Abstract
Microbial treatment of recycled concrete aggregate (RCA) may improve the quality of the aggregate, and enhance its use in the production of structural concrete and promote the recycling of concrete waste. The mortar phase of the RCA is responsible for the poor performance of the aggregate. Treating the old adhered mortar or removing it from the natural aggregate (NA) is an option to make RCA beneficial for the production of quality recycled aggregate concrete (RAC). Removing the adhered mortar from recycled concrete aggregate using silicate-solubilizing bacteria was investigated. The bacteria could synthesize the silicates in the calcium silicate hydrate phase of the cement paste leading to the breakdown of the old adhered mortar. Four SSB strains were tested for survivability and activity in an alkaline medium to simulate the concrete environment. The Serratia marcescens bacterial strain, which survived the environment, was inoculated into screw-cap glass vials containing recycled concrete aggregate fragments and glucose-enhanced nutrient broth and then incubated for 14 days. Partial removal of the old adhered mortar was observed based on the weight lost from the RCA. The S. marcescens bacterial strain could survive the alkaline concrete environment and solubilize the silicates present in cement paste resulting in the removal of the old adhered mortar.
1. Introduction
Concrete is the most popular construction material, with annual global production of about 32 billion tons [1]. The growth in concrete production indicates a substantial increase in the consumption of virgin natural aggregates (NA) and the generation of concrete waste, which poses economic and environmental problems.
The consumption of NA is expected to grow proportionally to the growth in concrete production. In 2020, global aggregate production was about 50 billion tons [2], and this amount is expected to grow annually [3]. Mining and transporting large quantities of aggregate creates large quarry pits and causes heavy traffic and high energy consumption, negatively impacting the environment and economy [4].
Further, concrete waste generated from construction and demolition (C&D) significantly contributes to solid waste that pollutes the environment. The global C&D waste generated in 2018 was over 3 billion tons [5], with a significant portion ending up in landfill. Using recycled concrete aggregate (RA) in the production of recycled aggregate concrete (RAC) is a sustainable alternative in the construction industry worldwide [6,7,8].
The use of RA in producing RAC for structural applications has been investigated for over two decades. However, the poor properties and performance of the RA due to the adhered old mortar have limited its use for unbounded applications such as filler material in shorelines and roads, and base and subbase material in pavement construction [5,9,10,11]. The presence of the adhered mortar makes RA more porous with a higher water absorption capacity than NA and reduces its strength by about 24% [9]. As a result, RAC has a higher absorption capacity and shrinkage and lower density and strength [12,13] relative to ordinary concrete. Since the porosity of RAC is about twice that of ordinary concrete made of NA (NAC) [14], the former is prone to damage caused by several factors: ingression of aggressive chemicals, such as sulfates and chlorides, alkali–silica reaction, and freeze–thaw cycles.
For RAC to be accepted as a structural material, its properties should be comparable to that of ordinary concrete. This could be achieved by either improving the quality of the old mortar or removing it from the NA. Several studies reported that the performance of RAC could be improved by treating RA. Pre-socking RA in acid could reduce water absorption and improve the strength and resistance to the freeze–thaw effects of RAC [15,16,17,18]. A reduction in water absorption and improvement in the strength of RAC was also observed using a thermal treatment of RA in which the aggregate was heated to a maximum temperature of 800 °C or microwaved to induce thermal stress that reduced the layer of the old mortar [19,20]. However, this method is energy intensive. Impregnation of RA with polymers such as polyvinyl alcohol [21,22], siloxane emulsion [23,24,25], paraffin wax [24], bituminous emulsion [26,27], and waste cooking oil residue [28] were investigated, and permeability reduction was observed in the RAC. Another treatment method investigated includes the immersion of RA in a pozzolanic slurry to enhance the pozzolanic reaction in the old mortar and improve the stiffness and strength of the aggregate when the reaction product fills up the pores and heals the microcracks. Some of the pozzolanic materials investigated include silica fume solution [29,30,31,32], sulphoaluminate cement paste [33,34], cement slurry containing slag [35], fly ash [32,33,34,35,36], and nanomaterials [22,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. Generally, slump and porosity reduction and strength gain were reported. Porosity reduction in RAC was also reported when the RA was subjected to accelerated carbonation curing, in which the aggregate is cured in CO2 under pressure [42,53,54,55,56]. Biotreatment of RA relies on the ability of bacteria to survive the concrete environment and synthesize calcium carbonate to fill up the pores in the old mortar [57,58,59]. Some treatment methods could improve a specific property of the RAC but could also have serious side effects. Silicate treatment could harm the durability of the RAC by enhancing the alkali–silica reaction [60]. Some treatment methods have prolonged curing time and compatibility issues or can promote corrosion in reinforcing bars by lowering the alkalinity of the concrete pore solution.
Generally, these studies have made considerable progress in improving the performance of the treated RAC relative to that of the untreated. Since the properties of the treated RAC are not comparable to the natural aggregate concrete (NAC), the desired outcome to recycle concrete in abundance and use RAC for structural applications has not yet been fully achieved. Therefore, more research is needed to overcome the barriers to recycling concrete waste in larger quantities. Complete replacement of NA with RA could be envisioned if the old mortar could be removed using a simple, cost-effective, and environmentally friendly method. This paper presents the results of a study conducted to remove the old mortar from the NA in RA using bacterial treatment. Unlike the bio-deposition method discussed above, which improves the old mortar by depositing solid calcium carbonate into the pores, this paper presents the use of bacteria in removing the old mortar. This method, defined as a process of surface modification using microorganisms to enhance the separation of one mineral from another [61,62,63], was applied to remove the adhered mortar from the RA as the result of the bacterial effect in breaking down the calcium silicate hydrate (CSH) present in cement paste.
Microorganisms play a significant role in the process of weathering rocks and the formation and biodegradation of minerals [64]. In biogeochemical cycles of silicates, some microorganisms actively transform polymerized silica into the simplest form [65]. Various silicate-solubilizing bacteria (SSB) such as Bacillus mucilaginosus, Bacillus megaterium, Bacillus flexus, Pseudomonas sp., and Pseudomonas fluorescens dissolve silicates present in soil and release minerals such as potassium, calcium, and magnesium [66]. Several studies have reported on the effectiveness of SSB in removing silicate from various minerals, such as bauxite [67,68], nickel ore [69], and pyrolusite [70]. Though the exact mechanism of desiliconization of materials by SSB remains unclear, acid hydrolysis, enzymatic hydrolysis, and formation of extracellular polysaccharides are some suggested mechanisms [71,72,73]. In an experimental study on the effect of silicate bacteria on magnesite ore, it was observed that the concentration of silicate in the ore was significantly reduced [74], indicating the effectiveness of the bacteria under favorable conditions. Bacteria are ubiquitous in nature and can be found in extreme environments, of which concrete should be considered one, due to the increased alkalinity and low water activity. A study found that concrete of varying composition and location was found to have predominantly bacterial DNA [75]. This suggests endolithic bacteria are able to survive in concrete and could be a source of SSB. Based on the above statement, the authors investigated the effect of SSB in removing the adhered mortar of the RA by dissolving the silicate present in the C-S-H phase of the cement paste.
2. Materials and Methods
2.1. Materials
2.1.1. Concrete
Since the main objective of this investigation was to test if the SSB could solubilize the silicate in the CSH phase of the cement paste, any strength but uncontaminated Portland cement concrete was used. Thus, concrete samples were collected from a local concrete waste plant. The concrete waste samples were washed, air-dried and crushed into a maximum aggregate size of 1 inch. Some aggregates were crushed into powder.
2.1.2. Bacterial Strains
The bacterial strains used in this experiment include Serratia marcescens, Citrobacter freundii, Pseudomonas stutzeri, and Bacillus megaterium. These commercially available bacterial strains are capable of solubilizing silicate minerals. They were selected because their biosafety level was assigned to BSL-1, the lowest level of health concern. The bacterial strains were grown in glucose-enriched nutrient broth (GNB) (Table 1).

Table 1.
Composition of glucose-enriched nutrient media.
2.1.3. Bunt and Rovira Medium
Bunt and Rovira medium (BR) was used to identify SSB, and the composition is shown in Table 2.

Table 2.
Composition of Bunt and Rovira Medium, pH 7.0 ± 0.5.
