Abstract
This study investigates the valorization of refuse-derived fuel (RDF), waste glass, and mill scale for sustainable ferrosilicon alloy production, contributing to zero-waste practices. RDF was blended with anthracite at ratios of 100, 90, 80, 70, 60 and 50 wt% (designated R1–R6) and applied as a reducing agent in the carbothermic reduction of SiO2 and Fe2O3, thereby decreasing reliance on conventional fossil-based reductants. Ferrosilicon synthesis was conducted at 1550 °C using glass–mill scale blends with reducing agents R1–R6, producing samples named blends A–F. XRD analysis confirmed that the metallic products consisted predominantly of the FeSi intermetallic phase, with characteristic (110) and (310) peaks at 2θ ≈ 45.02° and 78°. The metallic products appeared as numerous small, shiny droplets, with yields ranging from 14.85 to 19.47 wt%; blends D–F exhibited the highest yields. In contrast, blends A–C produced metals with higher Si contents (23.34–27.11 wt%) due to enhanced SiO2 reduction and efficient Si incorporation into the Fe matrix. Gas analysis and oxygen removal showed that blend B achieved the highest CO generation and reduction extent. Cl removal during RDF heat treatment indicated minimal potential for dioxin and furan formation. Overall, blends A and C were identified as optimal, providing high Si content, satisfactory metallic yield, and reduced CO/CO2 emissions, demonstrating the effectiveness of RDF-based carbons for environmentally friendly ferrosilicon production.
1. Introduction
Modern industrial development and urban expansion have resulted in extensive natural resource depletion, increased environmental pollution, and rising volumes of both industrial and municipal waste. Upcycling has emerged as a promising approach to transform such wastes into higher-value products, thereby supporting environmental protection and reducing reliance on natural resources. Refuse-derived fuel (RDF) is a solid fuel produced from municipal waste, consisting mainly of combustible fractions such as paper, textiles, wood, organic matter, and plastics (including PVC). RDF production plants are generally integrated with waste separation and recycling centers in metropolitan areas. However, the presence of chlorine in PVC poses a critical challenge, as RDF pyrolysis can release harmful chlorinated compounds such as dioxins and furans, which are known carcinogens. RDF has been widely utilized as a substitute for fossil fuels in power plants and cement kilns, as well as a feedstock for syngas production [1,2,3,4,5]. Therefore, RDF must undergo appropriate pre-treatment to reduce moisture, enhance calorific value, and remove undesirable components, particularly chlorine and heavy metals [4,5]. Expanding alternative applications of RDF is essential to advance circular economy goals and promote zero-waste practices. Waste glass, a non-combustible component of municipal waste, is a post-consumer material composed primarily of SiO2. Although it is commonly recycled through remelting, it can also be upcycled as a raw material for ferrosilicon production. Mill scale, a by-product generated during the hot-rolling process in steel mills, is another major industrial residue, along with slag and dust. Its high Fe2O3 content makes it a widely utilized secondary source of iron oxide in metallurgical applications. By integrating waste glass and mill scale as alternative raw materials, these wastes can be upcycled for ferrosilicon production, thereby reducing the reliance on natural resources and supporting sustainable metallurgical practices.
Ferrosilicon (FeSi) is an important iron–silicon alloy, typically containing 10–90% silicon. The Fe–Si phase diagram shows several possible phases, including FeSi, FeSi2 and Fe2Si5, with FeSi being the most stable and widely encountered [6]. Its main application is in steelmaking, where it serves as a deoxidizer and alloying agent to refine melt properties, regulate solidification, and improve product performance. Beyond steel industries, Fe–Si alloys are also used in silicon steels for electrical devices such as transformer cores and electromotors, as well as in thermoelectric applications [7]. Among these, β-FeSi2 has attracted attention as a thermoelectric material due to its low toxicity, thermal stability, and cost efficiency [7,8,9,10]. Traditionally, Fe–Si alloys can be synthesized by melting techniques, which often yield ε-FeSi dispersed in an α-Fe2Si5 matrix [11], or by mechanical alloying, which produces phases such as FeSi and Fe5Si3 at elevated temperatures [12]. Industrial production of ferrosilicon, however, relies on carbothermic reduction in silica and iron oxides at 1550–1750 °C in blast furnaces, electric arc furnaces, or submerged furnaces [12,13,14,15]. Silica is commonly supplied from quartz, sand, or high-silica biomasses like rice husk [13,14], while iron sources include ores, scrap metal, and by-products such as mill scale. Carbon, essential for the reduction reactions, is typically provided by coal, coke, or graphite. Several studies have investigated the use of waste-derived materials for ferrosilicon and related alloy production. Farzanz et al. [15] synthesized ferrosilicon at 1550 °C using waste glass, Fe2O3, and bakelite as silica, iron, and carbon sources, achieving a maximum silicon recovery of 16 wt%. Wang et al. [16] reported that coal gasification fine slag (CGFS) could be reduced in an electric arc furnace to produce a Si–Fe–Al–Ca alloy containing 63.83 wt% Si. However, this method required operating temperatures above 2000 °C, which poses serious challenges for industrial feasibility. Chen et al. [17] demonstrated that adjusting the Fe/Si ratio in coal fly ash reduction improved both the reduction in silica phases and Fe–Si alloy recovery, with magnetic separation efficiency rising from 79.68% to 89.03% as the Fe/Si ratio increased from 1.0 to 1.4. Overall, while these approaches demonstrate the potential of using waste materials in alloy production, their limitations—such as low silicon recovery, dependency on precise compositional control, and high energy demand–highlight the need for more practical, cost-effective, and sustainable methods of ferrosilicon synthesis.
The present study investigates the upcycling of RDF as a reducing agent for ferrosilicon production, aiming to reduce fossil fuel consumption. RDF was blended with anthracite at six ratios (100–50 wt%, R1–R6) and preheated to produce Cl-free chars. These chars were then mixed with waste glass and mill scale powders to prepare six different blends (A–F). Ferrosilicon alloy formation was examined at 1550 °C through carbothermic reduction of SiO2 from glass and Fe2O3 from mill scale. This approach demonstrates a sustainable pathway for utilizing wastes and byproducts within a zero-waste framework. By substituting fossil-based reductants, it not only decreases the use of natural resources but also contributes to lowering carbon emissions and mitigating environmental impacts.
2. Results and Discussion
2.1. Metallic Products
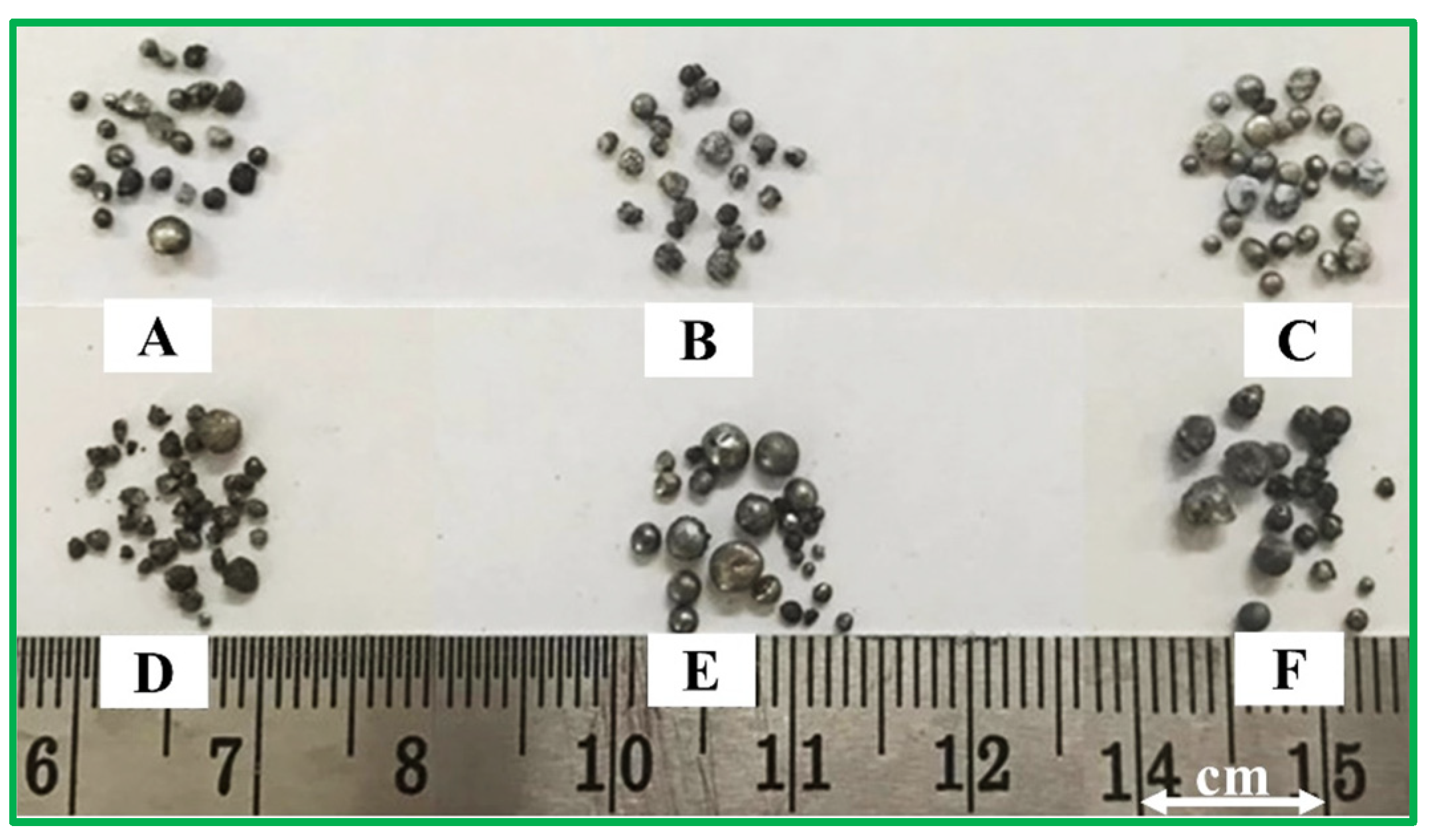
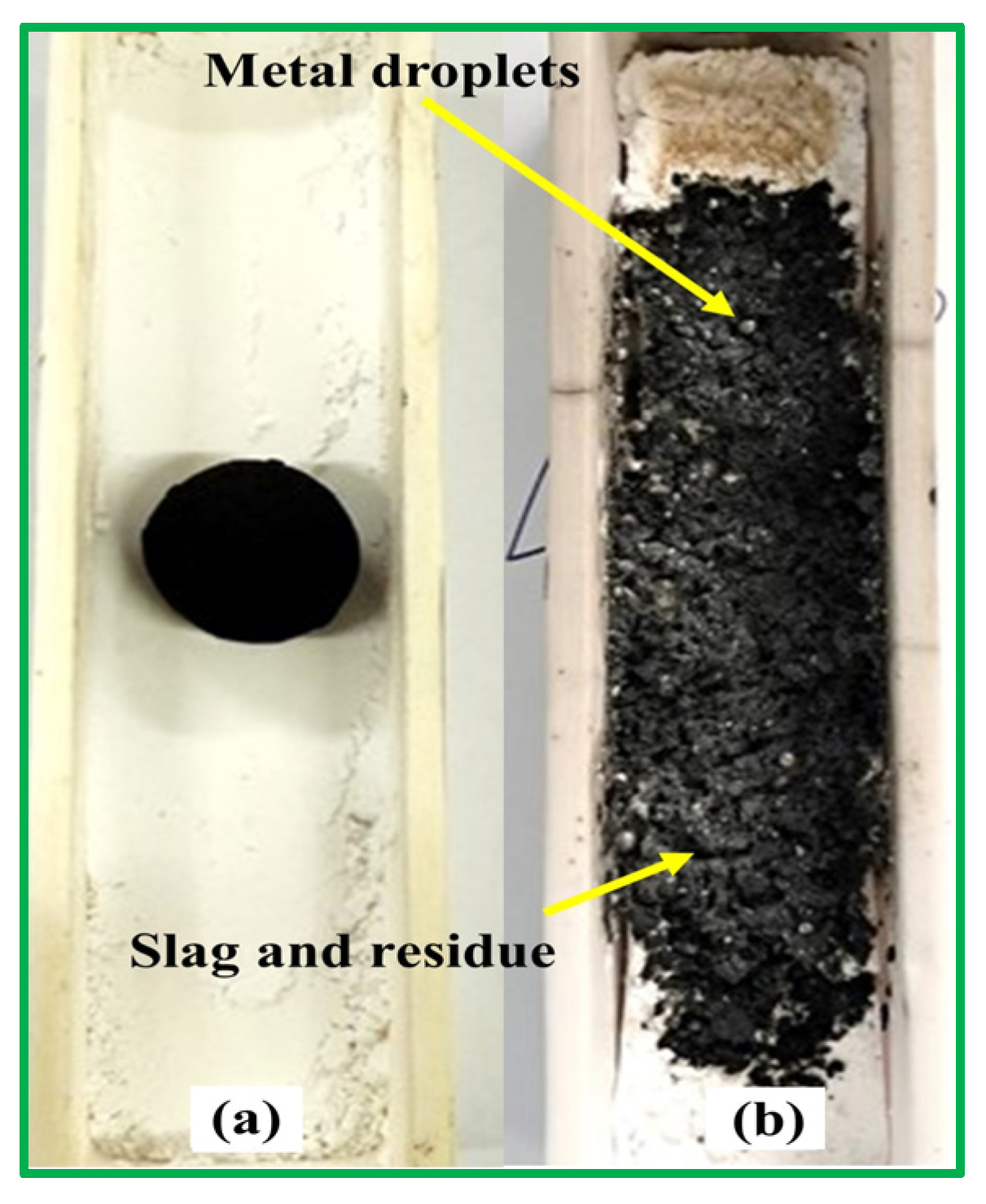
Metallic products were separated from the black residue and cleaned in an ultrasonic bath with ethanol to remove surface debris (Figure 1). The presence of metallic droplets confirmed the occurrence of carbothermic reduction in the glass–mill scale system for all reducing agents (R1–R6). Phase, morphology, yield, and chemical composition analyses were performed to characterize the metallic products. Table 1 shows the metallic yield of pellets (Blends A–F) after heating at 1550 °C for 1 h under an argon atmosphere. The initial mass of raw pellets was ~5 g. Post-heating mass ranged from 2.99 to 3.61 g, corresponding to a maximum mass loss of ~40 wt%. Pellet mass after heating was calculated by subtracting the empty crucible mass from that of the crucible containing the pellet. Metallic droplets weighed 0.45–0.69 g, corresponding to yields of 14.85–19.47 wt%. The lowest yield was obtained for blend A (100% RDF), while the highest was for blend E (60% RDF–anthracite). Manual collection may have introduced minor errors in yield determination.

