Abstract
The rapid consumption and disposal of electronic waste due to technological innovations and changes in living commodities are causing the development of a significant environmental challenge. Among the components of these wastes, spent printed circuit boards are particularly considered to be among the most valuable owing to their content of precious metals, such as gold first and potentially platinum, which may be available in a lower proportion. Effective methods as part of gold recovery strategies by industries and policymakers are developed and envisioned from economic and environmental perspectives. Currently, cyanidation dominates global gold production from e-waste due to its selectivity for gold. The high toxicity of cyanide, however, poses serious environmental issues, leading thiosulphate leaching to emerge as a non-toxic and promising alternative for gold extraction. Its industrial viability has been demonstrated by Barrick Gold Corporation at the Goldstrike site with the pretreatment of acidic or alkaline pressure oxidation. This review introduces bioleaching as a promising economic and environmentally friendly process for gold extraction. This review explores thiosulphate leaching of gold as an alternative to conventional cyanidation, with a particular focus on biothiosulphate production by adapted microorganisms. The factors that affect the pretreatment, chemical reaction mechanism, and design engineering are discussed. The consumption of thiosulphate was identified as one of the main challenges, restricting the reliability of the process. Various solutions for the reduction of its consumption and relevant process costs were discussed, with a particular examination from the engineering aspect of the process design and scalability to industrially relevant operating conditions by using bioreactors adapted to large pulp density loads of electrical waste.
1. Introduction
Nowadays, the increasing reliance of the modern lifestyle on electronic devices with relatively short replacement cycles has led to a substantial rise in the accumulation of electrical waste (e-waste) [1]. According to statistics by [2], 40 million tons of e-waste are produced annually, which makes up around 5% of global solid waste. In 2019, Asia produced 46.6% of the world’s total e-waste, with America ranking the second highest with 24.4% e-waste generation followed by Europe (22.4%), Africa (5.4%), and Oceania (1.3%) [3]. Ref. [4] reported the production of 52.2 Mt global e-waste in 2021, which is expected to exceed 74.7 Mt by 2030 and 120 Mt by 2050. Despite this rise, only a proportion of 10–15% of generated e-waste is currently recycled, and the rest is stored or deposited in landfills, often with reduced strategies for sustainable recovery of the metals they contain, leading to unprecedented contamination of the environment and risks of diseases such as cancer, kidney and heart malfunctions, and brain swelling [5]. As a result, landfilling is considered the worst option as an e-waste destination, and there is an urgent need to recover metals from e-waste in order to mitigate environmental contamination, promote the economic advantages as a secondary source of precious metals, and save the consumption of natural ores [6].
Among e-waste, printed circuit boards (PCBs) contribute to approximately 3% of the total e-waste weight, while we note that mobile phones and computer PCBs have been widely studied owing to their high disposal volumes and precious metal contents [7]. They are utilised as random-access memory motherboards and network interface cards to facilitate electrical and mechanical connections, and their compositions can vary depending on the technology, year of fabrication, and manufacturer brand; however, they are typically composed of a metallic portion (40%) and non-metallic components such as plastics (30%) and ceramics (30%) [8]. They consist of a lamination layer of copper-clad fiberglass reinforced with epoxy resin material and other essential components such as capacitors, resistors, microchips, and diodes. The hazardous components of PCBs include heavy metals (Pb, Hg, Cd, As, and Cr), polychlorinated biphenyls, polybrominated biphenyls, and epoxy resins, which pose environmental challenges. Generally, PCBs contain about 10–20% Cu, 7% Fe, 5% Al, 3% Sn, 1–3% Ni, 1.5% Pb, 25% organic compounds, and precious metals such as 200–3000 ppm Ag, 20–250 ppm Au, and 10–200 ppm Pt, which are used because of their favourable electrical conductivity, chemical stability, and resistance to oxidation, corrosion, and acids [9]. The worth of Au and Ag in discarded PCBs in 2022 was estimated at USD 54,514.97/kg–USD 65,714.28/kg and USD 592.22/kg–USD 866.13/kg, respectively. Although their total weight is less than 1% of PCBs, they are still precious and financially important [10].
Every year, around 17 million PCBs are discarded, which is equivalent to the disposal of nearly 0.5 million tons of waste PCBs. In computer PCBs, the concentrations of Cu and Au reach 20–40 times and 25–250 times, respectively, more than natural ores, have the potential to be recovered from e-waste with less energy consumption, and are seen as alternative sources to natural ores [11]. As an example, 210 kg of Cu and 1.5 kg of Au can be recovered from one metric ton of waste PCBs, while only around 5 g and 5.25 kg of Au and Cu can be recovered from relevant natural ores, respectively [3]. Thus, waste PCBs have drawn interest from industries and academia as a secondary metal resource to create efficient, sustainable, and economical metal recovery technologies [12]. Au is one of the first metals used by human civilisation, even before 3400 BC, and has captivated attention for its wide-ranging applications (i.e., jewellery, high-tech industries, chemical processes, and medical applications) [13,14]. According to the U.S. Geological Survey, China leads the world in Au production (420 tons), followed closely by Australia and Russia [15]. As a result, the growing demand for Au in different sectors and the depletion of natural resources make it essential to recover Au from alternative sources such as PCBs [14].
