Abstract
This study investigates the performance of biodiesel produced from distilled waste tire pyrolytic oil through transesterification as a lubricant additive for aqueous drilling fluid systems. Aqueous-based drilling fluids have a high coefficient of friction as compared to oil-based drilling fluids. The inclusion of a biodiesel additive was for smooth application/operation. The friction-reducing physicochemical properties of the additive were analyzed and compared with the guidelinesof the United States specification (ASTM Standard) and the European specification (EN Standard). The chemical structure of the produced biodiesel was analyzed using gas chromatography–mass spectrometry (GC-MS). The results show that the distilled waste tire pyrolytic oil contains aliphatic, naphthenic, and aromatic hydrocarbons. The free fatty acid value reduced from 5.6% (for pyrolytic oil) to 0.64% after the transesterification process. A saponification value of 203.36 mg/g was recorded for the pyrolytic oil, and this value was also reduced to 197.35 mg/g after the transesterification process. The kinematic viscosity was reduced from 11.2 to 5.3 mm2/s for the obtained biodiesel, and this value is within the ASTM D6751 and EN 14214 standard values (1.9 to 6 and 3.5 to 5 mm2/s, respectively). The cetane number (47.75) was obtained for the biodiesel, and this is within the minimum range stipulated in ASTM D6751 guidelines. The produced biodiesel’s chemical structure analysis using GC-MS shows that it comprises of decanoic acid methyl ester and methyl ester. Furthermore, comparative analysis of the quantified friction-reducing physicochemical properties of the additive shows that the biodiesel produced from the distilled pyrolytic oil is a suitable additive for the improved lubrication of the friction-prone metallic parts of drill bits when water-based drilling fluids are employed for drilling oil and gas wells.
1. Introduction
The success of hydrocarbon-well drilling operations depends on a variety of factors, but one of the most important factors, often controllable is the drilling fluid system used for the operation. During drilling, many problems related to drilling fluid properties arise, and they include a slow drilling rate or excessive drill pipe torque and drag, which merely render the drilling operation less efficient, causing non-productive time (NPT). The cost associated with drilling operation NPT can be extraordinary [1]. Practically, there are no accurate, uniform vertical holes, because the rotating drill pipes (flexible in nature) are held in different points along the wellbore. The friction resistance produced during these scenarios may require significantly, more torque than is necessary for turning the drill bit [2]. Similarly, significant friction can occur when raising and lowering the drill strings (tripping in and out of a hole), a problem called drag. Under certain conditions (substantial deviation, being under gauge holes, or the dynamics of the drill string being weak), the torque and drag can be enormous, leading to an unacceptable loss [3]. Adding a small amount of lubricant to the sludge can cut this energy loss, and it is common engineering practice to minimize friction by placing a layer of oil or grease between moving metal parts. When drilling, the practical way of freeing a stuck pipe is to spot oil around the stuck section. The capillary pressure of the oil on the aqueous filter cake runs into thousands of pounds, so the pressure of the mud column on the filter cake’s surface compresses it and reduces the contact angle [3].
Lubricants are added as additives to the drilling fluid to increase the effect of lubrication and reduce friction. High-lubricity drilling fluids can also increase the penetration rate during drilling, resulting in significant cost savings. Poor lubrication, however, can lead to wear on the drill bearing, drag, problems of torque, and differential adhesion [4]. Lubricants are mainly applied to aqueous-based drilling fluids due to inadequate lubricity properties. Aqueous-based drilling fluid is often preferred in drilling operations because it is non-toxic, relatively inexpensive, and biodegradable [5]. However, if these drilling fluid systems do not meet the required lubrication, the synthetic-based mud system (SBM), which is an oil-based mud system, will be used instead of the aqueous mud system. Aqueous-based drilling fluids possess a very high coefficient of friction when compared to oil-based mud, and this can be reduced by adding lubricating additives [6]. Liquid lubricants show enhanced performance when compared to solid lubricants in deep wells [7]. A small quantity of lubricant is adequate to provide effective lubricity for an aqueous drilling fluid system. According to Teng et al. [8], “one percent volume of lubricant in an aqueous mud system can reduce the torque during drilling operations by 20 percent”. Lubricating additives are obtained from fatty acids, vegetable oils, triglycerides, petroleum-based oil, polypropylene glycol, and much more.
Waste tire pyrolytic oil is composed, essentially, of hydrocarbons with 5–20 carbon atoms, hetero-compounds and aromatic fractions [9]. The hetero-compounds contain chlorine, nitrogen, oxygen, fluorine, and sulfur due to the initial tire composition [10,11]. The aromatic fraction constitutes mainly single-ring alkylbenzenes and polycyclic compounds, such as indene and naphthalene derivatives [12]. The elemental composition of the pyrolytic oil is also dependent on the heating rate, tire type, pyrolysis reactor type, particle size, and pressure [13,14]. Nevertheless, most of the oils produced by pyrolysis processes require an extra improvement stage before being used directly or as a feedstock. According to Breeden and Meyer [15], “in addition to recycling, lubricant formulations containing feedstocks that are obtained from the by-products of other processes will make an important contribution to art and economics”. Genuyt et al. [16] proposed that branched-chain ester-based oils are suitable as lubricants. The waste tire pyrolytic oil can be transesterified to produce an appropriate ester-based oil that will serve as an aqueous-based drilling fluid lubricant additive [17]. Mikulski et al. [18] also highlighted the advantage of utilizing pyrolytic oils from waste products such as plastics and rubbers.
There is consensus in the literature that many waste materials can be used as feedstocks for other processes, by recovering their active and chemical components, avoiding the environmental and human health problems associated with improper disposal. However, waste streams usually consist of several components, of which only a few are valuable materials. If there is a positive balance between usability and availability, the required component can be economically recovered for use as a raw material [19]. Thus, this study investigates transesterified waste tire pyrolytic oil’s suitability as a lubricant additive for aqueous-based drilling fluid. Pyrolysis has been a target technology for thermal conversion from residues into valuable products due to operation and control. The liquid fraction derived from waste tire pyrolysis (pyrolytic oil) was further treated and analyzed to achieve the objective of this study. The influence of the functional groups, characterized by Fourier Transform Infrared Spectroscopy (FTIR), and the molecular structure, characterized by gas chromatography coupled with mass spectroscopy (GC-MS), on lubricating properties was investigated.
2. Results
2.1. Pyrolytic Oil Chemical Content Analysis
Hentschel [20] and Minami [21] highlighted that the chemical structure of the components of an oil exerts a profound influence on its physical and chemical characteristics. Thus, the identified compound structures from the waste tire pyrolytic oil are tabulated as Table 1. Table 1 shows that the pyrolytic oil from the waste tire pyrolysis process contains more benzene, limonene, butene, indene, tetradecance, cyclohexane, propenal, naphthalene, bicyclo, acorenol, and pentadecane and the presence of sulfur. The result shows that the waste tire pyrolytic oil is made up of saturated and unsaturated compounds with sulfur presence. It is necessary to consider this trend when using these saturated and unsaturated compounds as feedstock for producing lubricant additives with the appropriate viscosity. The sulfur content was reduced using the distillation process as proposed by Tsietsi et al. [22].

Table 1.
Identified compounds and their structures.
2.2. Distilled Pyrolytic Oil and Biodiesel Analysis
The distilled pyrolytic oil functional groups were analyzed to identify the functional groups present, as this would help to determine the possibility of the pyrolytic oil corroding any contact surfaces and the effect of a functional group on the lubricant additive properties. Absorption measurements in the infrared region were obtained by FTIR analysis using a spectrophotometer. Table 2 shows the different functional groups present in the distilled pyrolytic oil.

Table 2.
Pyrolytic oil functional groups.
