Abstract
Polypropylene and polystyrene are petroleum-based thermoplastics which are commonly used and disposed of in the environment after their service life, leading to environmental degradation. There is a need to recycle polypropylene and polystyrene, but the effect of recycling on thermo-mechanical properties is not well understood. This study aims to determine thermo-mechanical properties of the recycled polypropylene and recycled polystyrene and compare them with corresponding virgin polypropylene and newly produced polystyrene (general purpose polystyrene 1540 and high impact polystyrene 7240). The study was carried out by preparing bar-shaped samples of recycled polypropylene, recycled polystyrene, general purpose polystyrene 1540, and high impact polystyrene 7240 by compression molding using a hot press and thermally characterizing them to determine glass transition temperature and melting temperature using differential scanning calorimetry. The changes in Young’s modulus, tensile strength, hardness, and toughness due to recycling activities were determined at room temperature (24 °C), 40 °C, 60 °C, and 80 °C. The thermo-mechanical properties of recycled polystyrene (PS) were found to be comparable to those of high impact polystyrene (HIPS) 7240. The study revealed that the hardness and toughness for the recycled polymers were higher than those of corresponding virgin polymers. On the other hand, tensile strength and Young’s modulus for the recycled polymers were lower than those of the virgin polymers. Understanding the thermo-mechanical properties of the recycled polymers will contribute to more industrial applications hence increase the rate of recycling, resulting in a reduction in environmental pollution.
1. Introduction
Polymers are categorized into thermoplastics, thermosets, and elastomers according to their thermal and mechanical properties. Scientists prefer polymer thermoplastics more than thermosets because of their low production cycle, lower processing costs, and high thermoplastic reparability [1,2]. Global polymer production has increased enormously to more than 330 million tons over the last five decades because polymers are widely used in industry [3,4,5,6]. In 2015, there were around 322 million tons produced worldwide, which was about 90 times higher than polymers produced in 1960. It was also reported that in the European Union, 58 million tons of polymers were produced and utilized, and only around 30% were recycled [3,4]. As a result of the growth in polymer production and utilization, large quantities of polymer waste are generated [7,8]. Polymers guarantee high durability, low density, high resistance-to-weight ratio, and low cost properties. However, many types of polymers cause long-term environmental pollution due to low biodegradability and chemical reactivity in the natural environment. The polymers break into smaller fragments termed as micro or macro-plastics that have a considerable set of ecosystem impacts and may harm animal and human health. Furthermore, other toxic compounds are released slowly into the soil, water, and air under various conditions [8,9].
Rapid growth of polymer use coincided with an increase in what is commonly called the green movement. The awareness of the environment means that the industry needs to be more concerned with waste disposal management. Also, there has been a need to increase the use of recycled polymers due to growing public concern for environmental degradation and strong government incentives to increase the rate of recycling of polymer wastes [7,10]. Apart from recycling, the disposal of waste polymers has been managed by incineration, reuse, and disposal. Disposal and incineration are methods which create the environmental problems of depleting landfill space and releasing massive amounts of carbon dioxide into the atmosphere, respectively. Recycling is the preferred option in reducing waste polymers since it makes it possible to reduce the volume of waste dumped in the environment in a much more environmental-friendly way, save energy, and reduce the need for natural resources because most polymer materials are obtained from gas and oil [7,11]. There are two methods of recycling, namely: chemical recycling and mechanical recycling. While these methods aim for the same objectives, they differ significantly. Mechanical recycling is when a polymer is ground, reprocessed, and re-granulated without changing the fundamental structure of the polymer to produce new polymer components. On the other hand, chemical recycling is the process where the polymer waste is converted back into its basic hydrocarbon/oil components which can be used as raw materials for production of new polymers using appropriate chemical solvents. Therefore, mechanical recycling is the most appropriate method of recycling from an industrial point of perspective because it is low cost, reliable, and an environmentally friendly process [12,13,14].
Polypropylene (PP) is a semi-crystalline and polymorphic thermoplastic with several crystal modifications such as hexagonal (β), orthorhombic (γ), monoclinic (α), etc. PP has been widely used in various fields of engineering, such as medical equipment, automotive, packaging products, etc., because of its cost which is relatively low with excellent properties such as good processing performance, low density, resistance to moisture, high resistance to ageing, good recyclability, excellent chemical and corrosion resistance, and high thermal stability [3,15,16,17].
Polystyrene (PS) is an aromatic polymer produced from the styrene monomer. There are two main polystyrene groups: General purpose polystyrene (GPPS) and high impact polystyrene (HIPS). However, HIPS and other types of polystyrenes, such as expanded polystyrene (EPS) are accepted for recycling [18,19,20]. HIPS is a copolymer of styrene and acrylonitrile, toughened with polybutadiene and shows the right balance between elasticity and rigidity. The incorporation of a rubber phase into glassy polymers is acknowledged as an efficient technique to enhance the toughness of polymer products. HIPS is widely employed in various fields including housewares, packaging, automobiles, electrical appliances, and light-duty industrial components due to its low cost, ease of processing, versatility, coloring, relative thermal stability, and good rigidity [20,21,22].
PS and PP are among the five most widely used thermoplastic polymers in terms of share of the market [10,12,23]. HIPS and PP were discovered to be the primary components of the plastics fraction of waste [24,25]. Therefore, recycling of PP and PS has attracted considerable attention in recent years due to a decreased supply of petroleum resources, increased environmental concerns, increased need for sustainability, and energy security. Various strategies for recycling programs for PP have been developed. Fortunately, a large proportion of PP residues are recovered using industrial recycling and recycled PP can be used in various ways, such as industrial parts, composite matrix, reinforcements in the construction industry, etc. [3,14]. Several scholars have researched and studied changes in the mechanical and rheological characteristics of PP after repeated injection molding cycles. Molecular weight variations, the polymer’s chemical structure, and some mechanical properties at room temperature have been analyzed as a result of degradation due to continuous processing operations. However, limited research studies have been conducted and published on the effect of recycling activities on the thermo-mechanical properties of PP as a function of temperature. Also, despite the availability of PS recycling technology, recycled polystyrenes’ market is characterized by relatively decreasing demand, overcapacity, and underuse due to limited knowledge on the effect of recycling on thermo-mechanical properties [19,26,27,28,29].
