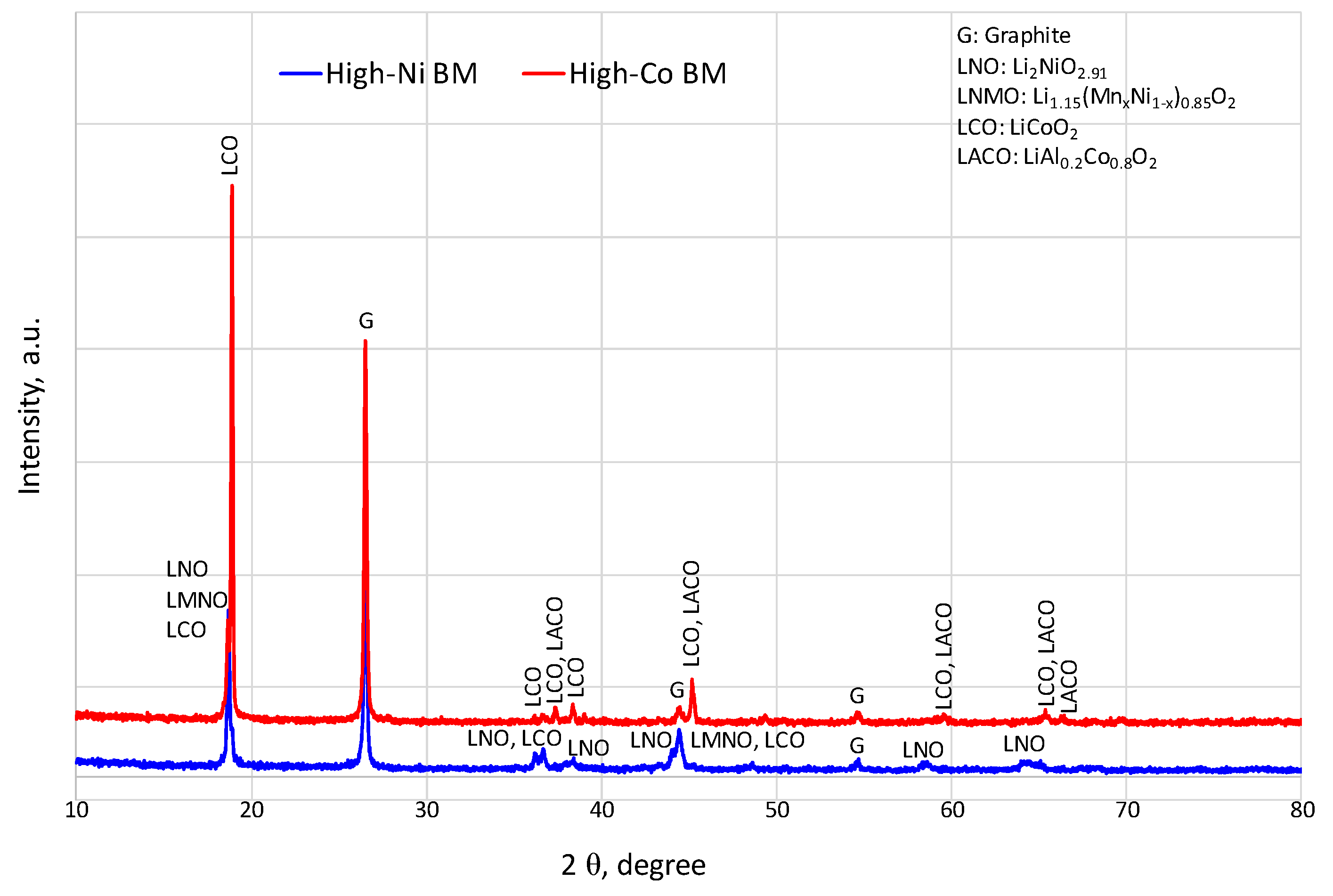
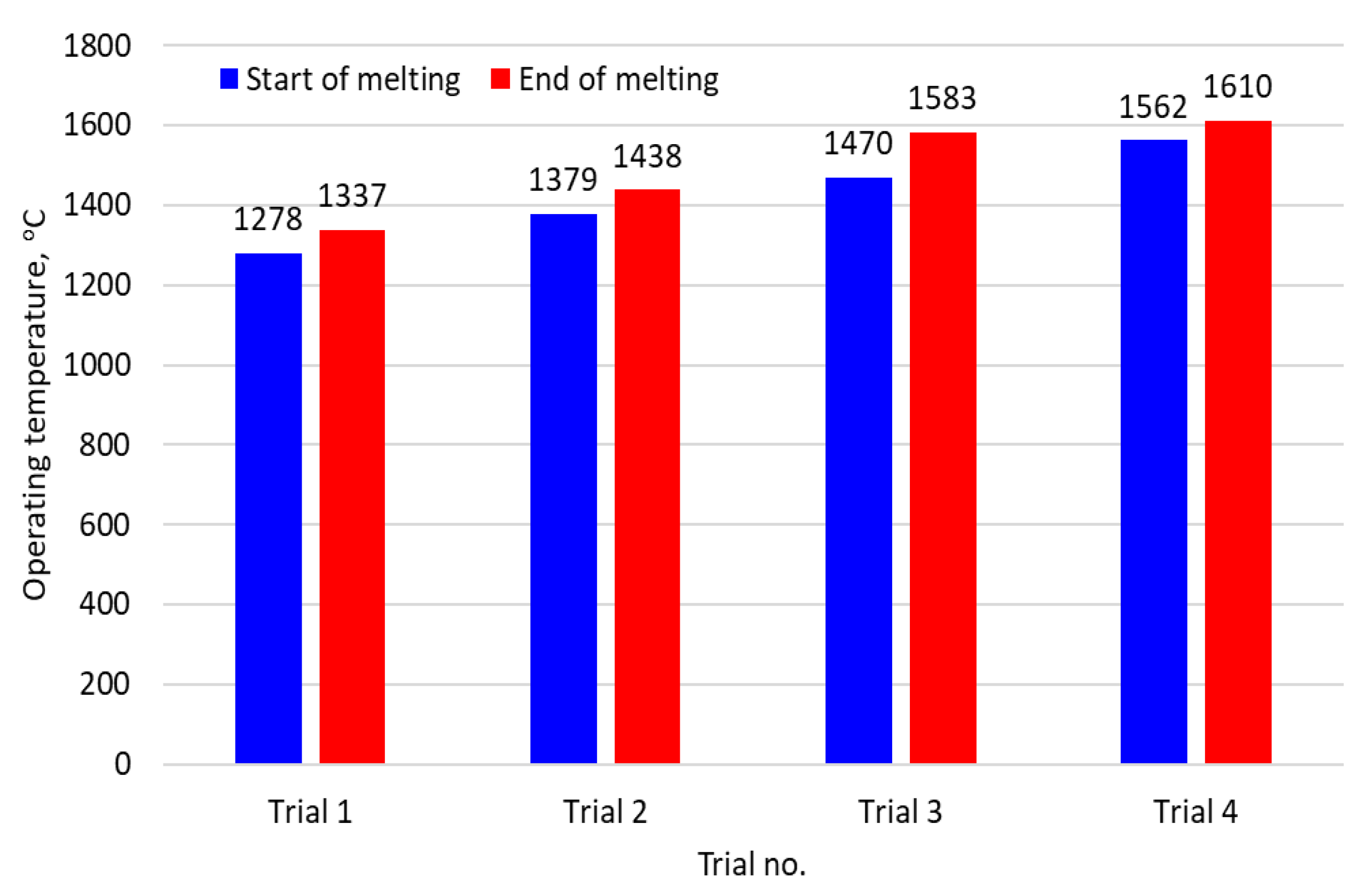
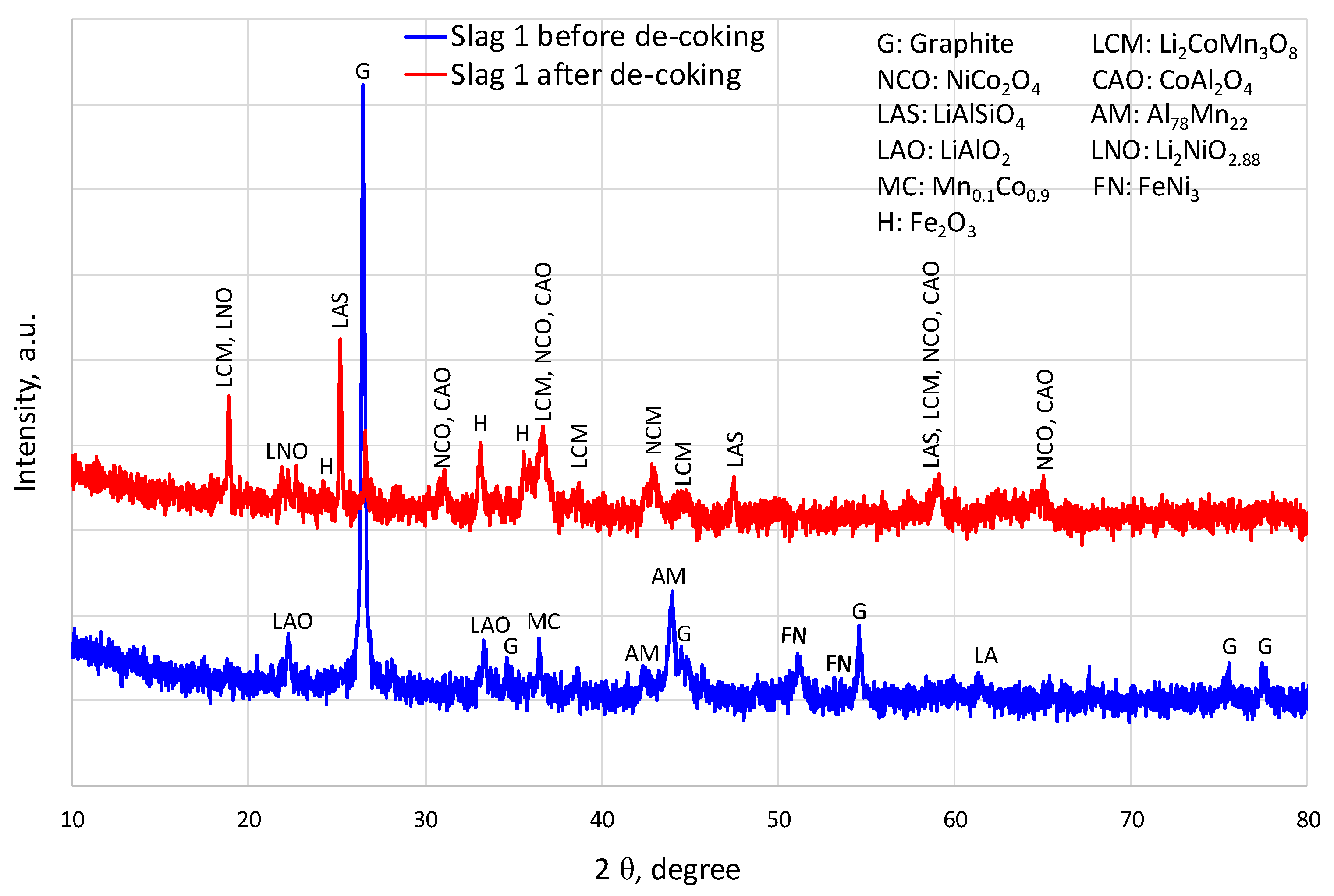
1. Introduction
Lithium-ion batteries (LIBs) are one of the most promising energy sources for various applications due to their high operating voltage, high specific energy, high power density and long cycle life [
1,
2]. Accordingly, the market value of global LIBs is sharply increasing [
3] and consequently, the need for solutions dealing with end-of-life LIBs is crucially required [
4]. The industry analysts predict that by 2030, the annual worldwide generated spent LIBs will be over 2 million tons [
5] and it could even reach about 4 million tons [
6]. The improper treatment of LIBs, either by landfill or incineration, can lead to serious risks to the environment and human health due to the presence of heavy metals and toxic electrolytes [