The results show the different absorption energy bands of various functional groups/compounds depending on their molecular structure. An average of twelve (12) functional groups was identified during the analysis by the molecular vibrations inferred by the surrounding groups, and these are similar to the results obtained by Dos Santos et al. [23]. These functional groups are responsible for the oil sample’s chemical reactions. Tsyurupa et al. [24] and Xiao et al. [25] highlighted that a compound’s specific properties can be improved by multiple functional groups. Table 2 shows the presence of both saturated and unsaturated compounds from the group frequencies. Other compounds such as carbonyls, nitrogen, and alcohols were also identified in the sample oil analysis. The different vibrations of the various functional groups in the molecule gave rise to bands of differing intensity. The most intense band in the spectrum tabulated in Table 2 is at 3420.15 and 3218.15 cm−1, which could be due to the stretching of the nitrogen and -OH bond. In any sample where hydrogen bonding occurs, the number and strength of intermolecular interactions vary significantly within the sample, causing the bands to be broad [26]. On the other hand, 750.62 cm−1 had the weakest bands in the spectrum, and this can be attributed to the carbon–carbon bonds. When intermolecular interactions are weak, the number of chemical environments is small, and narrow infrared bands are observed.
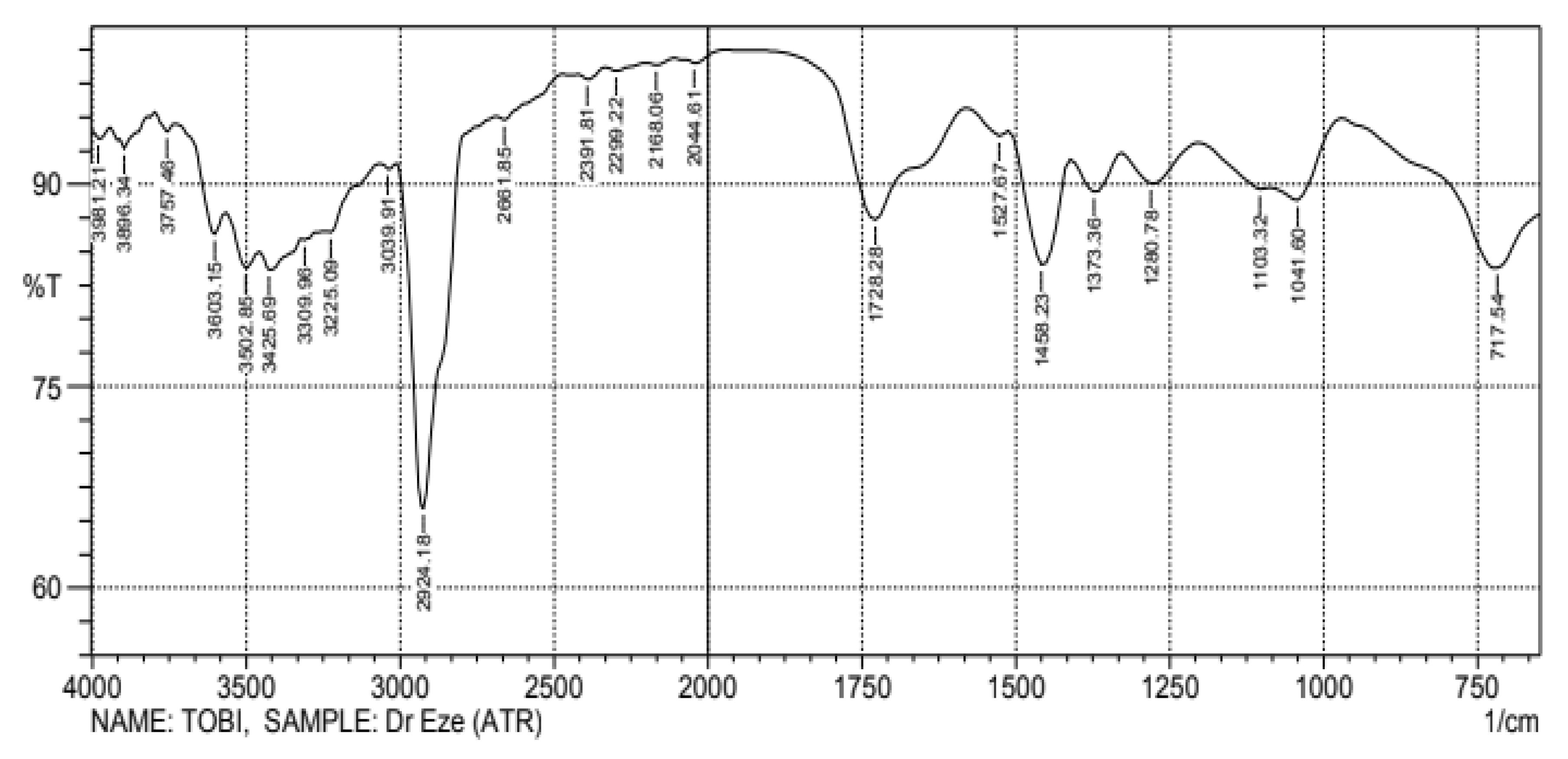
Figure 1 shows the relative peak intensities and absorption location of the compounds present in the produced biodiesel. Using the wavenumber, the identified compounds’ electromagnetic spectrum can be categorized as Mid to Near infrared using the relationship between the frequency, wavelength, and wavenumber [27]. The peak intensity in the infrared spectra during compound identification can be influenced by the concentration of molecules in the sample. The absorptivity is an absolute measure of infrared absorbance intensity for a specific molecule at a specific wavenumber. Thus, two peaks may have different intensities when considering a mixture because there are molecules present in different concentrations. Three distinct bonds were found within the fingerprint region (1200 to 700 cm−1). This region identified some vibrations such as C-H bend (aromatic) and C-O stretches for the produced biodiesel. The biodiesel infrared spectrum had more C-O, C-H, C=O, C=C, =C-H, O-H, and M-H stretches compared to the distilled waste tire pyrolytic oil spectrum. The 1280 cm−1 band was due to C-O stretching, and the 1373, 1458, and 1728.28 cm−1 bands were due to C-H, C=C, and C=O stretching. The 3603.15 cm−1 band corresponded to alcohol or phenol (O-H).
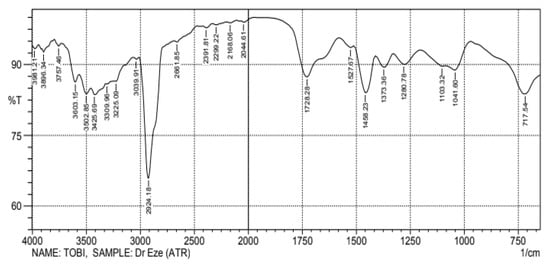
Figure 1.
Produced biodiesel FTIR analysis for functional groups.
2.3. Produced Biodiesel and Distilled Waste Tire Pyrolytic Oil Physicochemical Properties
Studies on the transesterification of waste tire pyrolytic oil are few [28], but the reaction is necessary for ease of application in the formulation of an aqueous drilling fluid system without any secondary emulsifier/surfactant. Lubricant additives are added to the aqueous drilling fluid to reduce friction and torque between the drilling pipes and the rock formations. Table 3 shows the distilled waste tire pyrolytic oil and biodiesel physicochemical properties and the standard test methods adopted for this study. According to Giakoumis and Sarakatsanis [29], the peculiar aspect of biodiesels is that they are produced from various feedstocks possessing different chemical compositions. Physicochemical property tests are integral to the verification of the produced biodiesel oil’s suitability as a lubricant additive.

Table 3.
Physicochemical analysis of distilled pyrolytic and biodiesel oil samples.
3. Discussions
Table 3 shows that the acid and free fatty acid values for the distilled pyrolytic oil were higher than those for the produced biodiesel. The acid value shows how well the esterification reaction was followed using chemical analysis, and an acid value of <3 mg KOH/g is deemed to indicate full esterification [30]. Thus, the value of 1.27 mg KOH/g obtained in this study for the produced biodiesel is a good fit. The free fatty acid is the percentage by weight of the uncombined fatty acid in the oil sample. The free fatty acids in the distilled pyrolytic oil underwent transformation that changed the degree of unsaturation and the carbon chain’s average length. The free fatty acid value was reduced from 5.6% (for pyrolytic oil) to 0.64% after the transesterification process, and this trend was also observed by Wahyudi et al. [31]. This shows that fatty acid structure influences oil properties, and Kilonzi et al. [32] observed that free fatty acids can lead to corrosion.
