Managing Cd Containing Waste—Caught by the Past, the Circular Economy Needs New Answers
Abstract
1. Introduction
- Technical requirements can vary from one application to another and this also holds true for toxicity thresholds, as has been intensively discussed for the migration of solvents from cardboard made from printed paper to food (cf. [18] and literature cited there).
- Technical standards for materials may change during the lifetime of a product. For secondary resources originating from a recycling process only current requirements are valid. This may turn out to be a crucial problem for material recovery in cases where certain contaminants are involved: Some important chemicals previously used in products have either been restricted to certain applications (e.g., compounds containing mercury, cadmium, lead) or banned completely (e.g., asbestos, PCBs, certain brominated diphenylethers). This is partially due to international conventions (e.g., POPs are regulated by the Stockholm Convention, mercury is regulated by the Minamata Convention) or is a result of regulations at European level based on specific directives (e.g., RoHS Directive [19]) or general chemicals regulations (e.g., restricted chemicals under the REACH regulation [20]).
- As the use of chemicals varies from region to region, widely depending on different regulations, secondary materials including certain additives from the first use of the material in question (plastics, fibres from textiles, cardboard …) may be allowed in some countries but not everywhere. Global trade and transport of secondary materials may therefore lead to a proliferation of contaminants in parts of the world where these contaminants have already been banned [21,22].
2. Scope and Structure of the Study
- Some hazardous compounds are specifically regulated in products. To what extent separate collection and recycling and / or safe disposal of these products can be ensured?
- Will those hazardous compounds already regulated today appear in waste streams other than those intended?
- the successful implementation and enforcement of regulations for the reduction of emissions from point sources in Europe
- the increasing relevance of diffuse sources in Europe in parallel to the restriction of emissions from point sources
- the growing importance of the waste sector, that means opportunities for recycling Cd, but
- risks from new diffuse sources due to cross-contamination from recycling operations or leaching from landfills
- Cd in NiCd batteries and accumulators
- Cd (used as an organic salt) in window frames made from PVC
3. Cadmium (Cd)
3.1. Important Regulations
- Severe restriction of Cd and its compounds in products intended to be diffused or used in close contact with the environment
