In the Search for Sustainable Processing in Compounds Containing Recycled Natural Rubber: The Role of the Reversion Process
Abstract
Highlights:
- -
- Blends raw natural rubber (NR)/residue of NR.
- -
- Recycling of NR at low cost.
- -
- Milling at room temperature.
- -
- Possible solution to the problem of the final disposal of solid residues, concerning NR.
- -
- Relationship among vulcanization characteristics, dynamic-mechanical, morphology and mechanical properties of blends containing recycled NR.
- -
- Green chemistry.
1. Introduction
2. Experimental
2.1. Materials
2.2. Mixing and Preparation of the Tensile Tests Samples
2.3. Characterization of the Blends
3. Results and Discussion
3.1. Vulcanization Characteristics
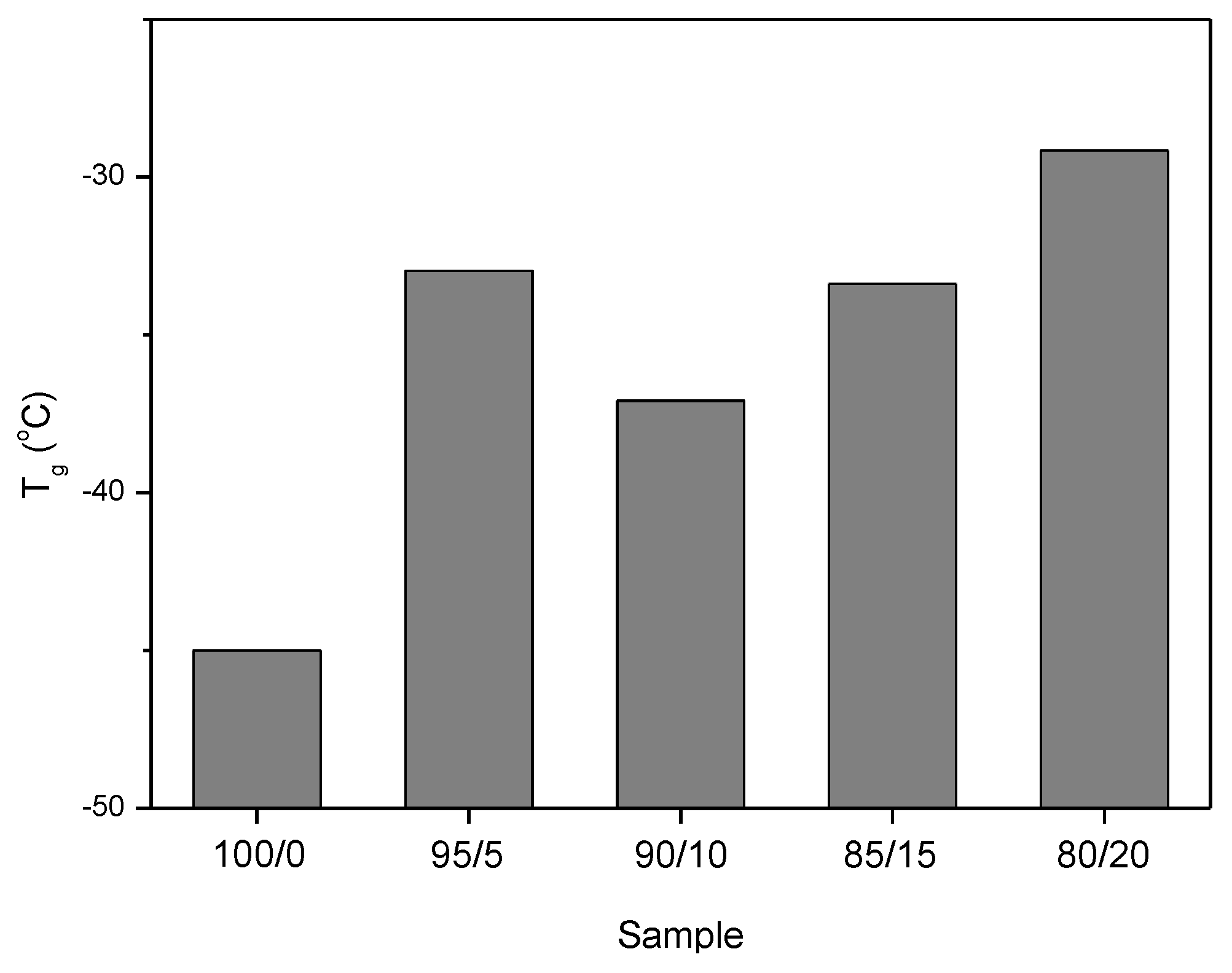
3.2. Dynamic-Mechanical Properties
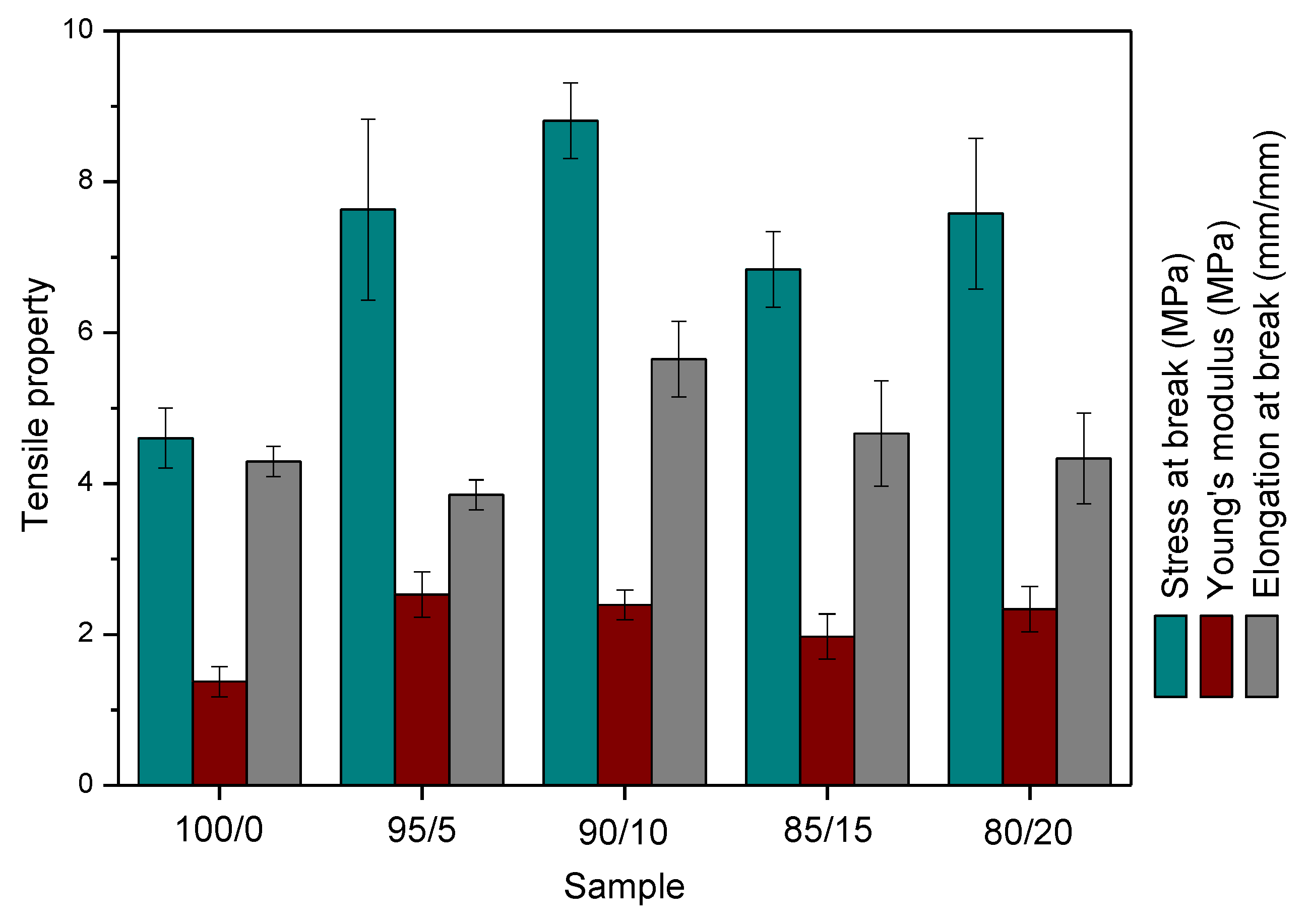
3.3. Mechanical Properties
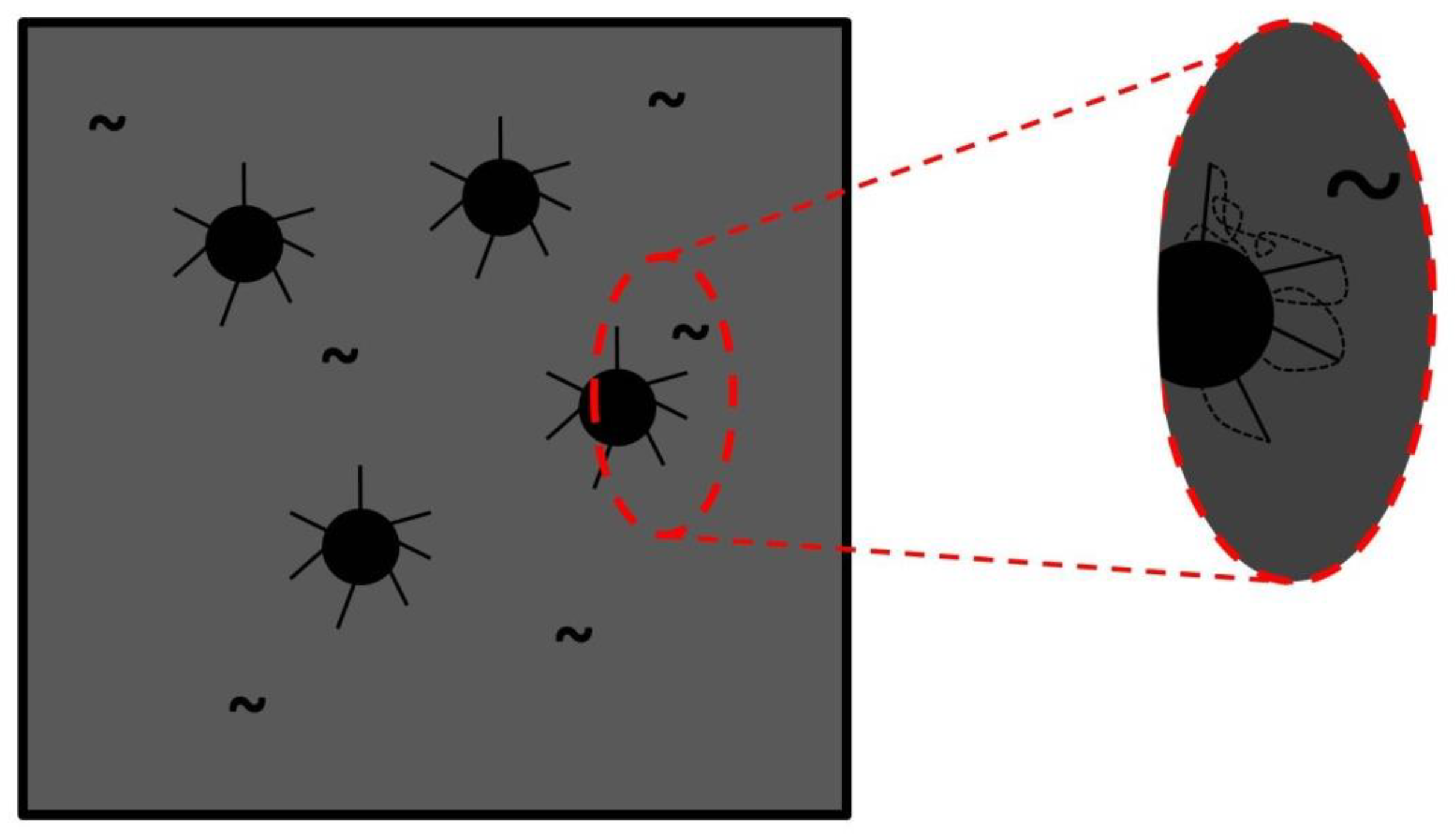
3.4. Morphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Sousa, F.D.B. Devulcanization of elastomers and applications. In Elastomers; Çankaya, N., Ed.; Intech: Rijeka, Croatia, 2017; Chapter 10; pp. 209–230. [Google Scholar]
- Mandal, S.K.; Alam, N.; Debnath, S.C. Reclaiming of ground rubber tire by safe multifunctional rubber additives: I. Tetra benzylthiuram disulfide. Rubber Chem. Technol. 2012, 85, 629–644. [Google Scholar] [CrossRef]
- Ramarad, S.; Khalid, M.; Ratnam, C.T.; Luqman Chuah, A.; Rashmi, W. Waste tire rubber in polymer blends: A review on the evolution, properties and future. Prog. Mater. Sci. 2015, 72, 100–140. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Siqueira, D.D.; Araújo, E.M.; Morais, D.D.S.; Bezerra, E.B. Toughening of polystyrene using styrene-butadiene rubber (SBRr) waste from the shoe industry. REM Int. Eng. J. 2018, 71, 253–260. [Google Scholar] [CrossRef]
- Zanchet, A.; De Sousa, F.D.B.; Crespo, J.S.; Scuracchio, C.H. Activator from sugar cane as a green alternative to conventional vulcanization additives. J. Clean. Prod. 2017, 174, 437–446. [Google Scholar] [CrossRef]
