A Comprehensive Review of Lithium-Ion Battery (LiB) Recycling Technologies and Industrial Market Trend Insights
Abstract
1. Introduction
2. Literature Review Summary
3. Spent Li-Batteries Recycling Technologies
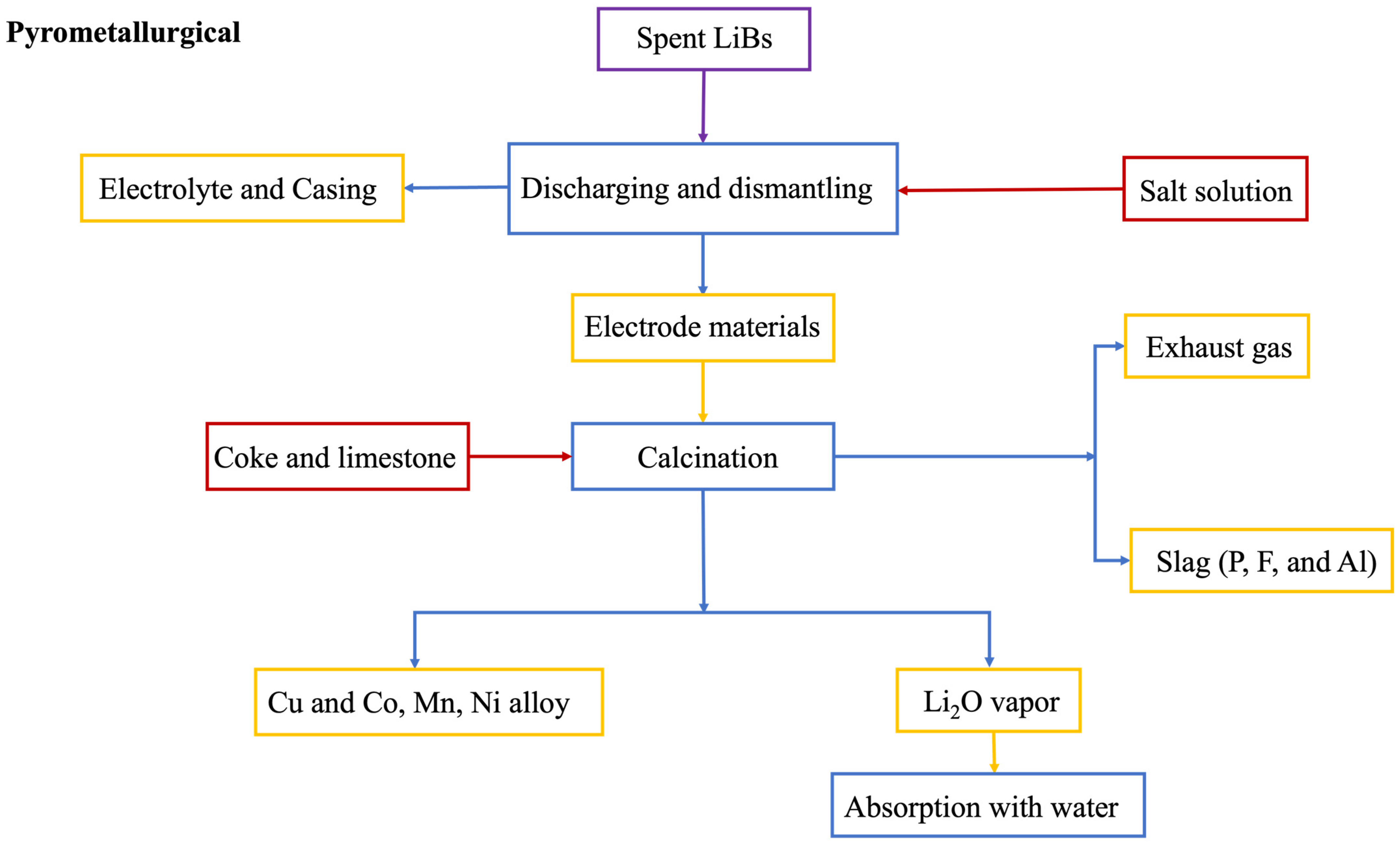
3.1. Pyrometallurgical Process
3.2. Hydrometallurgical Process
3.2.1. Discharging and Dismantling
3.2.2. Pretreatment
3.2.3. Leaching of Valuable Metals
3.2.4. Extraction of Metals from Leaching Solution
3.2.5. Synthesis of Electrode Materials and Inorganic Compounds from Leaching Solution
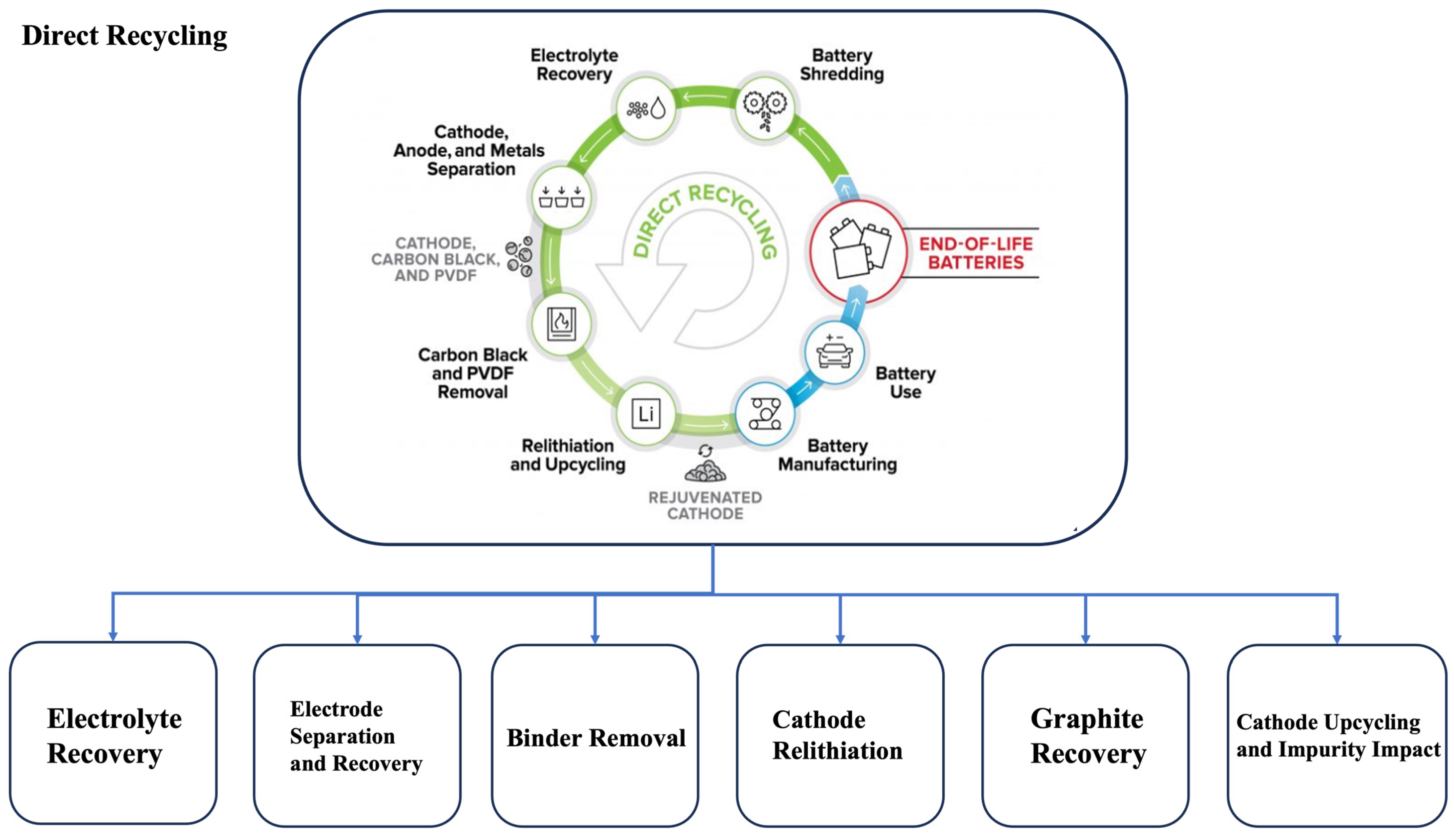
3.3. Direct Recycling
3.3.1. Binder Removal
3.3.2. Cathode Separation
3.3.3. Direct Upcycling
3.3.4. Electrolyte Extraction
3.3.5. Graphite Anode Recycling
3.3.6. Direct Recycling Challenges
- (1)
- Knowledge Transfer: Facilitate better communication and collaboration between academic researchers and industry professionals to share knowledge, findings, and technological advancements.
- (2)
- Business Models: Explore sustainable business models for LiB direct recycling, which may include incentives, subsidies, or extended producer responsibility programs.
- (3)
- Scalability: Continue to make efforts on scaling recycling technologies for industrial application, meeting the high demand of the LiB recycling market.
- (4)
- Environmental Impact: Prioritize environmentally friendly methods for LiB direct recycling to minimize the ecological footprint of the whole process.
- (5)
- Life Cycle Assessment: Develop the life cycle analysis of LiBs direct recycling to optimize sustainability of the whole procedure.