2.2. Tests
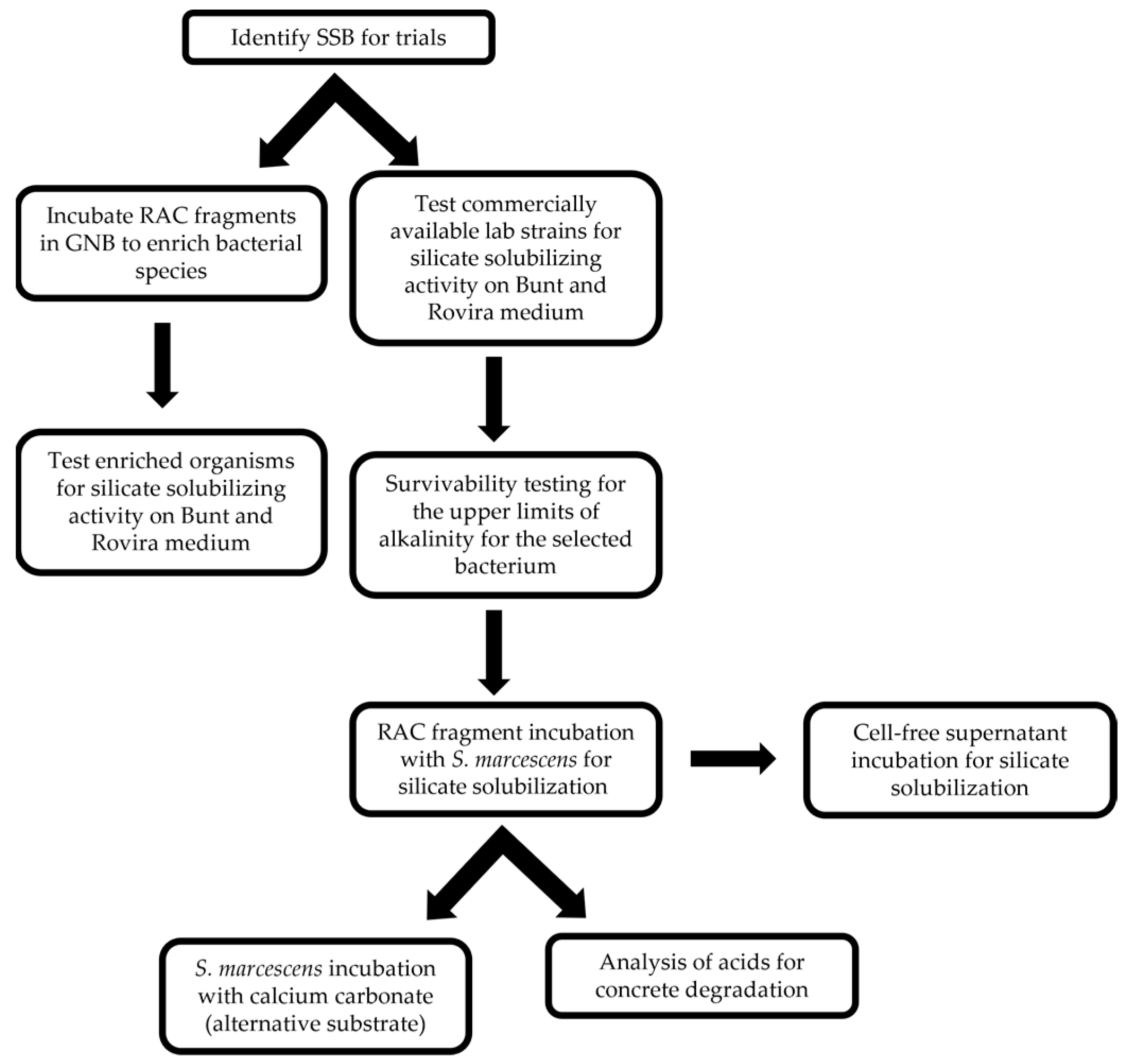
The experimental procedure followed in the study is summarized in the flowchart shown in Figure 1.
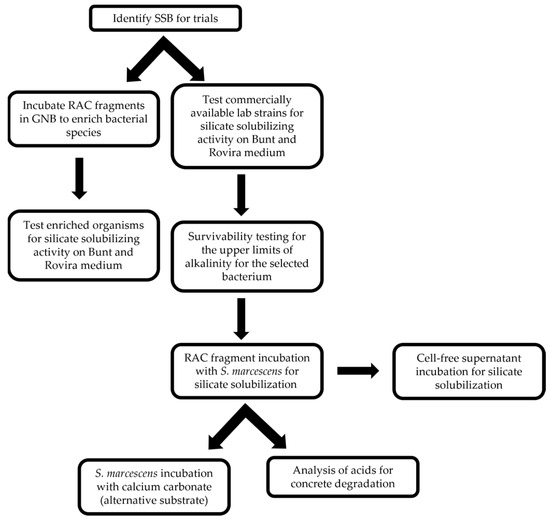
Figure 1.
Flowchart depicting methodological pipeline.
2.2.1. Identifying Silicate-Solubilizing Bacteria (SSB)
Bunt and Rovira medium was used to visually differentiate bacteria that can solubilize the silicate compound found in the agar plates. The first step was to determine if there were any environmental SSB on the recycled concrete aggregate (RA) fragments or if lab strains would be necessary to achieve the desired effect. Unsterilized RA was introduced into the nutrient broth and incubated for 24 h. Then, 0.1 mL of the solution was inoculated to Bunt and Rovira agar, and the plates were incubated for 24 h at 30 °C. Silicate solubilization is detected as a clearing effect on the agar medium, and any such organisms can be isolated and identified. Based on the results from the RA fragments, lab strains of bacteria were used. The bacteria S. marcescens, Pseudomonas stutzeri, Citrobacter freundii, and Bacillus megaterium were tested. The lab strains were chosen due to their ecological niche and potential to solubilize silicate. Each organism was streaked onto the Bunt and Rovira medium and incubated at 30 °C for 24 h. Although the final incubation temperature was set at 25 °C for the experimental trials, 30 °C was used initially to allow for faster growth due to the temperature preference of the samples used. After incubation, the media was evaluated for the zone of clearance (CZ) around each colony. Any clearing would indicate the solubilization of the magnesium trisilicate in the medium.
2.2.2. Selection of Bacteria
Bacterial strains that were able to produce the cleared zone were then reisolated and used for further testing to determine efficacy. Serratia marcescens, C. freundii, and B. megaterium were used for this next step. Each of the three silicate-solubilizing lab isolates was then grown on five Bunt and Rovira agar plates to determine the relative sizes of the cleared zones. The diameters of these zones including the colony were measured, and the largest average diameter relative to colony size was used to determine the isolate with the highest solubilization index as described in previous studies [76]. A time course was not considered, as all plates were incubated for 24 h. The organism with the highest ratio of solubilization would be used for further trials.
2.2.3. Identifying Favorable Environment
Since concrete is alkaline, the next step was to check if the introduction of the RA would raise the pH value of the solution to a level that exceeds the survival threshold for the chosen isolate. The lab strain S. marcescens can survive up to a pH of 9 [77]. The experiment was set up in 100 mL Erlenmeyer flasks containing nutrient broth with added glucose to a concentration of 10%. The medium was adjusted to a pH of 7 using 0.1 M hydrochloric acid, and bromothymol blue (BTB) was used as a pH indicator. Twenty flasks containing glucose medium were prepared. A total of 5 grams of mechanically powdered RA was added to the ten flasks, and 5–10 g of RA fragments was added to the other ten flasks. The flasks were sampled every hour for the first 8 h and then every 24 h for seven days. The pH values of the solutions were monitored via pH indicator dye with limited-range pH test strips. Serratia marcescens were then inoculated into the cultures to verify survivability in the medium. Growth was monitored via spectrophotometry and plate counts of colony-forming units (CFUs), which were taken every 24 h for 7 days.
2.2.4. Silicate Solubilization Test
To investigate whether the bacterial fermentative process was crucial for the degradation of the RA, 0.1 mL of S. marcescens was inoculated into 75 mL screw-cap glass vials containing 5 g of RA fragments and 50 mL of GNB with BTB; the vials were incubated aerobically and anaerobically at room temperature (22–25 °C), and the RA samples were weighed every 24 h. The testing period for each sample was set to 14 days. Each sample’s dry weight was measured before and at the end of each testing period. The sample was removed from the vial and weighed to avoid disturbing any bacterial attachment to the surface of the RA. The same procedure was followed for the control sample, which was prepared without introducing S. marcescens, to assess the effects of the medium and the sample removal technique on the results.
Due to the chemical makeup of cement paste with calcium silicate products, it was necessary to check whether the bacteria were solubilizing the calcium oxide or the silica components of the cement paste. Using testing procedures similar to RA treatments with S. marcescens, roughly 5 g samples of calcium carbonate were tested by replacing the RA. The measurements were taken every 24 h, and dry weights before and after the incubation were established. A cell-free control test of the calcium carbonate was run concurrently to ensure that the medium and measuring methods were not affecting the calcium carbonate.
2.2.5. Supernatant-Only Trial
A cell-free supernatant (CFS) was isolated from the medium to check that the bacteria were responsible for solubilizing the silicates. The supernatant would contain any soluble products, such as the acid produced by the bacteria without the cells. Thus, S. marcescens was added to 50 mL of GNB in vials with RA fragments of 5–10 g in weight. The RA was introduced to check if any specific products would precipitate in the presence of calcium silicate. The vials were then incubated without interruption at room temperature for seven days. On the seventh day, the medium was vacuum filtered with Millipore filter paper to prevent the bacteria from being transferred to the new sterile containers. Heat or chemical means were not used to preserve any proteins or other labile products that might be contained within the supernatant. After filtration, the CFS was added to sterile tubes with 5–10 g of sterile RA fragments. The pH of the CFS was, on average, 3.5 ± 0.5. An unpaired single-tailed t-test was performed to analyze whether the supernatant affected the no-supernatant control. This process was completed with six replicates to mirror the original testing parameters.
