Abstract
This article focuses on the problem of processing slag waste from non-ferrous metallurgy, in particular, the loss of copper, gold and silver with slag during autogenous smelting in the Vanyukov furnace at the Balkhash Copper Smelter (BMZ). An analysis of factors affecting metal losses, including electrochemical and mechanical components, is presented. This paper offers a comprehensive study of the distribution of Cu, Pb, As, Au and Ag between matte and slag, taking into account the unique characteristics of the raw material and the technological conditions of the copper smelter, which distinguishes it from previous studies. This paper establishes numerical values of dissolved and mechanical losses of valuable metals. It has been established that the most important quantitative result of smelting polymetallic raw materials in a Vanyukov furnace is the proportion of mechanical copper losses in the slag, which is approximately 75–80% of the total copper content in the slag. Mathematical models are proposed to predict the distribution of metals in the process of smelting and loss of copper, gold and silver with slag. It is proposed to integrate model representations into the technology control loop, which will optimize the process of metal recovery. This will lead to an increase in profitability and a reduction in the negative impact on the environment during copper production.
1. Introduction
One of the most significant tasks for non-ferrous metallurgy enterprises is the effective processing of accumulated and generated slag waste arising in the process of autogenous smelting. Significant volumes of this waste occupy large areas and have a significant negative impact on the environment and human health [1,2,3]. According to modern data, during the production of each ton of copper, from 2.2 to 3 tons of slag containing copper are formed. According to the analysis of experts, the annual volume of copper lost from metallurgical waste is estimated at about 4 million tons in the United States and 2 million tons in Japan [4]. Losses of non-ferrous metals with slag have a serious impact on the economic efficiency of their production, negatively affect the environment, and aggravate environmental problems for the enterprise as a whole. This problem has become global, and each enterprise strives to find a solution using available resources and technologies [5,6].
Modern autogenous processes of copper production require a thorough study of the problem of copper losses in slag. The use of advanced bubbling technologies, such as the Vanyukov furnace and Isa Smelt, is recognized as a very promising approach to slag waste processing.
Despite the innovation and uniqueness of the proposed options, none of the available methods is able to fully meet the requirements of of the complete extraction of non-ferrous metals from slag materials and the wide range of recoverable valuable elements. In the available scientific works, insufficient attention has been paid to the analysis of the distribution of gold and silver between products formed during autogenous smelting. Also, each of these methods is characterized by technical complexity and entails significant capital investments, as well as high energy costs.
In autogenous copper smelting, the losses of metal with slag are determined both by objective factors related to the characteristics of the initial materials and the composition of the smelting products, and subjective factors due to the control of the technological process [5].
At the Balkhash Copper Smelter (BMZ), a variety of sulfide materials with copper inclusions, characterized by various chemical compositions, are currently being processed in the Vanyukov furnace (PV). The mixture intended for smelting on average includes the following components (in wt.%): Cu—16–20; Pb—1–2; Zn—1.8–2.5; Fe—25–28; S—28–32; SiO2—14–16; Al2O3—2.5–3.5, as well as other elements. The content of the main components of slag fluctuates in a narrow interval, while maintaining an optimal ratio of components in %: SiO2—22–25; FeO—43–46; ZnO—1.5–2; CaO—2–4. The smelting process is organized in such a way that the output is comprised of matte with a copper share of up to 55%.
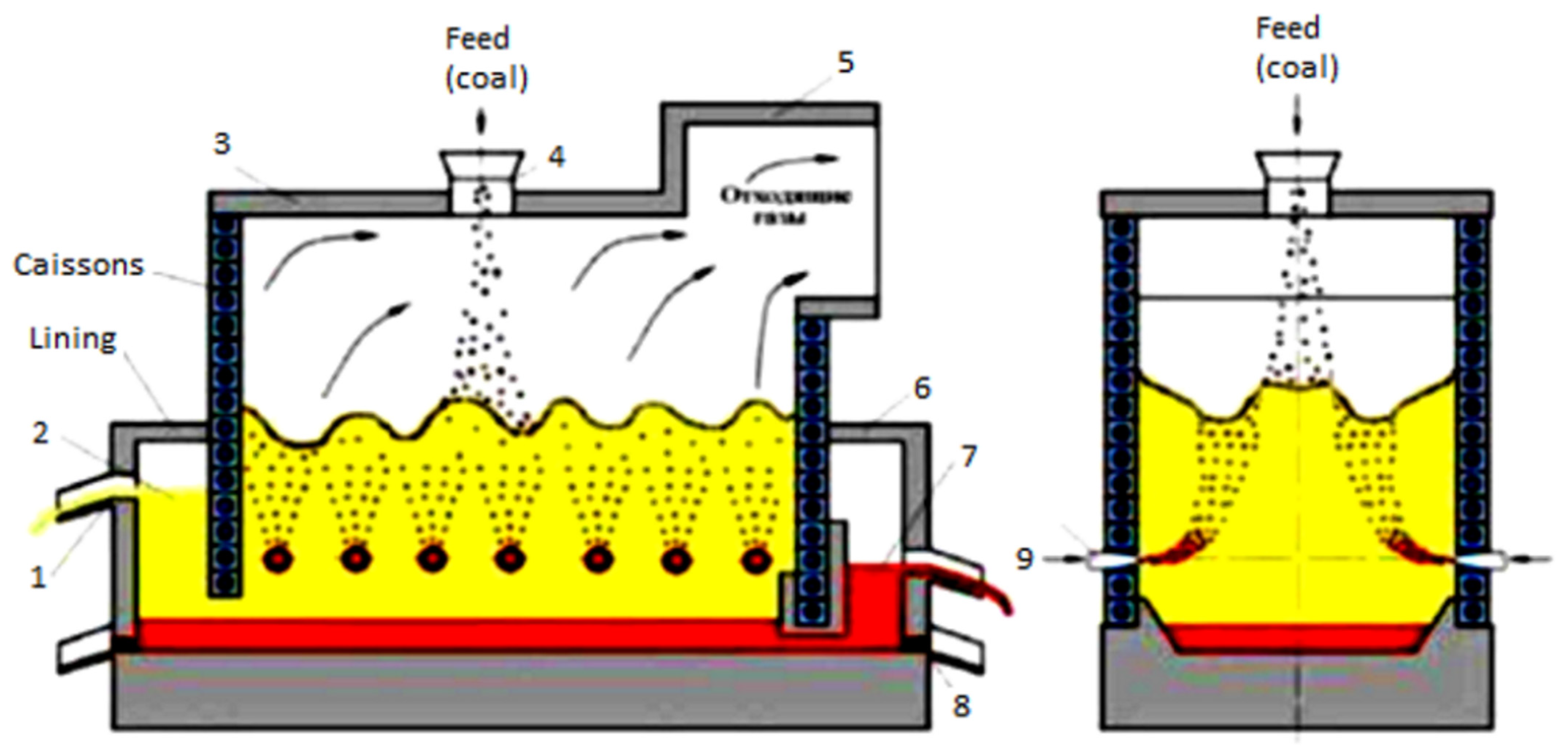
The main physical and chemical processes that bind the components of the mixture and slag include the process of oxidation of the components of the charge under the influence of oxygen coming with the blow; dissolution of silicon dioxide and other low-melting elements of the charge; and formation of slag and matte. All these processes mainly take place in the area above the tuyere of the VF, where bubbling is carried out (Figure 1).
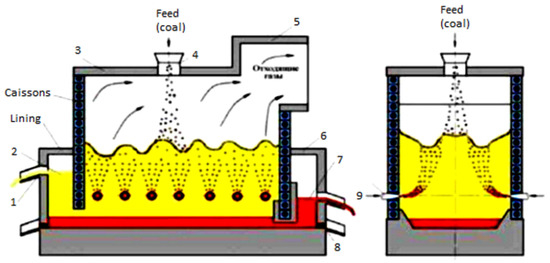
Figure 1.
General view of the Vanyukov furnace: 1—slag sedimentation tank; 2—slag; 3—furnace roof; 4—feeding hopper; 5—uptake; 6—matte sedimentation tank; 7—matte; 8—melt emergency release taphole; 9—bubbling tuyeres.
The oxygen–air mixture (OAI) is supplied to the slag melt through the side tuyere with a blast flow velocity at a tuyere cut of up to ~200 m/s and is accompanied by the formation of a matte–slag emulsion with a specific interfacial surface area of 600–1500 m2/m3 of melt. To maintain the thermal balance of the melting process in the optimal temperature range of 1270–1300 °C, carbon fuel is additionally burned in the melt.
Coal is used as the main fuel, which is charged into the furnace together with the charge (feed) with a metered flow rate. Coal combustion is carried out under conditions of intensive mixing of the melt bath, which ensures uniform distribution of coal particles over the volume of the tuyere zone.
In the tuyere (reaction) zone, which is an apparatus for perfect mixing (with uniform distribution of blast over the bath), matte and slag melts are in a state close to thermodynamic equilibrium.
Active mixing and blowing of the slag–matte mixture with gases in the area of the furnace tuyeres significantly increase the intensity of heat and mass transfer. Due to this, the homogeneity of temperature and chemical composition of melting products within the bubbling zone is quickly achieved. Blowing the melt with gases near the tuyeres promotes the process of coalescence of matte particles, thereby improving the separation of matte and slag.
The material loaded into the tuyere zone is quickly distributed over the entire volume of slag in the bubbled zone due to intensive mixing.
The slag melt formed as a result of oxidative smelting and matte droplets move from top to bottom—from the tuyere zone to the relatively calm sub-tuyere zone, where the final separation of matte and slag takes place due to the density difference.
Metal droplets drain through the slag layer, which ensures that the slag is repeatedly purified by the metal and further reduces the amount of metal impurities in the slag coming out of the furnace.
A key aspect is the absence of an oxidizing environment (oxygen blast insulation) in the area under the tuyere where the melt is located. This allows the reducing conditions to be maintained while avoiding undesirable oxidation reactions.
Siphon slag drainage during smelting in the Vanyukov furnace prevents the loss of metals contained in the floating charge and small metal droplets retained on the surface of the slag bath due to surface tension forces. Thus, factors that can lead to losses of non-ferrous metals in other technological processes are excluded.
The matte droplets that have passed through the slag phase form a layer of matte melt (about 700 mm high) on the furnace hearth, which is continuously discharged from the furnace through the matte siphon.
Slag, after separating the main number of matte drops from it, continuously leaves the furnace through a slag siphon.
In the Vanyukov furnace (VF), the total losses of copper in the slag consist of electrochemical (chemical and physical) and mechanical components [7,8]. At the same time, electrochemical losses dominate, accounting for 65–80% of the total copper content in the slag, while mechanical losses are 20–35% [7]. However, the current practice of operating VF at BMZ when melting complex copper charge demonstrates significant losses of copper with slag, reaching 3%, which is associated with a change in the ratio of dissolved and mechanical losses of copper [9,10].