- Growing restrictions for products that remain on the market, in the case of batteries combined with waste management aimed at closing the loop through take-back and recycling targets
- Threshold limits for emissions from point sources
- Quality standards for air, water and agricultural soil
- Limits for food and drinking water contamination
3.1.1. Use of Cd and Cd-Bearing Products
- Cd compounds must not be used in polymers with a limit of 0.01% w/w; an exception is valid for mixtures and articles containing recovered PVC to facilitate the recycling of used PVC profiles from the building sector (see Section 3.3).
- Cd pigments must not be used in paints, with the general exception of mixtures where Cd is used for safety reasons and with the exception of zinc-based products (>10% w/w Zn).
- Cd must no longer be used for plating (this does not apply to articles put on market before 10 December 2011), with the exception of articles used in the aeronautical, aerospace, mining, offshore and nuclear sectors as far as high safety standards are required. The ban does not apply to safety devices in road and agricultural vehicles, railway rolling stock, and vessels as well as to electrical contacts if needed to ensure the reliability of the apparatus in question.
- Cd in brazing fillers must not exceed 0.01% w/w, with the exception of aerospace, military and safety applications.
- Cd is prohibited in jewellery put on market after 10 December 2011 (0.01% w/w threshold limit).
3.1.2. Waste and Waste Classification Regulations
3.1.3. Environmental Standards
3.2. Recycling of NiCd Batteries and Accumulators
- Electrical and electronic devices: 7.0 years
- Construction (e.g., emergency systems): 9.0 years
- Transportation (e.g., locomotives): 9.8 years
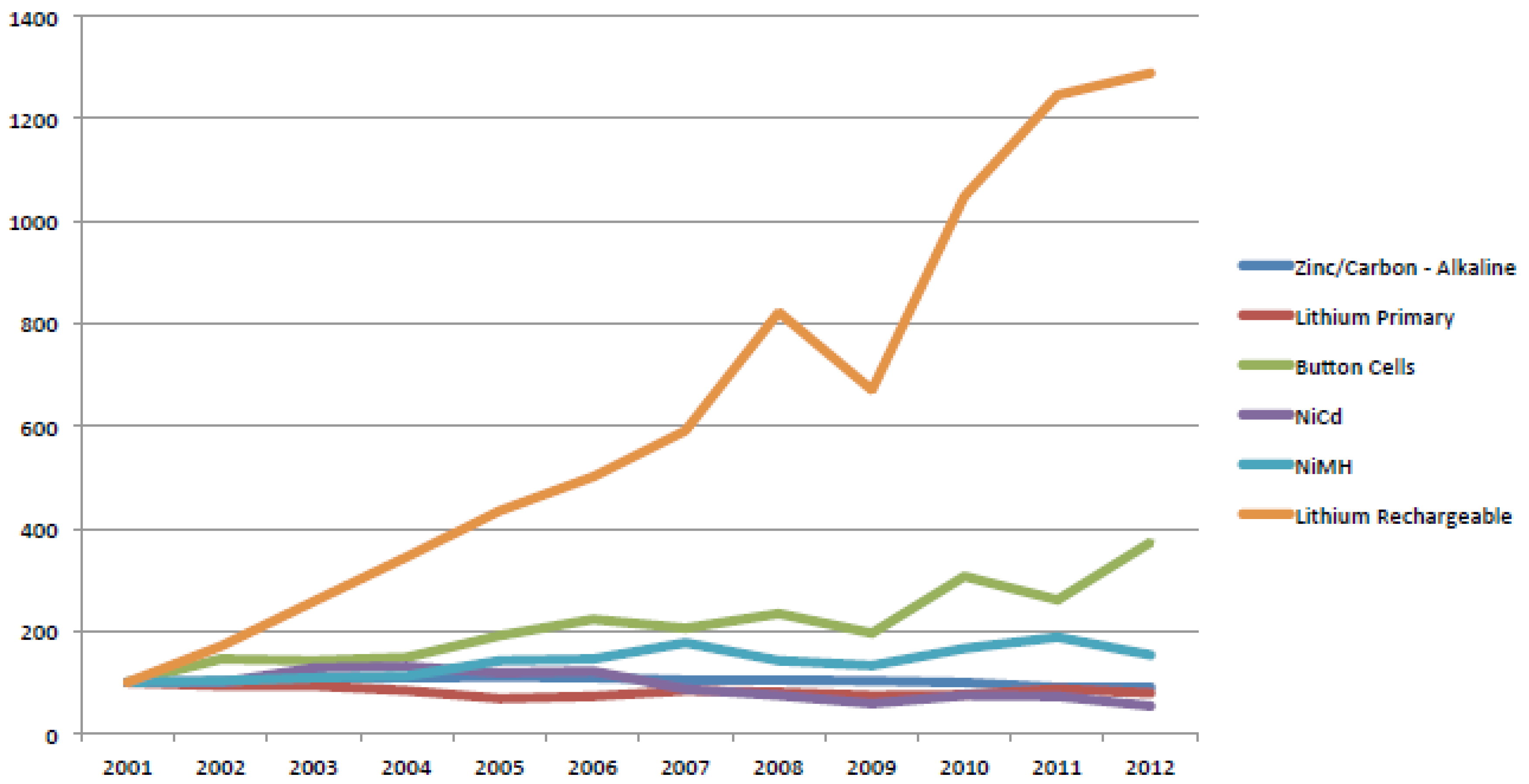
3.2.1. Production and Marketing
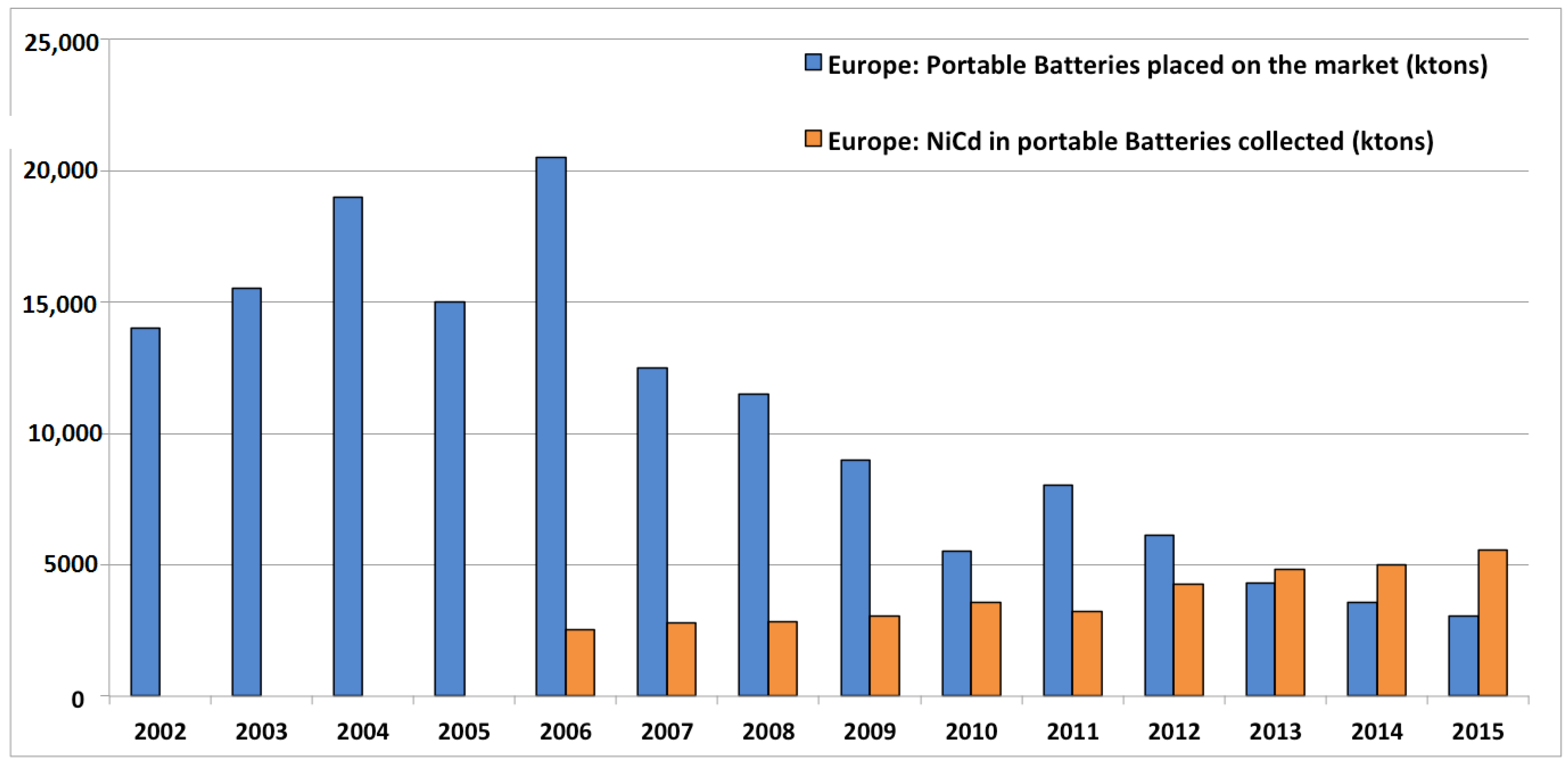
3.2.2. Collection
3.2.3. Recycling
3.2.4. Batteries in WEEE
- In integrated copper and other non-ferric metal mills, Cd is not separated for recovery. Due to its fugacity, Cd and its compounds form part of the flue dust (No 10 06 03*, European Waste Catalogue) (One of the major European non-ferric metal mills informed us on request that this waste is disposed of in a former salt mine suitable for hazardous waste.) or are emitted via the flue gas. In 2015 in Germany, 60% (=903 kg) of the Cd emissions reported in the German e-PRTR (1516 kg) were released by the metal industry (“Production of pig iron or steel (primary or secondary melting) including continuous casting >2.5 Mg h−1”), with three emitters (ThyssenKrupp Steel Europe AG, Schwelgern plant: 257 kg, Salzgitter Flachstahl GmbH, Salzgitter plant: 135 kg, ThyssenKrupp Steel Europe AG, Beeckerwerth plant: 121 kg) all above 100 kg year−1 [83].
