Sugarcane Industry By-Products: A Decade of Research Using Biotechnological Approaches
Abstract
1. Introduction
2. Methodology
3. Biotechnological Tools for the Valorization of Agroindustrial By-Products
3.1. Solid State Fermentation
3.2. Anaerobic Digestion
3.3. Biocatalysis
3.4. Submerged Fermentation
3.5. Comparative Economic Aspects of Biotechnological Applications
4. Main By-Products of the Sugarcane Industry
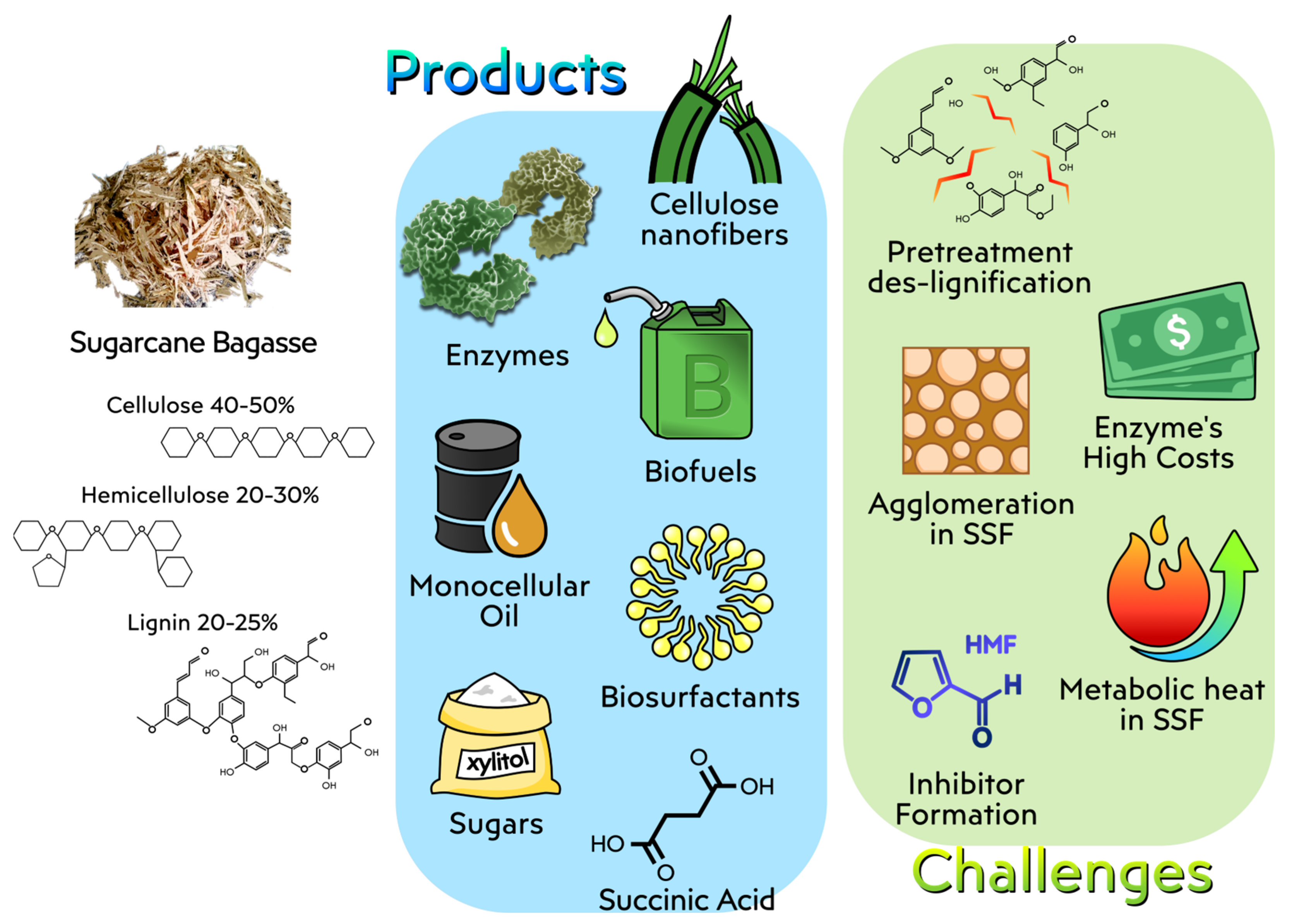
4.1. Sugarcane Bagasse
4.1.1. Biotechnological Approaches for the Utilization of Sugarcane Bagasse
4.1.2. Challenges in the Use of Sugarcane Bagasse
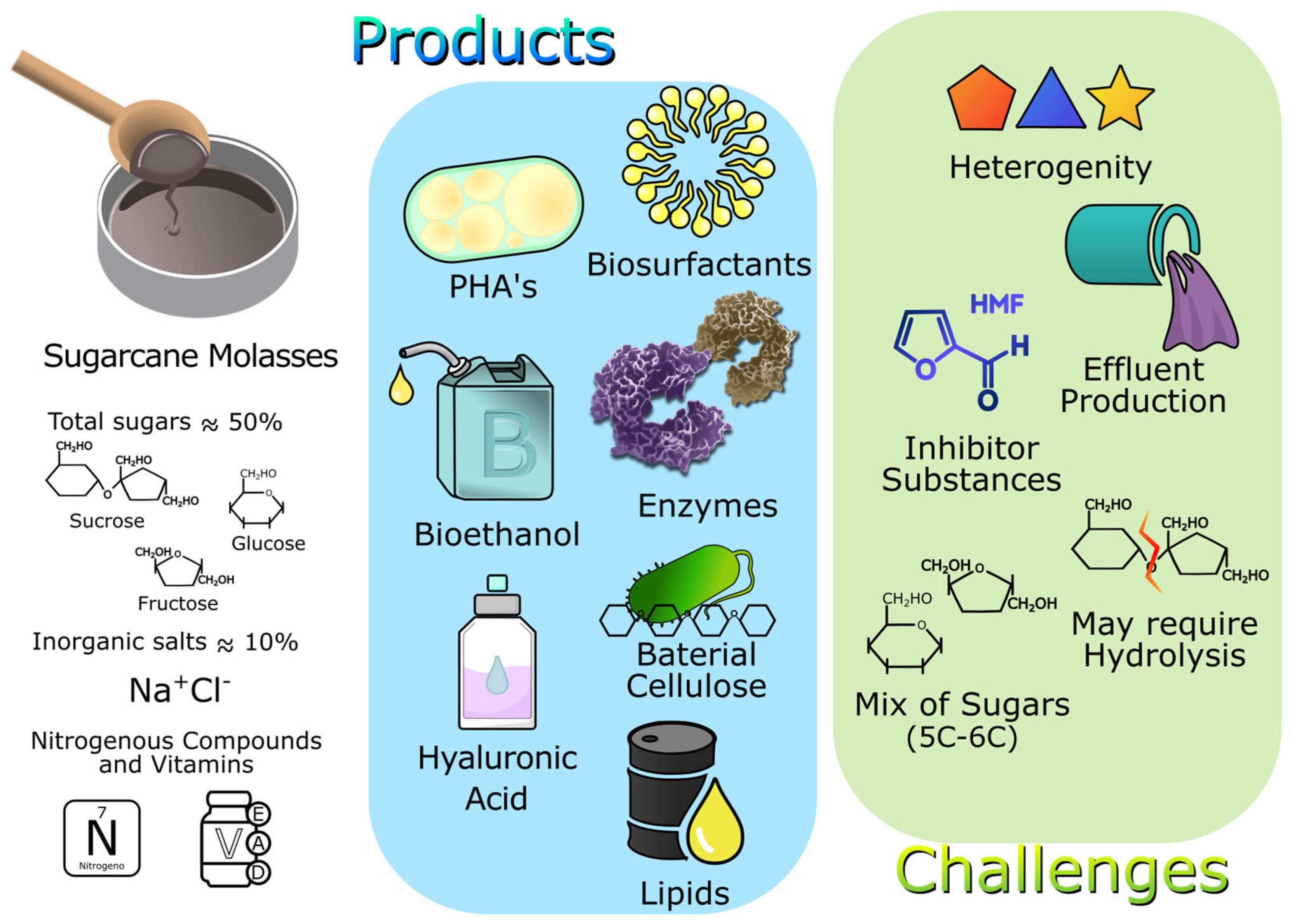
4.2. Sugarcane Molasses
4.2.1. Biotechnological Approaches for the Utilization of Sugarcane Molasses
4.2.2. Challenges in the Use of Sugarcane Molasses
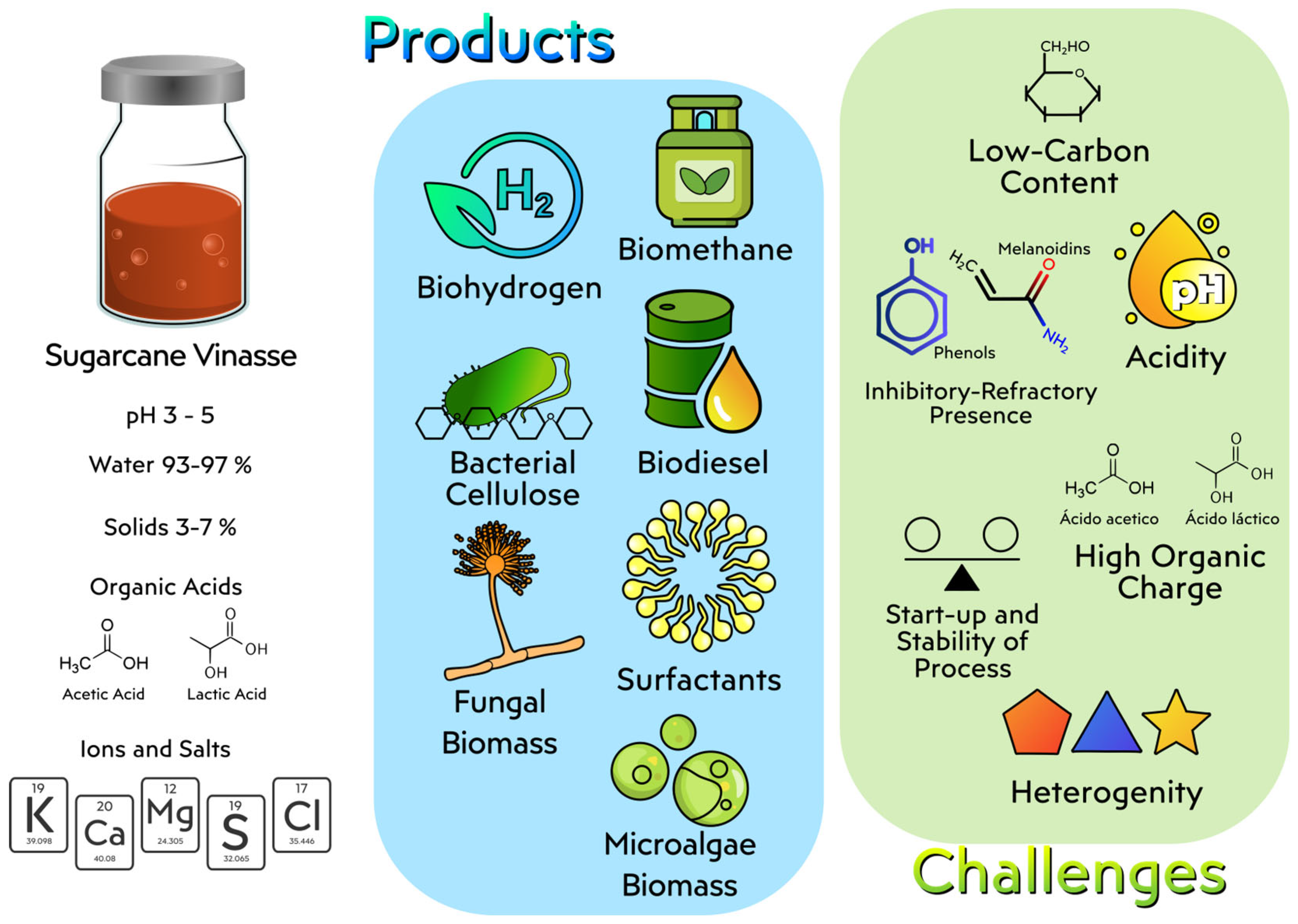
4.3. Sugarcane Vinasse
4.3.1. Biotechnological Approaches to Sugarcane Vinasse Utilization
4.3.2. Challenges in the Use of Sugarcane Vinasse
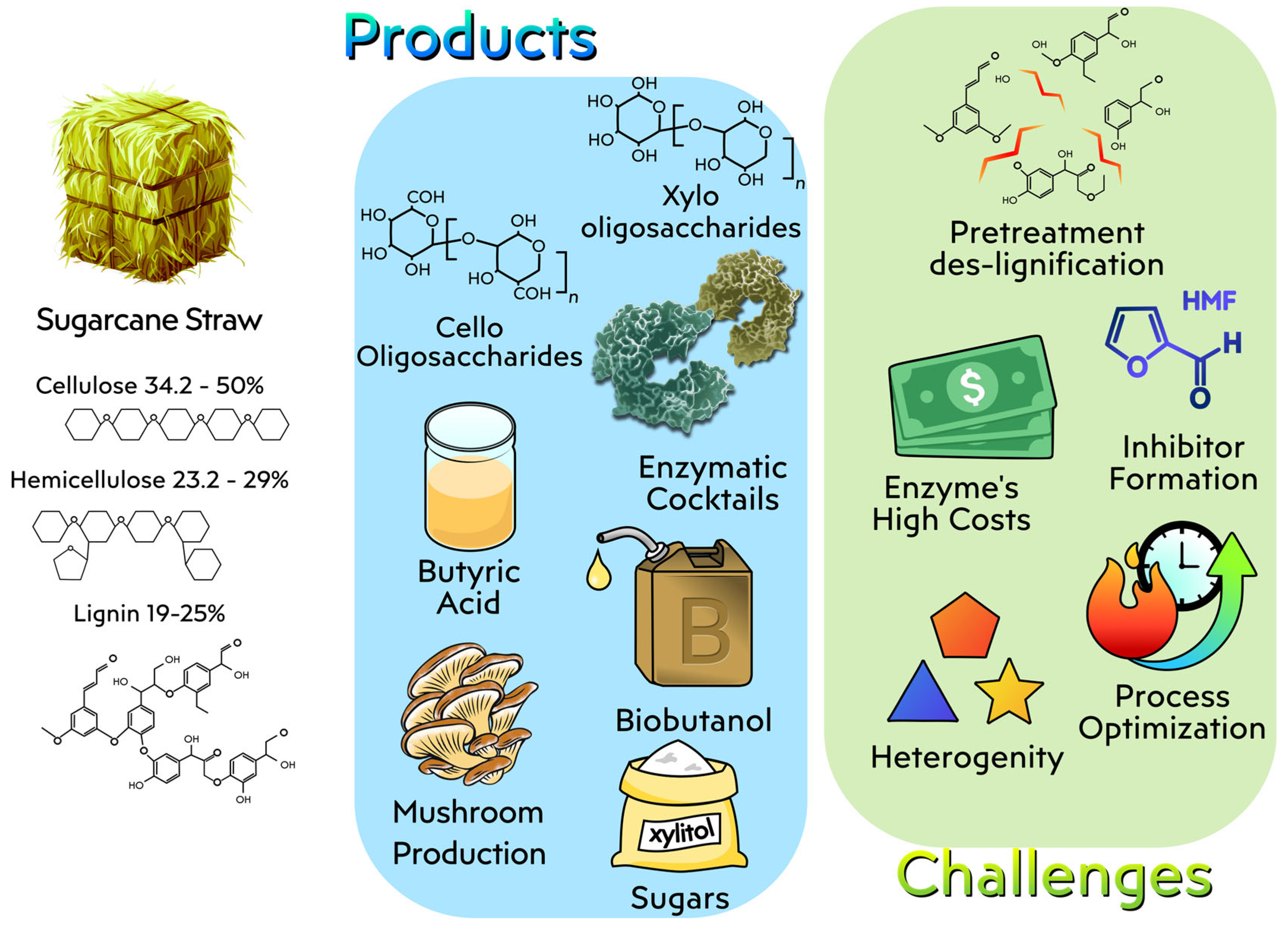
4.4. Sugarcane Straw
4.4.1. Biotechnological Approaches for the Utilization of Sugarcane Straw
4.4.2. Challenges in the Use of Sugarcane Straw
5. Strategic Approaches to Overcoming Biotechnological Challenges and Transitioning to Sustainable Alternatives
5.1. Mitigation of Inhibitory Compounds During Biotechnological Processing
5.2. Traditional Products vs. Bio-Based Products
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehrzad, S.; Taban, E.; Soltani, P.; Samaei, S.E.; Khavanin, A. Sugarcane Bagasse Waste Fibers as Novel Thermal Insulation and Sound-Absorbing Materials for Application in Sustainable Buildings. Build. Environ. 2022, 211, 108753. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Yu, L.; Shu, Y.; Tan, F.; Gou, Y.; Luo, S.; Yang, W.; Li, Z.; Wang, J. Sugarcane/Soybean Intercropping with Reduced Nitrogen Input Improves Crop Productivity and Reduces Carbon Footprint in China. Sci. Total Environ. 2020, 719, 137517. [Google Scholar] [CrossRef]
- Anur, R.M.; Mufithah, N.; Sawitri, W.D.; Sakakibara, H.; Sugiharto, B. Overexpression of Sucrose Phosphate Synthase Enhanced Sucrose Content and Biomass Production in Transgenic Sugarcane. Plants 2020, 9, 200. [Google Scholar] [CrossRef]
- Das, S.; Shakya, D.; Kaur, R.; Sevda, S.; Selvasembian, R.; Prabhu, A.A. Application of Cellulase from Mutated Aspergillus sp. for the Production of Sustainable 2G Ethanol from Sugarcane Bagasse. BioEnergy Res. 2025, 18, 25. [Google Scholar] [CrossRef]
- Khandelwal, N.; Kumari, S.; Poduval, S.; Behera, S.K.; Kumar, A.; Gedam, V.V. Life-Cycle Assessment of Three Biorefinery Pathways across Different Generations. Sci. Rep. 2025, 15, 13135. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Almeida, F.C.R.; Sales, A.; Moretti, J.P.; Mendes, P.C.D. Sugarcane Bagasse Ash Sand (SBAS): Brazilian Agroindustrial by-Product for Use in Mortar. Constr. Build. Mater. 2015, 82, 31–38. [Google Scholar] [CrossRef]
- Fregolente, L.G.; Miguel, T.B.A.R.; De Castro Miguel, E.; De Almeida Melo, C.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Toxicity Evaluation of Process Water from Hydrothermal Carbonization of Sugarcane Industry By-Products. Environ. Sci. Pollut. Res. 2018, 26, 27579–27589. [Google Scholar] [CrossRef]
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent Advances in Sugarcane Industry Solid By-Products Valorization. Waste Biomass Valorization 2016, 8, 241–266. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S.-Ș. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- Antunes, F.; Mota, I.F.; Da Silva Burgal, J.; Pintado, M.; Costa, P.S. A Review on the Valorization of Lignin from Sugarcane By-Products: From Extraction to Application. Biomass Bioenergy 2022, 166, 106603. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Ighalo, J.O.; Eshiemogie, S.; Omuku, P.E.; Adeniyi, A.G. Valorization of Sugar Industry’s By-Products: A Perspective. Sugar Tech 2022, 24, 1052–1078. [Google Scholar] [CrossRef]
- Bano, S.; Singh, K.; Chaudhary, A.; Chandra, R. Innovative Methods for the Valorisation of Solid Wastes from Sugar Mill and Refineries for Sustainable Development: A Review. Clean. Waste Syst. 2025, 2025, 100230. [Google Scholar] [CrossRef]
- Kamboj, A.; Sadh, P.K.; Yadav, B.; Kumari, A.; Kumar, R.; Surekha, N.; Saharan, B.S.; Brar, B.; Kumar, D.; Goyal, C.; et al. Unravelling the Potential of Sugarcane Bagasse: An Eco-Friendly and Inexpensive Agro-Industrial Waste for the Production of Valuable Products Using Pretreatment Processes for Sustainable Bio-Economy. J. Environ. Chem. Eng. 2024, 12, 114461. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Datta, S.C.; Biswas, D.R.; Dotaniya, C.K.; Meena, B.L.; Rajendiran, S.; Regar, K.L.; Lata, M. Use of Sugarcane Industrial By-Products for Improving Sugarcane Productivity and Soil Health. Int. J. Recycl. Org. Waste Agric. 2016, 5, 185–194. [Google Scholar] [CrossRef]