In the article [11], a comprehensive study of the microstructure of industrial slags from various zones of the VF (siphon, electric sedimentation tank) and converter slags under the conditions of the BMZ was carried out, with an emphasis on the distribution of metals between the phases. The authors found that in the richest slags (converter and from the siphon VF) up to 80–85% of copper is contained in the form of matte suspension. The measures used at BMZ to reduce the loss of copper with slag, including electrically heated sedimentation tanks and flotation, do not give the desired effect.
Modern research [12] focuses on the problems associated with learning and reducing the mechanical carryover of copper along with slag in the process of autogenous smelting. However, they do not address the comprehensive behavior of a diverse set of elements, including precious metals.
In the field of metallurgical research, the creation of mathematical devices for predicting the distribution of metals between the products of smelting in the Vanyukov furnace (VF) is relevant [13]. Modern scientific works are increasingly focusing on the integration of mathematical models into process control systems [14,15]. In the paper [14], the idea of using thermodynamic calculations to optimize melting in the converter, taking into account the specifics of the BMZ, is put forward. Such an approach can lead to improved productivity and cost reduction.
Despite the fact that the scientific literature widely covers the results of studies on copper leakage into slag [16,17], as well as works analyzing the thermodynamics of these losses [18], data on the solubility and behavior of gold and silver in the process of converter melting of charge to matte, especially in the processing of multicomponent raw materials, are not sufficiently presented.
The study focuses on a detailed analysis of the behavior of Au and Ag in the specific conditions of the Vanyukov process in the processing of polymetallic raw materials consisting of a mixture of materials of different types and compositions (sulfide copper concentrates, circulating dusts, pyrite concentrate, etc.). Such detailed studies have not been carried out for this system before.
In contrast to previous works that considered the general regularities of the distribution of precious metals, the paper proposes a detailed thermodynamic model that takes into account the influence of specific impurities and slag components characteristic of the Vanyukov process. This model predicts the behavior of Au and Ag more accurately.
The approach adopted in the work differs from previous work, where a combination of experimental studies (changes in the composition of melt products depending on various parameters) and thermodynamic modeling for a more complete understanding of the mechanism of distribution of Au and Ag was used.
Previous studies have focused on the equilibrium distribution of metals. In this work, attention is focused on the kinetic aspects of the transition of Au and Ag into slag, taking into account the cooling rate and the composition of the resulting products.
As a result of the study, new factors affecting the loss of Au and Ag in slag were identified, in particular, the effect of copper and lead content in matte and slag, which was not noticed in previous work.
We have developed a new method for calculating the coefficients of distribution of copper and gold between matte and slag, which gives similar values to the data on mechanical losses of copper obtained as a result of mineralogical studies. This method for determining the content of Au and Ag in slag with increased accuracy makes it possible to more correctly assess the losses of precious metals with slag.
The paper proposes specific recommendations for optimizing the technological process to reduce Au and Ag losses based on the results obtained. These recommendations take into account the specifics of the Vanyukov process and can be implemented in practice.
In contrast to copper loss studies, which focus on physical carry-over and dissolution of copper in slag, the focus is on the effect of chemical reactions and the formation of intermetallic compounds on Au and Ag losses with slag. This allows us to identify new mechanisms that have not been considered in previous studies on copper losses.
Previous studies of copper losses have mainly relied on the analysis of industrial data. Instead, we offer detailed process simulations that make it possible to control the parameters and identify the key factors that affect the loss of Au and Ag in slag.
In connection with the above, the following tasks are set and solved in this article:
- (1)
- A study of the material composition of slags from the VF siphon was carried out in order to determine the forms of occurrence of metals, including precious metals.
- (2)
- The effect of slag composition on the redistribution of metals between matte and slag in the process of melting copper raw materials of complex composition in the Vanyukov furnace was investigated.
- (3)
- Quantitative ratios of dissolved and mechanical losses of copper and precious metals with slag have been determined and recommendations for the development of a technology for the complex extraction of metals from slag have been formulated.
- (4)
- Mathematical models have been created to predict the distribution of metals during smelting.
The novelty of this scientific study lies in the thorough study of the distribution of Cu, Pb, As, Au and Ag between matte and slag. The study was carried out under the conditions of smelting polymetallic copper-containing materials in the Vanyukov furnace at the BMZ. Unlike previous studies, which mainly focused on the behavior of copper, this is the first time that the behavior of a wide range of valuable and harmful elements, including precious metals, has been analyzed in detail. The features of raw materials and technological parameters of the BMZ were taken into account.
Mathematical models developed to predict the distribution of metals (Cu, Pb, As, Au, Ag) between matte and slag take into account the influence of the composition of the slag (iron oxides, silicon dioxide, calcium oxide and other elements) under smelting conditions in the Vanyukov furnace. These models not only predict metal losses but also provide an opportunity to optimize process parameters to increase the recovery of valuable elements.
The models developed in this work are empirical in nature and are not based on strict thermodynamic calculations. The complexity and multicomponent nature of the slag–matte system under study (the presence of a wide range of associated impurity metals) make it extremely difficult to create an accurate thermodynamic model at this stage. Nevertheless, empirical models make it possible to establish a quantitative relationship between the technological parameters of the process (matte, slag composition) and the distribution of Au and Ag, which is an important step for process optimization. In the future, we plan to include thermodynamic calculations for individual subsystems in the model, using the available data and the results of additional studies.
Despite their empirical nature, the proposed models have a high practical value, since they make it possible to predict the distribution of Au and Ag with sufficient accuracy depending on the composition of melting products for the purpose of real-time process control. Traditional thermodynamic models require significant computational resources and precise knowledge of the composition of the system, which is not always possible in industrial settings. Our models make it easy to quickly assess the loss of precious metals and adjust the process parameters to minimize them.
The integration of the created models into the process control system at the Balkhash Copper Smelter will make it possible to move from empirical smelting control to control based on scientific data. This allows the process parameters to be quickly adapted to the changing feedstock composition and the required matte characteristics. This approach guarantees stable operation of the Vanyukov furnace and will minimize deviations from the best operating modes.
Ultimately, the results of the work will create a tool for making informed decisions in the field of matte smelting process control, which will lead to an increase in the economic efficiency and environmental safety of copper production. In the future, the improvement of modeling may include consideration of hydrodynamic processes and the development of three-dimensional models. These models will allow describing the distribution of temperature indicators and concentrations of various substances in the entire interior of the Vanyukov furnace.
2. Materials and Methods
Materials. The studies were carried out on the basis of the results of the compositions of industrial melt products (mattes and slags) obtained during the processing of a multicomponent copper-containing charge (feed) to produce matte in VF under the conditions of BMZ.
In the course of preparation of the statistical study, the data of factory analyses of replaceable samples of mattes and slags were used, obtained at the following average daily parameters of the melting unit:
The volume of copper matte produced was 1145 tons, with a copper content of 53–55%. This matte production was sent for conversion. The volume of slag formed reached 2073 tons, with the content of metals: copper up to 2%, lead—0.6–1.2% and zinc about 2.5%. The temperature in the furnace above the surface of the slag melt was maintained at 1573 K. These parameters guaranteed the uninterrupted operation of the furnace.
The statistical sample of processed matte and slag paired samples included 125 values for each component. This made it possible to perform a reliable assessment of the distribution of elements between matte and slag, as well as to determine the significant relationships of their distribution coefficients with the composition of the slag.
The slag–matte system is heterogeneous. To minimize the influence of heterogeneity on the results of the analysis, a large sample volume was used for analysis, with multiple measurements for the content of the desired elements in it. The results obtained were averaged, which made it possible to reduce the error associated with local fluctuations in composition.
Sampling was carried out every shift during 2024. As part of the study, changes in the composition of raw materials and technological parameters (matte and slag compositions) and their impact on the distribution of Au and Ag were recorded and analyzed.
Due to the large amount of data and the desire to identify general patterns, we used the average values of technological parameters and compositions. To take into account possible fluctuations, when building regression models, the method of robust regression was used, which minimizes the impact of outliers and non-stationary data. A more detailed analysis of the effect of individual fluctuations on the distribution of Au and Ag is the subject of further research.
Research methods. The following instruments were used to determine the concentration of non-ferrous and rare-earth metals with a fraction of less than 0.1% in materials obtained after melting: the Optima 2000 DV spectrometer (manufactured by Perkin Elmer Inc., New York, NY, USA) for control measurements; the D8 Advance X-ray diffractometer; and the Venus 200 PANalyical B.V. X-ray fluorescence spectrometer with wave dispersion (PANalytycal B.V., Almelo, The Netherlands). to achieve a high degree of accuracy and reliability of the experimental data obtained.
The gold and silver content in industrial slags was determined using an AA-7000 Shimadzu atomic absorption spectrophotometer (Shimadzu Corporation, Kyoto, Japan), within the range of 0.25 to 0.37 g/t and 7 to 8.9 g/t, respectively. Test conditions: temperature—22 °C, relative humidity—67%. In addition, the content of precious metals in the slags was confirmed by assay. The results obtained demonstrated a high degree of consistency, which allowed them to be used for further analysis.
The full composition of the array of industrial mattes and slags subjected to control measurements of elemental composition is given in Table 1.

Table 1.
Full composition of industrial matte and slag array.
Table 2 shows the statistics of industrial data.

Table 2.
Industrial data statistics.
Linear and multiple regression analyses were widely used in this study to predict the distribution of copper (Cu), gold (Au) and silver (Ag) between slag and matte, taking into account the composition of matte and slag. These approaches made it possible to establish and quantify the correlation between the components of the slag (as independent variables) and the distribution of metals (the dependent variable).
Previous studies [7,8,11,12] have shown that the behavior of copper, precious metals and impurities in the melting of copper raw materials is influenced by the copper content in the matte composition.
An increase in copper leads to more oxidizing conditions, and as a result, an increase in its losses in the form of oxides. There is also an increase in the concentration of copper in matte droplets, which increases mechanical losses with the same proportion of matte droplets in slag. An increase in the basicity of slag, as determined by the ratio of iron oxide to silica, also contributes to an increase in oxide loss. These factors, as well as the influence of the CaO modifier, are key to copper losses with slag and were therefore chosen to build regression models.
The regression analysis was based on the principles of linear and multiple regression, as well as the technique of least squares. A linear relationship was used to establish the optimal line showing the relationship of the copper, gold, and silver partition coefficient with each individual slag element. In turn, multiple regression was used to determine the effect of a complex of independent factors (matte and slag components) on the distribution of the metal. To minimize the discrepancies between the observed and predicted metal distributions, the least squares method was used.
To ensure the reliability of the results of the regression analysis, the following conditions were met:
- The existence of a linear relationship between the predictors and the target variable;
- Lack of relationship between residuals (difference between observed and model values);
- Uniformity of residue dispersion regardless of the values of predictor variables;
- Normal distribution of residues.
To assess the significance of the model, the correlation coefficient r was used, reflecting the proportion of variance of the target variable (metal distribution) explained by regression.