The distilled pyrolytic oil’s saponification value was 203.36 mg/g, and this value reduced to 197.35 mg/g after the transesterification process. The saponification value pertains to all fatty acids present in the oil sample, and this reduction can be attributed to the observed fatty acid value reduction after the transesterification process. According to Gopinath et al. [33], its value depends on the molecular weight and the percentage concentration of the fatty acid components present in the oil. The iodine value of the pyrolytic oil also reduced from 137.05 to 93.91 g of iodine/100 g after transesterification. The iodine value is a measure of the unsaturation of oils, fats, and fatty acid derivatives, and the required limit is 120 g of iodine/100 g according to standard EN 14214. The reduction trend is because of a decrease in the degree of unsaturation in the oil after the transesterification process.
Both oil samples have approximately equal specific gravity, but the variation in density values at ambient temperature can be attributed to the unsaturation degree. Demirbas and Al-Ghamdi [34] studied the relationship between the specific gravities and higher heating values (HHVs) of petroleum components, and they observed that an increase in specific gravity decreases the HHVs and that the decrease is highly regular. This study recorded an HHV value of 52.83, corresponding to a produced biodiesel specific gravity of 0.8039. Distilled pyrolytic oil has a high density and free fatty acid content (0.927 g/cm3 and 5.6%, respectively) compared to the produced biodiesel (0.884 g/cm3 and 0.64%, respectively). Thus, the higher the oil sample’s unsaturation degree, the higher the density [35,36].
A biodiesel yield of 56.83% was obtained from the distilled waste tire pyrolytic oil under the experimental conditions (transesterification process), with a molecular weight of 851.42. This yield is greater than the yields realized for some seed crude oil extractions [37,38]. An API gravity of 25.04 was recorded for the produced biodiesel, and this shows that the biodiesel is a light oil that floats on water (API gravity < 10 indicates light oil). Umar et al. [38] also obtained an API gravity of 27.7, and the API gravity essentially measures the relative density of oil and water. It is primarily used to evaluate and contrast the relative densities of petroleum liquids. The boiling, fire, and flash points of the produced biodiesel are within the U.S. specification (ASTM Standard) and the European specification (EN Standard). The cloud and pour points of the distilled waste tire pyrolytic oil were below the standard guideline ranges, but these values improved for the produced biodiesel (−3 and −15, respectively) and are within the standard guidelines (−3 to −12 and −15 to −16, respectively). The cloud point provides a rough idea of the temperature above which the oil can be safely handled without wax coagulation, which causes filter clogging. The pour point of the oil is the lowest temperature at which the oil is observed to flow under the test conditions.
The kinematic viscosity of the biodiesel was measured using ASTM D445, and it was observed that the kinematic viscosity of the distilled pyrolytic oil reduced from 11.2 to 5.3 mm2/s for the production of the biodiesel; the biodiesel kinematic viscosity value is within the ASTM D6751 and EN 14214 standard values (1.9 to 6 and 3.5 to 5 mm2/s, respectively). The kinematic viscosity is the ratio of the viscosity of a fluid to its density. The kinematic viscosity, according to Phankosol and Krisnangkura [39], is an essential physical property of biodiesel. From the literature, typical immersion oils have a refractive index of 1.51 and a dispersion similar to glass coverslips. Light rays passing through the specimen encounter a homogeneous medium between the coverslip and immersion oil, and they are not refracted as they enter the lens but only as they leave its upper surface [40]. The refractive index of both oil samples are with the range stated in the literature (1.62 for pyrolytic oil and 1.56 for produced biodiesel). The calorific value of the produced biodiesel is 51.19 kJ/kg. It was observed that this calorific value in this study was higher than the biodiesel calorific values reviewed by Ozcanli et al. [41] for alkali-catalyst-transesterified biodiesels (the calorific range was between 36.50 for Maclura pomifera seed oil and 45.30 for non-edible neem oil). The produced biodiesel in this study has a cetane number of 47.75, and this number measures the combustion quality of the produced biodiesel. The obtained value is within the minimum range stipulated in the ASTM D6751 guidelines. Ramirez-Verduzco et al. [42] observed that cetane number and higher heating value have a linear inter-relationship with molecular weight, that is, high molecular weights contribute to an increase in cetane number and higher heating value.
Torque and drag are often associated with drilling challenges, such as stuck pipes and sticking; the addition of lubricant additives in the drilling fluid system is used in addressing this drilling instability. The coefficient of rolling friction is an indication of how excellent the rolling resistance is for a given normal force between the drill strings and the subsurface formation upon which it is rolling [43]. The coefficient of rolling friction value for the produced biodiesel is 0.010, and it was determined experimentally. Rolling friction is commonly associated with deformation of the rolling drill strings, particularly on the soft subsurface formation [44]. According to Blau [45], the coefficient of friction is the ratio of two forces acting, respectively, perpendicular and parallel to an interface between two bodies under relative motion or impending relative motion; this dimensionless quantity turns out to be convenient for depicting the relative ease with which materials slide over one another under particular circumstances. Mills [46] highlighted that the values of static and dynamic coefficients of friction for common materials are 0.3 and 0.6. However, these values can drop to about 0.15 when oil is added as a lubricant. Esan et al. [47] highlighted the chemical versatility of biodiesel and its application in various industries. Still, the produced biodiesel in this study is suitable as a lubricant additive employed in the formulation of drilling fluids to reduce issues of torque and drag during the drilling of oil and gas hydrocarbons. Friction resistance to the rotation of the drill string is called torque, and one efficient way, according to Dong et al. [48], of dealing with torque is to add lubricant in water-based drilling fluid. Water-based drilling fluids possess a very high coefficient of friction as compared to oil-based drilling fluids. This high coefficient of friction can be reduced by adding lubricating additives to the water-based mud system. Thus, the proposed biodiesel from the waste tire pyrolytic oil is formed.