The use of recycled polymers instead of virgin polymers for industrial purposes is one of the most promising techniques for lowering the environmental impact and the expenses associated with scrapping components [14]. However, intensive melting due to prolonged exposure to high temperatures during the reprocessing process (melt degradation), ageing due to harmful effects of the environment, long-term heat ageing, and chain scission during service life of the polymers affect the thermo-mechanical properties of the recycled polymers. Furthermore, polymers are subjected to physical stresses (mechanical forces, heat, radiation), and chemical changes due to chemical deteriogens (humidity, oxygen, atmospheric pollutants). The conditions cause structural deterioration of the polymeric components [2,27,30]. Also, as a side effect to consider, contamination by residues such as inorganic, organic, or biological residues cause deterioration of the polymers during recycling. Besides, the mechanical recycling of polymers introduces further problems owing to the complexity of the morphological structures that occur between the polymeric components [3,31,32,33]. Based on the various mechanisms involved in the life cycle of polymers, the changes significantly alter the mechanisms of stabilization, mechanical, thermal, and rheological properties of recycled polymers [14,33,34]. The thermoplastic nature of these recycled thermoplastics raises essential questions about mechanical performance at high temperatures and low strain rates [35]. Therefore, manufacturing industries have not widely adopted the recycled polypropylene (rec PP), and recycled polystyrene (rec PS) obtained from various sources such as industrial wastes, environmental damping etc. due to a lack of sufficient knowledge on their thermo-mechanical properties which have caused their market to be relatively small and shrinking. The main aim of this study was to determine if the recycling activities affect the thermo-mechanical properties of recycled PP and recycled PS obtained from post-consumer and post-industrial waste polymers and compare them with those of corresponding virgin polymers (polypropylene, high impact polystyrene 7240, and general purpose polystyrene 1540). Also, recycled polymers were characterized thermally to determine properties such as the degree of crystallinity, melting temperature (), and glass transition temperature () where differential scanning calorimetry (DSC) was used. Thermal characterization was useful in evaluating any modifications in the mechanical performance of recycled polymers due to various factors such as degradation of polymers during their life service, recycling, or blending. Also, the mechanical properties that are frequently used to monitor polymer performance such as tensile strength, hardness, and toughness at elevated temperatures were determined and analyzed.
The knowledge gained will assist manufacturers in formulating applications for recycled PP and recycled PS to fit their products as a substitute for virgin polymers where applicable. As a result of expected increase in the applications, there would be an increase in the rate of recycling of PP and PS, which will contribute to the minimization of degradation of the environment and enhance environmental sustainability.
2. Materials and Methods
2.1. Materials
General purpose polystyrene (GPPS 1540) was purchased from Tabriz Petrochemical Company (Tabriz, Iran) with a density of 1.04 g/cm3, an approximate shrinkage in the mold of 0.4–0.7% (ASTM D 955), and a melt flow index of 11 g/10 min tested at 200 °C using a 5 kg load. High impact polystyrene (HIPS 7240) was purchased from Tabriz Petrochemical Company (Tabriz, Iran) with a melt flow index of 4.5 g/10 min tested at 200 °C using a load of 5 kg. The HIPS 7240 grade can be diluted with polystyrene crystal (such as GPPS 1240 or 1540). Recycled polypropylene (rec PP) and recycled polystyrene (rec PS) were supplied from Kaskada Ltd. (Plovdiv, Bulgaria) and used as received.
2.2. Experimental Methods
2.2.1. Sample Preparation
Recycled PS, HIPS 7240, and GPPS 1540 in the form of pellets were hot-pressed and molded into square-shaped samples with dimensions of 200 × 200 × 4 mm using a hot press (Servitec Polystat 400S, Wustermark, Germany) according to the European standards (EN) ISO 527-1:1995. The hot press had a dimension of 350 × 350 mm, and a working area of 300 × 300 mm. The processing parameters were: maximum press force of 450 kN, a maximum working temperature of 300 °C, the pressure of 234.3 N/mm2, the temperature of 160 °C, and time of 10 min. After cooling the samples to 35 °C between plates refrigerated with cold running water, they were taken out from the mold. Also, samples of recycled PP were prepared using the same hot press under the same pressure parameters but with a temperature of 170 °C for 10 min. After cooling the samples to 35 °C, they were taken out from the mold. Finally, the square-shaped samples were cut into bar-shaped samples with dimensions of 200 × 10 × 4 mm using a band saw.
2.2.2. Thermal Characterization
Melting temperature and glass transition temperature was determined using a differential scanning calorimeter (DSC, 204 Phoenix, Netzsch, Germany). The transitions were studied by heating in a range of -100 °C to 300 °C at a heating rate of 10 K/min. The samples were cooled under a nitrogen atmosphere. The measurements were performed with 6–10 mg materials cut from the core layer, which was sealed in an aluminum specimen pan for DSC. The temperature of the sample was controlled in the DSC cell.
2.2.3. Mechanical Testing
The tensile strength, Young’s modulus, and elongation at yield and breakpoints were determined. The tensile properties for all samples were measured with a tensile tester (Zwick Z 020 GmbH & Co. KG, Ulm, Germany) according to the British standards (BS) EN ISO 527-1:1996. The tensile tester had a maximum load of 20 kN with a maximum testing range of 1700 mm. The holding grips of the test specimen were attached to the machine so that the test specimen’s main axis coincided with the pull direction through the grip assembly centerline. It was ensured that the clamping system did not cause premature fracture at the grips. The distance between the clamps was adjusted to obtain a gauge length of 80 mm. The grip extended with a constant crosshead speed of 50 mm/min along its major longitudinal axis until the specimen fractured. Four tensile test samples were tested at measuring temperatures of 24 °C, 40 °C, 60 °C, and 80 °C, and the results averaged. The standard deviation was calculated as a measure of statistical uncertainty.
The impact strengths were measured using a Charpy edgewise un-notched test according to the ISO 179-1:2010(E) standard using 10 bar-shaped unnotched samples with dimensions of 100 × 10 × 4 mm at different temperatures of 24 °C, 40 °C, 60 °C, and 80 °C. The impact testing used an impact tester (PSW 4J, Kennesaw, USA) and a 5.5 J pendulum hammer. A standard deviation of 5% (drop weight) due to air resistance was subtracted in each test to calculate the Charpy impact strength. For each test, values were calculated as the average of 10 independent measurements and a standard deviation calculated as a measure of statistical uncertainty.
Hardness was measured using a Shore D hardness tester (PCE-DX-DS (D), Southampton, UK) according to the EN ISO 868:2004 standard. The sample pieces of the materials were tested at 24 °C, 40 °C, 60 °C, and 80 °C by placing them on a solid base inside a controlled hot chamber where the gauge was placed on a spot of the sample at least 12 mm away from the edge. Then the tester was pressed against the surface of the sample, and the durometer reading recorded 1 s after indenter application. The measurements for each specimen were repeated five times at different spots, which were at least 6 mm apart from each other. An average was done and a standard deviation calculated as a statistical uncertainty measure.