The statistical significance of the regression coefficients was determined using p-values. Small p-values (p < 0.05) for equations that predict the coefficient of distribution of copper, gold, and silver from slag composition indicate the statistical significance of the coefficient, i.e., its difference from zero.
Using regression analysis in the context of industrial data makes it possible to:
- (a)
- Evaluate the effect of matte and slag composition on the recovery of Cu, Au and Ag into matte or slag;
- (b)
- Determine the optimal matte and slag compositions for maximum recovery of valuable metals;
- (c)
- Predict metal losses in slag when the composition of smelting products or process parameters changes;
- (d)
- Optimize smelting parameters to increase the recovery of valuable metals and minimize losses.
Calculation of metal losses with slag based on equilibrium solubility in the matte–slag–gas phase system is a traditional problem. There are a number of calculation schemes of varying degrees of complexity. The most common scheme is the calculation of the metal distribution coefficient between matte and slag.
The second widespread scheme is the calculation of equilibrium constants of exchange reactions of the following type:
which are then simplified into the following form:
[MeS] + (FeO) ↔ [FeS] + (MeO),
K(1) = [FeS] × (MeO)/[MeS] × (FeO).
Reaction (1) leads to a change in the content of individual metals in the matte produced. Depending on the initial composition of the slag and matte, the reaction (1) can proceed from left to right and vice versa, until the counter transition of metals from one phase to another leads to their equilibrium distribution. Such an approach, despite its formal similarity with the calculation of equilibrium, is essentially empirical.
The third approach is related to the construction of equilibrium models, which has been intensively developed in the last decade, although the method of equilibrium models is the most logically justified and formally consistent, a large amount of reference data is needed to create numerical models. Such models, based entirely on reference data, are designed to predict the distribution of copper and related elements in the smelting of copper, copper-nickel ores and concentrates, as well as autogenous lead smelting.
The total copper content in slag is usually represented as the sum of its oxide (CuO0.5) and sulfide (CuS0.5) solubility. This representation corresponds to the structure of the silicate melt and is consistent with the stoichiometric relationships between the copper and sulfur content in slags, in which some copper ions are in coordination with oxygen and sulfur ions, and some are only with oxygen ions.
To describe the oxide solubility of copper in the slag of copper melts, it is customary to use the formulas Cu2O [19,20,21,22] and CuO0.5 [23,24]. The use of the CuO0.5 form seems more appropriate, since in this case Henry’s law for an infinitely diluted solution is satisfied [22]. This was taken into account by authors in further studies to establish quantitative ratios of the sulfide and oxide components of copper in slags.
3. Results and Discussion
The quantitative ratios of copper and precious metals in slags were determined on the basis of the results of the compositions of industrial mattes and slags given in Table 1.
The content of gold in mattes varies from 13 to 15 g/t, silver from 213 to 215 g/t. Slags from the Vanyukov furnace are characterized by a low gold content—0.2–0.4 g/t. The silver content in slags, unlike gold, is significant, and varies in a wider range—from 6 to 8 g/t.
Increased content of non-ferrous and precious metals in slags requires the organization of measures aimed at reducing their losses with slags. At the same time, along with the specific conditions of the process, first of all, it is necessary to take into account and clarify the mechanisms of the transition of metals into slag.
Calculations carried out using the model of M. Nagamori [25] showed that in industrial slags of copper smelting, the share of sulfide copper losses varies from 30 to 45% of the total copper content in slags. However, a wide range of variations in copper sulfide losses against the background of the constancy of the compositions of the initial charge and insignificant changes in the composition of the resulting smelting products indicates that the choice and application of this model for predicting copper losses in slags in contact with copper matte is not entirely successful. This is especially true for the case of melting polymetallic raw materials, which is typical for the conditions of BMZ. The obtained results are only predictive in nature and require additional research in order to clarify them.
3.1. Mineralogy and Morphology of Hardened Industrial Slags
For the study, air-hardened slags obtained from various sources were selected: industrial slags from the VF siphon and dump slags from the electric sedimentation tank.
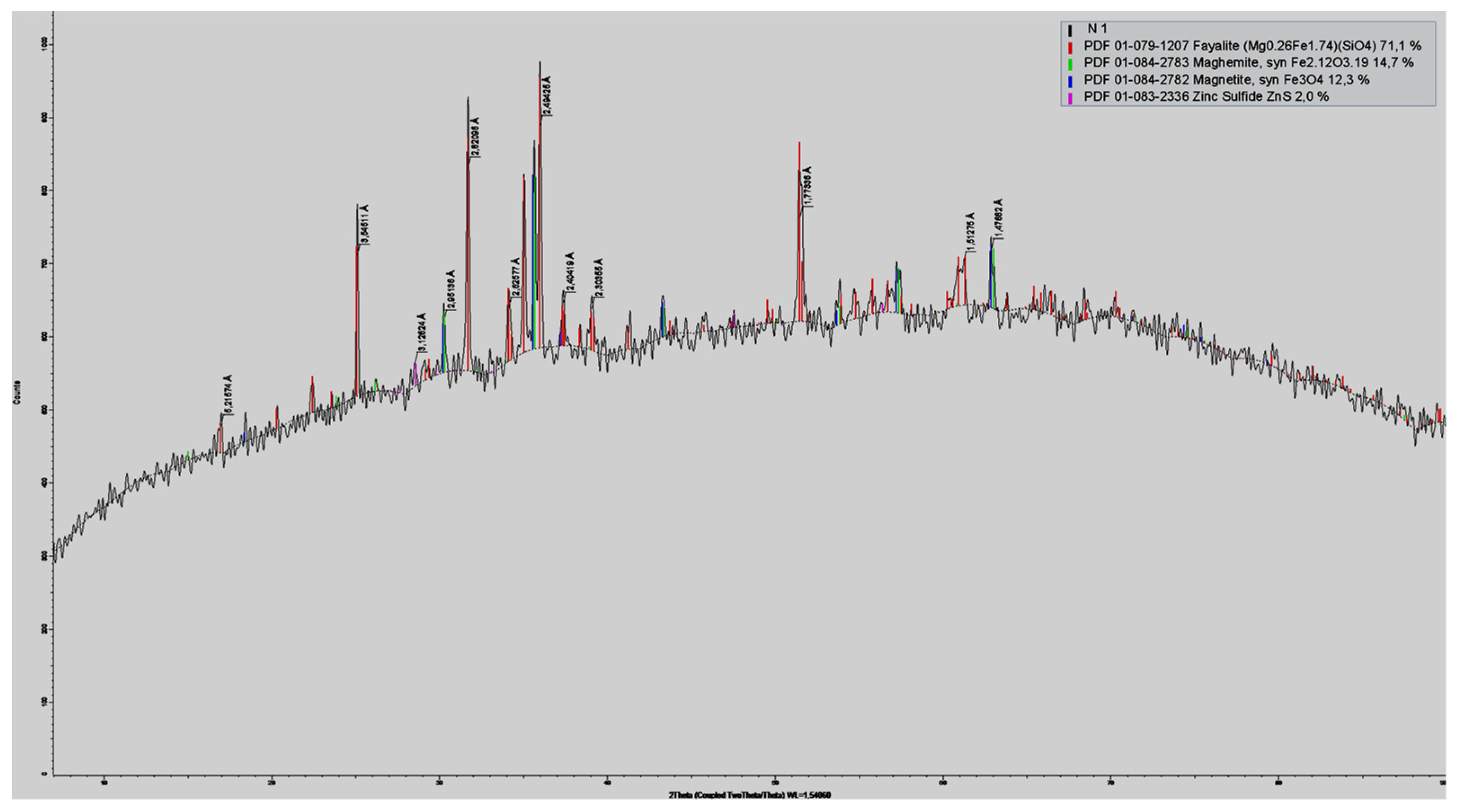
Slag from the VF siphon has a matrix, the basis of which is fayalite. The high concentration of copper in VF slags indicates a complex redistribution of metals between products and indicates a complex structure of the slag. The results of X-ray phase analysis (XRD) of the slag presented in Figure 2 show a significant magnetite content of up to 15%.
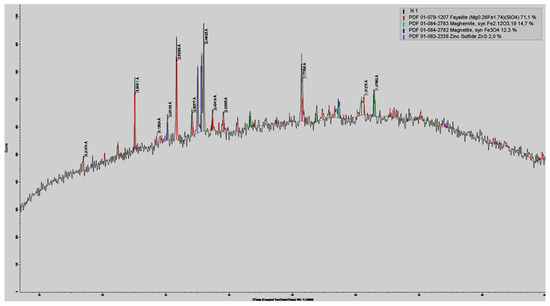
Figure 2.
Results of XRD analysis of slag from the VF siphon.
With such magnetite content, it is not possible to achieve a minimum copper content due to an increase in mechanical losses of copper.
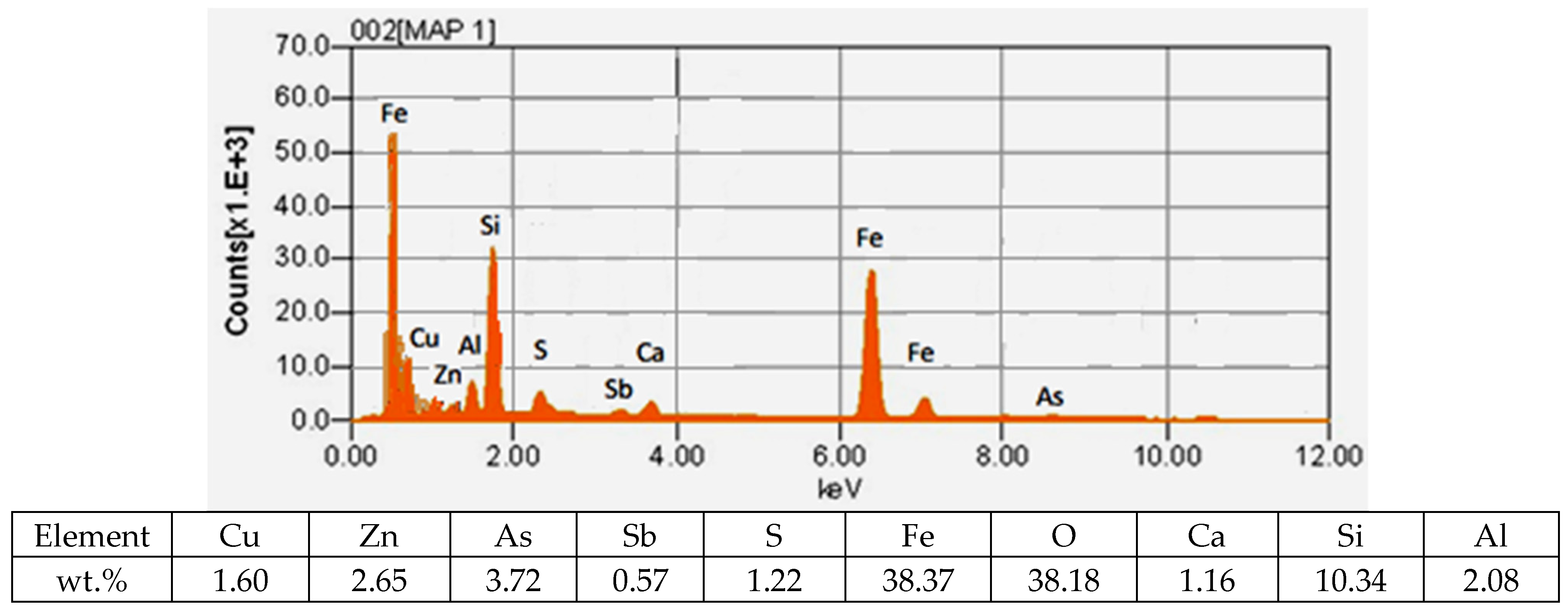
In the studied slag samples from the siphon, various metal particle associations were found from the submicron size to about 450 μm. The overwhelming majority of particles relate to the combination of iron oxides with impurities. Copper in slags is mainly found in the form of oxide and fine sulfide suspensions that did not have time to settle into the bottom phase of the matte. Moreover, as the results of the SEM-EDS analysis showed, the compositions of fine suspensions of mattes are identical to the compositions of mattes of the bottom phase. Also, in small particles of mattes, the so-called thin inclusions of copper with its maximum content, more than 85% by weight, were detected.