3.4. Comparison between Pyrometallurgical, Hydrometallurgical, and Direct Recycling Processes
4. Spent Li-Battery Recycling Market Trend Analysis
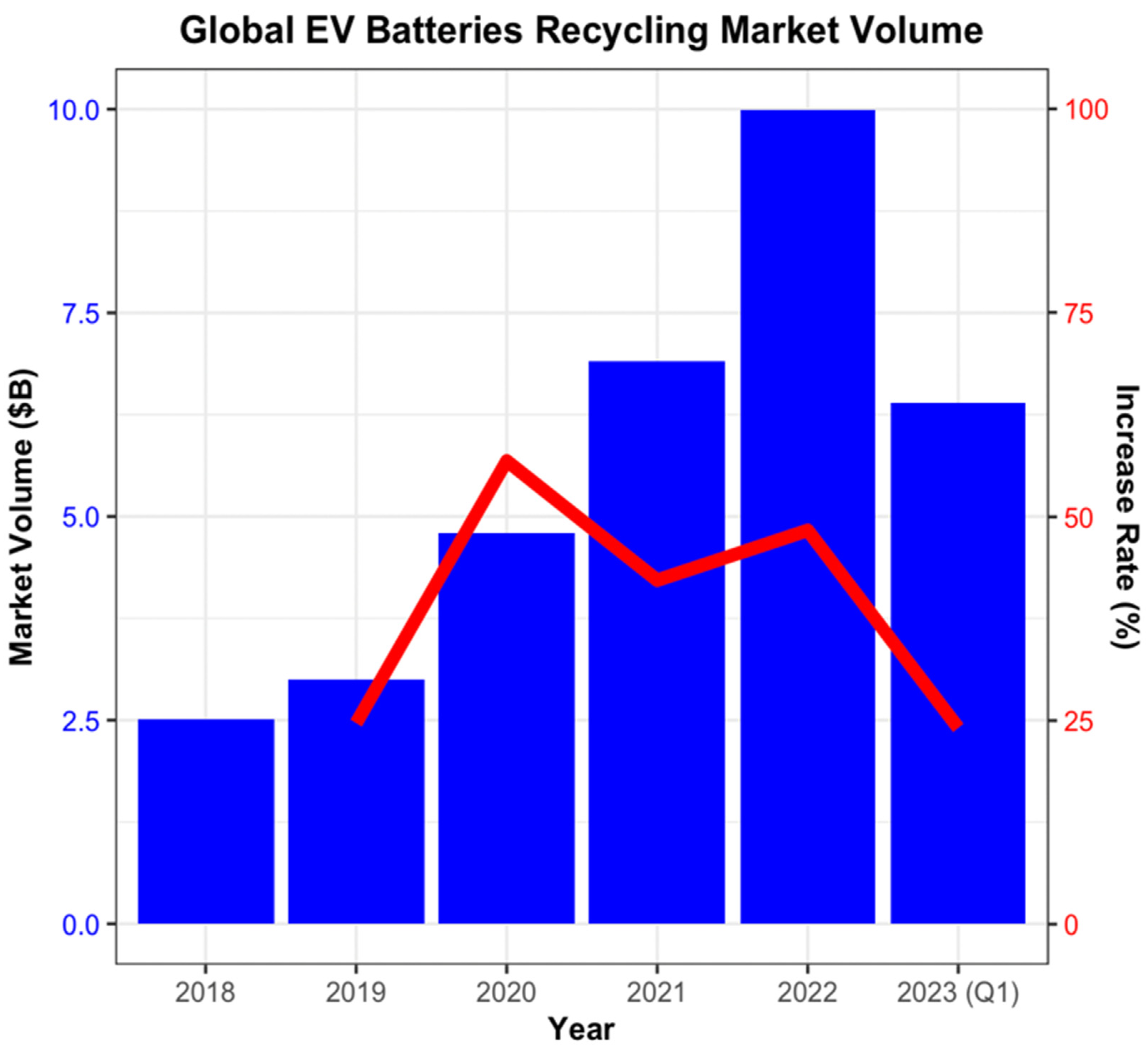
4.1. LiB Recycling Market Volume
4.2. LiB Recycling Market Current Trend
4.3. Major LiB Recycling Magnates Industrial Layout and Recent Actions
- SungEel HiTech (Gunsan, Republic of Korea)
- Umicore (Brussels, Belgium)
- Retriev Technologies Inc. (Lancaster, PA, USA)
- Redwood Materials (Carson City, NV, USA)
- Primobius GmbH (Hilchenbach, Germany)
- BASF (Ludwigshafen, Germany)
- GS Engineering and Construction Corp (Seoul, Republic of Korea)
- Northvolt (Stockholm, Sweden)
- Tesla (Austin, TX, USA)
- Brunp Recycling (Foshan, China)
- Ascend Elements (Westborough, MA, USA)
5. Conclusions and Future Direction
- (1)
- Safely and efficiently disassemble using AI-powered automation. Currently, most research and industry operations adopt manual dismantling spent LiBs by laborers. Although manual dismantling can simplify the recycling process, the processing efficiency can be very low. Thus, automated disassembly approach of spent LiB packs is in urgent need to tackle this challenge. For example, Zorn et al. [107] proposed a computer vision pipeline to enable the automated disassembly of various battery packs. Additionally, manually dismantling can cause damage and hazard to unskilled workers. Meanwhile, after the LiB is disassembled, the electrolyte is exposed to the air, which will have an impact on the environment and might cause significant harm to people. Thus, the focus of future research is to develop an artificial intelligent (AI) powered automatic process to dismantle the spent EV LiBs in a more efficient and safer manner.
- (2)
- Holistic recycling of valuable elements of spent EV LiBs. Currently, most research has focused on investigating the recycling of LiCoO2 batteries. Nonetheless, with more applications of LiNixCoyMn2O2, LiMn2O4, and LiFePO4 materials in the LiB cathode, the recycling waste stream is expected to receive more complicated types of spent EV LiBs. Unfortunately, the current recycling process of LiCoO2 LiBs is unsuitable for recycling other types of spent LiBs. Specifically, the profitability of business models that depend on pyrometallurgical and hydrometallurgical processes may become increasingly challenging for the current spent EV LiBs stream since they trend toward lower and lower cobalt concentrations [13]. Thus, new studies on developing profitable recycling technology associated with spent EV LiBs with low cobalt cathode are in urgent need. Additionally, there are few studies on the recycling of negative electrode materials and electrolytes. Thus, future research can focus on developing a comprehensive recycling framework for the holistic recycling of all types of valuable elements of spent EV LiBs.
- (3)
- Avoid secondary pollution during the recycling process. It should be noted that some recycling processes can produce toxic gases and potentially harmful waste liquids, posing a significant threat to public environmental health. Thus, paying attention to developing pollution-free, clean, and green closed-loop spent EV LiB recycling process is imperative.