Pyrometallurgy and hydrometallurgy technologies are conventional methods that are still employed but face growing challenges due to issues linked to economic and environmental outlooks, including the emission of harmful gases, costs, energy requirements, and chemical contamination. Therefore, it is important to develop energy-efficient technologies, such as those that rely on bioprocessing technology, operating at moderate conditions in terms of chemical and energy consumption [16,17,18]. Bioleaching, as one of the bioprocess technologies, relies on the ability of microorganisms to produce essential leaching agents such as organic acids (i.e., oxalic acid, citric acid, malic acid, gluconic acid, succinic acid, and formic acid); amino acids (i.e., L-valine, glycine, DL-alanine, and L-histidine); biosurfactants such as rhamnolipids (Rhls) produced from the bacterium P. aeruginosa CVCM to remove 11% Fe and 25% Zn at low concentration (0.4 mg/mL) and 19% Fe and 52% Zn at high concentration (1 mg/mL) [19]; siderophores such as hydroxamate and catecholate mixed-type pyoverdine PyoPpC-3B produced by the bacterium P. putida PpF1 to selectively extract Zn and Mn from primary and secondary mineral residues [20]; chelating; and complexing agents, and it is becoming a promising technology for recycling e-waste because it is straightforward, requires less skilled labour, reduced capital and operating costs, and can be controlled under mild conditions. This process also reduces the costs of final disposal and residue treatment, creating an environmentally friendly waste stream while ensuring selective metal extraction [21]. Currently, about 15–25% of the world’s copper production, 5% of Au production, and smaller proportions of Co, Ni, U, and Zn are produced by the bacteria-assisted leaching method [22]. For over a century, cyanide has been used as a cost-effective and efficient agent for gold leaching. However, due to its high toxicity, thiosulphate has been proposed as a safer alternative. Past review papers have explored the principles of bioleaching, including the methods and mechanisms, types of e-waste and microorganisms, and their role in the extraction of heavy and precious metals [23,24]. This review presents an update on bioleaching methods for the extraction of Au from waste PCBs by revisiting the fundamental concepts and discussions involved in the most recent findings regarding the design methodologies and mechanisms, as well as industrial perspectives and applications, contributing as a starting point to future directions. Thiosulphate-based leaching and associated challenges, the importance of engineering design and scale-up in the bioleaching process, and its impact on cost reductions are discussed.
2. Preprocessing of PCBs for Bioleaching
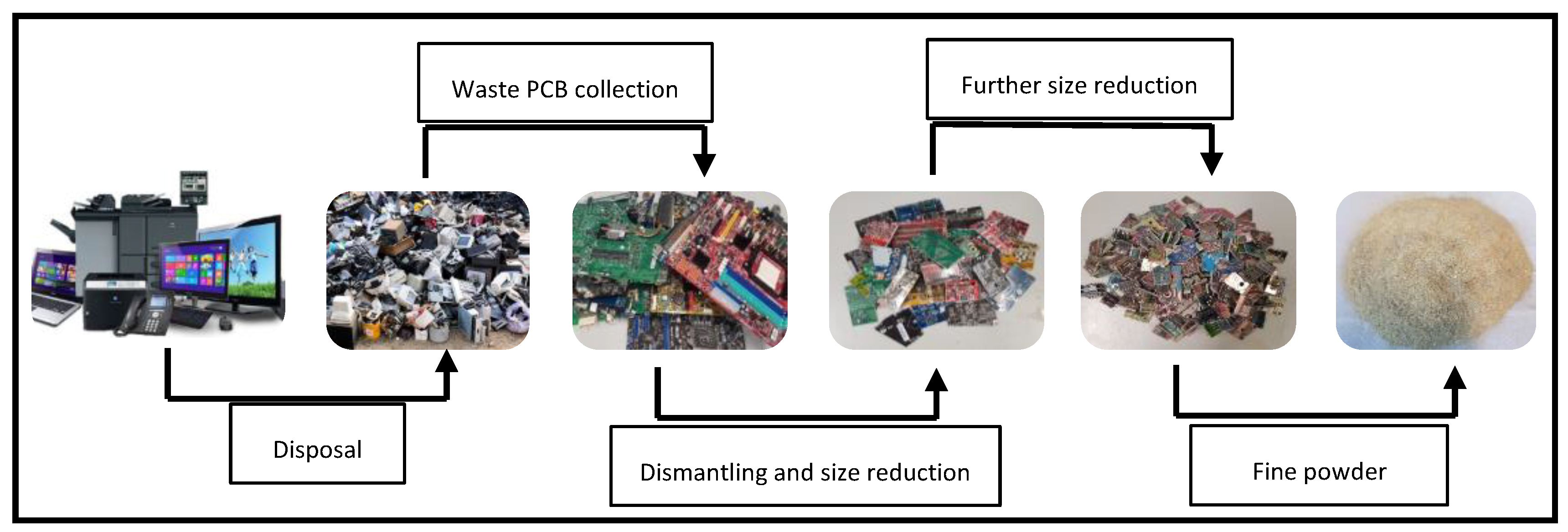
Typically, preprocessing is the initial step before recovering metals from waste PCBs. Dismantling various electronic components from PCBs is a crucial step in the recycling process. During dismantling, priority is given to the removal of reusable or hazardous components such as batteries and cathode ray tubes in order to make the recovery of metals easier [10]. Selective removal of hazardous components from PCBs helps prevent toxic elements from entering the recycling process. Manual dismantling, oven heating, and open burning are some common techniques for dismantling. However, some of these techniques are hazardous and might change the properties of waste PCBs and pose risks. For example, in the oven-heating technique, certain toxic substances may be generated as a result of the high melting point of lead-free solders (270–280 °C). The development of semi-automatic and automatic machineries to be used instead of manual dismantling methods is increasing the efficiency of the process and reducing the negative environmental impacts compared to oven heating, but the associated expenses remain high owing to the intricate geometries of PCBs of non-uniform structures, which highlights the application of manual dismantling. Ref. [25] estimated the costs for manual, mechanical, and heating dismantling techniques, as illustrated in Table 1. Manual dismantling proved to be the most economical option when the quantity of waste PCBs was below 1000 tons, but as the quantity was increased, mechanical dismantling and heating became more advantageous due to the high labour costs of manual dismantling. When the amount of waste PCBs exceeds 5000 tons, heating dismantling is considered the most cost-effective method [10,24]. A crushing stage is necessary to make the further handling of PCB waste easier. Size reduction follows and is performed by cutting PCBs into small pieces (1–2 cm2) by means of shredders or granulators. Further reduction of PCBs (5–10 mm) is achieved using ball mills, centrifugal mills, cutting mills, and ring mills [26]. The next step, which follows preprocessing, involves the recycling of waste materials in an economical and environmentally sustainable manner [24]. The production of fine dust during crushing and grinding poses a significant difficulty, and it can be challenging to control this process [27]. After removal of the