Figure 1.
The metallic products obtained after heating the pellets of blends A–F for 1 h at 1550 °C.

Table 1.
Mass balance of the pellets following heating at 1550 °C for 1 h in an inert argon atmosphere for blends A–F.
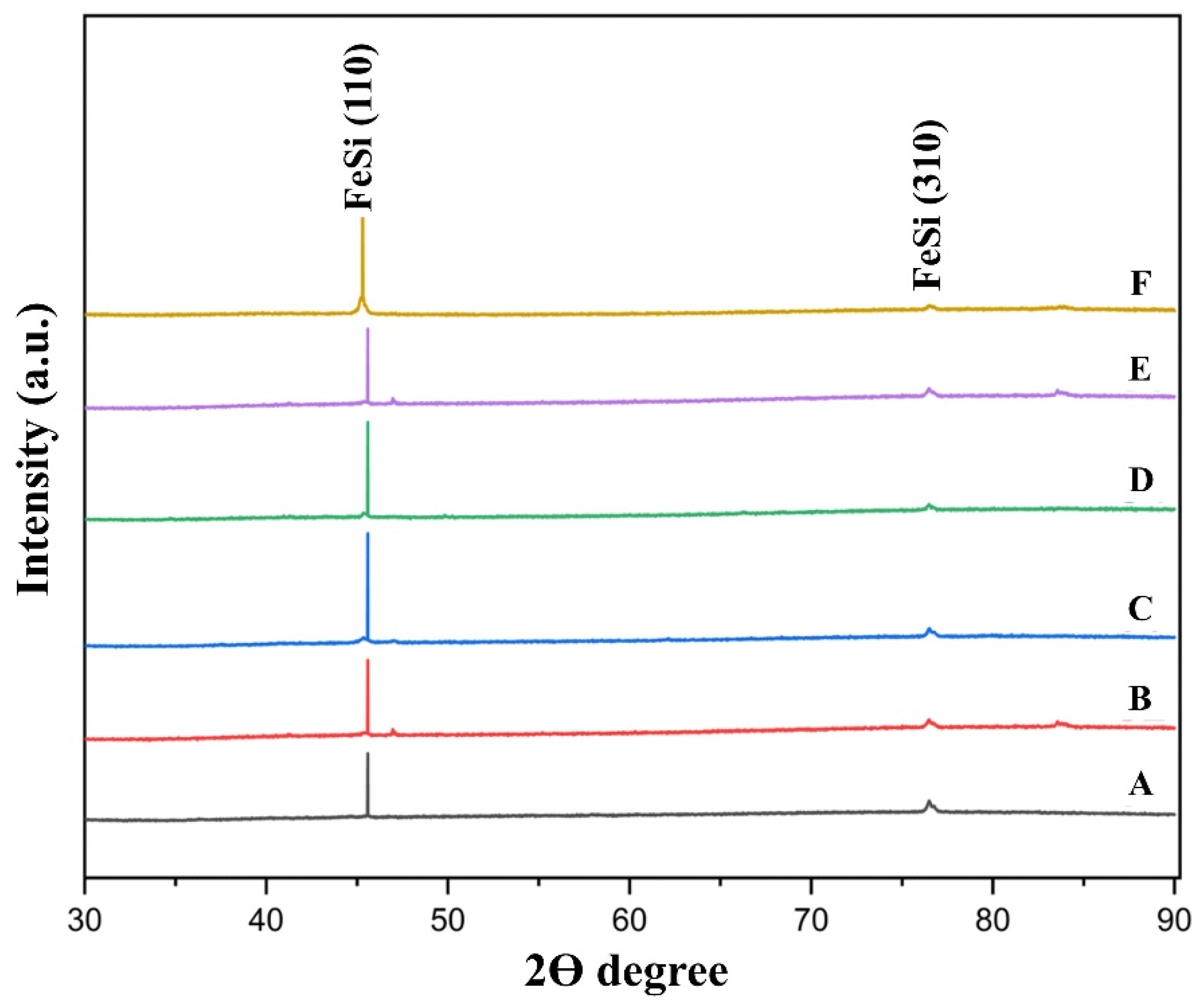
Several metallic droplets were cold-mounted in epoxy resin, ground with SiC paper, and polished with diamond suspension to obtain a reflective bulk surface for XRD analysis. Figure 2 shows the XRD patterns of metallic products from pellets heated at 1550 °C for 1 h. All samples consisted of the ferrosilicon intermetallic compound iron monosilicide (FeSi), exhibiting a primary peak at ~45.02° (2θ) corresponding to the (110) plane and a secondary peak near 78° (2θ) corresponding to the (310) plane [18,19]. No other inclusion phases were detected, confirming effective Fe–Si alloy formation via carbothermic reduction of SiO2 from waste glass and Fe2O3 from mill scale by carbon derived from RDF (R1–R6).