- As a trace metal in ferrous metal recycling, Cd is separated into electric arc furnace dust (together with Zn), which should be treated in order to remove the Cd. Zn can then be recovered in an imperial smelting furnace [84].
3.2.5. Fate of Batteries Not Collected Separately
3.3. Recycling of Cd Compounds in PVC Profiles
3.3.1. Production and Use
- profiles and rigid sheets for building applications
- doors, windows, fences, shutters, walls, blinds, roof gutters, cable ducts
- pipes for non-drinking water, if the secondary PVC is only used in the middle layer of the pipe surrounded by layers made of virgin PVC in compliance with the 100 mg kg−1 Cd limit
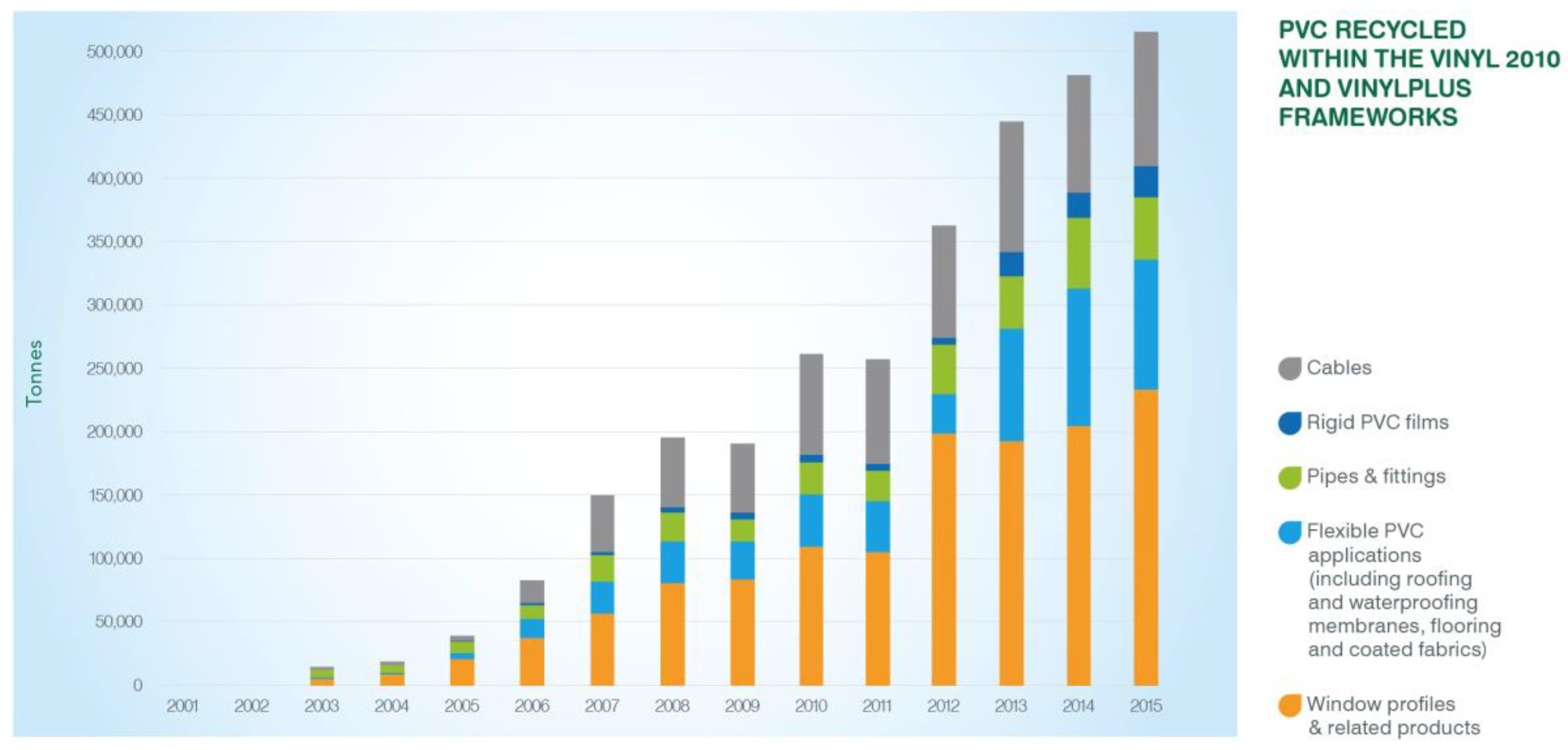
3.3.2. Recycling of PVC Profiles
3.3.3. Fate of PVC Profiles Not Collected Separately
4. Discussion
- The unknown number of portable batteries used or stored in households impedes a reliable calculation of the amount stored in the technosphere.
- The number of batteries integrated in electric appliances, which are not separated from WEEE, is unknown.
- There is no information concerning the number of industrial batteries imported into the EU and their recycling in Europe or elsewhere.