hazardous components, different mineral processing unit operations, such as shredding, crushing, and grinding, can be used to liberate metals from cladding materials such as resin, fiberglass, and plastics. Various types of hammer crushers, rotary crushers, disc crushers, shredders, and cutters equipped with a bottom sieve are used for liberation. As PCBs are made of reinforced resin, copper wires, and glass fibres (multilayer), conventional crushers may not achieve good liberation. In contrast, shredding or cutting, which work on the principle of shearing, are found to be more useful. Unlike mineral ores, PCBs do not have a particular size fraction for liberation; instead, different types of elements are liberated at different size fractions. Figure 1 outlines the preprocessing steps involved in preparing PCBs for bioleaching.

Table 1.
Cost of dismantling methods [25].
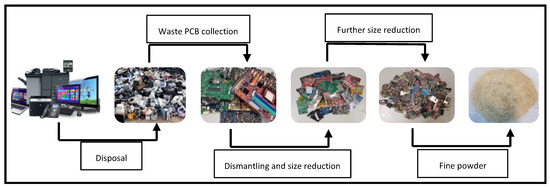
Figure 1.
Preprocessing of waste PCBs before bioleaching.
3. Bioleaching
3.1. Bacteria Used for Bioleaching and Mechanism
The primary difference in how autotrophic and heterotrophic microorganisms leach metals lies in their energy sources and metabolic activities. Autotrophic bacteria, commonly known as sulphur-oxidising and iron-oxidising bacteria, which are capable of growing in acidic conditions, are the most prevalent [28]. During the bioleaching process, these microorganisms facilitate the oxidation of ferrous ions to ferric ions and elemental sulphur to sulphuric acid (as shown in Equations (1) and (2)). Biogenic ferric iron and sulphuric acid act as oxidising agents (lixiviants) for the solubilisation of metals from e-wastes (Equations (3) and (4)) [29]. A. ferrooxidans and A. thiooxidans are the most used bacteria because they are resistant to contamination by other microorganisms, tolerate high concentrations of heavy metals, have a low nutrient requirement, and can grow under extremely acidic pH conditions [5]. These bacteria are able to capture electrons from substrates for their metabolic activities to release heat and solubilise metals without relying on external energy sources to facilitate the process [30]. Heterotrophic microorganisms, including fungi and bacteria, are extensively used in bioleaching due to their ability to metabolise organic compounds as energy sources and their adaptability to high pH levels and complex metal contents. Fungal species, such as Penicillium, Aspergillus, Trichoderma, Saccharomyces, and Phanerochaete, produce organic acids (i.e., citric acid, gluconic acid, oxalic acid, and formic acid) as leaching agents, which facilitate the mobilisation of metals from e-waste (Equation (5)). Additionally, these organic acids bind with metals to form stable complexes that significantly enhance metal solubility in the leaching solution [31,32]. There are three main mechanisms that occur between microorganisms and e-waste during bioleaching, namely, acidolysis (acid formation), redoxolysis (microbial-driven oxidation and reduction reactions), and complexolysis (biogenic complexing agents). In acidolysis, the oxygen atoms present in a metal oxide form interact with water molecules to facilitate the solubilisation of metals into a leaching solution. During acidolysis, a number of sulphur-oxidising autotrophs, such as A. thiooxidans, A. caldus, and S. thermosulfidooxidans, as well as heterotrophic bacteria species like Bacillus, Pseudomonas, and Chromobacterium and fungi including Aspergillus and Penicillium, consume nutrients to produce a range of acids, such as sulphuric, gluconic, acetic, malonic, oxalic, lactic, pyruvic, succinic, and formic acids, which facilitate metal solubilisation by maintaining low pH and reducing anion availability. Acidolysis is a fast and effective method commonly used to extract metals like Zn, Ni, and Cu from waste [18,33]. In the redoxolysis mechanism, the metals are dissolved via oxidation–reduction reactions. Through electron transfer, redoxolysis facilitates the supply of energy required for microbial growth [2]. Iron plays a major role as an electron carrier. After the microbial oxidation of Fe2+, Fe3+ acts as an oxidising agent that is able to solubilise metals such as Cu into Cu2+ and then can be chemically reduced to Fe2+ through redox reactions. Subsequently, Fe2+ is reoxidised to Fe3+ by the metabolic activity of the microorganisms [34]. Fe3+ ion is recognised to be an affordable oxidising agent in hydrometallurgical processes and has been proven to be a promising choice for the extraction of various metals from PCBs. Commercial-scale bioleaching commonly uses a combination of redoxolysis by biogenic Fe3+ and acidolysis by biogenic sulphuric acid for metal recovery of e-waste. In complexolysis, the target metals interact with ligands like cyanide, organic acids, or siderophores to form stable metal–ligand complexes. Complexolysis is used to leach metals such as Au, Ag, Fe, Al, and Pt by heterotrophic bacteria and fungi like C. violaceum, P. fluorescens, B. megaterium, P. aeruginosa, A. niger, etc. Common examples of these metal–ligand complexes include citric acid with Mg, oxalic acid with Al and Fe, and cyanide with Au, Ag, and Pt [35].
