Upcycling of Cupric Chloride Waste Solution from PCB Manufacturing for Antibacterial Copper Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Cupric Chloride Waste Solution
2.2. Recovery of Copper Sulfate from Spent Copper Etching Solution
2.3. Synthesis of Copper Nanoparticles from Copper Sulfate Precursor
3. Results and Discussion
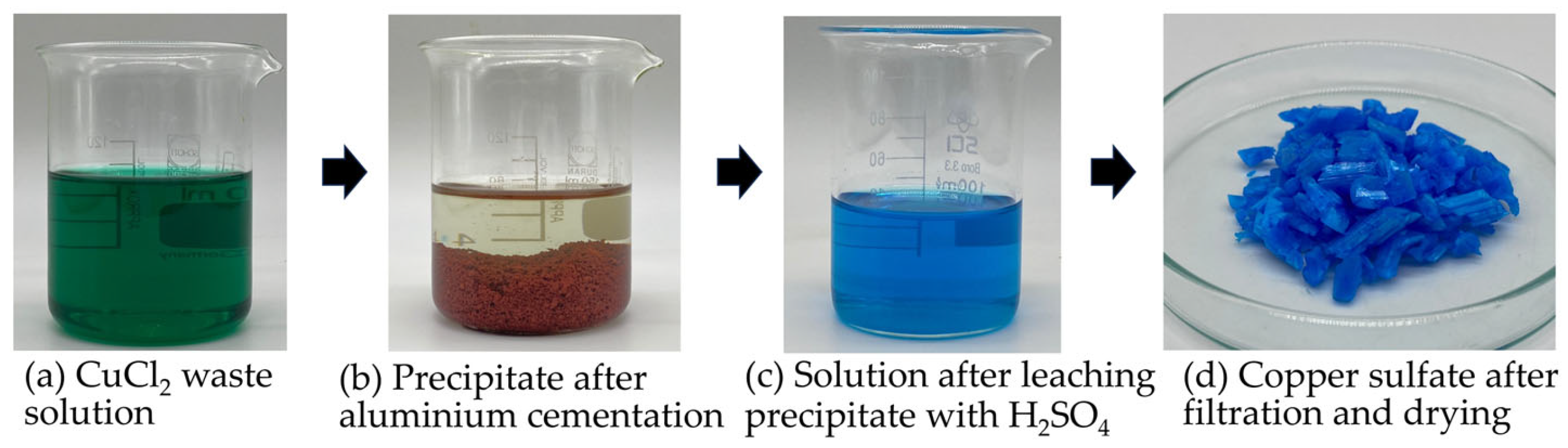
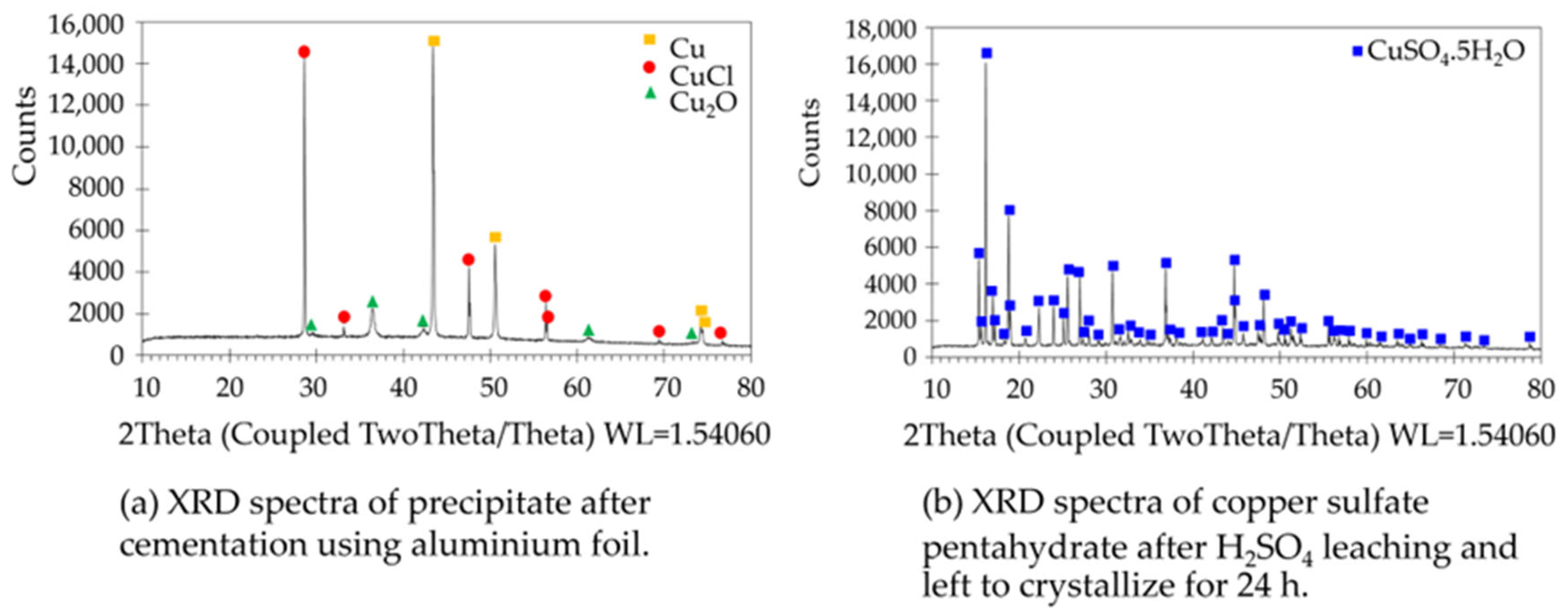
3.1. Copper Sulfate Recovered from the Spent Copper Etching Solution
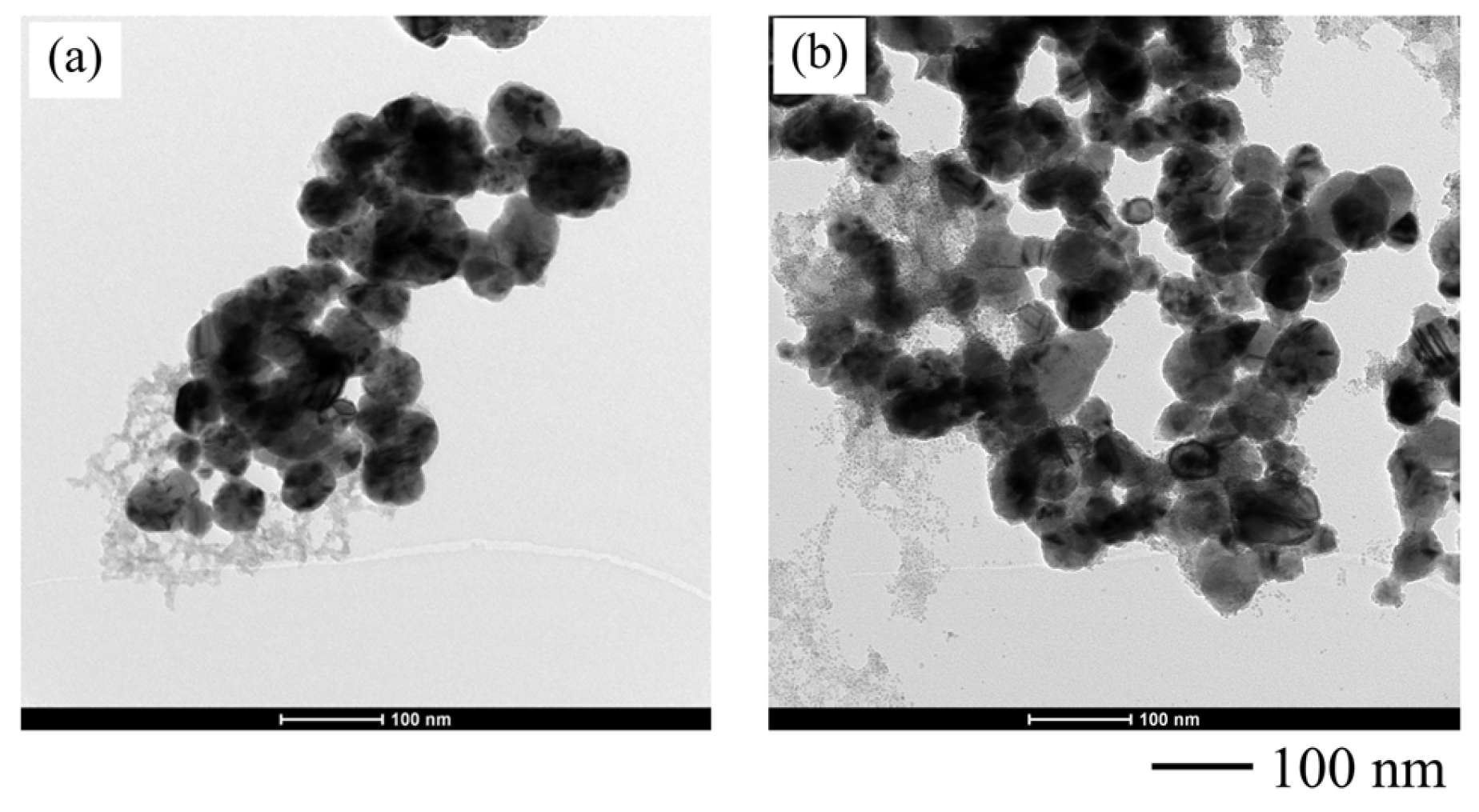
3.2. Effects of Synthesis Parameters on Size, Shape, and Composition of Copper Nanoparticles
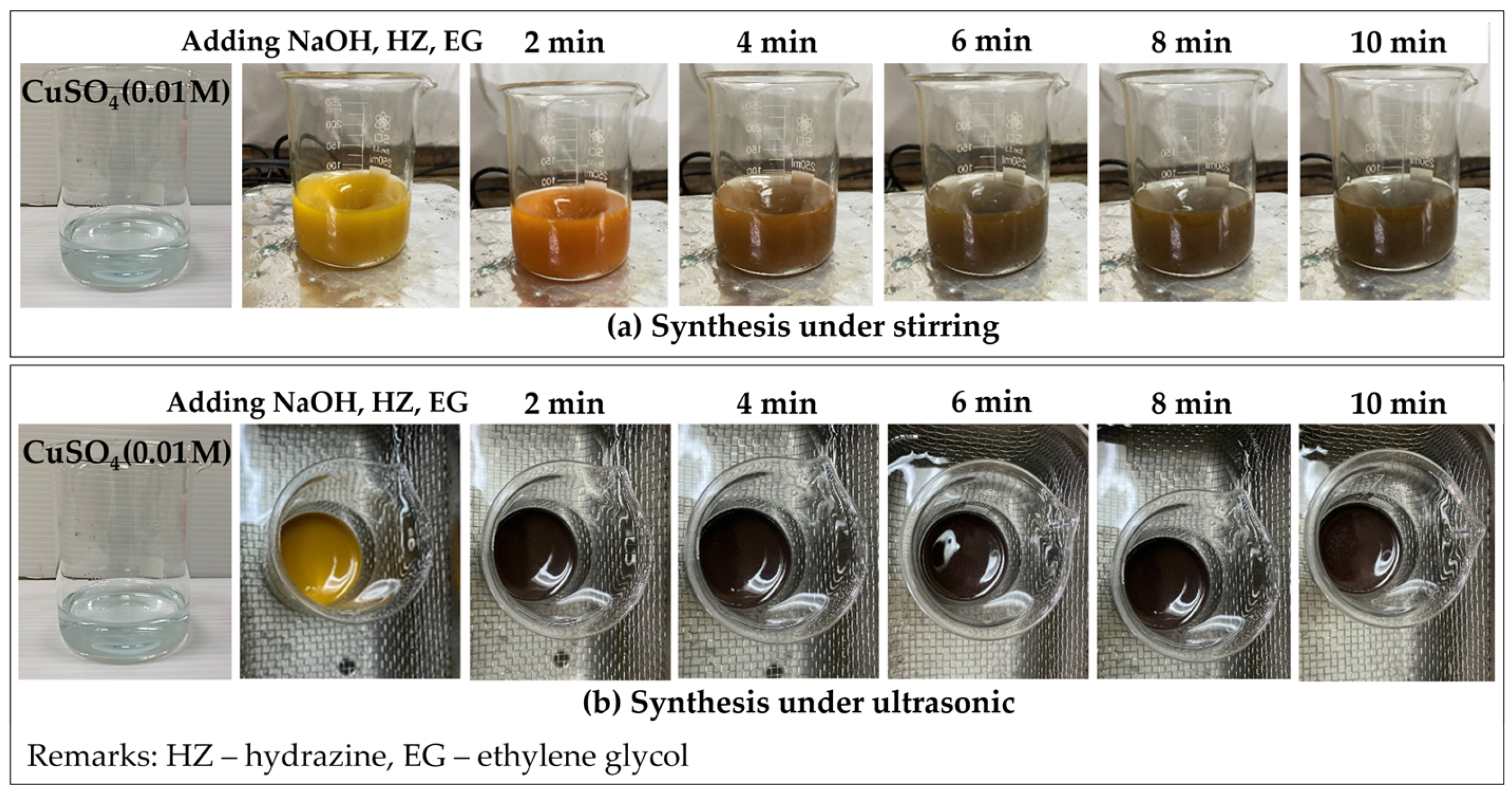
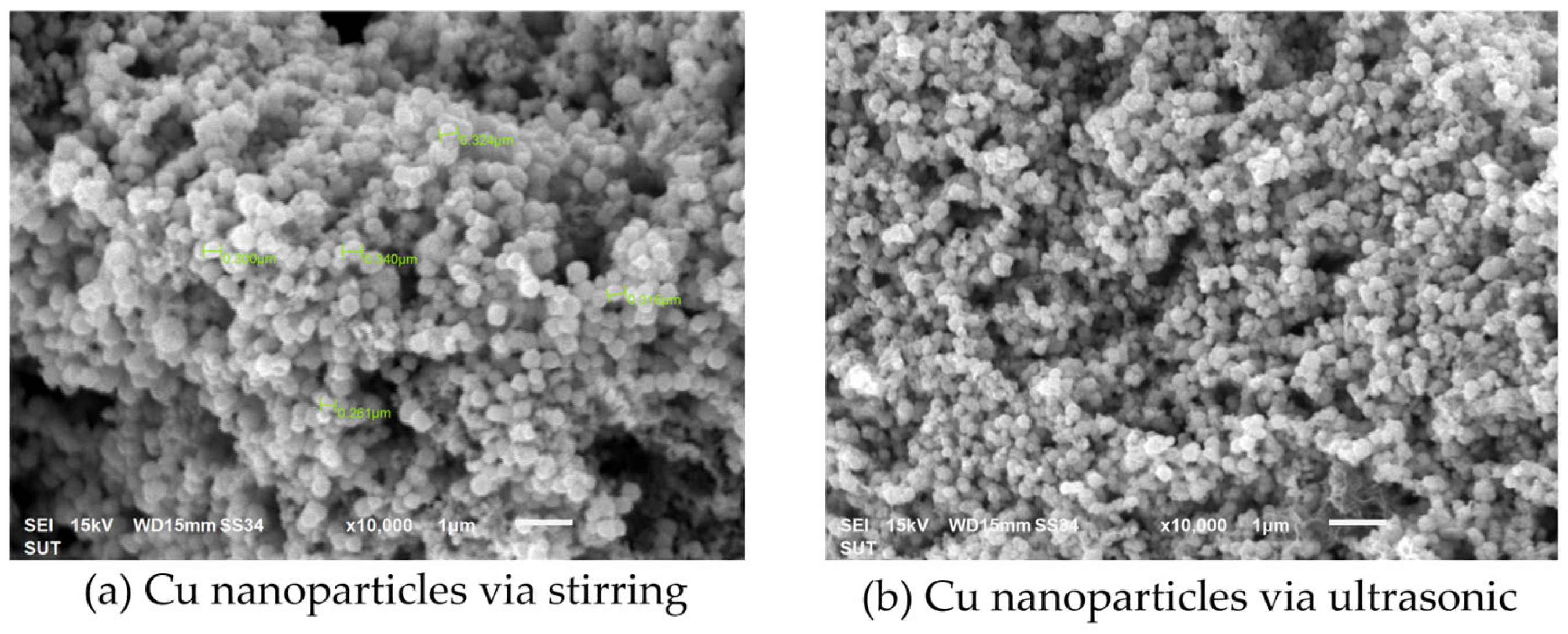
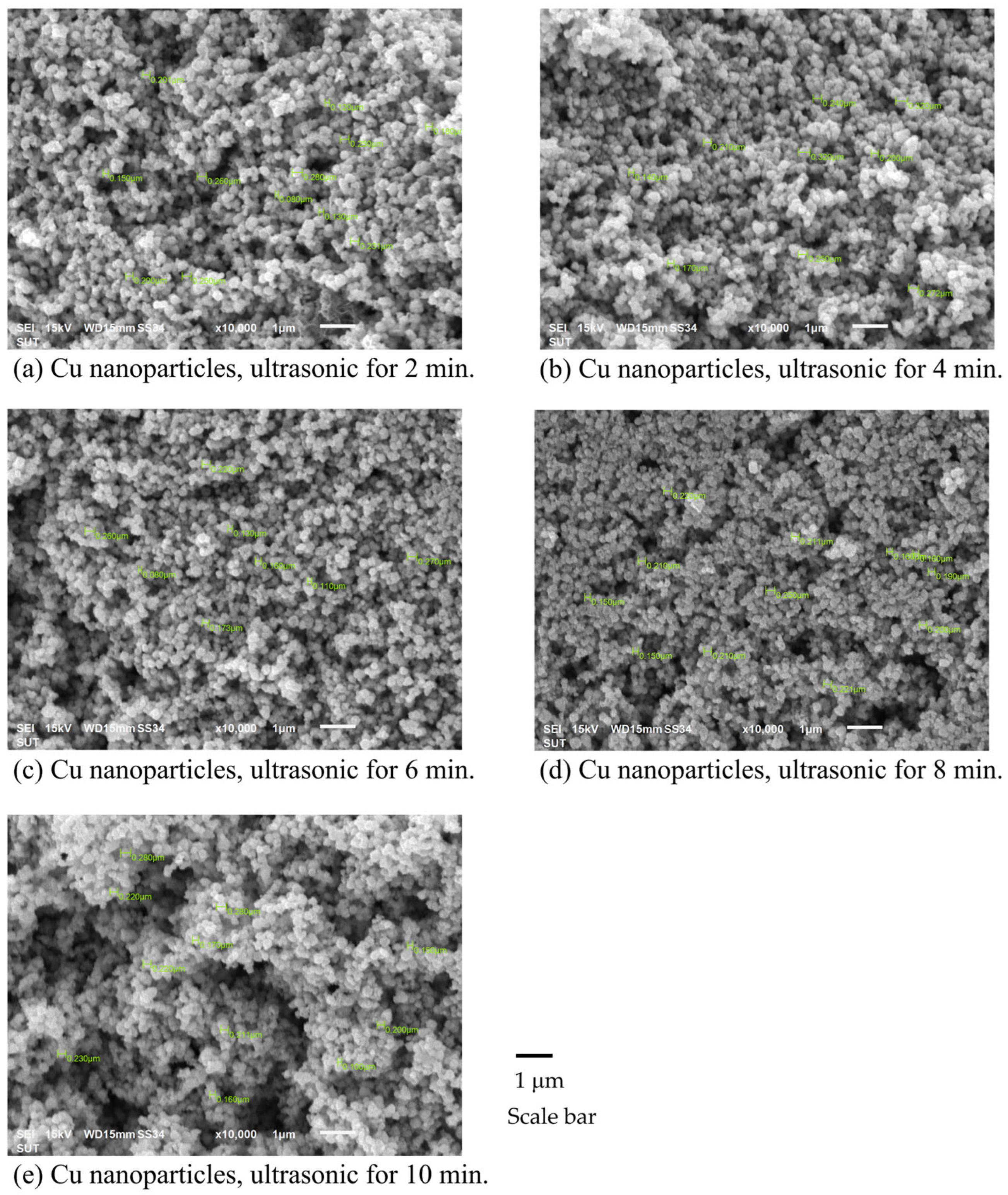
3.2.1. Effects of Synthesis Techniques and Time
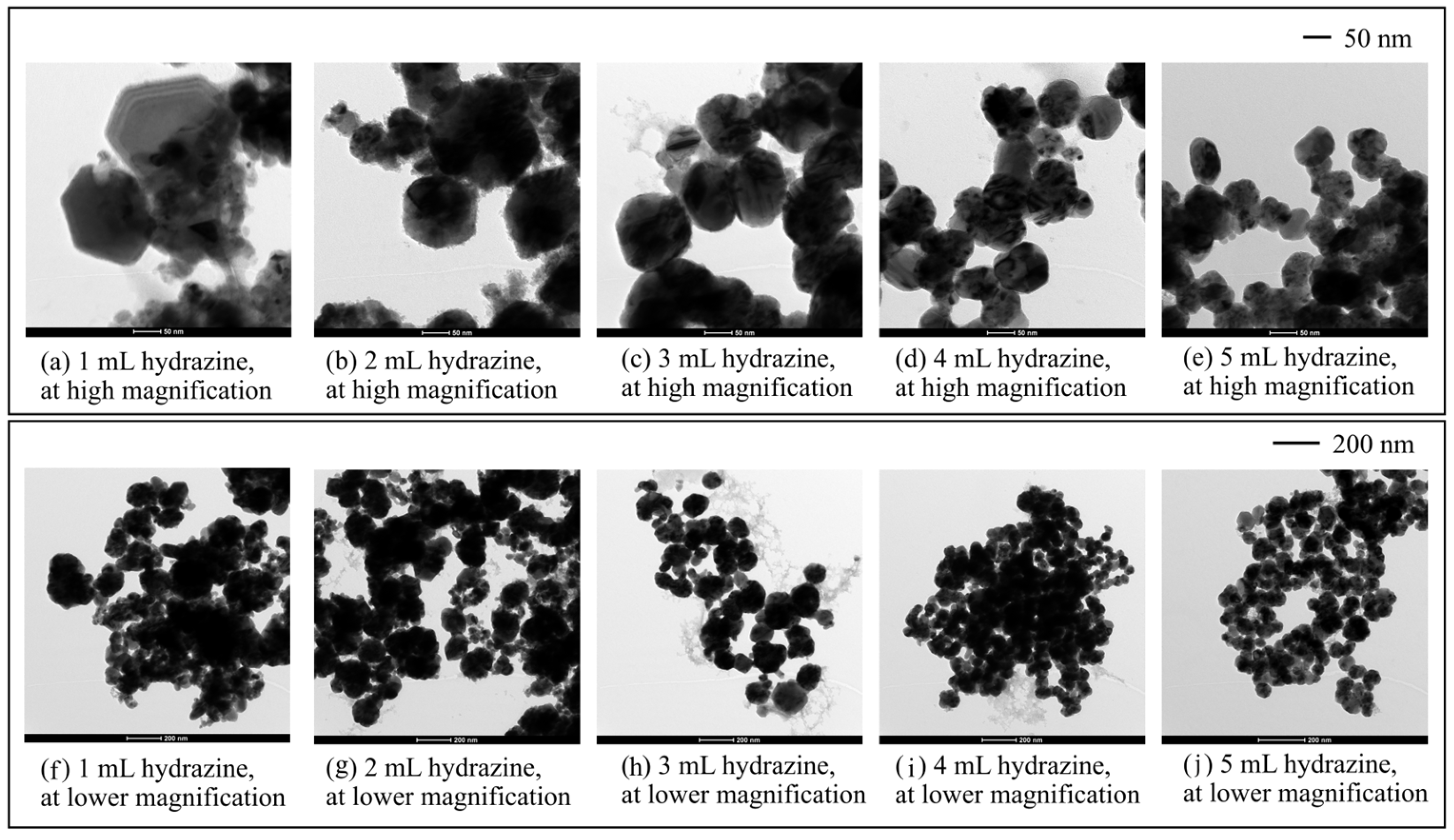
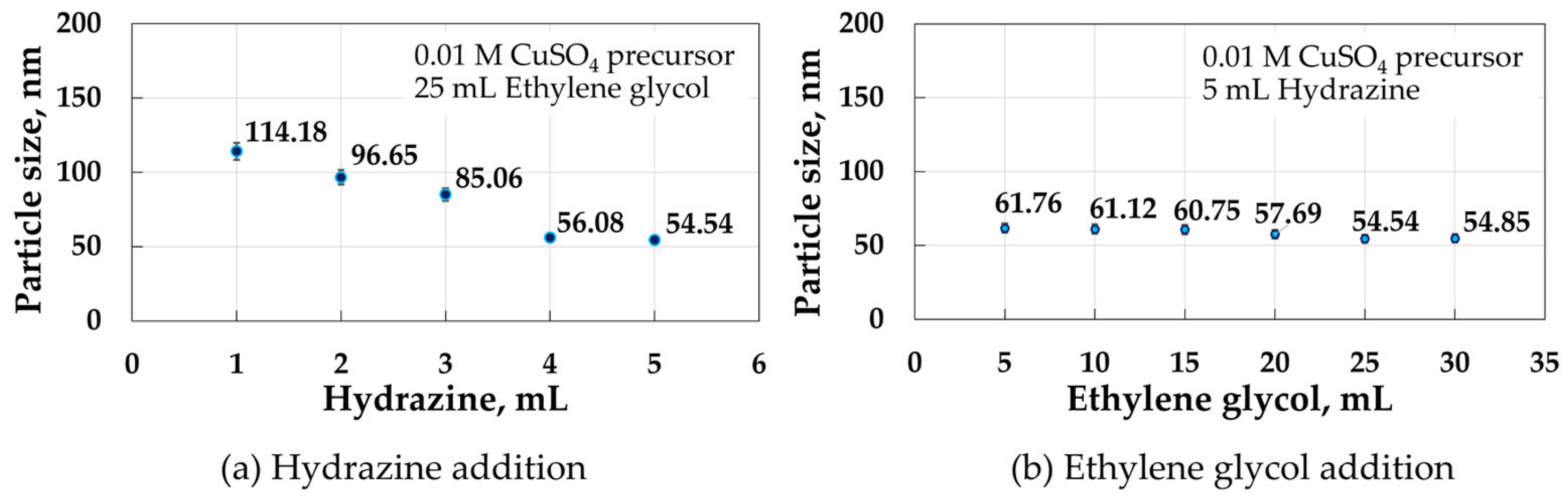
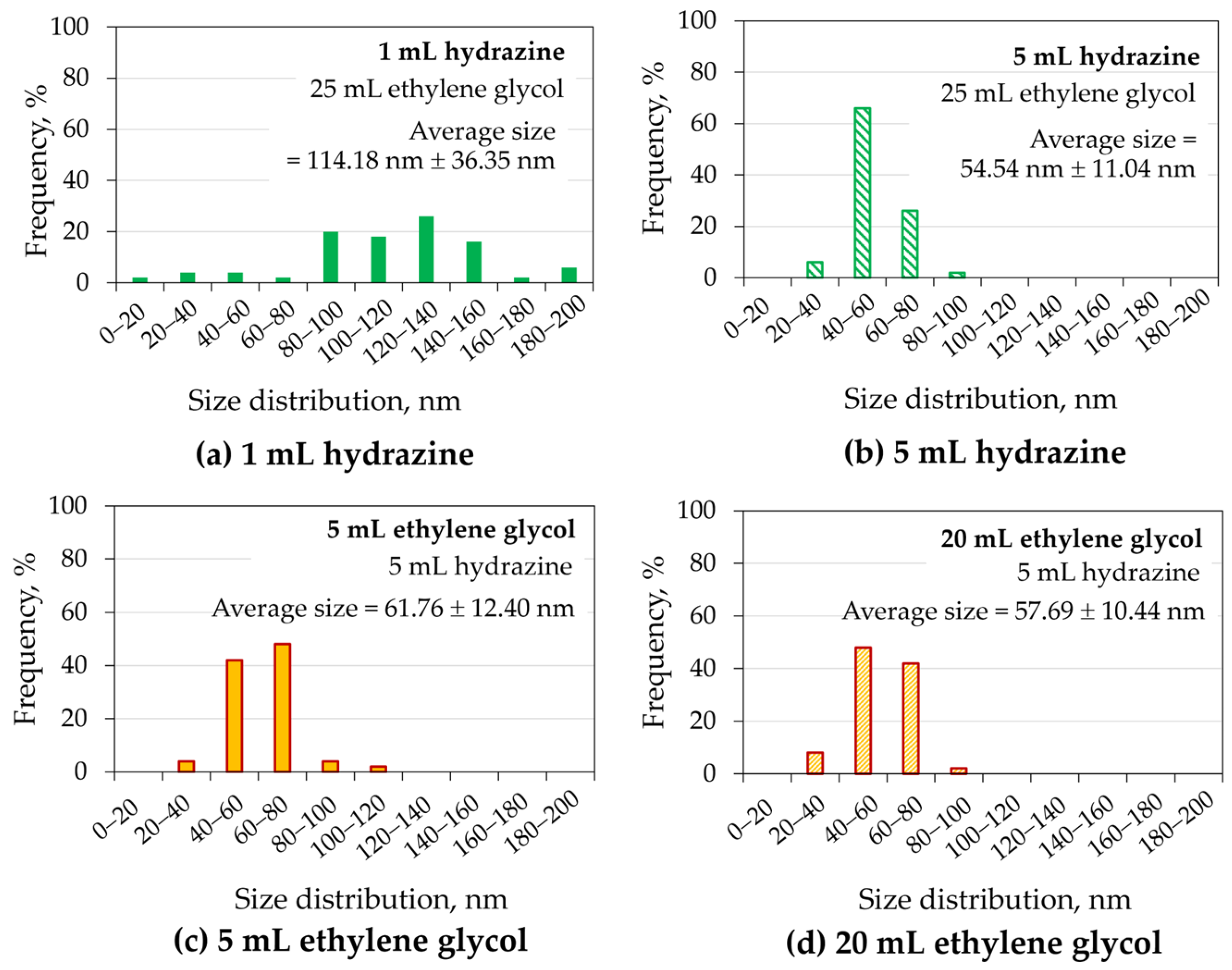
3.2.2. Effects of Hydrazine and Ethylene Glycol Additions
3.2.3. Effects of Precursor Concentrations
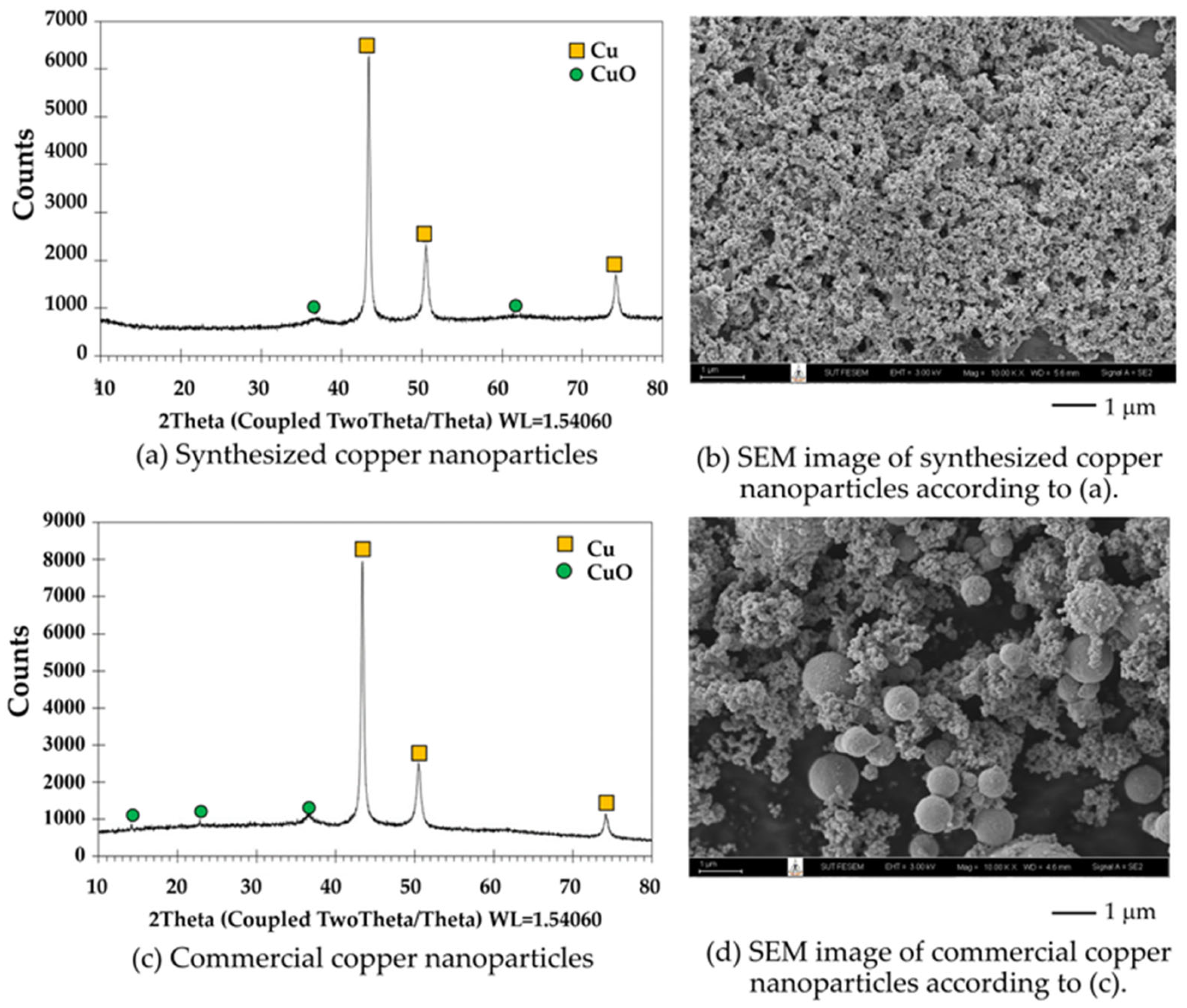
3.2.4. Phase and Composition of the Synthesized Copper Nanoparticles
3.3. Antibacterial Activity of the Synthesized Copper Nanoparticles
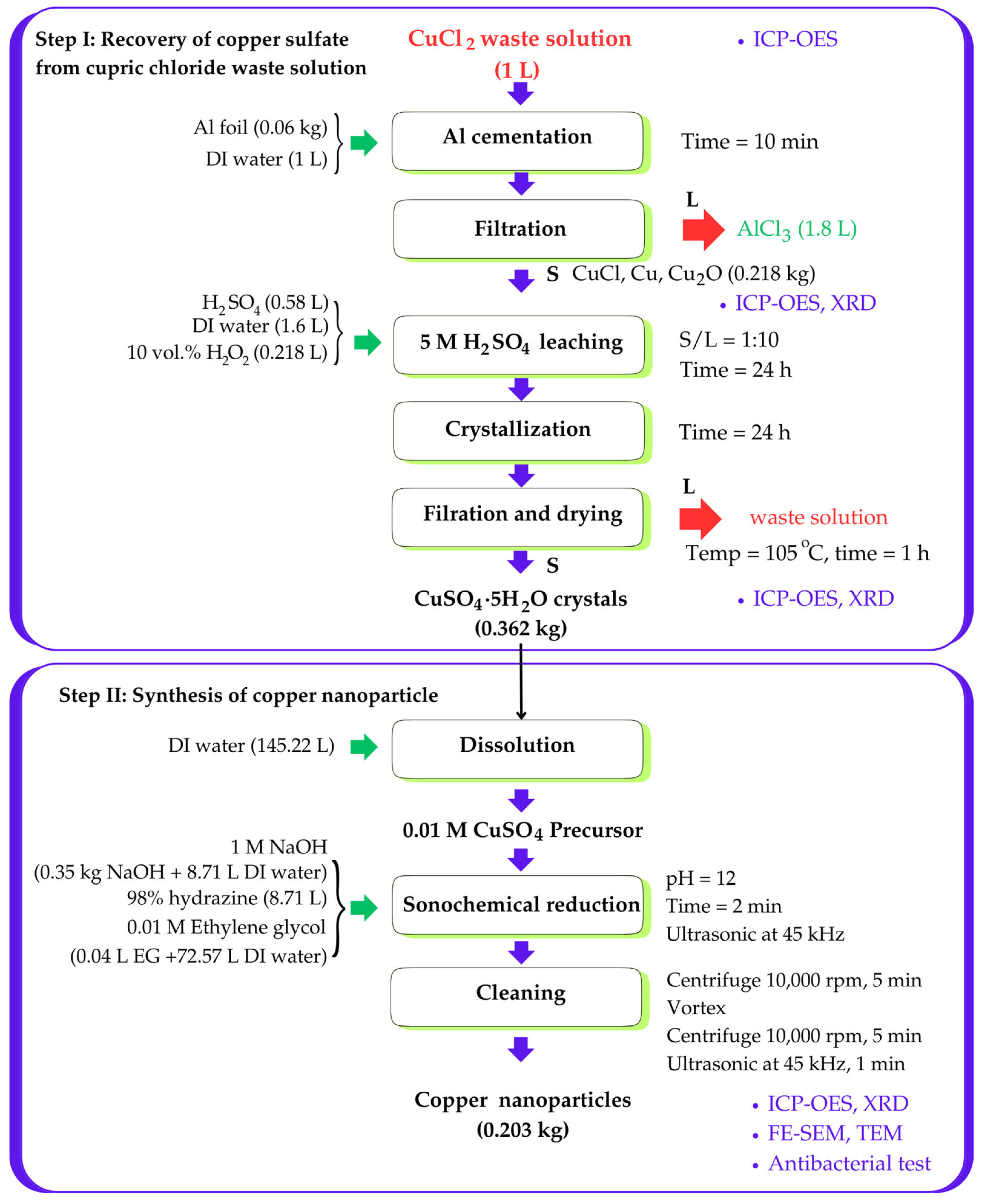
3.4. Process Flow and Material Balance of Copper Nanoparticles from Spent Copper Etching Solution
3.5. Chemical Reactions of Copper Nanoparticle Synthesis from Copper Etching Waste Solution
3.6. Nucleation and Growth Mechanisms of Copper Nanoparticle via Sonochemical Synthesis
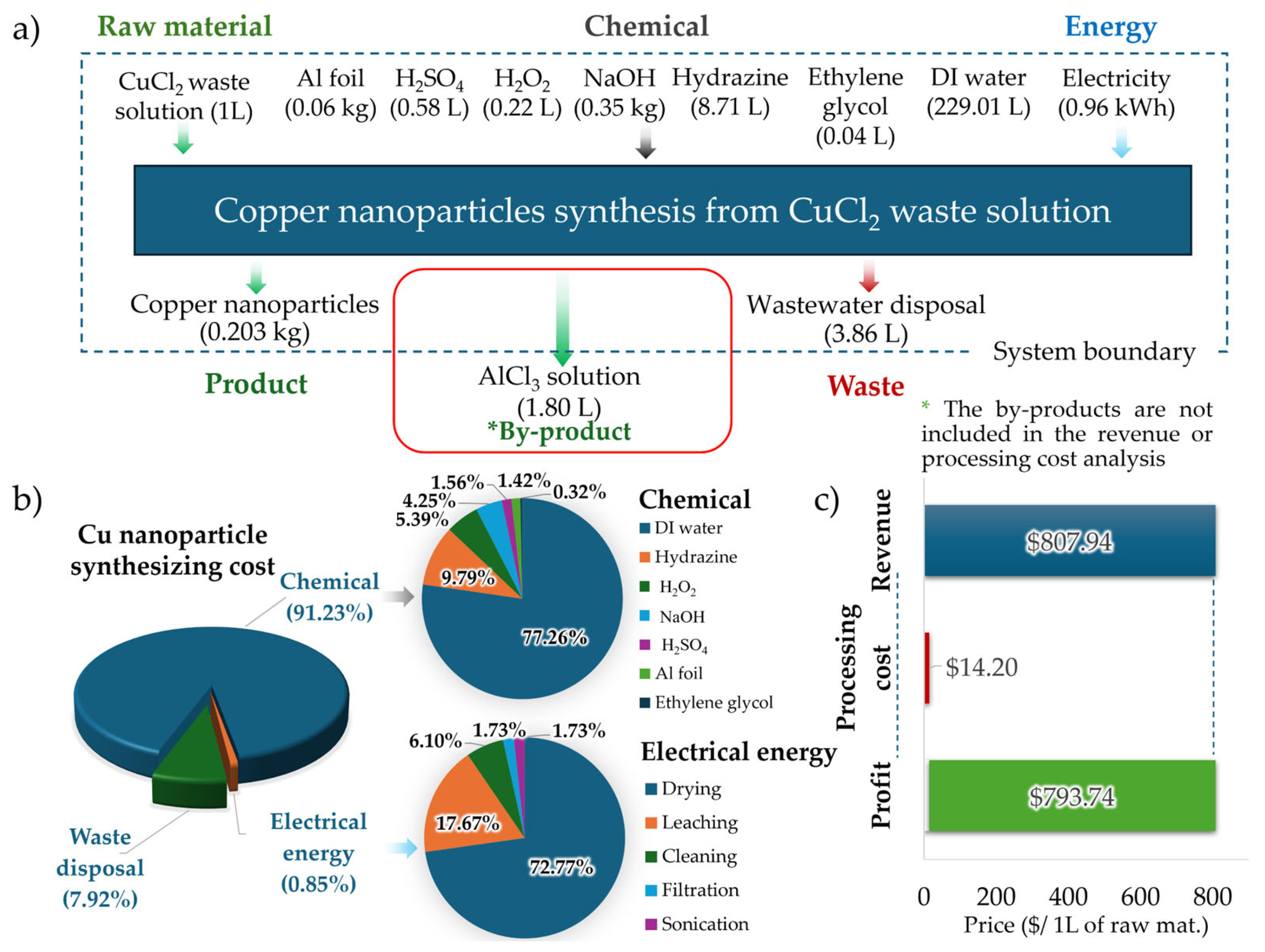
3.7. Feasibility Studies of Copper Nanoparticle Synthesis from Copper Etching Waste Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Business Research Company. Printed Circuit Board Global Markets Report 2025; The Business Research Company: London, UK, 2025; ID 5939373. [Google Scholar]
- Cakir, O. Copper etching with cupric chloride and regeneration of waste etchant. J. Mater. Process. Technol. 2006, 175, 63–68. [Google Scholar] [CrossRef]
- Keskitalo, T.; Tanskanen, J.; Kuokkanen, T. Analysis of key patents of the regeneration of acidic cupric chloride etchant waste and tin stripping waste. Resour. Conserv. Recycl. 2007, 49, 217–243. [Google Scholar] [CrossRef]
