Abstract
Hermetia illucens is an important species for waste management and the circular economy. The aim of this study was to analyze the effects of Trichoderma reesei C2A and Pleurotus sp. as pretreatments of brewer spent grain (BSG). BSG was inoculated with fungal solution or distilled water (control). After seven days, this was used for H. illucens larvae cultivation. At the end of bioconversion process, parameters of substrate reduction and H. illucens larval development were evaluated. Chemical properties of BSG, frass and larvae were also analyzed. With T. reesei C2A pretreatment, highest substrate reduction (46.3 ± 0.9%) was achieved, but larval growth rate was lower (1.0 ± 0.1 mg/d) than that of control (2.8 ± 0.2 mg/d). Larvae of Pleurotus sp. pretreatment had limited development, reflected in their negative growth rate (−0.6 ± 0.2 mg/d). In conclusion, cultivation of H. illucens larvae (six day old) on BSG pretreated with Pleurotus sp. is not recommended. On the other hand, T. reesei C2A pretreatment enhance BSG reduction, and its potential use for lignocellulosic waste management should be more explored.
1. Introduction
Brewer’s spent grain (BSG) is the main by-product of beer-brewing industry, it represents about 85% of the solid by-products [1,2]. Around 20 kg of wet BSG are obtained per hectoliter of beer produced [3]. According to this, the world annual production of BSG is 40 million tons [4], with almost 120,000 tons generated by Ecuador [5]. BSG is a lignocellulosic material with a diverse composition, containing fiber (30–70%), protein (20–30%), lipids (≈10), and minerals [2,6,7].
Traditionally, BSG has been used as cattle feed, however, due to their high moisture (77–81%) and sugar content is susceptible to microbial spoilage and so often discarded improperly with negative impacts on the environment [6,7,8]. During the last ten years, some studies have been conducted with the aim to develop alternative strategies for its recycling and revalorization. The most basic strategy could be composting, although due to its high moisture and physicochemical properties, it must be mixed with other organic materials to achieve optimal conditions for its biodegradation [9]. Moreover, BSG has demonstrated to be a good substrate for lignocellulolytic enzymes production [2], and cultivation of edible mushrooms [10,11] and insects such as Hermetia illucens larva [12,13].
H. illucens has captured great attention throughout the world due to its bio converting capacity of organic residues into value added bioproducts. H. illucens larvae can grow on several organic residues coming from animal, plants, and human sources, attaining high proportions of protein (37–63% dry matter; DM) and fat (37–63% DM) useful for animal feeding [14]. Additionally, H. illucens larva manure (frass), a byproduct of the bioconversion process, is a potential source of macro and micronutrients [15], and so currently marketed as a soil amendment.
Though H. illucens larva cultivation, BSG can be reduced by 45% DM basis, but non-lignocellulosic substrates, such as food remains, could be reduced up to 82% DM basis [13]. In some studies, it has been seen that pretreatments are a key part in biotechnological applications for breaking down lignocellulose into fermentable simple sugars [16].
Recent studies related to H. illucens bioconversion have shown that a biological pre-treatment alone or in combination with a mechanical pretreatment improves the bioconversion of different organic residues, such as maize straw [17], banana peels [18], chicken manure [19], and coconut pulp [20]. In these studies, biological pretreatment with Aspergillus niger [17], Rhizopus oligosporus [18], B. subtilis [19], and yeast [20] improved the substrate reduction and H. illucens larval development. Additionally, fungi, such as Trichoderma reesei, and Pleurotus sp., are well recognized for their capacity to produce extracellular enzymes which favor lignocellulose biomass decomposition [21,22].
Due to the capacity of T. reesei and Pleurotus sp. to breakdown fibrose organic residues, the hypothesis of this study is based on the fact that pretreatment of the substrate improves the bioconversion of BSG. The objective of this study is to analyze the effects of their use on the reduction of BSG and H. illucens larval development.
2. Results
2.1. Experiment One
To limit proliferation of opportunistic microbes during pretreatment, BSG was sterilized. Then, the effects of fungi on sterilized and non-sterilized BSG were evaluated both in the absence and presence of H. illucens larvae.