- US EPA. Available online: http://www.epa.gov/greenchemistry (accessed on 21 September 2018).
- De Sousa, F.D.B.; Zanchet, A.; Scuracchio, C.H. Influence of reversion in compounds containing recycled natural rubber: In search of sustainable processing. J. Appl. Polym. Sci. 2017, 134, 45325. [Google Scholar] [CrossRef]
- Imbernon, L.; Norvez, S. From landfilling to vitrimer chemistry in rubber life cycle. Eur. Polym. J. 2016, 82, 347–376. [Google Scholar] [CrossRef]
- Myhre, M.; Saiwari, S.; Dierkes, W.; Noordermeer, J. Rubber recycling: Chemistry, processing, and applications. Rubber Chem. Technol. 2012, 85, 408–449. [Google Scholar] [CrossRef]
- Da Costa, H.M.; Ramos, V.D.; Da Silva, W.S.; Sirqueira, A.S. Analysis and optimization of polypropylene (PP)/ethylene-propylene-diene monomer (EPDM)/scrap rubber tire (SRT) mixtures using RSM methodology. Polym. Test. 2010, 29, 572–578. [Google Scholar] [CrossRef]
- Bhadane, P.A.; Cheng, J.; Ellul, M.D.; Favis, B.D. Decoupling of reactions in reactive polymer blending for nanoscale morphology control. J. Polym. Sci. 2012, 50, 1619–1629. [Google Scholar] [CrossRef]
- Zanchet, A.; Carli, L.N.; Giovanela, M.; Crespo, J.S.; Scuracchio, C.H.; Nunes, R.C.R. Characterization of microwave-devulcanized composites of ground SBR scraps. J. Elastom. Plast. 2009, 41, 497–507. [Google Scholar] [CrossRef]
- Gujel, A.A.; Bandeira, M.; Veiga, V.D.; Giovanela, M.; Carli, L.N.; Mauler, R.S.; Brandalise, R.N.; Crespo, J.S. Development of bus body rubber profiles with additives from renewable sources: Part I—Additives characterization and processing and cure properties of elastomeric compositions. Mater. Des. 2014, 53, 1112–1118. [Google Scholar] [CrossRef]
- Fang, Y.; Zhan, M.; Wang, Y. The status of recycling of waste rubber. Mater. Des. 2001, 22, 123–128. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Azura, A.R. Comparison of thermo-oxidative ageing and thermal analysis of carbon black-filled NR/Virgin EPDM and NR/Recycled EPDM blends. Polym. Test. 2013, 32, 631–639. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Azura, A.R. Optimisation of accelerators and vulcanising systems on thermal stability of natural rubber/recycled ethylene-propylene-diene-monomer blends. Mater. Des. 2014, 53, 651–661. [Google Scholar] [CrossRef]