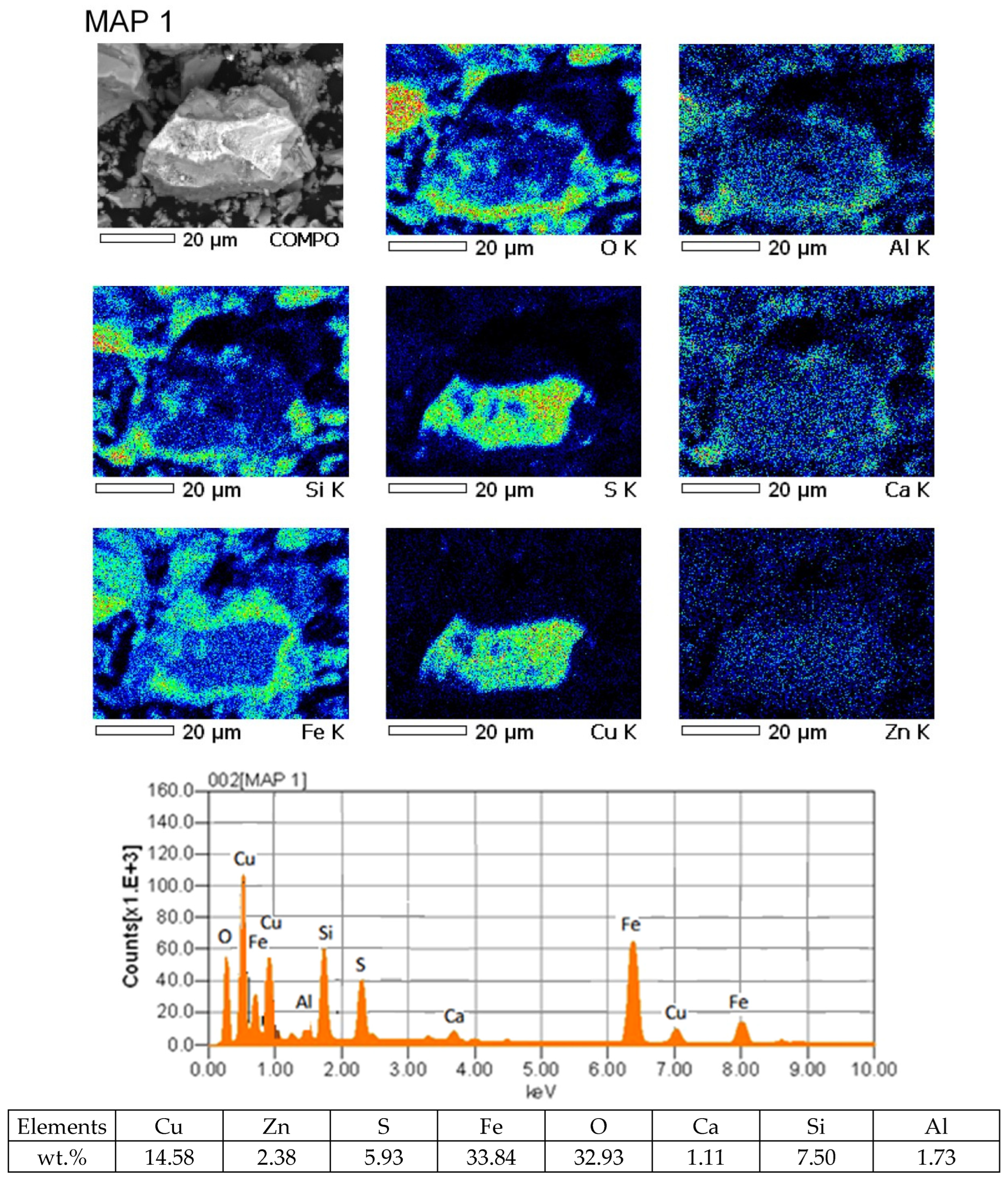
It was established that in the slags from the VF siphon, there is a large proportion of mattes lost in the slag, which is confirmed by the results of mapping the slag sample.
In Figure 3, it is not difficult to see pronounced matte particles, the compositions of which indicate the presence of a high sulfur content of ~5% and slag components in them.
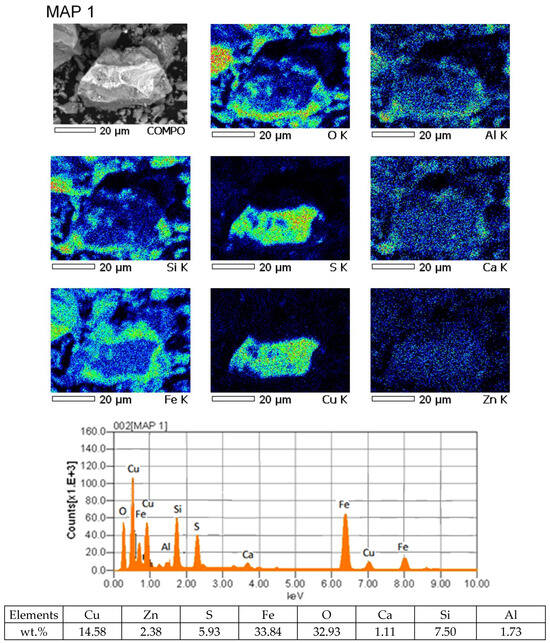
Figure 3.
Results of mapping the slag sample from the VF siphon.
This indicates that in the process of smelting in the VF slag siphon, the separation of products—matte and slag—is difficult. The presence of a proportion of matte inclusions leads to an increase in mechanical losses of copper with slag. The copper content in such associations is about 15%.
3.2. Forms of Copper and Associated Metals in Slags
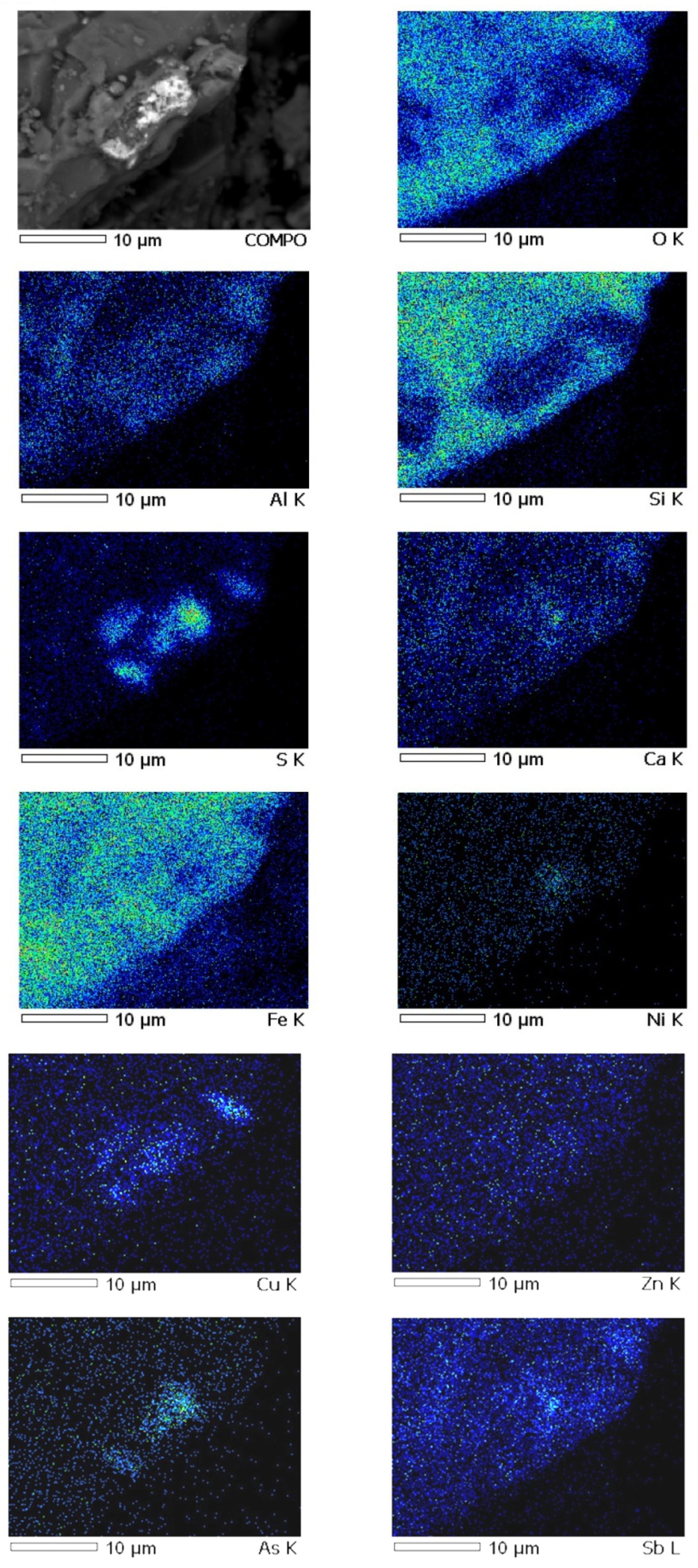
Analysis of the mineral composition showed that lead and zinc in the slag are present mainly in the fayalite fraction of the slag and are closely related to quartz in the form of oxide compounds. Sulfide inclusions of lead and zinc mixed with matte particles trapped in the slag were found in the slag samples. Arsenic is present in a stable oxidized form (As5+).
The results of mapping slag samples from the VF siphon (Figure 4) demonstrate that arsenic oxides are uniformly distributed between lead, zinc oxides and their compounds to varying degrees.