3. Results
3.1. Identification of Bacteria
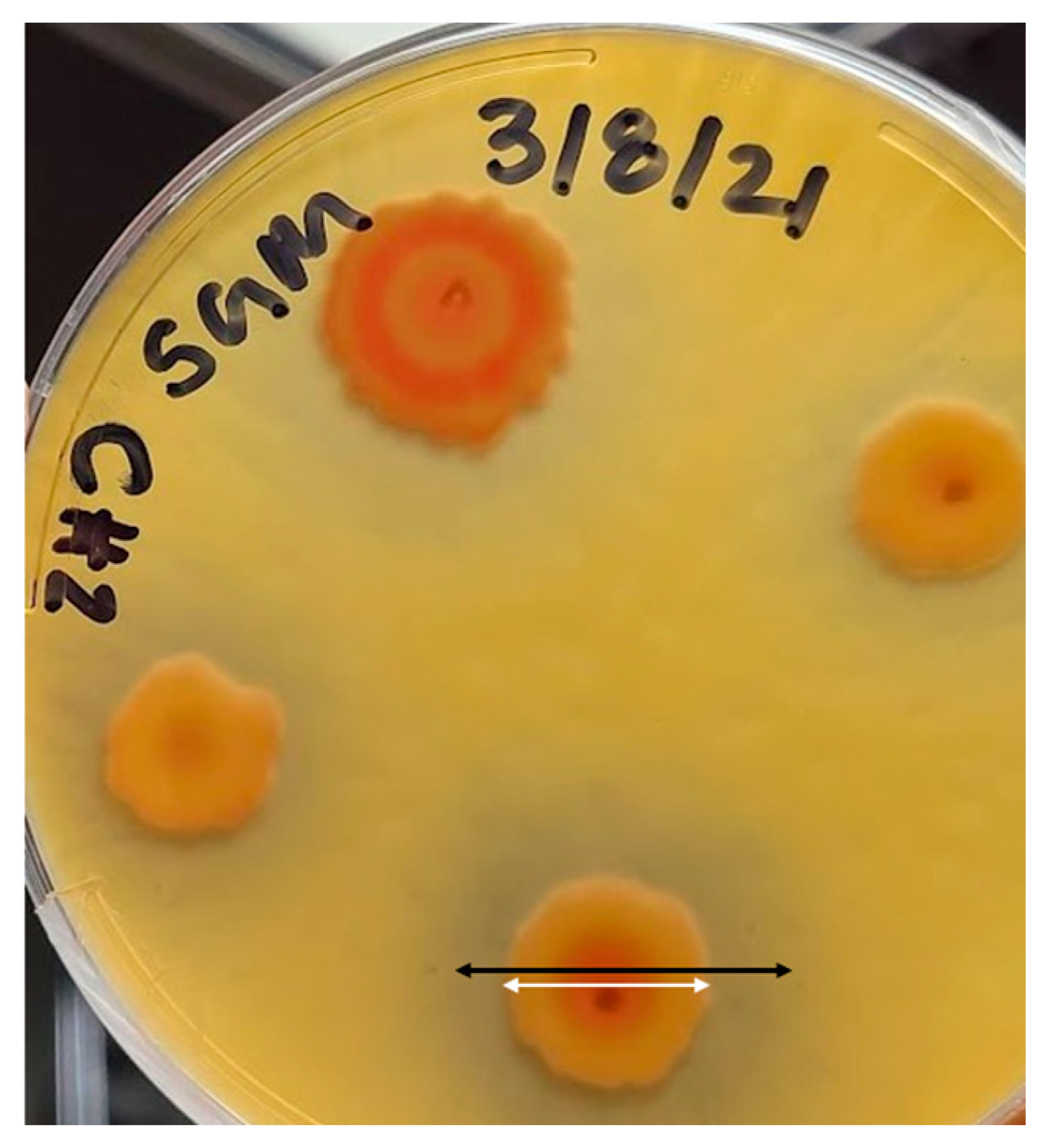
The four bacterial strains inoculated onto Bunt and Rovira agar plates and then incubated for 24 h at 30 °C were visually assessed for silicate solubilization [78]. Pseudomonas stutzeri did not produce any cleared zone, so it was characterized as incapable of solubilizing silicate and excluded from the list. Bacillus megaterium, Citrobacter freundii, and Serratia marcescens produced a clearing effect on the media. Each bacterium was inoculated via a needle to produce single-point colonies and then incubated for 24 h to provide a quantitative metric to differentiate the three bacteria. Each replicate was then measured via the diameters of the colony and the cleared zone, as shown in Figure 2. All colonies grew. As the bacterial cells multiplied, a clearance zone was produced, indicating silicate solubilization. As shown in the figure, to determine the solubilizing index (SI) the diameter of the cleared zone (black line) was then divided by the diameter of the colony (white line) to obtain an average metric for comparing bacterial samples. The average of each bacterium was taken, and the bacteria with the highest solubilizing index was determined to be S. marcescens. Citrobacter freundii and B. megaterium were excluded from further investigation.
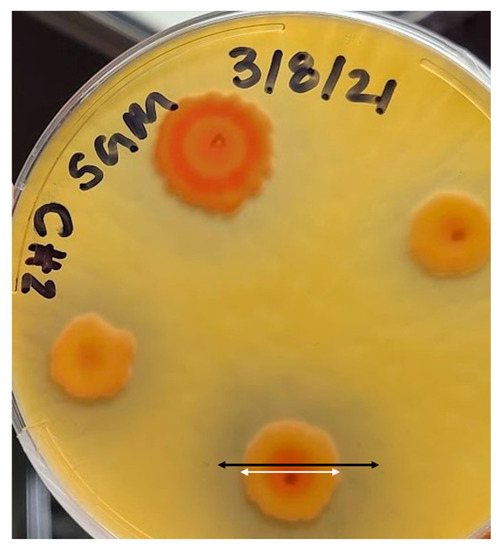
Figure 2.
Clearing effect of Serratia marcescens in Bunt and Rovira medium. The black arrow indicates the diameter of the cleared zone. The white arrow indicates the diameter of the bacterial colony. These measurements are used to determine the solubilizing index of the bacteria.
3.2. Favorable Environment for Bacteria
The pH in the flasks that received the powdered RA immediately reached 11 ± 0.5 (Figure 3) and maintained the range for 7 days; however, the pH in the flasks that received fragmented RA reached 9 ± 0.5 after 1 h and maintained the range for 7 days. After the S. marcescens were inoculated into the cultures, the spectrophotometry and plate count of colony-forming units (CFUs) revealed that the bacteria survived in the flasks containing the fragmented RA but not in those with the powdered RA. In the flasks where S. marcescens was reisolated, the average pH dropped to 3–5 due to bacterial acid production from glucose.

Figure 3.
pH survivability test of S. marcescens in an environment containing powdered RA.
