1. Introduction
The recycling of rare earth permanent magnets, especially NdFeB, has received widespread scientific attention, and consequently has also been addressed by different European Acts, prominently the Critical Raw Materials Act [
1].
A suitable recycling method that offers direct recycling with reduced environmental impact is the hydrogen processing of magnetic scrap (HPMS) process, which was developed and patented by the University of Birmingham [
2,
3,
4,
5]. This method, as well as different preparation and separation processes, has been researched in European Commission-funded projects and is on track to be implemented on an industrial level to promote sustainability and the resilience of the supply chain [
6]. However, there is an open question remaining, which shall be addressed in this paper: What are the tolerable levels of contamination of common contaminants from residual coatings, adhesives, and corrosion for recycling via HPMS and re-sintering? This especially includes some well-researched contaminating elements but also Ni, where prior evidence on the effects has been insufficient to judge the impact.
As HPMS employs direct recycling in a short loop, the possibilities to alter the chemical composition of the to-be-recycled magnets are rather limited, especially compared to traditional methods like hydrometallurgical and pyrometallurgical recycling where unwanted contaminants can be removed in the process. This necessitates the separation of possible contaminants prior to the production of the final recycled magnet. However, ideal separation may require extensive efforts and lower the economic viability of the recycling operation, whereas it is sensible to first determine how the properties of the magnet deteriorate in conjunction with the presence of the contaminants.
Possible contaminant sources are corrosion, residues from coatings, adhesives, and other adjacent materials like coolants and fillers, among others. The main contaminating elements researched in this paper are C, O, Ni, Cu, and Zn. The literature research is expanded by experiments regarding the effect of adding Ni to the material.
2. Literature Review: Effects of Common Contaminants on Sintered NdFeB Magnets
2.1. Carbon Contamination
Carbon uptake can happen through various sources of contamination. A possible source is that polymer coatings are commonly applied for their protective and electrically isolating nature [
7,
8], especially in high-speed electric machines, for example, electric traction motors. Furthermore, adhesives, used for segmenting and attaching magnets to the periphery, are usually based on polymer materials as well [
9,
10]. Carbon-rich fillers used in both polymer coatings and adhesives, for example, carbon black or some types of fiber reinforcement, are also commonly employed. Carbon can also be introduced if the magnet-containing component includes any other polymeric features, which are not removed before the magnetic material is crushed or milled. Other potential sources are solvents, coolants, binders used in metal injection molding and powder extrusion molding, and other gaseous and liquid carbon-rich materials potentially coming into contact with the magnet material during its lifetime or recycling [
11]. According to Hartwig et al. [
12], the presence of solid carbon-rich impurities in the material during sintering seems more detrimental than the exposure to carbon-rich atmospheres at higher temperatures. In the following
Figure 1, several studies where multiple carbon values are reported in conjunction with magnetic properties are compiled to illustrate the effect of carbon contamination.
A high C content causes the formation of tetragonal and bcc-Nd carbides, accumulating in the triple junctions, which reduce the amount of active α-Nd in the grain boundaries and thus also the coercivity [
13,
17]. Within the actual grain boundaries, no carbon is found [
13]. C can partially substitute B in the hard magnetic Nd
2Fe
14B phase, also referred to as the φ-phase [
17,
18]. However, the relative amount of C found in the φ-phase compared to the triple points is minimal [
13]. If C-rich residues are still present during sintering, where the amount of free Nd is greatly increased due to the solubility of Nd from the φ-phase in the Nd-rich phase, it can lead to disproportionation, as the Nd is bound in the form of carbides. This leads to the formation of α-Fe proportionally in accordance with the C content, greatly reducing coercivity [
12,
15,
17]. It is also seen that densification does not properly take place in the presence of significant amounts of α-Fe [
12,
15].
Overall, it is known that C is more sensitive to the magnetic properties than O and N [
11]. Commonly, 0.1 wt.% is referred to as the limit of tolerable C content, which is supported by data from several studies, which have been compiled in
Figure 1. Conversely to the reported detrimental effects, mostly regarding coercivity, Lukin and Szymura [
14] report that the corrosion resistance, remanence, and the maximum energy product can be increased with carbon addition. The effects of carbon contamination on the remanence are less prominent and rather inconsistent across studies, including our own experiments.
It is evident that an elevated carbon content should be avoided by properly removing carbon-rich substances like the coating, which can, for example, be removed by grinding, sandblasting, or chemically from the bulk magnet, as shown recently by Dickinson-Lomas et al. [
8].
2.2. Oxygen Contamination
NdFeB is highly prone to corrosion due to a high electric potential difference between the matrix φ-phase and the intergranular Nd-rich phase, leading to the formation of corrosion batteries and intergranular corrosion [
19]. Furthermore, rare earths like Nd are highly reactive and thus oxidize quickly [
20]. Oxidation of powdered material is especially critical, as the alloy has a high affinity to oxidize [
21], rendering the material potentially pyrophoric [
22]. The effect of oxygen contamination on the coercivity is shown in
Figure 2, where different studies that report oxygen values in conjunction with magnetic properties are compiled.
Oxidation of the Nd-rich phase can lead to the very common bcc-Nd
2O
3 or the less abundant fcc-NdO, as well as its polymorphs [
25]. Furthermore, Nd hydroxides and other metalorganic corrosion products can form based on the media to which the magnet is exposed [
26].
Like contamination with C, contamination with a critical level of O also results in a reduction in coercivity, due to hindering the formation of a continuous Nd-rich phase [
21,
27]. With regard to the studies compiled in
Figure 2, no clear tolerable limit that applies across alloy compositions can be determined. According to Zakotnik and Tudor [
28], the presence of oxides inhibits excessive grain growth which speaks to possible benefits when having a moderate O content if there is no sufficient alloy addition to mitigate grain growth. Furthermore, Fukagawa et al. [
29] report that cubic Nd oxides effectively suppress the nucleation of reverse domains, whereas a certain amount of oxygen would be beneficial for the development of high coercivity.
Furthermore, the presence of oxides can also affect processing during recycling, since when hydrogen processed, oxidized areas do not properly fall apart into fine, friable powder but larger aggregates [
28]. These have also been shown not to mill properly, whereas they can be removed by sieving and processed separately [
30], for example, to reduce the oxygen content by applying the Neoleach method [
31] as an extension of HPMS, or in severe cases, long-loop hydrometallurgical [
32] or pyrometallurgical [
33] processing can be applied. When applying the Neoleach method or jet milling, the oxidized Nd-rich phase is removed and can be replaced by the addition of fresh NdH
x. When a thermal process introduces oxidation, for example, thermal demagnetization, a superficial oxide layer is formed which can be removed simply by grinding off the discolored surface [
34].
2.3. Cu Contamination
Copper uptake is possible from coatings, as Ni-Cu-Ni coatings are a popular, highly protective variant [
35]. Similarly to Ni, Cu is rather ductile and can be removed alongside the Ni layers by milling and sieving of the powder; however, mechanical removal can be troublesome [
30]. It is questionable, though, if Cu removal itself is necessary, as it is deliberately used as an alloying element in NdFeB magnets. Most NdFeB alloys contain some amount of copper. According to an internal analysis of ICP-OES data derived from 195 end-of-life NdFeB magnets, the magnets most commonly contain up to 0.2 wt.% Cu, as 165 of the 195 magnets fit into that range. Few samples contain more, with the highest amounts measured ranging around 1–3 wt.% of Cu.