- De Sousa, F.D.B.; Gouveia, J.R.; De Camargo Filho, P.M.F.; Vidotti, S.E.; Scuracchio, C.H.; Amurin, L.G.; Valera, T.S. Blends of ground tire rubber devulcanized by microwaves/HDPE—Part A: Influence of devulcanization process. Polím. Ciênc. Tecnol. 2015, 25, 256–264. [Google Scholar] [CrossRef]
- De Sousa, F.D.B.; Gouveia, J.R.; De Camargo Filho, P.M.F.; Vidotti, S.E.; Scuracchio, C.H.; Amurin, L.G.; Valera, T.S. Blends ground tire rubber devulcanized by microwaves/HDPE—Part B: Influence of clay addition. Polím. Ciênc. Tecnol. 2015, 25, 382–391. [Google Scholar] [CrossRef]
- De Sousa, F.D.B.; Scuracchio, C.H.; Hu, G.H.; Hoppe, S. Effects of processing parameters on the properties of microwave-devulcanized ground tire rubber/polyethylene dynamically revulcanized blends. J. Appl. Polym. Sci. 2016, 133, 43503. [Google Scholar] [CrossRef]
- Hirayama, D.; Scuracchio, C.H.; Saron, C. Microwave devulcanization of SBR containing carbon black. J. Res. Updat. Polym. Sci. 2016, 5, 52–59. [Google Scholar]
- De Sousa, F.D.B.; Scuracchio, C.H.; Hu, G.H.; Hoppe, S. Devulcanization of waste tire rubber by microwaves. Polym. Degrad. Stab. 2017, 138, 169–181. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Seghar, S.; Aït Hocine, N. Recycling of rubber wastes by devulcanization. Resour. Conserv. Recycl. 2018, 133, 250–262. [Google Scholar] [CrossRef]
- Jin, K.; Li, L.; Torkelson, J.M. Recyclable crosslinked polymer networks via one-step controlled radical polymerization. Adv. Mater. 2016, 28, 6746–6750. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Zanetti, M.C.; Santagata, E.; Blengini, G.A. Life cycle assessment applied to bituminous mixtures containing recycled materials: Crumb rubber and reclaimed asphalt pavement. Res. Conserv. Recycl. 2017, 117, 204–212. [Google Scholar] [CrossRef]