- (4)
- Research on solid-state LiB recycling: future LiB is transitioning to solid-state because solid-state LiBs have a higher energy density and are generally safer in operation. However, a new challenge has come up: lithium metal is very difficult to handle and process. For instance, lithium metal can react with water aggressively so discharging solid-state LiBs using a salt solution to release the remaining energy becomes unpractical. Additionally, lithium metal can easily adhere to the shredder due to its soft nature, making the shredding process hard to control. As there is not much research on investigating the recycling technology associated with solid-state spent LiBs, future innovations should make an effort to fill this gap.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camp, J.; Gilligan, J.; He, B. The Unintended Consequences of Flood Mitigation along Inland Waterways—A Look at Resilience and Social Vulnerabilities through a Case Study Analysis. 2023. Available online: https://rosap.ntl.bts.gov/view/dot/67296 (accessed on 10 October 2023).
- He, B. Efficient Computational Evaluation Tools to Accelerate the Planning of Vulnerability, Resilience, and Sustainability of the Social-Environmental Systems in the City of Nashville. Ph.D. Thesis, Vanderbilt University, Nashville, TN, USA, 2023. [Google Scholar]
- He, B.; Gilligan, J.M.; Camp, J.V. An index of social fabric for assessing community vulnerability to natural hazards: Model development and analysis of uncertainty and sensitivity. Int. J. Disaster Risk Reduct. 2023, 96, 103913. [Google Scholar] [CrossRef]
- He, B.; Zheng, H.; Guan, Q. Evaluation of Future-Integrated Urban Water Management Using a Risk and Decision Analysis Framework: A Case Study in Denver-Colorado Metro Area (DCMA). Water 2023, 15, 4020. [Google Scholar] [CrossRef]
- He, B.; Ding, K.J. Localize the Impact of Global Greenhouse Gases Emissions under an Uncertain Future: A Case Study in Western Cape, South Africa. Earth 2021, 2, 111–123. [Google Scholar] [CrossRef]
- He, B.; Ding, K.J. Global greenhouse gases emissions effect on extreme events under an uncertain future: A case study in Western Cape, South Africa. PLoS Clim. 2023, 2, e0000107. [Google Scholar] [CrossRef]
- He, B.; Guan, Q. A Risk and Decision Analysis Framework to Evaluate Future PM2.5 Risk: A Case Study in Los Angeles-Long Beach Metro Area. Int. J. Environ. Res. Public Health 2021, 18, 4905. [Google Scholar] [CrossRef]
- He, B.; Guan, Q. Analysis and prediction of the correlation between environmental ecology and future global climate change. J. HFUT Nat. Sci. 2022, 6, 818–824. [Google Scholar]
- He, B.; Guan, Q. The statistical analysis and prediction associated with nuclear meltdown accidents risk evaluation. Int. J. Nucl. Saf. Secur. 2022, 1, 104–123. [Google Scholar] [CrossRef]
- Li, C.; Cao, Y.; Zhang, M.; Wang, J.; Liu, J.; Shi, H.; Geng, Y. Hidden benefits of electric vehicles for addressing climate change. Sci. Rep. 2015, 5, 9213. [Google Scholar] [CrossRef]
- International Energy Agency. Electric Vehicles. 2023. Available online: https://www.iea.org/reports/global-ev-outlook-2023/executive-summary (accessed on 11 November 2023).
- NPR. All New Cars in the EU Will Be Zero-Emission by 2035. Here’s Where the U.S. Stands. 2023. Available online: https://www.npr.org/2023/03/30/1166921698/eu-zero-emission-cars#:~:text=on%20March%2023.-,Starting%20in%202035%2C%20all%20cars%20sold%20in%20the%20European,will%20be%20zero%2Demission%20vehicles.&text=European%20Union%20member%20states%20gave,starting%20in%20the%20year%202035 (accessed on 19 November 2023).
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling end-of-life electric vehicle lithium-ion batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, L.; Zhang, Y.; Tan, Q.; Li, J. An overview of global power lithium-ion batteries and associated critical metal recycling. J. Hazard. Mater. 2022, 425, 127900. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Ren, Y. Prediction of various discarded lithium batteries in China. In Proceedings of the 2012 IEEE International Symposium on Sustainable Systems and Technology (ISSST), Boston, MA, USA, 16–18 May 2012. [Google Scholar]
- Ordonez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- He, L.; Sun, S.; Yu, J. Research progress on valuable metal recovery from spent Lithium-ion batteries. J. Chem. Eng. 2018, 69, 327–340. [Google Scholar]
- Barbieri, E.M.S.; Lima, E.P.C.; Cantarino, S.J.; Lelis, M.F.F.; Freitas, M.B.J.G. Recycling of spent ion-lithium batteries as cobalt hydroxide, and cobalt oxide films formed under a conductive glass substrate, and their electrochemical properties. J. Power Sources 2014, 269, 158–163. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Manag. 2016, 51, 245–251. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J. Clean Prod. 2016, 112, 247923. [Google Scholar] [CrossRef]