3.3. Silicate Solubilization
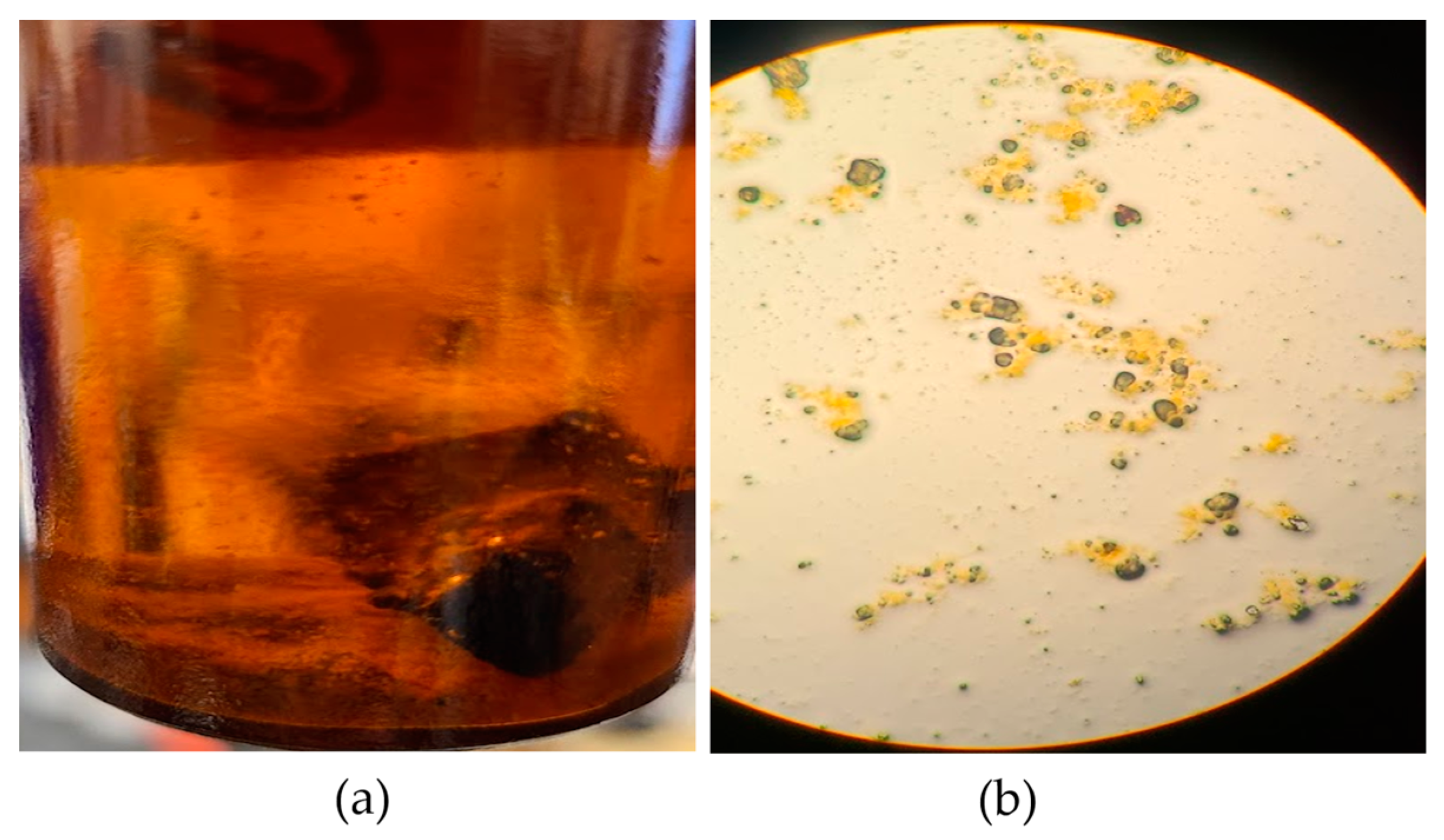
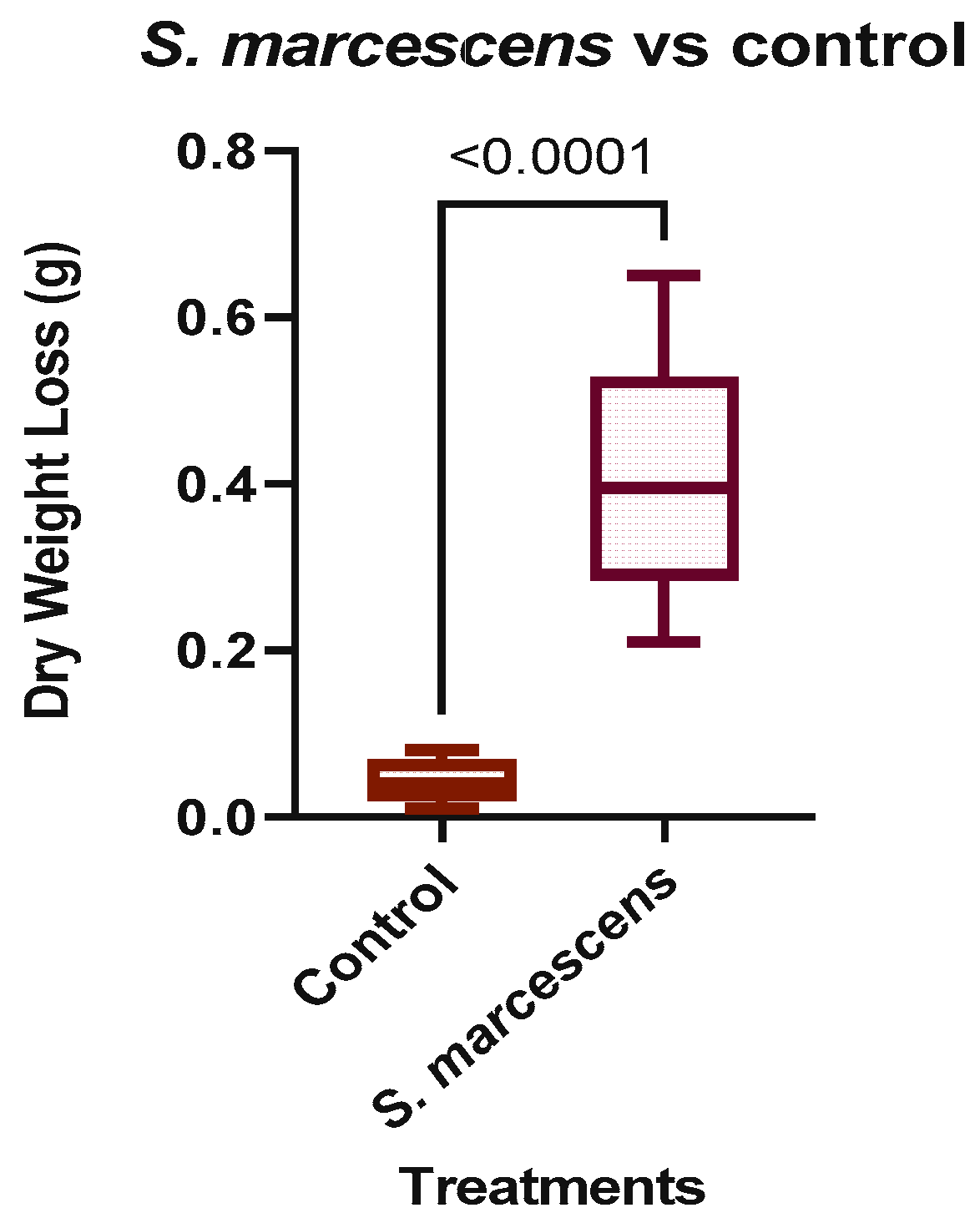
The RA fragments that were inoculated with S. marcescens gained an average of 0.5 g during the initial weighing, possibly due to the RA’s moisture. The wet weights of the RA fragments were measured daily, and the dry weights at the end of the test. During incubation, sediment was observed in the bottom of the treatment vials, as shown in Figure 4a. It was necessary to determine whether the sediment was from the RA or cell debris precipitated from the media. Using a Pasteur pipette, the sediment was observed in a glass slide without stains at 400X magnification (Figure 4b) and determined to be from the fragmented RA. Based on the recorded measurements (Table 3), the average weight loss in the vials inoculated with S. marcescens was 0.409 g over 14 days, equivalent to a 7.87% loss in weight. The average rate of weight loss was determined by dividing the average weight loss by the total testing period of 14 days, which resulted in about 0.03 g per day.
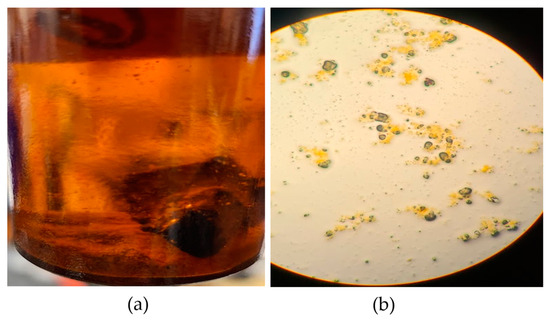
Figure 4.
Concrete solubilization micrograph: (a) Unidentified sediment in the bottom of the flask; (b) RA sediment identified using light microscopy.

Table 3.
S. marcescens experimental data.
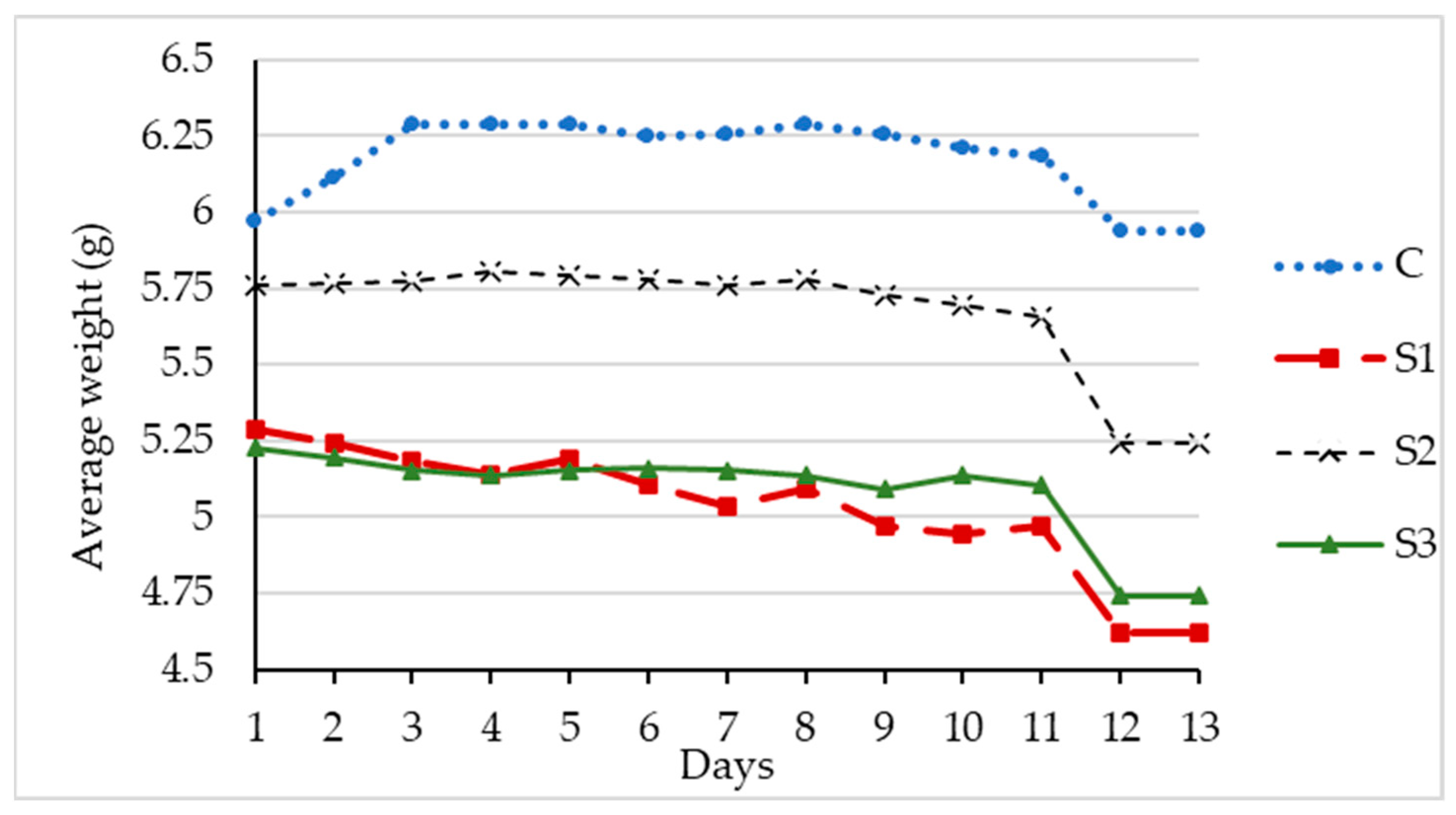
Similarly, the uninoculated control samples without S. marcescens had an average weight loss of 0.04 g over the 14 days or 0.003 g per day, as shown in Table 4. The average percentage lost in control was less than 1%. Figure 5 shows the average daily weight of three replicate samples with S. marcescens and the control sample. At the end of the testing period, the weight loss was higher in the samples with the bacterial strain.