- Pandey, A. Solid-State Fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in Solid-State Fermentation for Bioconversion of Agricultural Wastes to Value-Added Products: Opportunities and Challenges. Bioresour. Technol. 2021, 343, 126065. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2007, 99, 4044–4064. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic Digestion of Organic Solid Wastes. An Overview of Research Achievements and Perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Forti, L.; Cramarossa, M.R.; Filippucci, S.; Tasselli, G.; Turchetti, B.; Buzzini, P. Nonconventional Yeast-Promoted Biotransformation for the Production of Flavor Compounds. In Handbook of Food Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–187. [Google Scholar]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial Biocatalysis Today and Tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Van Den Brink, J.; De Vries, R.P. Fungal Enzyme Sets for Plant Polysaccharide Degradation. Appl. Microbiol. Biotechnol. 2011, 91, 1477–1492. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C. Forest Bioresources for Bioethanol and Biodiesel Production with Emphasis on Mohua (Madhuca latifolia L.) Flowers and Seeds. In Sustainable Sources, Interventions, and Challenges; Elsevier: Amsterdam, The Netherlands, 2018; pp. 233–247. [Google Scholar]
- Singhania, R.R.; Sukumaran, R.K.; Patel, A.K.; Larroche, C.; Pandey, A. Advancement and Comparative Profiles in the Production Technologies Using Solid-State and Submerged Fermentation for Microbial Cellulases. Enzym. Microb. Technol. 2010, 46, 541–549. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of Industrial Waste and By-Product Streams via Fermentation for the Production of Chemicals and Biopolymers. Chem. Soc. Rev. 2014, 43, 2587. [Google Scholar] [CrossRef] [PubMed]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid State Fermentation (SSF): Diversity of Applications to Valorize Waste and Biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef]
- Rehman, M.L.U.; Iqbal, A.; Chang, C.; Li, W.; Ju, M. Anaerobic Digestion. Water Environ. Res. 2019, 91, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2020, 60, 88–119. [Google Scholar] [CrossRef]
- De Moraes Rocha, G.J.; Nascimento, V.M.; Gonçalves, A.R.; Silva, V.F.N.; Martín, C. Influence of Mixed Sugarcane Bagasse Samples Evaluated by Elemental and Physical–Chemical Composition. Ind. Crops Prod. 2014, 64, 52–58. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of Sugarcane Bagasse in Semi Batch Reactor: Effects of Process Parameters on Product Yields and Characterization of Products. Ind. Crops Prod. 2016, 95, 704–717. [Google Scholar] [CrossRef]
- Saelee, K.; Yingkamhaeng, N.; Nimchua, T.; Sukyai, P. An Environmentally Friendly Xylanase-Assisted Pretreatment for Cellulose Nanofibrils Isolation from Sugarcane Bagasse by High-Pressure Homogenization. Ind. Crops Prod. 2015, 82, 149–160. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-Doped Activated Carbon Derived from Sugarcane Bagasse for CO2 Adsorption. Ind. Crops Prod. 2018, 128, 290–297. [Google Scholar] [CrossRef]
- Del Río, J.C.; Lino, A.G.; Colodette, J.L.; Lima, C.F.; Gutiérrez, A.; Martínez, Á.T.; Lu, F.; Ralph, J.; Rencoret, J. Differences in the Chemical Structure of the Lignins from Sugarcane Bagasse and Straw. Biomass Bioenergy 2015, 81, 322–338. [Google Scholar] [CrossRef]
- De Oliveira, F.B.; Bras, J.; Pimenta, M.T.B.; Da Silva Curvelo, A.A.; Belgacem, M.N. Production of Cellulose Nanocrystals from Sugarcane Bagasse Fibers and Pith. Ind. Crops Prod. 2016, 93, 48–57. [Google Scholar] [CrossRef]
- Pereira, S.C.; Maehara, L.; Machado, C.M.M.; Farinas, C.S. 2G Ethanol from the Whole Sugarcane Lignocellulosic Biomass. Biotechnol. Biofuels 2015, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, S.A.; Cruz, E.; Delatorre, A.B.; Barbosa, J.B.; Martins, M.L.L. Cellulase Production by Thermophilic bacillus sp. SMIA-2 and Its Detergent Compatibility. Electron. J. Biotechnol. 2015, 18, 110–115. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, C.; Zhang, Q.; Zhang, K.; Zhang, Y.; Tao, P.; Wang, S. Enzymatic and Cold Alkaline Pretreatments of Sugarcane Bagasse Pulp to Produce Cellulose Nanofibrils Using a Mechanical Method. Ind. Crops Prod. 2018, 124, 435–441. [Google Scholar] [CrossRef]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of Pectinases by Solid-State Fermentation of a Mixture of Citrus Waste and Sugarcane Bagasse in a Pilot-Scale Packed-Bed Bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Marques, N.P.; De Cassia Pereira, J.; Gomes, E.; Da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and Xylanases Production by Endophytic Fungi by Solid State Fermentation Using Lignocellulosic Substrates and Enzymatic Saccharification of Pretreated Sugarcane Bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Marcelino, P.R.F.; Peres, G.F.D.; Terán-Hilares, R.; Pagnocca, F.C.; Rosa, C.A.; Lacerda, T.M.; Santos, J.C.D.; Da Silva, S.S. Biosurfactants Production by Yeasts Using Sugarcane Bagasse Hemicellulosic Hydrolysate as New Sustainable Alternative for Lignocellulosic Biorefineries. Ind. Crops Prod. 2018, 129, 212–223. [Google Scholar] [CrossRef]