Figure 2.
XRD analysis of the metallic products after heating the pellets of blends A–F for 1 h at 1550 °C.
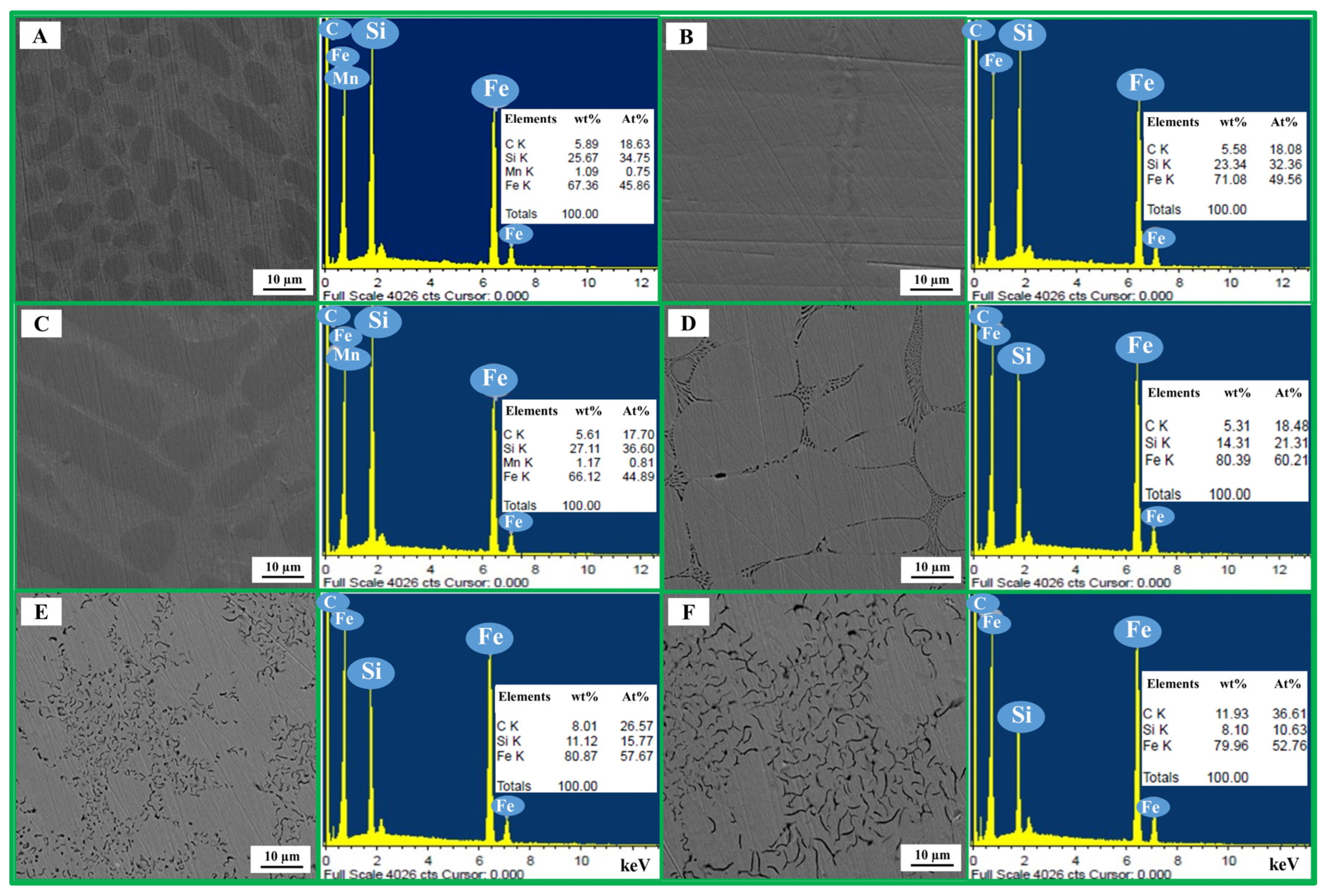
SEM micrographs (×500) of bulk metallic products from blends A–F are shown in Figure 3. All samples exhibited dendritic morphologies, with noticeable size variations particularly in blends A–D. Graphite flakes (small black lines) were observed in blends E and F, while no inclusion phases were detected in any sample. Higher-magnification SEM images (×1500) with EDS whole-area analysis are presented in Figure 4. The alloys primarily consisted of Fe–Si–C, with no detectable oxygen in any case; trace Mn was found in blends A and C. EDS results showed the highest Si content (27.11 wt%) in blend C, followed by 25.67 wt% in blend A (100% RDF) and 23.34 wt% in blend B. Si content decreased progressively to 14.31, 11.12 and 8.10 wt% in blends D–F as RDF content declined from 70 to 50 wt%. Carbon levels were ~5 wt% for blends A–D, increasing to ~8 wt% in blends E and F, consistent with the graphite flakes observed in their microstructures. Instead of EDS analysis, the Inductively Coupled Plasma (ICP) technique and the C–S analyzer are required for precise determination of Si and C contents in the metallic products, respectively. Unfortunately, due to the limitations of our laboratory, these techniques could not be performed on the metallic products produced in the present study.

Figure 3.
SEM (×500) micrographs of bulk metallic products from blends A–F.

Figure 4.
SEM micrographs (×1500) and EDS whole-area analysis of bulk metallic products from blends A–F.
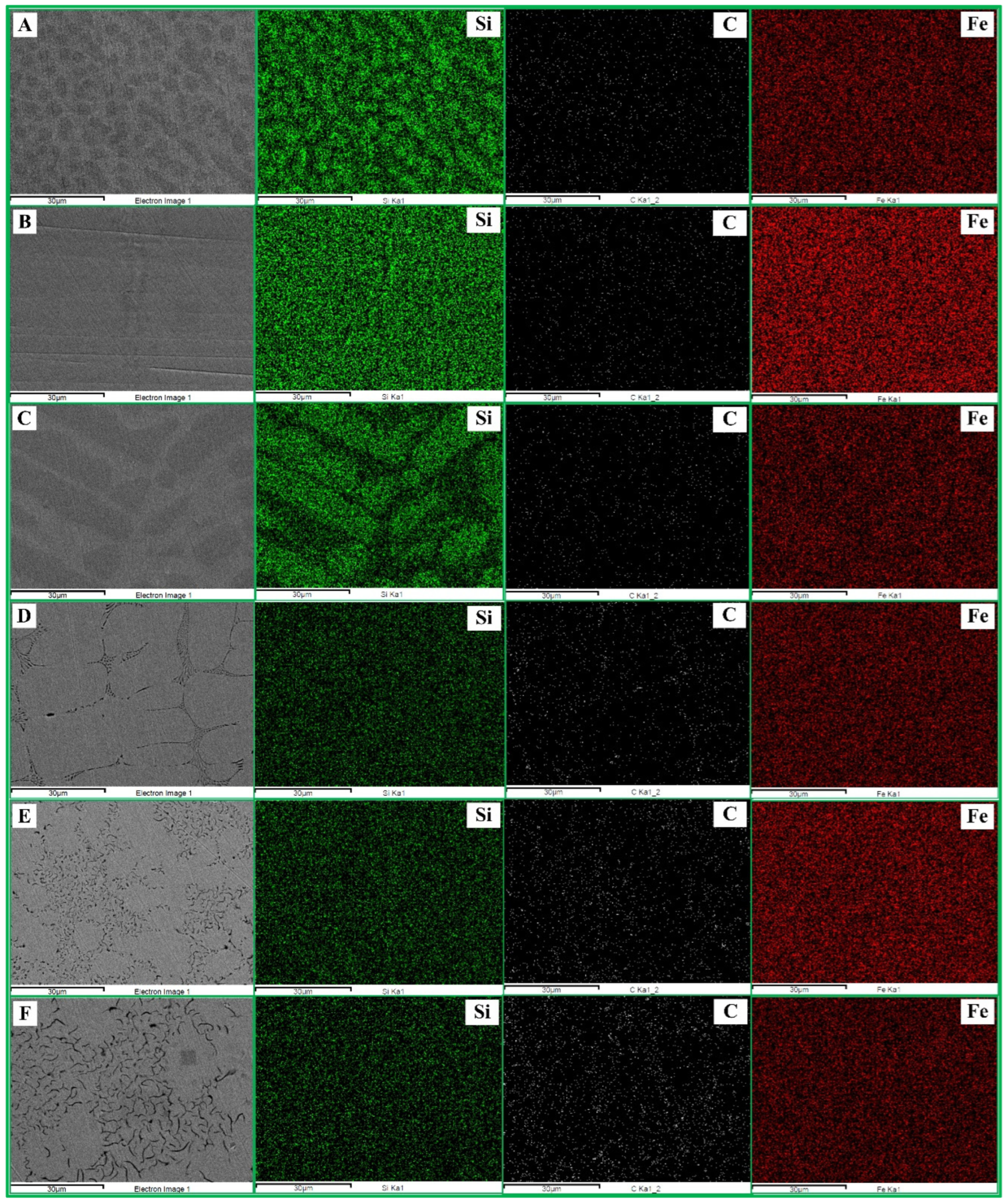
Figure 5 presents the SEM micrographs with EDS elemental mapping of the bulk metallic products for blends A–F. The green, white, and red dots correspond to Si, C, and Fe, respectively. A strong and widespread distribution of Si was observed in blends A–C, which gradually decreased with lower RDF content in blends D–F. In contrast, carbon distribution increased as the RDF content decreased. Meanwhile, Fe appeared uniformly distributed throughout the bulk metal, serving as the matrix phase. These observations indicate variations in the Si content of the metallic products and confirm the formation of FeSi intermetallic phases across all blends, which is consistent with the XRD results.