According to Sagawa et al. [
18], Cu has a very limited solubility in the φ-phase, being able to replace 0.2 of 14 Fe atoms. Further, Cu is soluble in the Nd-rich phase and Nd oxides, and it can form new phases. Different binary and ternary systems are reported for Nd, Cu, and Fe [
36,
37].
It is well known that Cu can be added to improve the coercivity of permanent magnets; however, there is a trade-off of a lowered Curie temperature and remanence reported by Pandian et al. [
38]. Several research groups investigate grain boundary modification with Cu-rich alloys. For example, Wan et al. [
39] introduced a Pr-Cu alloy via the dual alloy method into a NdFeB sintered magnet matrix, adding a total of 6.48 wt.% of Cu, improving the coercivity significantly, and reducing the remanence. Therefore, it seems that even a higher Cu contamination may be tolerable as the magnetic properties are changed but may be beneficial to some applications.
2.4. Zn Contamination
Zn uptake is possible from coatings, both the coatings of permanent magnets and some parts attached to them, as Zn is a popular metallic coating due to its anodic nature. Zn can generally be removed from bulk material by grinding or sandblasting; however, prior thermal demagnetization, which can be employed in conjunction with or prior to demounting, can lead to complications. Zn infiltrates the grain boundaries of the NdFeB magnet at elevated temperatures and extended times [
34,
40], reaching deep into the magnet material. This can be avoided by keeping the demagnetization time relatively short [
34]. Mechanical removal from the powder, like achieved with Ni-Cu-Ni coatings, was not viable in prior trials [
30]. However, in preliminary tests that need further investigation, the Neoleach method [
31] appeared promising to be able to remove Zn residues from the φ-phase grains along with rare earth oxides.
Regarding the effects of Zn addition on sintered NdFeB magnets, not much is known, likely due to Zn evaporating below the sintering temperature, at 907 °C, in atmospheric pressure [
41]. Horikawa et al. [
42] produced Zn-coated NdFeB powders made from milled sintered magnets by exposing the powders to Zn vapor at 550 °C, which led to the amorphization of the powder surface and formation of α-Fe, which differed from the effects of other coating elements like Yb and Mg. This decreased the magnetic properties of the powder, which were not re-sintered in the study [
42]. However, in hot deformed magnets Zn can be integrated to improve the magnetic properties [
43].
The evaporation of Zn during the process of sintering NdFeB powder should be seen as rather critical, as the equipment can be damaged by the deposition of a Zn layer and due to the high vapor pressure of Zn, which may cause structural damage to the brown part and a lack of densification. Preliminary tests were conducted, sintering samples with low Zn addition, which led to deposition of Zn on the inside of the sintering equipment, leading to the tests being discontinued immediately. The results of the test are shown in
Appendix A.
2.5. Ni Contamination
Ni can be introduced into the magnet matrix due to the widely used Ni and Ni-Cu-Ni coatings, as well as some more uncommon types with additional layers of Au or Sn [
44]. In a prior study, it was found that the removal of Ni is possible by milling and sieving, as the coating flakes do not mill as finely as the magnet powder due to their ductility [
30]. However, there are two caveats: First of all, some high-phosphate Ni layers are rather brittle and can mill as finely as the magnet powder, leading to a small residue (0.07 wt.% in the fraction below 25 µm) [
30]. Secondly, with highly oxidized material, more magnet powder falls into the higher sieve fractions, where there is also more Ni present [
30]. This leads to the question of how much Ni is tolerable, especially after multiple recycling procedures, and if Ni accumulates in the material.
Ni has a partial solubility in the Fe-sublattice of the Nd
2Fe
14B elementary cell, substituting up to 1 out of 14 Fe atoms [
18], which is equal to ~5.41 wt.% of Ni. The addition of Ni leads to an increase in the Curie temperature; however, it also results in a steep decline of the magnetization, and consequently also the remanence [
18]. Theoretically, several intermetallic compounds of Ni and Nd are also possible, potentially forming in the grain boundaries [
45]. A study by Trujillo Hernández et al. [
46] produced powders meant for exchange spring magnets via ball-milling and heat treatment with a Nd
16(Fe
76−x Ni
x)B
8 system, detecting Nd
2Fe
14B (φ-phase), α-Fe, Nd
1.1Fe
4B
4, and NdNi
2. This shows both that there is a risk of introducing Ni at the milling stage aside from brittle Ni residues and that a NdNi phase is likely to be found. Furthermore, the study reaffirms the conclusion that the saturation magnetization is decreased and shows that Ni may preferentially replace Fe at the 16k
2, 8j
2, and 4c sites of the φ-phase [
46].
While there is currently no further data about the effects on the magnetic properties related to the nickel content available, it can be assumed that a higher Ni content will significantly deteriorate the magnetic properties.
Due to the relatively high thickness of Ni coatings, comparably high Ni levels can be introduced accidentally when the recycling procedure and/or the magnet design are unfavorable; thus, this has to be investigated further. In the following, the effects of Ni addition during NdFeB recycling via HPMS and re-sintering are investigated.
3. Results
After the preliminary experiments, screening with 0.025 wt.%–10 wt.% Ni, the relevant range to be tested extensively was determined.
Sintered compacts with a Ni addition of 0.25 wt.%, 0.5 wt.%, 0.75 wt.%, 1 wt.%, 2 wt.%, 3 wt.%, 4 wt.%, and 5 wt.% were produced along with reference samples without any Ni addition (0 wt.%). Four samples of each contamination level were produced; however, as some samples had to be excluded due to issues with the respective sintering run resulting in excessive oxidation or a lack of densification, some had to be repeated until the target number of acceptable samples was successfully produced. Oxygen levels after processing were found to be between 0.07 wt.% and 0.39 wt.%, with an average of 0.21 wt.% in the sintered (and partially annealed) parts for the acceptable samples, which were taken into account.
For all samples of the main tests, the density ranged between 91% and 99%, with no clear tendency to decrease or increase. In the preliminary test, the samples with 5% and 10% show a drop in density to 81% in the case of a 5% Ni sample and 67% for the 10% Ni sample. The density is given relative to the density measured in the original magnet, which was determined to be 7.59 g/cm3.