- Unrean, P.; Ketsub, N. Integrated Lignocellulosic Bioprocess for Co-Production of Ethanol and Xylitol from Sugarcane Bagasse. Ind. Crops Prod. 2018, 123, 238–246. [Google Scholar] [CrossRef]
- Reddy, S.S.; Krishnan, C. Production of High-Pure Xylooligosaccharides from Sugarcane Bagasse Using Crude β-Xylosidase-Free Xylanase of Bacillus subtilis KCX006 and Their Bifidogenic Function. LWT 2015, 65, 237–245. [Google Scholar] [CrossRef]
- Moran-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of Cellulases and Xylanases in Solid-State Fermentation by Different Strains of Aspergillus niger Using Sugarcane Bagasse and Brewery Spent Grain. Biochem. Eng. J. 2021, 172, 108060. [Google Scholar] [CrossRef]
- De Cassia Pereira, J.; Marques, N.P.; Rodrigues, A.; De Oliveira, T.B.; Boscolo, M.; Da Silva, R.; Gomes, E.; Martins, D.A.B. Thermophilic Fungi as New Sources for Production of Cellulases and Xylanases with Potential Use in Sugarcane Bagasse Saccharification. J. Appl. Microbiol. 2015, 118, 928–939. [Google Scholar] [CrossRef]
- Bonturi, N.; Crucello, A.; Viana, A.J.C.; Miranda, E.A. Microbial Oil Production in Sugarcane Bagasse Hemicellulosic Hydrolysate without Nutrient Supplementation by a Rhodosporidium Toruloides Adapted Strain. Process Biochem. 2017, 57, 16–25. [Google Scholar] [CrossRef]
- Ong, K.L.; Li, C.; Li, X.; Zhang, Y.; Xu, J.; Lin, C.S.K. Co-Fermentation of Glucose and Xylose from Sugarcane Bagasse into Succinic Acid by Yarrowia lipolytica. Biochem. Eng. J. 2019, 148, 108–115. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Chade, M.; Mereles, E.B.; Bengoechea, D.I.; Brizuela, J.G.; Felissia, F.E.; Area, M.C. Strategies of Detoxification and Fermentation for Biotechnological Production of Xylitol from Sugarcane Bagasse. Ind. Crops Prod. 2016, 91, 161–169. [Google Scholar] [CrossRef]
- Vanitjinda, G.; Nimchua, T.; Sukyai, P. Effect of Xylanase-Assisted Pretreatment on the Properties of Cellulose and Regenerated Cellulose Films from Sugarcane Bagasse. Int. J. Biol. Macromol. 2018, 122, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.C.; Pereira, H.C.B.; De Lourdes Corradi Da Silva, M.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2016, 182, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, Z.; Zheng, Y.; Zhou, J.; Xiu, Z. Efficient Production of Lactic Acid from Sugarcane Molasses by a Newly Microbial Consortium CEE-DL15. Process Biochem. 2019, 81, 132–138. [Google Scholar] [CrossRef]
- Tyagi, N.; Suresh, S. Production of Cellulose from Sugarcane Molasses Using Gluconacetobacter Intermedius SNT-1: Optimization & Characterization. J. Clean. Prod. 2015, 112, 71–80. [Google Scholar] [CrossRef]
- Hassan, S.H.A.; Zohri, A.E.N.A.; Kassim, R.M.F. Electricity Generation from Sugarcane Molasses Using Microbial Fuel Cell Technologies. Energy 2019, 178, 538–543. [Google Scholar] [CrossRef]
- Machado, R.T.A.; Meneguin, A.B.; Sábio, R.M.; Franco, D.F.; Antonio, S.G.; Gutierrez, J.; Tercjak, A.; Berretta, A.A.; Ribeiro, S.J.L.; Lazarini, S.C.; et al. Komagataeibacter Rhaeticus Grown in Sugarcane Molasses-Supplemented Culture Medium as a Strategy for Enhancing Bacterial Cellulose Production. Ind. Crops Prod. 2018, 122, 637–646. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, P.; Singh, K.; Purchase, D.; Chandra, R. Translocation of Heavy Metals in Medicinally Important Herbal Plants Growing on Complex Organometallic Sludge of Sugarcane Molasses-Based Distillery Waste. Environ. Technol. Innov. 2021, 22, 101434. [Google Scholar] [CrossRef]
- Wu, R.; Chen, D.; Cao, S.; Lu, Z.; Huang, J.; Lu, Q.; Chen, Y.; Chen, X.; Guan, N.; Wei, Y.; et al. Enhanced Ethanol Production from Sugarcane Molasses by Industrially Engineered Saccharomyces cerevisiae via Replacement of the PHO4 Gene. RSC Adv. 2020, 10, 2267–2276. [Google Scholar] [CrossRef]
- Ellilä, S.; Fonseca, L.; Uchima, C.; Cota, J.; Goldman, G.H.; Saloheimo, M.; Sacon, V.; Siika-Aho, M. Development of a Low-Cost Cellulase Production Process Using Trichoderma reesei for Brazilian Biorefineries. Biotechnol. Biofuels 2017, 10, 30. [Google Scholar] [CrossRef]
