1. Introduction
Styrofoam, which is expanded polystyrene foam, is used in containers such as fish boxes (
Figure 1). Its properties, such as its light weight and excellent insulation properties, make it ideal for transporting fresh fish. Although there is limited information on the production volume of fish boxes, according to the Japan Expanded Polystyrene Association (JEPSA), in 2009, the volume of containers for agricultural and marine use was 91,300 tons [
1] and the shipment volume of polystyrene was 141,000 tons [
2] in Japan. Styrofoam for fish and marine products accounts for 55.8% of styrofoam shipments, of which 70% of the containers are used to transport fish. At present, for hygienic purposes, fish boxes are used only once, thus generating a large volume of discarded fish boxes in Japan. For this reason, in large-scale markets, such as the Tokyo Metropolitan Central Wholesale Market, devices are installed to compress the fish boxes into plastic ingots. As fish boxes are used only for fresh food transportation, they are free of harmful substances and are suitable for recycling. However, fish-like odors limit the ingots to low-grade recycling. The ingots are only used as fuel in thermal recycling or as admixture in the production of styrene products for building materials [
3,
4]. In recent years in Japan, the remaining ingots have been exported to China and other Asian countries. However, China banned the import of plastic waste in December 2017 [
5]. Some waste plastics have been exported to other Asian countries, but they are also banning the import of plastic waste. Hence, a recycling process for waste fish boxes in Japan is urgently required.
One of the factors hindering the material recycling of discarded fish boxes is the fish-like odor. Fish boxes are manufactured by filling polystyrene beads treated with a foaming agent into a mold and expanding the foaming agent under high-pressure and high-temperature conditions, creating aggregate cells of expanded beads welded together. The fish-like odor permeates and adheres to the gap between the cells, and it is difficult to remove it with water. Methods for deodorization of fish boxes before recycling include a neutralization method using a dilute hydrochloric acid solution and a masking method using limonene extracted from citrus peel. However, these methods have drawbacks, such as the necessity of treating the used solution with waste liquid, and no practical method has been established.
The smell is caused by the reduction of trimethylamine oxide ((CH
3)
3NO) by bacteria, producing trimethylamine ((CH
3)
3N) [
6]. Trimethylamine is an alkaline volatile substance and can be removed by baking or neutralization with an acid. Notably, it is soluble in organic solvents. In addition, various other fats and oils in fish also cause an odor when oxidized.
At present, the following techniques are used for treating waste styrofoam: (1) styrofoam oiling equipment (equipment for producing styrene oil from styrofoam), (2) desalting and volume reduction technology using heated waste vegetable oil, (3) a melting treatment with an organic solvent, and (4) processing using a shear compression crusher.
The first method uses direct heat to convert styrofoam to styrene oil [
7] and is used in Japan to treat styrofoam waste found drifting along the coast. There are pilot projects involving this method in Japan such as Tsushima Island in Nagasaki Prefecture and Hatoma Island in Okinawa Prefecture.
The second method, also used for treating styrofoam waste drifting on the coast, is a technique for desalting and reducing the volume using heated vegetable oil. Kawahara et al. [
8] of the National Institute of Technology, Oshima College, reported that the volume could be reduced to approximately 1/50th and can be solidified to produce plastic ingots. This volume reduction method, called the Oshima College method (OCMT), uses waste vegetable oil as a solvent [
9]. Styrofoam is heated to 160–200 °C with oil as a solvent to expel the air in styrofoam. By heating, the trapped air expands, and the structure of the styrene foam also becomes soft simultaneously, which causes the air to be discharged to the outside. The cells of styrofoam are destroyed and the volume is reduced (
Figure 2 and
Figure 3). In addition, it was reported that the salt attached to the surface of the polystyrene foam was desorbed, and a desalting effect was observed [
8].
The third method is to dissolve styrofoam into an organic solvent at room temperature [
10] and is used for processing food containers in retail stores. However, the organic solvent used requires additional waste treatment.
The fourth method is a compression crusher and is used in wholesale markets to reduce the volume of containers transporting farm and marine products. One example of a compressor is STYROS, from Japanese company (ELCOM Co., Ltd., Sapporo City, JAPAN)[
11]. The cells in the polystyrene foam are broken with heat from compression, and polystyrene foam melts and reduces volume; however, the attached fish-like odor does not evaporate and stays in the polystyrene ingot. This makes it difficult to remove the components with a fish-like odor further downstream in the waste treatment process.