Table 4.
No-cell control experimental data.
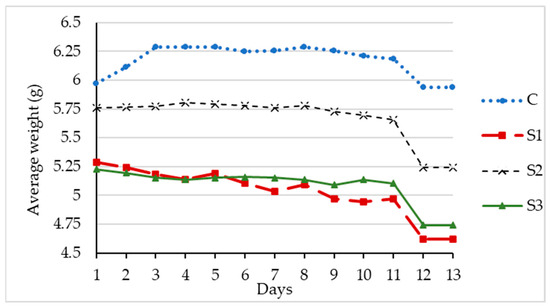
Figure 5.
Average daily weight of RA with S. marcescens.
Statistical analysis was performed on the results through GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Unpaired t-tests and analysis of variance (ANOVA) were performed to determine the effect that S. marcescens and its derivative tests had on the RA relative to the control. An unpaired one-tailed t-test was used to assess for a significant difference between the groups. The p-value was found to be significant at <0.0001 thresholds, suggesting that S. marcescens was affecting the RA fragments.
3.4. Testing Calcium Carbonate
Calcium carbonate was used as an alternative substrate control because it exists in a solid state like concrete, and any change in the mineral would likely be due to a change in its calcium content. The control calcium carbonate sample lost an average of 0.02 g or 0.001 g per day in dry weight. The S. marcescens treatment was observed to have an average loss of 0.07 g or 0.005 g per day in dry weight, which was much less than that of the fragmented RA. An unpaired one-tailed t-test was used to evaluate if there was a significant difference between the control and S. marcescens treatment. The p-value obtained was <0.0001, as shown in Figure 6. The values in the figure show the differences in the pre and post treatment dry weights from each replicate. The treatment that included S. marcescens had a larger incidence of variance across the replicates than the uninoculated control group. The p-value of the unpaired single-tailed t-test represents the difference between the two groups and is significantly different.
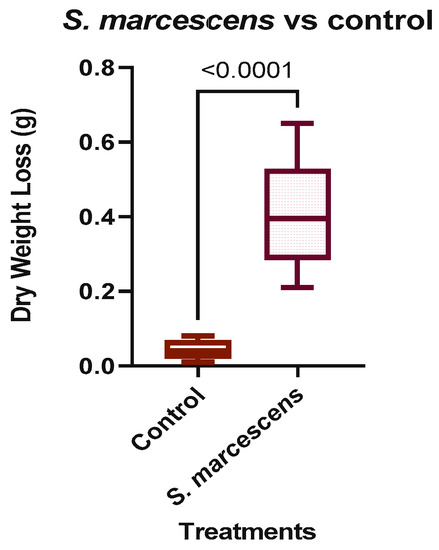
Figure 6.
Weight loss in S. marcescens treated and untreated (control) calcium carbonate.
3.5. Performance of Supernatant
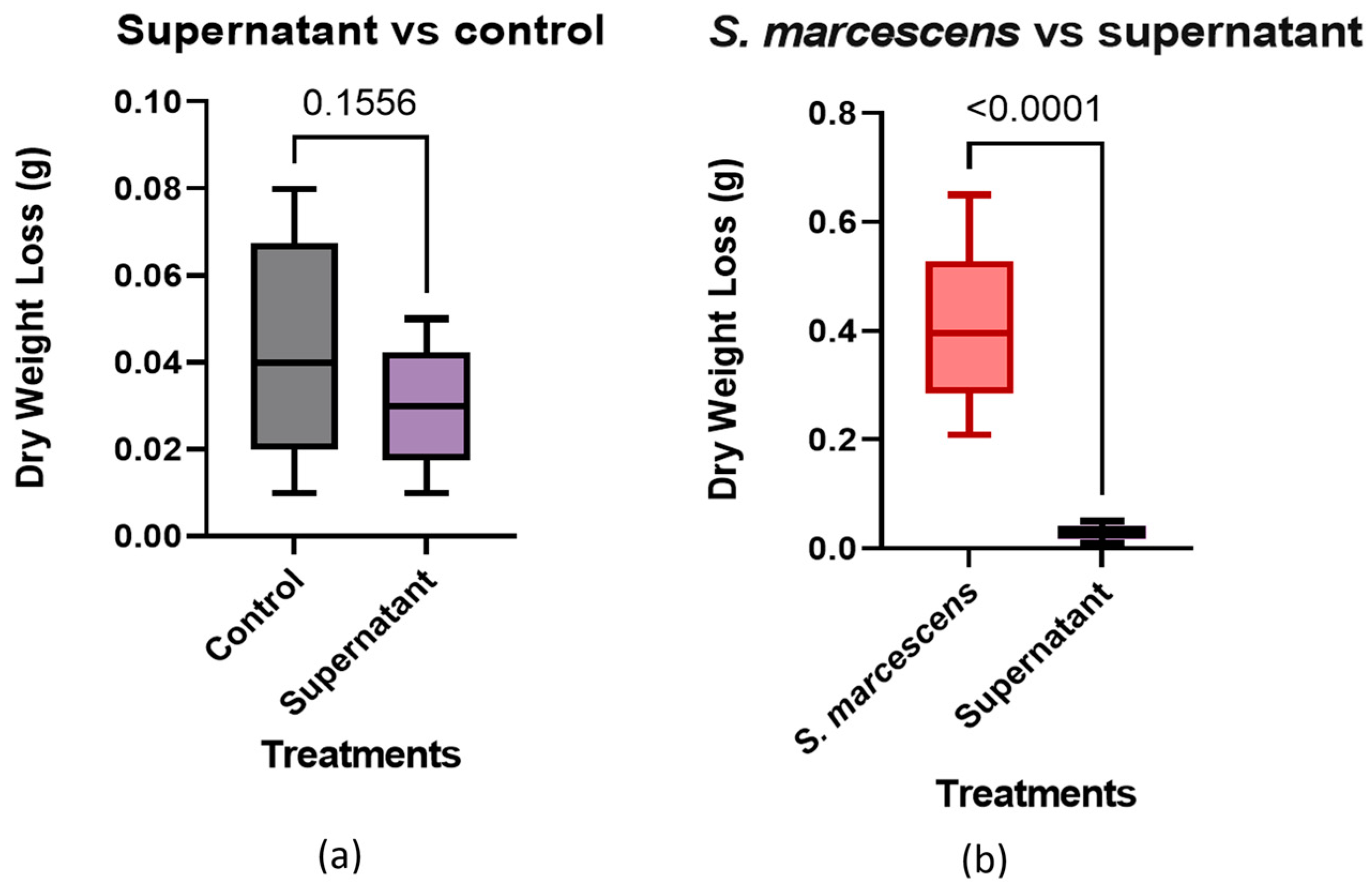
The supernatant-only control groups had an average change in weight of 0.03 g and a percentage loss of 0.5%, as shown in Table 5. This test was to check whether the bacteria produced any additional metabolic products that could influence the rate of breaking down of the silicates. No significant change in the weight of the samples was observed, indicating no other component beyond the effect observed in the standard tests.

Table 5.
Supernatant-only controls experimental data.