- United Nation. BASEL Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal, Protocol on Liability from Transboundary Movements of Hazardous Wastes and Their Disposal, Texts and Annexes; United Nation: New York, NY, USA, 2023. [Google Scholar]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Queneau, P.B.; Gruber, R.W. The U.S. production of copper chemicals from secondary and by-product sources. JOM 1997, 49, 34–37. [Google Scholar] [CrossRef]
- Zhao, G.; Richardson, H.W. Regeneration of Cupric Etchants and Recovery of Copper Sulfate. U.S. Patent No.20060196761 A1, 7 September 2006. [Google Scholar]
- Hung, Y.P.; Mohamed, N.; Darus, H. Recovery of copper from strong chloride-based solution. J. Appl. Sci. 2005, 5, 1328–1333. [Google Scholar] [CrossRef][Green Version]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Zhu, H.T.; Zhang, C.Y.; Yin, Y.S. Rapid synthesis of copper nanoparticles by sodium hypophosphite reduction in ethylene glycol under microwave irradiation. J. Cryst. Growth 2004, 70, 722–728. [Google Scholar] [CrossRef]
- Moghimi-Rad, J.; Zabihi, F.; Hadi, I.; Ebrahimi, S.; Isfahani, T.D.; Sabbaghzadeh, J. Effect of ultrasound radiation on the size and size distribution of synthesized copper particles. J. Mater. Sci. 2010, 45, 3804–3811. [Google Scholar] [CrossRef]
- Tang, A.; Wang, J. Electrochemical preparation of copper nanoparticles in an oscillatory belousov–zhabotinsky medium. J. Phys. Chem. 2022, 126, 11103–11110. [Google Scholar] [CrossRef]
- Ahmadi, H.; Khalaj, G.; Pourabdollah, M.; Zojaji, M.; Mahmoudan, M. Effect of electrode material, electrolyte composition, and surfactant on electrochemical synthesis of copper nanoparticles. J. Solid State Electrochem. 2024, 28, 2885–2897. [Google Scholar] [CrossRef]
- Togashi, T.; Nakayama, M.; Hashimoto, A.; Ishizaki, M.; Kanaizuka, K.; Kurihara, M. Solvent-free synthesis of monodisperse Cu nanoparticles by thermal decomposition of an oleylamine-coordinated Cu oxalate complex. Dalton Trans. 2018, 47, 5342–5347. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, F.B.; Sulca, M.A.; Machini, M.T.; Couto, R.A.; Kiyohara, P.K.; Machado, G.; Rossi, L.M. Copper nanoparticles synthesized by thermal decomposition in liquid phase: The influence of capping ligands on the synthesis and bactericidal activity. J. Nanopart. Res. 2014, 16, 2588. [Google Scholar] [CrossRef]