- Carli, L.N.; Bianchi, O.; Mauler, R.S.; Crespo, J.S. Crosslinking kinetics of SBR composites containing vulcanized ground scraps as filler. Polym. Bull. 2011, 67, 1621–1631. [Google Scholar] [CrossRef]
- Zanchet, A.; Dotta, A.L.B.; De Sousa, F.D.B. Relationship among vulcanization, mechanical properties and morphology of blends containing recycled EPDM. Recycling 2017, 2. [Google Scholar] [CrossRef]
- Weber, T.; Zanchet, A.; Brandalise, R.N.; Crespo, J.S.; Nunes, R.C.R. Grinding and characterization of scrap rubbers powders. J. Elastom. Plast. 2008, 40, 147–159. [Google Scholar] [CrossRef]
- Weber, T.; Zanchet, A.; Crespo, J.S.; Oliveira, M.G.; Suarez, J.C.M.; Nunes, R.C.R. Caracterização de artefatos elastoméricos obtidos por revulcanização de resíduo industrial de SBR (copolímero de butadieno e estireno). Polím. Ciênc. Tecnol. 2011, 21, 429–435. [Google Scholar] [CrossRef]
- Zanchet, A.; Dal’Acqua, N.; Weber, T.; Crespo, J.S.; Brandelise, R.N.; Nunes, R.C.R. Propriedades reométricas e mecânicas e morfologia de compósitos desenvolvidos com resíduos elastoméricos vulcanizados. Polím. Ciênc. Tecnol. 2007, 17, 23–27. [Google Scholar] [CrossRef]
- De Sousa, F.D.B. Vulcanization of natural rubber: Past, present and future perspectives. In Natural Rubber: Properties, Behaior and Applications; Hamilton, J.L., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 47–88. [Google Scholar]
- Zanchet, A.; Carli, L.N.; Giovanela, M.; Brandelise, R.M.; Crespo, J.S. Use of styrene butadiene rubber industrial waste devulcanized by microwave in rubber composites for automotive application. Mater. Des. 2012, 39, 437–443. [Google Scholar] [CrossRef]
- Morrison, N.J.; Porter, M. Temperature effects on the stability of intermediates and crosslinks in sulfur vulcanization. Rubber Chem. Technol. 1984, 57, 63–85. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; De, S.K.; Chakraborty, S.N. Effect of vulcanization temperature and vulcanization systems on the structure and properties of natural rubber vulcanizates. Polymer 1977, 18, 1243–1249. [Google Scholar] [CrossRef]
