [CrossRef]
- Jouenne, S.; Chakibi, H.; Levitt, D. Polymer Stability After Successive Mechanical-Degradation Events. SPE J. 2017, 23, 18–33. [Google Scholar] [CrossRef]
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. Additives in Polymers; Elsevier: Amsterdam, The Netherlands, 2016; pp. 87–108. ISBN 9780323443531. [Google Scholar]
- Tseng, W.; Lee, J.-S. Functional MBS Impact Modifiers for PC/PBT Alloy. J. Appl. Polym. Sci. 2000, 76, 1280–1284. [Google Scholar] [CrossRef]
- Titow, W.V. PVC PLASTICS Properties, Processing, and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Kazemi, Y.; Ramezani Kakroodi, A.; Rodrigue, D. Compatibilization Efficiency in Post-Consumer Recycled Polyethylene/Polypropylene Blends: Effect of Contamination. Polym. Eng. Sci. 2015, 55, 2368–2376. [Google Scholar] [CrossRef]
- Inoya, H.; Wei Leong, Y.; Klinklai, W.; Takai, Y.; Hamada, H. Compatibilization of Recycled Poly(Ethylene Terephthalate) and Polypropylene Blends: Effect of Compatibilization on Blend Toughness, Dispersion of Minor Phase, and Thermal Stability. J. Appl. Polym. Sci. 2012, 124, 5260–5269. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Liu, G.; Pu, S. Core/Shell Morphologies in Recycled Poly(Ethylene Terephthalate)/Linear Low-Density Polyethylene/Poly(Styrene-b-(Ethylene-Co-Butylene)-b-Styrene) Ternary Blends. Polym. Bull. 2017, 74, 4223–4233. [Google Scholar] [CrossRef]
- Santana, R.; Manrich, S. Studies on Morphology and Mechanical Properties of PP/HIPS Blends from Postconsumer Plastic Waste. J. Appl. Polym. Sci. 2003, 87, 747–751. [Google Scholar] [CrossRef]
- Tostar, S.; Stenvall, E.; Foreman, M.R.S.J.; Boldizar, A. The Influence of Compatibilizer Addition and Gamma Irradiation on Mechanical and Rheological Properties of a Recycled WEEE Plastics Blend. Recycling 2016, 1, 101–110. [Google Scholar] [CrossRef]
- Vazquez, Y.V.; Barbosa, S.E. Recycling of Mixed Plastic Waste from Electrical and Electronic Equipment. Added Value by Compatibilization. Waste Manag. 2016, 53, 196–203. [Google Scholar] [CrossRef]
- Tanrattanakul, V.; Bunkaew, P. Effect of Different Plasticizers on the Properties of Bio-Based Thermoplastic Elastomer Containing Poly(Lactic Acid) and Natural Rubber. Express Polym. Lett. 2014, 8, 387–396. [Google Scholar] [CrossRef]
- Wu, D.; Hakkarainen, M. Recycling PLA to Multifunctional Oligomeric Compatibilizers for PLA/Starch Composites. Eur. Polym. J. 2015, 64, 126–137. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Thongmee, J. The Effects of Cross-Linked Starch on the Properties of Thermoplastic Starch. Mater. Des. 2013, 45, 586–589. [Google Scholar] [CrossRef]
- Müller, K.; Zollfrank, C.; Schmid, M. Natural Polymers from Biomass Resources as Feedstocks for Thermoplastic Materials. Macromol. Mater. Eng. 2019, 304, 1800760. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Tawakkal, I.S.M.A.; Ilyas, R.A. Water Barrier and Mechanical Properties of Sugar Palm Crystalline Nanocellulose Reinforced Thermoplastic Sugar Palm Starch (TPS)/Poly(Lactic Acid) (PLA) Blend Bionanocomposites. Nanotechnol. Rev. 2021, 10, 431–442. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef]
- Kockott, D. Natural and Artificial Weathering of Polymers. Polym. Degrad. Stab. 1989, 25, 181–208. [Google Scholar] [CrossRef]
- Rabek, J.F. Photostabilization of Polymers: Principles and Applications; Elsevier Applied Science: London, UK, 1990. [Google Scholar]
- Silva Freitas, F.L.; Chinellato, A.C.; Pereira Filho, E.R.; Cruz, S.A. Evaluation of the Effect of Additives on Thermo-Oxidative and Hydrolytic Stabilization of Recycled Post-Consumer Poly (Ethylene Terephthalate) Using Design of Experiments. Polym. Test. 2020, 81, 106275. [Google Scholar] [CrossRef]