3. Results and Discussion
3.1. Thermal Properties
The thermal behavior of the recycled polymers and virgin polymers were probed via DSC to determine glass transition temperature ( and melting temperature (. Glass transition temperature and melting temperature are determined to compare any modifications due to recycling or blending. Figure 1 predicts of GPPS 1540, HIPS 7240, and recycled PS. Also, Figure 2 predicts of virgin polypropylene (VPP) and recycled PP. Thermo-mechanical properties of the materials such as hardness, toughness, tensile strength, and Young’s modulus depend on the values of and [36]. Also, of polymers is essential in order to create the best methods of processing the materials into finished products and to foresee performance during the lifetime of the product [37].
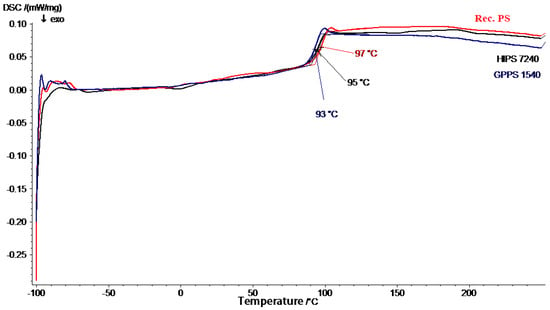
Figure 1.
Differential scanning calorimetry (DSC) plot for high impact polystyrene (HIPS) 7240, general purpose polystyrene (GPPS) 1540, and recycled polystyrene (PS).
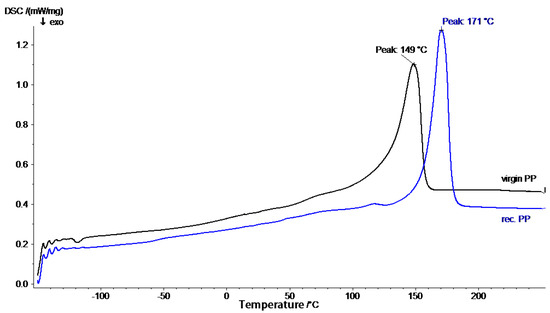
Figure 2.
DSC plot for virgin polypropylene (PP) and recycled PP.
The DSC analysis of GPPS 1540, HIPS 7240, and recycled PS showed similar thermographs where only one temperature corresponding to the glass transition temperature was observed. Virgin polystyrenes (GPPS 1540 and HIPS 7240) had a of 93 °C and 95 °C, respectively. Ahmed et al. [28] did a similar experiment for both GPPS and HIPS and got a common of 95 °C. The presence of phenyl groups and amorphous structure in GPPS 1540 and HIPS 7240 is responsible for the relatively high glass transition temperature without having a sharp melting point [38]. On the other hand, recycled PS had a of 97 °C. The higher of recycled PS than virgin polystyrenes (GPPS 1540 and HIPS 7240) was attributed to increased crosslinking and change of the chemical structure of the polybutadiene phase which is influenced by contaminants, embrittlement, and thermo-oxidation on both polybutadiene and PS during lifetime and reprocessing [39,40,41]. In addition, the crystallinity of recycled polymers increases as a result of molecular weight reduction and entanglements of chains from several thermal reprocessing cycles [12].
It was observed that recycled PP and virgin PP had similar thermographs. Also, only single melting peaks of 149 °C and 171 °C for recycled PP and virgin PP, respectively, were observed. It is well known that PP is a semi-crystalline and polymorphic material with several crystal modifications including at least monoclinic (α), hexagonal (β), and orthorhombic (γ), etc. The monoclinic α form is the most prevalent and stable modification observed in all types of solution-crystallized PP samples and also in most melt-crystallized specimens. In this case, the melting peaks of 149 °C and 171 °C suggest that recycled PP and virgin PP are the endothermic peaks of α crystals of PP [15,17,42]. Zdiri et al. [26] and Shayuti et al. [43] did a similar experiment and determined the melting point of virgin PP to be 166.3 °C and 163.3 °C, respectively. The for recycled PP was 12.9% lower than that of virgin PP due to several factors such as reprocessing conditions, thermal history factors, the presence of crystals which are less perfect, chain degradation which occurs during melt processing, and other changes in crystals [43,44]. The wide melting peak for recycled PP and virgin PP as shown in Figure 2 indicate that different crystalline structures coexist and that the distributions of the crystallite block length are wide in both the soft (amorphous, atactic PP) and hard (crystalline, isotactic PP) phases. The broad peak is also due to the melting of the crystallized phase made by the stereo-regular sequences co-crystallization [5].
Amorphous polymers generally have a glass transition temperature without a melting temperature, while semi-crystalline polymers may have a melting temperature, glass transition temperature, crystallization temperature with different crystallization and melting enthalpies [45]. The network structure of the granules of the sample thermoplastic materials would be destroyed during hot pressing where the temperature could slightly exceed the melting temperature. A polymer is ductile above the , while the polymer acts in a brittle manner below it. Table 1 summarizes the temperatures of glass transition and the temperatures at which melting occurs for the polymer materials.

Table 1.
Summary of the temperatures of glass transition and melting temperatures determined by DSC. , glass transition temperature; , melting temperature.
3.2. Thermo-Mechanical Properties
To get a better understanding of the mechanical properties to expect from recycled PP and recycled PS at different test temperature conditions, determination of tensile strength, toughness, and hardness were performed, analyzed graphically, and compared to virgin polymers (VPP, GPPS 1540, HIPS 7240). Tensile tests performed also enabled us to determine properties such as Young’s modulus and elongation at break for recycled and virgin polymers as a function temperature. The yield strength was not obtainable for GPPS 1540 since it was brittle, but the stress at break was determined.
3.2.1. Tensile Strength
Tensile Strengths and Strain Behaviors of Recycled Polypropylene and Virgin Polypropylene
The tensile strength of recycled PP at elevated temperature conditions of 24 °C, 40 °C, 60 °C, and 80 °C provides important data on the possible application temperature of recycled PP. The information which is shown in Figure 3a represents stress vs. strain curves for recycled PP at variable temperatures. Also, Figure 3b represents a comparison of tensile strengths between virgin PP and recycled PP at variable temperatures.
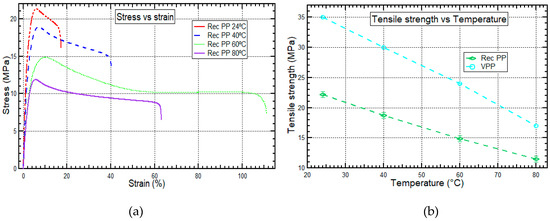
Figure 3.