3.2. Bioleaching Methods
The bioleaching process is carried out through direct and indirect methods. The direct method is classified into one-step and two-step methods, while the indirect method involves the spent medium [36]. The one-step method requires the addition of samples of e-waste and microorganisms directly into a sterile culture medium at the same time, which allows the bacteria to grow in the presence of waste. The intricate composition of e-waste and the presence of toxic metals, however, may hinder microbial activity during the leaching process, decreasing the bioleaching efficiency. A study, for instance, showed that in the one-step bioleaching method, the growth of Frankia spp. decreased as the concentration of waste PCBs increased due to the lack of secondary metabolites and the decreased toxic effects of e-waste [37]. In the two-step method, on the other hand, the microorganisms grow first without any addition of e-waste. In fact, microorganisms are cultivated in a culture medium under adapted environmental conditions suitable for their activity until they reach the logarithmic growth stage [33]. E-waste samples are then introduced into the microbial culture medium. In contrast to the one-step method, the two-step method can notably mitigate the inhibition of microbial growth caused by e-waste and enhance the bioleaching efficiency. Since the microorganisms are still present in the two-step method, the toxicity of e-waste may partially affect the microbial growth and constrain the flexibility of the process compared to the spent-medium method [34]. In the indirect leaching method, the microorganisms are cultivated until they reach the growth phase and produce their metabolites. After the metabolite production, the bacteria are filtered by centrifugation and removed from the culture medium to obtain a cell-free medium, while the e-waste samples are added to the spent medium. This method eliminates the harmful effects of e-waste on microorganisms and provides better control throughout operation conditions [35]. Unlike the one-step and two-step methods, the spent-medium method enables an independent optimisation of the biological and chemical processes, such as shortening the leaching process and increasing the temperature and rotation speed free of the shear limitation on bacteria, which enhances the mass transfer rate and metal recovery [36]. For example, in a study by [32], fungal leaching of metals from a spent lithium-ion phone mobile battery by A. niger was conducted under one-step, two-step, and spent-medium methods, and the maximum leaching efficiency of Cu (100%), Li (95%), Mn (70%), Al (65%), Co (45%), and Ni (38%) was obtained at a pulp density of 1% in the spent-medium method. Another study reported that increasing the pulp density led to a decline in the bioleaching efficiency of Au to 11.3% by the bacterium C. violaceum at 0.5% pulp density when using the two-step method compared to 18% by the spent-medium method [38].
3.3. Effective Factors During the Bioleaching Process
Many studies have shown that factors such as pH, temperature, pulp density, microorganism type, nutrient, and aeration affect bioleaching efficiency. Maintaining an optimal pH in the culture medium is crucial for enhancing microbial activity and metal solubilisation during bioleaching. The pH influences both the effectiveness of metal leaching and the stability of metal ions in solutions. Acidophilic bacteria perform best at a pH level 2.0–2.5. A. ferrooxidans can tolerate pH levels below 2.0, but higher pH levels (above 2.5) reduce the bioleaching efficiency due to the precipitation of the ferric iron as jarosite and the bacterial attachment to these compounds, which hinders their activity. Temperature plays a key role in bioleaching, as different microorganisms thrive at specific temperature ranges. Mesophilic acidophiles typically grow at a temperature in the range of 25–30 °C, while thermophiles are grown at 40–45 °C. Fungi generally grow at a temperature within a range of 25–35 °C, with A. niger showing optimal performance at 25 °C [18,35]. The microorganisms involved in the bioleaching process are typically aerobic, meaning they require oxygen for their metabolism and growth. In the laboratory, aeration is provided using a shaker incubator, with an agitation speed typically maintained between 120 and 145 rpm for bacterial cultures. An excessive agitation, however, can cause physical stress on the bacteria and negatively affect their activity [23,39]. The effectiveness of microbial leaching is significantly influenced by the composition of the culture medium, as it directly affects the metabolic activities of microorganisms. This medium includes both organic and inorganic nutrients that support microbial growth. For the cultivated cyanogenic bacteria used in leaching valuable metals from e-waste, a nutrient-rich medium containing organic components like peptone, yeast extract, glycine, and amino acids is typically employed. Acidophilic bacteria, however, are grown in a chemically defined medium that supplies essential nutrients such as ammonium sulphate, dipotassium hydrogen phosphate, magnesium sulphate, iron sulphate, and elemental sulphur [40]. The pulp density, which is defined as the weight ratio of a solid material (i.e., e-waste) to a liquid in a solution (i.e., leaching agent), is a key factor affecting the effectiveness of metal leaching. High pulp densities increase toxicity, reduce oxygen transfer, and inhibit microbial growth, leading to a reduced leaching efficiency. Although some microorganisms, like acidophilic bacteria, tolerate heavy metals, their activity decreases at high pulp densities due to the limited presence of oxygen and increased medium viscosity. Several studies identified 1% (w/v) as an optimal pulp density for e-wastes. For example, ref. [41] found that the consortium of C. violaceum, P. aeruginosa, and P. fluorescens were able to leach 69.3% Au from waste PCBs at a pulp density of 1%, but the leaching rate decreased to 20.28% when the pulp density was increased to 10%. Pulp density is also one of the important parameters that affects reactor design, culture media consumption, and operating cost. When the pulp density is, for instance, increased from 1% to 10%, the volume of the liquid phase and the size of the reactor decrease notably by 90%, leading to a substantial decline in the operation cost [2]. According to [42], there was a significant drop in operating costs when the pulp density was increased from 1% to 5% with pH adjustment, which led to an annual profit of AUD 2749 per ton of PCBs. In spent-medium bioleaching, the optimisation of operating parameters such as temperature, agitation speed, and pulp density are critical. Unlike the one-step and two-step methods, the absence of live microbial culture allows for broader flexibility in the parameter selection. In this context, maximising metal leaching efficiency becomes the primary focus independent of conditions required to sustain microbial viability. For example, high temperatures or agitation speeds may be used to accelerate reaction kinetics without concern for microbial tolerance, enabling greater adaptability and a potential for scale-up to industrial applications.