- Liu, L.-C.; Liang, W.-C.; Chen, C.-M. Manufacture of Recyclable Thermoplastic Polyurethane (TPU)/Silicone Blends and Their Mechanical Properties. Manuf. Lett. 2022, 31, 1–5. [Google Scholar] [CrossRef]
- Yang, H.; He, P.; Cheng, H.; Shentu, B. Preparation of Nano-TiO2 Loaded Antioxidant and Its Anti-Aging Performance against UV/O3 in Thermoplastic Vulcanizates. Ind. Eng. Chem. Res. 2019, 58, 12516–12524. [Google Scholar] [CrossRef]
- Utracki, L.A. Compatibilization of Polymer Blends. Can. J. Chem. Eng. 2002, 80, 1008–1016. [Google Scholar] [CrossRef]
- Azevedo, V.; Carvalho, R.; Borges, S.; Claro, P.; Hasegawa, F.; Yoshida, M.; Marconcini, J.M. Thermoplastic Starch/Whey Protein Isolate/Rosemary Essential Oil Nanocomposites Obtained by Extrusion Process: Antioxidant Polymers. J. Appl. Polym. Sci. 2019, 136, 47619. [Google Scholar] [CrossRef]
- Kuczenski, B.; Geyer, R. Material Flow Analysis of Polyethylene Terephthalate in the US, 1996–2007. Resour. Conserv. Recycl. 2010, 54, 1161–1169. [Google Scholar] [CrossRef]
- Piringer, O.G.; Baner, A.L. (Eds.) Plastic Packaging: Interactions with Food and Pharmaceuticals, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Moreno, D.D.P.; Saron, C. Influence of Compatibilizer on the Properties of Low-Density Polyethylene/Polyamide 6 Blends Obtained by Mechanical Recycling of Multilayer Film Waste. Waste Manag. Res. 2018, 36, 729–736. [Google Scholar] [CrossRef]
- Antón, N.; González-Fernández, Á.; Villarino, A. Reliability and Mechanical Properties of Materials Recycled from Multilayer Flexible Packages. Materials 2020, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic Flexible Films Waste Management—A State of Art Review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Pappa, G.; Boukouvalas, C.; Giannaris, C.; Ntaras, N.; Zografos, V.V.; Magoulas, K.; Lygeros, A.I.; Tassios, D.P. The Selective Dissolution/Precipitation Technique for Polymer Recycling: A Pilot Unit Application. Resour. Conserv. Recycl. 2001, 34, 33–44. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, Ε. V Chemical Recycling of Plastic Wastes Made from Polyethylene (LDPE and HDPE) and Polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulou, I.; Pappa, G.D.; Vouyiouka, S.N.; Magoulas, K. Recycling of Post-Consumer Multilayer Tetra Pak® Packaging with the Selective Dissolution-Precipitation Process. Resour. Conserv. Recycl. 2021, 165, 105268. [Google Scholar] [CrossRef]
- Walker, T.W.; Frelka, N.; Shen, Z.; Chew, A.K.; Banick, J.; Grey, S.; Kim, M.S.; Dumesic, J.A.; Van Lehn, R.C.; Huber, G.W. Recycling of Multilayer Plastic Packaging Materials by Solvent-Targeted Recovery and Precipitation. Sci. Adv. 2020, 6, eaba7599. [Google Scholar] [CrossRef]
- Mulakkal, M.C.; Castillo Castillo, A.; Taylor, A.C.; Blackman, B.R.K.; Balint, D.S.; Pimenta, S.; Charalambides, M.N. Advancing Mechanical Recycling of Multilayer Plastics through Finite Element Modelling and Environmental Policy. Resour. Conserv. Recycl. 2021, 166, 105371. [Google Scholar] [CrossRef]
- Payne, J.; Jones, M.D. The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities. ChemSusChem 2021, 14, 4041–4070. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Liew, M.S. Tertiary Recycling of Plastics Waste: An Analysis of Feedstock, Chemical and Biological Degradation Methods. J. Mater. Cycles Waste Manag. 2021, 23, 32–43. [Google Scholar] [CrossRef]