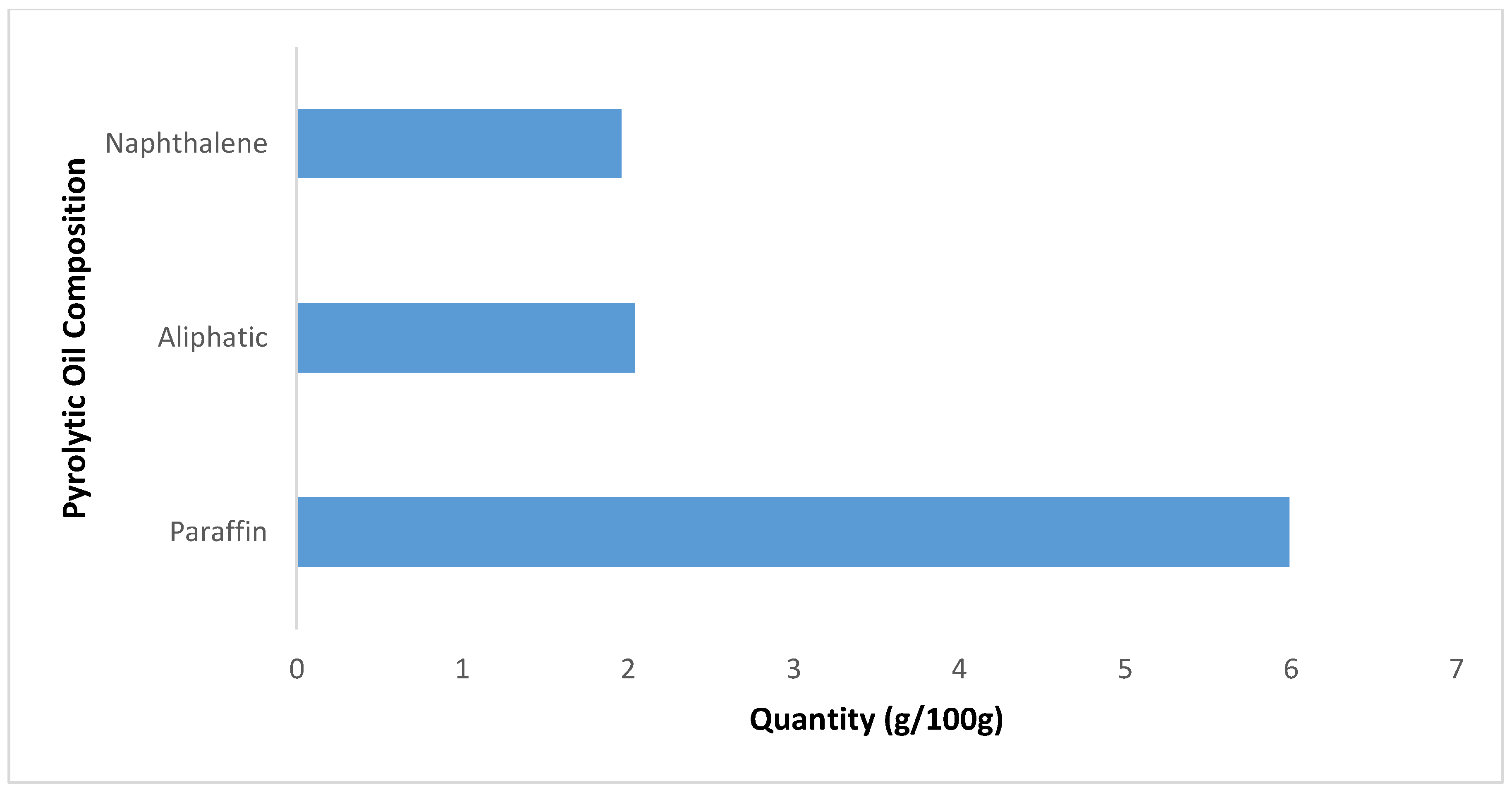
The biodiesel’s chemical properties were analyzed, as it has been identified as a required component for a good lubricant additive. Knox and Jiang [7] emphasized the importance of considering the chemical families when selecting the right lubricant for a water-based mud system. Literature has shown that a lubricating oil’s chemical structure consists of a mixture of aliphatic, naphthenic, and aromatic hydrocarbons in various proportions depending upon the nature and source of the oil [49]. Figure 2 shows the composition of aliphatic, naphthenic, and aromatic hydrocarbons in the distilled waste tire pyrolytic oil. Gas chromatography analysis was adopted to analyze the distilled waste tire oil sample; the paraffin and cyclic hydrocarbons were identified using whole oil-gas chromatography analysis. It is thought that a lubricating oil should contain a certain proportion of unsaturated hydrocarbons that it is compatible with [50].
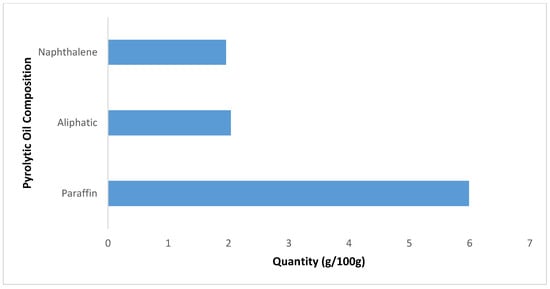
Figure 2.
Waste tire pyrolytic oil hydrocarbon compositions.
The produced biodiesel’s chemical structure was analyzed using gas chromatography–mass spectrometry (GC-MS). The analysis shows the presence of decanoic acid methyl ester and methyl ester. The GC-MS analysis results in this study are similar to what was obtained by Maulidiyah et al. [51] when they analyzed their biodiesel’s composition. Decanoic acid methyl ester is an ester form of decanoic acid, and it has been studied as a single-component biodiesel surrogate by Wang and Oehlschlaeger [52]. This ester is much lighter than the basic components of real biodiesel, which significantly facilitates its handling. Shettigaar et al. [53], in their study on eco-friendly lubricants for water-based drilling fluids, observed that lubricating oil properties depend upon the functional moieties present in the oil. They highlighted that oxygen-carrying moieties enhance the lubricity and film strength of the additive. Additionally, Uchoa et al. [54] observed that fatty compounds have better lubrication due to oxygen atoms, which provides polarity to the molecule and, consequently, adhesion to the metal surface; these oxygen-carrying moieties are present in the produced biodiesel.
The comparative analysis of several valuable friction-reducing physicochemical properties, including the specific gravity, acid value, iodine value, peroxide value, free fatty acids, low pour point, high flash point, kinematic viscosity, refractive index, and so on (Table 3), indicated that the biodiesel obtained from distilled pyrolytic oil is a suitable lubricant additive for water-based drilling fluid.
Economic Cost Analysis
This section focuses on obtaining useful information for the economic cost assessment of the production process for biodiesel using waste tire pyrolytic oil. It is a suitable indicator for the future development of biodiesel to reduce feedstock oil costs and, at the same time, increase the yield of valuable products with adequate plant capacity. The main obstacle to biodiesel’s commercialization compared to that of petroleum-based diesel is its high production costs, mainly due to the costs of raw materials. Economic considerations are an essential driver for the development of profitable raw materials and processes for biodiesel production. This study evaluates operating costs of using waste tire pyrolytic oil in a pilot-scale plant with a capacity of 5000 tons/year, and the biodiesel production expenses for the components considered are tabulated in Table 4. The result shows that most of the cost is associated with feedstock and supplies. The low cost of the pyrolytic oil used as feedstock shows that the proposed plant is viable compared to the propositions of other authors [55,56].

Table 4.
Biodiesel production expenses for the proposed plant.
4. Methods and Materials
4.1. Materials
The waste tire used in this study was collected from a landfill in the South-West region of Nigeria. The tires were cleaned to remove debris, and the wireless sidewalls of the used tires were cut into 10 to 20 mm dimensions in the Engineering Workshop at the College of Engineering, Covenant University. These waste tire samples were further used as feedstocks in the already-existing pyrolytic reactor in the department for the degradation of the weighty polymeric compounds in the waste tire. In most of the tire formulations, the elastomer is composed of natural rubber, synthetic rubbers, or blends [57,58].