4.1. Economic Perspective
4.2. Entropy Perspective
4.3. Application and Consumption
- identification of the type of battery, also in the case of accumulators integrated in electrical appliances
- take-back stations that are easily accessible for citizens
- public awareness and incentives for the return of batteries
4.4. Time Perspective
5. Conclusions
5.1. Management of Cd-Bearing Products and Waste
5.2. Circular Economy Approaches
Author Contributions
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme (UNEP); International Solid Waste Association (ISWA). Global Waste Management Outlook; Wilson, D.C., Ed.; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2015; ISBN 978-92-807-3479-9. [Google Scholar]
- Official Journal of the European Union. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Official Journal of the European Union, 19 November 2008; L 312/3–L 312/30. [Google Scholar]
- Proposal for a Directive of the European Parliament and of the Council Amending Directive 2008/98/EC on Waste. COM/2015/0595 Final—2015/0275 (COD). 2015. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015PC0595 (accessed on 6 September 2017).
- European Commission. Factsheet: Circular Economy: Closing the Loop. From Waste to Resources. 2015. Available online: https://ec.europa.eu/commission/sites/beta-political/files/circular-economy-factsheet-waste-to-resources_en.pdf (accessed on 13 February 2018).
- UNEP. Circular Economy. An Alternative Model for Economic Development. 2006. Available online: http://www.unep.org/chinese/documents/final_circulareconomy_wholedoc.pdf (accessed on 27 October 2016).
- European Commission. Tackling the Challenges in Commodity Markets and on Raw Materials. Communication from the Commissions. COM (2011) 25 Final; Brussels. 2011. Available online: http://www.europarl.europa.eu/meetdocs/2009_2014/documents/com/com_com(2011)0025_/com_com(2011)0025_en.pdf (accessed on 10 August 2017).
- European Commission (EC). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of Regions: Roadmap to a Resource Efficient Europe; COM (2011) 571 Final. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52011DC0571&from=EN (accessed on 27 July 2017).
- Ghisellini, P.; Cialani, C.; Ulgiati, P. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. Towards the Circular Economy. Opportunities for the Consumer Goods Sector. 2013. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/publications/TCE_Report-2013.pdf (accessed on 15 December 2016).
- Ellen MacArthur Foundation; McKinsey Center for Business and Environment. Growth within a Circular Economy Vision for a Competitive Europe. 2015. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/publications/EllenMacArthurFoundation_Growth-Within_July15.pdf (accessed on 18 August 2015).
- Spangenberg, B. Was hat Recycling mit Entropie zu tun? Müll und Abfall 2000, 32, 502–504. [Google Scholar]
- Ignatenko, O.; van Schaik, A.; Reuter, M.A. Exergy as a tool for evaluation of the resource efficiency of recycling systems. Miner. Eng. 2007, 20, 862–874. [Google Scholar] [CrossRef]
- Bartl, A. Barriers and limits for recycling and moving towards “Zero Waste”. Waste Manag. Res. 2014, 32, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Kuczenski, B.; Zink, T.; Henderson, A. Common Misconceptions about Recycling. J. Ind. Ecol. 2015, 20, 1010–1017. [Google Scholar] [CrossRef]
- De Man, R.; Friege, H. Circular Economy—European Policy on Shaky Ground. Waste Manag. Res. 2016, 34, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Pivnenko, K.; Olsson, M.E.; Götze, R.; Eriksson, E.; Astrup, T.F. Quantification of chemical contaminants in the paper and board fractions of municipal solid waste. Waste Manag. 2016, 51, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (No Longer in Force). Available online: http://eur-lex.europa.eu/eli/dir/2002/95/oj (accessed on 19 February 2018).
- Official Journal of the European Union. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Official Journal of the European Union, 30 December 2006; L 396/1–L 396/849. [Google Scholar]
- Bilitewski, B.; Darbra, R.M.; Barceló, D. Global Risk-Based Management of Chemical Additives I. In The Handbook of Environmental Chemistry; Springer: Heidelberg/Berlin, Germany, 2011; Volume 18, ISBN 978-3-642-248757. [Google Scholar]
- Bilitewski, B.; Darbra, R.M.; Barceló, D. Global Risk-Based Management of Chemical Additives II. In The Handbook of Environmental Chemistry; Springer: Heidelberg/Berlin, Germany, 2013; Volume 23, ISBN 978-3-642-34572-2. [Google Scholar]
- International Agency for Research on Cancer (IARC). Cadmium and Cadmium Compounds. 2012. Available online: https://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-8.pdf (accessed on 13 February 2018).
- Official Journal of the European Union. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Official Journal of the European Union, 31 December 2008; L 353/1–L 353/1355. [Google Scholar]
- European Food Safety Authority (EFSA). Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. [Google Scholar]
- UNEP. Final Review of Scientific Information on Cadmium. Geneva. 2010. Available online: http://drustage.unep.org/chemicalsandwaste/sites/unep.org.chemicalsandwaste/files/publications/GAELP_PUB_UNEP_GC26_INF_11_Add_2_Final_UNEP_Cadmium_review_and_apppendix_Dec_2010.pdf (accessed on 3 January 2017).
- USGS. Cadmium. Statistics and Information. 2016. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/cadmium/ (accessed on 27 March 2017).
- USGS. 2015 Minerals Yearbook: Cadmium [Advance Release]. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/cadmium/myb1-2015-cadmi.pdf (accessed on 27 March 2017).
- Wellington, T.A.; Mason, T.E. The effects of population growth and advancements in technology on global mineral supply. Resour. Policy 2014, 42, 73–82. [Google Scholar] [CrossRef]
- Hawkins, T.R.; Matthews, H.S.; Hendrickson, C. Closing the Loop on Cadmium. An Assessment of the Material Cycle of Cadmium in the U.S. Int. J. Life Cycle Assess. 2006, 11, 38–48. [Google Scholar] [CrossRef]
- Ellis, T.W.; Mirza, A.H. Battery Recycling: Defining the Market and Identifying the Technology Required to Keep High Value Materials in the Economy and Out of the Waste Dump; ResearchGate Publication 265220510; 2006. Available online: https://www.nist.gov/sites/default/files/documents/2017/04/28/245_battery_recycling_defining_the_market.pdf (accessed on 10 August 2017).