- Dhas, N.A.; Raj, C.P.; Gedanken, A. Synthesis, characterization, and properties of metallic copper nanoparticles. Chem. Mater. 1998, 10, 1446–1452. [Google Scholar] [CrossRef]
- Cheirmadurai, K.; Biswas, S.; Murali, R.; Thanikaivelan, P. Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv. 2014, 4, 19507–19511. [Google Scholar] [CrossRef]
- Mott, D.; Galkowski, J.; Wang, L.; Luo, J.; Zhong, C.-J. Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 2007, 23, 5740–5745. [Google Scholar] [CrossRef]
- Qi, L.; Ma, J.; Shen, J. Synthesis of copper nanoparticles in nonionic water-in-oil microemulsions. J. Colloid Interface Sci. 1997, 186, 498–500. [Google Scholar] [CrossRef]
- Song, X.; Sun, S.; Zhang, W.; Yin, Z. A method for the synthesis of spherical copper nanoparticles in the organic phase. J. Colloid Interface Sci. 2004, 273, 463–469. [Google Scholar] [CrossRef]
- Kanninen, P.; Johans, C.; Merta, J.; Kontturi, K. Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid Interface Sci. 2008, 318, 88–95. [Google Scholar] [CrossRef]
- Khanna, P.K.; More, P.; Jawalkar, J.; Patil, Y.; Rao, N.K. Synthesis of hydrophilic copper nanoparticles: Effect of reaction temperature. J. Nanopart. Res. 2008, 11, 793–799. [Google Scholar] [CrossRef]
- Lu, R.; Hao, W.; Kong, L.; Zhao, K.; Baic, H.; Liu, Z. A simple method for the synthesis of copper nanoparticles from metastable intermediates. RSC Adv. 2023, 13, 14361–14369. [Google Scholar] [CrossRef]
- Cerchier, P.; Dabalà, M.; Brunelli, K. Green synthesis of copper nanoparticles with ultrasound assistance. Green Process Synth. 2017, 6, 311–316. [Google Scholar] [CrossRef]
- Panda, R.; Pant, K.K.; Bhaskar, T.; Naik, S.N. Dissolution of brominated epoxy resin for environment friendly recovery of copper as cupric oxide nanoparticles from waste printed circuit boards using ammonium chloride roasting. J. Clean. Prod. 2021, 291, 125928. [Google Scholar] [CrossRef]
- Wang, T.; Berrill, P.; Zimmerman, J.B.; Hertwich, E.G. Copper recycling flow model for the United States economy: Impact of scrap quality on potential energy benefit. Environ. Sci. Technol. 2021, 55, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burn. Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef]