The results of the t-test found that the supernatant was not statistically different than the cell-free control, with a p-value of 0.1556 (Figure 7a). The same method was performed on data from the treatment with S. marcescens present. The p-value was <0.0001 (Figure 7b), with the cell-free supernatant showing a significant difference between the two groups. This suggests that the supernatant is missing a crucial component for the degradation of concrete. The values shown are the differences in the pre and post treatment dry weights from the trials. The results were evaluated using an unpaired one-tailed t-test.
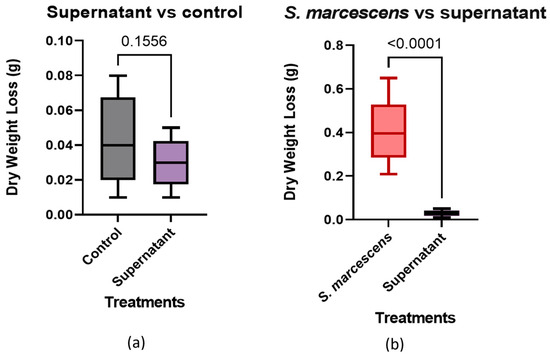
Figure 7.
Supernatant vs. cell-free control and bacterial trials: (a) Supernatant vs. control, (b) Supernatant vs. S. marcescens.
4. Discussion
Concrete encroaches on natural spaces due to development from the construction area to a landfill as waste. One possible solution for enhancing concrete recycling is the bioremediation of concrete waste using SSB. In this study, it was demonstrated that certain laboratory strains of bacteria were able to solubilize silicate compounds. Furthermore, these bacteria could reduce the weight of the RA by about 10% in 14 days.
Pastore et al. [79] studied the effects of SSB on silicon and phosphorus from bedrock and determined how the phosphorus concentration correlated to the microorganisms in the environment. They found that soil rich with SSB had an average lower pH value and higher concentrations of silicate, which conforms with the observation in this study that pH and acid production are essential to solubilizing silicate. When S. marcescens was introduced into the vials, the pH value changed from 7 to 3, which resulted in a more considerable weight loss of the RA fragments. It is currently unknown or not explored in this study whether the interaction between concrete and SSB is limited to producing acids that cause weathering.
In addition to enhancing concrete recycling by removing the adhered mortar, bacteria could benefit the environment by converting insoluble silicate minerals to a usable form that plants could use. Kang et al. [80] found that rice plants had more robust growth in fields with SSB. Higher silicate concentrations offered biotic and abiotic tolerances to crops. Chandrakala et al. [81] stated that most silicate compounds that plants use are introduced through irrigation water if not otherwise supplemented. The silicate concentration in soil biomass is depleted as the crop grows and is only partially replenished with watering. Adding more silicate sources to soil and SSB to help break it down could improve crop production, especially in areas that have been over-farmed to the point that the biomass cannot support growth. Concrete, which is currently seen as waste, could be utilized to replenish these environments depending on the rest of its makeup.
According to Solé et al. [77], when S. marcescens ferments glucose, it produces excess lactic, acetic, citric, and pyruvic acids. Their study aimed to identify whether production of prodigiosin, a tripyrrole bright-red pigment characteristic of this species, was inhibited through an abundance of glucose in the environment during fermentation. The results showed that, in a high-glucose environment, S. marcescens is capable of rapid extracellular acidification and that the primary end products were lactic acid and acetic acid. This explains the rapid decline in pH during the incubation and why S. marcescens had marginal success in solubilizing the concrete. The medium used in the experiments was nutrient broth enriched with a 10% concentration of glucose, which was an extremely high concentration that could inhibit the metabolism and growth of some bacteria [77]. S. marcescens might have rapidly fermented the additional glucose and converted it to acids that could solubilize the silicates in the RA.
Interestingly, the cell-free supernatant did not significantly affect the RA. This could be explained by the lower acid concentration found in the supernatant than in the acid-only trials. The RA and medium itself could have buffered the effects of the weak concentrations of acids found in the supernatant. Another possible explanation could be that the bacteria produced acid near the RA, allowing them to interact before being buffered. During the trials, it was observed that S. marcescens was making a biofilm on the RA fragments. Serratia marcescens has been demonstrated to produce biofilms, which can regulate pH within themselves [82]. The proximity of the biofilm to the RA fragment could mean an immediate release of the acid on the surface of the RA, leading to the weathering effect mentioned previously.
Shi et al. [83] tested different concrete and cement paste compositions with nitric and acetic acid to determine the corrosive effect of acid on the different cement compositions. In this research, a focus was made on the analysis reported by these researchers on Portland cement. They used nitric acid with a pH value of 3 and acetic acid with pH values of 3 and 5. They found that acetic acid with a pH value of 3 was the most corrosive to the cement and had the highest concentrations of calcium and silicate during and after treatment. This supports the results observed with the concrete samples treated with white vinegar at a pH of 3. The samples were rapidly degraded when compared to the bacterial trials. These data, in agreement with those reported by Solé et al. [77], support the idea that the silicate solubilization observed was likely due to bacterial production of acetic acid during fermentation.
This study aimed to determine if bacteria are viable for RA treatment and thus enhance concrete recycling. It was observed that S. marcescens can increase the rate of concrete degradation. Further investigations are necessary to support the idea of using SSB as a means of RA treatment. This study focused on small-scale samples; however, it is necessary to determine if the results can be replicated at a larger scale. An avenue for further research would be to investigate whether acetic acid production in bacteria could be manipulated to accelerate the breakdown of silicate minerals. Lee et al. [84] identified a strain of Enterobacter ludwigii with enhanced acid production, which supports this possibility.
The limitation of time also needs to be evaluated. Each test was run for 14 days, so time was a limiting factor. Tests that are not limited by time would give insight into the longevity of some of the treatments. The bacterial treatment may be a slower method, but the bacteria could continue to reduce the RA weight with time if they are self-sustaining with enough glucose and other factors. Finally, the most important aspect is the environmental impact of these methods. The bacterium that was chosen is ubiquitous but has links to nosocomial infections [85]. The benefits of recycling concrete could be outweighed by the effects non-native bacteria have on the environment. An environmental impact study should be completed prior to widespread implementation.
5. Conclusions
The following conclusions could be drawn from the results obtained in the experimental study on the effect of SSB on the removal of adhered mortar from the RA.
- S. marcescens bacterial strain can survive the alkaline environment in concrete and solubilize the silicates present in cement paste.
- SSB are capable of breaking down the CSH phase in cement paste by solubilizing the silicates.
- Cell-free supernatant did not significantly affect the RA, possibly due to the lower acid concentration in the supernatant.
- SSB treatment of RA to remove the adhered mortar is effective but a slow process by only reducing 10% in 14 days. Further investigation is required to speed up the process.
- The results of this research could serve as a basis for further investigation that could enhance the properties of RAC and the recycling of aggregate.
Author Contributions
Conceptualization, T.G. and R.J.; methodology, A.R. and R.J.; software, A.R.; validation, A.R., T.G. and R.J.; formal analysis, A.R. and T.G.; investigation, A.R.; resources, R.J. and T.G.; data curation, A.R.; writing—original draft preparation, T.G.; writing—review and editing, A.R. and T.G.; visualization, T.G.; supervision, R.J. and T.G.; project administration, R.J. All authors have read and agreed to the published version of the manuscript.
Funding
Publication funding provided by Tech American Society for Microbiology.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the space and equipment support provided by the Department of Biological Sciences and the Department of Civil, Environmental, and Construction Engineering at Texas Tech University and the technical support by John Zak, Tirhas Hailu, and Abdul Hamood.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GCCA. Global Cement and Concrete Industy Announces Roadmap to Achieve Groundbreaking ‘Net Zero’ CO2 Emission by 2050. 2021. Available online: https://gccassociation.org/news/global-cement-and-concrete-industry-announces-roadmap-to-achieve-groundbreaking-net-zero-co2-emissions-by-2050/ (accessed on 30 September 2022).
- GAIN. Global Aggregates Information Network. Available online: https://www.gain.ie/ (accessed on 8 March 2023).
- Business, A. Growing Global Aggregates Sustainability. Aggreg. Bus. 2021. Available online: https://www.aggbusiness.com/feature/growing-global-aggregates-sustainably (accessed on 30 September 2022).