- Kok, C.M. The effects of compounding variables on the reversion process in the sulphur vulcanization of natural rubber. Eur. Polym. J. 1987, 23, 611–615. [Google Scholar] [CrossRef]
- Ismail, H.; Anuar, H.; Tsukahara, Y. Effects of palm oil fatty acid on curing characteristics, reversion and fatigue life of various natural rubber compounds. Polym. Int. 1999, 48, 607–613. [Google Scholar] [CrossRef]
- Isayev, A.I.; Yushanov, S.P.; Kim, S.H.; Levin, V.Y. Ultrasonic devulcanization of waste rubbers: Experimentation and modeling. Rheol. Acta 1996, 35, 616–630. [Google Scholar] [CrossRef]
- Isayev, A.I.; Chen, J.; Tukachinsky, A. Novel ultrasonic technology for devulcanizatio of waste rubbers. Rubber Chem. Technol. 1995, 68, 267–280. [Google Scholar] [CrossRef]
- Oh, J.S.; Ghose, S.; Isayev, A.I. Effects of ultrasonic treatment on unfilled butadiene rubber. J. Polym. Sci. 2003, 41, 2959–2968. [Google Scholar] [CrossRef]
- Oh, J.S.; Isayev, A.I. Continuous ultrasonic devulcanization of unfilled butadiene rubber. J. Appl. Polym. Sci. 2004, 93, 1166–1174. [Google Scholar] [CrossRef]
- Li, S.Y.; Lamminmaki, J.; Hanhi, K. Effect of ground rubber powder and devulcanizates on the properties of natural rubber compounds. J. Appl. Polym. Sci. 2005, 97, 208–217. [Google Scholar] [CrossRef]
- Garcia, P.S.; De Sousa, F.D.B.; De Lima, J.A.; Cruz, S.A.; Scuracchio, C.H. Devulcanization of ground tire rubber: Physical and chemical changes after different microwave exposure times. Express Polym. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Ghosh, P.; Chakrabarti, A. Conducting carbon black filled EDPM vulcanizates: Assessment of dependence of physical and mechanical properties and conducting character on variation of filler loading. Eur. Polym. J. 2000, 36, 1043–1054. [Google Scholar] [CrossRef]
- Jacob, C.; Bhattacharya, A.K.; Bhowmick, A.K.; De, P.P.; De, S.K. Recycling of ethylene propylene diene monomer (EPDM) waste. III. Processability of EPDM rubber compound containing ground EPDM vulcanizates. J. Appl. Polym. Sci. 2003, 87, 2204–2215. [Google Scholar] [CrossRef]