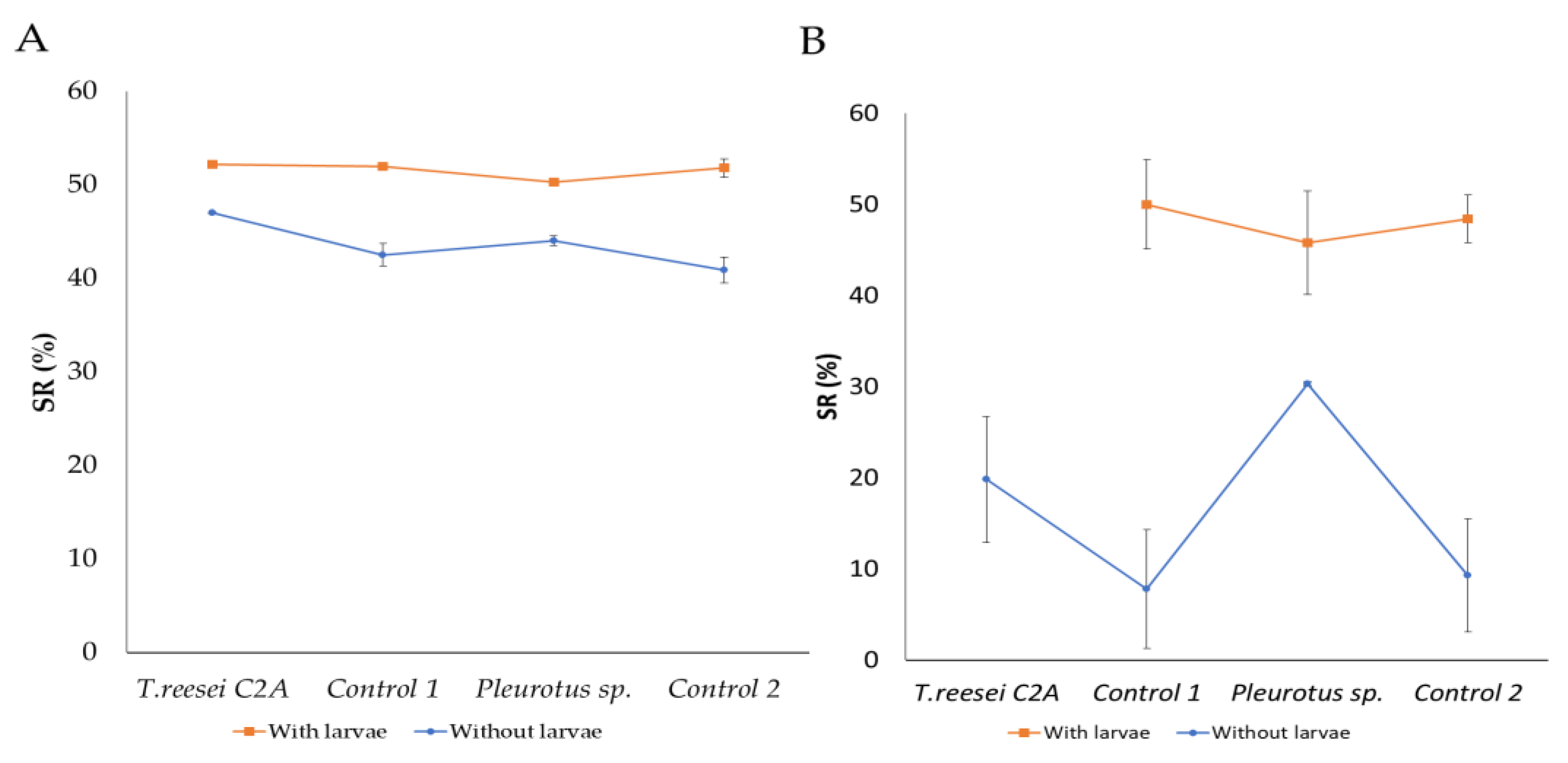
Higher substrate reduction (SR) percentages were obtained in the presence of H. illucens larvae no matter if BSG was sterilized or not (≈50%) (p < 0.0001). But, when BSG was sterilized and larvae were absent (Control 1 and 2), lower SR were obtained (7.8 and 9.3%) (p < 0.0001) (Figure 1).