4.2. Methods
Pyrolysis of Selected Waste Tire
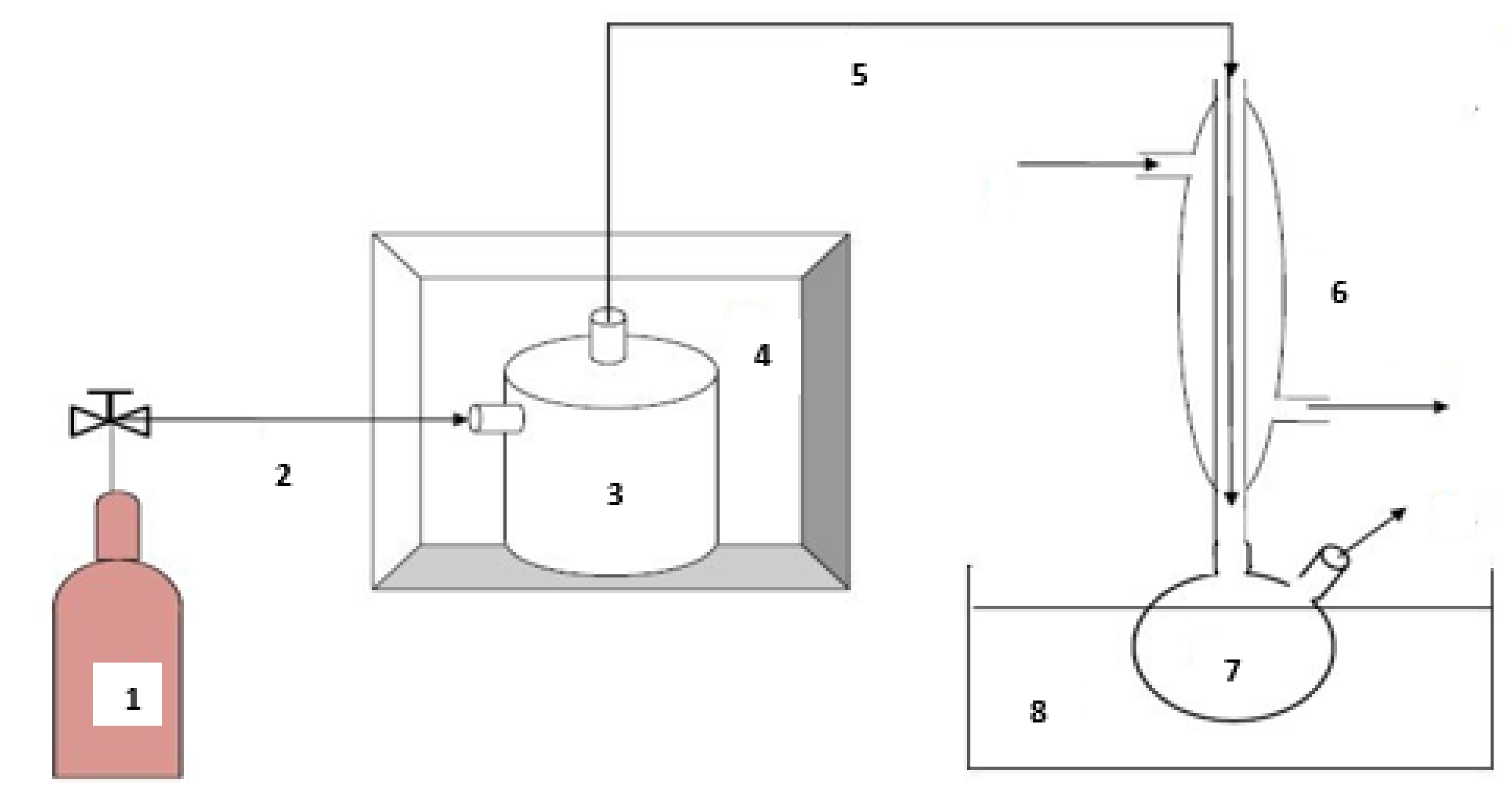
Pyrolysis is represented by molecular thermal cracking, which begins at a temperature of about 500 °C, which continues to increase above 800 °C. A high temperature is necessary to accelerate the chemical reactions inducing the molecular breakdown of large molecules to smaller molecules [59,60]. In this study, the waste tire pyrolysis was carried out in a fixed-bed reactor placed in a muffle furnace (J.P. Selesta, S.A., 582543 Serial number (S/N), 230 VAC, 00-C/2000367, 50/60 Hz, 3500 W, Barcelona, Spain) with a Pressure Integrated Differential (PID) temperature controller, which was attached to the heat source. The muffle furnace was powered electrically, and its temperature can reach up to 1100 °C. Figure 3 shows the pyrolysis process scheme for producing the waste tire pyrolytic oil.
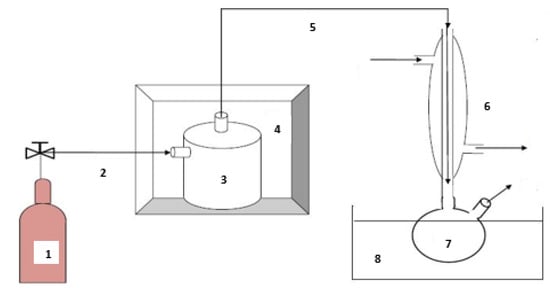
Figure 3.
Waste tire pyrolysis schematic [9]: 1—Nitrogen bottle; 2—Nitrogen inlet; 3—Fixed bed reactor; 4—Muffle furnace; 5—Vapor outlet; 6—Reflux condenser; 7—Pyrolytic oil collector; 8—Ice bath.
Nitrogen gas was used to provide an inert atmosphere and acts as a carrier gas during pyrolysis. After transferring the sample into the reactor, the system was cleaned with nitrogen gas to remove air from the system before pyrolysis. To facilitate the passage of gaseous matter in the reactor, the reactor was not completely filled up with the waste tire. After turning on the furnace’s heat source, the temperature controller was programmed to the specified value of 860 °C as the operating temperature, with a heat transfer rate of 18 °C/min. When the process had reached its set temperature, the reactor temperature was kept constant for 20 min and then cooled to 20 °C. This was to ensure that all possible liquid and vapor exited the reactor and reached the collection unit. The collection unit was removed from the setup, and the pyrolytic oil was collected and measured. In the process, solid, liquid, and gaseous products are formed. The gas phase was recovered by partial condensation, and the solid phase was stored for utilization as a potential absorbent material.
The liquid phase, termed pyrolytic oil, which is the main focus of this study, was further analyzed to determine the chemical composition and its properties.
4.3. Pyrolytic Oil Analysis
The collected liquid fraction from tire pyrolysis (pyrolytic oil) has a dark brown coloration and a strong odor. Literature has shown that pyrolytic oil is a multi-component liquid mixture possibly made up of heterocompounds, both aromatic and aliphatic compounds [61,62]. Most of the individual compounds in waste tire pyrolysis oils can be identified and quantified using conventional analytical methods [63]. Gas chromatography coupled with mass spectroscopy (GC-MS, Agilent, Santa Clara, CA, USA) and FTIR (SHIMADZY, Kyoto, Japan) were used to analyze the pyrolytic oil’s key compounds. Literature has identified sulfur as one of the heterocompounds present in tire pyrolytic oil, and it can exist in different chemical and oxidation states [64].
Sulfur compounds are undesirable due to the negative environmental impact associated with them; thus, it is a good idea to remove all possible traces of sulfur in the pyrolytic oil before its utilization as feedstock.
4.4. Distillation of the Waste Tire Pyrolytic Oil
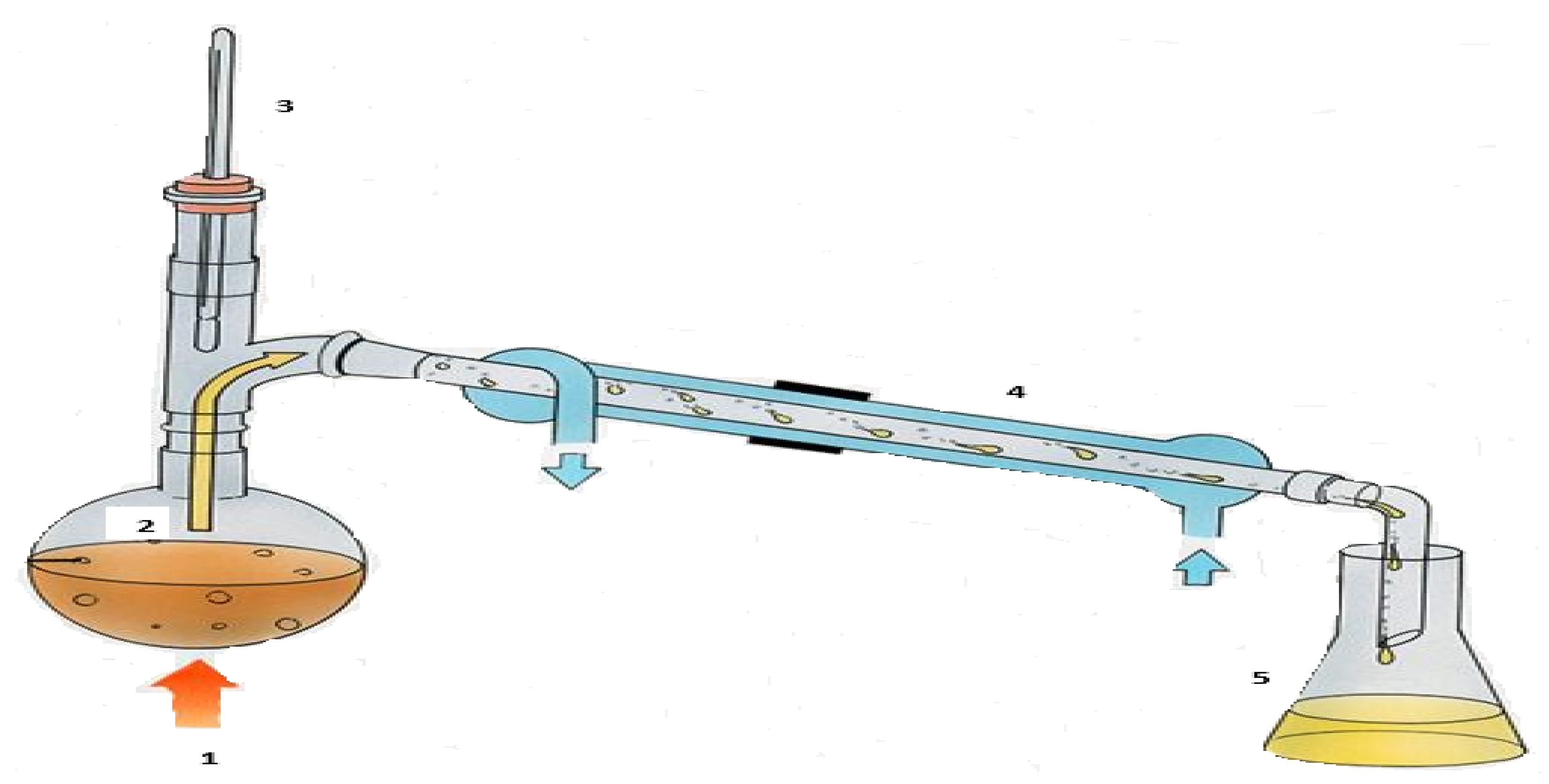
Hester and Harrison [65] observed that mercaptans, sulfides, and di-sulfides are typical sulfur compounds present in most crude tire-derived oils, because they contain cross-linked sulfur compounds. Their study further highlighted that the boiling point ranges of mercaptans, sulfides, and di-sulfides are 6.2–98.46 °C, 60.7–185 °C, and 46.3–193.5 °C, respectively. Tsietsi et al. [22], in the discussion of their results, emphasized the need to reduce the total sulfur content of pyrolytic oil. In this study, the pyrolytic oil collected was further distilled at a temperature of 205 °C, which is above the boiling point range of the three possible sulfur compounds present in the pyrolytic oil (Figure 4).