- Kosloski-Oh, S.C.; Wood, Z.A.; Manjarrez, Y.; de Los Rios, J.P.; Fieser, M.E. Catalytic Methods for Chemical Recycling or Upcycling of Commercial Polymers. Mater. Horiz. 2021, 8, 1084–1129. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Narro, G.; Hassan, S.; Phan, A.N. Chemical Recycling of Plastic Waste for Sustainable Polymer Manufacturing—A Critical Review. J. Environ. Chem. Eng. 2024, 12, 112323. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of Polypropylene Plastic Waste into Carbonaceous Char: Priority of Plastic Waste Management amidst COVID-19 Pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef] [PubMed]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics Waste Management: A Review of Pyrolysis Technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Singh, E.; Kumar, S. Recent Research Advancements in Catalytic Pyrolysis of Plastic Waste. ACS Sustain. Chem. Eng. 2023, 11, 2033–2049. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Impact of Fast and Slow Pyrolysis on the Degradation of Mixed Plastic Waste: Product Yield Analysis and Their Characterization. J. Energy Inst. 2019, 92, 1647–1657. [Google Scholar] [CrossRef]
- Honus, S.; Kumagai, S.; Fedorko, G.; Molnár, V.; Yoshioka, T. Pyrolysis Gases Produced from Individual and Mixed PE, PP, PS, PVC, and PET—Part I: Production and Physical Properties. Fuel 2018, 221, 346–360. [Google Scholar] [CrossRef]
- Bow, Y.; Rusdianasari, R.; Pujiastuti, L.S. Pyrolysis of Polypropylene Plastic Waste into Liquid Fuel. IOP Conf. Ser. Earth Environ. Sci. 2019, 347, 012128. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Ureel, Y.; Eschenbacher, A.; Vermeire, F.H.; Varghese, R.J.; Oenema, J.; Stefanidis, G.D.; Van Geem, K.M. Challenges and Opportunities of Light Olefin Production via Thermal and Catalytic Pyrolysis of End-of-Life Polyolefins: Towards Full Recyclability. Prog. Energy Combust. Sci. 2023, 96, 101046. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.K. Catalytic Pyrolysis of Polyethylene and Polypropylene over Desilicated Beta and AL-MSU-F. Catalysts 2018, 8, 501. [Google Scholar] [CrossRef]
- Oh, D.; Lee, H.W.; Kim, Y.M.; Park, Y.K. Catalytic Pyrolysis of Polystyrene and Polyethylene Terephthalate over Al-MSU-F. Energy Procedia 2018, 144, 111–117. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: Moving toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Khan, S.R.; Zeeshan, M.; Ahmed, A.; Saeed, S. Comparison of Synthetic and Low-Cost Natural Zeolite for Bio-Oil Focused Pyrolysis of Raw and Pretreated Biomass. J. Clean. Prod. 2021, 313, 127760. [Google Scholar] [CrossRef]
- Khazaal, R.M.; Abdulaaima, D.A. Valuable Oil Recovery from Plastic Wastes via Pressurized Thermal and Catalytic Pyrolysis. Energy Convers. Manag. X 2023, 20, 100430. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, L.; Wang, W.; Yu, J. Catalytic Pyrolysis of Waste Composite Plastics with Waste FCC Catalyst. J. Energy Inst. 2023, 110, 101338. [Google Scholar] [CrossRef]
- Bhatt, K.P.; Patel, S.; Upadhyay, D.S.; Patel, R.N. A Critical Review on Solid Waste Treatment Using Plasma Pyrolysis Technology. Chem. Eng. Process. Process Intensif. 2022, 177, 108989. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.; Zhang, L.; Pan, L. Disinfection Technology of Hospital Wastes and Wastewater: Suggestions for Disinfection Strategy during Coronavirus Disease 2019 (COVID-19) Pandemic in China. Environ. Pollut. 2020, 262, 114665. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Yansaneh, O.Y.; Zein, S.H. Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview. Processes 2022, 10, 332. [Google Scholar] [CrossRef]
- Ma, C.; Kumagai, S.; Saito, Y.; Yoshioka, T.; Huang, X.; Shao, Y.; Ran, J.; Sun, L. Recent Advancements in Pyrolysis of Halogen-Containing Plastics for Resource Recovery and Halogen Upcycling: A State-of-the-Art Review. Environ. Sci. Technol. 2024, 58, 1423–1440. [Google Scholar] [CrossRef]