7]. Meanwhile, the recycling of LIB components results not only in relieving the environmental contamination pressure but also in significant economic and social benefits. The spent LIBs usually contain 5–20% Co, 5–10% Ni, 5–7% Li, 5–10% other metals (e.g., Cu, Al, Fe, etc.), ~15% organic compounds and ~7% of plastic materials [
8]. The recovery of valuable components from the spent LIBs can significantly contribute towards: (i) a supply of raw material, particularly the critical elements, which are needed for sustainable new battery production or other highly valuable applications, (ii) environmental concerns as the improper disposal of waste battery materials can result in the release of toxic gases, corrosive liquids and dissolved metals that are poisonous to humans, plants, and animals, and (iii) economic profits and environmental gains as the recovery of valuable elements from secondary resources is more economic and generates fewer greenhouse emissions than its extraction from virgin resources. Therefore, from the resource, economic and environmental perspectives, it becomes urgent to find flexible and more efficient recycling routes for spent LIBs and for finding optimal methods and feasible techniques to recover the valuable components [
9]. From a geopolitical standpoint and according to the European Commission, cobalt and natural graphite (both are main components in the LIBs cell) are critical elements while lithium and copper are expected to suffer from supply risk in the near future [
10]. Other components in LIBs, such as Al, Ni and Mn, are economically important. At least one-third of the cost of the LIBs is related to the material cost [
11]. Therefore, recovery of these valuable metals can release the heavy burden of industrial raw material shortage, save energy, reduce waste generation, decrease environmental risks, and maintain the circular economic approach [
12,
13]. All these factors make the recycling of LIBs essential to secure the sustainable production of LIBs, boosting the circular potential of batteries, conserving natural resources and reducing the environmental impacts.