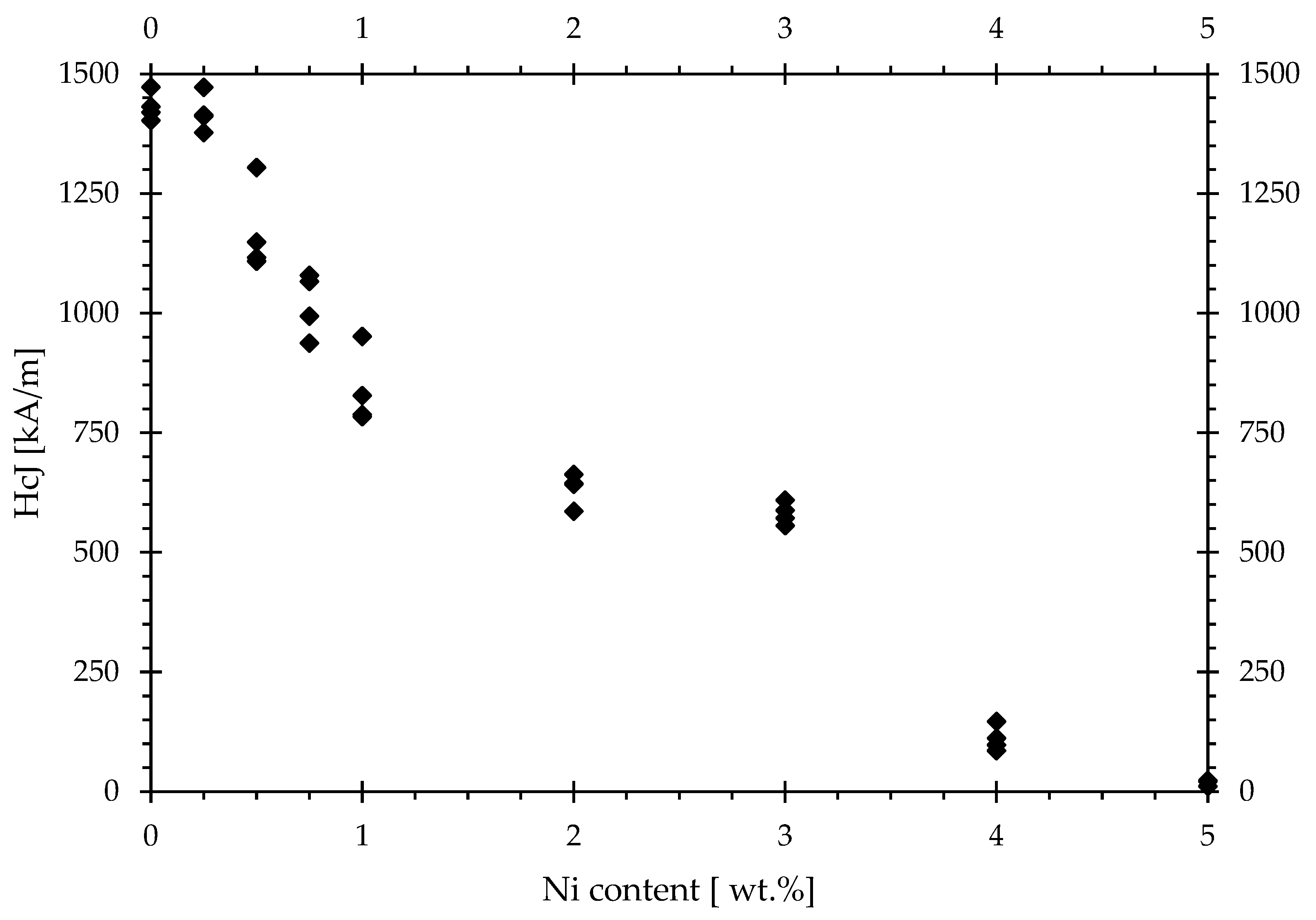
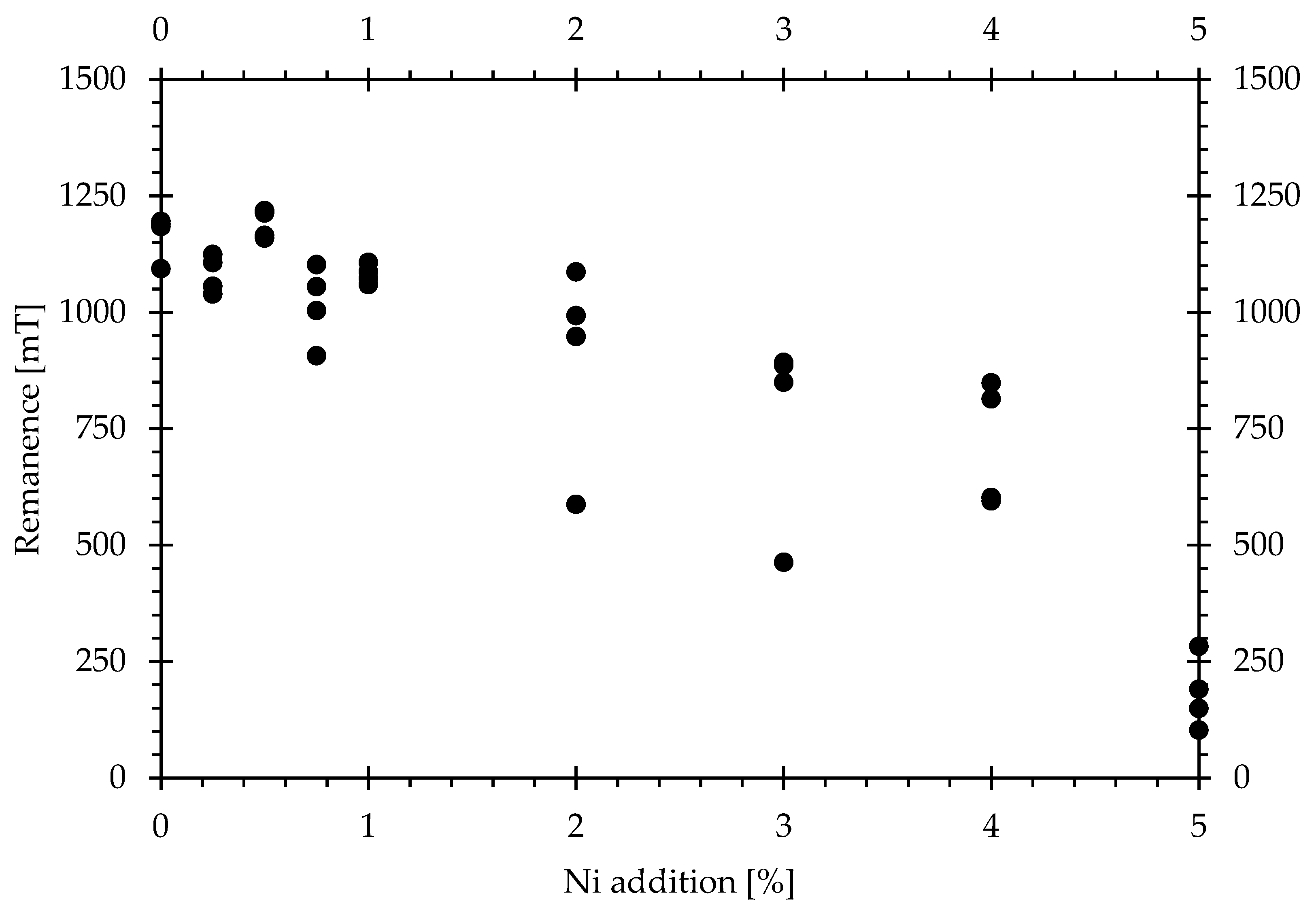
The effects of Ni addition on coercivity and remanence are shown in the following
Figure 3 and
Figure 4.