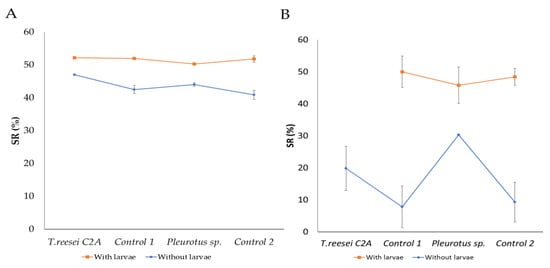
Figure 1.
Substrate reduction (SR) of (A) non-sterilized and (B) sterilized BSG, after pretreatments with T. reesei C2A and Pleurotus sp., and H. illucens larva cultivation. Symbols represent the mean (n = 3) and error bars the standard deviation of the mean (Tukey test, p < 0.0001). Control 1 and 2: BSG without inoculation of fungi.
2.2. Experiment Two
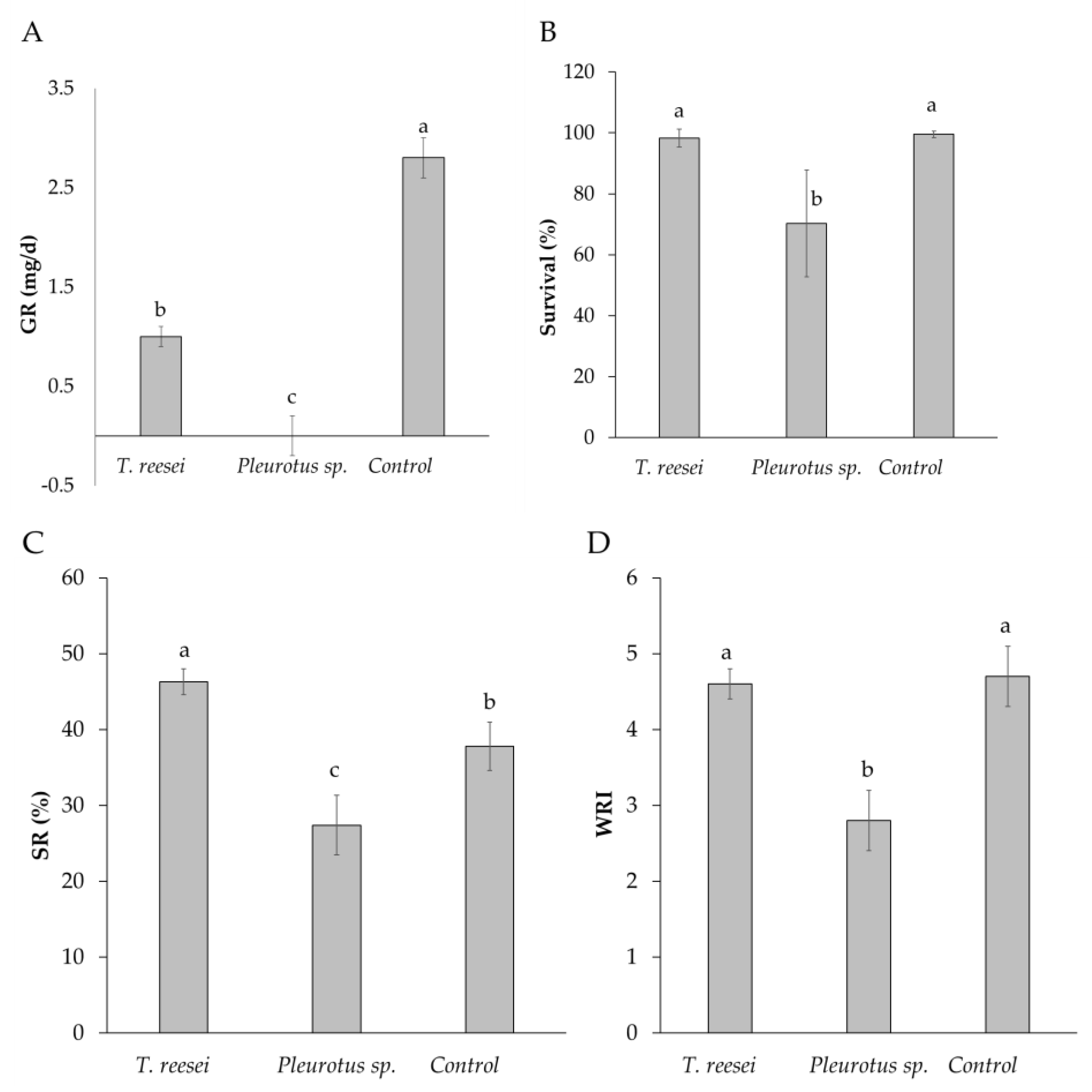
Larvae from T. reesei C2A treatment grew more slowly than the control (p < 0.0001) (1.0 ± 0.1 mg/d vs. 2.8 ± 0.2 mg/d, respectively) (Figure 2A), but the survival rate was similar on both cases (p > 0.05). (98.3 ± 2.9% and 99.5 ± 1%) (Figure 2B). Larvae fed on BSG that was pretreated with Pleurotus sp. did not develop normally. This treatment was suspended at tenth day with none prepupa, although 70% of the larvae survived (Figure 2B), they showed a negative growth rate (GR, −0.6 ± 0.2 mg/d) (Figure 2A). Regarding crude protein content (CP) of the larvae, there were no statistical differences between T. reesei C2A and the control (42.6 ± 1% and 43.1 ± 0.6%) (p = 0.34) (Table 1). CP of larva from Pleurotus sp. treatment was not analyzed since not enough larval biomass was obtained.