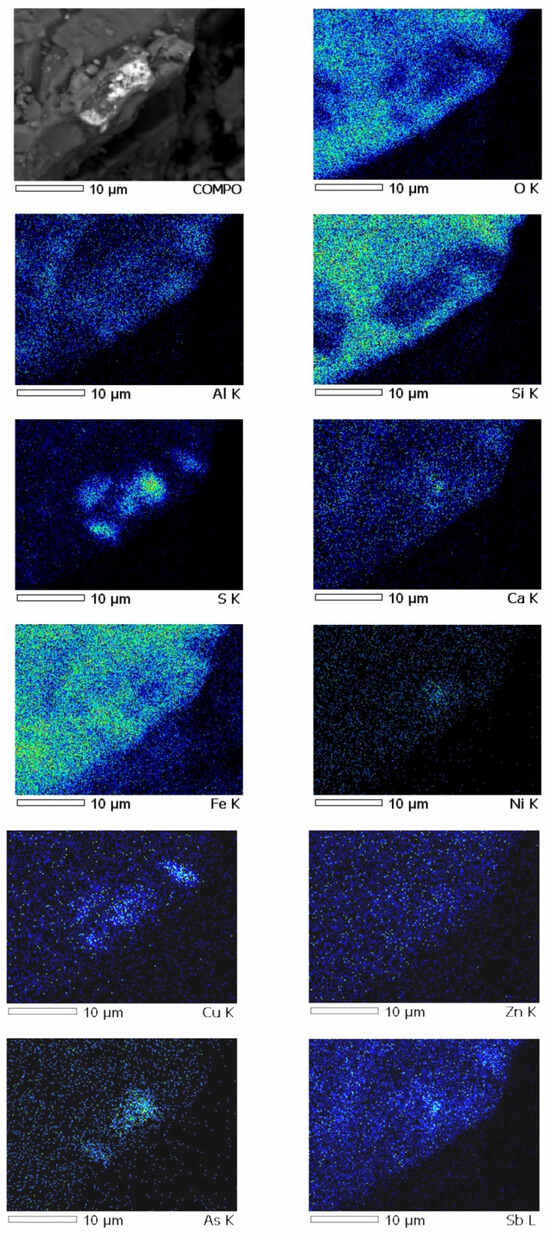
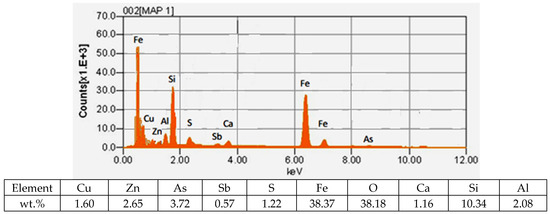
Figure 4.
Results of metal distribution in slag.
Chemical analysis of the identified phases confirms the high concentration of arsenic in the slag. This fact has fundamental importance and requires taking into account the effect of As2O5 on the redistribution of metals during the smelting process. In addition, the accounting and control of arsenic in slag has great importance in terms of environmental pollution. The results obtained on the forms of copper are in good agreement with the data of the work [11,12].
In contrast to slags from the VF siphon, dump slags from the electric sedimentation tank are characterized by a more homogeneous composition. Their structure is represented mainly by silicates, with a lower content of magnetite (about 10%), which is confirmed by the data of XRD analysis.
Copper in dump slag is mainly present in the form of larger sulfide inclusions, which makes it easier to recover. The size of these inclusions ranges from 50 to 200 μm, and they are often associated with other sulfides such as pyrite and chalcopyrite.
The results of the SEM-EDS analysis of dump slags showed that the copper content in sulfide inclusions is significantly higher than in slags from the VF siphon. This is due to the fact that the dump slags underwent a longer period of cooling and crystallization, which allowed copper to concentrate more efficiently in the sulfide phases. In addition, individual grains of native copper were found in the dump slags, which indicates the reduction of copper from oxides and sulfides in the process of natural sedimentation in an electric sedimentation tank.
Precious metals in slags are associated mainly with sulfide inclusions. If gold and silver are distributed more evenly in the slags from the VF siphon, in the dump slags, they are concentrated in separate large sulfide grains. This is due to the fact that in the process of long-term sedimentation, precious metals migrate and redistribute, concentrating in the most stable sulfide phases.
The obtained data allow us to conclude that slags from VF and dump slags have a different mineral composition and a different distribution of copper and precious metals. Slags from VF are characterized by a more complex structure and a high content of mechanically trapped mattes, which makes it difficult to extract copper. Dump slags, on the other hand, have a more homogeneous composition and contain larger sulfide inclusions, which makes them easier to further process.
Analysis of the obtained data proves that during the melting of a complex charge in the Vanyukov furnace under the conditions of BMZ, effective separation of matte and slag does not occur. The increased copper content in slag is mainly due to an increase in mechanical losses of copper. In this regard, it is logical to assume that a significant potential for reducing losses of both copper and precious metals is the reduction in mechanical losses of copper with slag.
3.3. Estimation of Losses of Copper, Lead and Precious Metals with Slag
As a result of mineralogical studies of VF slag samples, it was established that the nature and composition of sulfide inclusions present in the slag are identical to the compositions of real copper mattes. Therefore, it can be argued that slag sulfide inclusions are mechanical inclusions of matte that have not separated from the slag.
According to A.V. Vanyukov, the dissolution of precious metals in slag formations is insignificant. Losses of these metals, which are observed in slag, are mainly due to the presence of small mechanical particles of matte in it [10].
This hypothesis is supported by several factors: first, the low gold content of the slag; second, the similarity of the chemical composition of the sulfide inclusions in the slag to that of the bottom matte; and, finally, compliance with the LAu > LCu condition presented in Table 3. All these data strongly support the above statement.

Table 3.
Estimated values of the ratio of mechanical and dissolved copper losses in VF slags.
In this case, the calculation of the copper and gold partition coefficients between matte and slag should give similar values to the data on the mechanical losses of copper obtained as a result of mineralogical studies.
To estimate the contribution of the ratio of mechanically captured (dmech) and dissolved (ddis) copper to the total losses of the metal with slags, the assumption of a complete transition of gold into slag as part of the mechanical fraction of matte was used. In this case, the ratio of mechanical losses of copper can be determined from the expression:
dmech = 1 − ddis = LCu/LAu,
The results obtained during the calculations are shown in Table 3.
Table 4 shows the final data of error calculations for each of the evaluated parameters. Calculations were made taking into account percentage deviations in the measurements of copper, gold and silver content in both slag waste and matte.

Table 4.
Results of uncertainty calculations.
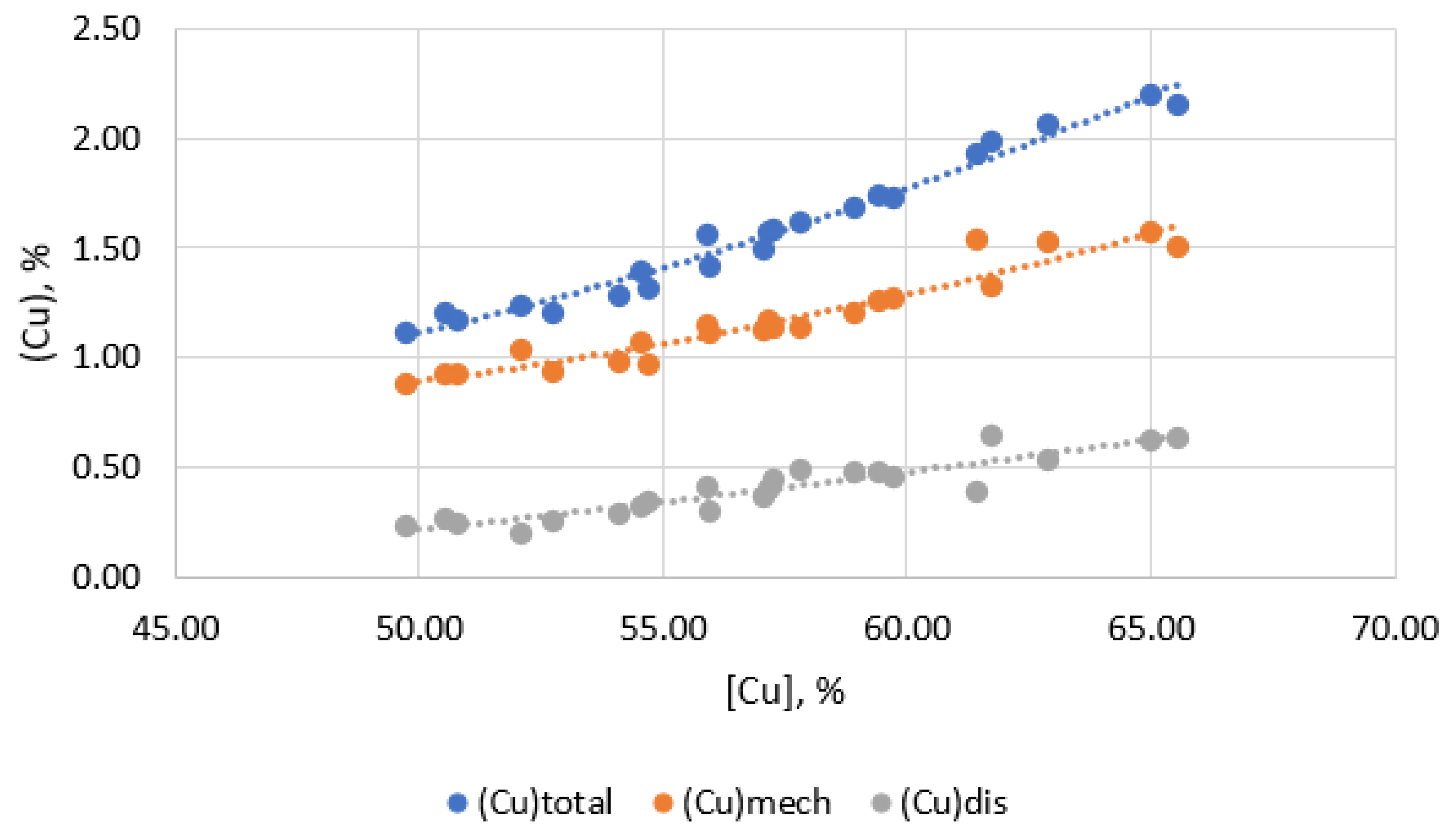
Analysis of the obtained results shows that the main part of copper losses, approximately 75–80% of its total content in slag, is due to its mechanical entrainment in the form of sulfides. At the same time, the average content of copper dissolved in the slag (Cudis) is ~0.41% (Table 2). Small fluctuations and relative constancy of the concentration of dissolved copper indicate the high reliability of the obtained results, which are in good agreement with theoretical models of autogenous smelting.