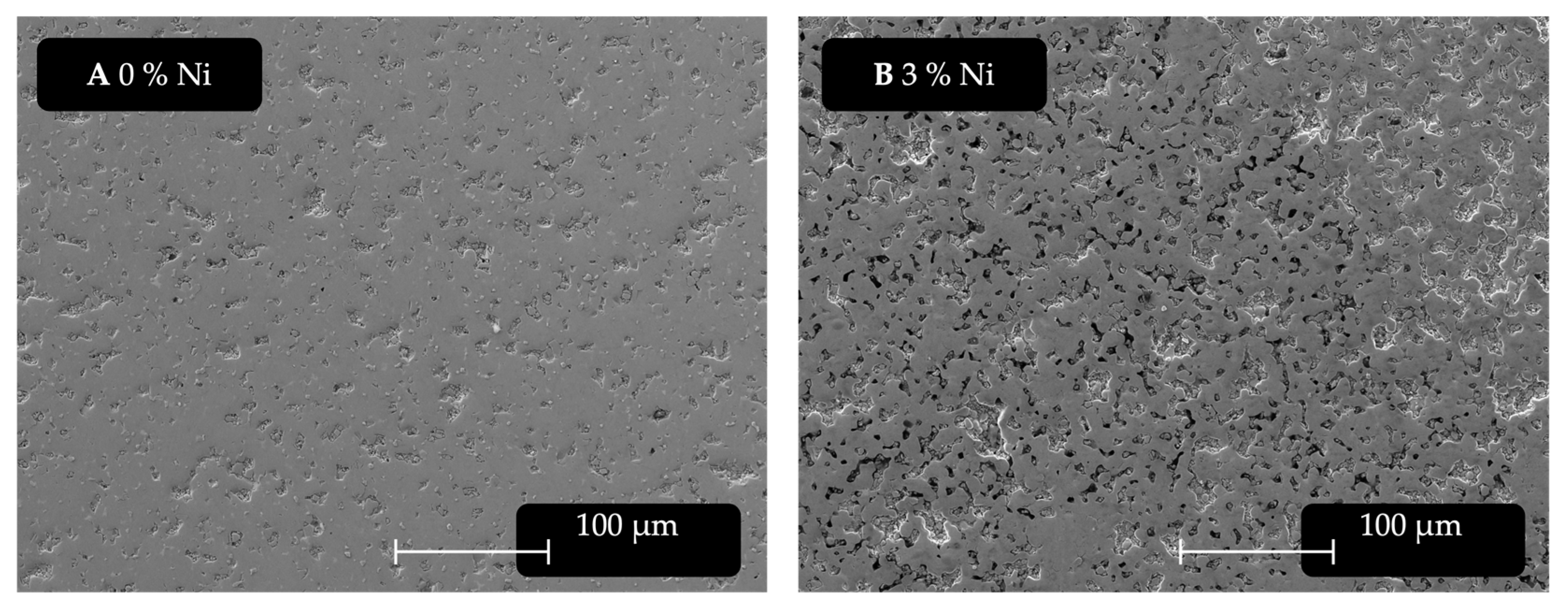
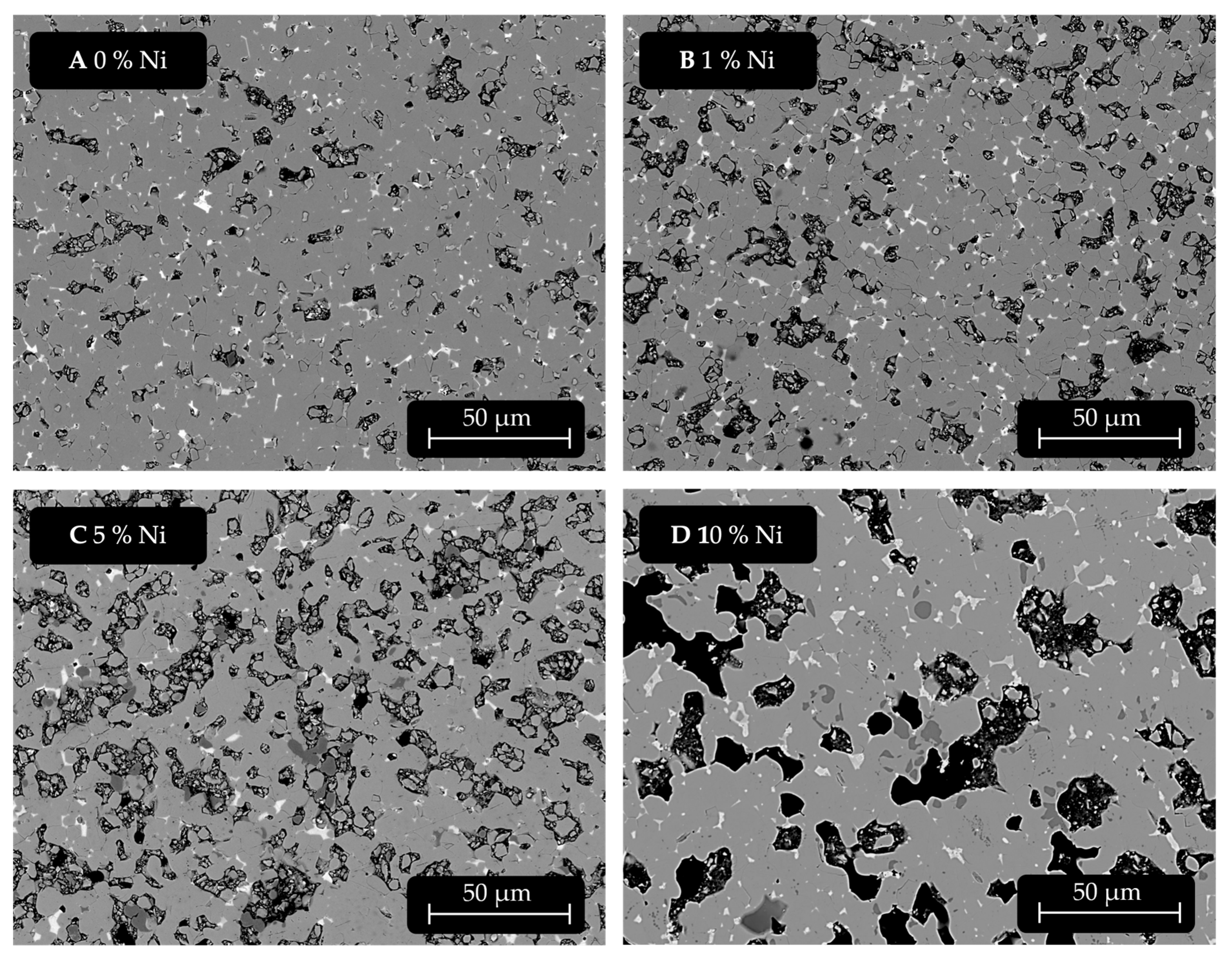
The samples were investigated via SEM-EDXS. The following
Figure 5 shows the changes in the microstructure with increasing Ni content of 0 wt.% (
Figure 5A), 1 wt.% (
Figure 5B), 5 wt.% (
Figure 5C), and 10 wt.% (
Figure 5D). It can be noted that between 0 wt.% and 0.25 wt.% there are no tangible differences in microstructure observable in SEM and no Ni detectable in EDXS; however, starting at 0.50 wt.%, some Ni can be measured by EDXS.
In
Figure 6 and
Table 1, some exemplary EDXS measurements are shown, where
Figure 6 indicates the measured spots and
Table 1 compiles the corresponding measured values in wt.%. The standard deviation is given in wt.% as well.
In
Appendix B, the microstructure of the original magnet and the recycled magnet without nickel addition are compared. Also in
Appendix C, SE mode images are shown to support the observations in
Figure 5 and
Figure 6 regarding polishing defects and porosity.
4. Discussion
The coercivities measured for different Ni additions show that there is barely any effect from the addition of 0.25 wt.%.
At 0.5 wt.% and higher, the coercivities drop significantly, and at 5 wt.%, which is almost equal to the maximum substitution rate in the φ-phase, barely any measurable coercivity remains. The effect of the Ni additions on the remanence initially yields a fairly consistent remanence with additions of up to 1 wt.%, after which the remanence starts to drop consistently; however, it does not drop not quite as drastically as seen for coercivity.