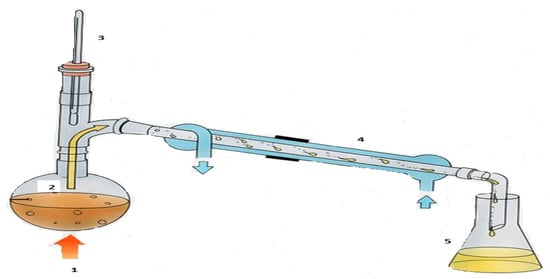
Figure 4.
Waste tire pyrolysis distillation schematic: 1—Heat source; 2—Waste tire pyrolytic oil; 3—Thermometer; 4—Water condenser; 5—Distilled pyrolytic oil.
4.5. Production of Biodiesel from Distilled Pyrolytic Oil as Lubricant Additive
Lubricants are added to drilling fluids as an additive to improve the lubricating effect and minimize friction. Unlike the continuous oil phase in the engine, the lubricants for water-based drilling fluid are emulsified and dispersed in the mud system [48]. Thus, to achieve this feature, the distilled waste tire pyrolytic oil must undergo a transesterification process. Transesterification and esterification are conducted at a temperature of 60 to 110 °C with basic catalysts.
4.5.1. Pre-Treatment of Oil (Esterification)
Sigma-Aldrich methanol (99.8%, J.T. baker, Philipsburg, PA, USA) and concentrated sulfuric acid were used to reduce the amount of impurities properly. Due to the high content of free fatty acids in the oil samples, pretreatment was introduced to improve the oil’s separation into the corresponding esters.
Procedure
1% of the sulfuric acid was mixed with 300 mL of methanol in an Erlenmeyer flask containing 1000 mL sample of oil. The mixture was vigorously stirred at approximately 1000 rpm for 30 min using a magnetic stirrer and heater at 55 °C. The mixture was then poured into a separator funnel for about 12 h. Impurities formed the upper layer, while the treated oil settled on the lower layer. The treated oil was collected and dried at a temperature of 105 °C to remove excess methanol for 2 h.
4.5.2. Transesterification Process
Alcohol and Catalyst Mixture
5 g of sodium hydroxide (99%) pellets were mixed with methanol (300 mL) in a 500 mL Pyrex beaker. Mixing was allowed to continue until all of the sodium hydroxide pellets dissolved in the methanol to form a methoxide solution.
Methyl Ether Mixture
The methoxide solution was mixed with 1000 mL of esterified oil in a beaker, and to obtain a homogeneous mixture, the solution was heated at a temperature of 55 °C for 1 h. The homogenized mixture was added while stirring the oil, as the reaction is initially very slow. Therefore, the stirring of the mixture and the esterified oil increases the reaction rate and conversion to ester.
Biodiesel and Glycerin Separation
The mixture was poured into a separator funnel and allowed to settle for 24 h. When the separation was completed, the two main products, glycerin, and biodiesel, came out of the separation process, with biodiesel at the top and glycerin at the bottom.
Washing
The biodiesel from the separation process was washed with water to remove soap residue and other contaminants. The water was allowed to settle before draining from the mixture. The washed biodiesel was collected and carefully heated in an oven at 105 °C to evaporate possible water and methanol traces in the produced biodiesel.
4.5.3. Mechanism of the Transesterification
The transesterification mechanism consists of a series of reversible sequential reactions in which the triglyceride is gradually converted to diglyceride, monoglyceride, and, finally, glycerol. One mole of an ester is released in each step. These are reversible reactions, although the balance tends towards the products, that is, glycerin and free fatty esters. In the case of the alkali-catalyzed reaction used in this study, the mechanism was formulated in three steps: In the first step, the alcohol anion attacks the carbonyl carbon atom of the triglyceride, and a tetrahedral intermediate is formed. The second step is to react the intermediate with the alcohol to regenerate the alcohol anion. Finally, in the third step, a rearrangement of the tetrahedral intermediate occurs, leading to the formation of an ester of a fatty acid and a diglyceride.
4.6. Analysis of the Distilled Pyrolytic Waste Tire Oil and Produced Biodiesel
Yield: The volume of the washed biodiesel produced was measured with a graduated cylinder, and the produced biodiesel yield in weight percent was calculated based on the amount of oil used in the process.
Specific Gravity and Density: The specific gravity and density of the produced biodiesel was calculated using Equations (2) and (3);
Free Acid or Fatty Acid (FFA) Number: The acid number is the amount in mg of potassium hydroxide that neutralizes the free acid in grams of the oil (mgKOH/g). A 25 mL volume of ethanol was mixed with the same volume of ether, and 4 drops of phenolphthalein indicator were added into the Erlenmeyer flask containing 2 g of the oil sample. The mixture was titrated with 0.1 M aqueous KOH and stirred vigorously until a pink color was obtained, which took about 10 s.
Iodine number: This is a measure of the level of unsaturation in an oil or fat. It is the mass of iodine absorbed by 100 parts of the sample, and it is expressed as mg/g. The oil sample was poured into a dry sealed glass bottle with a capacity of approximately 250 mL. The weight (g) of the oil was obtained by dividing the expected maximum iodine number by 20. A 20 mL volume of Wiji’s solution and 10 mL of carbon tetrachloride were added to the bottle. A plug moistened with potassium iodine solution was inserted and kept in the dark for 30 min. A 100 mL volume of water and 15 mL of potassium iodide solution were mixed and titrated with a 0.1 M thiosulfate solution, using starch as an indicator. At the same time, a blank value was obtained from 10 mL of carbon tetrachloride.