(a) Stress vs. strain behavior for recycled PP and (b) Tensile strength for recycled PP and virgin PP at 24 °C, 40 °C, 60 °C, and 80 °C. Data for virgin PP was obtained from Mallick [46].
From Figure 3a, it is shown that recycled PP’s stress vs. strain relationships are linear at strains lower than the maximum strain. The stress vs. strain relationships depicts recycled PP to have characteristics of tough thermoplastic materials without yield point. Stress of recycled PP continuously decreased after yielding until fracture occurred and recorded a total increase in elongation at break of 244.4%. Due to the development of residual thermal stresses in the recycled PP structure during cooling down from the melting temperature in the hot compression molding, the yield strains of recycled PP decreased as the test temperature increased [46]. Furthermore, the decrease in tensile strength was because of transitions related to an increase in molecular mobility within the crystalline phase caused by reduced shear strength of the crystals at high temperatures [35]. It was also observed that recycled PP has a high elongation to failure because recycled PP is like a semi-crystalline material which has a high ability to absorb more energy before it fails [27,47]. According to the study conducted by Hamad et al. [12], there is a reduction of the molecular weight with an increase in the number of processing cycles, which results in an increase in the flow activation energy of the PP samples. Also, the strain vs. stress relationship results showed that stress at break and strain at break of the PP samples decreased significantly after each processing cycle, which was attributed to lower cohesion in the polymer. The lower cohesion was because there was a decrease in molecular weight as the amount of reprocessing increased.
The tensile strength of recycled PP was lower than that of virgin PP by 33.0–38.2%, as shown in Figure 3b. Bourmaud et al. [47] did a similar experiment of investigating how maximum tensile strength related to the amount of recycling and found out that maximum tensile strength decreased as the number of injection cycles increased. This phenomenon is because recycled PP comes from diverse sources with a collection of different grades and properties, e.g., a blend of two PP homopolymers with different molecular weights due to a different lifetime [35]. Furthermore, recycled PP undergoes natural degradation due to chain scission and thermo-mechanical degradation because of high temperature, oxidation, and shearing during processing [48]. Nevertheless, the mismatching of properties such as phase size, phase structure, and miscibility of components due to contamination by residues such as inorganic, organic, or biological residues can affect the thermo-mechanical properties of the polymers significantly [15,31].
Tensile Strengths of Recycled Polystyrene and Virgin Polystyrenes (HIPS 7240 and GPPS 1540)
Figure 4a–d shows the tensile stress vs. strain curves of recycled PS and virgin polystyrenes (HIPS 7240 and GPPS 1540) under different test temperature conditions of 24 °C, 40 °C, 60 °C, and 80 °C. The behavior of elongation as a function of the stress applied to recycled PS, GPPS 1540, and HIPS 7240 at temperatures of 24 °C, 40 °C, 60 °C, and 80 °C are as shown in Figure 4a–d respectively.
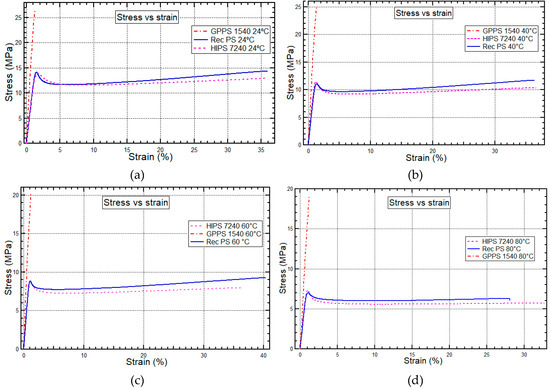
Figure 4.
Stress vs. strain behavior for HIPS 7240, GPPS 1540, and recycled PS at 24 °C, 40 °C, 60 °C, and 80 °C.
The materials’ elongation before breaking showed that recycled PS and HIPS 7240 have the characteristic of non-brittle materials and are classified as tough materials with yield points. Therefore, the thermo-mechanical properties of recycled PS and HIPS 7240 are comparable. HIPS 7240 and recycled PS had a decrease in elongation at break of 8.3% and 22.2%, respectively, when the temperature increased from 24 °C to 80 °C. The elongation to failure was considerably higher for the HIPS 7240 than for recycled PS at 80 °C as shown in Figure 4d, due to the presence of polybutadiene rubber in the HIPS 7240. On the other hand, GPPS 1540 was an exceptionally brittle thermoplastic with elongation to failure of 1% as shown in Figure 4a–d.
Figure 5 shows comparisons of the tensile strengths between recycled PS and virgin polystyrenes (HIPS 7240 and GPPS 1540) at different test temperatures of 24 °C, 40 °C, 60 °C, and 80 °C.
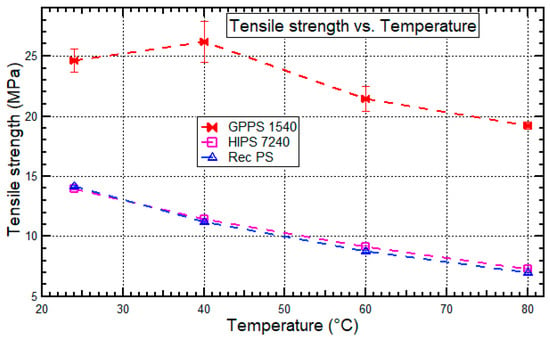
Figure 5.
Tensile strength for recycled PS, HIPS 7240, and GPPS 1540 at 24 °C, 40 °C, 60 °C, and 80 °C.
The stress level for the tested sample materials decreased with increasing temperature at the same strain rate. The trend observed is because the molecules of the thermoplastics possess sufficient thermal mobility to move [49], and subsequently lead to a reduction of the material’s tensile strength due to weakening forces of secondary valence bonding. Recycled PS had a slightly lower tensile strength than virgin PS (HIPS 7240) by 2.8–5.6% as the temperature increased from 24 °C to 80 °C. A similar experiment was done by Santana et al. [39] to determine how the amount of reprocessing affects tensile strengths of HIPS, and it was found that the tensile strength decreased with the amount of reprocessing. Thermo-mechanical degradation of the material produced by continuous grinding, drying, and hot pressing contributed to the decrease in tensile strength. Also, the decline was associated with other degrading agents such as water, light, oxidation, contamination by residues such as inorganic, organic or biological residues [39]. On the other hand, GPPS 1540 had higher tensile strengths than recycled PS by 45.0–62.6% because the crystallization behavior of amorphous GPPS 1540 increases yield stress [18,19,20,38].