When comparing the SEM images shown in
Figure 5, it can be seen that the samples with 1 wt.% and 5 wt.% Ni addition are more affected by sample preparation. This may indicate that a brittle phase was formed or the integrity of the material is otherwise impacted. The sample with 10 wt.% Ni addition shows what appears to be a darker phase forming, which looks similar to α-Fe as well as large cavities, reflected in a loss of density. Furthermore, grain growth can be observed. The dark grey phase α-(Fe, Ni) can be found increasingly with higher additions in samples with at least 3% Ni addition. Below this level, no such phase was spotted. This phase is very similar to the typical α-Fe phase resulting from any kind of disproportionation of the φ-phase.
The measured Ni content in the φ-phase tends to be lower than the added relative amount, but close to the originally introduced level. Ni appears to substitute and displace Fe in the φ-phase, as expected based on the research literature. However, some amount of Ni is also found in the α-(Fe, Ni) phase. Additionally, a high Ni content can be measured in the Nd-rich phase.
The presence of a soft magnetic Ni-containing phase in the grain boundaries, as well as the formation of α-(Fe, Ni), explains the drastic loss of coercivity. The loss of remanence can be attributed to the uptake of Ni into the φ-phase, as elaborated in
Section 2.5.
The variation in density is likely attributed to factors like oxygen uptake during recycling, excess Ni and Fe consuming active Nd and hindering re-densification, and possibly variation induced by the lab-scale equipment used for pressing and sintering. The variation in density most probably contributes significantly to the variance in the measured magnetic properties. However, the lack of density in some samples does not clearly correlate with the degree of variation observed.
It is known that, for example, excessive carbon contamination can lead to a lack of densification and formation of α-Fe in the NdFeB microstructure, which was reviewed in
Section 2.1 in more detail, of which some may be reiterated at this point: The reason for this is that during sintering, the liquid phase dissolves Nd from the hard magnetic phase, which is able to bond with contaminants. The active liquid phase is thus reduced and the re-distribution of material required for successful densification is hampered. In the case of Ni, it is suspected that a similar mechanism leads to a decrease in density and higher porosity as a result: Ni replaces Fe and excess Fe is precipitated and forms α-Fe. Excess Fe and Ni should form a regular or Ni-rich ϕ-phase with any excess Nd and B, the latter usually being added with some excess to avoid the formation of α-Fe. Further, some direct binding of Nd by Ni is likely as well, as expanded on in
Section 2.5 and supported by the EDS measurements in
Table 1.
It is concluded that the most prominent effect is on the coercivity, and the acceptable limit of Ni contamination should be set at around 0.25 wt.%. However, it must be kept in mind that the contamination accumulates in the φ-phase and is therefore irreversible. This can pose a limitation to employing short-loop recycling on already recycled material (as opposed to the first-time recycling of magnets from virgin material), even while utilizing supporting techniques like Neoleach or jet milling, which can only reverse contamination that has accumulated in the grain boundary phase, not in the φ-phase.
5. Materials and Methods
During preliminary experiments, excessive oxygen uptake during sample transport and equipment failure rendered some samples unusable which were therefore discarded; however, limited data of these trials was identified as acceptable and has been marked accordingly.
For the main experiments, production scrap magnets with exceptionally low oxygen contamination, excellent microstructure, and good consistency of magnetic properties were chosen and processed by removing the epoxy resin coatings via sandblasting, HPMS processing at 3 bar hydrogen and room temperature, partial degassing at 500 °C for 1 h, and ball milling to an average particle size Dv50 of 6.67 µm (measured dry with the laser diffraction particle size analyzer Mastersizer 3000 (Malvern Panalytical Ltd., Almelo, the Netherlands)).
The experimentally determined chemical composition of the magnets used is shown in
Table 2 and has been determined using the ICP-OES for metallic constituents. ICP samples are digested in aqua regia solution using a MARS6 microwave (CEM Corporation, Matthews, NC, USA), heating the digestive solution to 200 °C. The solution was then diluted with 5% nitric acid and measured in an iCAP 7400 ICP-OES (Thermo Fisher Scientific Inc., Bremen, Germany) against a custom tuning solution. For organic constituents, the LECO ONH836 (LECO Corporation, St. Joseph, MI, USA) and LECO CS744 (LECO Corporation, St. Joseph, MI, USA) were used. After processing, oxygen levels were measured again.
The recycled powder is modified by the addition of different amounts of nickel powder < 10 micron/99.9% by chemPUR Feinchemikalien und Forschungsbedarf GmbH (Karlsruhe, Germany), mixing, and preparing pressed and sintered magnets. The addition levels shown in the
Section 3 were chosen based on preliminary screening experiments to provide a more detailed overview in the more realistic lower-contamination range below 1 wt.% of Ni and a more broad overview in the higher-contamination range up to 5 wt.%.
The magnet powder was filled into a custom silicone mold and vacuum-sealed, aligned at 2000 V in a K-series pulse magnetizer from Magnet Physik Dr. Steingroever GmbH (Cologne, Germany), isostatically pressed at 10 t in a SJYP 40T manual CIP press by Xiamen Tmax Battery Equipments Limited, (Xiamen, China), and then sintered at 1100 °C, with a heating rate of 600 K/h up to 1030 °C and 200 K/h from 1030 °C to 1100 °C and a hold time of 30 min at 1100 °C. The sintering furnace is not externally cooled and slowly cools down to room temperature until the vacuum is reversed and the furnace tube is removed. A Nabertherm R 50/500/12 tube furnace with a C 550 Controller (Nabertherm GmbH, Lilienthal, Germany) with a custom Inconel 635 tube is used in conjunction with a vacuum system consisting of a Pfeiffer Vacuum HiScroll 6 (Asslar, Germany), and a Pfeiffer Vacuum HiCUBE TC 110 (Asslar, Germany) vacuum pump. The vacuum at sintering temperature is roughly 5 × 10−5 hPa.
Samples that did not properly densify or oxidize visibly and excessively due to leaks in the setup were excluded.
The produced samples were characterized with SEM-EDXS using the Hitachi Flex-SEM 1000 II (Hitachi High-Tech, Tokyo, Japan) with a W-cathode and an Oxford Instruments micsF+ x-stream-2 EDS (Oxford Instruments, Wycombe, UK); underwent density measurement via the Archimedean principle, using the Sartorius Entris124I-1S scale and the YDK Density determination kit (Sartorius GmbH & Co. KG, Göttingen, Germany); and their magnetic properties were recorded using the Hystograph HG 200 from Dr. Brockhaus Messtechnik GmbH & Co. KG (Lüdenscheid, Germany).
6. Conclusions
The effects of different contaminants have been reported in several studies and are reviewed in
Section 2 of this paper. Compiling the results of different studies reaffirms the known acceptable limit of carbon contamination of around 0.1 wt.%. For oxygen contamination, the results are less conclusive, and it is assumed that the effects strongly interact with other microstructural and/or chemical parameters; however, in the case of oxygen, most studies do not show a distinct drop (with the exception of Yamashita [
16]) but rather a linear decrease. Researching existing studies, a lack of data regarding the practical effects of contamination with metallic coating residues is found. It is likely that Zn does not remain in the magnet material after re-sintering. During preliminary trials, experiments involving Zn contamination were found to be impossible without significant damage to the sintering setup due to the evaporation of Zn. This is shortly elaborated on in the
Appendix A.