- Drew, L.J.; Langer, W.H.; Sachs, J.S. Environmentalism and Natural Aggregate Mining. Nat. Resour. Res. 2002, 11, 19–28. [Google Scholar] [CrossRef]
- Akhtar, A.; Sarmah, A.K. Construction and demolition waste generation and properties of recycled aggregate concrete: A global perspective. J. Clean. Prod. 2018, 186, 262–281. [Google Scholar] [CrossRef]
- Wang, R.; Yu, N.; Li, Y. Methods for improving the microstructure of recycled concrete aggregate: A review. Constr. Build. Mater. 2020, 242, 118164. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Skaf, M.; Faleschini, F.; Manso, J.M.; Ortega-López, V. Self-compacting concrete manufactured with recycled concrete aggregate: An overview. J. Clean. Prod. 2020, 262, 121362. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Saffar, D.M.A.; Alyousef, R. The Utilization of Recycled Aggregate in High Performance Concrete: A Review. J. Mater. Res. Technol. 2020, 9, 8469–8481. [Google Scholar] [CrossRef]
- Cakir, O. Experimental analysis of properties of recycled coarse aggregate (RCA) concrete with mineral additives. Constr. Build. Mater. 2014, 68, 17–25. [Google Scholar] [CrossRef]
- Hansen, T.C. Recycling of Demolished Concrete Masonry; CRC Press: London, UK, 1992. [Google Scholar]
- Collins, R.J. The use of recycled aggregates in concrete. BRE Rep. 1994, 5, 49. [Google Scholar]
- Sagoe-Crentsil, K.K.; Brown, T.; Taylor, A.H. Performance of concrete made with commerically produced coarse recycled concrete aggregate. Cem. Concr. Res. 2001, 31, 707–712. [Google Scholar] [CrossRef]
- Sri Ravindrarajah, R.; Loo, Y.H.; Tam, C.T. Recycled Concrete as Fine and Coarse Aggregate in Concrete. Mag. Concr. Res. 1987, 39, 214–220. [Google Scholar] [CrossRef]
- Gomez-Soberon, J.M.V. Porosity of recycled concrete with substitution of recycled concrete agregate. Cem. Concr. Res. 2002, 32, 1301–1311. [Google Scholar] [CrossRef]
- Saravanakumar, P.; Abhiram, K.; Manoj, B. Properties of treated recycled aggregates and its influence on concrete strength characteristics. Constr. Build. Mater. 2016, 111, 611–617. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M.; Le, K.N. Removal of cement mortar remains from recycled aggregate using pre-soaking approaches. Resour. Conserv. Recycl. 2007, 50, 82–101. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.L.; Qian, X.; Chen, P.Y.; Xu, Y.; Guo, J.X. An environmentally friendly method to improve the quality of recycled concrete aggregates. Constr. Build. Mater. 2017, 144, 432–441. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Munir, M.J.; Wu, Y.-F.; Patnaikuni, I.; Zhou, Y.; Xing, F. Effect of different aggregate treatment techniques on the freeze-thaw and sulfate resistance of recycled aggregate concrete. Cold Reg. Sci. Technol. 2020, 178, 103126. [Google Scholar] [CrossRef]
- Akbarnezhad, A.; Ong, K.C.G.; Zhang, M.H.; Tam, C.T.; Foo, T.W.J. Microwave-assisted beneficiation of recycled concrete aggregates. Constr. Build. Mater. 2011, 25, 3469–3479. [Google Scholar] [CrossRef]
- Larbi, J.A.; Heijnen, W.M.M.; Brouwer, J.P.; Mulder, E. Preliminary laboratory investigation of thermally treated recycled concrete aggregate for general use in concrete. In Waste Management Series; Woolley, G.R., Goumans, J.J.J.M., Wainwright, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 1, pp. 129–139. [Google Scholar]
- Kou, S.C.; Poon, C.S. Properties of concrete prepared with PVA-impregnated recycled concrete aggregates. Cem. Concr. Comp. 2010, 32, 649–654. [Google Scholar] [CrossRef]
- Lei, B.; Li, W.; Tang, Z.; Li, Z.; Tam, V.W.Y. Effects of environmental actions, recycled aggregate quality and modification treatments on durability performance of recycled concrete. J. Mater. Res. Technol. 2020, 9, 13375–13389. [Google Scholar] [CrossRef]
- Shi, C.J.; Li, Y.K.; Zhang, J.K.; Li, W.G.; Chong, L.L.; Xie, Z.B. Performance enhancement of recycled concrete aggregate—A review. J. Clean. Prod. 2016, 112, 466–472. [Google Scholar] [CrossRef]
- Santos, W.F.; Quattrone, M.; John, V.M.; Angulo, S.C. Roughness, wettability and water absorption of water repellent treated recycled aggregates. Constr. Build. Mater. 2017, 146, 502–513. [Google Scholar] [CrossRef]
- Spaeth, V.; Tegguer, A.D. Improvement of recycled concrete aggregate properties by polymer treatment. Int. J. Sustain. Built Environ. 2013, 2, 143–152. [Google Scholar]
- Giri, J.P.; Panda, M.; Sahoo, U.C. Performance of Bituminous Mixes Containing Treated Recycled Concrete Aggregates and Modified by Waste Polyethylene. J. Mater. Civ. Eng. 2018, 30, 04018184. [Google Scholar] [CrossRef]
- Kareem, A.I.; Nikraz, H.; Asadi, H. Application of Double-Coated Recycled Concrete Aggregates for Hot-Mix Asphalt. J. Mater. Civ. Eng. 2019, 31, 04019036. [Google Scholar] [CrossRef]
- Ma, J.; Sun, D.; Pang, Q.; Sun, G.; Hu, M.; Lu, T. Potential of recycled concrete aggregate pretreated with waste cooking oil residue for hot mix asphalt. J. Clean. Prod. 2019, 221, 469–479. [Google Scholar] [CrossRef]
- Esen, Y.; Orhan, E. Investigation of the effect on the physical and mechanical properties of the dosage of additive in self-consolidating concrete. KSCE J. Civ. Eng. 2016, 20, 2849–2858. [Google Scholar] [CrossRef]
- Katz, A. Treatments for the Improvement of Recycled Aggregate. J. Mater. Civ. Eng. 2004, 16, 597–603. [Google Scholar] [CrossRef]
- Arora, S.; Singh, S.P. Flexural fatigue performance of concrete made with recycled concrete aggregates and ternary blended cements. J. Sustain. Cem. Based Mater. 2018, 7, 182–202. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Cao, Z.; Ling, T.C.; Zheng, J. Performance of mortar prepared with recycled concrete aggregate enhanced by CO2 and pozzolan slurry. Cem. Concr. Compos. 2018, 86, 130–138. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, T.; Liu, H.; Su, S. Modifying recycled aggregate concrete by aggregate surface treatment using sulphoaluminate cement and basalt powder. Constr. Build. Mater. 2018, 192, 526–537. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Lu, L.; Gong, C. Evaluation of pre-coated recycled aggregate for concrete and mortar. Constr. Build. Mater. 2013, 43, 191–196. [Google Scholar] [CrossRef]
- Lee, C.-H.; Du, J.-C.; Shen, D.-H. Evaluation of pre-coated recycled concrete aggregate for hot mix asphalt. Constr. Build. Mater. 2012, 28, 66–71. [Google Scholar] [CrossRef]
- Esen, Y.; Kurt, A. Effect of High Temperature in Concrete for Different Mineral Additives and Rates. KSCE J. Civ. Eng. 2018, 22, 1288–1294. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Tam, V.W.Y.; Xiao, J.; Shah, S.P. Current progress on nanotechnology application in recycled aggregate concrete. J. Sustain. Cem. Based Mater. 2019, 8, 79–96. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Meng, T.; Shah, S.P. Surface Treatment on Recycled Coarse Aggregates with Nanomaterials. J. Mater. Civ. Eng. 2016, 28, 04015094. [Google Scholar] [CrossRef]
- Mukharjee, B.B.; Barai, S.V. Influence of incorporation of nano-silica and recycled aggregates on compressive strength and microstructure of concrete. Constr. Build. Mater. 2014, 71, 570–578. [Google Scholar] [CrossRef]
- Mukharjee, B.B.; Barai, S.V. Influence of Nano-Silica on the properties of recycled aggregate concrete. Constr. Build. Mater. 2014, 55, 29–37. [Google Scholar] [CrossRef]
- Li, W.; Luo, Z.; Long, C.; Wu, C.; Duan, W.H.; Shah, S.P. Effects of nanoparticle on the dynamic behaviors of recycled aggregate concrete under impact loading. Mater. Des. 2016, 112, 58–66. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.-S.; Xie, Z. Performance Enhancement of Recycled Concrete Aggregates through Carbonation. J. Mater. Civ. Eng. 2015, 27, 04015029. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhao, Y.X.; Meng, T.; Shah, S.P. The modification effects of a nano-silica slurry on microstructure, strength, and strain development of recycled aggregate concrete applied in an enlarged structural test. Constr. Build. Mater. 2015, 95, 721–735. [Google Scholar] [CrossRef]
- Shaikh, F.; Chavda, V.; Minhaj, N.; Arel, H.S. Effect of mixing methods of nano silica on properties of recycled aggregate concrete. Struct. Concr. 2018, 19, 387–399. [Google Scholar] [CrossRef]