More than 50 companies around the world are working on the recycling of spent LIBs on scales varying from small laboratory plants to industrial full-scale production [
14]. The present global recycling rate of spent LIBs is estimated to be only 5–7% [
6,
15], which is far from the required recycling capacity that is significantly required for sustainable LIB production. Besides the low collection rate and safety concerns [
14], the recycling of LIBs is challenged by other issues such as the complex structure of batteries, variety of battery types, shapes, sizes, and chemistries. The LIBs consist of compacted cells and each cell contains a cathode, anode, separator, electrolyte, and binder in a complex structure, which further complicates the recycling efforts of spent LIBs in order to recover all of the valuable components at a high yield and quality, meanwhile achieving profits compared to those of virgin minerals. Currently, there are three main recycling approaches for the recycling of spent LIBs: pyrometallurgy, hydrometallurgy, and direct recycling [
16]. Pyrometallurgy applies high temperature to smelt the valuable metals in LIBs and form alloys [
17,
18] while hydrometallurgy employs multiple steps of acid-based leaching for individual metal separations [
19,
20]. On the other hand, direct recycling applies physical treatment processes with minimal destruction that enable the recovery of valuable metals without causing chemical changes [
21,
22]. Some recycling processes combine pyro- and hydrometallurgical steps and are often integrated with pre-processing and mechanical treatment [
12,
23]. The pre-processing includes steps of discharging, sorting, and classifications that do not alter the battery cells structure while mechanical, thermal, and physical processing involves dismantling, crushing, sieving, magnetic separation, flotation and others, which are used to liberate, classify, and concentrate the materials without altering their chemistry [
24]. The pre-processing of spent LIBs generates fractions called “black mass, BM”, which is composed of ~40–50% graphite, ~45% active materials, NMC, in the form of Li(Ni
x, Mn
y, Co
(1−x−y))O
2, and traces of Cu, Al, F, etc. [
25,
26,
27].
In the pyrometallurgical process, the black mass is subjected to smelting reduction at high temperatures to recover Co, Ni, and Mn in the form of a metal alloy [
28,
29]. The recovered alloy can be used as a master alloy for producing special steels or it can be further processed to hydrometallurgy treatment to extract Co, Ni, and Mn in a pure state for new LIB production. Although the recycling of spent LIBs by pyrometallurgical routes is characterised by flexibility for different types and sizes of batteries and is able to achieve profits from the recovery of Co, Ni, and Cu at a large industrial scale, it suffers from the loss of Li and Al and partially Mn to the slag and the burning of graphite [
30,
31]. Moreover, only part of the graphite is used in the reduction of metal oxides, meanwhile, the rest is burnt and generates CO
2 or is entrapped in the slag. Recent newly developed concepts, based on a pyrometallurgical approach, called InduRed [
32] and InduMelt [
33] showed promising results in the recovery of Li. Most of Li is recovered in the gas phase and collected in the top dust while less than 10% is lost in the slag. In the ReLion pyrometallurgical process developed at SWERIM [
34,
35], it was possible to recover >95% of Co, Ni, and Mn in metal alloys and ~70% of Li, recovered in the flue dust as Li
2CO
3, but still, graphite has not been recovered. In hydrometallurgical processes, the black mass could be subjected to multiple steps of acid leaching, solvent extraction, and precipitation to extract Co, Ni, and Mn from the black mass as corresponding sulfates, which can be further purified and used as the precursors for manufacturing of new LIBs [
27,
28]. However, the presence of high-volume graphite in the black mass restricts the efficiency of the leaching process and makes the process more costly and time-consuming. Although hydrometallurgy is characterised by lower energy consumption compared to pyrometallurgy, the long chain of metals recovery, wastewater generation, and acid consumption are the main drawbacks of hydrometallurgy for spent LIB recycling. The direct recycling process is another promising route to recover the useful components from spent LIBs without high energy consumption or chemicals unlike pyro- and hydrometallurgical routes, respectively, however, the performance of the recycled materials could be affected by the presence of impurities in the long term. In addition, direct recycling has not been demonstrated at a large scale as it required single-cathode input and good control of battery quality [
16,
36].
In this context, the pyrometallurgical processes can perform the simplest, profitable, and highest production routes among other routes on an industrial scale, however, the excess graphite is not only burnt and generates CO
2 emissions but also generates unnecessary extra heat in the reactor and the off-gas system. Additionally, it could affect the full recovery of valuable metals from the black mass (BM) [
31]. Therefore, this paper aims at understanding the smelting behaviour of the BM and the effect of graphite on metal recovery by melting the BM in a carbon-saturated molten bath of hot metal (pig iron) or in a low-carbon molten bath of steel scrap. Currently, there are two mainstream LIBs in the market for almost all EVs; lithium iron phosphate (LFP) batteries and NMC lithium batteries. The paper demonstrates knowledge on the smelting of NMC from spent LIBs and the behaviour of graphite and evaluates the metal recovery and the slag formation in molten iron and steel baths. The trials were executed on a technical scale in view of the results obtained at the lab scale [
34], meanwhile, the results of the current work contributed to the designing and setting of the operating parameters of the pilot-scale trials in EAF, as discussed elsewhere [
35].