- Da Costa, A.J.; Matos, J.F.; Bernardes, A.M.; Müller, I.L. Beneficiation of cobalt, copper and aluminum from wasted lithium-ion batteries by mechanical processing. Int. J. Miner. Process. 2015, 145, 77–82. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Fouad, O.A.; Farghaly, F.I.; Bahgat, M.A. A novel approach for synthesis of nanocrystalline γ-LiAlO2 from spent lithium-ion batteries. J. Anal. Appl. Pyrolysis 2007, 78, 65–69. [Google Scholar] [CrossRef]
- Bahgat, M.; Farghaly, F.E.; Basir, S.M.A. and Fouad, O.A. Synthesis, characterization, and magnetic properties of microcrystalline lithium cobalt ferrite from spent lithium-ion batteries. J. Mater. Process. Technol. 2007, 183, 117–121. [Google Scholar] [CrossRef]
- Chen, S.; He, T.; Lu, Y.; Su, Y.; Tian, J.; Li, N.; Chen, G.; Bao, L.; Wu, F. Renovation of LiCoO2 with outstanding cycling stability by thermal treatment with Li2CO3 from spent Li-ion batteries. J. Energy Eng. 2016, 8, 262–273. [Google Scholar]
- Li, J.; Wang, G.; Xu, Z. Environmentally friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Yang, S.; Li, Y.; Zhao, D.; Lai, Y.; Chen, Y. Oxidizing roasting behavior and leaching performance for the recovery of spent LiFePo4 batteries. Minerals 2020, 10, 949. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.S. and Kwade, A. Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes. J. Electrochem. Soc. 2016, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Zhang, J.; Jing, Q.; Ma, B.; Chen, Y.; Zhang, W. Graphite recycling from the spent lithium-ion batteries by sulfuric acid curing–leaching combined with high-temperature calcination. ACS Sustain. Chem. Eng. 2020, 8, 9447–9455. [Google Scholar] [CrossRef]
- Gupta, V.; Yu, X.; Gao, H.; Brooks, C.; Li, W.; Chen, Z. Scalable Direct Recycling of Cathode Black Mass from Spent Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203093. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, P.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium-ion battery cycle life in electrical vehicle: Aging mechanism identification. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Raugei, M.; Steubing, B.; Hartwell, A.; Anderson, P.A.; Heidrich, O. Life cycle assessment of lithium-ion battery recycling using pyrometallurgical technologies. J. Ind. Ecol. 2021, 25, 1560–1571. [Google Scholar] [CrossRef]
- Liu, Z.; Sederholm, J.G.; Lan, K.W.; Cho, E.J.; Dipto, M.J.; Gurumukhi, Y.; Rabbi, K.F.; Hatzell, M.C.; Perry, N.H.; Miljkovic, N.; et al. Life cycle assessment of hydrometallurgical recycling for cathode active materials. J. Power Sources 2023, 580, 233345. [Google Scholar] [CrossRef]
- Zhang, Z.; He, W.; Li, G.; Zhang, Z.; He, W.; Li, G.; Xia, J.; Hu, H.; Huang, J. Ultrasound-assisted hydrothermal renovation of LiCoO2 from the cathode of spent lithium-ion batteries. Int. J. Electrochem. Sci. 2014, 9, 3691–3700. [Google Scholar] [CrossRef]
- Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar] [CrossRef]
- Shin, S.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- He, L.; Sun, S.Y.; Song, X.F.; Yu, J.G. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Manag. 2015, 46, 523–528. [Google Scholar] [CrossRef]
- Xu, Y.; Song, D.; Li, L.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. A simple solvent method for the recovery of LixCoO2 and its applications in alkaline rechargeable batteries. J. Power Sources 2014, 252, 286–291. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Ferreira, D.; Prados, L.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminum, cobalt, copper, and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Nie, H.; Shi, H.; Wang, D.; Guo, F.; Shi, X.; Zhang, L. Heat treatment of LiCoO2 recovered from cathode. scraps with solvent method. J. Power Sources 2014, 249, 137–141. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Wen, J.; Wang, H.; Guan, R.; Zhang, J.; Zeng, G. Regeneration and reutilization of cathode materials from spent lithium-ion batteries. Chem. Eng. J. 2020, 383, 123089. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Marrani, A.G.; Russina, O.; D’Annibale, L.; Amato, F.; Pagnanelli, F.; Altimari, P. Aqueous electrochemical delithiation of cathode materials as a strategy to selectively recover lithium from waste lithium-ion batteries. J. Energy Chem. 2024, 88, 144–153. [Google Scholar] [CrossRef]
- Yang, L.; Xi, G.; Xi, Y. Recovery of Co, Mn, Ni, and Li from spent lithium-ion batteries for the preparation of LiNixCoyMnzO2 cathode materials. Ceram. Int. 2015, 41, 11498–11503. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Dong, P. Use of glucose as reductant to recover Co from spent lithium ions batteries. Waste Manag. 2017, 64 (Suppl. C), 214–218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ge, J.; Chen, R.; Wu, F.; Chen, S.; Zhang, X. Environmentally friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag. 2010, 30, 2615–2621. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.; Zhang, X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using L-tartaric acid as a leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Pant, D.; Dolker, T. Green and facile method for the recovery of spent lithium nickel manganese cobalt oxide (NMC) based lithium-ion batteries. Waste Manag. 