Peroxide value: It is a measure of oxygen content, and it is expressed in mol/kg. A 2 g amount of the oil sample and 1 g of potassium iodide (powdered) were placed in 2 test tubes containing 20 mL of a mixture of solvents (2 parts by volume of glacial acetic acid + 1 part by volume of chloroform ice cream) in the ratio 60:30. Firstly, this was performed in an empty test tube (no sample). The tubes were placed in a water bath and vigorously boiled for 30 s. The contents were quickly poured into an Erlenmeyer flask containing 10 mL of a 5% potassium iodide solution. The tubes were each washed with 5 mL of water, the contents were poured into each Erlenmeyer flask, and then, four drops of phenolphthalein were added to each Erlenmeyer flask and titrated with 0.01 M thiosulfate until the color changed.
Saponification value: This is the number of milligrams of potassium hydroxide needed to neutralize the free fatty acids after 1 g of the sample has been completely hydrolyzed. It is measured in mg/g.
Flash point and fire point (ASTMD 93): Flash points were determined using a small heat-resistant beaker and a heating blanket. The sample was poured into a beaker and gradually heated with stirring to evenly distribute the heat in the beaker, and the temperature was monitored with a thermometer. At regular temperature intervals, the beaker was exposed to an open flame. The temperature was recorded at which the biodiesel raised flame-like lightning but did not aid combustion, which is the flash point of the biodiesel product.
5. Conclusions
Alternative lubricant additives must have friction-reducing physicochemical properties and moderate costs comparedto those of petroleum oils before they can become widely accepted in the marketplace. Waste tire pyrolytic oil was adopted as a feedstock for this study. Lubricant additives are added to drilling fluids to lower the drag and torque between the drill strings and rock formation. A 56.83% yield was obtained from the feedstock using transesterification. The physicochemical properties of the produced biodiesel were compared with the guidelines of the U.S. specification (ASTM Standard) and the European specification (EN Standard). Most of the valuable friction-reducing properties were within the standard ranges for lubricant additives. The chemical structure of the produced biodiesel was identified using gas chromatography-mass spectrometry (GC-MS). The comparative analysis of several valuable friction-reducing physicochemical properties indicated that the biodiesel obtained from distilled pyrolytic oil is a suitable lubricant additive for water-based drilling fluid.
Author Contributions
E.E.O.: conceptualization, supervision, investigation, methodology, and writing—original draft. S.I. and S.E.S.: conceptualization, investigation, project administration, writing—review and editing, and methodology. E.E.O. and S.I.: formal analysis, data curation, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Covenant University Centre for Research Innovation and Discovery (CUCRID) Ota, Nigeria, for its support in making the publication of this research possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- York, P.A.; Prichard, D.M.; Dodson, J.K.; Dodson, T.; Rosenberg, S.M.; Gala, D. Eliminating non-productive time associated with drilling through trouble zones. In Proceedings of the Offshore Technology Conference Paper, Houston, TX, USA, 4–7 May 2009. [Google Scholar]
- Sönmez, A.; Kök, M.V.; Özel, R. Performance analysis of drilling fluid liquid lubricants. J. Petrol. Sci. Eng. 2013, 108, 64–73. [Google Scholar] [CrossRef]
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Drilling problems related to drilling fluids. In Composition and Properties of Drilling and Completion Fluids; Gulf Professional Publishing: Houston, TX, USA, 2017; pp. 367–460. [Google Scholar] [CrossRef]
- Okoro, E.E.; Dosunmu, A.; Iyuke, S. Silicon Ethoxide as reversible surfactant in reversible drilling mud and the mud’s effect on permeability. J. King Saud Univ. Eng. Sci. 2019, 32, 402–406. [Google Scholar] [CrossRef]
- Kania, D.; Yunus, R.; Omar, R.; Rashis, S.A.; Jan, B.M. A review of bio-lubricants in drilling fluids: Recent research, performance, and applications. J. Petrol. Sci. Eng. 2015, 135, 177–184. [Google Scholar] [CrossRef]
- Shettigar, R.R.; Misra, N.M.; Naik, B.; Patel, K. Eco-friendly extreme pressure lubricants for water based drilling fluids. Int. Proc. Chem. Biol. Environ. Eng. 2015, 90. [Google Scholar] [CrossRef]
- Knox, D.; Jiang, P. Drilling further with water based fluids–selecting the right lubricant. In SPE International Symposium on Oil Field Chemistry; SPE Paper: Houston, TX, USA, 2005. [Google Scholar]
- Teng, J.; Espagne, B.J.-L.; Degouy, D.; Lescure, J. Development and field trial of a non-aqueous-based mud lubricant. Soc. Pet. Eng. 2013. [Google Scholar] [CrossRef]
- Okoro, E.E.; Erivona, N.O.; Sanni, S.E.; Orodu, K.B.; Igwilo, K.C. Modification of waste tire pyrolytic oil as base fluid for synthetic lube oil blending and production: Waste tire utilization approach. J. Mater. Cycle Waste Manag. 2020, 22, 1258–1259. [Google Scholar] [CrossRef]
- Banar, M.; Akyildiz, V.; Ozkan, A.; Çokaygil, Z.; Onay, O. Characterization of pyrolytic oil obtained from pyrolysis of TDF (tire-derived fuel). Energy Convers. Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Umeki, E.R.; Oliveira, C.F.; Torres, R.B.; Santos, R.G. Physicochemistry properties of fuel blends composed of diesel and tire pyrolysis oil. Fuel 2016, 185, 236–242. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Rocha, C.L.; Felipe, F.L.S.; Cezario, F.T.; Correia, P.J.; Rezaei-Gomari, S. Tire waste management; an overview from chemical compounding to the pyrolysis derived fuels. J. Mater. Cycle Waste Manag. 2020. [Google Scholar] [CrossRef]
- Conesa, J.A.; Martın-Gullon, I.; Font, R.; Juauhiainen, J. Complete study of the pyrolysis and gasification of scrap tires in a pilot plant reactor. Environ. Sci. Technol. 2004, 38, 3189–3194. [Google Scholar] [CrossRef]
- Quek, A.; Balasubramanian, R. Liquefaction of waste tires by pyrolysis for oil and chemicals—A review. J. Anal. Appl. Pyrol. 2013, 101, 1–16. [Google Scholar] [CrossRef]
- Breeden, D.L.; Meyer, R.L. Ester-Containing Downhole Drilling Lubricating Composition and Processes Therefor and Therewith. U.S. Patent 6,884,762, 26 April 2005. Available online: http://www.freepatentsonline.com/6884762.html (accessed on 28 March 2020).