3.4. Progress in Culture Growth
The toxic metals present in e-waste can negatively impact microbial growth, metabolism, and survival during the leaching process. To maintain a sufficient and active microbial population in the solution, it is often necessary to use microorganisms that are resistant or adapted to these toxic conditions. Therefore, before starting bioleaching, the microorganisms should undergo a gradual adaptation process. This involves serial sub-culturing over time with a gradually increasing pulp density of the waste material, allowing microbes to increase their tolerance to the levels of metal toxicity [17,43]. For instance, Ref. [30] studied the bioleaching of base metals from PCBs using adapted A. ferrooxidans. The adaptation process involved stepwise increases in waste concentration from 1 to 15 g/L over 187 days in a flask, followed by further adaptation in a bubble column bioreactor with waste concentrations raised to 40 g/L over 44 days. Under optimal conditions with a solid content of 20 g/L, this approach resulted in 54% of Cu, 75% of Ni, and 55% of Fe recovered after 9 days. Although microorganisms have been widely used across various scientific and industrial fields, most research on bioleaching has focused on pure microbial cultures, which are often lacking in terms of adaptability, efficiency, and the ability to manage complex substrates. Mixed cultures, which consist of two or more microorganisms in a consortium of culture media, offer a more effective approach for bioleaching compared to pure cultures. Species like A. thiooxidans, A. ferrooxidans, A. caldus, L. ferrooxidans, and L. ferriphilum have been combined for the extraction of metals in bioleaching processes to form biofilms that generate microenvironments, enhancing leaching kinetics. Although the precise mechanisms that illustrate the interaction within these communities remain weakly known, the growing prevalence of mixed cultures in bioleaching is partly due to the presence of diverse metal ions in ores and soils, which cannot be effectively processed by pure cultures. The applications of mixed cultures of microorganisms in bioleaching demonstrated that these cultures can bioleach the concentrate more rapidly and extensively than pure cultures [44,45]. For example, in one study, Cu leaching in a mixed culture of A. ferrooxidans and A. thiooxidans was 10% higher than in pure cultures [46].
The use of genetically modified organisms (GMOs) has emerged as a promising approach to improving the efficiency of the bioleaching process. Through genetic engineering, microorganisms can be designed to tolerate and adapt to high metal concentrations, enhancing the metabolic activity under optimal conditions and significantly reducing the time needed for metal extraction. Recent advancements in genetic engineering, along with progress in DNA synthesis, sequencing, and integrated -omics technologies (such as genomics, proteomics, transcriptomics, and metabolomics), are providing new opportunities to develop high-performing engineered microorganisms suitable for enhanced leaching performance and resilience in harsh environments [40]. For example, genetic engineering has been successfully used to enhance arsenic bioleaching by A. ferrooxidans TFBk [47]. In another study, Tay et al. observed improved Au recovery using two genetically modified strains of C. violaceum, named pBAD and pTAC, with a leaching of 30% and 25% of Au, respectively, compared to only an 11% leaching rate by the non-modified microorganisms [48].