- Hansen, E.; Christensen, F.M.; Lassen, C.; Jeppesen, C.N.; Wrming, M.; Kjølholt, J. Review and Survey of Cadmium and Cadmium Compounds; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2013; ISBN 978-87-92903-05-1. [Google Scholar]
- Metalpedia Cadmium Resources, Reserves and Production. Available online: http://metalpedia.asianmetal.com/metal/cadmium/resources&production.shtml (accessed on 7 May 2017).
- Lig, R.; Held, M. Cadmium flows in Europe. Rev. Métall. 2009, 106, 559–565. [Google Scholar] [CrossRef]
- International Cadmium Association (ICdA). 2017. Available online: http://www.cadmium.org (accessed on 15 June 2017).
- European Commission. Socio-Economic Impact of a Potential Update of the Restrictions on the Marketing and Use of Cadmium. Risk & Policy Analyst for Directorate-General Enterprise and Industry. Brussels. 2010. Available online: https://publications.europa.eu/en/publication-detail/-/publication/482e491d-3b83-4532-9127-896c598006d0/language-en (accessed on 4 May 2018).
- European Environment Agency (EEA). European Pollutant Release and Transfer Register (E-PRTR). Available online: http://prtr.ec.europa.eu/#/pollutantreleases (accessed on 5 August 2017).
- Cheng, K.; Tian, H.Z.; Zhao, D.; Lu, L.; Wang, Y.; Chen, L.; Liu, X.G.; Jia, W.X.; Huang, Z. Atmospheric emission inventory of cadmium from anthropogenic sources. Int. J. Environ. Sci. Technol. 2014, 11, 605–616. [Google Scholar] [CrossRef]
- Shao, X.; Cheng, H.; Li, Q.; Lin, C. Anthropogenic atmospheric emissions of cadmium in China. Atmos. Environ. 2013, 79, 155–160. [Google Scholar] [CrossRef]
- Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der guten fachlichen Praxis beim Düngen (Düngeverordnung—DüV). Available online: https://www.gesetze-im-internet.de/d_v_2017/D%C3%BCV.pdf (accessed on 7 May 2018).
- European Commission: Proposal for a Regulation on the Making Available on the Market of CE Marked Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009. 2016. Available online: http://ec.europa.eu/DocsRoom/documents/15949 (accessed on 09 November 2017).
- European Parliament Think Tank. CE-Marked Fertilising Products. 23 October 2017. Available online: http://www.europarl.europa.eu/thinktank/en/document.html?reference=EPRS_ATA(2017)608762 (accessed on 9 November 2017).
- Official Journal of the European Union. Council Directive 86/287/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Official Journal of the European Union, 4 July 1986; L 181/6–L 186/12. [Google Scholar]
- Official Journal of the European Union. Regulation (EC) No 689/2008 of the European Parliament and of the Council of 17 June 2008 concerning the export and import of hazardous chemicals. Official Journal of the European Union, 1 March 2008; L 204/1–L 204/35. [Google Scholar]
- Official Journal of the European Union. Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Official Journal of the European Union. 1 July 2017, pp. L 174/88–L 174/110. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011L0065&from=DE (accessed on 10 August 2017).
- Official Journal of the European Union. Directive 2000/53/EC of the European Parliament and of the Council on end-of-life vehicles. Official Journal of the European Union, 21 October 2000; L 269/34–L 269/42. [Google Scholar]
- Official Journal of the European Union. Commission Directive (EU) 2017/2096 of 15 November 2017 amending Annex II to Directive 2000/53/EC of the European Parliament and of the Council on end-of life vehicles. Official Journal of the European Union. 16 November 2017, pp. L 299/24–L 299/30. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017L2096 (accessed on 13 February 2018).
- Official Journal of the European Union. Directive 2009/48/EC of the European Parliament and of the Council on the safety of toys. Official Journal of the European Union, 18 June 2009; L 170/1–L 170/37. [Google Scholar]
- Official Journal of the European Union. Official Journal of the European Union. Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on batteries and accumulators and waste batteries and accumulators. Official Journal of the European Union, 26 September 2006; L 266/1–L 266/14. [Google Scholar]
- European Portable Battery Association (EPBA). The Collection of Waste Portable Batteries in Europe in View of the Achievability of the Collection Targets Set by Batteries Directive 2006/66/EC. August 2013, update December 2016. Available online: https://www.epbaeurope.net/wp-content/uploads/2017/02/Report-on-the-portable-battery-collection-rates-Update-Dec-16-full-version-FINAL-rev.1.pdf (accessed on 29 August 2017).
- Official Journal of the European Union. Directive 94/62/EC of the European Parliament and of the Council Directive of 20 December 1994 on packaging and packaging waste. Official Journal of the European Union, 31 December 1994; L 365/10–L 365/23. [Google Scholar]
- Official Journal of the European Union. Commission Decision of 18 December 2014 amending Decision 2000/532/EC on the list of waste pursuant to Directive 2008/98/EC of the European Parliament and of the Council No 2014/955/EU. Official Journal of the European Union, 30 December 2014; L 370/44–L 370/86. [Google Scholar]
- Basel Convention. Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal. 1989. Available online: http://www.basel.int/portals/4/basel%20convention/docs/text/baselconventiontext-e.pdf (accessed on 19 March 2017).
- Behrend, S. Einstufung von Abfällen nach Ihrer Gefährlichkeit anhand typischer Schadstoffparameter (Classification of Waste as Hazardous on the Basis of Typical Pollutants); Müll-Handbuch; Kz. 8033, Lfg. 1/15; Erich Schmidt Verlag: Berlin, Germany, 2015. [Google Scholar]
- Official Journal of the European Union. Directive 2013/56/EU of the European Parliament and of the Council of 20 November 2013 amending Directive 2006/66/EC of the European Parliament and of the Council on batteries and accumulators and waste batteries and accumulators as regards the placing on the market of portable batteries and accumulators containing cadmium intended for use in cordless power tools, and of button cells with low mercury content, and repealing Commission Decision 2009/603/EC. Official Journal of the European Union, 10 December 2013; L 329/5–L 329/9. [Google Scholar]
- Integrated Pollution Prevention and Control: Reference Document on the Best Available Techniques for Waste Incineration. 2006. Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/wi_bref_0806.pdf (accessed on 6 November 2017).