- Xu, S.; Han, Z.; Yuan, K.; Qin, P.; Zhao, W.; Lin, T.; Zhou, T.; Huang, F. Upcycling Chlorinated Waste Plastics. Nat. Rev. Methods Primers 2023, 3, 44. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Jiang, S.; Hou, H. Carbonization: A Feasible Route for Reutilization of Plastic Wastes. Sci. Total Environ. 2020, 710, 136250. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, L.; Noor, T.; Iqbal, N. Conversion of Plastic Waste to Carbon-Based Compounds and Application in Energy Storage Devices. ACS Omega 2022, 7, 13403–13435. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, S.; Asasian-Kolur, N. Polyethylene Terephthalate (PET) Waste to Carbon Materials: Theory, Methods and Applications. J. Anal. Appl. Pyrolysis 2022, 163, 105496. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Garciá, J.M. Chemical Recycling of Waste Plastics for New Materials Production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical Recycling of PET: A Stepping-Stone toward Sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- del Mar Castro López, M.; Ares Pernas, A.I.; Abad López, M.J.; Latorre, A.L.; López Vilariño, J.M.; González Rodríguez, M.V. Assessing Changes on Poly(Ethylene Terephthalate) Properties after Recycling: Mechanical Recycling in Laboratory versus Postconsumer Recycled Material. Mater. Chem. Phys. 2014, 147, 884–894. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current Developments in Chemical Recycling of Post-Consumer Polyethylene Terephthalate Wastes for New Materials Production: A Review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and Approaches for Chemical Recycling of Plastic Waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Martín, A.J.; Mondelli, C.; Jaydev, S.D.; Pérez-Ramírez, J. Catalytic Processing of Plastic Waste on the Rise. Chem 2021, 7, 1487–1533. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Chen, H.; Liu, H. Towards Recycling Purpose: Converting PET Plastic Waste Back to Terephthalic Acid Using PH-Responsive Phase Transfer Catalyst. Chin. J. Chem. Eng. 2022, 51, 53–60. [Google Scholar] [CrossRef]
- Hu, H.; Wu, Y.; Zhu, Z. Optimization of Microwave-Assisted Preparation of TPA from Waste PET Using Response Surface Methodology. J. Polym. Environ. 2018, 26, 375–382. [Google Scholar] [CrossRef]
- Ügdüler, S.; van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; de Meester, S. Towards Closed-Loop Recycling of Multilayer and Coloured PET Plastic Waste by Alkaline Hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Bengtsson, J.; Peterson, A.; Idström, A.; de la Motte, H.; Jedvert, K. Chemical Recycling of a Textile Blend from Polyester and Viscose, Part II: Mechanism and Reactivity during Alkaline Hydrolysis of Textile Polyester. Sustainability 2022, 14, 6911. [Google Scholar] [CrossRef]
- Güçlü, G.; Yalçinyuva, T.; Özgümüş, S.; Orbay, M. Hydrolysis of Waste Polyethylene Terephthalate and Characterization of Products by Differential Scanning Calorimetry. Thermochim. Acta 2003, 404, 193–205. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Pan, Z. Catalytic Depolymerization of Polyethylene Terephthalate in Hot Compressed Water. J. Supercrit. Fluids 2012, 62, 226–231. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Y.; Zhang, D. Effects of Temperature on Catalytic Hydrolysis of PET by Zinc Sulfate under Microwave Irradiation. Adv. Mater. Res. 2011, 233–235, 1628–1631. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Song, H.; Wang, Y.; Deng, T.; Hou, X. Zinc-Catalyzed Ester Bond Cleavage: Chemical Degradation of Polyethylene Terephthalate. J. Clean. Prod. 2019, 208, 1469–1475. [Google Scholar] [CrossRef]
- Liu, F.; Cui, X.; Yu, S.; Li, Z.; Ge, X. Hydrolysis Reaction of Poly(Ethylene Terephthalate) Using Ionic Liquids as Solvent and Catalyst. J. Appl. Polym. Sci. 2009, 114, 3561–3565. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into Terephthalic Acid in Neutral Media Catalyzed by the ZSM-5 Acidic Catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Islam, M.S.; Islam, Z.; Hasan, R.; Jamal, A.H.M.S.I.M. Acidic Hydrolysis of Recycled Polyethylene Terephthalate Plastic for the Production of Its Monomer Terephthalic Acid. Prog. Rubber Plast. Recycl. Technol. 2023, 39, 12–25. [Google Scholar] [CrossRef]