Figure 5.
SEM micrographs and elemental mapping of the bulk metallic product from blends A–F.
2.2. Formation of the Metallic Products
The results presented in Section 2.1 confirmed that the metallic products are ferrosilicon (FeSi) alloys. These alloys were successfully synthesized from waste glass and mill scale through carbothermic reduction at 1550 °C, using carbon derived from RDF (R1–R6) as the reducing agent. In the glass–scale–RDF system, ferrosilicon formation occurs via the reduction of SiO2 in glass powder and Fe2O3 in mill scale. Since the reduction of SiO2 typically proceeds at temperatures above 1400 °C [20], the chosen operating temperature (1550 °C) was sufficient to promote silicon reduction. The possible reaction pathways for SiO2 reduction are given in Equations (1)–(7), involving both solid–solid and solid–gas reactions. Specifically, SiO2 can be reduced by solid carbon to form SiC, liquid Si, SiO and CO, as in Equations (1)–(3). SiO gas can also be further reduced by solid carbon, yielding similar products, Equations (4) and (5). In addition, the SiC formed in the system can further participate in reduction reactions: it may reduce SiO2 to produce solute Si, SiO and CO, or react with SiO to yield solute Si and CO gas, as represented in Equations (6) and (7), respectively.
SiO2 (s) + 3C (s) = SiC (s) + 2CO (g)
SiO2 (s) + 2C (s) = Si (l) + 2CO (g)
SiO2 (s) + C (s) = SiO (g) + CO (g)
SiO (g) + 2C (s) = SiC (s) + CO (g)
SiO (g) + C (s) = Si (l) + CO (g)
SiO2 (s) + SiC (s) = Si + SiO (g) + CO (g)
SiO (g) + SiC (s) = 2Si + CO (g)
Fe2O3 in mill scale can be directly reduced by solid carbon, as shown in Equations (8)–(10), generating CO gas in the system. The produced CO can subsequently act as a reducing agent for Fe2O3 through the reactions in Equations (11)–(13), which lead to the formation of CO2. The CO2 formed may then oxidize solid carbon within the pellets to regenerate CO, as described in Equation (14). This cyclic CO/CO2–C mechanism further enhances the overall reduction of Fe2O3. The calculated standard Gibbs free energies (ΔG°) at 1550 °C for Equations (10), (13) and (14) are −124.46 kJ, −276.71 kJ, and −145.63 kJ, respectively, confirming a strong thermodynamic driving force for these reduction reactions [21,22].
3Fe2O3 (l) + C (s) = 2Fe3O4 (l) + CO (g)
Fe3O4 (l) + C (s) = 3FeO (l) + CO (g)
FeO (l) + C (s) = Fe (l) + CO (g)
3Fe2O3 (l) + CO (g) = 2Fe3O4 (l)+ CO2 (g)
Fe3O4 (l) + CO (g) = 3FeO (l) + CO2 (g)
FeO (l) + CO (g) = Fe (l) + CO2 (g)
C (s) + CO2 (g) = 2CO (g)
SiC (s) + Fe (l) = FeSi (l) + C
2SiO2 (s) + Fe2O3 (l) + 7C (s) = 2FeSi (l) + 7CO
The formation of the FeSi intermetallic phase can proceed through two primary pathways. First, solute Si atoms generated in the reaction zone can diffuse and dissolve into the liquid Fe matrix. Second, SiC can directly interact with liquid Fe to form FeSi, as expressed in Equation (15). In addition, SiO2 may react simultaneously with Fe2O3 and solid carbon to yield FeSi and CO, representing the overall reaction pathway shown in Equation (16). Consistent with the EDS results, the carbon detected in the bulk metallic products is likely associated with the dissolution and transfer of carbon atoms from the RDF/anthracite blends into the liquid iron during solid–liquid contact.
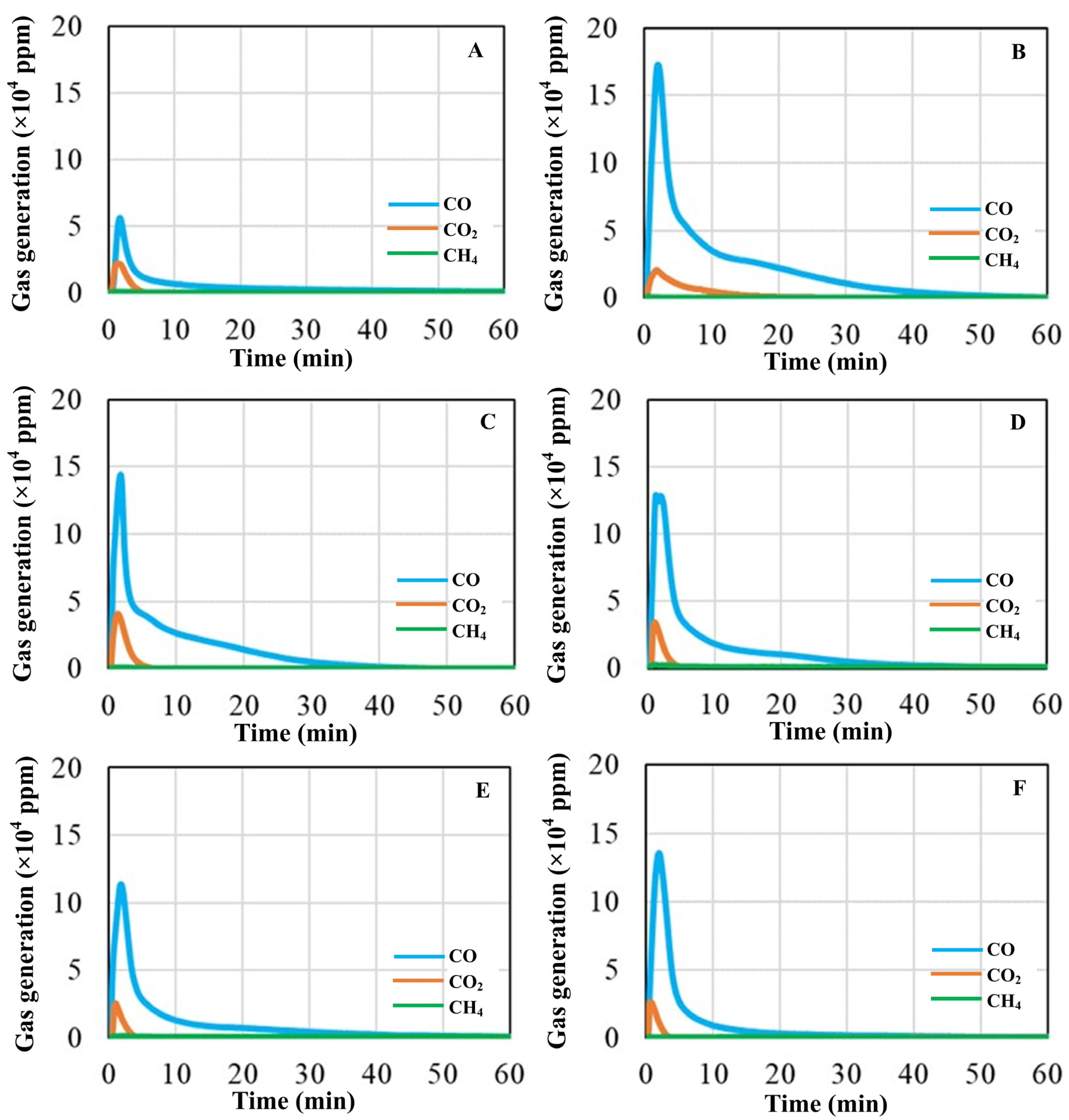
Figure 6 presents the gas emissions generated during the pyrolysis of glass–mill scale–carbon pellets at 1550 °C, analyzed using an IR gas analyzer. For all blends, CO was identified as the predominant gaseous product, followed by CO2, with trace amounts of CH4. The amount of gas evolution showed a sharp rise within the first 5 min of reaction, followed by a gradual decline over time. Among the blends (A–F), the pellet of blend B exhibited the highest CO release (172,900 ppm), followed by blend C (143,800 ppm), while blend A produced the lowest CO concentration (56,200 ppm).

Figure 6.
Variation in CO, CO2 and CH4 generation during carbothermic reduction in the pellets of blends A–F.
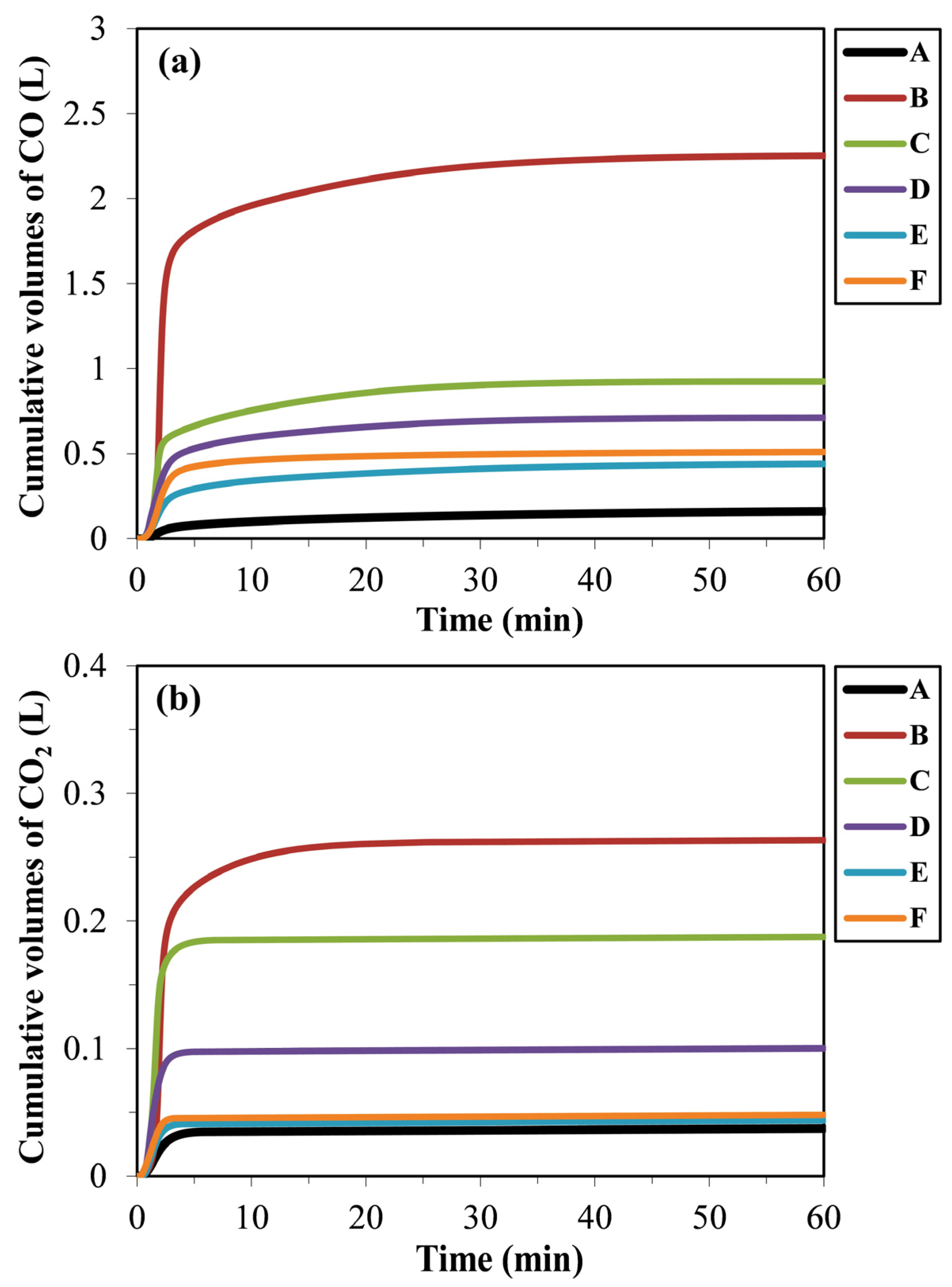
The generation of CO is particularly significant, as it reflects the extent of carbothermic reduction of SiO2 and Fe2O3 by RDF/anthracite blends, which in turn promotes the formation of FeSi alloy. In contrast, CO2 primarily originated from the carbothermic reduction of Fe2O3 in the mill scale. Moreover, CO plays a dual role in the system: not only as a product of reduction but also as an auxiliary reductant that complements the reducing action of carbon derived from RDF. Figure 7 shows cumulative volume of CO and CO2 generation during carbothermic reduction in the pellets of blends A–F. The cumulative gas generation during pyrolysis revealed that blend B produced the highest CO volume (~2.25 L), followed by blend C (~0.92 L), while the lowest value was obtained for blend A (~0.16 L). The other blends exhibited comparable CO volumes in the range of ~0.44–0.71 L. In contrast, CO2 evolution was significantly lower than CO in all cases, confirming CO as the major gaseous product. The maximum cumulative CO2 generation was also observed in blend B (~0.26 L), followed by blends C (~0.19 L) and D (~0.10 L), while the remaining blends showed similar CO2 volumes of ~0.04 L.