- Chaprão, M.J.; Ferreira, I.N.S.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of Bacterial and Yeast Biosurfactants for Enhanced Removal and Biodegradation of Motor Oil from Contaminated Sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; De Aragão, G.M.F.; Poletto, P. Polyhydroxybutyrate (PHB) Production by Cupriavidus Necator from Sugarcane Vinasse and Molasses as Mixed Substrate. Process Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Almeida, D.G.; Soares da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Response Surface Methodology for Optimizing the Production of Biosurfactant by Candida tropicalis on Industrial Waste Substrates. Front. Microbiol. 2017, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wen, H.; Xing, D.; Pei, X.; Zhu, J.; Ren, N.; Liu, B. Molasses Wastewater Treatment and Lipid Production at Low Temperature Conditions by a Microalgal Mutant Scenedesmus sp. Z-4. Biotechnol. Biofuels 2017, 10, 111. [Google Scholar] [CrossRef]
- Rane, A.N.; Baikar, V.V.; Kumar, V.R.; Deopurkar, R.L. Agro-Industrial Wastes for Production of Biosurfactant by Bacillus subtilis ANR 88 and Its Application in Synthesis of Silver and Gold Nanoparticles. Front. Microbiol. 2017, 8, 492. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.Y.; Hussin, M.H.; Baidurah, S. Biosynthesis of Poly(3-Hydroxybutyrate) (PHB) by Cupriavidus Necator from Various Pretreated Molasses as Carbon Source. Biocatal. Agric. Biotechnol. 2018, 17, 51–59. [Google Scholar] [CrossRef]
- De Vrieze, J.; Raport, L.; Willems, B.; Verbrugge, S.; Volcke, E.; Meers, E.; Angenent, L.T.; Boon, N. Inoculum Selection Influences the Biochemical Methane Potential of Agro-industrial Substrates. Microb. Biotechnol. 2015, 8, 776–786. [Google Scholar] [CrossRef]
- Simair, A.A.; Qureshi, A.S.; Khushk, I.; Ali, C.H.; Lashari, S.; Bhutto, M.A.; Mangrio, G.S.; Lu, C. Production and Partial Characterization of α-Amylase Enzyme from Bacillus sp. BCC 01-50 and Potential Applications. BioMed Res. Int. 2017, 2017, 9173040. [Google Scholar] [CrossRef]
- Gießelmann, G.; Dietrich, D.; Jungmann, L.; Kohlstedt, M.; Jeon, E.J.; Yim, S.S.; Sommer, F.; Zimmer, D.; Mühlhaus, T.; Schroda, M.; et al. Metabolic Engineering of Corynebacterium glutamicum for High-Level Ectoine Production: Design, Combinatorial Assembly, and Implementation of a Transcriptionally Balanced Heterologous Ectoine Pathway. Biotechnol. J. 2019, 14, 1800417. [Google Scholar] [CrossRef]
- Jones, M.P.; Lawrie, A.C.; Huynh, T.T.; Morrison, P.D.; Mautner, A.; Bismarck, A.; John, S. Agricultural By-Product Suitability for the Production of Chitinous Composites and Nanofibers Utilising Trametes versicolor and Polyporus brumalis Mycelial Growth. Process Biochem. 2019, 80, 95–102. [Google Scholar] [CrossRef]
- Arshad, M.; Hussain, T.; Iqbal, M.; Abbas, M. Enhanced Ethanol Production at Commercial Scale from Molasses Using High Gravity Technology by Mutant S. cerevisiae. Braz. J. Microbiol. 2017, 48, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A Comprehensive Overview and Recent Advances on Polyhydroxyalkanoates (PHA) Production Using Various Organic Waste Streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and Characterization of Bacterial Cellulose Produced by Komagatacibacter xylinus PTCC 1734 Using Vinasse as a Cheap Cultivation Medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Filamentous Fungal Biomass Cultivated on Vinasse as an Alternative Nutrient Source of Fish Feed: Protein, Lipid, and Mineral Composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef]
- Parnaudeau, V.; Condom, N.; Oliver, R.; Cazevieille, P.; Recous, S. Vinasse Organic Matter Quality and Mineralization Potential, as Influenced by Raw Material, Fermentation and Concentration Processes. Bioresour. Technol. 2007, 99, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- De Barros, V.G.; Duda, R.M.; De Oliveira, R.A. Biomethane Production from Vinasse in Upflow Anaerobic Sludge Blanket Reactors Inoculated with Granular Sludge. Braz. J. Microbiol. 2016, 47, 628–639. [Google Scholar] [CrossRef]
- Júnior, A.D.N.F.; Etchebehere, C.; Zaiat, M. Mesophilic Hydrogen Production in Acidogenic Packed-Bed Reactors (APBR) Using Raw Sugarcane Vinasse as Substrate: Influence of Support Materials. Anaerobe 2015, 34, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Pitombo, L.M.; Carmo, J.B.D.; De Hollander, M.; Rossetto, R.; López, M.V.; Cantarella, H.; Kuramae, E.E. Exploring Soil Microbial 16S rRNA Sequence Data to Increase Carbon Yield and Nitrogen Efficiency of a Bioenergy Crop. GCB Bioenergy 2015, 8, 867–879. [Google Scholar] [CrossRef]
- Del Nery, V.; Alves, I.; Damianovic, M.H.R.Z.; Pires, E.C. Hydraulic and Organic Rates Applied to Pilot Scale UASB Reactor for Sugar Cane Vinasse Degradation and Biogas Generation. Biomass Bioenergy 2018, 119, 411–417. [Google Scholar] [CrossRef]