4. Precious Metal Leaching
4.1. Cyanide-Based Leaching
The recovery of precious metals like Au and Ag from PCBs has been given much attention, as they are utilised in different industries such as electronic industries owing to their stability and conductivity [49]. Ref. [50] reported a global demand of Au in electronic industries of 254 tons in 2015, which highlights the importance of the recovery of Au through sustainable methods. Cyanidation was introduced by John Stewart MacArthur in the 1880s, and since then, it has been the primary method for Au leaching due to its efficiency for the selective recovery of Au [51]. Cyanide refers to inorganic compounds containing a cyano group (C-N), which are highly toxic, particularly at temperatures above 25.6 °C. The total reaction of precious metal dissolution by cyanide is represented in Equation (6). Although cyanide has been the main leaching agent for Au extraction, it has some drawbacks, such as absence of possible solutions for cyanide regeneration as well as environmental concerns owing to cyanide leakage into groundwater and the overall management of hazards associated with its toxic nature [52]. According to statistics by [53], cyanide leakage from metallurgical plants caused some serious accidents. For instance, in 1995 in Guyana, a tailings dam at the Omai mine collapsed and discharged approximately 2.9 million m3 of cyanide-laced tailings, which contaminated the nearby Omai River. Another accident happened in 2000 in Baia Mare, Romania, when the collapse of the Aurul S.A. tailings dam released a swage containing up to 100 tons of cyanide, which finally flowed into the Danube River. Gold leaching with a biocyanide solution has emerged as a cost-effective, simple, and efficient alternative to traditional cyanidation methods. Recent studies have shown that bacteria such as C. violaceum, Pseudomonas species, E. coli, and B. megaterium can produce biological cyanide. But still, the high consumption of cyanide by Cu poses challenges. To address this issue, certain autotrophic bacteria have been employed to target base metals and remove them selectively in the first step in order to facilitate the leaching of precious metals [35]. Several studies have reported high Au extraction rates after Cu removal. For instance, in the study by [54], 98.4% of Cu was extracted by A. ferrivorans and A. thiooxidans under acidic condition (pH 1–1.6) and room temperature after 7 days. In the second step, P. putida was employed, and 44% Au was solubilised under alkaline condition (pH 7.3–8.6) after 2 days. In another study, PCBs were pretreated with biooxidation using A. ferrooxidans, and over 80% of Cu removal enhanced the Au/Cu ratio in the residual PCBs. Before the pretreatment, the Au leaching rate was only 20.8%, which doubled to 40.1% after the treatment [55]. Table 2 summarises some previous studies on the bioleaching of precious metals from e-waste by different microorganisms. Cyanide-producing microorganisms are the main source for the selective leaching of Au and Ag from waste PCBs.

Table 2.
Overview of the previous studies on bioleaching of precious metals under different conditions.
4.2. Thiosulphate-Based Leaching
Thiosulphate (S2O32−), a sulphur oxyanion with a tetrahedral structure, is considered among the most promising alternatives to cyanide, as it has numerous benefits compared to various cyanide and non-cyanide leaching agents, including low corrosivity, stability, rapid leaching kinetics, operability in a safe workplace, and lower risk of environmental pollution [61]. Because of its efficiency, thiosulphate is recognised as a highly effective reagent for extracting Au from waste PCBs [51]. The first report on the use of thiosulphate in precious metal recovery dates back to 1905, when Au was extracted from ores by employing ammonia–thiosulphate leaching [62]. The overall reaction for the thiosulphate leaching of Au is represented by Equation (7). After the formation of the Au(I)–thiosulphate complex, a slightly acidic to highly alkaline condition is needed to prevent the decomposition of the complex and ensure its stability in solution [14]. Thiosulphate-based leaching requires an oxidant to facilitate Au solubilisation. Cu2+ is one of the prevalent oxidants, which accelerates Au dissolution by 17–20 times. The high redox potential of Cu2+/Cu+, however, results in high thiosulphate consumption, which is often moderated by the presence of ammonia to form a cupric ammonia complex (Cu (NH3)42+) as a stable catalyst to weaken the interaction between Cu2+ and thiosulphate as well as to reduce copper hydroxide precipitation [63,64,65]. Additionally, ammonia has a non-negligible role in reducing the formation of a passivation layer on the Au surface and enhancing the leaching kinetics [66].
4.2.1. Thiosulphate Leaching Methods
Ammonia-Based Method
There are two main methods of thiosulphate leaching: non-ammonia- and ammonia-based methods. The ammonia-based methods consist of copper ammonia, nickel ammonia, and cobalt ammonia leaching. Ammonia accelerates the dissolution of Au, stabilises thiosulphate, and maintains the pH level [64]. In an ammonia-based thiosulphate system, Cu2+ acts as a redox mediator to facilitate Au oxidation through both anodic and cathodic reactions [15]. In the anodic area (Equations (8)–(10)), NH3 moves toward the Au surface and forms a complex with Au+ (Au(NH3)2+). NH3 is then replaced by S2O32−, and a more stable complex is formed (Au(S2O3)23−). Although Au can form different complexes with ammonia and thiosulphate, Au(S2O3)23− is the most stable complex in an ammonia-based system. In the cathodic area, Cu(NH3)42+ is reduced to Cu(S2O3)35− and rapidly oxidised back to Cu(NH3)42+ by dissolved oxygen (Equations (11) and (12)). The values of associated Gibbs free energy offer an overview of the level of spontaneous reactions occurring with reference to the chemical equilibrium. While most values support the forward path, the spontaneous solubilisation of the metal gold, for instance (Equation (8)), is not favoured. In one study, around 98% of Au was solubilised from 1% (w/v) PCBs by using ammonium thiosulphate as the leaching agent [55]. In another study, 1 M ammonium thiosulphate was utilised to extract around 91% Au from 1% (w/v) PCBs after 24 h [67]. The authors of [68] compared thiosulphate and thiourea for Au dissolution and reported that approximately 70% of Au was dissolved from PCBs with different concentrations of ammonium thiosulphate (0.08 to 0.12 M) at 20 °C and a pH of 10.5, while only 40% dissolved Au was achieved with thiourea. Table 3 summarises some key information on the ammonia-based thiosulphate leaching of Au reported in previous studies, noting the boundary conditions of typical operations (i.e., thiosulphate 0.1–1 M, ammonia 0.1–4 M, and Cu2+ < 0.1 M, with leaching rates ranging from 15% to 99%). Despite the substantial number of lab-scale experiments and advances in understanding the chemical mechanisms and fundamental principles, the development toward an ammonia-based method has been slow, partly due to the high volatility of ammonia and the environmental issues related to the leakage of ammonia during storage or transportation [62]. Table 4 summarises the advantages and disadvantages of employing ammonia-based systems in thiosulphate leaching.