Figure 7.
Cumulative volume of (a) CO and (b) CO2 generation during carbothermic reduction in the pellets of blends A–F.
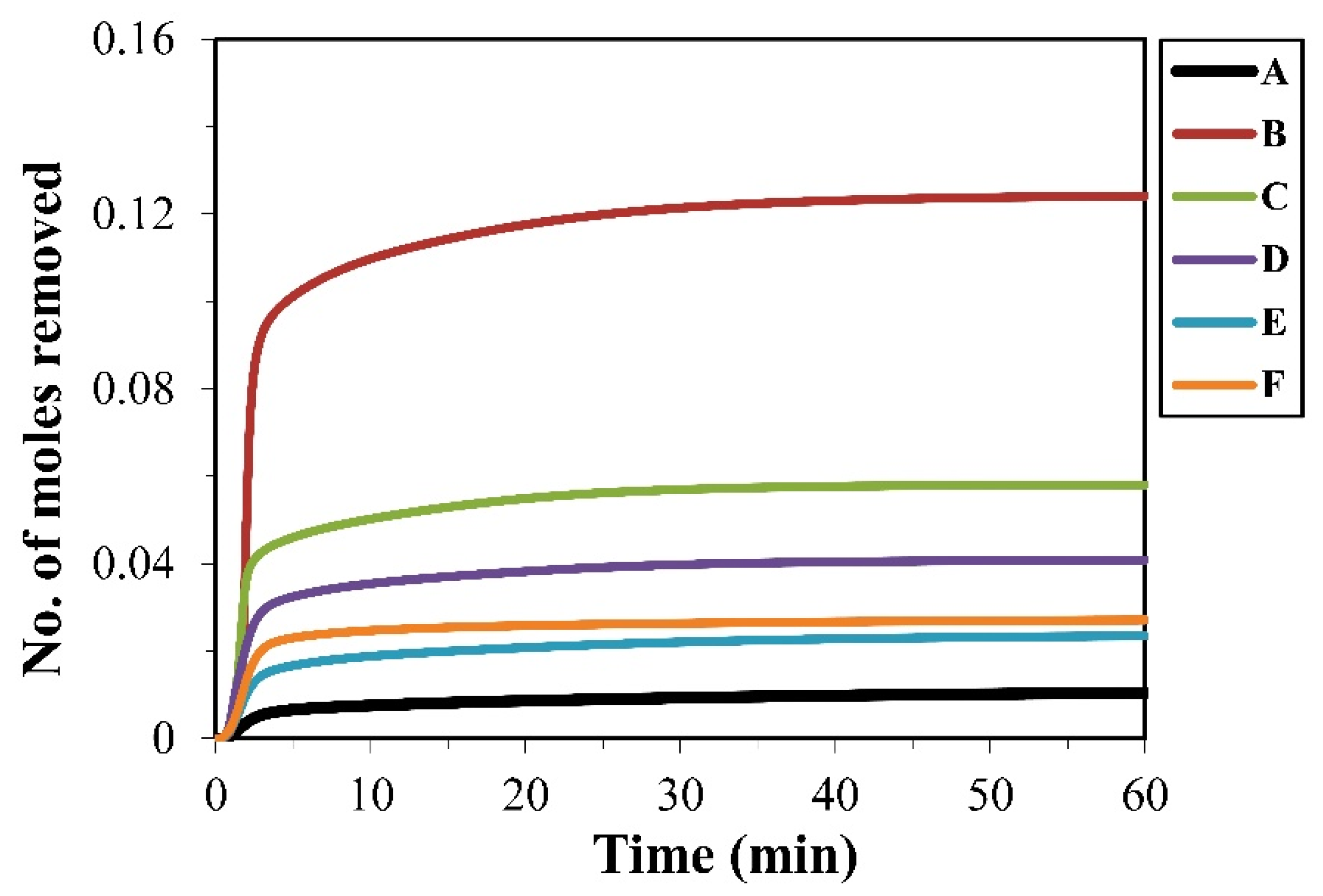
To evaluate the extent of carbothermic reduction of SiO2 and Fe2O3 in the glass–mill scale–carbon systems, the moles of oxygen removed during the reactions were calculated and are presented in Figure 8. A similar trend to gas evolution was observed, with blend B showing the highest oxygen removal (0.12 mol), followed by blend C at about half this value (0.06 mol), while the lowest was recorded for blend A (0.01 mol). The other blends exhibited intermediate values in the range of 0.02–0.04 mol. The greater oxygen removal in blend B indicates the highest extent of carbothermic reduction, which is consistent with its higher CO generation and can be attributed to the greater reactivity of carbon and enhanced interaction between the carbon and oxide phases in this blend.

Figure 8.
Moles of oxygen removed during carbothermic reduction in the pellets of blends A–F.
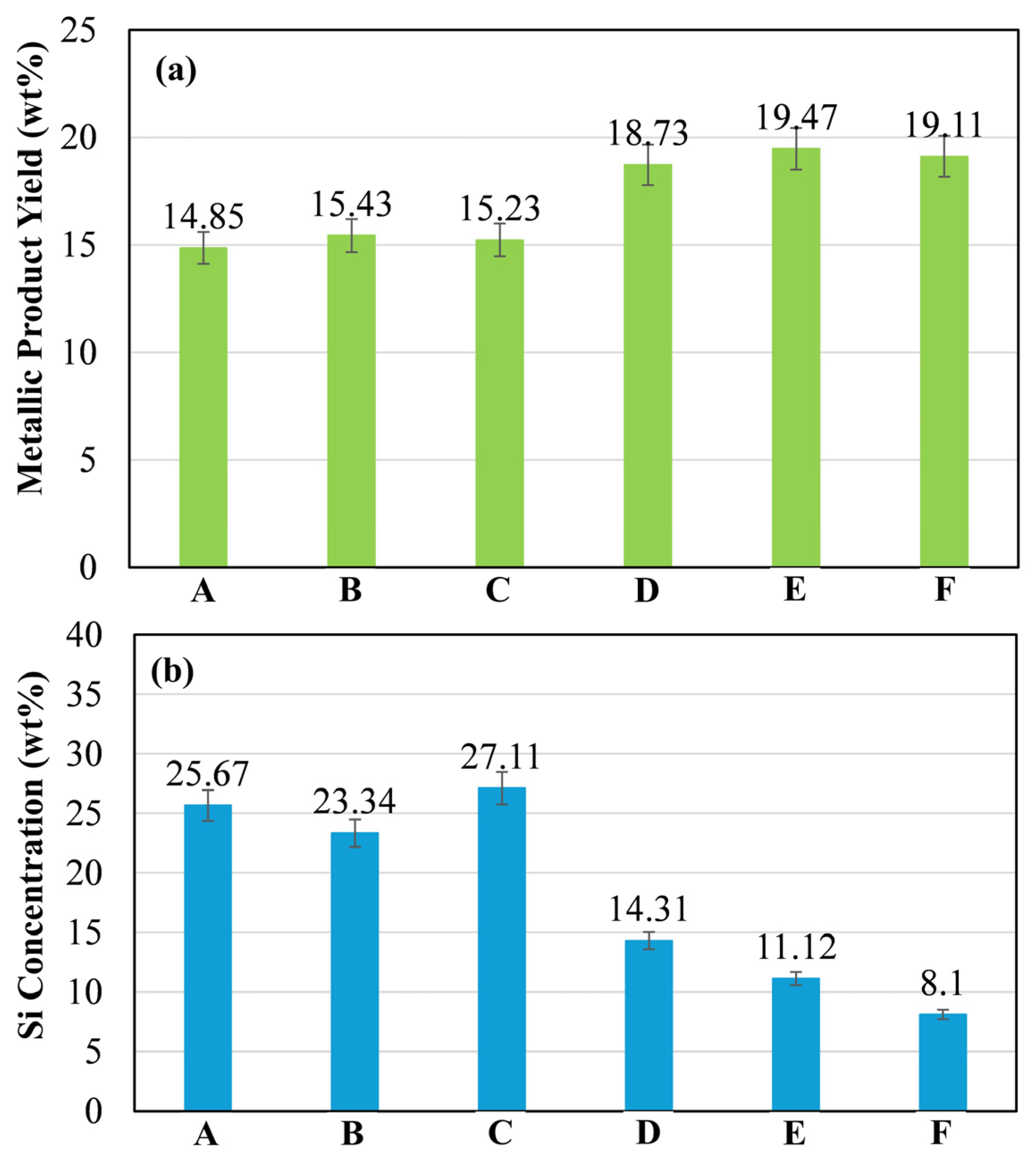
However, the gas analysis results did not fully correspond with the metallic yield and Si concentration of the metallic products. As shown in Figure 9, blends D, E and F exhibited the high metallic yields (18.73–19.47 wt%), suggesting a greater extent of Fe2O3 reduction. In contrast, blends A, B, and C produced metals with lower metallic yield (14.85–15.43 wt%) and higher Si contents (23.34–27.11 wt%). These findings suggest that the CO/CO2 generated in the system may have originated not only from the reduction of SiO2 and Fe2O3, but also from the combustion of the carbonaceous materials present in the blends. The higher Si content observed in the FeSi alloys of blends A–C is likely attributed not only to the greater extent of SiO2 reduction, but also to the mass transfer and diffusivity of Si atoms into the Fe matrix phase. Thus, the extent of carbothermic reduction and the formation of the FeSi intermetallic alloy appear to be strongly governed by both the compositional makeup and the intrinsic characteristics of the blends.