- Sandoval, C.; Ríos, G.; Sepúlveda, N.; Salvo, J.; Souza-Mello, V.; Farías, J. Effectiveness of copper nanoparticles in wound healing process using in vivo and in vitro studies: A systematic review. Pharmaceutics 2022, 14, 1838. [Google Scholar] [CrossRef]
- Lemus, M.; González, C.; Quilaqueo, M.; García, A.; Hassan, N.; Estay, H. Copper nanoparticles production by a novel non-dispersive membrane nanoprecipitation process. J. Mater. Res. Technol. 2024, 28, 2962–2968. [Google Scholar] [CrossRef]
- Shïrïnzadeh, H. Antimicrobial activities of naphthalenylmethylen hydrazine derivatives. J. Sci. Technol. 2021, 14, 464–471. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Ye, T.; Ye, Y. Method and Apparatus for Recycling Acidic Copper Chloride Etching Waste Solution by Using Composite Electrolytic Cell. Patent No. WO2024/179517, 28 February 2024. [Google Scholar]
- Ahmadi, T.S.; Wang, Z.L.; Green, T.C.; Henglein, A.; El-Sayed, M.A. Shape-controlled synthesis of colloidal platinum nanoparticles. Science 1996, 272, 1924–1926. [Google Scholar] [CrossRef]
- Alam, M.A.; Tabassum, M.; Mostofa, S.; Bishwas, R.K.; Sarkar, D.; Jahan, S.A. The effect of precursor concentration on the crystallinity synchronization of synthesized copper nanoparticles. J. Cryst. Growth 2023, 621, 127386. [Google Scholar] [CrossRef]
- Lai, M.-J.; Huang, Y.-W.; Chen, H.-C.; Tsao, L.-I.; Chien, C.-F.C.; Singh, B.; Liu, B.R. Effect of size and concentration of copper nanoparticles on the antimicrobial activity in Escherichia coli through multiple mechanisms. Nanomaterials 2022, 12, 3715. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, T.; Zamani, A.; Froushani, S.M.A. Preparation of Cu nanoparticles fixed on cellulosic walnut shell material and investigation of its antibacterial, antioxidant and anticancer effects. Heliyon 2020, 6, e03528. [Google Scholar] [CrossRef]
- Galal, G.F.; Abd-Elhalim, B.T.; Abou-Taleb, K.A.; Haroun, A.A.; Gamal, R.F. Toxicity assessment of green synthesized Cu nanoparticles by cell-free extract of Pseudomonas silesiensis as antitumor cancer and antimicrobial. Ann. Agric. Sci. 2021, 66, 8–15. [Google Scholar] [CrossRef]