- JRC. Best Available Techniques (BAT) Reference Document for the Non-Ferrous Metals Industries. Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Prepared by Cusano, G.; Gonzalo, M.R., Farrell, F., Remus, R., Roudier, S., Delgado, S.; L. EUR 28648 EN. 2017. Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/NFM/JRC107041_NFM_bref2017.pdf (accessed on 9 August 2017).
- Official Journal of the European Union. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Official Journal of the European Union, 26 January 2005; L 23/3–L 23/16. [Google Scholar]
- Official Journal of the European Union. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water. Official Journal of the European Union, 24 December 2008; L 348/84–L 348/97. [Google Scholar]
- Cha, K.; Son, M.; Matsuno, Y.; Fthenakis, V.; Hur, T. Substance flow analysis of cadmium in Korea. Resour. Conserv. Recycl. 2013, 71, 31–39. [Google Scholar] [CrossRef]
- Bräutigam, K.R.; Achternbosch, M.; Hartlieb, N.; Kupsch, C.; Sardemann, G. Ressourcen- und Abfallmanagement von Cadmium in Deutschland; FZKA-Bericht 7315; Forschungszentrum Karlsruhe: Karlsruhe, Germany, 2008; Available online: https://publikationen.bibliothek.kit.edu/270074956/3815438 (accessed on 9 August 2017).
- Garcia, J.P. Aspects of Battery Recycling Legislation. Recyclia. 2015. Available online: https://de.slideshare.net/Recyclia/ma-bi-c15-pilasbateriasenglish-version (accessed on 8 August 2017).
- Official Journal of the European Union. Council Directive 91/157/EEC of 18 March 1991 on batteries and accumulators containing certain dangerous substances. Official Journal of the European Union, 26 March 1991; L 78/38–L 78/41. [Google Scholar]
- European Commission. Recast of the WEEE Directive. 2012. Available online: http://ec.europa.eu/environment/waste/weee/index_en.htm (accessed on 21 June 2012).
- European Commission. Frequently Asked Questions on Directive 2006/66/EU on Batteries and Accumulators and Waste Batteries and Accumulators. Updated Version. 2014. Available online: http://ec.europa.eu/environment/waste/batteries/pdf/faq.pdf (accessed on 22 May 2017).
- Chanson, C.; (Recharge Brussels, Belgium). Personal communication, 2017.
- Petrikowski, F.; (Umweltbundesamt, Dessau, Germany). Personal communications, 2017.
- Federal Ministry for the Environment. Nature Conservation, Building and Nuclear Safety (Bundesministerium für Umwelt, Naturschutz, Bau und Reaktorsicherheit, BMUB). Statistik Altbatterien. 2017. Available online: http://www.bmub.bund.de/themen/wasser-abfall-boden/abfallwirtschaft/statistiken/statistik-altbatterien/ (accessed on 1 August 2017).
- DPA-System. WEEE, BAT, and ELV Statistics 2014; Copenhagen, Denmark, 2015. Available online: https://www.dpa-system.dk/en/DPA/Documents?id=7854eb59-7b8d-4fcc-b58a-221f6d0b9ad5 (accessed on 21 August 2017).
- Bio Intelligence Service; ARCADIS; Institute for European Environmental Policy (IEEP). Ex-Post Evaluation of Certain Waste Stream Directives; Final Report. European Commission—DG Environment, 2014. Available online: http://ec.europa.eu/environment/waste/pdf/target_review/Final%20Report%20Ex-Post.pdf (accessed on 11 November 2017).
- Matsuno, Y.; Hur, T.; Fthenakis, V. Dynamic modelling of cadmium substance flow with zinc and steel demand in Japan. Resour. Conserv. Recycl. 2012, 61, 83–90. [Google Scholar] [CrossRef]
- European Commission; Joint Research Centre; Institute for Prospective Technological Studies. Study on the Selection of Waste Streams for End of Waste Assessment. 2009. Available online: https://www.prognos.com/uploads/tx_atwpubdb/090213_Prognos_EU-Kommission_Studie_Selection_waste_streams_01.pdf (accessed on 14 February 2017).
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M. Characterization and leaching of NiCd and NiMH spent batteries for the recovery of metals. Waste Manag. 2005, 25, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Commission Regulation No 493/2012 of 11 June 2012. Detailed rules regarding the calculation of recycling efficiencies of the recycling processes of waste batteries and accumulators. Official Journal of the European Union, 12 June 2012; L 151/9–L 151/21. [Google Scholar]
- Accurec Recycling GmbH. NiCd-Battery Recycling; Accurec Recycling GmbH: Mülheim/Ruhr, Germany; Available online: http://www.accurec.de/treatment-and-recycling/technologies/nicd-batteries (accessed on 19 February 2018).
- Weyhe, R. Stoffliches Recycling moderner Batteriesysteme. In Recycling und Rohstoffe 3; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; TK Verlag: Neuruppin, Germany, 2010; pp. 663–674. ISBN 978-3935317504. [Google Scholar]
- Espinosa, D.C.R.; Tenório, J.A.S. Fundamental aspects of recycling of nickel-cadmium batteries and accumulators through vacuum distillation. J. Power Sources 2004, 135, 320–326. [Google Scholar] [CrossRef]
- Huang, K.; Li, J.; Xu, Z. A Novel Process for Recovering Valuable Metals from Waste Nickel-Cadmium Batteries. Environ. Sci. Technol. 2009, 43, 8974–8978. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT. Waste Statistics—Electrical and Electronic Equipment. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Waste_statistics_-_electrical_and_electronic_equipment (accessed on 20 February 2016).