Figure 9.
(a) Metallic yield and (b) Si concentration of the metallic product from Blends A–F.
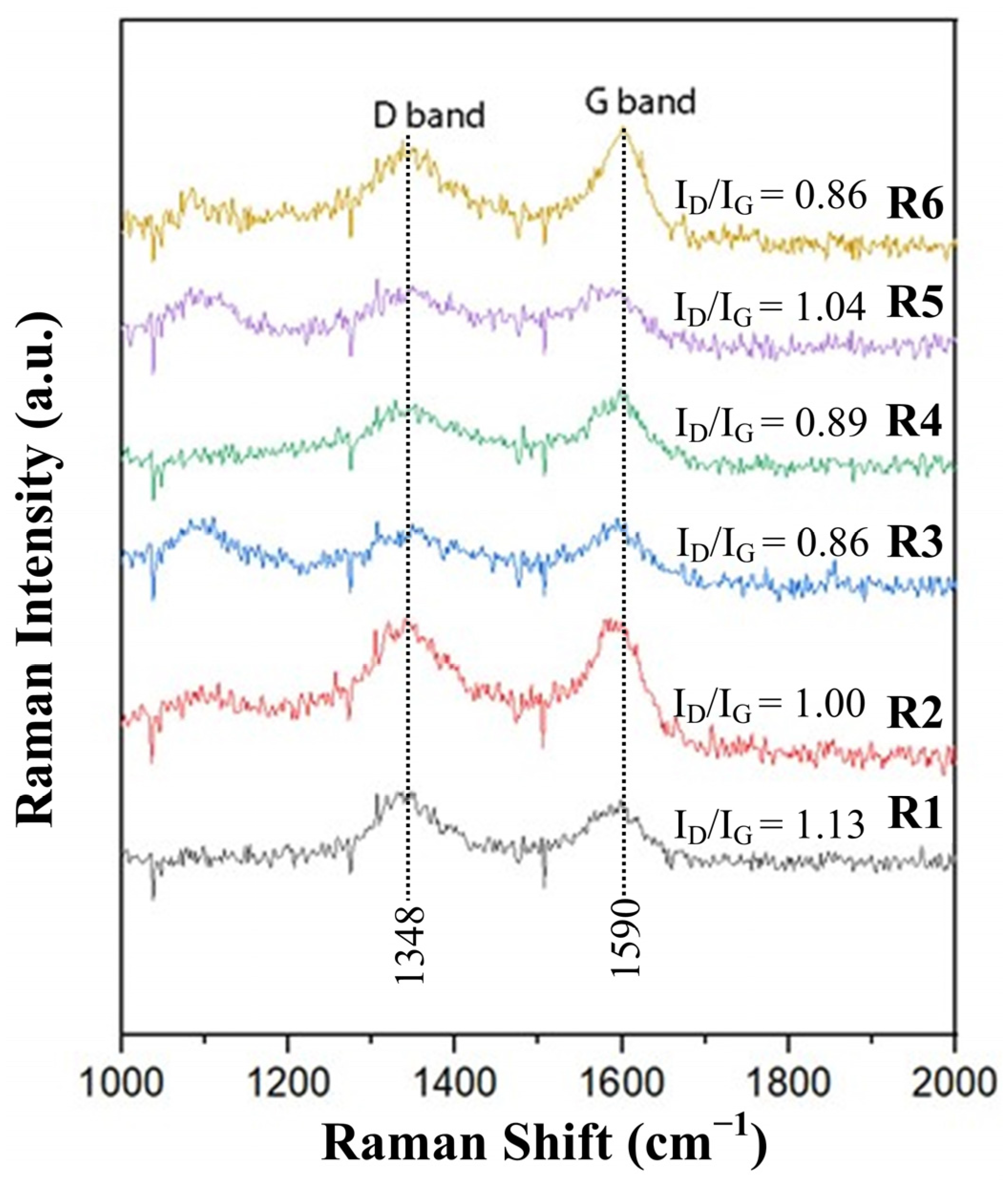
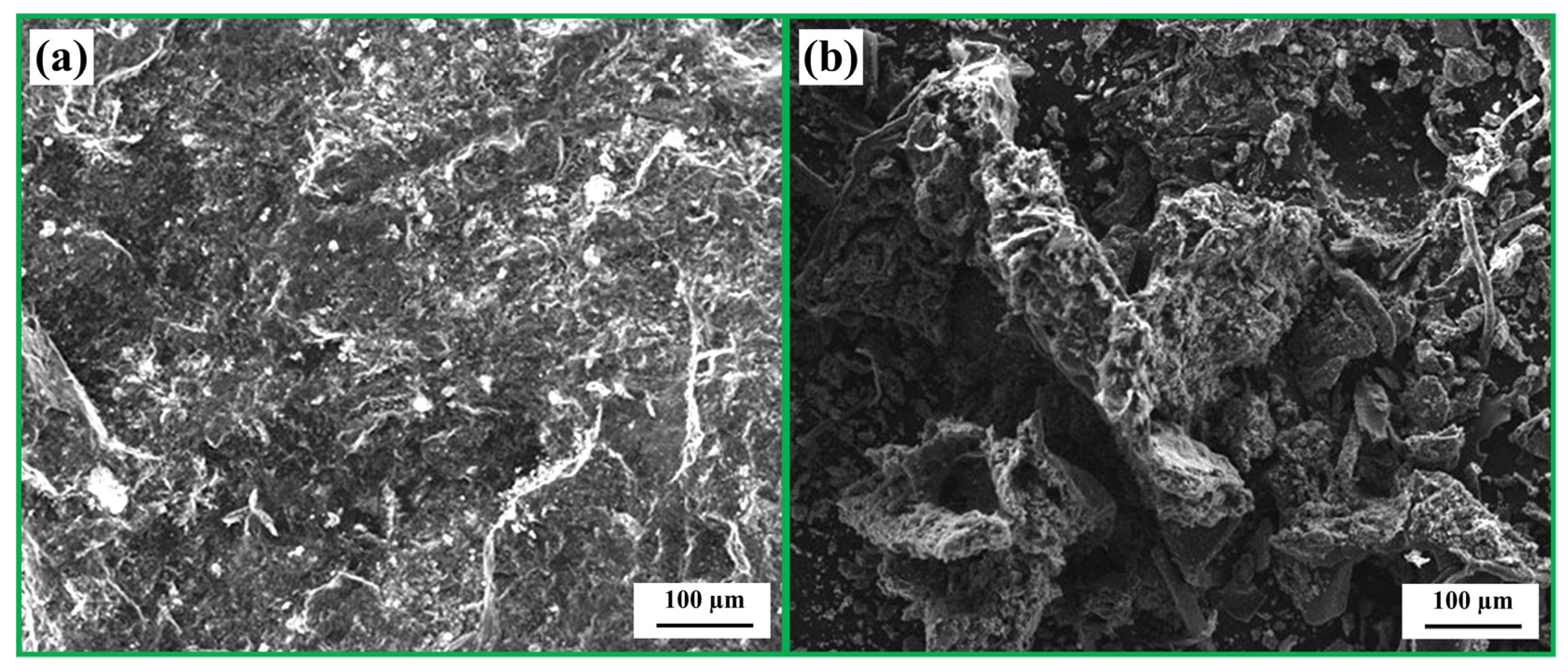
The characteristics of carbon in the glass–scale–carbon system (blends A–F), particularly surface area and structural order, can influence FeSi formation. Figure 10 shows the Raman spectra of carbons derived from RDF/anthracite blends (R1–R6). The lowest ID/IG ratio was 0.86 for R3 and R6, indicating a higher fraction of crystalline carbon. In contrast, R1 exhibited the highest ID/IG ratio of 1.13, suggesting a more disordered structure. Figure 11 compares the surface morphology of anthracite and RDF char particles. RDF char displayed a rough and porous surface, which corresponds to a larger surface area, while anthracite showed a dense and smooth texture. As a result, RDF/anthracite blends with higher RDF content (R1–R3) are expected to have higher surface areas compared to R4–R6.

Figure 10.
Raman spectra of the derived carbons from RDF/anthracite blends R1–R6.