In the matte-slag system, under conditions close to equilibrium, the content of dissolved copper in the slag on its content in the matte is described by the dependence of the type: (Cu) − f[Cu]. When smelting to obtain matte with a copper content of 50–55%, the level of copper dissolved in the slag, according to this dependence, is 0.45%, which gives a good match with our result. Based on this, it can be concluded that during the melting of polymetallic materials in the Vanyukov furnace at BMZ, the losses of copper with slag are mainly formed due to the increase in mechanical losses due to mechanical entrainment in the form of matte inclusions with slag.
Figure 5 shows the dependence of the quantitative ratios of copper losses with slag in dissolved and mechanical forms on the copper content in matte.
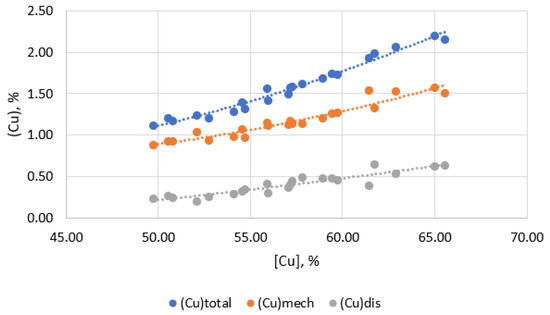
Figure 5.
Dependence of mechanical and dissolved copper losses on slag from copper content in matte.
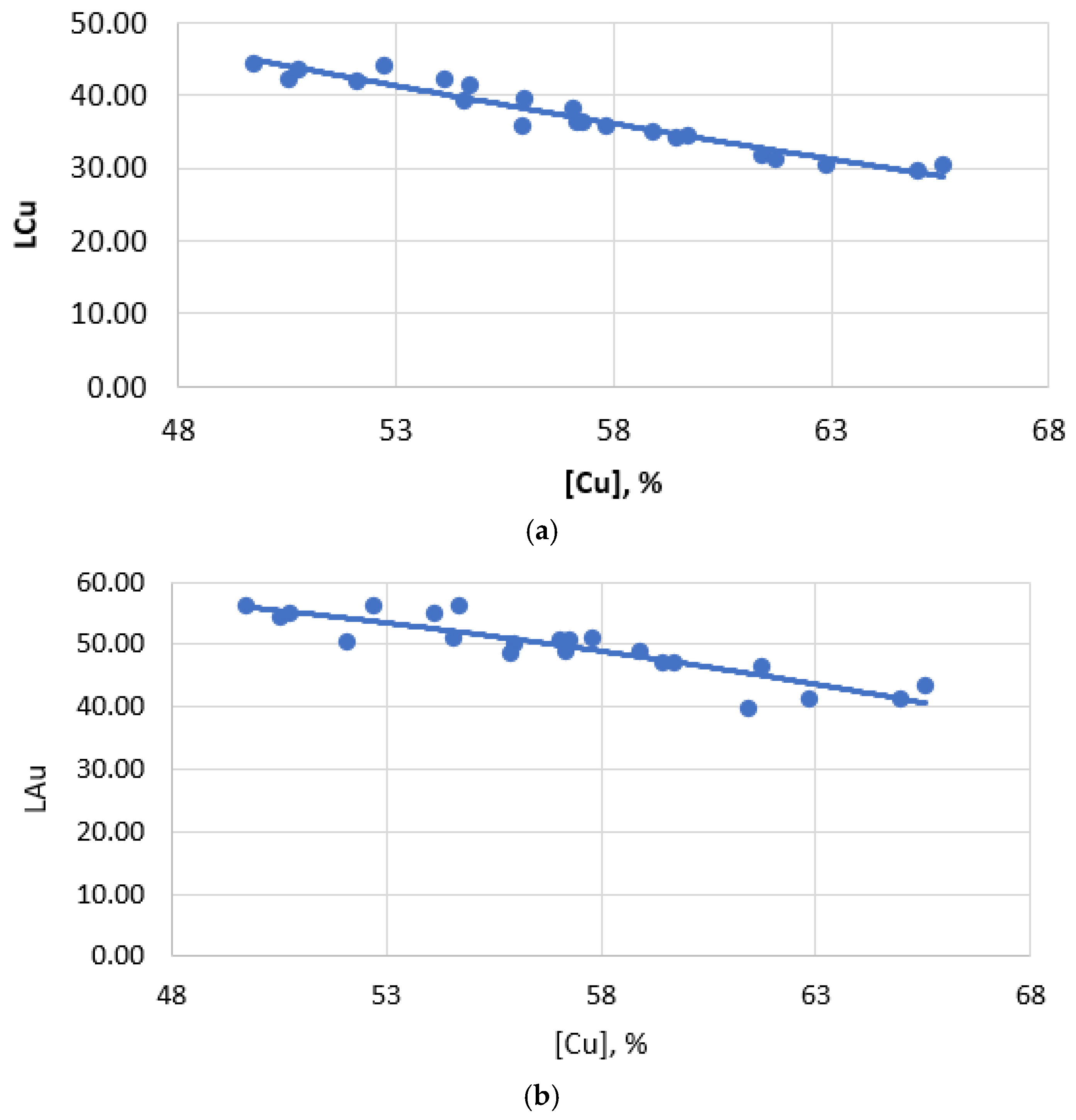
When smelting to obtain copper-rich mattes (55–60% Cu), the total losses of copper with slag increase and copper losses are formed mainly due to an increase in mechanical losses of copper (Figure 4). At the same time, the dissolved losses of copper with slag change insignificantly. This is because the dissolved losses of copper with slag are determined primarily by the composition of the slag and the temperature of the process, rather than by the copper content of the matte. Mechanical losses, on the contrary, directly depend on the copper content in the matte, since an increase in the copper content in the matte leads to the formation of a larger number of small droplets of matte, which are difficult to separate from the slag. And indeed, the analysis of the dependence of the copper-gold partition coefficient between matte and slag on the copper content in matte shows a strong relationship between them (Figure 6).
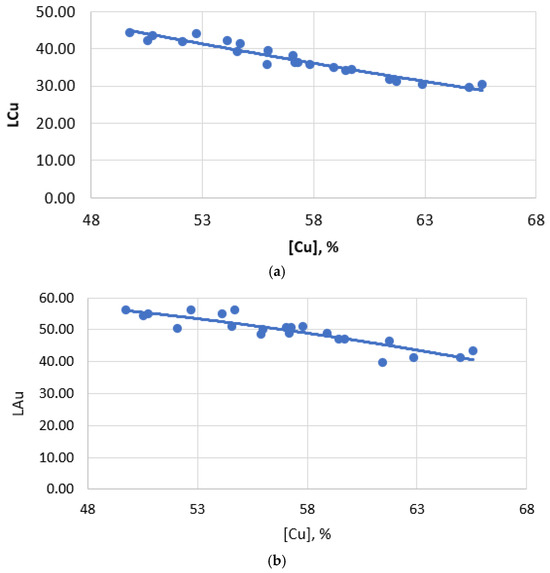
Figure 6.
Dependence of the distribution coefficient on the copper content of matte: (a) copper; (b) gold.
The results obtained are well confirmed by the results of statistical processing of industrial data. Thus, a close relationship has been established between gold and copper in the matte phase, which is described by the pair correlation equation:
[Au] = 8.12 + 0.12 [Cu], r = 0.96.
Regression analysis data showed a high R-squared value of 0.94. This indicates that 96% of the variance [Au] is explained by the change in [Cu] and indicates a high degree of dependence between the concentrations of gold and copper, as well as good consistency of the model with industrial data.
The close value of the normalized R-squared equal to 0.935 to the R-squared value indicates the stability of the model.
The average deviation of the observed values [Au] from the values predicted by the model (standard error) is 0.1285 units. This characterizes the accuracy of the model’s prediction.
Significance by the F-test, estimated by the p-value, is significantly less than the standard level of significance and less than p < 0.05. Consequently, we reject the null hypothesis that the model (regression) is generally irrelevant. This means that there is a statistically significant relationship between the copper [Cu] content and the silver [Au] content in the matte phase, and the model generally describes the industrial data well.
The Y-intersection coefficient (constant) is 8.123. Standard error: 0.355; T-stats: 22.88. A p-value of 1.63 × 10−5 is much smaller than p < 0.05 and means that the constant is statistically significantly different from zero.
The coefficient for the variable [Cu] is 0.108. Standard error: 0.0062; T-stats: 17.33. A p-value of <0.05 means that the coefficient at [Cu] is statistically significantly different from zero. This is a key result confirming that [Cu] has a statistically significant effect on [Au].
The data on confidence intervals (bottom 95% and top 95%) for both ratios do not contain zero, which further confirms their statistical significance.
Thus, the regression analysis data demonstrate a strong and statistically significant linear relationship between the concentrations of copper [Cu] and gold [Au] in the total array studied. The Equation [Au] = 8.12 + 0.1 [Cu] is an adequate model to describe this relationship within the observed range of [Cu] values.
The dependence of the distribution coefficient of copper (LCu), gold (LAu) and silver (LAg) on their content in matte is described by the equations:
LCu = 95.93 − 1.01 [Cu], r = 0.95.
LAu = 192.86 − 10.14 [Au], r = 0.94.
LAg = 891.21 − 4.02 [Ag], r = 0.94.
All three values of the coefficient of determination R2 are quite high and close to 1: for Equation (5), R2 = (0.95)2 = 0.902, or 90.2%; for Equation (6), R2 = (0.94)2 = 0.883, or 88.3%; for Equation (7), R2 = (0.94)2 = 0.883, or 88.3%. The obtained values indicate a strong relationship between the content of copper, gold and silver in matte and the corresponding values of their distribution coefficients.
The particularly high R2 value of 92.16% indicates a strong variation in the LCu value, which explains very well the variation in the copper content of matte.
For Equations (6) and (7), the R2 values are also high, but explain somewhat less variability than in the case of copper.
The results show that there is a strong correlation between the copper, gold and silver contents in matte and their corresponding distribution coefficient values. This may indicate that the measurement of the distribution coefficient values of copper, gold and silver confirms the quantitative measurements of Cu, Au and Ag and can be a useful tool for estimating the contents of these elements in matte.
Table 5 shows the metric of the values of a simple quantitative comparison of the distribution of gold and silver by instrument and by model.

Table 5.
Metrics of the values of quantitative comparison of the distribution of gold and silver by instrument and by model.
The MAE (Mean Absolute Error) value of 1.776 for gold indicates that, on average, the model’s predictions deviate from the true values by about 1.78 units, which indicates good model accuracy.
The root of the RMSE (Root Mean Squared Error) of 2.114 is slightly larger than the MAE, which indicates the presence of some more significant errors, since the RMSE is more likely to penalize large deviations. However, a value around 2.11 also indicates acceptable accuracy.
A coefficient of determination R2 of 0.844 shows that the model explains about 84.4% of the variance in the data. This is a fairly high result and indicates a good quality of predictions. The linear regression model of Equation (6) shows good results with a fairly high accuracy and is able to effectively explain the dependence on the data. Prediction errors are relatively small, and R2 indicates a strong relationship between predicted and real values. The model can be considered reliable enough for practical use.