The effects of Ni contamination on short-loop recycled and re-sintered magnets have been researched methodically in this work, and Ni was found in the φ-phase, the Nd-rich phase, and the α-(Fe, Ni) phase. A tolerable limit of approximately 0.25 wt.% can be identified when the goal is to produce magnets that show almost identical properties to uncontaminated ones. Beyond that level, a nearly linear decline can be seen. Therefore, the acceptable limit can be increased, depending on the individual requirements. However, due to irreversible accumulation in the φ-phase, the long-term effects in multiple recycling cycles should not be underestimated. It is therefore recommended to recyclers to declare the Ni content of their recycled magnets if a full removal is not possible within technical and economic means. This allows future recyclers to choose the correct treatment option for Ni-contaminated scrap, as the contamination could be reversed with long-loop recycling or the material could be blended with Ni-free material to lower the total content.
Regarding the effects of different, deliberately added alloying elements and also the interaction of these with the contaminants reviewed and researched in this work, more scientific work is essential. Similarly, the influence of the metallic contaminants over multiple recycling cycles including non-short-loop routes is an important topic for future research as already achieved for organic contaminants by Zakotnik et al. [
47]. Furthermore, it could be researched whether contaminants like Ni can be compensated for by the addition of other material, for example, as performed with the addition of fresh Nd to compensate for Nd bound by organic contaminants [
47]. NdFeB-recycling-related research groups are highly encouraged to follow up and reproduce the presented results with different base magnets.
Author Contributions
Conceptualization, L.G. and F.B.; methodology, L.G. and F.B.; software, L.G.; validation, L.G., F.B. and N.M.; formal analysis, L.G.; investigation, L.G. and N.M.; resources, C.B. and S.K.; data curation, L.G. and S.R.; writing—original draft preparation, L.G.; writing—review and editing, F.B., N.M., S.R., S.K. and C.B.; visualization, L.G.; supervision, C.B., S.K. and F.B.; project administration, C.B. and S.K.; funding acquisition, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Commission under Horizon Europe, Grant. Agreement No. 101058598 (REEsilience), and EIT RawMaterials, Grant No. 20090 (INSPIRES).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank ZF Friedrichshafen AG for supplying us with excellent production scrap magnets which were used in these experiments. We also thank Laurence Schieren, Rosario Moreno Lopez, and Peter Fleissner for assistance in analyzing the initial material. Furthermore, heartfelt thanks are due to the colleagues at Institut Jožef Stefan K7 Nanostructured Materials Magnetics group for their support during the preliminary trials performed at IJS. During the preparation of this manuscript/study, the author(s) used Grammarly Premium for the purposes of correction of grammar and style. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BSE-COMP | Backscattered electron compositional imaging mode |
| CS | Carbon–sulfur analysis via hot gas extraction |
| EDXS | Energy-dispersive x-ray spectroscopy |
| HPMS | Hydrogen processing of magnetic scrap |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| ONH | Oxygen–nitrogen–hydrogen analysis via hot gas extraction |
| SE | Secondary electron imaging mode |
| SEM | Scanning electron microscopy |
Appendix A. Effects of Zn Contamination
No extensive study on the effects of Zn contamination could be performed due to the damage to the equipment which was evident even when processing pressed compacts with 0.025–0.075% Zn addition. In the sintering run, ~0.1 g of Zn was added; however, the entire glass furnace tube used in the preliminary tests was metallized after the treatment, and the samples showed a high porosity.
Figure A1 shows the metallized glass tube. Detrimental effects on the vacuum system are unfortunately expected to arise if similar experiments are carried out several times.
Figure A2 shows the microstructure of the Zn-contaminated magnets. No Zn was found remaining in the sintered magnets in ICP-OES; however, due to the very low initial addition, this may be an error or it may be different with higher additions. The experiments were discontinued immediately, and we do not recommend replicating them without sufficient protection of the vacuum system.
Figure A1.
Photograph of the glass sintering tube after sintering samples with a total Zn content of around 0.1 g.
Figure A1.
Photograph of the glass sintering tube after sintering samples with a total Zn content of around 0.1 g.
Figure A2.
Porosity seen in samples with small Zn addition, image from the preliminary trials. Hitachi FlexSEM, 15 kV acceleration voltage, BSE-COMP mode, 200×.
Figure A2.
Porosity seen in samples with small Zn addition, image from the preliminary trials. Hitachi FlexSEM, 15 kV acceleration voltage, BSE-COMP mode, 200×.
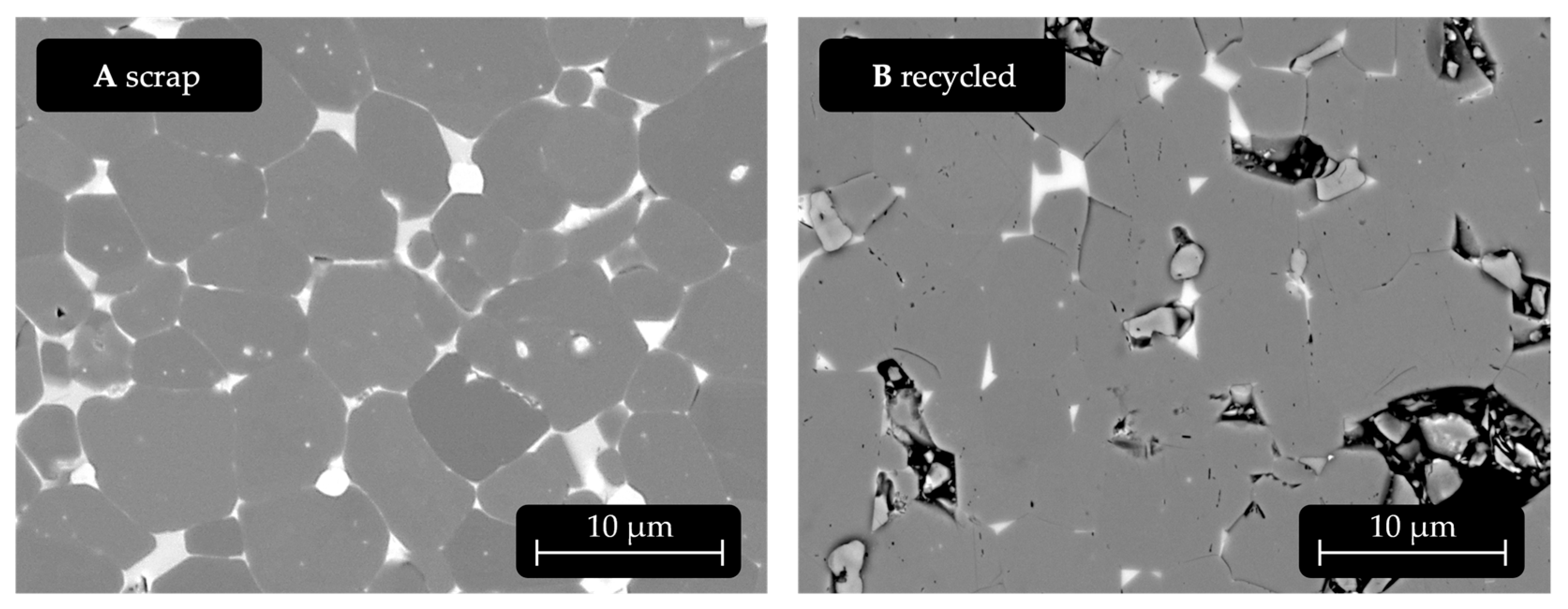
Appendix B. Microstructure of the Original Scrap Magnet Before Recycling
For comparison, the microstructures of the magnet prior to recycling and the recycled magnet without Ni addition are shown next to each other.