- Barredo, A.; Asueta, A.; Amundarain, I.; Leivar, J.; Miguel-Fernández, R.; Arnaiz, S.; Epelde, E.; López-Fonseca, R.; Gutiérrez-Ortiz, J.I. Chemical Recycling of Monolayer PET Tray Waste by Alkaline Hydrolysis. J. Environ. Chem. Eng. 2023, 11, 109823. [Google Scholar] [CrossRef]
- Onwucha, C.N.; Ehi-Eromosele, C.O.; Ajayi, S.O.; Schaefer, M.; Indris, S.; Ehrenberg, H. Uncatalyzed Neutral Hydrolysis of Waste PET Bottles into Pure Terephthalic Acid. Ind. Eng. Chem. Res. 2023, 62, 6378–6385. [Google Scholar] [CrossRef]
- Chen, J.; Lv, J.; Ji, Y.; Ding, J.; Yang, X.; Zou, M.; Xing, L. Alcoholysis of PET to Produce Dioctyl Terephthalate by Isooctyl Alcohol with Ionic Liquid as Cosolvent. Polym. Degrad. Stab. 2014, 107, 178–183. [Google Scholar] [CrossRef]
- Laldinpuii, Z.T.; Khiangte, V.; Lalhmangaihzuala, S.; Lalmuanpuia, C.; Pachuau, Z.; Lalhriatpuia, C.; Vanlaldinpuia, K. Methanolysis of PET Waste Using Heterogeneous Catalyst of Bio-Waste Origin. J. Polym. Environ. 2022, 30, 1600–1614. [Google Scholar] [CrossRef]
- Samuilov, A.Y.; Korshunov, M.V.; Samuilov, Y.D. Methanolysis of Polycarbonate Waste as a Method of Regenerating Monomers for Polycarbonate Synthesis. Polym. Sci. Ser. B 2020, 62, 411–415. [Google Scholar] [CrossRef]
- Gupta, P.; Bhandari, S. Chemical Depolymerization of PET Bottles via Ammonolysis and Aminolysis. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–134. [Google Scholar]
- Shirazimoghaddam, S.; Amin, I.; Faria Albanese, J.A.; Shiju, N.R. Chemical Recycling of Used PET by Glycolysis Using Niobia-Based Catalysts. ACS Eng. Au 2023, 3, 37–44. [Google Scholar] [CrossRef]
- Mo, S.; Guo, Y.; Liu, X.; Wang, Y. Efficient Depolymerization of PET over Ti-Doped SBA-15 with Abundant Lewis Acid Sites via Glycolysis. Catal. Sci. Technol. 2023, 13, 6561–6569. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Chen, C.; Li, H. Chemical Degradation of Thermoplastic Polyurethane for Recycling Polyether Polyol. Fibers Polym. 2011, 12, 857–863. [Google Scholar] [CrossRef]
- Ronkvist, Å.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-Catalyzed Hydrolysis of Poly(Ethylene Terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Pan, Y.; Qi, Z.; You, S.; Gao, Y.; Zhou, Y.; Jiang, N.; Wang, M.; Su, R.; Qi, W. Integrating Glycolysis and Enzymatic Catalyst to Convert Waste Poly(Ethylene Terephthalate) into Terephthalic Acid. Biochem. Eng. J. 2024, 207, 109328. [Google Scholar] [CrossRef]
- Tang, H.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Xu, G.; Wang, X.; Zhang, T. Synthesis of Gasoline and Jet Fuel Range Cycloalkanes and Aromatics from Poly(Ethylene Terephthalate) Waste. Green Chem. 2019, 21, 2709–2719. [Google Scholar] [CrossRef]
- Du, J.T.; Sun, Q.; Zeng, X.F.; Wang, D.; Wang, J.X.; Chen, J.F. ZnO Nanodispersion as Pseudohomogeneous Catalyst for Alcoholysis of Polyethylene Terephthalate. Chem. Eng. Sci. 2020, 220, 115642. [Google Scholar] [CrossRef]
- Scremin, D.M.; Miyazaki, D.Y.; Lunelli, C.E.; Silva, S.A.; Zawadzki, S.F. PET Recycling by Alcoholysis Using a New Heterogeneous Catalyst: Study and Its Use in Polyurethane Adhesives Preparation. Macromol. Symp. 2019, 383, 1800027. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, X.; Ju, Z.; Liu, B.; Yao, H.; Xu, J.; Zhou, Q.; Hu, Y.; Zhang, S. Alcoholysis of Polyethylene Terephthalate to Produce Dioctyl Terephthalate Using Choline Chloride-Based Deep Eutectic Solvents as Efficient Catalysts. Green Chem. 2019, 21, 897–906. [Google Scholar] [CrossRef]