- Laha, D.; Pramanik, A.; Laskar, A.; Jana, M.; Pramanik, P.; Karmakar, P. Shape-dependent bactericidal activity of copper oxide nanoparticle mediated by DNA and membrane damage. Mater. Res. Bull. 2014, 59, 185–191. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Shen, Y. Synthesis and speciation of polyaluminum chloride for water treatment. Environ. Int. 1998, 24, 899–910. [Google Scholar] [CrossRef]
- Sulcius, A.; Griskonis, E.; Zmuidzinaviciene, N. Copper dissolution in concentrated sulfuric acid. World J. Chem. Educ. 2019, 7, 196–202. [Google Scholar] [CrossRef]
- Park, I.; Yoo, K.; Alorro, R.D.; Kim, M.; Kim, S. Leaching of copper from cuprous oxide in aerated sulfuric acid. Mater. Trans. 2017, 58, 1500–1504. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.F.; Chen, Y.B.; Wu, L.M. Hydrazine reduction of metal ions to porous submicro-structures of Ag, Pd, Cu, Ni, and Bi. J. Solid State Chem. 2012, 191, 19–26. [Google Scholar] [CrossRef]
- Chen, J.P.; Lim, L.L. Key factors in chemical reduction by hydrazine for recovery of precious metals. Chemosphere 2002, 49, 363–370. [Google Scholar] [CrossRef]
- Audrieth, I.F.; Ogg, B.A. The Chemistry of Hydrazine; John Wiley and Sons: New York, NY, USA, 1951. [Google Scholar]
- Begletsova, N.N.; Shinkarenko, O.A.; Selifonova, E.I.; Tsvetkova, O.Y.; Al-Alwani, A.J.K.; Zakharevich, A.M.; Chernova, R.K.; Glukhovskoy, E.G. Synthesis of copper nanoparticles using hydrazine in micellar solutions of sodium dodecyl sulfate. Mosc. Univ. Chem. Bull. 2019, 74, 14–19. [Google Scholar] [CrossRef]
- Salzemann, C.; Lisiecki, I.; Urban, J.; Pileni, M.-P. Anisotropic copper nanocrystals synthesized in a supersaturated medium: Nanocrystal growth. Langmuir 2004, 20, 11772–11777. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, A.P.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of copper nanoparticles in ethylene glycol by chemical reduction with vanadium (+2) salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- El-Berry, M.F.; Sadeek, S.A.; Abdalla, A.M.; Nassar, M.Y. Microwave-assisted fabrication of copper nanoparticles utilizing different counter ions: An efficient photocatalyst for photocatalytic degradation of safranin dye from aqueous media. Mater. Res. Bull. 2021, 133, 111048. [Google Scholar] [CrossRef]