Figure A3.
Comparison of the scrap magnet and the recycled magnet microstructure. The recycled magnet is without Ni addition. No NdHx addition or annealing. Hitachi FlexSEM, 15 kV acceleration voltage, BSE-COMP mode, 2500×.
Figure A3.
Comparison of the scrap magnet and the recycled magnet microstructure. The recycled magnet is without Ni addition. No NdHx addition or annealing. Hitachi FlexSEM, 15 kV acceleration voltage, BSE-COMP mode, 2500×.
Appendix C. SE Images of the Microstructure
Supporting the evaluation of the microstructural topography, an SE image of recycled magnets without Ni contamination and with 3 wt.% Ni contamination are shown to complement the BSE images used throughout the publication.
Figure A4.
SE-images of recycled magnet containing 0 wt.% and 3 wt.% addition of Ni. Please note that the bright areas in C1B are caused by charge on the borders of defects and porosities. Hitachi FlexSEM, 15 kV acceleration voltage, SE mode, 2500×.
Figure A4.
SE-images of recycled magnet containing 0 wt.% and 3 wt.% addition of Ni. Please note that the bright areas in C1B are caused by charge on the borders of defects and porosities. Hitachi FlexSEM, 15 kV acceleration voltage, SE mode, 2500×.
References
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020, and Directives 2000/53/EC and 2005/64/EC: Critical Raw Materials Act (CRMA). 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32024R1252 (accessed on 3 August 2025).
- Harris, I.R.; Walton, A.; Speight, J.D. Magnet Recycling. U.S. Patent 13/907,166, 31 May 2013. [Google Scholar]
- Burkhardt, C.; van Nielen, S.; Awais, M.; Bartolozzi, F.; Blomgren, J.; Ortiz, P.; Xicotencatl, M.B.; Degri, M.; Nayebossadri, S.; Walton, A. An overview of Hydrogen assisted (Direct) recycling of Rare earth permanent magnets. J. Magn. Magn. Mater. 2023, 588, 171475. [Google Scholar] [CrossRef]
- Walton, A.; Yi, H.; Rowson, N.A.; Speight, J.D.; Mann, V.; Sheridan, R.S.; Bradshaw, A.; Harris, I.R.; Williams, A.J. The use of hydrogen to separate and recycle neodymium–iron–boron-type magnets from electronic waste. J. Clean. Prod. 2015, 104, 236–241. [Google Scholar] [CrossRef]
- Zakotnik, M.; Tudor, C.O.; Peiró, L.T.; Afiuny, P.; Skomski, R.; Hatch, G.P. Analysis of energy usage in Nd–Fe–B magnet to magnet recycling. Environ. Technol. Innov. 2016, 5, 117–126. [Google Scholar] [CrossRef]
- Rizos, V.; Righetti, E.; Kassab, A. Developing a Supply Chain for Recycled Rare Earth Permanent Magnets in the EU: Challenges and Opportunities; CEPS In-Depth Analysis: Brussels, Belgium, 2020; Available online: https://circulareconomy.europa.eu/platform/en/knowledge/developing-supply-chain-recycled-rare-earth-permanent-magnets-eu-challenges-and-opportunities?utm_source=chatgpt.com (accessed on 3 August 2025).
- Cheng, C.W.; Man, H.C.; Cheng, F.T. Magnetic and corrosion characteristics of Nd-Fe-B magnet with various surface coatings. IEEE Trans. Magn. 1997, 33, 3910–3912. [Google Scholar] [CrossRef]
- Dickinson-Lomas, A.M.; Keith, M.J.; Brown, D.N.; Jenkins, M.J. The removal of epoxy resins from NdFeB mag-nets for recycling—A review. Resour. Conserv. Recycl. 2025, 215, 108113. [Google Scholar] [CrossRef]
- Krings, A.; Monissen, C. Review and Trends in Electric Traction Motors for Battery Electric and Hybrid Vehicles. In Proceedings of the 2020 International Conference on Electrical Machines (ICEM), Gothenburg, Sweden, 23–26 August 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1807–1813. [Google Scholar]
- Bomatec, A.G. Handhabung von Wirbelströmen in einem Hochgeschwindigkeits Dauermagnet Motor-Generator. 2023. Available online: https://content.bomatec.com/en/case-study-eddy-current-reduction#download (accessed on 14 April 2025).
- Minowa, T.; Shimao, M.; Honshima, M. Microstructure of Nd-rich phase in Nd-Fe-B magnet containing oxygen and carbon impurities. J. Magn. Magn. Mater. 1991, 97, 107–111. [Google Scholar] [CrossRef]
- Hartwig, T.; Lopes, L.; Wendhausen, P.; Ünal, N. Metal Injection Molding (MIM) of NdFeB Magnets. EPJ Web Conf. 2014, 75, 4002. [Google Scholar] [CrossRef]
- Sasaki, T.T.; Ohkubo, T.; Une, Y.; Kubo, H.; Sagawa, M.; Hono, K. Effect of carbon on the coercivity and micro-structure in fine-grained Nd–Fe–B sintered magnet. Acta Mater. 2015, 84, 506–514. [Google Scholar] [CrossRef]
- Lukin, A.; Szymura, S. Sintered (Nd, Tb)–(Fe, Ti)–B+C permanent magnets. Intermetallics 2001, 9, 169–171. [Google Scholar] [CrossRef]
- Lopes, L.U.; Costa Santos, E.; Hartwig, T.; Wendhausen, P.A. Investigation of the influence of carbon on the magnetic properties of powder injection molded Nd-Fe-B magnet. In Proceedings of the IEEE Magnetics Conference (INTERMAG), Beijing, China, 11–15 May 2015; IEEE: Piscataway, NJ, USA, 2015; p. 1, ISBN 978-1-4799-7322-4. [Google Scholar]
- Yamashita, O. Magnetic Properties of Nd-Fe-B Magnets Prepared by Metal Injection Molding. J. Jpn. Soc. Powder Powder Metall. 1995, 42, 1073–1078. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, M.; Zhao, R.F.; Liu, B.X.; Tokunaga, M. Some new Nd-rich carbides formed by solid state reaction of and carbon. J. Phys. D Appl. Phys. 1998, 31, 488–493. [Google Scholar] [CrossRef]
- Sagawa, M.; Hirosawa, S.; Yamamoto, H.; Fujimura, S.; Matsuura, Y. Nd–Fe–B Permanent Magnet Materials. Jpn. J. Appl. Phys. 1987, 26, 785–800. [Google Scholar] [CrossRef]
- Jakubowicz, J. Corrosion protection of nanocomposite Nd–Fe–B/α-Fe magnets. J. Alloys Compd. 2001, 314, 305–308. [Google Scholar] [CrossRef]
- Bala, H.; Trepak, N.M.; Szymura, S.; Lukin, A.A.; Gaudyn, V.A.; Isaicheva, L.A.; Pawłowska, G.; Ilina, L.A. Cor-rosion protection of Nd-Fe-B type permanent magnets by zinc phosphate surface conversion coatings. Intermetallics 2001, 9, 515–519. [Google Scholar] [CrossRef]