- Genuyt, B.; Janssen, M.; Reguerre, R.; Cassiers, J.; Breye, F. Biodegradable Lubricating Composition and Uses Thereof, in Particular in a Bore Fluid [Composition Lubrifiante Biodegradable et ses Utilisations, Notamment Dans un Fluide de Forage]. WO Patent 0,183,640, 8 November 2001. [Google Scholar]
- Runov, V.A.; Mojsa, Y.N.; Subbotina, T.V.; Pak, K.S.; Krezub, A.P.; Pavlychev, V.N. Lubricating Additive for Clayey Drilling Solution—Obtained by Esterification of Tall Oil or Tall Pitch with Hydroxyl Group Containing Agent. SU Patent 1,700,044, 23 December 1991. [Google Scholar]
- Mikulski, M.; Ambrosewicz-Walacik, M.; Duda, K.; Hunicz, J. Performance and emission characterization of a common-rail compression-ignition engine fueled with ternary mixtures of rapeseed oil, pyrolytic oil and diesel. Renew. Energy 2020, 148, 739–755. [Google Scholar] [CrossRef]
- Talavera-Prieto, N.M.C.; Ferreira, A.G.M.; Moreira, R.J.; Portugal, A.T.G. Monitoring of the transesterification reaction by continuous off-line density measurements. Fuel 2020, 264, 116877. [Google Scholar] [CrossRef]
- Hentschel, K.-H. The influence of molecular structure on the frictional behaviour of lubricating fluids. J. Synth. Lubr. 1985, 2, 143–165. [Google Scholar] [CrossRef]
- Minami, I. Molecular science of lubricant additives. Appl. Sci. 2017, 7, 445. [Google Scholar] [CrossRef]
- Tsietsi, P.; Edison, M.; Mukul, S. Reduction of sulphur in crude tyre oil by gas-liquid phase oxidative adsorption. S. Afr. J. Chem. Eng. 2014, 19, 22–30. [Google Scholar]
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrog. Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Davidovich, Y.A.; Lyubimov, S.E.; Naumkin, A.V.; Davankov, V.A. On the nature of “functional groups” in non-functionalized hypercrosslinked polystyrenes. React. Funct. Polym. 2012, 72, 973–982. [Google Scholar] [CrossRef]
- Xiao, G.; Meng, Q.; Wen, R. Adsorption of aspirin on the macropore resin with six functional group sites: Multiple functional group sites in macropore resin versus the micropore filling in hypercrosslinked resin. React. Funct. Polym. 2020, 151, 104581. [Google Scholar] [CrossRef]
- Atuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2004. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product application. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A. Synthesis and physical properties of estolide-based functional fluids. Ind. Crop. Prod. 2003, 18, 183–196. [Google Scholar] [CrossRef]
- Wahyudi, W.; Wardana, I.N.G.; Widodo, A.; Wijayanti, W. Improving vegetable oil properties by transforming fatty acid chain length in jatropha oil and coconut oil blends. Energies 2018, 11, 394. [Google Scholar] [CrossRef]
- Kilonzi, F.M.; Kumar, A.; Namango, S.S.; Kiriamiti, H.K.; Some, D.K. Optimization of transesterification of sunflower oil with ethanol using eggshell as heterogeneous catalyst. Chem. Proc. Eng. Res. 2015, 30, 2224–2267. [Google Scholar]
- Gopinath, A.; Puhan, S.; Nagarajan, G. Theoretical modeling of iodine value and saponification value of biodiesel fuels from their fatty acid composition. Renew. Energy 2009, 34, 1806–1811. [Google Scholar] [CrossRef]
- Demirbas, A.; Al-Ghamdi, K. Relationships between specific gravities and higher heating values of petroleum components. Petrol. Sci. Technol. 2015, 33, 732–740. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Awogbemi, O.; Onuh, E.I.; Inambao, F.L. Comparative study of properties and fatty acid composition of some neat vegetable oils and waste cooking oils. Int. J. Low-Carbon Technol. 2019, 14, 417–425. [Google Scholar] [CrossRef]
- Sokoto, M.A.; Hassan, L.G.; Salleh, M.A.; Dangoggo, S.M.; Ahmed, H.G. Quality assessment and optimization of biodiesel from lageneria vulgaris (calabash) seeds oil. Int. J. Pure Appl. Sci. Technol. 2013, 15, 55–66. [Google Scholar]
- Umar, A.; Uba, A.; Mohammed, M.L.; Almustapha, M.N.; Muhammad, C.; Sani, J. Microwave assisted biodiesel production from Lagenaria vulgaris seed oil using amberlyst 15 ion exchange resin and eggshell as catalysts. Nig. J. Basic Appl. Sci. 2018, 26, 88–96. [Google Scholar] [CrossRef]
- Phankosol, S.; Krisnangkura, K. Estimation kinematic viscosity of biodiesel produced by ethanolysis. Eng. Trans. 2015, 18, 39. [Google Scholar]
- Khodier, S.A. Refractive index of standard oils as a function of wavelength and temperature. Opt. Laser Technol. 2002, 34, 125–128. [Google Scholar] [CrossRef]
- Ozcanli, M.; Gungor, C.; Aydin, K. Biodiesel fuel specifications: A review. Energy Sources Part A 2013, 35, 635–647. [Google Scholar] [CrossRef]
- Ramirez-Verduzco, L.F.; Rodriguez-Rodriguez, J.E.; Jaramillo-Jacob, A.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Cross, R. Effects of surface roughness on rolling friction. Euro. J. Phys. 2015, 36, 065029. [Google Scholar] [CrossRef]
- Liu, J.; Yun, B.; Zhao, C. Identification and validation of rolling friction models by dynamic simulation of sandpile formation. Int. J. Geomech. 2012, 12, 484–493. [Google Scholar] [CrossRef]
- Blau, P.J. The significance and use of the friction coefficient. Tribol. Int. 2001, 34, 585–591. [Google Scholar] [CrossRef]
- Mills, A. The coefficient of friction, particularly of ice. Phys. Edu. 2008, 43, 392–395. [Google Scholar] [CrossRef]
- Esan, A.O.; Adeyemi, A.D.; Ganesan, S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production. J. Clean. Prod. 2020, 257, 120561. [Google Scholar] [CrossRef]
- Dong, X.; Wang, L.; Yang, X.; Lin, Y.; Xue, Y. Effect of ester based lubricant SMJH-1 on the lubricity properties of water based drilling fluid. J. Petrol. Sci. Eng. 2015, 135, 161–167. [Google Scholar] [CrossRef]
- Amorin, R.; Dosunmu, A.; Amankwah, R. Local plant seed oils (esters): The frontier of geothermal drilling applications – A Review. Ghana J. Technol. 2017, 1, 62–72. [Google Scholar]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef]
- Maulidiyah, M.; Nurdin, M.; Fatma, F.; Natsir, M.; Wibowo, D. Characterization of methyl ester compound of biodiesel from industrial liquid waste of crude palm oil processing. Anal. Chem. Res. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Oehlschlaeger, M.A. A shock tube study of methyl decanoate autoignition at elevated pressures. Combust. Flame 2013, 159, 476–481. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Peng, H.; Guo, J.; Ji, T.; Chen, B.; You, Z.; Liu, L. A novel environmentally friendly lubricant for water-based drilling fluids as a new application of biodiesel. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference, Singapore, 22–24 August 2016. [Google Scholar] [CrossRef]
- Uchôa, I.M.A.; Neto, A.A.D.; Santos, E.S.; De Lima, L.F.; Neto, E.L.B. Evaluation of lubricating properties of diesel based fuels micro emulsified with glycerin. Mater. Res. 2017, 20, 701–708. [Google Scholar] [CrossRef]
- Acevedo, J.C.; Hernandez, J.A.; Valdes, C.F.; Khanal, S.K. Analysis of operating costs for producing biodiesel from palm oil at pilot-scale in Colombia. Biores. Tech. 2015, 188, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tasic, M.B.; Stamenkovic, O.S.; Veljkovic, V.B. Cost analysis of simulated base-catalyzed biodiesel production processes. Energy Conv. Manag. 2014, 84, 405–413. [Google Scholar] [CrossRef]
- Alsaleh, A.; Sattler, M.L. Waste Tire Pyrolysis: Influential parameters and product properties. Curr. Sustain. Renew. Energy Rep. 2014, 1, 129–135. [Google Scholar] [CrossRef]
- Barbin, W.W.; Rodgers, M.B. The science of rubber compounding. In Science and Technology of Rubber, 2nd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Laresgoiti, M.F.; Caballero, B.M.; Marco, I.; Torres, A.; Cabrero, M.A.; Chomon, M.J. Characterization of the liquid products obtained in tyre pyrolysis. J. Anal. Appl. Pyrol. 2004, 71, 917–934. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G.M. Prospects of pyrolysis technologies in bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Williams, P.T.; Bottrill, R.P.; Cunliffe, A.M. Combustion of tyre pyrolysis oil. Trans IChemE Part B 1998, 76, 291–301. [Google Scholar] [CrossRef]
- Vihar, R.; Seljak, T.; Oprešnik, S.R.; Katrašnik, T. Combustion characteristics of tire pyrolysis oil in turbo charged compression ignition engine. Fuel 2015, 150, 226–235. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrol. 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Murugan, S.; Ramaswamy, M.C.; Nagarayan, G. A comparative Study on the performance, emissions and combustion studies of DI Engine using distilled tyre pyrolysis oil-diesel fuel blends. Fuel 2008, 87, 2111–2121. [Google Scholar] [CrossRef]
- Hester, R.E.; Harrison, R.M. Waste as a Resource; The Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 978-1-84973-668-8. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).