Table 3.
Ammonia-based thiosulphate leaching of Au under typical operating conditions.

Table 4.
The advantages and disadvantages of using ammonia in thiosulphate leaching systems [62,66].
Non-Ammonia Method
Many studies have employed thiosulphate leaching by eliminating ammonia. Alternative non-ammonia methods in thiosulphate leaching include copper thiosulphate, oxygen thiosulphate, ferric ethylenediaminetetraacetic acid (EDTA) thiosulphate, copper EDTA thiosulphate, and ferric oxalate thiosulphate to mitigate the issues associated with ammonia. Ref. [66] employed a copper–citrate–thiosulphate method for Au leaching from a refractory carbonaceous gold concentrate, and the findings demonstrated that substituting citrate with ammonia had a similar extraction capability but reduced the thiosulphate consumption from 0.045 M to 0.025 M. Ref. [76] observed that copper ion–ethanediamine–thiosulphate leaching was more effective than copper ion–ammonia–thiosulphate leaching of Au from gold ore. The use of cetyltrimethyl ammonium bromide (CTAB) was found to increase the extraction rate to 94.3% and to decrease thiosulphate consumption to 1.12 kg/t in a leaching system containing 0.1 M sodium thiosulphate, 0.06 M ethanediamine, 0.005 M Cu2+, and 1.5 kg/t of CTAB. In this process, CTAB broke down into [CTA+], attracted the negatively charged particles of [Au(S2O3)23−]·nH2O and [CTA+][AuBr2−]·nH2O, and formed ion pairs that facilitated the stabilisation of [Au(S2O3)23−]. Overall, ethylenediamine can form a more stable complex with Cu2+ than ammonia. The cupric–ethylenediamine complex is considered an interesting agent in reducing the catalytic oxidation ability of Cu2+ over the cupric/cuprous redox equilibrium potential, which in turn lowers the consumption of thiosulphate. In some of the literature, the effect of external factors on the leaching rate was assessed. For example, in one experiment, ultrasound was applied in a cobalt ammonia thiosulphate leaching system (0.2 M S2O32−, 0.03 M Co2+, 1 M NH3, 50 °C, and 750 W ultrasonic power). The results showed that the leaching rate was 8 times faster and the extraction yield with ultrasound reached 89%, which was found to be 25% higher than the one without ultrasound. Temperature, ultrasonic power, and the reduction in activation energy from 22.65 kJ/mol to 13.86 kJ/mol were the main reasons for the high leaching rate [13].
4.2.2. Challenges with the Thiosulphate-Based Leaching
The primary challenge associated with the use of thiosulphate is the high cost of reagent consumption and its impacts on the generated sulphate, tetrathionate, and trithionate [63]. Addressing this challenge requires the implementation of strategies, including the control of key factors of operations such as temperature, flow rate and mixing speed, oxygen level, Eh, pH, and the concentration of Cu2+ and ammonia. Depending on pH and Eh, unstable compounds such as tetrathionate, sulphite, and trithionate may form; therefore, controlling the Eh and pH is essential to minimise thiosulphate loss [77]. Using inorganic additives like phosphate, sulphate, and chloride and organic additives such as EDTA, humic acid (HA), polyamine, CDTA, ethylenediamine (EDA), triethanolamine (TEA), ammonium alcohol polyvinyl phosphate, citric acid, carboxymethyl cellulose (CMC), and amino acids can reduce thiosulphate consumption as they have the potential to compete with thiosulphate anions and form complexes with Cu2+ that decrease the interaction between Cu2+ and thiosulphate [78]. For example, in a copper–ammonia–thiosulphate leaching system, 1 L of leach solution was applied to 400 g of sulphide ore, with an initial gold concentration of 4.3 mg/kg. The addition of a low concentration of EDTA (2.0 mM) enhanced the efficiency of Au leaching to 100% and decreased the consumption of ammonium thiosulphate from 9.63 kg/t to 3.85 kg/t after 24 h [79]. Ref. [65] employed TEA as an additive for Au leaching in a thiosulphate–copper–ammonia system. The results demonstrated that the dissolution rate increased by 50% while thiosulphate consumption was reduced by around 10%. In a study conducted by [80], ethydiaminedhephen acetic (EDDHA) was employed as a non-toxic, environmentally friendly, and cost-effective organic additive to form a stable complex with Cu2+ owing to the numerous coordination sites of carboxylic acid and amine functional groups in it, which led to a stable Cu-EDDHA complex ion and the dissolution of 82.84% Au, which was 56.02% higher than in cyanide leaching. Moreover, EDDHA reduced the thiosulphate consumption from 103.19 kg/t to 10.54 kg/t. Ref. [81] investigated the effect of different additives on Au oxidation and revealed that potassium ethyl xanthate (KEX), imidazole, sulphinic acid, sodium di-ethyl dithiocarbamate, pyridine, thioglycolic acid, and mercaptobenzothiazole completely passivated the Au surface, while a small amount (5 mM) of thiourea and thioacetamide improved the oxidation of Au. Another study found that Au dissolution in a thiosulphate–oxygen–copper medium was only 2% over 24 h and increased to 95% in the presence of activated carbon as an additive [77]. In some experiments, Cu2+ was substituted by Co as an oxidising agent. According to [82], in ammoniacal thiosulphate solutions, Co(III)-NH3 complexes were able to extract around 80% of Au, while they reduced thiosulphate consumption by 44.2% thiosulphate. Table 5 summarises the effects of different additives on Au leaching and the consumption of thiosulphate. As seen, adding additives increased the dissolution of Au by approximately 20% and decreased the thiosulphate consumption by 30%.