- Abbasi-Kesbi, F.; Rashidi, A.M.; Astinchap, B. Preparation of ultrafine grained copper nanoparticles via immersion deposit method. Appl. Nanosci. 2018, 8, 221–230. [Google Scholar] [CrossRef]
- Astaraki, H.; Masoudpanah, S.; Alamolhoda, S. Effects of ethylene glycol contents on phase formation, magnetic properties and photocatalytic activity of CuFe2O4/Cu2O/Cu nanocomposite powders synthesized by solvothermal method. J. Mater. Res. Technol. 2021, 14, 229–241. [Google Scholar] [CrossRef]
- Handayani, W.; Ningrum, A.S.; Imawan, C. The role of pH in synthesis silver nanoparticles using Pometia Pinnata (Matoa) leaves extract as bioreductor. J. Phys. Conf. Ser. 2020, 1428, 012021. [Google Scholar] [CrossRef]
- Rajesh, K.M.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Synthesis of copper nanoparticles and role of pH on particle size control. Mater. Today Proc. 2016, 3, 1985–1991. [Google Scholar] [CrossRef]
- Din, M.I.; Arshad, F.; Rani, A.; Aihetasham, A.; Mukhtar, M.; Mehmood, H.A. Single step green synthesis of stable copper oxide nanoparticles as efficient photo catalyst material. J. Optoelectron. Biomed. Mater. 2017, 9, 41–48. [Google Scholar]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Ikeda, S.; Akamatsu, K.; Nawafune, H.; Nishino, T.; Deki, S. Formation and growth of copper nanoparticles from ion-doped precursor polyimide layers. J. Phys. Chem. B 2004, 108, 15599–15607. [Google Scholar] [CrossRef]
- Vaseema, M.; Leeb, K.M.; Kima, D.Y.; Hahna, Y.-B. Parametric study of cost-effective synthesis of crystalline copper nanoparticles and their crystallographic characterization. Mater. Chem. Phys. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Sharma, S.; Trivedi, R. Review on biogenic synthesis of copper nanoparticles and its potential applications. Inorg. Chem. Commun. 2023, 149, 110448. [Google Scholar] [CrossRef]
- Pathak, R.; Punetha, V.D.; Bhatt, S.; Punetha, M. A review on copper-based nanoparticles as a catalyst: Synthesis and applications in coupling reactions. J. Mater. Sci. 2024, 59, 6169–6205. [Google Scholar] [CrossRef]
- Wang, Z.; Susha, A.S.; Chen, B.; Reckmeier, C.; Tomanec, O.; Zboril, R.; Zhong, H.; Rogach, A.L. Poly(vinylpyrrolidone) supported copper nanoclusters: Glutathione enhanced blue photoluminescence for application in phosphor converted light emitting devices. Nanoscale 2016, 8, 7197–7202. [Google Scholar] [CrossRef]