3.2.2. Young’s Modulus
Figure 6 provides a comparison of Young’s modulus behavior of recycled PS with virgin polystyrenes (HIPS 7240 and GPPS 1540) at different temperatures. Also, Young’s modulus of recycled PP and virgin PP were compared. It was observed that Young’s modulus for all the materials tested decreased significantly with the increase in temperature because the polymers become more ductile at higher temperatures due to Brownian mobility of the molecules [50]. The low Young’s modulus at a higher temperature means that when the polymer is loaded, it increases deflections within the structure, which can limit the product in the requirements of serviceability [49].
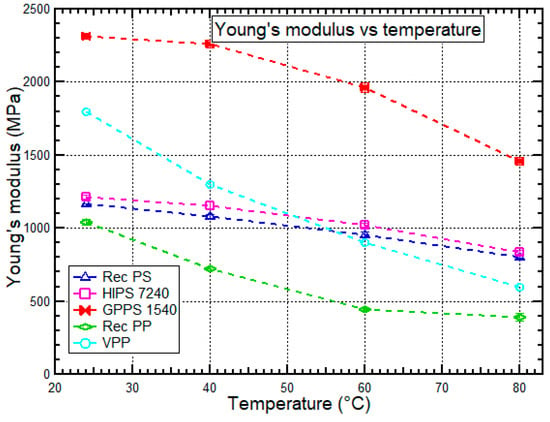
Figure 6.
Young’s modulus vs. temperature behavior for HIPS 7240, GPPS 1540, recycled PS, recycled PP, and virgin PP at 24 °C, 40 °C, 60 °C, and 80 °C. Data for virgin PP was obtained from Mallick et al. [46].
Recycled PS recorded a lower Young’s modulus than HIPS 7240 and GPPS 1540 by 5.2–9.8% and 46.0–53.2%, respectively. Santana et al. [39] did a similar experiment to determine how the amount of reprocessing affects the Young’s modulus of HIPS and found out that the Young’s modulus decreased with the number of reprocessing cycles. The reduced Young’s modulus for recycled PS compared to HIPS 7240 after recycling was due to the reduced polymer cohesion resulting from a reduction in molecular weight [12]. GPPS 1540 is more rigid than HIPS 7240 and recycled PS since the crystallization behavior of amorphous GPPS 1540 increases the Young’s modulus [18,19,20,38]. Also, it was also observed that recycled PP had a lower Young’s modulus than virgin PP by 39.2–52.0%. The result was similar to a study conducted by Zdiri et al. [26] and Bourmaud et al. [47], which showed that Young’s modulus reduced slightly after recycling. In this study, the lower Young’s modulus of recycled PS and recycled PP than corresponding virgin polymers could be due to thermal degradation, which occurred due to physical ageing and thermo-oxidative degradation. The thermo-mechanical degradation is a temperature-dependent process which occurs due to a decrease of molecular weight [12]. Santana et al. [39] demonstrated that successful mechanical recycling is primarily dependent on sufficient melt processing stability. Processing causes oxidation, thermal, and mechanical stress to both virgin and recycled polymers. These three stress types are a permanent processing feature and can induce changes in mechanical properties.
3.2.3. Toughness
Toughness is one of the main parameters that affect the performance of polymers in different engineering applications [51]. The results obtained for the toughness of HIPS 7240 and GPPS 1540 was compared to recycled PS. Also, the recycled PP’s results were compared to virgin PP as a function of temperature, as shown in Figure 7.
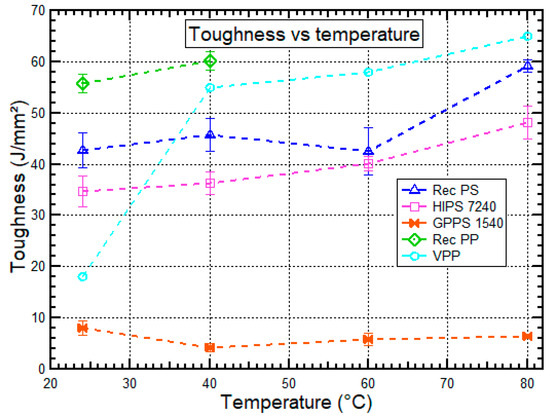
Figure 7.
Toughness vs. temperature behavior for HIPS 7240, GPPS 1540, recycled PS, recycled PP, and virgin PP at 24 °C, 40 °C, 60 °C, and 80 °C. Data for virgin PP was obtained from Sahin et al. [52].
The results indicated that the amount of energy absorbed by recycled PS, HIPS 7240, recycled PP, and virgin PP increased as temperature increased from 24 °C to 80 °C because, at low temperatures, the thermoplastic materials are more brittle, causing the impact toughness to be small. At higher temperatures, the polymers are more ductile, and consequently, the impact toughness becomes higher. However, GPPS 1540 had exceptionally low toughness with no change as temperature increased due to its brittleness characteristics.
The toughness for recycled PS during impact was higher than that of HIPS 7240 by 21.3–44.0%. The higher impact strength for the recycled PS is caused by a reduction in molecular weight of the polymers due to chain scission, thermal stress, ionizing radiation during different recycling conditions, and chemical ageing of the polymer as a result of oxidative ageing and hydrolytic ageing. The presence of lower molecular weight chains between macromolecules is likely to act as a plasticizer, resulting in increased impact strength [39,53]. However, in the thermo-oxidative degradation of HIPS during lifetime and recycling processes, the polybutadiene phase undergoes chemical changes that result in the polymer’s mechanical failure [54].
Due to its high fragility, virgin PP has low impact properties at low temperatures due to the intrinsic low crystallinity, which is one of the main concerns with frequent use of this material [51]. As the temperature increased beyond 40 °C, recycled PP did not break because recycled PP was becoming ductile as the temperature increased, which caused molecular movement, thus requiring more energy to break it. According to the research done by Alcock et al. [35], the flexibility of a polymer depends basically on the rotation ability of its segments due to the mobility of the molecules within the crystalline phase. However, crystalline structures in semi-crystalline polymers (PP) impede such rotations; therefore, in its amorphous condition, the crystalline material is considerably more rigid than the plastic equivalent [27]. The results further showed that toughness for recycled PP was higher than that of virgin PP by 11.0–215.7%, which was similar to the results obtained by Bourmaud et al. [47] in which it was shown that impact energy increased as the number of injection cycles increased. The higher toughness of recycled PP than virgin PP could be due to the condition of a decrease of the molecular weight during recycling which increases the mobility and the ability of the chains to fold, facilitating thick lamellae formation and a high degree of crystallinity (elastomeric phase). The thick molecules were able to cross over to more than one crystal plate and tie the whole structure together hence increase the toughness. Matrix characteristics are, therefore, the main variables that determine the toughness of recycled PP [16,42].