- Gomide, R. Operações Unitárias: Operações com Sistemas Sólidos Granulares; Câmara Brasileira do Livro: São Paulo, Brazil, 1983. [Google Scholar]
- Jacob, C.; De, P.P.; Bhowmick, A.K.; De, S.K. Recycling of EPDM waste. I. Effect of ground EPDM vulcanizate on properties of EPDM rubber. J. Appl. Polym. Sci. 2001, 82, 3293–3303. [Google Scholar] [CrossRef]
- Bezerra, A.; Santos, A.C.; Costa, H.; Ramos, V.D. Efeito do óleo de linhaça e do óleo de amendoim sobre a vulcanização da borracha natural (NR). Parte I: Modelo generalizado. Polím. Ciênc. Tecnol. 2013, 23, 395–401. [Google Scholar] [CrossRef]
- Shibulal, G.S.; Jang, J.; Yu, H.C.; Huh, Y.I.; Nah, C. Cure characteristics and physico-mechanical properties of a conventional sulphur-cured natural rubber with a novel anti-reversion agent. J. Polym. Res. 2016, 23, 237. [Google Scholar] [CrossRef]
- De Sousa, F.D.B.; Scuracchio, C.H. The role of carbon black on devulcanization of natural rubber by microwaves. Mater. Res. 2015, 18, 791–797. [Google Scholar] [CrossRef]
- Cao, L.M.; Cao, X.D.; Jiang, X.J.; Xu, C.H.; Chen, Y.K. In situ reactive compatibilization and reinforcement of peroxide dynamically vulcanized polypropylene/ethylene-propylene-diene monomer tpv by zinc dimethacrylate. Polym. Compos. 2013, 34, 1357–1366. [Google Scholar] [CrossRef]
- Gibala, D.; Laohapisitpanich, K.; Thomas, D.; Hamed, G.R. Cure and mechanical behavior of rubber compounds containing ground vulcanizates. Part II-Mooney viscosity. Rubber Chem. Technol. 1996, 69, 115–119. [Google Scholar] [CrossRef]
- Ghorai, S.; Bhunia, S.; Roy, M.; De, D. Mechanochemical devulcanization of natural rubber vulcanizate by dual function disulfide chemicals. Polym. Degrad. Stab. 2016, 129, 34–46. [Google Scholar] [CrossRef]
- Milani, G.; Milani, F. Curing degree prediction for S-TBBS-DPG natural rubber by means of a simple numerical model accounting for reversion and linear interaction. Polym. Test. 2016, 52, 9–23. [Google Scholar] [CrossRef]