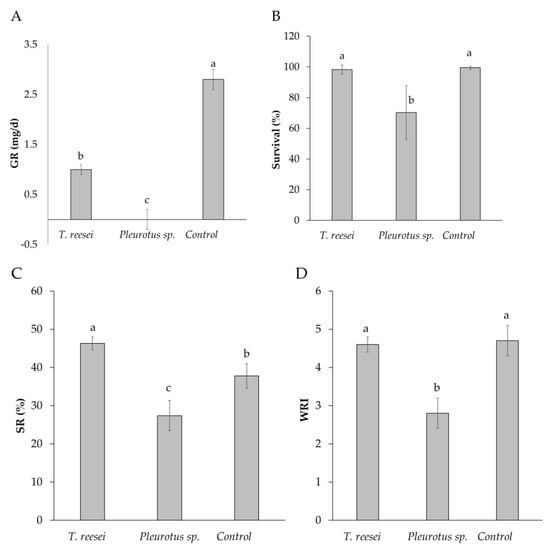
Figure 2.
(A) Larval growth rate (GR), (B) Survival, (C) Substrate reduction and (D) Waste reduction index after cultivation of H. illucens larvae on BSG pretreated with T. reesei C2A and Pleurotus sp. Means with different letters are significantly different according to Tukey or Dunn test, p < 0.05 (n = 4, mean ± SD). Control: without inoculation of fungi.

Table 1.
Chemical properties of larvae and frass from H. illucens (n = 4, mean ± SD).
Higher substrate reduction (SR) was achieved with T. reesei C2A when compared to the control C1 (46.3 ± 0.9% vs. 37.8 ± 1.6%) (p = 0.003) (Figure 2C). When waste reduction index (WRI) was analyzed, there were no statistical differences between T. reesei and Control 1 (4.7 ± 0.4 and 4.6 ± 0.2 respectively) (p < 0.0001) (Figure 2D). Lowest SR and WRI were obtained with Pleurotus sp. (27.4 ± 3.9% and 2.8 ± 0.4, respectively) (p < 0.0001) (Figure 2C,D).
There were not statistical differences in carbon nitrogen (C/N) ratio nor pH of the frass between treatments and control (p > 0.05), but the C/N ratio and pH of frass were higher than those of the substrate (Table 1).
3. Discussion
This study evaluated for the first time, the effects of T. reesei C2A and Pleurotus sp. pretreatments on substrate reduction and larval development of H. illucens cultivated on the BSG pretreated. Experiment number one showed substrate reduction of BSG was higher due to the presence and activity of H. illucens larvae whether BSG was sterilized or not. In the absence of H. illucens larvae, SR of non-sterilized BSG was higher than that of sterilized BSG, probably due to the proliferation of opportunistic microbes that showed up during the cultivation period. Because of non-inoculated fungi were observed on the controls, sterilized substrate was employed for a second experiment.