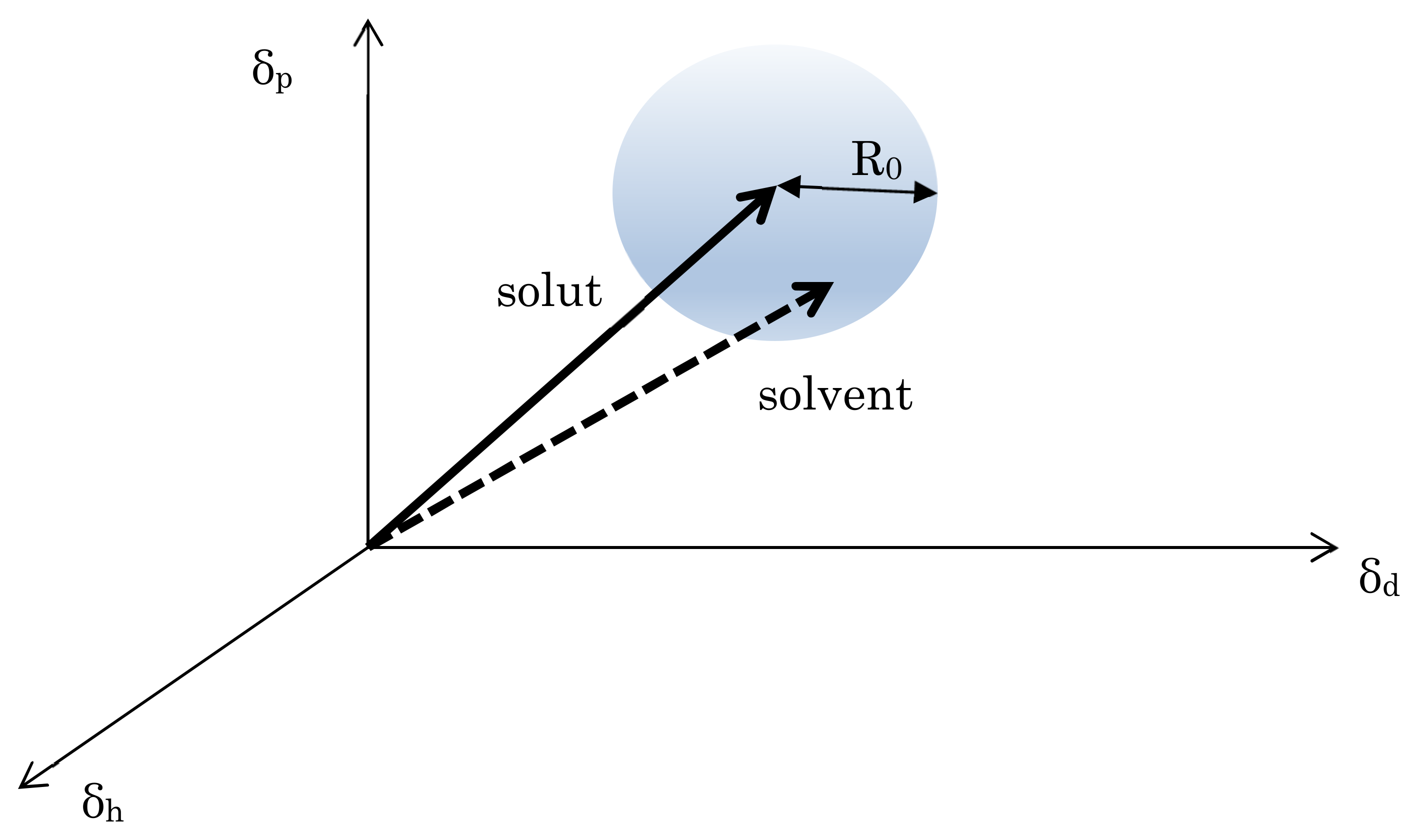
In this study, we investigated the solubility of trimethylamine in vegetable oil using the Hansen solubility parameter (HSP) to confirm whether OCMT can remove the fish-like odor. It is known that trimethylamine is soluble in various organic solvents, such as toluene, but its solubility in vegetable oil is not known. If trimethylamine can be easily desorbed from the styrofoam surface using vegetable oil, OCMT performs a deodorizing function in addition to volume reduction and desalination. To our knowledge, there have been no reports on methods for deodorizing trimethylamine or dimethyl sulfide from polystyrene. Although there is a report on the deodorizing effect of Eriobotrya Japonica seed oil [
12], there is no literature on the solubility of trimethylamine and dimethyl sulfide in vegetable oils.
5. Conclusions
In this study, the results obtained using HSPs predict that trimethylamine and dimethyl sulfide can be dissolved in vegetable oil. These results show that OCMT is able to perform the function of deodorizing waste styrofoam found on the coast and used for fish boxes in wholesale markets. This is in addition to desorbing the salt attached to the surface of the polystyrene foam. To confirm the results of this paper, experimental data are needed to ensure the evaluation values by calculation. The planning and preparing for such an experiment shall be conducted in a further study.