- Adarme, O.F.H.; Baêta, B.E.L.; Filho, J.B.G.; Gurgel, L.V.A.; De Aquino, S.F. Use of Anaerobic Co-Digestion as an Alternative to Add Value to Sugarcane Biorefinery Wastes. Bioresour. Technol. 2019, 287, 121443. [Google Scholar] [CrossRef]
- Dionizio, B.S.; Rabelo, C.A.B.S.; Rodrigues, C.V.; Camargo, F.P.; Silva, E.L.; De Souza, D.H.F.; Varesche, M.B.A. Co-Fermentation of Sugar-Alcohol Industry Waste to Produce Hydrogen and Value-Added Metabolites. Bioresour. Technol. Rep. 2025, 2025, 102211. [Google Scholar] [CrossRef]
- Lazaro, C.Z.; Perna, V.; Etchebehere, C.; Varesche, M.B.A. Sugarcane Vinasse as Substrate for Fermentative Hydrogen Production: The Effects of Temperature and Substrate Concentration. Int. J. Hydrogen Energy 2014, 39, 6407–6418. [Google Scholar] [CrossRef]
- Sánchez, F.E.; Fuess, L.T.; Cavalcante, G.S.; Adorno, M.Â.T.; Zaiat, M. Value-Added Soluble Metabolite Production from Sugarcane Vinasse within the Carboxylate Platform: An Application of the Anaerobic Biorefinery beyond Biogas Production. Fuel 2020, 286, 119378. [Google Scholar] [CrossRef]
- Greses, S.; Llamas, M.; Kaoutar, A.; González-Fernández, C. Vinasses Valorization into Short-Chain Fatty Acids: Microbiome Robustness against Process Variations. Bioresour. Bioprocess. 2025, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, G.; Srinikethan, G. Bacterial Cellulose Production by K. Saccharivorans BC1 Strain Using Crude Distillery Effluent as Cheap and Cost Effective Nutrient Medium. Int. J. Biol. Macromol. 2019, 138, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, V.E.; Michelin, M.; Vici, A.C.; De Almeida, P.Z.; De Lourdes Teixeira De Moraes Polizeli, M. Trametes versicolor Laccase Production Using Agricultural Wastes: A Comparative Study in Erlenmeyer Flasks, Bioreactor and Tray. Bioprocess Biosyst. Eng. 2019, 43, 507–514. [Google Scholar] [CrossRef]
- Vera, E.C.S.; De Souza De Azevedo, P.O.; Domínguez, J.M.; De Souza Oliveira, R.P. Optimization of Biosurfactant and Bacteriocin-like Inhibitory Substance (BLIS) Production by Lactococcus Lactis CECT-4434 from Agroindustrial Waste. Biochem. Eng. J. 2018, 133, 168–178. [Google Scholar] [CrossRef]
- Chuppa-Tostain, G.; Hoarau, J.; Watson, M.; Adelard, L.; Sing, A.S.C.; Caro, Y.; Grondin, I.; Bourven, I.; Francois, J.-M.; Girbal-Neuhauser, E.; et al. Production of Aspergillus niger Biomass on Sugarcane Distillery Wastewater: Physiological Aspects and Potential for Biodiesel Production. Fungal Biol. Biotechnol. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Náthia-Nevesorcid, G.; De Alencar Neves, T.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Start-up Phase of a Two-Stage Anaerobic Co-Digestion Process: Hydrogen and Methane Production from Food Waste and Vinasse from Ethanol Industry. Biofuel Res. J. 2018, 5, 813–820. [Google Scholar] [CrossRef]
- Albanez, R.; Chiaranda, B.C.; Ferreira, R.G.; França, A.L.P.; Honório, C.D.; Rodrigues, J.A.D.; Ratusznei, S.M.; Zaiat, M. Anaerobic Biological Treatment of Vinasse for Environmental Compliance and Methane Production. Appl. Biochem. Biotechnol. 2015, 178, 21–43. [Google Scholar] [CrossRef]
- Naspolini, B.F.; De Oliveira Machado, A.C.; Cravo, W.B., Jr.; Freire, D.M.G.; Cammarota, M.C. Bioconversion of Sugarcane Vinasse into High-Added Value Products and Energy. BioMed Res. Int. 2017, 2017, 8986165. [Google Scholar] [CrossRef]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella Vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef]
- Olguín, E.J.; Dorantes, E.; Castillo, O.S.; Hernández-Landa, V.J. Anaerobic Digestates from Vinasse Promote Growth and Lipid Enrichment in Neochloris Oleoabundans Cultures. J. Appl. Phycol. 2015, 27, 1813–1822. [Google Scholar] [CrossRef]
- Ávila, P.F.; Martins, M.; De Almeida Costa, F.A.; Goldbeck, R. Xylooligosaccharides Production by Commercial Enzyme Mixture from Agricultural Wastes and Their Prebiotic and Antioxidant Potential. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100234. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.F.; De Arruda, P.V.; De Almeida Felipe, M.D.G. Sugarcane Straw as a Feedstock for Xylitol Production by Candida guilliermondii FTI 20037. Braz. J. Microbiol. 2016, 47, 489–496. [Google Scholar] [CrossRef]
- Pratto, B.; Chandgude, V.; De Sousa, R.; Cruz, A.J.G.; Bankar, S. Biobutanol Production from Sugarcane Straw: Defining Optimal Biomass Loading for Improved ABE Fermentation. Ind. Crops Prod. 2020, 148, 112265. [Google Scholar] [CrossRef]