The pretreatment with T. reesei C2A for seven days prior to the culture of H. illucens led to a 22% greater SR when compared to the control. This interesting finding results very important for managing BSG residue of the beer producing process, which is commonly discarded to the environment with subsequent polluting effects. Although higher amount of BSG was reduced, it was not necessarily due to the addition of BSF larvae, as they showed low evidenced in their lower growth rate.
Taking into consideration high CP of BSG (26.9%), pretreatments with mentioned fungi seems not improve larval development. It seems that a pretreatment application of less nutritive substrates could be more efficient. This observation agrees with results obtained by Isibika et al. who found that banana peels (68.6% of fiber, 5.5% of protein), had 50% less crude fiber content after seven days of fermentation with a T. reesei strain [18]. In the mentioned study, the pretreatment aided the decomposition of the substrate as well as the increase of H. illucens larval weight under continuous feeding regimen. However, in our study, substrate was supplied at once, so water or nutrients could be loss during the process.
The treatment with T. reesei C2A did not influence the protein content of H. illucens larvae. The amount of protein found was similar to values reported by Nyakeri et al. (43%) but inferior to those reported by Liu et al. (49.9–54.1%) for the same type of substrate [13,23]. A possible explanation for the variation in content of protein of the larval biomass among studies is the variability of the nutritional profile of the BSG as a subproduct.
As for Pleurotus sp. treatment, larvae presented abnormal behavior, migrating to the perimeter of the tray. None reached the prepupae stage, therefore the treatment was suspended at day 10 of the experiment, by this time the substrate looked dry and compact. On a previous study undertaken by the same authors, older H. illucens larvae were fed BSG pretreated for three days with Pleurotus sp., completing their larval phase with a survival rate of 100%. A possible explanation for this different performance is that the larvae consumed the substrate before it was completely colonized by the fungi mycelium. This study corresponds to the first report of co-culture of Pleurotus sp. and Hermetia illucens on BSG.
Based on the results obtained in the second experiment, it can be clearly seen that the rise of C/N ratio and pH was mainly due to H. illucens larval activity rather than the inoculated fungal activity. This observation agrees with results from the studies of Meneguz et al. and Rehman at al., where H. illucens larva was able to increase pH from 4 to 9 [24] and from 5.7 to 8.6 [25]. Regarding C/N ratio, contrast results were obtained by other authors, which may be due to substrate properties, especially nitrogen content [25,26].
Finally, it is important to mention that the protocols to determine harvest age, post-harvest larval and the factor for protein quantification influence the results. Method standardization is key in H. illucens bioconversion studies to compare results among studies, using, when possible, procedures suggested by Bosch et al. [27].
4. Materials and Methods
4.1. Collection and Rearing of Hermetia illucens
Wild H. illucens larvae were collected in an urban sector of the city of Guayaquil, Guayas province, Ecuador. The collected larvae were used to establish a colony in a nursery garden at the Life Sciences Faculty of the Escuela Superior Politécnica del Litoral (ESPOL) (108 m.a.s.l.). Resulting flies were housed in a metallic structure (0.75 × 0.75 × 1.5 m) covered with mosquito net and kept under ambient conditions (temperature 21–31 °C). A tray with fermented fruits (mainly mango) served as an attractant substrate, and cardboard pieces (5 × 3 cm) located above the feeding tray aided oviposition. Eggs were daily collected and transferred to plastic containers until hatching (3rd to 4th d after collection). Neonate larvae were fed ad libitum with chicken feed meal (60% humidity) for five days according to Bosch et al. [27]. On the sixth day, larvae were separated from the feed using a fine paintbrush. Larvae assigned to the experiments corresponded to the first and second generation produced under laboratory conditions. Unused larvae were used for maintenance of the colony.
4.2. Fungi Culture
The fungus, T. reesei C2A and Pleurotus sp. were provided by Biotechnology Research Center of Ecuador (CIBE). T. reesei C2A was cultured in Potato Dextrose Agar (PDA media), and Pleurotus sp. was cultured in PDA media supplemented with 15 g of malt extract and 5 g of mycological peptone per 1,000 mL of distilled water. After incubation for seven days at 28 °C, spores were rinsed with a sterile saline solution (NaCl 0.9%) and diluted to a final concentration of 1 × 108 CFU/mL [18]. Spores were counted using a Neubauer chamber, and resulting suspensions were stored at 4 °C for later use.