3.2.4. Hardness
Hardness is the degree of permanent deformation of a material under a force applied to it using a durometer. Figure 8 displays the analyzed results of Shore D hardness for recycled PS compared to HIPS 7240 and GPPS 1540 as a function of test temperatures. Similarly, hardness for recycled PP was compared to virgin PP. Hardness is always related to the crystallinity of the polymer such that amorphous polymers are harder than crystalline polymers.
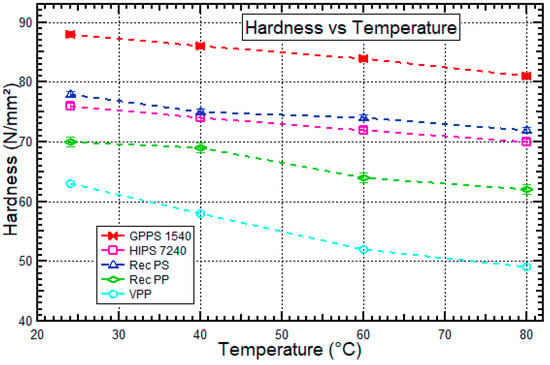
Figure 8.
Hardness vs. temperature behavior for HIPS 7240, GPPS 1540, recycled PS, recycled PP, and virgin PP at 24 °C, 40 °C, 60 °C, and 80 °C. Data for virgin PP was obtained from Sahin et al. [52].
A decreasing tendency of hardness for the polymers tested was observed as the temperature increased from 24 °C to 80 °C. Polymers consist of long-chain molecules in which the degree of entanglement varies with the length and exact shape of the polymer molecule. As the temperature increased, molecular mobility of polymers also increases, causing the polymer to become soft elastic hence decrease in hardness [55].
Recycled PS had a higher hardness than HIPS 7240 by 2.2–5.1%. A similar experiment was done by Tamer et al. [56] and showed that hardness for HIPS decreased with time because butadiene rubber in HIPS tends to embrittle due to weathering. GPPS 1540 recorded a higher hardness than that of recycled PS by 11.4–13.2% because GPPS 1540 is an amorphous polymer with brittle characteristics as observed from the stress–strain curves (Figure 4). Amorphous polymers have characteristics of high hardness due to their molecules which are irregularly arranged. When the molecules are irregularly arranged and bulky, an amorphous polymer is produced which consists of enmeshed chains of polymer which are disordered. Typically, such polymers are optically transparent, hard, and often fragile. Therefore, there is a tendency of materials such as GPPS 1540 with high tensile strength and modulus, as shown in Figure 5 and Figure 6, respectively, to show higher hardness as shown in Figure 8. The high mechanical properties are also directly related to their crystallinity [27,57]. Recycled PP had improved hardness compared to that of virgin PP by 11.7–26.5% because recycled PP could have less flexibility matrix than virgin PP as explained by Homkhiew et al. [58]. The results were contrary to the explanations given by Ratanawilai et al. [59] that thermal degradation due to chain scission of virgin PP contributes cracks and hence lowers the hardness of recycled PP. Table 2 shows the summarized, analyzed results obtained from the thermo-mechanical characterization of the polymers (recycled PS, HIPS 7240, GPPS 1540, recycled PP). However, the thermo-mechanical properties of virgin PP were obtained from a literature review.

Table 2.
Summary of the results for the polymers obtained from thermo-mechanical characterization.
4. Conclusions
The thermal and mechanical analysis was used in this study to determine thermo-mechanical properties of the recycled PP and recycled PS and compare them to those of corresponding virgin PP and newly produced polystyrenes (GPPS 1540 and HIPS 7240). Also, the behavior of the mechanical properties of the polymers as a function of temperature was studied. From the DSC thermographs, the glass transition temperatures and melting temperatures for the studied recycled polymers were determined.
It was found that Young’s modulus, tensile strength, and hardness for recycled PP, virgin PP, recycled PS, HIPS 7240, and GPPS 1540 decreased as temperature increased. However, toughness for the recycled PP, virgin PP, recycled PS, and HIPS 7240 increased as the temperature increased, differing from GPPS whose toughness was maintained. HIPS 7240 and recycled PS showed characteristics of semi-crystalline and tough thermoplastic materials with a yield point while GPPS 1540 showed characteristics of an amorphous, brittle, and highly transparent material. On the other hand, recycled PP showed characteristics of a semi-crystalline and tough thermoplastic material without a yield point. The distinct characteristics of GPPS 1540 of being amorphous in terms of crystallinity made it unfit to be directly compared with recycled PS since it had a much higher Young’s modulus, tensile strength, hardness, and very low toughness compared to that of HIPS 7240. It was established from the comparative analysis that recycled PS and recycled PP had a lower Young’s modulus by 5.2–9.8% and 39.2–52.0%, respectively, than that of corresponding HIPS 7240 and virgin PP. Also, tensile strengths for recycled PP and recycled PS were 33.0–38.2% and 2.8–5.6% lower, respectively, than those of corresponding virgin PP and HIPS 7240. On the other hand, toughness for recycled PP and recycled PS was 11.0–215.7% and 21.3–44.0% higher, respectively, than that of corresponding virgin PP and HIPS 7240. Also, hardness for recycled PP and recycled PS was 11.7–26.5% and 2.2–5.1% higher, respectively, than those of corresponding virgin PP and HIPS 7240.
This work has demonstrated that recycled PP and recycled PS collected from polymeric wastes from different sources are promising mechanical recyclates. The results presented in this study allow a better understanding of the thermo-mechanical properties of recycled PP and recycled PS and will assist manufacturers in formulating applications for recycled PP and recycled PS to fit their products as a substitute for virgin polymers where applicable. As a result, there will be an increase in the need for recycling, leading to a decrease in environmental degradation.
Author Contributions
Conceptualization, J.M. and M.H.; Data collection, J.M.; Methodology, J.M.; Formal analysis, J.M.; investigation, J.M.; writing—original draft preparation, J.M.; writing—review and editing, M.H. and P.M.; visualization, J.M.; Supervision, M.H. and P.M.
Funding
This research was funded by Technical University of Applied Science Wildau.