- Scott, D.W.; Ma, B.M.; Liang, Y.L.; Bounds, C.O. The effects of average grain size on the magnetic properties and corrosion resistance of NdFeB sintered magnets. J. Appl. Phys. 1996, 79, 5501–5503. [Google Scholar] [CrossRef]
- Adler, B.; Müller, R. Seltene Erdmetalle: Gewinnung, Verwendung und Recycling; Universitätsverlag Ilmenau: Ilmenau, Germany, 2014; ISBN 978-3-86360-093-8. [Google Scholar]
- Kim, A.S. Effect of oxygen on magnetic properties of Nd-Fe-B magnets. J. Appl. Phys. 1988, 64, 5571–5573. [Google Scholar] [CrossRef]
- Rathfelder, S.; Schuschnigg, S.; Kukla, C.; Holzer, C.; Suess, D.; Burkhardt, C. Production of Anisotropic NdFeB Permanent Magnets with In-Situ Magnetic Particle Alignment Using Powder Extrusion. Materials 2025, 18, 3668. [Google Scholar] [CrossRef]
- Sasaki, T.T.; Ohkubo, T.; Hono, K.; Une, Y.; Sagawa, M. Correlative multi-scale characterization of a fine grained Nd-Fe-B sintered magnet. Ultramicroscopy 2013, 132, 222–226. [Google Scholar] [CrossRef]
- Kovačič, T.P.; Kovačević, N.; Milošev, I. Corrosion of Sintered NdFeB Permanent Magnets. J. Electrochem. Soc. 2025, 172, 71501. [Google Scholar] [CrossRef]
- Li, W.F.; Ohkubo, T.; Hono, K. Effect of post-sinter annealing on the coercivity and microstructure of Nd–Fe–B permanent magnets. Acta Mater. 2009, 57, 1337–1346. [Google Scholar] [CrossRef]
- Zakotnik, M.; Tudor, C.O. Commercial-scale recycling of NdFeB-type magnets with grain boundary modification yields products with ‘designer properties’ that exceed those of starting materials. Waste Manag. 2015, 44, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, T.; Hirosawa, S.; Ohkubo, T.; Hono, K. The effect of oxygen on the surface coercivity of Nd-coated Nd–Fe–B sintered magnets. J. Appl. Phys. 2009, 105, 07A724. [Google Scholar] [CrossRef]
- Grau, L.; Fleissner, P.; Kobe, S.; Burkhardt, C. Processability and Separability of Commercial Anti-Corrosion Coatings Produced by In Situ Hydrogen-Processing of Magnetic Scrap (HPMS) Recycling of NdFeB. Materials 2024, 17, 2487. [Google Scholar] [CrossRef]
- Schieren, L.; Semsari Parapari, S.; Tomše, T.; Žužek, K.; Sturm, S.; Kobe, S.; Burkhardt, C. (Eds.) Development of a Process to Remove the Neodymium-Rich Phase from Recycled NdFeB Magnet Powder Through Leaching with Organic Acid; REPM: Birmingham, UK, 2023. [Google Scholar]
- Önal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; van Gerven, T. Hydrometallurgical recycling of NdFeB magnets: Complete leaching, iron removal and electrolysis. J. Rare Earths 2017, 35, 574–584. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Saguchi, A.; Takahashi, W.; Yagura, T.; Ono, K. Recycling of Rare Earth Magnet Scraps: Part II Oxygen Removal by Calcium. Mater. Trans. 2001, 42, 2492–2498. [Google Scholar] [CrossRef]
- Grau, L.; Moreno López, R.; Kubelka, P.; Burkhardt, F.; Tomše, T.; Kobe, S.; Burkhardt, C. Effects of Thermal Demagnetization in Air on the Microstructure and Organic Contamination of NdFeB Magnets. Materials 2024, 17, 5528. [Google Scholar] [CrossRef]
- Rampin, I.; Bisaglia, F.; Dabalà, M. Corrosion Properties of NdFeB Magnets Coated by a Ni/Cu/Ni Layer in Chloride and Sulfide Environments. J. Mater. Eng. Perform. 2010, 19, 970–975. [Google Scholar] [CrossRef]
- Zheng, J.-X.; Nong, L.-Q. A phase diagram of the alloys of the Nd-Cu binary system. Acta Phys. Sin. 1983, 32, 1449. [Google Scholar] [CrossRef]
- Müller, C.; Reinsch, B.; Petzow, G. Phase Relations in the System Nd-Fe-Cu. Int. J. Mater. Res. 1992, 83, 845–852. [Google Scholar] [CrossRef]
- Pandian, S.; Chandrasekaran, V.; Markandeyulu, G.; Iyer, K.J.L.; Rama Rao, K.V.S. Effect of Al, Cu, Ga, and Nb additions on the magnetic properties and microstructural features of sintered NdFeB. J. Appl. Phys. 2002, 92, 6082–6086. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, Y.; Han, J.; Liu, S.; Liu, T.; Zhou, L.; Fu, J.; Zhou, D.; Zhang, X.; Yang, J.; et al. Coercivity enhancement in Dy-free Nd–Fe–B sintered magnets by using Pr-Cu alloy. J. Appl. Phys. 2014, 115, 203910. [Google Scholar] [CrossRef]
- Pickering, L. Investigation into the Influence of Zn Coatings on Recycling of Sintered NdFeB-type Magnets. In Proceedings of the REPM 2023 Proceedings 27th International Workshop on Rare Earth and Future Permanent Magnets and Their Applications, Birmingham, UK, 3–10 September 2023. [Google Scholar]
- Royal Society of Chemistry. Periodic Table: Zinc. Available online: https://www.rsc.org/periodic-table/element/30/zinc (accessed on 26 April 2024).
- Horikawa, T.; Itoh, M.; Suzuki, S.; Machida, K. Magnetic properties of the Nd–Fe–B sintered magnet powders recovered by Yb metal vapor sorption. J. Magn. Magn. Mater. 2004, 271, 369–380. [Google Scholar] [CrossRef]
- Li, Y.; Kim, Y.B.; Yoon, T.S.; Suhr, D.S.; Kim, T.K.; Kim, C.O. Coercivity enhancement by Zn addition in hot de-formed NdFeB magnets. J. Magn. Magn. Mater. 2002, 242–245, 1369–1371. [Google Scholar] [CrossRef]
- Burkhardt, C.; Lehmann, A.; Fleissner, P.; Grau, L.; Trautz, M.; Mungenast, M.; Podmiljšak, B.; Kobe, S. Comparative Evaluation of Anti-Corrosion Coatings for NdFeB-Type Magnets with Respect to Performance and Recyclability via Hydrogen-Assisted Recycling (HPMS). Mater. Proc. 2021, 5, 87. [Google Scholar] [CrossRef]
- Okamoto, H. Nd-Ni (Neodymium-Nickel). J. Phase. Equilib. 1998, 19, 290. [Google Scholar] [CrossRef]
- Trujillo Hernández, J.S.; Tabares, J.A.; Pérez Alcázar, G.A. Comparative Magnetic and Structural Properties Study of Micro- and Nanopowders of Nd2Fe14B Doped with Ni. J. Supercond. Nov. Magn. 2017, 30, 3423–3430. [Google Scholar] [CrossRef]
- Zakotnik, M.; Harris, I.R.; Williams, A.J. Multiple recycling of NdFeB-type sintered magnets. J. Alloys Compd. 2009, 469, 314–321. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).