4.3. Study One
Glass bottles were filled with hydrated BSG (9 g of BSG: 12 mL of water) and covered with aluminum foil. Then, half of the bottles were subjected to sterilization by autoclaving at 121 °C for 15 min. Some of the bottles with sterilized and non-sterilized BSG were inoculated with T. reesei C2A and Pleurotus sp. Covered with cotton plugs and incubated for three days at 28 °C. After the incubation period, ten H. illucens larvae were added to some bottles prior to incubation for 14 days at 28 °C. Then, larvae were harvested, and residues dehydrated for substrate reduction evaluation. All treatments had three replicates.
4.4. Study Two
4.4.1. Substrate Preparation
BSG was mixed with water at a ratio of 70 mL: 30 g of BSG and autoclaved at 121 °C for 15 min. This material was stored at 4 °C until the next day.
4.4.2. Experimental Design
This study was designed to determine the effects of T. reesei C2A and Pleurotus sp., pretreatments of BSG with the subsequent cultivation of H. illucens larvae. All treatments were run using four replicates.
Hence, 100 g of sterilized BSG (70% humidity) was placed in plastic trays (Φ 10 cm, h 6 cm), immediately one ml of spore solutions (1 × 108 CFU/mL), or distilled water (control) was added to the trays. After seven days of fermentation, 100 six-day old larvae (average weight 2.7–8.7 mg) where added to trays (density of 1.27 larvae/cm2). Each unit was covered with mosquito mesh netting (0.5 mm) and incubated at 28 °C. As soon as the first prepupa was identified, larvae were harvested using entomological tweezers. Larval weight was measured following the methodology described by Somroo et al. [28] with slight modifications. Collected larvae were kept unfed for 24 h to empty their intestinal tract, after which they were rinsed with tap water and dried with paper towels. Once clean, they were weighed and inactivated at 105 °C for 10 min. The dry weight of larvae and residues of the process were determined after drying the samples for two days at 60 °C.
4.4.3. Larval Development and Substrate Reduction Parameters
Larval growth rate (GR), survival, substrate reduction (SR), and waste reduction index (WRI) were calculated as in the work of Bosch et al. [27] and Diener et al. [29], using the following formulas:
where: GR = larval growth rate, FLW = Final weight of larval biomass, ILW = Initial weight of larval biomass, SR = Substrate reduction, Rearing time = larval development time measured from the beginning of the experiment to harvest, S = total substrate weight administrated at the start of the experiment, and R = weight of residues collected at the end of experiment. All parameters were measured on dry basis.
4.4.4. Chemical Analysis
Dried samples of larvae, substrate, and residues were grinded with a coffee mill (Krups®® F203) for 20 s. Total nitrogen of all samples was determined by combustion using an elemental organic analyzer (Elementar®® Vario MACRO cube). To obtain the crude protein content (CP) of larvae, the resulting nitrogen value was multiplied by the protein conversion factor of 4.76 proposed by Janssen et al. [30]. Substrate and residues were additionally analyzed for total carbon with the same equipment (Elementar®® Vario MACRO cube). pH was measured with a potentiometer (Thermo Scientific ®® ORION STAR A215).
The crude protein content, C/N ratio, and pH of BSG (dry matter basis) was 26.9 ± 1, 10.1 ± 0.2, and 4.2, respectively.
4.5. Statistical Analysis
All data were examined with Shapiro–Wilk, Levene, and Durbin–Watson test, for normality, homoscedasticity, and variance independence assumption check compliment, respectively. A one-way analysis of variance (ANOVA) test was used to analyze the resulting data. Survival parameter did not comply with normality assumption, so Kruskal-Wallis nonparametric test was applied. GR and WRI did not fulfill with homogeneity of variance assumption, so Welch’s correction was applied to the ANOVA test. When significant differences were found, the means were compared using Tukey test (parametric) or Dunn test (non-parametric) with a significance level of 0.05 (p < 0.05). Statistical analyses were performed using R 4.2.2 software.