- Hosseini, P.; Booshehrian, A.; Delkash, M.; Ghavami, S.; Zanjani, M.K. Use of Nano-SiO2 to Improve Microstructure and Compressive Strength of Recycled Aggregate Concretes. In Nanotechnology in Construction 3; Springer: Berlin, Heidelberg, 2009; pp. 215–221. [Google Scholar] [CrossRef]
- Wang, Y.; Hughes, P.; Niu, H.; Fan, Y. A new method to improve the properties of recycled aggregate concrete: Composite addition of basalt fiber and nano-silica. J. Clean. Prod. 2019, 236, 117602. [Google Scholar] [CrossRef]
- Meng, R.; Tao, M.; Huang, M.; Xu, Q. Research on Composite Strengthening Nano-Technique of Recycled Aggregate. Appl. Mech. Mater. 2012, 357–360, 2211–2214. [Google Scholar] [CrossRef]
- Li, W.; Long, C.; Tam, V.W.Y.; Poon, C.-S.; Hui Duan, W. Effects of nano-particles on failure process and microstructural properties of recycled aggregate concrete. Constr. Build. Mater. 2017, 142, 42–50. [Google Scholar] [CrossRef]
- Gao, C.; Huang, L.; Yan, L.; Jin, R.; Chen, H. Mechanical properties of recycled aggregate concrete modified by nano-particles. Constr. Build. Mater. 2020, 241, 118030. [Google Scholar] [CrossRef]
- Ying, J.; Zhou, B.; Xiao, J. Pore structure and chloride diffusivity of recycled aggregate concrete with nano-SiO2 and nano-TiO2. Constr. Build. Mater. 2017, 150, 49–55. [Google Scholar] [CrossRef]
- Moro, C.; Francioso, V.; Velay-Lizancos, M. Nano-TiO2 effects on high temperature resistance of recycled mortars. J. Clean. Prod. 2020, 263, 121581. [Google Scholar] [CrossRef]
- Mohammed, R.; Aladdin, A.; Farhad, M. Effect of Nano-Alumina on Microstructure And Mechanical Properties of Recycled Concrete; Journal of Engineering and Sustainable Development, 2018, 2, 90–103. J. Eng. Sustain. Dev. 2018, 2, 90–103. [Google Scholar]
- Fernández Bertos, M.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Thiery, M.; Villain, G.; Dangla, P.; Platret, G. Investigation of the carbonation front shape on cementitious materials: Effects of the chemical kinetics. Cem. Concr. Res. 2007, 37, 1047–1058. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Wattage, H.; Le, K.N.; Buteraa, A.; Soomro, M. Methods to improve microstructural properties of recycled concrete aggregate: A critical review. Constr. Build. Mater. 2021, 270, 121490. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Klama, J.; Zawal, D.; Krupa, D. Modification of recycled concrete aggregate by calcium carbonate biodeposition. Constr. Build. Mater. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- García-González, J.; Rodríguez-Robles, D.; Wang, J.; De Belie, N.; Morán-del Pozo, J.M.; Guerra-Romero, M.I.; Juan-Valdés, A. Quality improvement of mixed and ceramic recycled aggregates by biodeposition of calcium carbonate. Constr. Build. Mater. 2017, 154, 1015–1023. [Google Scholar] [CrossRef]
- Wu, C.-R.; Zhu, Y.-G.; Zhang, X.-T.; Kou, S.-C. Improving the properties of recycled concrete aggregate with bio-deposition approach. Cem. Concr. Compos. 2018, 94, 248–254. [Google Scholar] [CrossRef]
- Cheng, H.L.; Wang, C.Y. The influence of sodium silicate on the properties of recycled aggregates. Gypsum Cem. Build. 2005, 12, 12–14. [Google Scholar]
- Patra, P.; Natarajan, K.A. Role of mineral specific bacterial proteins in selective flocculation and flotation. Int. J. Miner. Process. 2008, 88, 53–58. [Google Scholar] [CrossRef]
- Santhiya, D.; Subramanian, S.; Natarajan, K.A.; Rao, K.H.; Forssberg, K.S.E. Biomodulation of galena and sphalerite surfaces using Thiobacillus thiooxidans. Int. J. Miner. Process. 2001, 62, 121–141. [Google Scholar] [CrossRef]
- Sharma, P.K.; Rao, K.H. Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: Surface thermodynamics and extended DLVO theory. Colloid. Surf. B 2003, 29, 21–38. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Lauwers, A.M.; Heinen, W. Biodegradation and Utilization of Silica and Quartz. Arch. Microbiol. 1974, 95, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, N.; Saleena, L.M.; Raj, S.A. Silica Solubilization Potential of Certain Bacterial Species in the Presence of Different Silicate Minerals. Silicon 2016, 10, 267–275. [Google Scholar] [CrossRef]
- Rashid, H.; Nawaz, H.; Bhatti, T.M. Bioleaching studies of bauxite ore using Aspergillus niger. J. Biol. Sci. 2001, 1, 501–504. [Google Scholar]
- Zhan, S.F.; Liu, J.J.; Chen, Y.; Sun, D.S. Single and Coorperative Bauxite Bioleaching by Silicate Bacteria. In Proceedings of the 2013 International Conference on Agricultural and Natural Resources Engineering (Icanre 2013), Singapore, 1–2 May 2013; Volume 5, p. 172. [Google Scholar] [CrossRef]
- Krylova, L.N.; Gusakov, M.S.; Adamov, E.V.; Vainshtein, M.B. Application of bacterial-chemical oxidation for processing of nickel-containing raw materials. Russ. J. Non-Ferr. Met. 2011, 52, 410–415. [Google Scholar] [CrossRef]
- Yang, Z.C.; Feng, Y.L.; Li, H.R.; Wang, W.D.; Teng, Q. Effect of biological pretreatment on flotation recovery of pyrolusite. Trans. Nonferrous Met. Soc. China 2014, 24, 1571–1577. [Google Scholar] [CrossRef]
- Vandevivere, P.; Welch, S.A.; Ullman, W.J.; Kirchman, D.L. Enhanced Dissolution of Silicate Minerals by Bacteria at near-Neutral Ph. Microb. Ecol. 1994, 27, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Santelli, C.M.; Welch, S.A.; Westrich, H.R.; Banfield, J.F. The effect of Fe-oxidizing bacteria on Fe-silicate mineral dissolution. Chem. Geol. 2001, 180, 99–115. [Google Scholar] [CrossRef]
- Banfield, J.F.; Barker, W.W.; Welch, S.A.; Taunton, A. Bilogical impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. USA 1999, 96, 3404–3411. [Google Scholar]
- Teng, Q.; Feng, Y.L.; Li, H.R. Effects of silicate-bacteria pretreatment on desiliconization of magnesite by reverse flotation. Colloid. Surf. A 2018, 544, 60–67. [Google Scholar] [CrossRef]
- Brown, J.; Chen, C.; Fernández, M.; Carr, D. Urban endoliths: Incidental microbial communities occurring inside concrete. AIMS Microbiol. 2023, 2, 277–312. [Google Scholar] [CrossRef]
- Mohamed, E.; Farag, A.; Youssef, S. Phosphate Solubilization by Bacillus subtilis and Serratia marcescens Isolated from Tomato Plant Rhizosphere. J. Environ. Prot. 2018, 9, 266–277. [Google Scholar] [CrossRef]
- Solé, M.; Rius, N.; Lorén, J.G. Rapid extracellular acidification induced by glucose metabolism in non-proliferating cells of Serratia marcescens. Int. Microbiol. 2000, 3, 39–43. [Google Scholar]
- Bunt, J.S.; Rovira, A.D. Microbiological Studies of Some Subantarctic Soils. J. Soil Sci. 1955, 1, 119–128. [Google Scholar] [CrossRef]
- Pastore, G.; Kernchen, S.; Spohn, M. Microbial solubilization of silicon and phosphorus from bedrock in relation to abundance of phosphorus-solubilizing bacteria in temperate forest soils. Soil Biol. Biochem. 2020, 151, 108050. [Google Scholar] [CrossRef]
- Kang, S.-M.; Waqas, M.; Shahzad, R.; You, Y.-H.; Asaf, S.; Khan, M.A.; Lee, K.-E.; Joo, G.-J.; Kim, S.-J.; Lee, I.-J. Isolation and characterization of a novel silicate-solubilizing bacterial strain Burkholderia eburnea CS4-2 that promotes growth of japonica rice (Oryza sativa L. cv. Dongjin). Soil.Sci. Plant Nutr. 2017, 63, 233–241. [Google Scholar] [CrossRef]
- Chandrakala, C.; Voleti, S.R.; Bandeppa, S.; Sunil Kumar, N.; Latha, P.C. Silicate Solubilization and Plant Growth Promoting Potential of Rhizobium sp. Isolated from Rice Rhizosphere. Silicon 2019, 11, 2895–2906. [Google Scholar] [CrossRef]
- Hollmann, B.; Perkins, M.; Chauhan, V.M.; Aylott, J.W.; Hardie, K.R. Fluorescent nanosensors reveal dynamic pH gradients during biofilm formation. npj Biofilms Microbiomes 2021, 7, 50. [Google Scholar] [CrossRef]
- Shi, C.; Stegemann, J.A. Acid corrosion resistance of different cementing materials. Cem. Concr. Res. 2000, 30, 803–808. [Google Scholar] [CrossRef]
- Lee, K.-E.; Adhikari, A.; Kang, S.-M.; You, Y.-H.; Joo, G.-J.; Kim, J.-H.; Kim, S.-J.; Lee, I.-J. Isolation and Characterization of the High Silicate and Phosphate Solubilizing Novel Strain Enterobacter ludwigii GAK2 that Promotes Growth in Rice Plants. Agronomy 2019, 9, 144. Available online: https://www.mdpi.com/2073-4395/9/3/144 (accessed on 8 August 2021). [CrossRef]
- Grimont, F.; Grimont, P.A.D. The Genus Serratia. In The Prokaryotes: A Handbook on the Biology of Bacteria Volume 6: Proteobacteria: Gamma Subclass; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 219–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).