- Fang, P.; Lu, X.; Zhou, Q.; Yan, D.; Xin, J.; Xu, J.; Shi, C.; Zhou, Y.; Xia, S. Controlled Alcoholysis of PET to Obtain Oligomers for the Preparation of PET-PLA Copolymer. Chem. Eng. J. 2023, 451, 138988. [Google Scholar] [CrossRef]
- Fang, P.; Xia, S.; Lu, X. Rapid Alcoholysis of PET Enhanced by Its Swelling under High Temperature. J. Environ. Chem. Eng. 2022, 10, 107823. [Google Scholar] [CrossRef]
- Dębowski, M.; Iuliano, A.; Plichta, A.; Kowalczyk, S.; Florjańczyk, Z. Chemical Recycling of Polyesters. Polimery 2021, 64, 764–776. [Google Scholar] [CrossRef]
- Verma, A.; Soni, R.K.; Teotia, M. Prevention of Poly(Vinyl Chloride) Degradation through Organic Terephthalamides Generated from Poly(Ethylene Terephthalate) Waste. J. Appl. Polym. Sci. 2019, 136, 48022. [Google Scholar] [CrossRef]
- Teotia, M.; Chauhan, M.; Khan, A.; Soni, R.K. Facile Synthesis, Characterization, and Ab-Initio DFT Simulations of Energy Efficient NN′ Dialkyl 1,4 Benzene Dicarboxamide Monomers Recovered from PET Bottle Waste. J. Appl. Polym. Sci. 2020, 137, 49321. [Google Scholar] [CrossRef]
- Jamdar, V.; Kathalewar, M.; Sabnis, A. Depolymerization Study of PET Waste Using Aminoethylethanolamine and Recycled Product Application as Polyesteramide Synthesis. J. Polym. Environ. 2018, 26, 2601–2618. [Google Scholar] [CrossRef]
- More, A.P.; Kute, R.A.; Mhaske, S.T. Chemical Conversion of PET Waste Using Ethanolamine to Bis(2-Hydroxyethyl) Terephthalamide (BHETA) through Aminolysis and a Novel Plasticizer for PVC. Iran. Polym. J. (Engl. Ed.) 2014, 23, 59–67. [Google Scholar] [CrossRef]
- Kárpáti, L.; Fejér, M.; Kalocsai, D.; Molnár, J.; Vargha, V. Synthesis and Characterization of Isophorondiamine Based Epoxy Hardeners from Aminolysis of PET. Express Polym. Lett. 2019, 13, 618–631. [Google Scholar] [CrossRef]
- Parab, Y.S.; Shukla, S.R. Microwave Synthesis and Antibacterial Activity of 1,4-Bis (5-Aryl-1,3,4-Oxadiazole-2-Yl) Benzene Derivatives from Terephthalic Dihydrazide Obtained through Aminolysis of PET Bottle Waste. Waste Biomass Valorization 2013, 4, 23–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, F.; Wu, Z.; Li, X.; Liu, X.; He, Y. Chemical Conversion of Waste PET to Valued-Added Bis(2-Hydroxyethyl) Terephthalamide through Aminolysis. Mater. Today Commun. 2022, 32, 104045. [Google Scholar] [CrossRef]
- Dutt, K.; Soni, R.K. Synthesis and Characterization of Bis-Amino Ethyl Terephthalamide from PET Waste and Its Applications as Hardener in DGEBA. Int. J. Plast. Technol. 2014, 18, 16–26. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, D.O.; In Shim, K.; Kim, J.K.; Pelton, J.G.; Ryu, M.H.; Joo, J.C.; Han, J.W.; Kim, H.T.; Kim, K.H. One-Pot Chemo-Bioprocess of PET Depolymerization and Recycling Enabled by a Biocompatible Catalyst, Betaine. ACS Catal. 2021, 11, 3996–4008. [Google Scholar] [CrossRef]
- Alikin, M.B.; Alekseeva, K.D.; Panfilov, D.A.; Dvorko, I.M.; Lavrov, N.A. The properties of epoxy compositions cured by aminolytic splitting products of a secondary polyethylene terephthalate and polycarbonate. Mech. Compos. Mater. 2022, 58, 999–1010. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Conversion of Waste Bottles’ PET to a Hydrogel Adsorbent via PET Aminolysis. J. Environ. Chem. Eng. 2021, 9, 106129. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Aminolysis-Assisted Hydrothermal Conversion of Waste PET Plastic to N-Doped Carbon Dots with Markedly Enhanced Fluorescence. J. Environ. Chem. Eng. 2022, 10, 107749. [Google Scholar] [CrossRef]