- Campioni, T.S.; De Jesus Moreira, L.; Moretto, E.; Nunes, N.S.S.; De Oliva Neto, P. Biobleaching of Kraft Pulp Using Fungal Xylanases Produced from Sugarcane Straw and the Subsequent Decrease of Chlorine Consumption. Biomass Bioenergy 2018, 121, 22–27. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; De Queiroz, J.H.; Colodette, J.L. Assessment of Alkaline Pretreatment for the Production of Bioethanol from Eucalyptus, Sugarcane Bagasse and Sugarcane Straw. Ind. Crops Prod. 2016, 94, 932–941. [Google Scholar] [CrossRef]
- Fonseca, B.C.; Reginatto, V.; López-Linares, J.C.; Lucas, S.; García-Cubero, M.T.; Coca, M. Acetic Acid as Catalyst for Microwave-Assisted Pretreatment of Sugarcane Straw Aids Highly Specific Butyric Acid Bioproduction. Ind. Crops Prod. 2020, 157, 112936. [Google Scholar] [CrossRef]
- Martins, M.; Ávila, P.F.; De Andrade, C.C.P.; Goldbeck, R. Synergic Recombinant Enzyme Association to Optimize Xylo-Oligosaccharides Production from Agricultural Waste. Biocatal. Agric. Biotechnol. 2020, 28, 101747. [Google Scholar] [CrossRef]
- Vieira, F.R.; De Andrade, M.C.N. Optimization of Substrate Preparation for Oyster Mushroom (Pleurotus ostreatus) Cultivation by Studying Different Raw Materials and Substrate Preparation Conditions (Composting: Phases I and II). World J. Microbiol. Biotechnol. 2016, 32, 190. [Google Scholar] [CrossRef]
- Magalhães, B.L.; Grassi, M.C.B.; Pereira, G.A.G.; Brocchi, M. Improved N-Butanol Production from Lignocellulosic Hydrolysate by Clostridium Strain Screening and Culture-Medium Optimization. Biomass Bioenergy 2017, 108, 157–166. [Google Scholar] [CrossRef]
- Barbosa, F.C.; Kendrick, E.; Brenelli, L.B.; Arruda, H.S.; Pastore, G.M.; Rabelo, S.C.; Damasio, A.; Franco, T.T.; Leak, D.; Goldbeck, R. Optimization of Cello-Oligosaccharides Production by Enzymatic Hydrolysis of Hydrothermally Pretreated Sugarcane Straw Using Cellulolytic and Oxidative Enzymes. Biomass Bioenergy 2020, 141, 105697. [Google Scholar] [CrossRef]
- Piccinni, F.E.; Ontañon, O.M.; Ghio, S.; Sauka, D.H.; Talia, P.M.; Rivarola, M.L.; Valacco, M.P.; Campos, E. Secretome Profile of Cellulomonas sp. B6 Growing on Lignocellulosic Substrates. J. Appl. Microbiol. 2018, 126, 811–825. [Google Scholar] [CrossRef]
- Vieira, M.M.; Kadoguchi, E.; Segato, F.; Da Silva, S.S.; Chandel, A.K. Production of Cellulases by Aureobasidium pullulans LB83: Optimization, Characterization, and Hydrolytic Potential for the Production of Cellulosic Sugars. Prep. Biochem. Biotechnol. 2020, 51, 153–163. [Google Scholar] [CrossRef]
- Martins, M.; Silva, K.C.G.; Ávila, P.F.; Sato, A.C.K.; Goldbeck, R. Xylo-Oligosaccharide Microparticles with Synbiotic Potential Obtained from Enzymatic Hydrolysis of Sugarcane Straw. Food Res. Int. 2020, 140, 109827. [Google Scholar] [CrossRef]
- Ávila, P.F.; De Mélo, A.H.F.; Goldbeck, R. Cello-Oligosaccharides Production from Multi-Stage Enzymatic Hydrolysis by Lignocellulosic Biomass and Evaluation of Prebiotic Potential. Innov. Food Sci. Emerg. Technol. 2023, 85, 103335. [Google Scholar] [CrossRef]
- Da Silva, D.S.; Dantzger, M.; Assis, M.A.; Gallardo, J.C.M.; Teixeira, G.S.; Missawa, S.K.; Domingues, R.R.; Carazzolle, M.F.; Lunardi, I.; Leme, A.F.P.; et al. Lignocellulolytic Characterization and Comparative Secretome Analysis of a Trichoderma erinaceum Strain Isolated from Decaying Sugarcane Straw. Fungal Biol. 2019, 123, 330–340. [Google Scholar] [CrossRef]
- Fit, C.G.; Clauser, N.M.; Felissia, F.E.; Area, M.C. Biorefinery Design from Agroindustrial By-Products and Its Scaling-up Analysis. Bioresour. Technol. Rep. 2025, 2025, 102175. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(Lactic Acid) (PLA) and Polyhydroxyalkanoates (PHAs), Green Alternatives to Petroleum-Based Plastics: A Review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef] [PubMed]
- Farahani, S.S.; Asoodar, M.A. Life Cycle Environmental Impacts of Bioethanol Production from Sugarcane Molasses in Iran. Environ. Sci. Pollut. Res. 2017, 24, 22547–22556. [Google Scholar] [CrossRef] [PubMed]
- Parsaee, M.; Kiani, M.K.D.; Karimi, K. A Review of Biogas Production from Sugarcane Vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Hong, K.; Zhang, H.; Han, M.; Nie, X.; Fu, X.; Lei, F.; He, D. A Novel Four-Species Microbial Consortium for Nutritional Value Improvement of Rapeseed Meal. Food Chem. 2025, 478, 143712. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Nakamurakare, E.C.; Robles, C.A.; Carmaran, C.C.; Rojas, N.L. An Unexplored Enzymatic Source for Brewer’s Spent Grain Valorization: The Role of Wood-Substrate Beetle-Associated Fungi and Endophytes. Biomass Bioenergy 2025, 197, 107788. [Google Scholar] [CrossRef]