- Friege, H.; Reutter, L.; Gnutzmann, N.; Klöffer, A.; Mohrlok, M.; de la Sauce, A.; Wons, W.; Kross, S. Examination of batteries remaining in used electric and electronic devices. Insights gained from a transdisciplinary project. Recycling 2016, 1, 321–327. [Google Scholar] [CrossRef]
- WEEE Forum. WEEELABEX Normative Document on Treatment V10.0. 2013. Available online: http://www.weeelabex.org/wp-content/uploads/2015/10/968606_0dbec6e7617cd83ae8307684f59d4244.pdf (accessed on 31 December 2016).
- Chryssos, G. Ordnungsgemäße und sichere Altbatterierücknahme im Rahmen ADR, BattG und ElektroG. Presented at Mitgliederversammlung des Abfallwirtschaftsvereins Rhein-Wupper, Düsseldorf, Germany, 15 May 2014. [Google Scholar]
- Federal Environment Agency. PRTR Data (as Status 20.09.2017) is Available in Different File Formats. 2017. Available online: https://www.thru.de/3/thrude/downloads/ (accessed on 10 November 2017).
- Reuter, M.A.; Hudson, C.; van Schaik, A.; Heiskanen, K.; Meskers, C.; Hagelüken, C. Metal Recycling. Opportunities, Limits, Infrastructure; Report of the Working Group on the Global Metal Flows to the International Resource Panel, Ed.; United Nations Environment Program: Nairobi, Kenya, 2013; ISBN 978-92-807-3267-2. [Google Scholar]
- Bartl, A. Withdrawal of the circular economy package: A wasted opportunity or a new challenge? Waste Manag. 2015, 44, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and Long-Term Composition of MSW Landfill Leachate: A Review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Aucott, M. The fate of heavy metals in landfills: A review. Industrial Ecology, Pollution Prevention and the NY-NJ Harbor. Project of the New York Academy of Sciences. 2006. Available online: www.researchgate.net (accessed on 25 November 2017).
- Morf, L.S.; Gloor, R.; Haag, O.; Haupt, M.; Skutan, S.; Di Lorenzo, F.; Böni, D. Precious metals and rare earth elements in municipal solid waste–Sources and fate in a Swiss incineration plant. Waste Manag. 2013, 33, 633–643. [Google Scholar] [CrossRef] [PubMed]
- The International Solid Waste Association (ISWA). Management of APC Residues from W-t-E Plants, 2nd ed.; Working Group on Thermal Treatment of Waste; ISWA: Copenhagen, Denmark, 2008; Available online: http://www.iswa.org/uploads/tx_iswaknowledgebase/Management_of_APC_residues_from_W-t-E_Plants_2008_01.pdf (accessed on 10 August 2017).
- Official Journal of the European Union. Commission Regulation (EU) No 494/2011 of 20 May 2011 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Annex XVII (Cadmium). Official Journal of the European Union. 21 May 2011, pp. L 134/2–L 134/5. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0494&from=EN (accessed on 10 August 2017).
- Statista. Produktion von Fenstern mit Kunststoffrahmen in Deutschland in den Jahren 1995 bis 2013. Available online: https://de.statista.com/statistik/daten/studie/291360/umfrage/produktion-von-fenstern-mit-kunststoffrahmen-in-deutschland/ (accessed on 5 May 2017).
- VinylPlus. Progress Report 2016; Reporting on 2015 Activities; 2016. Available online: http://www.vinylplus.eu/uploads/160826_VINYPLUS_2016_WEB_PS_Singlepage_version.pdf (accessed on 9 August 2017).
- CONSULTIC. Produktion, Verarbeitung und Verwertung von Kunststoffen in Deutschland 2015—Kurzfassung; CONSULTIC Marketing & Industrieberatung GmbH: Alzenau, Germany, 2016; Available online: http://www.plasticseurope.org/documents/document/20161018113129-consultic-studie_2015_kurzfassung.pdf (accessed on 9 August 2017).
- Kelly, A.L.; Rose, R.M.; Spares, R.; Coates, P.D.; Weston, R. Recycling of uPVC Window Profile Waste. J. Vinyl Addit. Technol. 2005, 11, 119–126. [Google Scholar] [CrossRef]
- Rewindo. Kunststofffenster-Recycling in Zahlen 2013; Rewindo GmbH: Bonn, Germany, 2014; Available online: http://www.rewindo.de/rewindo-downloads/downloads/Rewindo_Mengenstromnachweis_2013.pdf (accessed on 9 August 2017).
- Rewindo. Kunststofffenster-Recycling in Zahlen 2015; Rewindo GmbH: Bonn, Germany, 2016; Available online: http://www.rewindo.de/rewindo-downloads/downloads/Rewindo_Mengenstromnachweis_2016.pdf (accessed on 9 August 2017).
- Friege, H.; Fendel, A. Competition of different methods for recovering energy from waste. Waste Manag. Res. 2011, 29, 30–38. [Google Scholar] [CrossRef] [PubMed]
- European Commission. European Commission DGXI.E.3: The Behaviour of PVC in Landfill; Final Report; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Reisinger, H.; Schöller, G.; Jakl, T.; Quint, R.; Müller, B.; Riss, A.; Brunner, P.H. Lead, Cadmium and Mercury Flow Analysis—Decision Support for Austrian Environmental Policy. Österreichische Wasser-und Abfallwirtschaft 2009, 61, 63–69. [Google Scholar] [CrossRef][Green Version]
- Friege, H. Resource recovery from used electric and electronic equipment: Alternative options for resource conservation. Waste Manag. Res. 2012, 30, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Friege, H. Ressourcenschonung am Beispiel der Elektro- und Elektronikaltgeräte. I. Grenzen des WEEE-Ansatzes. Müll und Abfall 2012, 44, 80–93. [Google Scholar]
- Friege, H. Ressourcenschonung am Beispiel der Elektro- und Elektronikaltgeräte. II. Ansätze für einen effizienteren Umgang mit nicht erneuerbaren Ressourcen. Müll und Abfall 2012, 44, 307–317. [Google Scholar]
- Friege, H. Nachhaltiges Ressourcenmanagement als abfallwirtschaftliches Leitbild. Müll und Abfall 2015, 47, 500–508. [Google Scholar]
- Chryssos, G.; (Stiftung Gemeinsames Rücknahmesystem Batterien, Hamburg, Germany). Personal communication, 2015.