2016, 60, 689. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z.H.I. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery—A closed-loop process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Anantharaj, S.; Tayade, R.J.; Bajaj, H.C.; Kundu, S. Recovered spinel MnCo2O4 from spent lithium-ion batteries for enhanced electrocatalytic oxygen evolution in alkaline medium. Dalton T. 2017, 46, 14382–14392. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ralph, D.E.; Ahn, J.G.; Rhee, Y.H. Bioleaching of metals from spent lithium-ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. J. Clean Prod. 2016, 116, 249–258. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium-ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium-ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of Leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical process enhanced cobalt and lithium recycling from wasted lithium-ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351 (Suppl. C), 192–199. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F.S. Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dichlorination of polyvinyl chloride in subcritical water. J. Hazard. Mater. 2016, 316, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, G.; Barik, S.P.; Kumar, N.; Kumar, L. Electrochemical process for electrode material of spent lithium-ion batteries. Waste Manag. 2017, 68 (Suppl. C), 527–533. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.C. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, P.G.; Altimari, P.; Sturabotti, E.; Giacomo Marrani, A.; Simonetti, G.; Pagnanelli, F. Decomposition of Deep Eutectic Solvent Aids Metals Extraction in Lithium-Ion Batteries Recycling. ChemSusChem 2022, 15, e202200966. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, P.G.; Altimari, P.; Branchi, M.; Zanoni, R.; Simonetti, G.; Navarra, M.A.; Pagnanelli, F. Selective recovery of cobalt from mixed lithium-ion battery wastes using deep eutectic solvent. Chem. Eng. J. 2021, 417, 129249. [Google Scholar] [CrossRef]
- Torkaman, R.; Asadollahzadeh, M.; Torab-mostaedi, M.; Maragheh, M.G. Recovery of cobalt from spent lithium-ion batteries by using acidic and basic extractants in solvent extraction process. Sep. Purif. Technol. 2017, 186, 318–325. [Google Scholar] [CrossRef]
- Mantuano, D.P.; Dorella, G.; Elias, R.C.A.; Mansur, M.B. Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid–liquid extraction with Cyanex 272. J. Power Sources 2006, 159, 1510–1518. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Huang, G.; Tian, Q.; Sun, H. Recovery of lithium from the effluent obtained in the process of spent lithium-ion batteries recycling. J. Environ. Manag. 2017, 198 Pt 1, 84–89. [Google Scholar] [CrossRef]
- Lemaire, J.; Svecova, L.; Lagallarde, F.; Laucournet, R.; Thivel, P.X. Lithium recovery from aqueous solution by sorption/desorption. Hydrometallurgy 2014, 143, 1–11. [Google Scholar] [CrossRef]
- Iizuka, A.; Yamashita, Y.; Nagasawa, H.; Yamasaki, A.; Yanagisawa, Y. Separation of lithium and cobalt from waste lithium-ion batteries via bipolar membrane electrodialysis coupled with chelation. Sep. Purif. Technol. 2013, 113, 33–41. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Yu, J.G. Performance of LiNi1/3Co1/3Mn1/3O2 prepared from spent lithium-ion batteries by a carbonate co-precipitation method. Ceram. Int. 2018, 44, 351–357. [Google Scholar] [CrossRef]
- Xiao, J.L.; Sun, S.Y.; Wang, J.; Li, P.; Yu, J.G. Synthesis and adsorption properties of Li1.6Mn1.6O4 spinel. Ind. Eng. Chem. Res. 2013, 52, 11967–11973. [Google Scholar] [CrossRef]
- Grossman, G.; Sonin, A.A. Membrane fouling in electrodialysis: A model and experiments. Desalination 1973, 12, 107–125. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Sun, F.; Wu, F.; Liu, J. Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process. Hydrometallurgy 2011, 108, 220–225. [Google Scholar] [CrossRef]
- Kim, D.S.; Sohn, J.S.; Lee, C.K.; Lee, J.H.; Han, K.S.; Lee, Y.I. Simultaneous separation and renovation of lithium cobalt oxide from the cathode of spent lithium-ion rechargeable batteries. J. Power Sources 2004, 132, 145–149. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Wang, F.; Ge, L.; Zhu, X.; Li, H. Chemical and process mineralogical characterizations of spent lithium-ion batteries: An approach by multi-analytical techniques. Waste Manag. 2014, 34, 1051–1058. [Google Scholar] [CrossRef]
- Sa, Q.; Gratz, E.; He, M.; Lu, W.; Apelian, D.; Wang, Y. Synthesis of high performance LiNi1/3Mn1/3Co1/3O2 from lithium-ion battery recovery stream. J. Power Sources 2015, 282, 140–145. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef]
- Yang, L.; Xi, G.; Lou, T.; Wang, X.; Wang, J.; He, Y. Preparation and magnetic performance of Co0.8Fe2.2O4 by a sol-gel method using cathode materials of spent Li-ion batteries. Ceram. Int. 2016, 42 Pt B, 1897–1902. [Google Scholar] [CrossRef]
- ReCell. Research. 2023. Available online: https://recellcenter.org/research/ (accessed on 16 December 2023).