- Zhang, X.X.; Lu, C.H.; Liang, M. Properties of natural rubber vulcanizates containing mechanochemically devulcanized ground tire rubber. J. Polym. Res. 2009, 16, 411–419. [Google Scholar] [CrossRef]
- Boonkerd, K.; Deeprasertkul, C.; Boonsomwong, K. Effect of sulfur to accelerator ratio on crosslink structure, reversion, and strength in natural rubber. Rubber Chem. Technol. 2016, 89, 450–464. [Google Scholar] [CrossRef]
- Oliveira, M.G.; Soares, B.G. Influência do sistema de vulcanização nas propriedades da mistura NBR/EPDM. Polím. Ciênc. Tecnol. 2002, 12, 11–19. [Google Scholar] [CrossRef]
- Rabiei, S.; Shojaei, A. Vulcanization kinetics and reversion behavior of natural rubber/styrene-butadiene rubber blend filled with nanodiamond—The role of sulfur curing system. Eur. Polym. J. 2016, 81, 98–113. [Google Scholar] [CrossRef]
- Sun, X.; Isayev, A.I. Continuous ultrasonic devulcanization: Comparison of carbon black filled synthetic isoprene and natural rubbers. Rubber Chem. Technol. 2008, 81, 19–46. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; De, S.K. Kinetics of crosslinking and network changes in natural rubber vulcanizates with a dithiodimorpholine based accelerator system. Rubber Chem. Technol. 1980, 53, 1015–1022. [Google Scholar] [CrossRef]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Vulcanization of natural rubber modified with cashew nut shell liquid and its phosphorylated derivative-a comparative study. Polymer 1998, 39, 4033–4036. [Google Scholar] [CrossRef]
- Isayev, A.I.; Sujan, B. Nonisothermal vulcanization of devulcanized GRT with reversion type behavior. J. Elastom. Plast. 2006, 38, 291–318. [Google Scholar] [CrossRef]
- Sui, G.; Zhong, W.H.; Yang, X.P.; Yu, Y.H. Curing kinetics and mechanical behavior of natural rubber reinforced with pretreated carbon nanotubes. Mater. Sci. Eng. 2008, 485, 524–531. [Google Scholar] [CrossRef]
- Milani, G.; Leroy, E.; Milani, F.; Deterre, R. Mechanistic modeling of reversion phenomenon in sulphur cured natural rubber vulcanization kinetics. Polym. Test. 2013, 32, 1052–1063. [Google Scholar] [CrossRef]