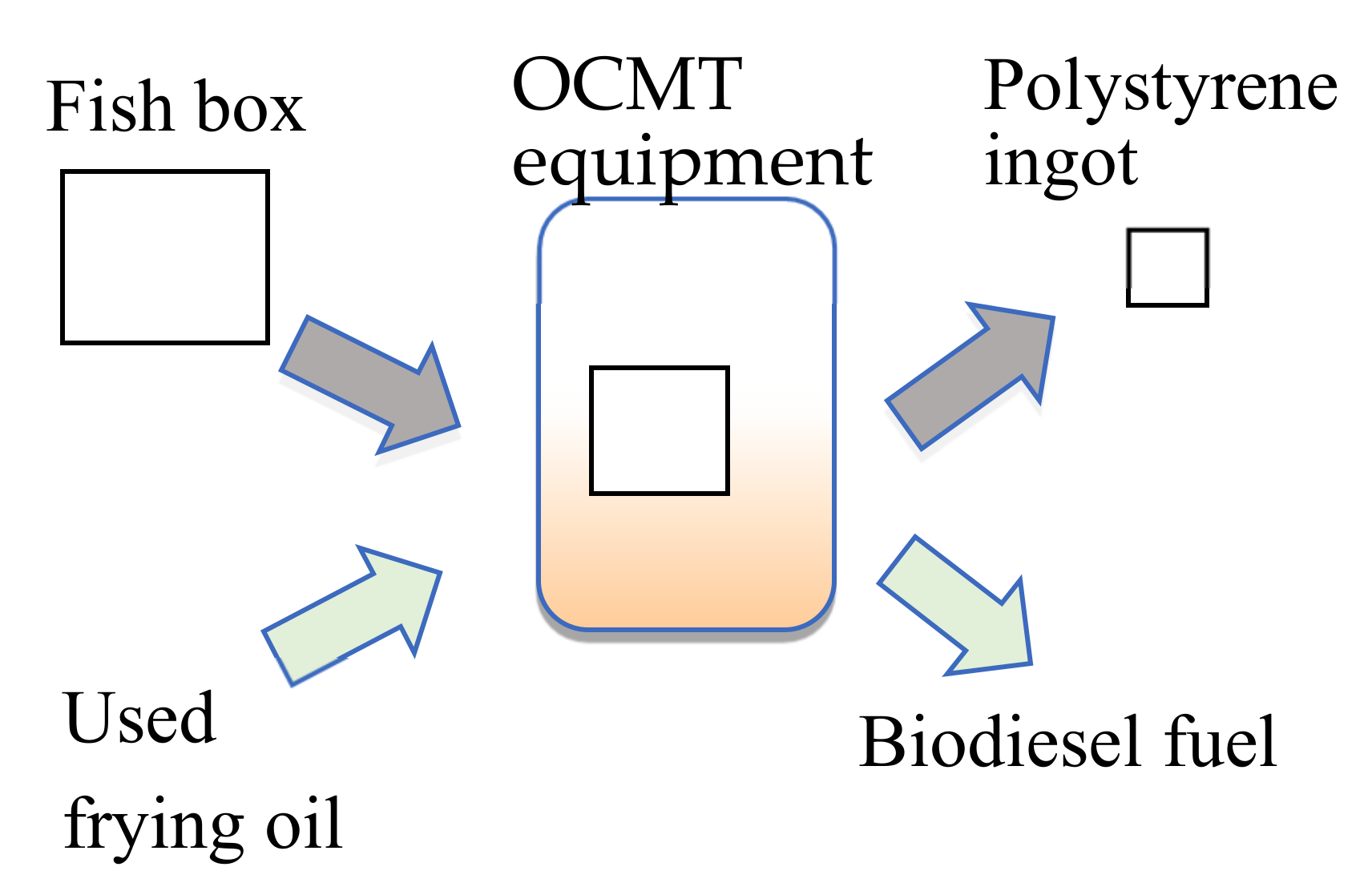
Furthermore, the deodorized fish boxes are more likely to be recycled into high-grade raw materials, and the vegetable oil used as a solvent can be converted into biodiesel fuel. In the future, as shown in
Figure 5, it will be possible to construct a closed system that does not generate waste liquid. Regarding concerns about the effects of amines from trimethylamine in biodiesel oil, there are cases where amines are used as antioxidants for biodiesel [
17], and it is thought that mixing of amines is not a problem.
In addition, a large amount of waste can be found drifting on the shores of the islands facing the East China Sea, such as Tsushima in Nagasaki, Japan. Most of this waste is styrofoam-based, and if the proposed process is realized, it is thought that it can contribute to the treatment of this waste. The cause of the sea-like odor attached to the drifting polystyrene foam is dimethyl sulfide, and it is necessary to experimentally evaluate its deodorizing effect in the future. This will open the possibility of high-quality recycling for drifting styrofoam, which will contribute to the environmental protection of the oceans and coastlines.