- Tahir, H.; Saad, M.; Latif, M.; Hyder, S.T.; Ahmed, R. Synthesis of metal oxide nano particles by sol-gel method and investigation of its biomedical applications. Nanochem. Res. 2024, 9, 19–27. [Google Scholar] [CrossRef]
- Patel, M.; Mishra, S.; Verma, R.; Shikha, D. Synthesis of ZnO and CuO nanoparticles via Sol gel method and its characterization by using various techniques. Discov. Mater. 2022, 2, 1. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Deep, A.; Szulejko, J.E.; Vellingiri, K.; Kumar, S.; Kwon, E.E.; Yun, S.-T. Recovery of nanomaterials from battery and electronic wastes: A new paradigm of environmental waste management. Renew. Sustain. Energy Rev. 2018, 82, 3694–3704. [Google Scholar] [CrossRef]
- Pavoski, G.; Martins, T.A.G.; Marinatto, Y.; Espinosa, D.C.R.; Tenório, J.A.S. Synthesis of metallic nanoparticles from the recovery of secondary sources: An opportunity within the circular economy process. JOM 2025, 77, 431–450. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Othmani, A.; Ghanei, J.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Pandey, A. Green route for recycling of low-cost waste resources for the biosynthesis of nanoparticles (NPs) and nanomaterials (NMs)-A review. Environ. Res. 2022, 207, 112202. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Holghoomi, R.; Shamsabadipour, A.; Maleki-baladi, R.; Rahdar, A.; Pandey, S. Copper nanoparticles from chemical, physical, and green synthesis to medicinal application: A review. Plant Nano Biol. 2024, 8, 100070. [Google Scholar] [CrossRef]





| Waste | Metal Concentration (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Sn | Ag | Fe | Ni | Mn | S | Na | |
| CuCl2 solution | 199.6 | n/a | n/a | n/a | n/a | n/a | n/a | 0.011 | 2.548 |
| Solution | Metal Concentration (g/L) in Solution | % Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Sn | Pb | Al | Fe | Na | Ni | S | ||
| Before | 99.80 | <limit | <limit | <limit | <limit | 0.013 | <limit | <limit | 99.95 * |
| After | 0.053 | <limit | <limit | 92.55 | <limit | 0.005 | <limit | <limit | |
| Solution | Metal Concentration (g/L) in Solution | % Recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Sn | Pb | Al | Fe | Na | Ni | S | ||