- Plasticker. Raw Materials & Prices. Constantly Updated Price Overviews—Based on Offers from the plasticker Material Exchange. Available online: http://plasticker.de/preise/preise_monat_single_en.php (accessed on 20 February 2018).
- Brunner, P.H.; Rechberger, H. Practical Handbook of Material Flow Analysis; CRC Press: Boca Raton, FL, USA, 2004; ISBN 1-5667-0604-1. [Google Scholar]
- Dahmus, J.B.; Gutowski, G.T. What Gets Recycled: An Information Theory Based Model for Product Recycling. Environ. Sci. Technol. 2007, 41, 7543–7550. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Mella, J.; Pongrácz, E. Drivers and Constraints of Critical Materials Recycling: The Case of Indium. Resources 2016, 5, 34. [Google Scholar] [CrossRef]
- Spohn, C.; Treder, M. Betreiber von Abfallverbrennungsanlagen im Spannungsfeld des BVT-Merkblatts. In Proceedings of the Berliner Abfallwirtschafts- und Energiekonferenz, Berlin, Germany, 25–26 January 2016; Available online: https://www.itad.de/information/studien/BREFITADTKBerlinJan2016.pdf (accessed on 16 June 2017).

| Batteries for Industrial Use | Portable Batteries |
|---|---|
| Emergency or back-up power supply (hospitals, airports, offices) | Emergency and alarm systems, including emergency lighting |
| Use in trains or aircraft | Cordless power tools (expired) |
| Use on offshore oil rigs or in lighthouses | Medical equipment |
| Hand-held payment terminals, bar code readers | |
| Professional video equipment | |
| Miners’ and diving lamps attached to helmets | |
| Electrical vehicles (cars, wheelchairs, bicycles …) | |
| Back-up for electric doors to prevent blocking | |
| Use in connection with renewable energy applications |
| Year | NiCd Battery Sales in Germany (Mg) | NiCd Batteries Collected in Germany (Mg) | Collection Rate |
|---|---|---|---|
| 2004 | 3961 | 1182 | |
| 2005 | 3132 | 1078 | |
| 2006 | 4185 | 1022 | 27% |
| 2007 | 2687 | 1089 | 33% |
| 2008 | 2476 | 1230 | 39% |
| 2009 | 803 | 1141 | 57% |
| 2010 | 1191 | 1034 | 69% |
| 2011 | 1336 | 1013 | 91% |
| 2012 | 1009 | 1183 | 100% |
| 2013 | 775 | 1349 | 130% |
| 2014 | 568 | 1416 | 181% |
| 2015 | 501 | 1383 | 225% |
| 2016 | 415 | 1442 | 292% |
| Collection of Used Windows (Pure PVC) | 42,740 Mg | 100% |
|---|---|---|
| Production of windows | 24,810 Mg | 58% |
| Demolition | 8890 Mg | 21% |
| Disposal companies | 6760 Mg | 16% |
| Residential construction | 2280 Mg | 5% |
| Whereabouts of used windows (pure PVC) | 42,740 Mg | 100% |
| Re-use | 2850 Mg | 7% |
| Recycling (pure PVC) | 39,890 Mg | 93% |
| Recycling of used windows (pure PVC) | 39,890 Mg | 93% |
| Material recycling by Rewindo | 22,330 Mg | 52% |
| Material recycling by other companies | 3264 Mg | 8% |
| Energetic recovery by other companies | 14,296 Mg | 33% |
| For comparison: Production waste (pure PVC) | 75,030 Mg |
| Year | Cadmium | Nickel | Copper | Zinc |
|---|---|---|---|---|
| 2006 | 3.0 | 24.2 | 89.0 | 3.5 |
| 2007 | 7.6 | 37.2 | 82.0 | 3.4 |
| 2008 | 5.9 | 21.1 | 210.0 | 2.0 |
| 2009 | 2.9 | 14.6 | 158.0 | 1.7 |
| 2010 | 3.9 | 21.8 | 221.0 | 2.3 |
| 2011 | 2.8 | 22.9 | 349.0 | 2.3 |
| 2012 | 2.0 | 17.5 | 150.0 | 2.1 |
| 2013 | 1.9 | 15.0 | 112.0 | 2.1 |
| 2014 | 1.9 | 16.9 | 119.0 | 2.4 |
| 2015 | 1.5 | 11.8 | 77.0 | No data |
| 7 May 2017 | 1.9 | 10.7 | 58.8 | 3.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friege, H.; Zeschmar-Lahl, B.; Borgmann, A. Managing Cd Containing Waste—Caught by the Past, the Circular Economy Needs New Answers. Recycling 2018, 3, 18. https://doi.org/10.3390/recycling3020018
Friege H, Zeschmar-Lahl B, Borgmann A. Managing Cd Containing Waste—Caught by the Past, the Circular Economy Needs New Answers. Recycling. 2018; 3(2):18. https://doi.org/10.3390/recycling3020018
Chicago/Turabian StyleFriege, Henning, Barbara Zeschmar-Lahl, and Andreas Borgmann. 2018. "Managing Cd Containing Waste—Caught by the Past, the Circular Economy Needs New Answers" Recycling 3, no. 2: 18. https://doi.org/10.3390/recycling3020018
APA StyleFriege, H., Zeschmar-Lahl, B., & Borgmann, A. (2018). Managing Cd Containing Waste—Caught by the Past, the Circular Economy Needs New Answers. Recycling, 3(2), 18. https://doi.org/10.3390/recycling3020018