Figure 11.
SEM micrographs present surface morphology of (a) anthracite and (b) RDF char particles.
The formation of ferrosilicon alloy occurs in two main steps: (1) reduction of Fe2O3 and SiO2 by carbon to form Fe and Si phases, and (2) diffusion and mass transfer of Si atoms into the Fe matrix [11]. Therefore, the balance between surface area and carbon structure is likely responsible for the higher Si content observed in the FeSi products produced from blends containing R1–R3 carbons. Ferrosilicon alloys typically contain 15–90 wt% Si, with commercial grades at 15, 45, 75 and 90 wt%. The metallic products from blends A–C contained 25–27 wt% Si, falling between FeSi15 and FeSi45, and are suitable for steelmaking and non-ferrous metallurgy applications.
2.3. Environmental Aspect
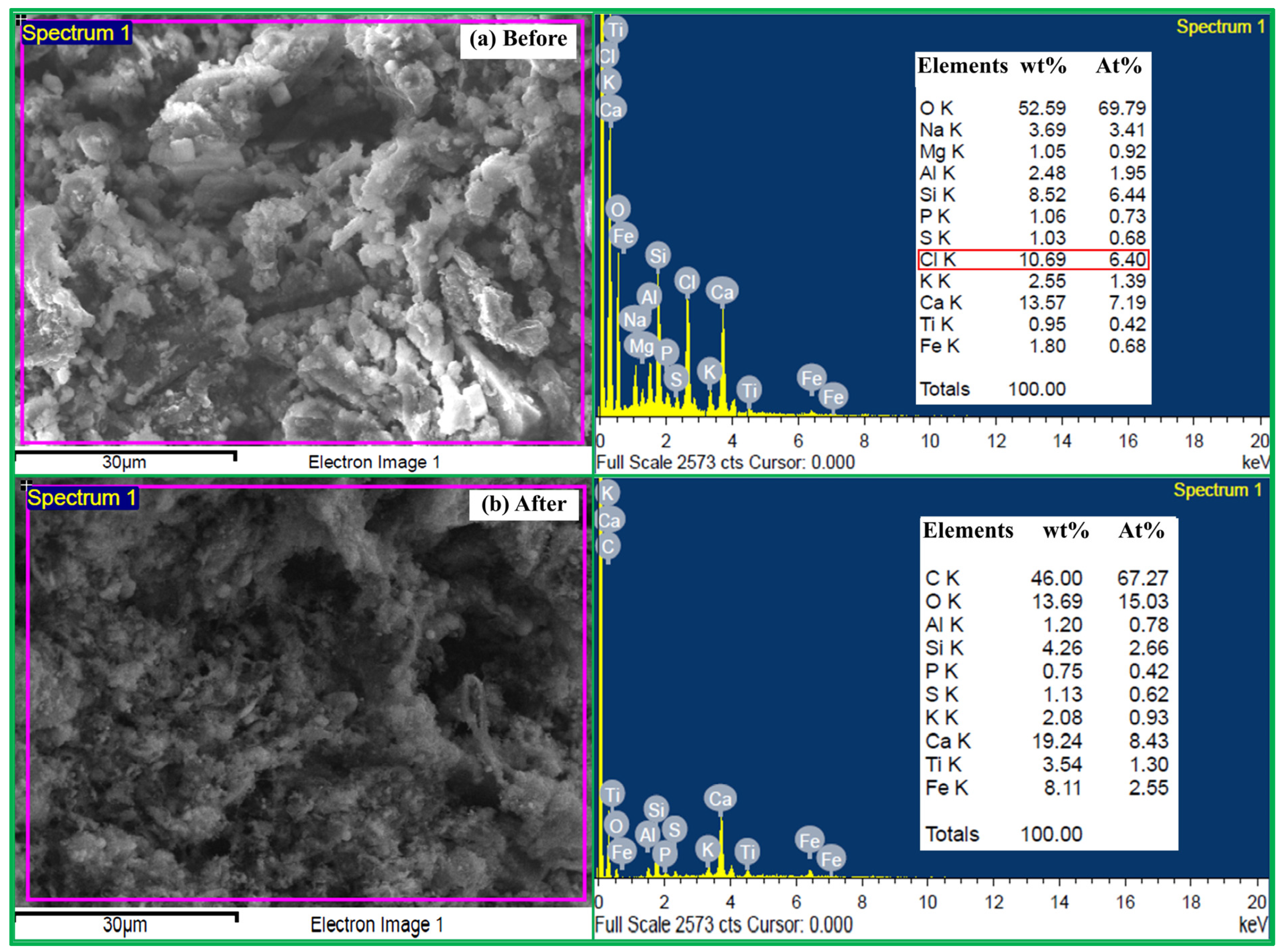
Figure 12 shows the SEM micrographs and EDS analysis of raw RDF and RDF char used in this study. Both samples contained inorganic residues such as CaO, SiO2, Al2O3 and TiO2, originating from the RDF composition. A notable difference was the detection of chlorine (Cl) in the raw RDF, which was completely absent in the RDF char. The detection of Cl in RDF reflects the contribution of plastics, PVC, and other chlorine-bearing waste fractions present in the feedstock. This is significant because chlorine compounds are precursors for toxic dioxins and furans. Therefore, the removal of Cl during heat treatment indicates that carbon from RDF/anthracite blends (R1–R6), when applied as reducing agents in the glass–mill scale system for ferrosilicon alloy production, is unlikely to generate these hazardous pollutants. The enhanced environmental compatibility of RDF-based carbons highlights their suitability as sustainable alternatives to conventional reductants—anthracite, coal, and coke—used in metallurgical operations. Among the glass–scale–carbon blends, A and C were identified as the optimum conditions for ferrosilicon alloy production, offering high Si content, acceptable metallic yield, and reduced CO/CO2 emissions.

Figure 12.
SEM micrographs present morphology and EDS analysis of RDF particles: (a) before and (b) after heated treatment. The red box highlights the presence of Cl in the RDF.
3. Materials and Methods
3.1. Materials
Anthracite (3–8 mm, Thailand Anthracite Co., Ltd., Rayong, Thailand) and refuse-derived fuel (RDF) powder (Nakhon Ratchasima, Thailand) were used as carbon sources. RDF was dried at 90 °C for 48 h, pyrolyzed from room temperature to 1000 °C at 5 °C/min under argon with a 1 h dwell, and reheated at 1550 °C for 30 min. RDF char alone and blends with anthracite (90–50 wt% RDF) were prepared (samples R1–R6; Table 2). Chemical composition was analyzed using a LECO CHN628 analyzer (LECO Corporation, St. Joseph, MI, USA).

Table 2.
Composition of the derived carbons from RDF/anthracite blends.
Waste glass powder from Glass Bridge Co., Ltd. (post-consumer glass recycler, Bangkok, Thailand) was sieved to <180 µm. Mill scale from UMC Metal Co., Ltd. (EAF steel mill, Chonburi, Thailand) was ground and sieved to <180 µm. XRF analysis (Table 3) showed that glass powder was predominantly SiO2 (73.6 wt%), mill scale was rich in Fe2O3 (93.7 wt%), and RDF ash contained 32.51 wt% SiO2 as its major component.

Table 3.
Chemical analysis of raw materials used in the present study.
The derived carbons from RDF/anthracite (R1–R6) were blended with glass powder and mill scale in a rolling mill for 30 min at a C/O molar ratio of 1, producing blends A–F. Blend compositions are given in Table 4. Here, C represents the total moles of carbon in the derived carbons, and O represents the total moles of oxygen from SiO2 in glass powder and Fe2O3 in mill scale.

Table 4.
Components of the Glass-Scale-Carbon blends.
3.2. Experimental
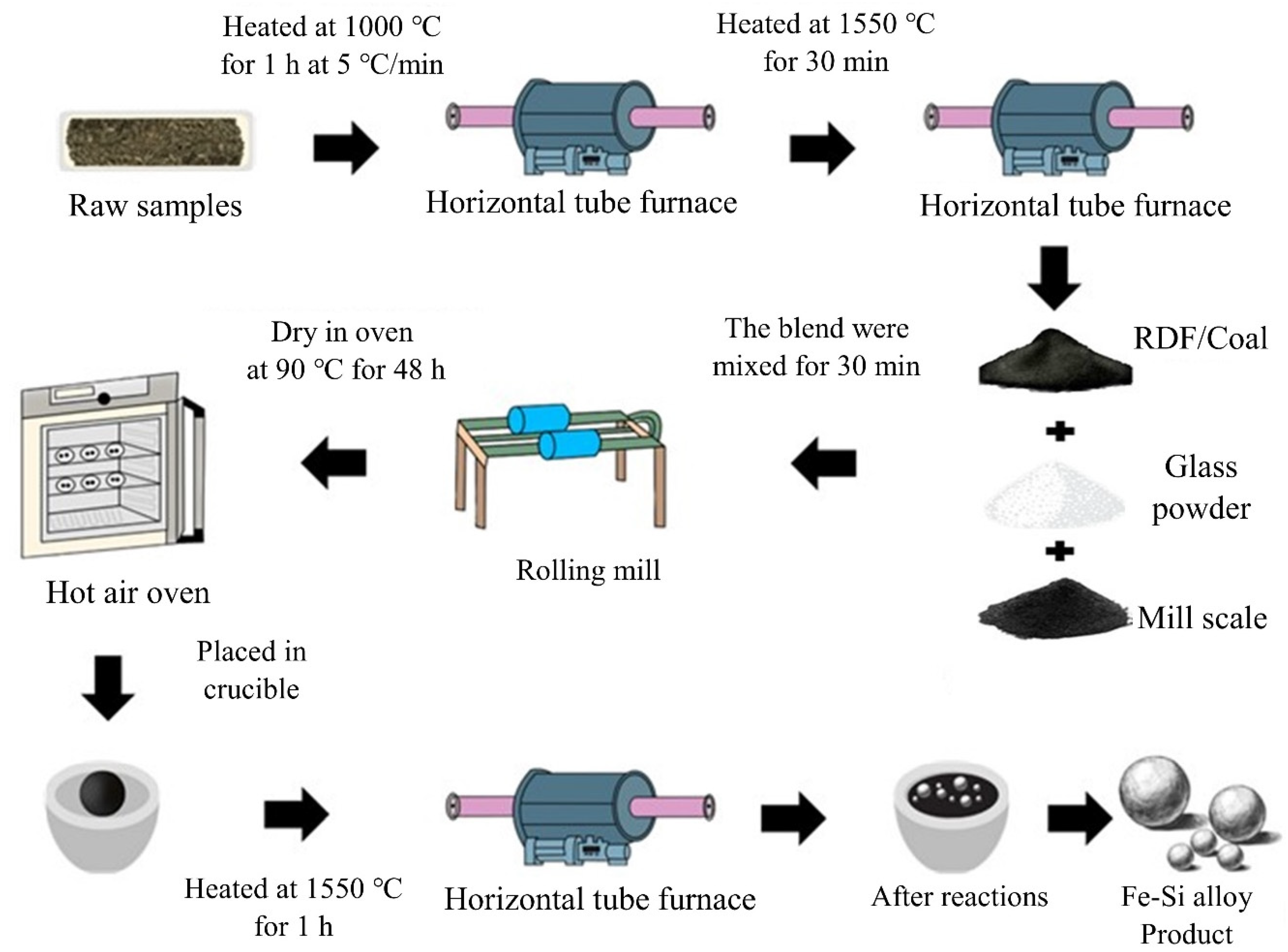
Blends A–F were hand-rolled into spherical pellets with the addition of water and dried at 90 °C for 48 h. Each pellet was placed in a separate refractory crucible and heated in a horizontal tube furnace under high-purity argon (99.998%) flowing at 2 L/min. To minimize thermal shock, the crucible was held in the cold zone for 5 min before being pushed into the hot zone at 1550 °C for 1 h, then returned to the cold zone to terminate reactions. The resulting metal droplets were collected for analysis. Phase identification was performed by XRD (Bruker D8 Advance, Karlsruhe, Germany), while morphology and elemental composition were examined using SEM and EDS (JEOL JSM-7800F, Tokyo, Japan). An overview of sample preparation and experimental procedures is shown in Figure 13, and pellets before and after heating are presented in Figure 14. After heating at 1550 °C for 1 h, pellets of blends A–F produced small metal balls with a metallic sheen accompanied by black residue covering the crucible base.