- Sethuraj, M.R.; Mathew, N.M. Natural Rubber: Biology, Cultivation and Technology; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Nasir, M.; Teh, G.K. The effects of various types of crosslinks on the physical properties of natural rubber. Eur. Polym. J. 1988, 24, 733–736. [Google Scholar] [CrossRef]
- Levin, V.Y.; Kim, S.H.; Isayev, A.I.; Massey, J.; VonMeerwall, E. Ultrasound devulcanization of sulfur vulcanized SBR: Crosslink density and molecular mobility. Rubber Chem. Technol. 1996, 69, 104–114. [Google Scholar] [CrossRef]
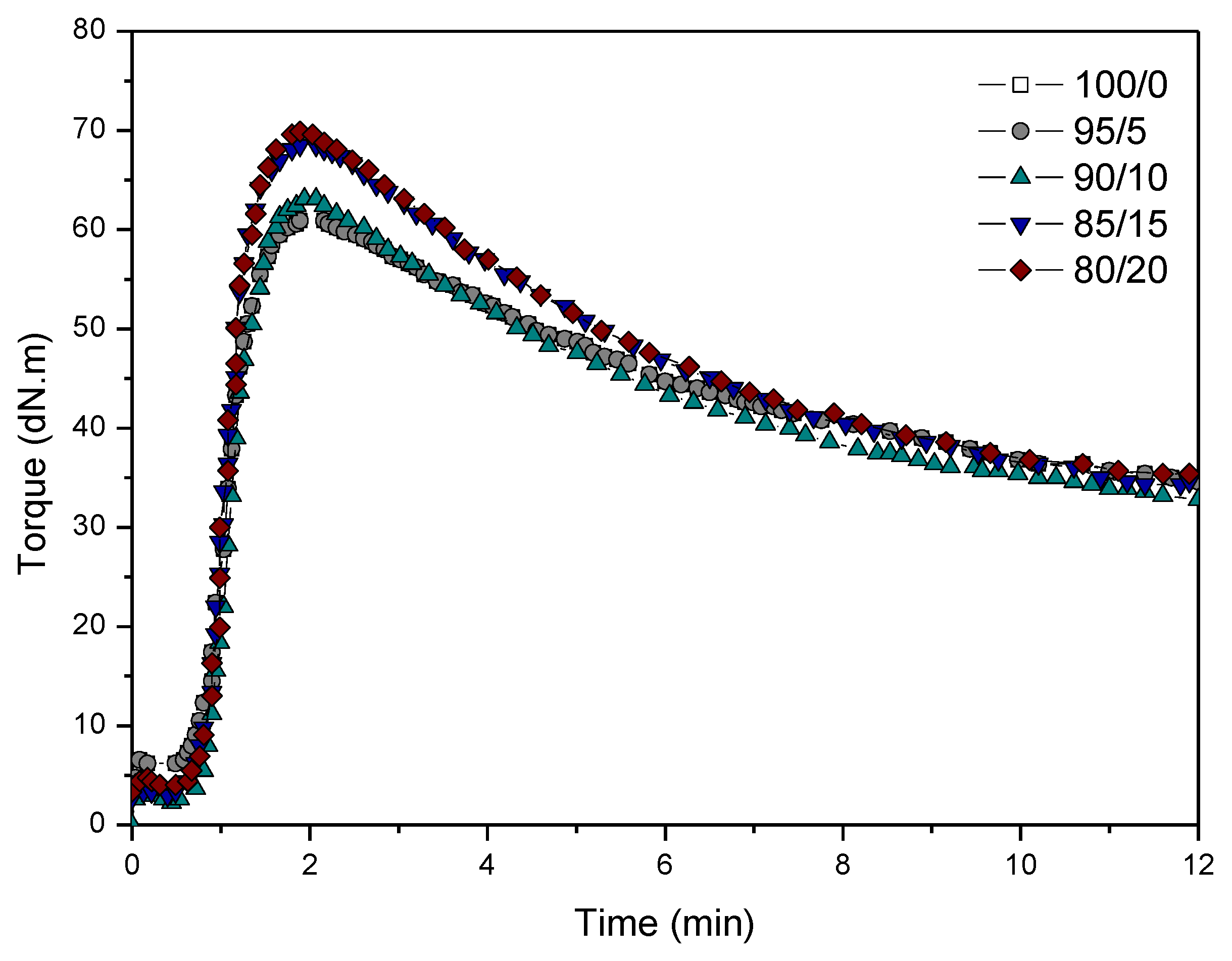


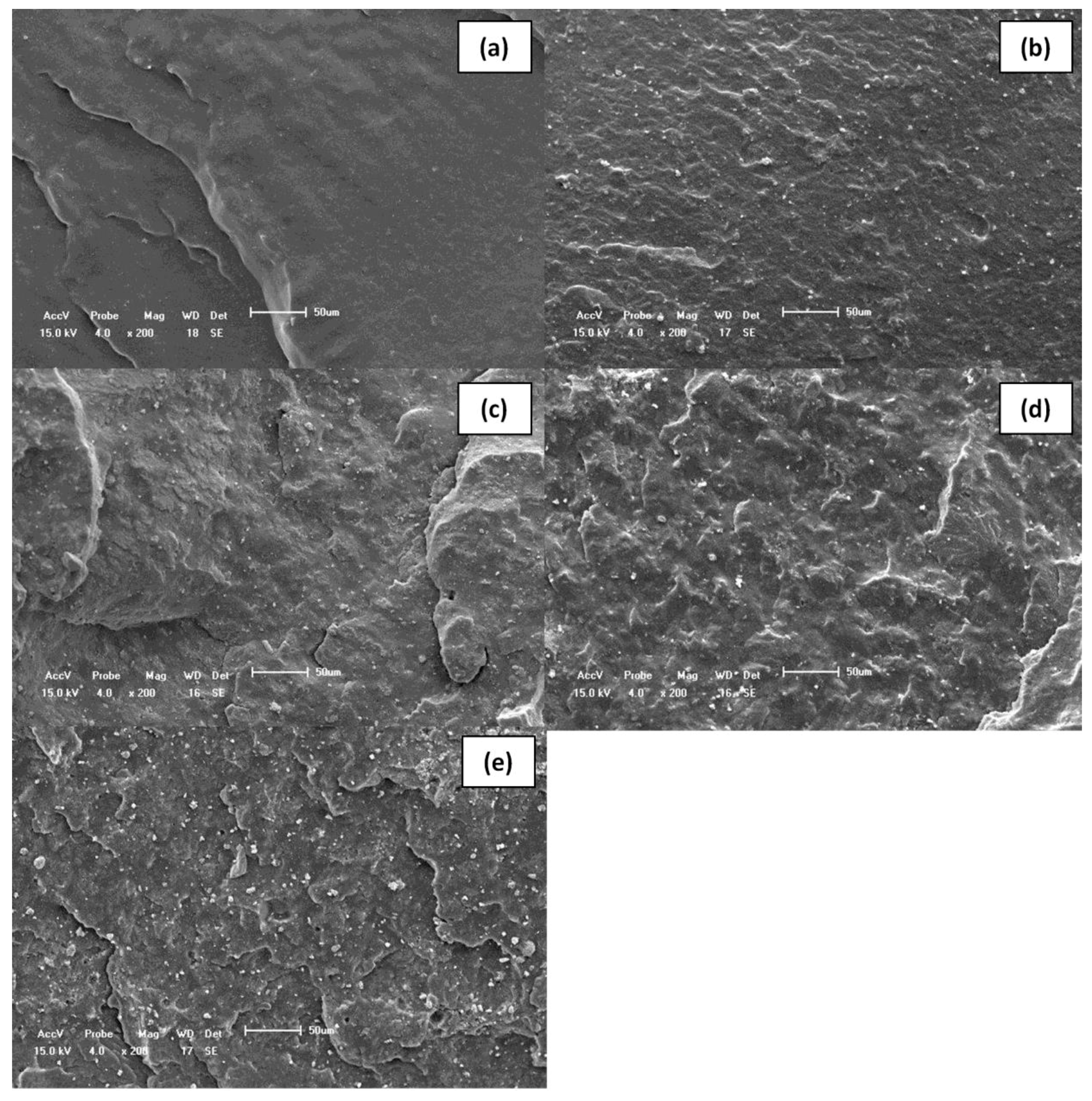
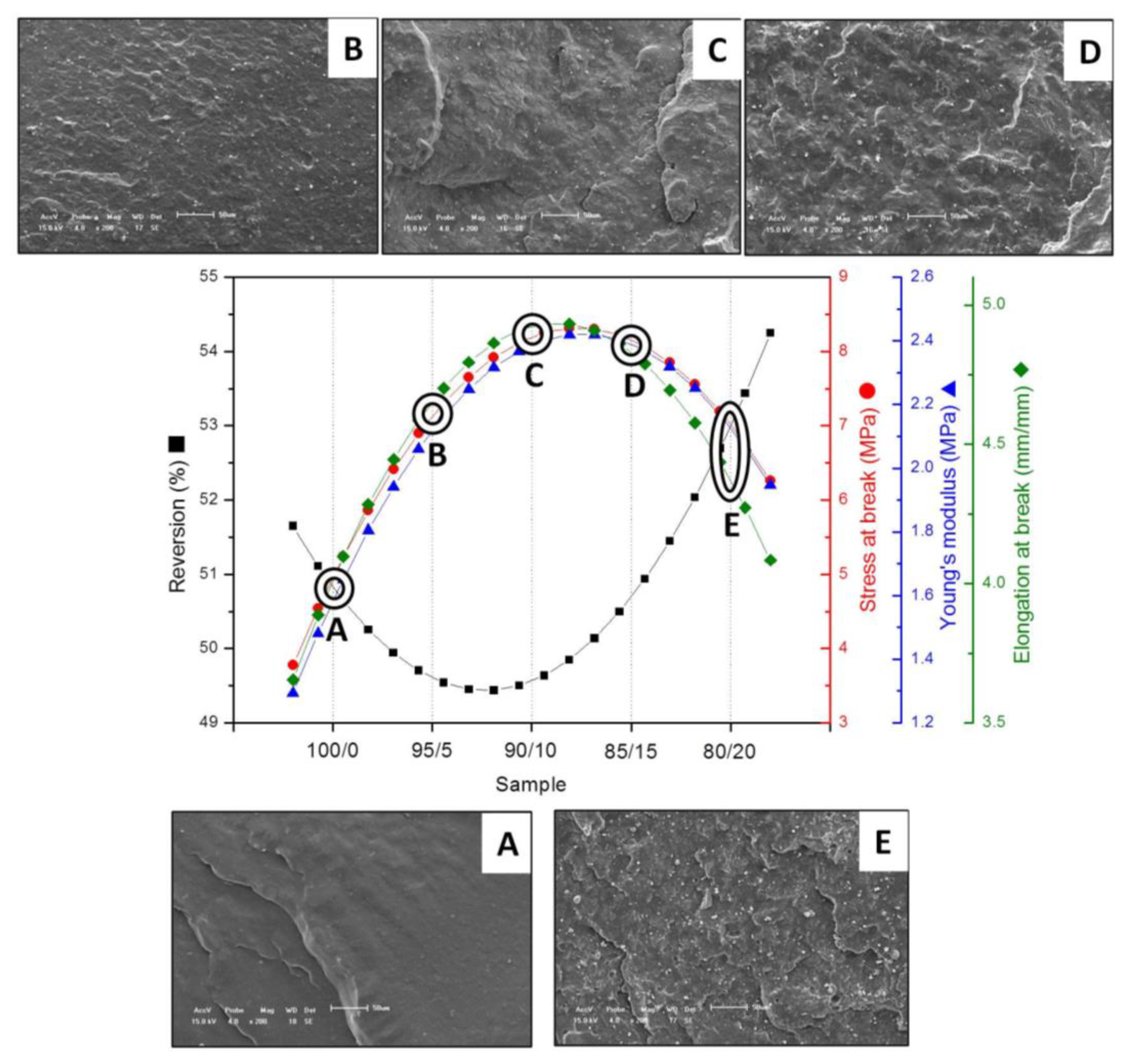
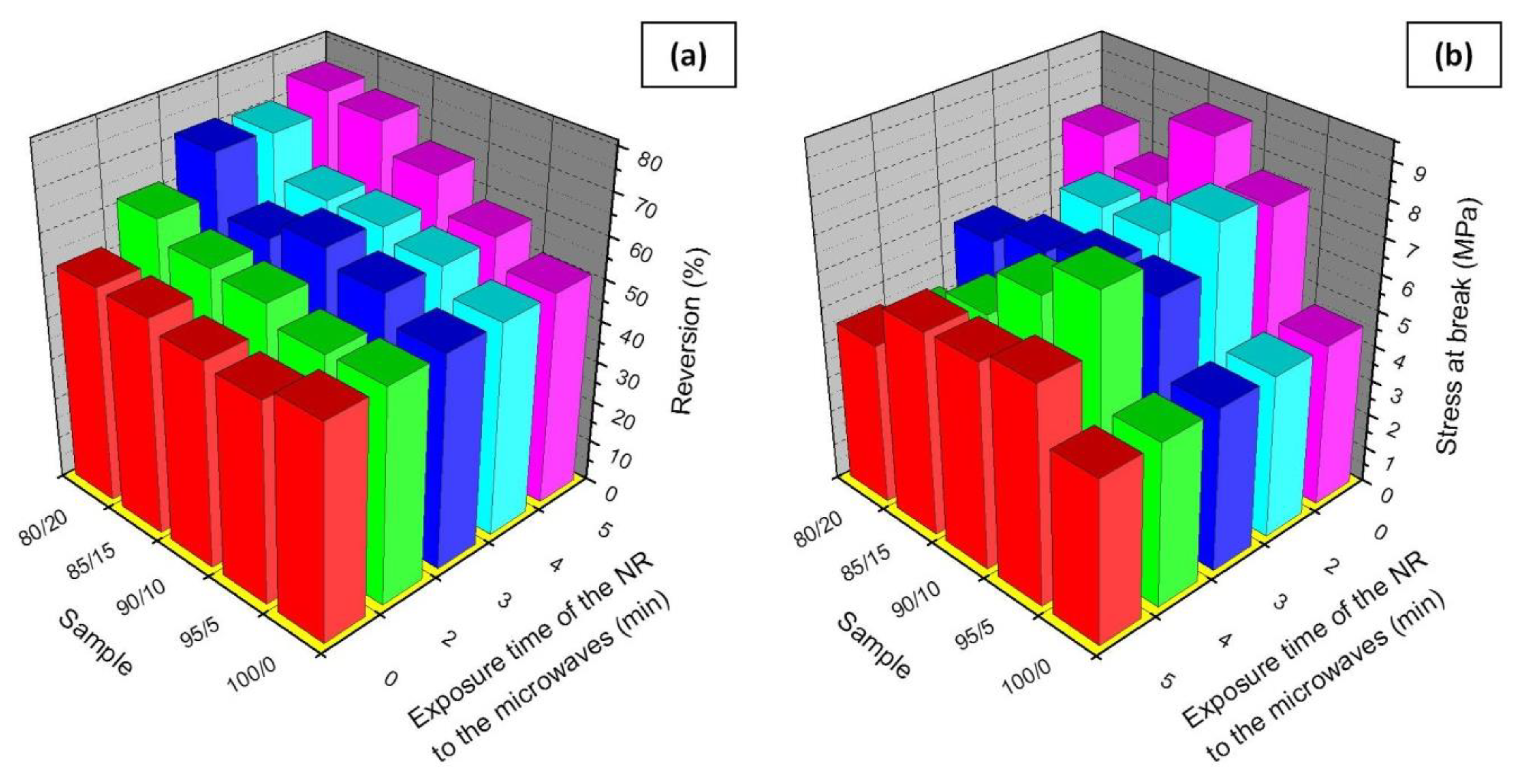
| Sample | ts1 (min) | t90 (min) | ML (dN·m) | MH (dN·m) | ΔM (dN·m) | R (%) |
|---|---|---|---|---|---|---|
| 100/0 | 0.63 | 1.41 | 6.30 | 60.92 | 54.62 | 51.50 |
| 95/5 | 0.62 | 1.40 | 6.28 | 60.89 | 54.61 | 48.27 |
| 90/10 | 0.66 | 1.50 | 2.20 | 63.10 | 60.90 | 49.54 |
| 85/15 | 0.57 | 1.38 | 3.01 | 68.45 | 65.44 | 52.05 |
| 80/20 | 0.66 | 1.41 | 4.15 | 69.83 | 65.68 | 52.32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sousa, F.D.B.; Zanchet, A. In the Search for Sustainable Processing in Compounds Containing Recycled Natural Rubber: The Role of the Reversion Process. Recycling 2018, 3, 47. https://doi.org/10.3390/recycling3040047
De Sousa FDB, Zanchet A. In the Search for Sustainable Processing in Compounds Containing Recycled Natural Rubber: The Role of the Reversion Process. Recycling. 2018; 3(4):47. https://doi.org/10.3390/recycling3040047
Chicago/Turabian StyleDe Sousa, Fabiula Danielli Bastos, and Aline Zanchet. 2018. "In the Search for Sustainable Processing in Compounds Containing Recycled Natural Rubber: The Role of the Reversion Process" Recycling 3, no. 4: 47. https://doi.org/10.3390/recycling3040047
APA StyleDe Sousa, F. D. B., & Zanchet, A. (2018). In the Search for Sustainable Processing in Compounds Containing Recycled Natural Rubber: The Role of the Reversion Process. Recycling, 3(4), 47. https://doi.org/10.3390/recycling3040047