Acknowledgments
This paper forms part of the author’s MSc research supported by the Technical University of Applied Science Wildau, Germany, and Dedan Kimathi University of Technology, Nyeri, Kenya. Contributions of supervisors are highly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geels, K.; Kopp, W.; Rückert, M. Mounting. Metallographic and Materialographic Specimen Preparation, Light Microscopy. In Image Analysis and Hardness Testing; Nester, R.C., Petzow, G., Eds.; ASTM International: West Conshohocken, PA, USA, 2007; pp. 54–79. [Google Scholar]
- Awad, S.A.; Khalaf, E.M. Investigation of improvement of properties of polypropylene modified by nano silica composites. Compos. Commun. 2019, 12, 59–63. [Google Scholar] [CrossRef]
- Bertomeu, D.; Arrieta, M.P.; Ferri, M.; Juan, L. Interference of Biodegradable Plastics in the Polypropylene Recycling Process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy 2017, 131, 179–185. [Google Scholar] [CrossRef]
- Zander, A.N.E.; Gillan, M.; Gardea, F. Recycled polypropylene blends as novel 3D printing materials. Addit. Manuf. 2019, 25, 122–130. [Google Scholar] [CrossRef]
- Mourad, A.I. Thermo-mechanical characteristics of thermally aged polyethylene/polypropylene blends. Mater. Des. 2010, 31, 918–929. [Google Scholar] [CrossRef]
- Stenvall, E.; Boldizar, A. Mechanical and Thermal Characterization of Post-Consumer WEEE Thermoplastics. Recycling 2016, 1, 89. [Google Scholar] [CrossRef]
- Minelgaite, A.; Liobikiene, G. Waste problem in European Union and its infl uence on waste management behaviours. Sci. Total Environ. 2019, 667, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, A.; Ozbakkaloglu, T. Recycled plastic. In New Trends in Eco-efficient and Recycled Concrete; Woodhead Publishing: Sawston, UK, 2019; pp. 59–79. [Google Scholar]
- Valencia, C.; Franco, J.M. Effect of amorphous/recycled polypropylene ratio on thermo-mechanical properties of blends for lubricant applications. Polym. Test. 2013, 32, 516–524. [Google Scholar] [CrossRef]
- Abdullah, M.Z.; Haziq, N.; Aslan, C. Performance Evaluation of Composite from Recycled Polypropylene Reinforced with Mengkuang Leaf Fiber. Resources 2019, 8, 97. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Grigore, M.E. Methods of Recycling, Properties and Applications of Recycled Thermoplastic Polymers. Recycling 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Galve, E.; Elduque, D.; Pina, C. Dimensional Stability and Process Capability of an Industrial Component Injected with Recycled Polypropylene. Polymers 2019, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, W.; Bao, R. Toughening of Polypropylene with β-Nucleated Thermoplastic Vulcanizates Based. Mater. Des. 2013, 51, 536–543. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Song, B.; Fan, X. Nucleating agent induced impact fracture behavior change in PP/POE blend. Polym. Bull. 2009, 62, 405–419. [Google Scholar] [CrossRef]
- Krache, R.; Benavente, R.; Lo, J.M.; Peren, M. Competition between R and γ Polymorphs in a β-Nucleated Metallocenic Isotactic Polypropylene. Macromolecules 2007, 40, 6871–6878. [Google Scholar] [CrossRef]
- Kulkarni, G.S. Introduction to Polymer and Their Recycling Techniques. In Recycling of Polyurethane Foams; William Andrew Publishing: Halle(Saale), Germany, 2018; pp. 1–16. [Google Scholar] [CrossRef]
- Taylor, P.; Maharana, T.; Negi, Y.S.; Mohanty, B. Recycling of Polystyrene. Polym.-Plast. Technol. Eng. 2007, 46, 729–736. [Google Scholar] [CrossRef]
- Satterthwaite, M.K. Plastics Based on Styrene. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 311–328. [Google Scholar]
- Wang, F.; Chang, L.; Wu, G. Synthesis and Properties of In-Situ Bulk High Impact polystyrene Toughened by high cis-1,4 Polybutadiene. Polymers 2019, 11, 791. [Google Scholar] [CrossRef]
- Ding, L.; Jia, G.; Sun, H. Estimation of Mechanical Performance, Thermal Stability and Flame Retardancy of High-Impact Polystyrene/Surface-Modified APP/Carboxylic-Functionalized MWCNTs Nanocomposites. Polymers 2019, 11, 615. [Google Scholar] [CrossRef]
- Muñoz-pascual, S.; Lopez-gonzalez, E.; Saiz-arroyo, C. Effect of Mold Temperature on the Impact Behavior and Morphology of Injection Molded Foams Based on Polypropylene Polyethylene—Octene. Polymers 2019, 11, 894. [Google Scholar] [CrossRef]
- Dimitrakakis, E.; Janz, A.; Bilitewski, B.; Gidarakos, E. Small SWEE: Determining recyclables and hazardous substances in plastics. J. Hazard. Mater. 2009, 161, 913–919. [Google Scholar] [CrossRef]
- Martinho, G.; Pires, A.; Saraiva, L.; Ribeiro, R. Composition of plastics from waste electrical and electronic equipment (WEEE) by direct sampling. Waste Manag. 2012, 32, 1213–1217. [Google Scholar] [CrossRef]
- Zdiri, K.; Elamri, A.; Hamdaoui, M. Reinforcement of recycled PP polymers by nanoparticles incorporation. Green Chem. Lett. Rev. 2018, 11, 296–311. [Google Scholar] [CrossRef]
- Wang, K.; Bahlouli, N.; Adddiego, F. Elastic and yield behaviours of recycled polypropylene-based composites: Experimental and modelling study. Compos. Part B Eng. 2016, 99, 132–152. [Google Scholar]
- Ahmed, M.R. Effect of Recycling in Post-Consumer Polystyrene Cups; Arcada University of Applied Sciences: Helinski, Finland, 2016; pp. 7–60. Available online: https://www.theseus.fi/bitstream/handle/10024/113847/theseus%20mehnaz.pdf?sequence=1&isAllowed=y (accessed on 27 January 2019).