| Before | 55.010 | <limit | <limit | 1.840 | <limit | <limit | <limit | <limit | 94.76 ** |
| After | 2.830 | <limit | <limit | 0.167 | <limit | <limit | <limit | <limit | |
| Solution | Metal Concentration (g/L) in Solution | % Purity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sn | Pb | Ag | Fe | Ni | Na | Mn | Al | S | ||
| CuSO4·5H2O | <limit | <limit | <limit | <limit | <limit | <limit | <limit | <limit | <limit | >99.95 |
| Cu Nanoparticles (Cu NPs) | Hydrazine (mL) | pH | Metal Concentration (mg/L) | % Purity | |
|---|---|---|---|---|---|
| Na | S | ||||
| Commercial | - | - | <limit | <limit | >99.95 |
| 0.01 M CuSO4 precursor | 5 | 12 | <limit | <limit | >99.95 |
| 0.01 M CuSO4 precursor | 5 | 13 | <limit | <limit | >99.95 |
| 0.10 M CuSO4 precursor | 5 | 12 | <limit | <limit | >99.95 |
| 0.10 M CuSO4 precursor | 5 | 13 | <limit | <limit | >99.95 |
| 0.20 M CuSO4 precursor | 5 | 12 | <limit | <limit | >99.95 |
| 0.20 M CuSO4 precursor | 5 | 13 | <limit | <limit | >99.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patcharawit, T.; Kansomket, C.; Mahiwan, N.; Chailoi, S.; Chandakhiaw, T.; Yingnakorn, T.; Tunnukij, T.; Khumkoa, S. Upcycling of Cupric Chloride Waste Solution from PCB Manufacturing for Antibacterial Copper Nanoparticles. Recycling 2025, 10, 97. https://doi.org/10.3390/recycling10030097
Patcharawit T, Kansomket C, Mahiwan N, Chailoi S, Chandakhiaw T, Yingnakorn T, Tunnukij T, Khumkoa S. Upcycling of Cupric Chloride Waste Solution from PCB Manufacturing for Antibacterial Copper Nanoparticles. Recycling. 2025; 10(3):97. https://doi.org/10.3390/recycling10030097
Chicago/Turabian StylePatcharawit, Tapany, Chatisa Kansomket, Napat Mahiwan, Sumita Chailoi, Thanapon Chandakhiaw, Tanongsak Yingnakorn, Teerawut Tunnukij, and Sakhob Khumkoa. 2025. "Upcycling of Cupric Chloride Waste Solution from PCB Manufacturing for Antibacterial Copper Nanoparticles" Recycling 10, no. 3: 97. https://doi.org/10.3390/recycling10030097
APA StylePatcharawit, T., Kansomket, C., Mahiwan, N., Chailoi, S., Chandakhiaw, T., Yingnakorn, T., Tunnukij, T., & Khumkoa, S. (2025). Upcycling of Cupric Chloride Waste Solution from PCB Manufacturing for Antibacterial Copper Nanoparticles. Recycling, 10(3), 97. https://doi.org/10.3390/recycling10030097