The metric of the values of a simple quantitative comparison of the distribution of silver by device and by model also shows high results.
The average absolute error of the MAE is 1.23, which means that on average, the model is only 1.23 units wrong when predicting values. This is a noticeable improvement over the previous version of the model (MAE ≈ 1.78), indicating an increase in accuracy.
The root of the RMSE error has also decreased.
Despite the deletion of some data, R2 is still 0.783, i.e., the model explains 78.3% of the variance of the target feature. This high value confirms that the model captures the data structure well.
The results of cross-checking the linear regression model of Equations (6) and (7) for gold and silver showed stable and high values of the coefficient of determination (R2) under 5 times cross-validation (Table 6).

Table 6.
Determination coefficient (R2) values for gold with 5x cross-validation.
The mean R2 value of 0.826 for Equation (6) indicates a high degree of variance: the model explains ~82.6% of the variation in the target variable.
The R2 values for folds are close to each other and show a slight variation. The difference between the high and low is: R2max − R2min = 0.866 − 0.784 = 0.082. The results obtained confirm the correctness of the constructed predictive model for Equation (6) and its suitability for practical use, taking into account the available data.
The linear regression model of Equation (7) also demonstrated stable and high values of the coefficient of determination (R2) when cross-validating 5 times (Table 7).

Table 7.
Determination coefficient (R2) values for silver with 5x cross-validation.
The mean R2 was 0.805 (Table 7), which is 81% of the variance in the dependent variable explained by the model based on the selected variable.
R2 values do not fluctuate much between folds. The difference between the high and low is: R2max − R2min = 0.824 − 0.794 = 0.030.
This indicates the stability of the model and the fact that the results do not depend significantly on the splitting into a training/test sample.
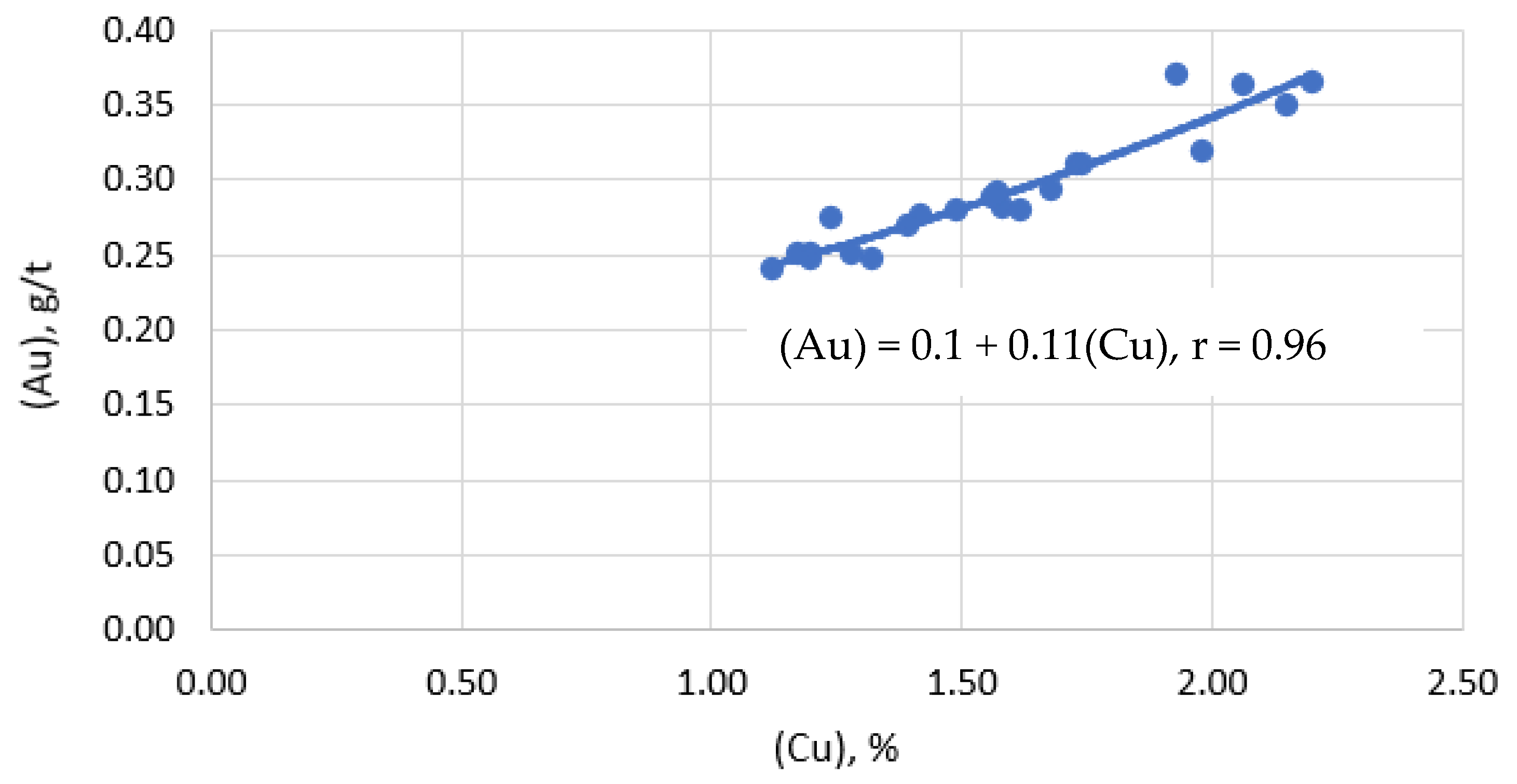
Visualization of the dependence of the gold content in the slag on the copper content in the slag is shown in Figure 7.
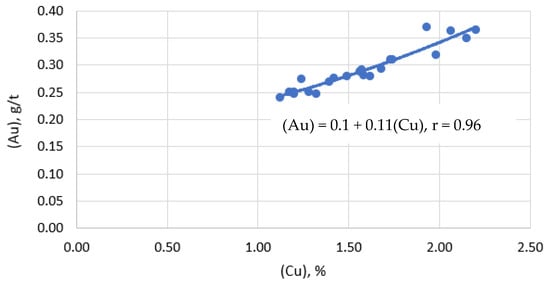
Figure 7.
Dependence of gold content in slag on copper content in slag.
Direct interpolation to zero gold content corresponds to the value of 0.43% Cu dissolved in the slag. This fact suggests that the main part of gold in slag is represented in the form of mechanically captured matte particles. The increase in gold recovery into matte is directly due to a decrease in mechanical losses of copper along with slag.
Silver in the matte phase correlates with both copper and lead, and its relationship with lead is more pronounced. This pattern holds true for the analysis of silver in slag. In particular, knowledge of the closer relationship of silver with lead makes it possible to predict its distribution between different phases (matte, slag, metal) depending on the composition of the charge and the technological parameters of the melt. This, in turn, can be used to optimize the processes of silver recovery and increase the efficiency of its extraction.
The presence of correlations between silver, copper, and lead in slag is also of practical importance. Slag analysis for these elements can serve as an indicator of smelting efficiency and silver losses. High concentrations of silver in slag, especially in combination with a high lead content, may indicate the need to adjust the technological regime to reduce the loss of the valuable metal.
In addition, studying the correlations between the elements in matte and slag can provide information about the mechanisms of silver’s transition from one phase to another. For example, if there is a sharp increase in the concentration of silver in the slag at a certain ratio of copper and lead in the matte, this may indicate that under these conditions, there is an active displacement of silver from the matte to the slag.
Thus, the comprehensive study of the distribution and correlations of silver with other elements in matte and slag is an important tool for optimizing metallurgical processes and improving the efficiency of valuable metal recovery. The data obtained can be used to develop new technological solutions and improve the economic efficiency of production.
Multiple correlation equations describing the dependence of the silver content in matte and slag on the content of copper and lead in the corresponding phases are as follows:
[Ag] = 206.91 + 0.13 [Cu] − 0.45 [Pb], r = 0.95;
(Ag) = 5.1 + 2.83 (Cu) − 3.21 (Pb), r = 0.79.
The transition of silver into slag is mainly determined by the mechanism of dissolution of lead in slag. Physically, this means that lead, as a more “slag-forming” element, passes into slag, involving silver with it due to their mutual solubility or the formation of complex oxide phases at the matte-slag interface. Obviously, the amount of silver lost directly correlates with the loss of lead carried into the slag and is determined by how the silver is distributed between the matte and the slag.
The relation describing the distribution of silver between matte and slag, which reflects the thermodynamic equilibrium of this distribution between the two phases, obeys the following equation:
LAg = 58.94 − 0.46 [Cu] − 3.0 [Pb], r = 0.72.
Equations (8)–(10) can be used to predict the quantitative losses of silver with slag. Understanding the physicochemical mechanisms underlying these equations allows you to optimize the process parameters of the melt to minimize the loss of valuable metal.
The assumptions made in the work about the proportion of mechanical inclusion are indirect in nature. Of course, for a more accurate quantitative description of the presence of precious metals in the slag phase, detailed studies will be carried out using modern research methods.
At the same time, it can already be argued that further study of slags as a potential source of extraction of valuable components is promising. Visual analysis and preliminary results of elemental analysis suggest an uneven distribution of inclusions, which is probably due to the peculiarities of crystallization and phase transformations occurring during melt cooling.
For a deeper understanding of the mechanisms of inclusions formation, it is necessary to conduct a series of experiments simulating the processes occurring in the smelting unit. It is important to consider factors such as temperature, cooling rate, charge composition, and gas environment. These parameters have a significant impact on the solubility of the noble metals in the slag and thus on their distribution in the different phases.
It is planned to carry out thermodynamic modeling of the “slag—noble metal” system in order to determine the optimal conditions for the concentration of valuable components in certain phases. The data obtained during the experiments will be used to verify and refine thermodynamic models. This will make it possible to predict the behavior of precious metals in various technological processes and develop effective methods for their extraction.
Particular attention will be paid to the study of the effect of various additives on the processes of flotation and gravity enrichment of slags. The goal is to develop economically feasible and environmentally friendly technologies for processing slag with maximum extraction of precious metals. The results of these studies will be of practical importance for the mining and metallurgical industry, contributing to a more rational use of secondary raw materials and reducing the burden on natural resources.
4. Conclusions
Based on the established patterns and indirect data, it can be assumed that it is necessary to apply a set of measures aimed at reducing both mechanical and dissolved copper losses. For smelting into rich matte, measures to minimize mechanical copper losses are of importance. Based on the studies and indirect data, it can be preliminarily concluded that when smelting polymetallic raw materials in VF, the share of mechanical copper losses in slag is predominant and is approximately 75–80% of its total content in slag. Also, all gold in slags is contained in the mechanical suspension of matte, which indicates the need to reduce mechanical copper losses with slag to increase gold extraction into matte. Improved silver extraction in matte is indirectly associated with a decrease in lead content in slags.