- Jmal, H.; Bahlouli, N.; Wagner-Kocher, C.; Ruch, F. Influence of the grade on the variability of the mechanical properties of polypropylene waste. Waste Manag. 2018, 1–13. [Google Scholar] [CrossRef]
- Pospõâsï, J.; Horak, Z.; Krulis, Z. Degradation and aging of polymer blends I. Thermomechanical and thermal degradation. Polym. Degrad. Stab. 2009, 65, 405–414. [Google Scholar]
- Brems, A.; Baeyens, J.; Dewil, R. Recycling And Recovery of Post-Consumer Plastic. Therm. Sci. 2012, 16, 669–685. [Google Scholar] [CrossRef]
- Kwon, D.E.; Park, B.K. Solid-State Foaming of Acrylonitrile-Butadiene-Styrene/Recycled Polyethylene Terephthalate Using Carbon Dioxide as a Blowing Agent. Polymers 2019, 11, 291. [Google Scholar] [CrossRef]
- Lago, E.D.; Boaretti, C.; Piovesan, F. The Effect of Different Compatibilizers on the Properties of a Post-Industrial PC/PET Blend. Materials 2019, 12, 49. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Alcock, B.; Cabrera, N.O.; Reynolds, C.T. The effect of temperature and strain rate on the mechanical properties of highly oriented polypropylene tapes and all-polypropylene composites. Compos. Sci. Technol. 2007, 67, 2061–2070. [Google Scholar] [CrossRef]
- Schmidt, P.N.S.; Cioffi, M.O.H.; Voorwald, H.J.C. Flexural Test On Recycled Polystyrene. Procedia Eng. 2011, 10, 930–935. [Google Scholar] [CrossRef]
- EAG LABORATORIES. Characterization of Polymers Using Differential Scanning Calorimetry (DSC). Available online: https://www.eag.com/resources/whitepapers/characterization-of-polymers-using-differential-scanning-calorimetry-dsc/ (accessed on 3 February 2019).
- Brun, N.; Bourson, P.; Margueron, S. Study of the thermal behavior of syndiotactic and atactic polystyrene by Raman spectroscopy. AIP Conf. Proc. 2011, 1353, 856–860. [Google Scholar] [CrossRef]
- Santana, R.C.; Manrich, S. Studies on Thermo-Mechanical Properties of Post-Consumer High Impact Polystyrene in Five reprocessing steps. SAGE J. Prog. Rubber Plast. Recycl. Technol. 2002, 18, 99–110. [Google Scholar] [CrossRef]
- Biswal, M.; Mohanty, S.; Nayak, S.K. Recycling of engineering plastics from waste electrical and electronic equipment: Influence of virgin polycarbonate and impact modifier on the final perfprmance of blends. Waste Manag. Res. 2014, 371–387. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Analytical strategies for the quality assessment of recycled high-impact polystyrene: A combination of thermal analysis, vibrational spectroscopy, and chromatography. Anal. Chim. Acta 2007, 604, 18–28. [Google Scholar] [CrossRef]
- Matei, E.; Rapa, M.; Pica, A. Recycled Polypropylene Improved with Thermoplastic Elastomers. Hindawi Int. J. Polym. Sci. 2017, 2017. [Google Scholar] [CrossRef]
- Mat-Shayuti, M.S.; Abdullah, M.Z.; Megat-Yusoff, P.S.M. Thermal properties and morphology of Polypropylene/Polycarbonate/Polypropylene-Graft-Maleic anhydride blends. MATEC Web Conf. 2016, 69, 03001. [Google Scholar] [CrossRef]
- Rajan, G.S.; Vu, Y.T.; Mark, J.E.; Myers, C.L. Thermal and mechanical properties of polypropylene in the thermoplastic elastomeric state. Eur. Polym. J. 2004, 40, 63–71. [Google Scholar] [CrossRef]
- Gregorova, A.; Safia, A. Application of Differential Scanning Calorimetry to the Characterization of Biopolymers. In Application of Calorimetry in a Wide Context: Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalometry, 2nd ed.; Amal, A., Ed.; IntechOpen: Rijeka, Croatia, 2013; Volume 3, pp. 85–164. [Google Scholar]
- Mallick, P.K.; Zhou, Y. Effects of Temperature and Strain Rate on the Tensile Behavior of Unfilled and Talc-Filled Polypropylene. Polym. Eng. Sci. 2002, 42, 2449–2459. [Google Scholar] [CrossRef]
- Duigou, A.; Bourmaud, A.; Baley, C. What is the technical and environmental interest in reusing a recycled polypropylene-hemp fibre composite? Polym. Degrad. Stab. 2011, 96, 1732–1739. [Google Scholar] [CrossRef]
- Brachet, P.; Hinrichsen, E.L.; Melum, F. Modification of mechanical properties of recycled polypropylene from post-consumer containers. Waste Manag. 2008, 28, 2456–2464. [Google Scholar] [CrossRef]
- Bajracharya, R.M.; Manalo, A.C. Effect of elevated temperature on the tensile properties of recycled mixed plastic waste. In Proceedings of the 23rd Australasian Conference on the Mechanics of Structures and Materials, Byron Bay, Australia, 9–12 December 2014; Volume 1, pp. 281–286. [Google Scholar]
- Bayer, S.; Delale, F.; Liaw, M. Effect of Temperature On Mechanical Properties of Nanoclay Reinforced Polymeric Nanocomposites. J. Compos. Mater. 2012, 27, 491–504. [Google Scholar]
- Younesi, M.; Bahrololoom, M.E. Producing toughened PP/HA-LLDPE ternary bio-composite using a two-step blending method. Mater. Des. 2009, 30, 4253–4259. [Google Scholar] [CrossRef]
- Sahin, S.; Yayla, P. Effects of testing parameters on the mechanical properties of polypropylene random copolymer. Polym. Test. 2005, 24, 613–619. [Google Scholar] [CrossRef]
- Benzarti, K.; Collin, X. Understanding the durability of advanced fibre-reinforced polymer (FRP) composites for structural applications. In Advanced Fibre-Reinforced Polymer (FRP) Composites for Structural Applications; Woodhead Publishing Limited-Elsevier: Sawston, UK, 2013; pp. 361–439. [Google Scholar]
- Hirayama, D.; Saron, C. Morphologic and mechanical properties of blends from recycled acrylonitrile-butadiene-styrene and high-impact polystyrene. Polymer 2018, 135, 271–278. [Google Scholar] [CrossRef]
- Othman, M.H.; Tun, U.; Onn, H. Recycled Polypropylene-Nanoclay Composites—Mechanical Properties. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2019; 4195p. [Google Scholar]
- Tamer, S.; Sahin, T.; Senol, S. The effect of natural weathering on the mechanical, morphological and thermal properties of high impact polystyrene (HIPS). Mater. Des. 2007, 28, 2303–2309. [Google Scholar] [CrossRef]
- Jiménez, A.; Torre, L.; Kenny, J.M. Processing and properties of recycled polypropylene modified with elastomers. Plast. Rubber Compos. 2003, 32, 357–367. [Google Scholar] [CrossRef]
- Homkhiew, C.; Ratanawilai, T.; Thongruang, W. Time-temperature and stress-dependent behaviors of composites made from recycled polypropylene and rubberwood flour. Constr. Build. Mater. 2014, 66, 98–104. [Google Scholar] [CrossRef]
- Ratanawilai, T.; Homkhiew, C.; Thongruang, W. Effects of natural weathering on the properties of recycled polypropylene composites reinforced with rubberwood flour. Ind. Crops Prod. 2014, 56, 52–59. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).