Figure 13.
Overview of sample preparation and experimental procedure.

Figure 14.
Glass-Scale-Carbon pellets (a) before and (b) after heating process.
4. Conclusions
With the aim of achieving zero-waste practices, this study explored the upcycling of waste-derived materials—refuse-derived fuel (RDF), waste glass, and mill scale—as raw resources for ferrosilicon production. The experiments were conducted at 1550 °C, focusing on ferrosilicon alloy formation under different RDF/anthracite blending ratios. The key findings are summarized below.
- Carbon derived from RDF, blended with anthracite, was used as a reductant in the carbothermic reduction of SiO2 from waste glass and Fe2O3 from mill scale, reducing dependence on fossil-based reductants. XRD confirmed that the metallic products were predominantly iron monosilicide (FeSi), with characteristic peaks at 2θ ≈ 45.02° (110) and 78° (310).
- The metallic products appeared as small metal balls with a metallic sheen with yields of 14.85–19.47 wt%. Blends D–F showed the highest yields (18.73–19.47 wt%), reflecting greater Fe2O3 reduction.
- SEM–EDS revealed that the metallic products were mainly Fe–Si–C with Si contents of 8.1–27.11 wt%. Blends A–C, though yielding less metal, contained higher Si (23.34–27.11 wt%), while blends D–F had lower Si (8.1–14.31 wt%) due to reduced RDF content. Elemental mapping confirmed Si and C dispersion within the Fe phase, with stronger Si incorporation in blends A–C, indicating more pronounced FeSi formation.
- Pyrolysis gas analysis showed that blend B produced the highest CO (~2.25 L) and CO2 (~0.26 L), followed by blend C, while blend A had the lowest gas evolution. The other blends yielded intermediate volumes. Blend B had the highest oxygen removal (0.12 mol), followed by C (0.06 mol) and A (0.01 mol), with the others in between (0.02–0.04 mol), indicating the greatest carbothermic reduction in blend B.
- The higher Si content in blends A–C results from more extensive SiO2 reduction and efficient Si diffusion into Fe, indicating that FeSi formation is strongly influenced by blend composition and properties.
- Cl removal during heat treatment indicates that RDF/anthracite carbon (R1–R6) is unlikely to generate toxic dioxins or furans. Blends A and C were optimal, offering high Si content, satisfactory yield, and lower CO/CO2 emissions.
Future work should focus on process optimization to maximize FeSi yield and quality. Pilot-scale trials are recommended to assess industrial feasibility and energy efficiency.
Author Contributions
Conceptualization, K.S. and S.K.; methodology, K.S., T.C., S.A. and S.K.; software, K.S. and S.K.; validation, K.S., T.C., S.A. and S.K.; formal analysis, K.S. and S.K.; investigation, K.S., T.C., S.A. and S.K.; resources, S.K.; data curation, K.S., T.C., S.A. and S.K.; writing—original draft preparation, K.S. and S.K.; writing—review and editing, S.A. and S.K.; visualization, K.S. and S.K.; supervision, S.K.; project administration, S.K.; funding acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
Thailand Science Research and Innovation Fundamental Fund fiscal year 2024, Contract No. TUFF 21/2567.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
This work was supported by the Thailand Science Research and Innovation Fundamental Fund fiscal year 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haydary, J. Gasification of Refuse-Derived Fuel (RDF). Geosci. Eng. 2016, 62, 37–44. [Google Scholar] [CrossRef]
- Sharma, P.; Sheth, P.N.; Chourasia, M.; Mohapatra, B.N. Chemical characterization of refuse derived fuel (RDF) using Py-GC/MS. J. Anal. Appl. Pyrolysis 2024, 179, 106456. [Google Scholar] [CrossRef]
- Nobre, C.; Vilarinho, C.; Alves, O.; Mendes, B.; Gonçalves, M. Upgrading of refuse derived fuel through torrefaction and carbonization: Evaluation of RDF char fuel properties. Energy 2019, 181, 66–76. [Google Scholar] [CrossRef]
- Násner, A.M.L.; Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Restrepo, J.C.; Venturini, O.J.; Ratner, A. Refuse Derived Fuel (RDF) production and gasification in a pilot plant integrated with an Otto cycle ICE through Aspen plus™ modelling: Thermodynamic and economic viability. Waste Manag. 2017, 69, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Aluri, S.; Syed, A.; Flick, D.W.; Muzzy, J.D.; Sievers, C.; Agrawal, P.K. Pyrolysis and gasification studies of model refuse derived fuel (RDF) using thermogravimetric analysis. Fuel Process. Technol. 2018, 179, 154–166. [Google Scholar] [CrossRef]
- Okamoto, H. (Ed.) Desk Handbook Phase Diagrams for Binary Alloys, 2nd ed.; ASM International, Materials Park: Novelty, OH, USA, 2010; p. 389. [Google Scholar]
- Yoshisato, K.; Masashi, Y.; Yaw, W.C. Thermoelectric properties of nearly single-phase ꞵ- FeSi2 alloys fabricated by gas-atomized powder sintering. Mater. Trans. 2019, 60, 652–661. [Google Scholar]
- Kiatgamolchai, S.; Sakulkalavek, A. Distribution of elements in a Cu-Added FeSi2 alloy under peritectoid and eutectoid reactions. J. Electron. Mater. 2011, 40, 1029–1034. [Google Scholar] [CrossRef]
- Yamadaa, H.; Katsumata, H.; Yuasa, D.; Uekusa, S.; Ishiyama, M.; Souma, H.; Azumaya, I. Structural and electrical properties of ȕ-FeSi2 bulk materials for thermoelectric applications. Phys. Procedia 2012, 23, 13–16. [Google Scholar] [CrossRef]
- Cherigui, M.; Guessasma, S.; Fenineche, N.; Hamzaoui, R.; El-Kedimb, O.; Coddet, C. Studies of magnetic properties of iron-based coatings produced by a high-velocity oxy-fuel process. Mater. Chem. Phys. 2005, 92, 419–423. [Google Scholar] [CrossRef]
- Method for Producing Ferrosilicon at Low Cost. Available online: https://patents.google.com/patent/CN102517446A/en (accessed on 1 July 2025).
- Piamba, J.F.; Ortega, C.; Hernández-Bravo, R.; González Carmona, J.M.; Tabares, J.A.; Pérez Alcázar, G.A.; Alvarado-Orozco, J.M. Theoretical and experimental study of FeSi on magnetic and phase properties. Appl. Phys. A 2020, 126, 849. [Google Scholar] [CrossRef]
- Kongkarat, S.; Boonyaratchinda, M.; Chobtham, C. Formation of ferrosilicon alloy at 1550 °C via carbothermic reduction of SiO2 by coal and graphite: Implication for rice husk ash utilization. Solid State Phenom. 2021, 315, 16–24. [Google Scholar] [CrossRef]
- Boonyaratchinda, M.; Kongkarat, S. Fundamental investigation of ferrosilicon production Using rice husk and rubber tree bark at 1550 °C: Implication for utilization of agricultural waste in steelmaking industry. Mater. Sci. Forum 2020, 977, 171–177. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Sahajwalla, V. Characteristics of waste automotive glasses as silica resource in ferrosilicon synthesis. Waste Manag. Res. 2016, 34, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Li, L.; Guo, Z.; Wei, D.; Kong, J.; Du, H.; Wang, H.; Zhuang, Y.; Xing, P. A novel process to recycle coal gasification fine slag by preparing Si-Fe-Al-Ca alloy. J. Environ. Manag. 2023, 337, 117681. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, W.; Jiang, W.; Jiang, W.; Wei, P.; Nyarko-Appiah, J.E. Fe-Si alloys production and alumina extraction from coal fly ash via the vacuum thermal reduction and alkaline leaching. Fuel Process. Technol. 2023, 224, 107702. [Google Scholar] [CrossRef]
- Banerjee, P.; Kumar, N.S.; Franco, A.; Swain, A.K.; Naidu, K.C.D. Insights into the dielectric loss mechanism of bianisotropic FeSi/SiC composite materials. ACS Omega 2020, 5, 25968–25972. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yuan, Y.; Jiang, J.T.; Gong, Y.X.; Zhen, L. Microwave absorption properties of FeSi flaky particles prepared via a ball-milling process. J. Magn. Magn. Mater. 2015, 395, 152–158. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.V.; Ikram-ul-hag, M.; Burmistrov, I.; Cayumil, R.; Belov, V.A.; Rogachev, O.; Leybo, D.V.; Mukherjee, P.S. An innovative route for velarizing iron and aluminium oxide rich industrial wastes: Recovery of multiple metals. J. Environ. Manag. 2021, 295, 113035. [Google Scholar] [CrossRef] [PubMed]
- Dankwah, J.R.; Koshy, P.; Saha-Chaudhury, N.; O’Kane, P.; Skidmore, C.; Knights, D.; Sahajwalla, V. Reduction of FeO in EAF steelmaking slag by metallurgical coke and waste plastics blends. ISIJ Int. 2011, 51, 498–507. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P.; O’Kane, P.; Sahajwalla, V. Reduction of FeO in EAF steelmaking slag by blends of metallurgical coke and end-of-life tire. Steel Res. Int. 2012, 83, 766–774. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).