For the practical implementation of the proposed measures to reduce mechanical losses of copper and increase the recovery of precious metals, it is necessary to introduce a number of technological improvements in the process of melting in the VF. First of all, it is necessary to optimize the hydrodynamic mode of the furnace, aimed at reducing flow turbulence and preventing intensive mixing of slag and matte. This can be achieved by changing the design of the tuyeres, adjusting the blast rate and optimizing the geometry of the furnace space.
An important aspect is the control of the particle size distribution of the charge. The use of finer fractions of raw materials contributes to a more complete release of gold and silver, as well as a decrease in the mechanical capture of copper particles by slag. At the same time, the influence of particle size on dust formation and the overall efficiency of the process must be taken into account.
To reduce the lead content in slag, it is advisable to use selective fluxes that contribute to the formation of lead compounds that easily transform into matte. An alternative approach is to use a reducing atmosphere in the furnace, which will result in the recovery of lead and its transition into sublimations.
Regular monitoring of the chemical composition of slag and matte is a prerequisite for prompt adjustment of process parameters and maintenance of optimal smelting conditions. The use of modern analytical methods, such as X-ray fluorescence analysis and atomic emission spectrometry, will make it possible to quickly and accurately determine the content of valuable components and impurities in smelting products. The introduction of automated smelting process control systems based on monitoring data will ensure stable recovery of copper, gold and silver and minimize losses with dump slag.
The shortcomings identified in the existing technology make it possible to recommend two directions for improving the technology for processing VF slag in the conditions of BMZ:
- (1)
- Optimize phase separation in the electric sedimentation tank by intensifying the processes of selective extraction of copper into the bottom matte phase with the concentration of Au and Ag, and deep sublimation of impurities (Pb, Zn, As) into dust.
- (2)
- Develop fundamentally new solutions to increase the productivity of the depletion of slags and the selective extraction of valuable metals and impurities into targeted products.
The second direction, in our opinion, seems to be the most promising. The development and creation of an independent, separate technology for the processing of current liquid slag will make it possible to involve additional converter and accumulated solid slags in smelting, which will significantly improve the technological, economic and environmental performance of copper production as a whole.
The economic analysis of the proposed technological improvements in the copper smelting process has identified key areas for increasing process efficiency, namely reducing copper, gold, and silver losses in slags. The economic benefits from implementing these technological improvements consist of the following components:
- Benefits:
- Increased copper recovery by reducing mechanical copper losses in slag (up to 75–80%) and optimizing furnace hydrodynamics and feed granulometry control. This will lead to higher output of marketable copper and, consequently, increased revenue.
- Improved gold and silver recovery by reducing mechanical copper losses and lowering lead content in slags, which will positively impact the extraction of these valuable metals and further increase revenue.
- Reduced volume of waste slags due to more efficient extraction of valuable components from the feed, resulting in lower volumes of waste slags, reduced storage and disposal costs, and decreased negative environmental impact.
- Improved environmental performance by reducing the content of lead and other harmful impurities in slags and dust, which will enhance the environmental situation around the facility and lower environmental protection costs.
- Processing of additional slags through the development of new technology will allow for the treatment of converter and accumulated solid slags, generating additional profit.
- 2.
- Costs:
- Implementation of technological improvements will require modernization of existing equipment, including electric furnaces, tuyeres, air supply systems, and process control and management systems.
- Development and implementation of new technologies, such as selective fluxes and X-ray fluorescence analysis systems, will require significant investment.
- Optimization of furnace hydrodynamics: costs for designing and implementing changes to tuyere construction, regulating air supply rates, and optimizing furnace geometry.
- Development of new slag processing technologies will require additional research and development, including labor costs for scientific staff and the purchase of equipment and materials.
- Implementation of new technologies will require personnel training, which will also incur costs.
- During the implementation period of new technologies, there may be a temporary decrease in productivity.
To conduct an accurate assessment of economic efficiency, the following data are required:
- The cost of implementing each proposed technological improvement.
- The forecasted increase in copper, gold, and silver recovery resulting from each proposed technological improvement.
- Current prices for copper, gold, and silver.
- The cost of storage and disposal of waste slags.
- Costs for electricity, fluxes, and other consumables.
The proposed technological improvements have significant potential to enhance the efficiency of the copper smelting process. Their implementation will increase the recovery of valuable metals, reduce costs for storage and disposal of waste slags, improve the environmental situation, and enhance the competitiveness of the enterprise. To realize this potential, a detailed techno-economic analysis and a phased implementation plan for technological improvements will be developed in the future.
Author Contributions
Conceptualization, N.D.; methodology, N.D. and L.D.; investigation, B.S. and Y.Z.; writing—review and editing, B.S., Y.Z. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out within the framework of grant funding from the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan for 2023–2025 in the priority area “Geology, production and processing of mineral and hydrocarbon raw materials, new materials, technologies, safe products and structures” of the project AP19676951: “Development of a resource-saving, combined technology for the integrated processing of multicomponent dust of non-ferrous metallurgy with the production of marketable products”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Leonid Dityatovskiy was employed by the IStalproekt Institute. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ash, C.; Borůvka, L.; Tejnecký, V.; Šebek, O.; Nikodem, A.; Drábek, O. Temporal dissolution of potentially toxic elements from silver smelting slag by synthetic environmental solutions. J. Environ. Manag. 2013, 129, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Radojevic, A.A.; Serbula, S.M.; Kalinovic, T.S.; Kalinovic, J.V.; Steharnik, M.M.; Petrovic, J.V. Metal/metalloid content in plant parts and soils of Corylus spp. influenced by mining–metallurgical production of copper. Environ. Sci. Pollut. Res. 2017, 24, 10326–10340. [Google Scholar] [CrossRef] [PubMed]
- Rönnlund, I.; Reuter, M.; Horn, S.; Aho, J.; Aho, M.; Päällysaho, M.; Ylimäki, L.; Pursula, T. Eco-efficiency indicator framework implemented in the metallurgical industry: Part 2—A case study from the copper industry. Int. J. Life Cycle Assess 2016, 21, 1719–1748. [Google Scholar] [CrossRef]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resour. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Coursol, P.; Valencia, N.C.; Mackey, P.; Bell, S.; Davis, B. Minimization of copper losses in copper smelting slag during electric furnace treatment. JOM 2012, 64, 1305–1313. [Google Scholar] [CrossRef]
- Demetrio, S.; Ahumada, S.A.J.; Durán, M.Á.; Mast, E.; Rojas, U.; Sanhueza, J.; Reyes, P.; Morales, E. Slag cleaning: The Chilean copper smelter experience. JOM 2000, 52, 20–25. [Google Scholar] [CrossRef]
- Vanyukov, A.V.; Zaitsev, V.Y. Slags and Mattes of Non-Ferrous Metallurgy; Metallurgy: Moscow, Russia, 1969. [Google Scholar]
- Esin, O.A.; Geld, P.V. Physical Chemistry of Pyrometallurgical Processes; Metallurgy: Moscow, Russia, 1965; Part 2. [Google Scholar]
- Medikhanov, D.G. Involvement of technogenic deposits of BGMK in the processing of raw materials. In Proceedings of Scientific Works on the Problems of the BGMK, Dedicated to the 10th Anniversary of the Independence of the Republic of Kazakhstan; BGTI: Balkhash, Kazakhstan, 2001; pp. 137–142. [Google Scholar]
- Kvyatkovsky, A.N.; Bobrov, V.M.; Sitko, E.A. Search for ways to increase the complexity of the use of raw materials of the Kazakhmys Corporation. In Proceedings of Scientific Works on the Problems of the BGMK, Dedicated to the 10th Anniversary of the Independence of the Republic of Kazakhstan; BGTI: Balkhash, Kazakhstan, 2001; pp. 19–23. [Google Scholar]
- Karyaev, V.I.; Komkov, A.A.; Kuznetsov, A.V.; Plotnikov, I.P.; Sokolykh, V.A. Study of the microstructure of industrial copper-containing slags. Metallurgist 2020, 9, 90–99. [Google Scholar]
- Lukavy, S.L.; Fedorov, A.N.; Khabiev, R.P.; Min, M.G. Study of the dynamic viscosity of high-copper slag melts. Non-Ferr. Met. 2012, 2, 32–35. [Google Scholar]
- Vanyukov, A.V.; Bystrov, V.P.; Vaskevich, A.D.; Bruek, V.N. Flash Smelting; Metallurgiya: Moscow, Russia, 1988. [Google Scholar]
- Kaplan, V.A.; Vaskevich, A.D.; Zaitsev, V.Y. Study of equilibrium in the system of matte–slag–gas phase. Metals 1982, 2, 31–34. [Google Scholar]
- Swinbourne, D.R.; Kho, T.S. Computational thermodynamics modeling of minor element distributions during copper flash converting. Metall. Mater. Trans. B 2012, 43, 823–829. [Google Scholar] [CrossRef]
- Sarfo, P.; Das, A.; Wyss, G.; Young, C. Recovery of metals values from copper slag and reuse of residual secondary slag. Waste Manag. 2017, 70, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Dosmukhamedov, N.; Egizekov, M.; Zholdasbay, E.; Kaplan, V. Metal Recovery from converter slags using a sulfiding agent. JOM 2018, 70, 2400–2406. [Google Scholar] [CrossRef]
- Vaskevich, A.D.; Sorokin, M.L. General thermodynamic model of copper solubility in slags. Non-Ferr. Met. 1982, 10, 22–26. [Google Scholar]
- Ruddle, R.W.; Taylor, B.; Bates, A.P. The solubility of copper in iron silicate slags. Trans. Inst. Min. Met. 1966, 75, 1–12. [Google Scholar]
- Toguri, J.M.; Santander, N.H. The solubility of copper in fayalite slags at 1300 °C. Can. Metall. Q. 1969, 8, 167–171. [Google Scholar] [CrossRef]
- Toguri, J.M.; Santander, N.H. Distribution of copper between Cu-Au alloys and silica-saturated fayalite slags. Can. Metall. Q. 1972, 3, 586–588. [Google Scholar] [CrossRef]
- Altman, R.; Kellog, H.H. Thermodynamics of FeO-MnO-TiO2 melts saturated with iron at 1475 °C. Trans. Inst. Min. Met. 1972, 81, 163–175. [Google Scholar]
- Nagamori, M.; Mackey, P.J.; Tarassoff, P. The distribution of As, Sb, Bi, Se, and Te between molten copper and white metal. Metall. Trans. B 1975, 6, 295–301. [Google Scholar] [CrossRef]
- Taylor, J.P.; Jeffes, I.H.E. Activity of cuprous oxide in iron silicate slags of various compositions. Trans. Inst. Min. Met. 1975, 84, 18–24. [Google Scholar]
- Nagamori, M. Metal loss to slag: Part I. Sulfidic and oxidic dissolution of copper in fayalite slag from low grade matte. Metall. Trans. B 1974, 5, 531–538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).