- Liu, G.; Gong, H.; Tang, H.; Meng, Z.; Wang, Z.; Cui, W.; Zhang, K.; Chen, Y.; Yang, Y. Enhanced Lignocellulose Degradation in Bacillus subtilis RLI2019 through CRISPR/Cas9-Mediated Chromosomal Integration of Ternary Cellulase Genes. Int. J. Biol. Macromol. 2025, 306, 141727. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, Y.; Dong, Y.; Sun, Y.; Tang, R.; Xiao, Y.; Gao, B.; Zhu, D. The Enzyme Cocktail Produced by Endophytic Chaetomium globosum Unlocks in Situ Production of Sugars and Shows Great Potential Application in Biorefinery. Renew. Energy 2025, 2025, 122449. [Google Scholar] [CrossRef]
- Castilla-Marroquín, J.D.; Pacheco, N.; Herrera-Corredor, J.A.; Hernández-Rosas, F.; Jiménez-Morales, K.; Benítez-Salamanca, M.J.; Hernández-Martínez, R. Polyhydroxyalkanoates Production by Bacillus thuringiensis HA1 Using Sugarcane Molasses as Carbon Source. Rev. Mex. Ing. Química 2024, 23, bio24352. [Google Scholar] [CrossRef]
- De Carvalho, R.S.F.; Mahnke, L.C.; Palácio, S.B.; Barbosa, W.T.; Hodel, K.V.S.; Barbosa, J.D.V.; De Assis Dutra Melo, F.; Chorilli, M.; Meneguin, A.B.; Pinto, F.C.M.; et al. Bacterial Cellulose Hydrogel Produced by Gluconacetobacter Hansenii Using Sugarcane Molasses as Medium: Physicochemical Characterization for Wound Healing Applications. Carbohydr. Polym. Technol. Appl. 2024, 9, 100632. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Ng, I.-S. Biofabrication of Polyhydroxybutyrate (PHB) in Engineered Cupriavidus Necator H16 from Waste Molasses. J. Taiwan Inst. Chem. Eng. 2024, 167, 105843. [Google Scholar] [CrossRef]
- León, K.E.S.; Calderón, O.M.H.; García, C.A.; Gaxiola, M.E.C.; Castro, E.R.; Del Castillo, J.R.O.; Iribe, E.Y.R. Optimization of Bioethanol Production from Sorghum Green Malt and Cane Molasses Using Saccharomyces bayanus in Submerged Cultivation. Can. J. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- Borges, A.D.V.; Fuess, L.T.; Takeda, P.Y.; Rogeri, R.C.; Saia, F.T.; Gregoracci, G.B.; Damianovic, M.H.R.Z. Unleashing the Full Potential of Vinasse Fermentation in Sugarcane Biorefineries. Renew. Sustain. Energy Rev. 2024, 208, 115096. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Devens, K.U.; Camargo, F.P.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Harnessing the Energy Potential and Value-Added Products from the Treatment of Sugarcane Vinasse: Maximizing Methane Production Through Co-Digestion with Sugarcane Molasses and Enhanced Organic Loading. Appl. Biochem. Biotechnol. 2024, 197, 964–988. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, D.; Singh, S.; Cheng, G. Recent Developments in Ionic Liquid Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion. Sustain. Energy Fuels 2021, 5, 1655–1667. [Google Scholar] [CrossRef]
- Wang, S.; Sun, X.; Yuan, Q. Strategies for Enhancing Microbial Tolerance to Inhibitors for Biofuel Production: A Review. Bioresour. Technol. 2018, 258, 302–309. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, M.; Tang, Y.; Geng, B.; Qiu, M.; He, Q.; Chen, S.; Wang, X.; Yang, S. Progress and Perspective on Lignocellulosic Hydrolysate Inhibitor Tolerance Improvement in Zymomonas Mobilis. Bioresour. Bioprocess. 2018, 5, 6. [Google Scholar] [CrossRef]
- Shan, W.; Yan, Y.; Li, Y.; Hu, W.; Chen, J. Microbial Tolerance Engineering for Boosting Lactic Acid Production from Lignocellulose. Biotechnol. Biofuels Bioprod. 2023, 16, 78. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Product | Application | Process |
|---|---|---|---|
| [33] | Cellulose nanofibrils | Food, pharmaceutical, packaging, film, paper, and nanocomposites | Biocatalysis |
| [37] | Bioethanol | Biofuel | Biocatalysis and SmF |
| [38] | Cellulases | Detergent additive | SmF |
| [39] | Cellulose nanofibrils | High-performance products (electronics) | Biocatalysis |
| [40] | Pectinases | d-galacturonic acid production | SSF |
| [41] | Cellulases and xylanases | Enzymatic saccharification | SSF |
| [42] | Biosurfactants | Food and pharmaceutical industries | SmF |
| [43] | Ethanol and xylitol | Food and pharmaceutical industries | Biocatalysis and Simultaneous Saccharification and Fermentation |
| [44] | Xylo-oligosaccharides | Prebiotics | Biocatalysis |
| [45] | Cellulases and xylanases | Enzymatic saccharification/bioethanol | SSF |
| [46] | Cellulases and xylanases | Enzymatic saccharification | SSF |
| [47] | Single-cell oil | Biofuel (biodiesel) | SmF |
| [48] | Succinic acid | Food, agricultural, and pharmaceutical industries | SmF |
| [49] | Xylitol | Food industry (sweetener) | SmF |
| [50] | Cellulose films | Packaging and medicine | Biocatalysis |
| Ref. | Product | Application | Process |
|---|---|---|---|
| [58] | Cellulase | Bioethanol synthesis (biofuel) | SmF |
| [59] | Biosurfactant | Bioremediation | SmF |
| [60] | PHB | Biodegradable plastics | SmF |
| [61] | Biosurfactant | Medicine, household products, agriculture, food products, cosmetics, pharmaceuticals, and the petroleum industry | SmF |
| [62] | Lipids | Biodiesel (biofuel) | Microalgae Cultivation |