5. Conclusions
The application of T. reesei C2A as pretreatment for seven days with the subsequent inoculation of H. illucens larvae lead to a major BSG reduction. The percentage of substrate decomposed was apparently not consumed by the H. illucens larvae affecting the larval growth. Nevertheless, the larvae were able to coexist with T. reesei C2A, reaching the harvest age in a short period, which was not the case with the Pleurotus sp. treatment, in which a limited development of the larvae was observed. Further studies are necessary regarding the application of T. reesei C2A and other fungal strains as a pretreatment of lignocellulosic substrates with low nutritional value (protein content). It is advisable to evaluate different concentrations of microbial complexes as well as fermentation times as to establish optimal fermentation conditions according to the type of substrate studied. Cultivation of H. illucens larvae (six day old) on BSG pretreated with Pleurotus sp. is not recommended.
Author Contributions
Conceptualization, M.S.-Z., E.Á., E.J.C., J.O.-U. and L.G.; methodology, M.S.-Z., E.Á. and L.G.; formal analysis, M.S.-Z., E.Á. and O.R.-B.; investigation, M.S.-Z.; resources, L.G., M.I.J.-F. and M.T.-U.; writing—original draft preparation, M.S.-Z.; writing—review and editing, E.Á. and J.N.-W.; supervision, E.Á. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Escuela Superior Politécnica del Litoral (ESPOL).
Data Availability Statement
The data present in this study are available on request from the corresponding author.
Acknowledgments
The authors kindly acknowledge to CIBE for kindly provided the microorganisms used in the study. We are also grateful with Blanca Sumba for providing Hermetia illucens larvae and PRODAL S.A. for the BSG donation. This research was undertaken as part of a joint graduate program within the VLIR NETWORK from Ecuador.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Xiros, C.; Christakopoulos, P. Biotechnological Potential of Brewers Spent Grain and Its Recent Applications. Waste Biomass Valorization 2012, 3, 213–232. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Mudura, E.; Dulf, F.V.; Vodnar, D.C.; And, M.T.; Salanță, L.C. Exploitation of Brewing Industry Wastes to Produce Functional Ingredients. IntechOpen 2017, 32, 137–144. [Google Scholar] [CrossRef]
- Pabbathi, N.P.P.; Velidandi, A.; Pogula, S.; Gandam, P.K.; Baadhe, R.R.; Sharma, M.; Sirohi, R.; Thakur, V.K.; Gupta, V.K. Brewer’s Spent Grains-Based Biorefineries: A Critical Review. Fuel 2022, 317, 123435. [Google Scholar] [CrossRef]
- Jurado Morales, M.Á. Fraccionamiento Del Bagazo Cervecero Bajo El Concepto de Biorrefinería; PUCE: Quito, Ecuador, 2017. [Google Scholar]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain: Composition and Preservation of BSG. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of Brewery Wastes in Food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Assandri, D.; Pampuro, N.; Zara, G.; Cavallo, E.; Budroni, M. Suitability of Composting Process for the Disposal and Valorization of Brewer’s Spent Grain. Agriculture 2020, 11, 2. [Google Scholar] [CrossRef]
- Stoffel, F.; Santana, W.d.O.; Gregolon, J.G.N.; Kist, T.B.L.; Fontana, R.C.; Camassola, M. Production of Edible Mycoprotein Using Agroindustrial Wastes: Influence on Nutritional, Chemical and Biological Properties. Innov. Food Sci. Emerg. Technol. 2019, 58, 102227. [Google Scholar] [CrossRef]
- Wolters, N.; Schabronath, C.; Schembecker, G.; Merz, J. Efficient Conversion of Pretreated Brewer’s Spent Grain and Wheat Bran by Submerged Cultivation of Hericium erinaceus. Bioresour. Technol. 2016, 222, 123–129. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on Different Organic By-Products: Influence on Growth, Waste Reduction, and Environmental Impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef]