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of Polyethylene Terephthalate Wastes: A Review of Technologies, Routes, and Applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Jeya, G.; Dhanalakshmi, R.; Anbarasu, M.; Vinitha, V.; Sivamurugan, V. A Short Review on Latest Developments in Catalytic Depolymerization of Poly (Ethylene Terephathalate) Wastes. J. Indian Chem. Soc. 2022, 99, 100291. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the Catalytic Glycolysis of Polyethylene Terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhou, W.; Shen, Y.; Li, Z. Chemically Recyclable Polyurethanes Based on Bio-Renewable γ-Butyrolactone: From Thermoplastics to Elastomers. Polym. Degrad. Stab. 2022, 204, 110116. [Google Scholar] [CrossRef]
- Tu, Y.M.; Wang, X.M.; Yang, X.; Fan, H.Z.; Gong, F.L.; Cai, Z.; Zhu, J.B. Biobased High-Performance Aromatic-Aliphatic Polyesters with Complete Recyclability. J. Am. Chem. Soc. 2021, 143, 20591–20597. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Liu, Y.; Shen, Y.; Li, Z. Functionalizable and Chemically Recyclable Thermoplastics from Chemoselective Ring-Opening Polymerization of Bio-Renewable Bifunctional α-Methylene-δ-Valerolactone. Angew. Chem. Int. Ed. 2022, 61, e202207105. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, Y.; Li, Z. Chemical Upcycling of Poly(3-Hydroxybutyrate) into Bicyclic Ether Ester Monomer toward Value-Added, Degradable and Recyclable Poly(Ether-Ester). ACS Sustain. Chem. Eng. 2022, 10, 8228–8238. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, D.; Shen, Y.; Li, Z. Ring-Opening Polymerization of Enantiopure Bicyclic Ether-Ester Monomers toward Closed-Loop Recyclable and Crystalline Stereoregular Polyesters via Chemical Upcycling of Bioplastic. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302101. [Google Scholar] [CrossRef]
- Shi, C.; Clarke, R.W.; McGraw, M.L.; Chen, E.Y.X. Closing the “One Monomer-Two Polymers-One Monomer” Loop via Orthogonal (De)Polymerization of a Lactone/Olefin Hybrid. J. Am. Chem. Soc. 2022, 144, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rettner, E.M.; Harry, K.L.; Hu, Z.; Miscall, J.; Rorrer, N.A.; Miyake, G.M. Chemically Recyclable Polyolefin-like Multiblock Polymers. Science 2023, 382, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Semyonov, O.; Sviridova, E.; Gulyaev, R.; Gorbunova, A.; Kogolev, D.; Trelin, A.; Yamauchi, Y.; Boukherroub, R.; Postnikov, P. “Functional Upcycling” of Polymer Waste towards the Design of New Materials. Chem. Soc. Rev. 2023, 52, 4755–4832. [Google Scholar] [CrossRef]
- Saxon, D.J.; Gormong, E.A.; Shah, V.M.; Reineke, T.M. Rapid Synthesis of Chemically Recyclable Polycarbonates S1 of S36 Supporting Information Rapid Synthesis of Chemically Recyclable Polycarbonates from Renewable Feedstocks. ACS Macro Lett. 2021, 10, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, J.; Wu, Y.-C.; Chen, J.-X.; Shan, S.-Y.; Cai, Z.; Zhu, J.-B. Highly Reactive Cyclic Carbonates with Fused-Ring towards Functionalizable and Recyclable Polycarbonates. ACS Macro Lett. 2022, 11, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.K.; Chen, Y.-C.; Jeng, R.-J.; Abu-Omar, M.M.; Lin, C.-H. Upcycling Waste Polycarbonate to Poly(Carbonate Imine) Vitrimers with High Thermal Properties and Unprecedented Hydrolytic Stability. ACS Sustain. Chem. Eng. 2023, 11, 8580–8591. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Mayes, H.B.; Maurya, A.K.; Tassone, C.J.; Beckham, G.T.; Chen, E.Y.X. Redesigned Hybrid Nylons with Optical Clarity and Chemical Recyclability. J. Am. Chem. Soc. 2022, 144, 5366–5376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Truong, N.P.; Pei, Z.; Coote, M.L.; Anastasaki, A. Reversing RAFT Polymerization: Near-Quantitative Monomer Generation Via a Catalyst-Free Depolymerization Approach. J. Am. Chem. Soc. 2022, 144, 4678–4684. [Google Scholar] [CrossRef]
- Jacobsen, A.J. A Future Vision for More Sustainable Plastics. Nature Portfolio. 2023, Amazon. Available online: https://www.nature.com/articles/d42473-022-00488-1 (accessed on 14 March 2024).