| [63] | Biosurfactant | Immunological and medical applications | SmF |
| [55] | Bacterial cellulose (or nanocellulose) | Biomedical and pharmaceutical applications | SmF (static) |
| [64] | Chitin | Drug delivery carriers, antibacterial agents, and food stabilizers | SmF |
| [65] | PHB | Medical, surgical, and pharmacology (implant material and drug carrier) | SmF |
| [66] | Methane | Biogas | Anaerobic Digestion |
| [67] | Alpha-amylase | Saccharification of starchy materials, food, pharmaceutical, detergent, and textile industries. | SmF |
| [51] | Hyaluronic acid | Medical and cosmetic applications | SmF |
| [68] | Ectoine | Medicine, cosmetics, and biotechnology | SmF |
| [69] | Chitinous composites and nanofibers | Composites, cosmetics, pharmaceuticals, and water treatment applications | SSF and SmF |
| [70] | Ethanol | Biofuel | SmF |
| Ref. | Product | Application | Process |
|---|---|---|---|
| [60] | PHB | Plastic substitute | SmF |
| [76] | Biohydrogen | Biofuel | Anaerobic Digestion |
| [73] | Fungal biomass | Fish feed | SmF |
| [75] | Biomethane | Biofuel | Anaerobic Digestion |
| [72] | Bacterial cellulose | Biomedical | SmF |
| [78] | Methane | Biogas–Biofuel | Anaerobic Digestion |
| [84] | Bacterial cellulose | Textile industry, food processing, and pharmaceutical applications | SmF (Static) |
| [85] | Lacasse | Bioethanol, paper and cellulose, tissues, and animal feed | SmF |
| [86] | Biosurfactant and bacteriocin | Pharmaceutical/medicine, food, cosmetic, pesticide, oil, and biodegradation industries | SmF |
| [87] | Biomass | Bioremediation and biofuel | SmF |
| [88] | Hydrogen and methane | Biofuel | Anaerobic Digestion |
| [89] | Methane | Biofuel | Anaerobic Digestion |
| [90] | Biosurfactant (rhamnolipids) | Agriculture, cosmetics, pharmaceuticals, detergents, personal care products, food processing, textile manufacturing, laundry supplies, metal treatment and processing, pulp and paper processing, and paint industries | SmF |
| [91] | Chlorella vulgaris biomass | Value-added compounds for food, nutraceutical, cosmetic, and biofuel applications | Microalgae Cultivation (SmF) |
| [92] | Neochloris oleoabundans biomass | Biodiesel–Biofuel | Anaerobic Digestion |
| Ref. | Product | Application | Process |
|---|---|---|---|
| [97] | Bioethanol | Biofuel | Biocatalysis and SSF |
| [95] | Biobutanol | Biofuel | Biocatalysis and SSF |
| [93] | XOS | Food industries (sweeteners, stabilizers, emulsificants, prebiotics) | |
| [94] | Xylitol | Food, odontological, and pharmaceutical industries | SmF |
| [100] | Oyster mushroom | Food | SSF |
| [101] | n-butanol | Biofuel | SmF |
| [102] | Cellooligosaccharides | Biofuel, food, and feed | Biocatalysis |
| [96] | Xylanases | Paper industry (biobleaching) | SmF |
| [103] | Enzymatic cocktails | Food, paper-pulp, animal feed, laundry detergents, and second-generation bioethanol production | SmF |
| [104] | Cellulases | Lignocellulose saccharification | SmF |
| [99] | Enzymes and XOS | Functional foods (prebiotics) | SmF and Biocatalysis |
| [105] | Xylooligosaccharide microparticles | Functional foods (prebiotics) | Biocatalysis |
| [98] | Butyric acid | Chemical, pharmaceutical, food, and feed industries | Biocatalysis and SmF |
| [106] | Cellooligosaccharides | Food, chemical, and pharmaceutical industries | Biocatalysis |
| [107] | Enzymatic cocktail | Biofuel | SmF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Contreras, S.; Hernández-Rosas, F.; Lizardi-Jiménez, M.A.; Herrera-Corredor, J.A.; Baltazar-Bernal, O.; Avalos-de la Cruz, D.A.; Hernández-Martínez, R. Sugarcane Industry By-Products: A Decade of Research Using Biotechnological Approaches. Recycling 2025, 10, 154. https://doi.org/10.3390/recycling10040154
Pérez-Contreras S, Hernández-Rosas F, Lizardi-Jiménez MA, Herrera-Corredor JA, Baltazar-Bernal O, Avalos-de la Cruz DA, Hernández-Martínez R. Sugarcane Industry By-Products: A Decade of Research Using Biotechnological Approaches. Recycling. 2025; 10(4):154. https://doi.org/10.3390/recycling10040154
Chicago/Turabian StylePérez-Contreras, Serafín, Francisco Hernández-Rosas, Manuel A. Lizardi-Jiménez, José A. Herrera-Corredor, Obdulia Baltazar-Bernal, Dora A. Avalos-de la Cruz, and Ricardo Hernández-Martínez. 2025. "Sugarcane Industry By-Products: A Decade of Research Using Biotechnological Approaches" Recycling 10, no. 4: 154. https://doi.org/10.3390/recycling10040154
APA StylePérez-Contreras, S., Hernández-Rosas, F., Lizardi-Jiménez, M. A., Herrera-Corredor, J. A., Baltazar-Bernal, O., Avalos-de la Cruz, D. A., & Hernández-Martínez, R. (2025). Sugarcane Industry By-Products: A Decade of Research Using Biotechnological Approaches. Recycling, 10(4), 154. https://doi.org/10.3390/recycling10040154