- Ross, B.J.; LeResche, M.; Liu, D.; Durham, J.L.; Dahl, E.U.; Lipson, A.L. Mitigating the impact of thermal binder removal for direct Li-ion battery recycling. ACS Sustain. Chem. Eng. 2020, 8, 12511–12515. [Google Scholar] [CrossRef]
- Folayan, T.O.; Lipson, A.L.; Durham, J.L.; Pinegar, H.; Liu, D.; Pan, L. Direct recycling of blended cathode materials by froth flotation. Energy Technol. 2021, 9, 2100468. [Google Scholar] [CrossRef]
- Ma, X.; Hou, J.; Vanaphuti, P.; Yao, Z.; Fu, J.; Azhari, L.; Liu, Y.; Wang, Y. Direct upcycling of mixed Ni-lean polycrystals to single-crystal Ni-rich cathode materials. Chem 2022, 8, 1944–1955. [Google Scholar] [CrossRef]
- Bertilsson, S.; Larsson, F.; Furlani, M.; Albinsson, I.; Mellander, B.E. Lithium-ion battery electrolyte emissions analyzed by coupled thermogravimetric/Fourier-transform infrared spectroscopy. J. Power Sources 2017, 365, 446–455. [Google Scholar] [CrossRef]
- Grützke, M.; Kraft, V.; Weber, W.; Wendt, C.; Friesen, A.; Klamor, S.; Winter, M.; Nowak, S. Supercritical carbon dioxide extraction of lithium-ion battery electrolytes. J. Supercrit. Fluids 2014, 94, 216–222. [Google Scholar] [CrossRef]
- Mu, D.; Liu, Y.; Li, R.; Ma, Q.; Dai, C. Transcritical CO 2 extraction of electrolytes for lithium-ion batteries: Optimization of the recycling process and quality–quantity variation. New J. Chem. 2017, 41, 7177–7185. [Google Scholar] [CrossRef]
- He, K.; Zhang, Z.Y.; Alai, L.; Zhang, F.S. A green process for exfoliating electrode materials and simultaneously extracting electrolyte from spent lithium-ion batteries. J. Hazard. Mater. 2019, 375, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Divya, M.L.; Aravindan, V. Should we recycle the graphite from spent lithium-ion batteries? The untold story of graphite with the importance of recycling. J. Energy Chem. 2022, 71, 351–369. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. An urgent call to spent LIB recycling: Whys and wherefores for graphite recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Aravindan, V.; Jayaraman, S.; Tedjar, F.; Madhavi, S. From electrodes to electrodes: Building high-performance Li-ion capacitors and batteries from spent lithium-ion battery carbonaceous materials. ChemElectroChem 2019, 6, 1407–1412. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Chen, B.; Meng, Z.; Wang, Y. High-performance graphite recovered from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2019, 7, 19732–19738. [Google Scholar] [CrossRef]
- Ma, Z.; Zhuang, Y.; Deng, Y.; Song, X.; Zuo, X.; Xiao, X.; Nan, J. From spent graphite to amorphous sp2+sp3 carbon-coated sp2 graphite for high-performance lithium-ion batteries. J. Power Sources 2018, 376, 91–99. [Google Scholar] [CrossRef]
- Zorn, M.; Ionescu, C.; Klohs, D.; Zähl, K.; Kisseler, N.; Daldrup, A.; Hams, S.; Zheng, Y.; Offermanns, C.; Flamme, S.; et al. An approach for automated disassembly of lithium-ion battery packs and high-quality recycling using computer vision, labeling, and material characterization. Recycling 2022, 7, 48. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Jiao, B.; Chang, N.; Wu, M.; Liu, G.; Lin, X.; Weng, X.; Chen, J.; Zhang, L.; et al. Direct Recycling of Spent Li-ion Batteries: Challenges and Opportunities towards Practical Applications. Iscience 2023, 26, 107676. [Google Scholar] [CrossRef]
- Wang, J.; Kang, Q.; Yuan, J.; Fu, Q.; Chen, C.; Zhai, Z.; Liu, Y.; Yan, W.; Li, A.; Zhang, J. Dendrite-free lithium and sodium metal anodes with deep plating/stripping properties for lithium and sodium batteries. Carbon Energy 2021, 3, 153–166. [Google Scholar] [CrossRef]
- Kawamura, T.; Okada, S.; Yamaki, J.I. Decomposition reaction of LiPF6-based electrolytes for lithium-ion cells. J. Power Sources 2006, 156, 547–554. [Google Scholar] [CrossRef]
- Zhiyanzhan Industrial Research Institute. Profound Research and Investment Prospect Forecast Report on China’s Spent Battery Recycling Industry from 2023 to 2029. 2023. Available online: https://www.zhiyanzhan.cn/analyst/14990.html# (accessed on 1 November 2023).
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A review on battery market trends, second-life reuse, and recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Industrial and Information Technology and Eight Relevant Departments of China. Implementation Plan on Accelerating the Comprehensive Utilization oof Industrial Resources. Resour. Recycl. 2022, 2, 44–47. [Google Scholar]
- State Council of China. Implementation Plan of Carbon Peaking in Industrial Sector. 2022. Available online: https://www.gov.cn/zhengce/zhengceku/2022-08/01/5703910/files/f7edf770241a404c9bc608c051f13b45.pdf (accessed on 1 September 2023).
- Battery Industry. EcoGraf, Strategic Agreement with South Korean Battery Recycler. 2020. Available online: https://batteryindustry.tech/ecograf-strategic-agreement-with-south-korean-battery-recycler/ (accessed on 6 October 2023).
- BloombergNEF. U.S. Narrows Gap with China in Race to Dominate Battery Value Chain. 2021. Available online: https://about.bnef.com/blog/u-s-narrows-gap-with-china-in-race-to-dominate-battery-value-chain/ (accessed on 4 September 2023).
- Marubeni Corporation. Strategic Partnership for Developing New Circular Business for End-of-Life Lithium-Ion Batteries. 2021. Available online: https://www.marubeni.com/en/news/2021/release/00011.html (accessed on 19 September 2023).
- Lora, K. Redwood Materials Scores a New $2 Billion Loan to Build out Battery Recycling Facility in Nevada. 2023. Available online: https://www.cnbc.com/2023/02/09/redwood-materials-nabs-2-billion-loan-for-battery-recycling-in-nevada.html (accessed on 16 August 2023).
- SMS Group. Neometals and SMS Group Set Up Joint Venture for Recycling Lithium-Ion Batteries. 2020. Available online: https://www.sms-group.com/press-and-media/press-releases/press-release-detail/neometals-and-sms-group-set-up-joint-venture-for-recycling-lithium-ion-batteries (accessed on 19 September 2023).
- BASF. Finnish Battery Industry Intensifies Cooperation: Fortum, BASF, and Nornickel Sign Cooperation Agreement on Battery Recycling. 2020. Available online: https://www.basf.com/global/en/media/news-releases/2020/03/p-20-135.html (accessed on 8 August 2023).