- Yang, R.; Xu, G.; Dong, B.; Guo, X.; Wang, Q. Selective, Sequential, and “One-Pot” Depolymerization Strategies for Chemical Recycling of Commercial Plastics and Mixed Plastics. ACS Sustain. Chem. Eng. 2022, 10, 9860–9871. [Google Scholar] [CrossRef]
- García, J.L. Enzymatic Recycling of Polyethylene Terephthalate through the Lens of Proprietary Processes. Microb. Biotechnol. 2022, 15, 2699–2704. [Google Scholar] [CrossRef]
- Singh, A.; Rorrer, N.A.; Nicholson, S.R.; Erickson, E.; DesVeaux, J.S.; Avelino, A.F.T.; Lamers, P.; Bhatt, A.; Zhang, Y.; Avery, G.; et al. Techno-Economic, Life-Cycle, and Socioeconomic Impact Analysis of Enzymatic Recycling of Poly(Ethylene Terephthalate). Joule 2021, 5, 2479–2503. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Mavinkere Rangappa, S.; Siengchin, S.; Puttegowda, M.; Thiagamani, S.M.K.; Hemath Kumar, M.; Oladijo, O.P.; Fiore, V.; Moure Cuadrado, M.M. Sustainable Recycling Technologies for Thermoplastic Polymers and Their Composites: A Review of the State of the Art. Polym. Compos. 2022, 43, 5831–5862. [Google Scholar] [CrossRef]
- Then, J.; Wei, R.; Oeser, T.; Barth, M.; Belisário-Ferrari, M.R.; Schmidt, J.; Zimmermann, W. Ca2+ and Mg2+ Binding Site Engineering Increases the Degradation of Polyethylene Terephthalate Films by Polyester Hydrolases from Thermobifida Fusca. Biotechnol. J. 2015, 10, 592–598. [Google Scholar] [CrossRef]
- Sadler, J.C.; Wallace, S. Microbial Synthesis of Vanillin from Waste Poly(Ethylene Terephthalate). Green Chem. 2021, 23, 4665–4672. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.A.; Barbosa, R.; Mesquita, A.B.S.; Ferreira, J.H.L.; de Carvalho, L.H.; Alves, T.S. Fungal Degradation of Reprocessed PP/PBAT/Thermoplastic Starch Blends. J. Mater. Res. Technol. 2020, 9, 2338–2349. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Yao, S.-S.; Jin, F.-L.; Rhee, K.Y.; Hui, D.; Park, S.-J. Recent Advances in Carbon-Fiber-Reinforced Thermoplastic Composites: A Review. Compos. B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Almushaikeh, A.M.; Alaswad, S.O.; Alsuhybani, M.S.; AlOtaibi, B.M.; Alarifi, I.M.; Alqahtani, N.B.; Aldosari, S.M.; Alsaleh, S.S.; Haidyrah, A.S.; Alolyan, A.A.; et al. Manufacturing of Carbon Fiber Reinforced Thermoplastics and Its Recovery of Carbon Fiber: A Review. Polym. Test. 2023, 122, 108029. [Google Scholar] [CrossRef]
- Roux, M.; Eguémann, N.; Dransfeld, C.; Thiébaud, F.; Perreux, D. Thermoplastic Carbon Fibre-Reinforced Polymer Recycling with Electrodynamical Fragmentation: From Cradle to Cradle. J. Thermoplast. Compos. Mater. 2017, 30, 381–403. [Google Scholar] [CrossRef]
- Cousins, D.S.; Suzuki, Y.; Murray, R.E.; Samaniuk, J.R.; Stebner, A.P. Recycling Glass Fiber Thermoplastic Composites from Wind Turbine Blades. J. Clean. Prod. 2019, 209, 1252–1263. [Google Scholar] [CrossRef]
- Kiss, P.; Stadlbauer, W.; Burgstaller, C.; Stadler, H.; Fehringer, S.; Haeuserer, F.; Archodoulaki, V.-M. In-House Recycling of Carbon- and Glass Fibre-Reinforced Thermoplastic Composite Laminate Waste into High-Performance Sheet Materials. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).