- Nyakeri, E.M.; Ogola, H.J.O.; Ayieko, M.A.; Amimo, F.A. Valorisation of Organic Waste Material: Growth Performance of Wild Black Soldier Fly Larvae (Hermetia illucens) Reared on Different Organic Wastes. J. Insects Food Feed 2017, 3, 193–202. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional Value of the Black Soldier Fly (Hermetia illucens L.) and its Suitability as Animal Feed—A Review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Arabzadeh, G.; Delisle-Houde, M.; Tweddell, R.J.; Deschamps, M.-H.; Dorais, M.; Lebeuf, Y.; Derome, N.; Vandenberg, G. Diet Composition Influences Growth Performance, Bioconversion of Black Soldier Fly Larvae: Agronomic Value and In Vitro Biofungicidal Activity of Derived Frass. Agronomy 2022, 12, 1765. [Google Scholar] [CrossRef]
- Díaz-González, A.; Perez Luna, M.Y.; Ramírez Morales, E.; Saldaña-Trinidad, S.; Rojas Blanco, L.; de la Cruz-Arreola, S.; Pérez-Sariñana, B.Y.; Robles-Ocampo, J.B. Assessment of the Pretreatments and Bioconversion of Lignocellulosic Biomass Recovered from the Husk of the Cocoa Pod. Energies 2022, 15, 3544. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion Performance and Life Table of Black Soldier Fly (Hermetia illucens) on Fermented Maize Straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Isibika, A.; Vinnerås, B.; Kibazohi, O.; Zurbrügg, C.; Lalander, C. Pre-Treatment of Banana Peel to Improve Composting by Black Soldier Fly (Hermetia iIllucens (L.), Diptera: Stratiomyidae) Larvae. Waste Manag. 2019, 100, 151–160. [Google Scholar] [CrossRef]
- Yu, G.; Cheng, P.; Chen, Y.; Li, Y.; Yang, Z.; Chen, Y.; Tomberlin, J.K. Inoculating Poultry Manure with Companion Bacteria Influences Growth and Development of Black Soldier Fly (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2011, 40, 30–35. [Google Scholar] [CrossRef]
- Wong, C.Y.; Lim, J.W.; Chong, F.K.; Lam, M.K.; Uemura, Y.; Tan, W.N.; Bashir, M.J.K.; Lam, S.M.; Sin, J.C.; Lam, S.S. Valorization of Exo-Microbial Fermented Coconut Endosperm Waste by Black Soldier Fly Larvae for Simultaneous Biodiesel and Protein Productions. Environ. Res. 2020, 185, 109458. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological Pretreatment of Lignocellulosic Biomass - An Overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Sarsaiya, S.; Jain, A.; Kumar Awasthi, S.; Duan, Y.; Kumar Awasthi, M.; Shi, J. Microbial Dynamics for Lignocellulosic Waste Bioconversion and Its Importance with Modern Circular Economy, Challenges and Future Perspectives. Bioresour. Technol. 2019, 291, 121905. [Google Scholar] [CrossRef]
- Liu, Z.; Minor, M.; Morel, P.C.H.; Najar-Rodriguez, A.J. Bioconversion of Three Organic Wastes by Black Soldier Fly (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2018, 47, 1609–1617. [Google Scholar] [CrossRef]
- Meneguz, M.; Gasco, L.; Tomberlin, J.K. Impact of PH and Feeding System on Black Soldier Fly (Hermetia illucens, L; Diptera: Stratiomyidae) Larval Development. PLoS ONE 2018, 13, e0202591. [Google Scholar] [CrossRef]
- Rehman, K.u.; Rehman, A.; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of Mixtures of Dairy Manure and Soybean Curd Residue by Black Soldier Fly Larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373. [Google Scholar] [CrossRef]
- Rehman, K.u.; Rehman, R.U.; Somroo, A.A.; Cai, M.; Zheng, L.; Xiao, X.; Ur Rehman, A.; Rehman, A.; Tomberlin, J.K.; Yu, Z.; et al. Enhanced Bioconversion of Dairy and Chicken Manure by the Interaction of Exogenous Bacteria and Black Soldier Fly Larvae. J. Environ. Manag. 2019, 237, 75–83. [Google Scholar] [CrossRef]
- Bosch, G.; Oonincx, D.G.A.B.; Jordan, H.R.; Zhang, J.; van Loon, J.J.A.; van Huis, A.; Tomberlin, J.K. Standardisation of Quantitative Resource Conversion Studies with Black Soldier Fly Larvae. J. Insects Food Feed 2019, 6, 95–109. [Google Scholar] [CrossRef]
- Somroo, A.A.; ur Rehman, K.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus Buchneri on Soybean Curd Residue Co-Conversion by Black Soldier Fly Larvae (Hermetia illucens) for Food and Feedstock Production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of Organic Material by Black Soldier Fly Larvae: Establishing Optimal Feeding Rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio Molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).