- Kim, S.; Choi, H.; Cho, J. Korea’s GS E&C to Invest $86 Million on Li-ion Battery Recycling Plant in Pohang. 2020. Available online: https://pulsenews.co.kr/view.php?year=2020&no=33125 (accessed on 9 September 2023).
- Northvolt. Northvolt and Hydro Launch Joint Venture to Enable Electric Vehicle Battery Recycling. 2020. Available online: https://northvolt.com/articles/announcing-hydrovolt/ (accessed on 12 August 2023).
- Argus. Tesla Starts Battery Recycling in China. 2020. Available online: https://www.argusmedia.com/en/news/2139066-tesla-starts-battery-recycling-in-china (accessed on 2 September 2023).
- Gu, F.; Guo, J.; Yao, X.; Summers, P.A.; Widijatmoko, S.D.; Hall, P. An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China. J. Clean. Prod. 2017, 161, 765–780. [Google Scholar] [CrossRef]
- CATL. Yichang BRUNP-CATL Project Kicks off. 2022. Available online: https://www.catl.com/en/news/1028.html (accessed on 10 October 2023).
- Kannisto, A. Understanding the Tensions in the Lithium Recycling Industry in Europe: An Integration between Paradox Theory and Circular Economy Perspective. Master’s Thesis, Lahti University of Technology, Lappeenranta, Finland, 2023. [Google Scholar]
- Ascend Elements. Ascend Elements Raises $542 Million to Accelerate Production of U.S.-Engineered Lithium-Ion Battery Materials. 2023. Available online: https://www.prnewswire.com/news-releases/ascend-elements-raises-542-million-to-accelerate-production-of-us-engineered-lithium-ion-battery-materials-301918678.html (accessed on 3 October 2023).


| Topic | Literature | Remark |
|---|---|---|
| Hydrometallurgical process | Barbieri et al. [22] Barik et al. [23] Bertuol et al. [24] Chen et al. [25] Chen et al. [26] DaCosta et al. [27], etc. | Total of 55 selected papers covering the discharging and dismantling, pretreatment, leaching, extraction of metal from leaching solution, and synthesis of electrode materials from leaching solution |
| Pyrometallurgical process | Makuza et al. [28] Fouad et al. [29] Bahgat et al. [30] Chen et al. [31] Li et al. [32] Zhang et al. [33] Jie et al. [34] | Total of 7 selected papers covering previous pyrometallurgical process studies |
| Direct Recycling | Chen et al. [35] Diekmann et al. [36] Gao et al. [37] Gupta et al. [38] Harper et al. [39] Han et al. [40], etc. | Total of 24 selected papers covering binder removal, cathode separation and upcycling, and electrolyte and graphite recovery |
| Type | Pyrometallurgical Process | Hydrometallurgical Process | Direct Recycling Process |
|---|---|---|---|
| Operational Process | Short process | Lengthy and complicated process | Short but complicated process |
| Commercially viable | Commercially viable | Only at lab scale | |
| No presorting | Pretreatment required | Presorting required with more separation processes | |
| Flexible input stream | Flexible input stream | More requirements for input materials | |
| High economic cost | Medium economic cost | Low economic cost | |
| Energy Consumption | High energy consumption | Low Energy consumption | Low energy consumption |
| Embedded energy loss inside of material structure | Embedded energy inside of material structure loss | Embedded energy inside of material maintained | |
| Extra energy cost for treating gas pollution | Low energy cost for treating extra environmental pollution | Low energy cost for treating environmental pollution | |
| Environmental Pollution | Heavy gas pollution | Chemical pollutions caused by usage of chemicals | Less environmental pollution |
| Product and residue | Li and Al loss in slag | High recovery rate and high purity of valuable metals | Products can directly be used as cathode materials |
| Year | Market Volume (USD B) | Increase Rate (%) |
|---|---|---|
| 2018 | 2.5 | na |
| 2019 | 3 | 24.64 |
| 2020 | 4.8 | 56.83 |
| 2021 | 6.9 | 42.18 |
| 2022 | 10 | 48.30 |
| 2023 Q1 | 6.4 | 24.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Zheng, H.; Tang, K.; Xi, P.; Li, M.; Wei, L.; Guan, Q. A Comprehensive Review of Lithium-Ion Battery (LiB) Recycling Technologies and Industrial Market Trend Insights. Recycling 2024, 9, 9. https://doi.org/10.3390/recycling9010009
He B, Zheng H, Tang K, Xi P, Li M, Wei L, Guan Q. A Comprehensive Review of Lithium-Ion Battery (LiB) Recycling Technologies and Industrial Market Trend Insights. Recycling. 2024; 9(1):9. https://doi.org/10.3390/recycling9010009
Chicago/Turabian StyleHe, Bowen, Han Zheng, Karl Tang, Ping Xi, Muqing Li, Laiwei Wei, and Qun Guan. 2024. "A Comprehensive Review of Lithium-Ion Battery (LiB) Recycling Technologies and Industrial Market Trend Insights" Recycling 9, no. 1: 9. https://doi.org/10.3390/recycling9010009
APA StyleHe, B., Zheng, H., Tang, K., Xi, P., Li, M., Wei, L., & Guan, Q. (2024). A Comprehensive Review of Lithium-Ion Battery (LiB) Recycling Technologies and Industrial Market Trend Insights. Recycling, 9(1), 9. https://doi.org/10.3390/recycling9010009