Table 5.
Effects of additives on Au dissolution and thiosulphate consumption.
4.2.3. Biothiosulphate Produced by Microorganisms
Over the past two decades, efforts have been made to minimise the breakdown of thiosulphate during thiosulphate leaching of Au by using different types of additives and controlling the parameters affecting the process. It was discovered that producing thiosulphate by means of microorganisms (biothiosulphate) offers a potential pathway toward the development of a feasible, innovative, and sustainable metal recovery technology [51,85]. The benefits of using biogenic thiosulphate for Au leaching include, for instance, fewer toxic substances, less energy requirements associated with intermediate chemical productions, and scalability combined with e-waste recycling operations. Currently, an industrial thiosulphate leaching process is employed at Nevada Gold Mines that involves thermal treatment of sulphur and calcium hydroxide at 90 °C followed by oxidation of substances under pressure at 550 kPa. Therefore, developing a biogenic process in moderate conditions (e.g., ambient temperature and atmospheric pressure) can be recognised as an innovative alternative to reduce CAPEX and OPEX costs [86]. The two most common species that produce thiosulphate are cyanobacteria and proteobacteria. Table 6 summarises the ability of various microorganisms to produce thiosulphate under different environmental and substrate conditions [87]. The bacterium Microcoleus chtonoplastes produces thiosulphate (695 mg/L) using light, sulphide, and hydrogen and consumes carbonate as a carbon source at 22 °C and a pH around 8 [88]. Methylophaga sulfidovorans consumes methanol or dimethyl sulphide (DMS) as substrates to produce thiosulphate, as shown in Equation (13), and grows at a pH above 7.5 and a temperature of 22–30 °C. In the thiosulphate production by the bacterium Thiobacillus thioparus, sulphide, thiocyanate, elemental sulphur (S0), DMS, and polythionates can act as electron donors. Around 18.5% of thiosulphate production by this bacterium results from sulphide oxidation, which occurs under oxygen-limiting conditions and pH 7 [89]. Streptomyces fradiae produced up to 485 mg/L of Na2S2O3 as a main byproduct in a mineral medium at pH 7.5, which remained stable without degradation during 20 days of incubation [90]. Ref. [91] discovered that the addition of 1 mM sulphide to the medium containing recombinant E. coli resulted in the production of 0.45 mM sulphite, 0.2 mM thiosulphate, and 0.1 mM sulphur. Sulphurimonas sp. was observed to consume sulphide to produce 1 mM thiosulphate after intermediate production of elemental sulphur [89]. In a mixed culture with a neutral pH and temperature range of 20–32 °C, T. oxidans and T. neapolitanus produced sulphate and thiosulphate through sulphur oxidation. Sulphate was produced as a main product under atmospheric conditions and at pH 8.0; however, during oxygen-limited conditions, especially when the O2/S2 consumed ratio was around 0.5, thiosulphate formation (35 mg/L) occurred as a result of the increased sulphide–oxygen ratio [92].

Table 6.
Illustration of thiosulphate production by microorganisms [87].
Biogenic thiosulphate as an intermediate metabolite is extremely unstable under acidic conditions but can be stabilised through the adjustment of pH and presence of inhibitors [51]. For example, biogenic thiosulphate (500 mg/L) obtained from A. thiooxidans was able to leach 65% Au from 0.5% w/v of PCBs in the presence of 3.25 mg/L sodium azide (NaN3) as an inhibitor and 1 M ammonia at pH 6–7 after 36 h [93]. Ref. [94] investigated the thiosulphate leaching of Au from ore by the bacterium Methylophaga sulfidovorans using sodium sulphide as the substrate. The bacterium was able to produce thiosulphate in a rotating sealed flask of high-salinity medium at pH 7–8 and a temperature of 30 °C. A maximum of 61.9% of Au was leached in 24 h under conditions of 10% pulp density, air flow rate of 0.1 L/min, and 50 °C. The biothiosulphate approach was found to be a viable substitute for chemical thiosulphate, opening the door to further investigation of other thiosulphate-producing microorganisms from inexpensive substrates. This is considered a promising area in bioprocess engineering for metal recovery in an efficient and environmentally friendly way.
5. Engineering Perspective and Scaling up
The phase of scaling up the operation of the laboratory scale has been an important challenge in bioprocessing to achieve representative microbiological and technological enhancements and bring it as close as possible to industrial applications [22]. Currently, industrial-scale metal extraction is performed using methods such as dump bioleaching, heap bioleaching, and tank bioleaching. Among these, dump leaching is the oldest technique used in the industry, primarily for extraction of Cu, Ni, Zn, Au, and U from ores. In this method, a lixiviant generated by bacteria is sprayed onto ores piled in a dump, and the leachate solution is collected in ditches at the bottom of the dump, recovered, and then directed to an oxidation basin, where bacteria are regenerated for reuse in the process. This technique generally has low metal recovery efficiency and requires several days to complete the process. Moreover, there is a risk of leachate seeping into nearby water sources, with a potential risk of water pollution. Appropriate safety measures must therefore be undertaken to control the potential runoff [95]. Heap bioleaching is another method suitable for low-grade ores that are not economically viable for leaching through grinding and smelting and is particularly effective, for instance, for treating complex sulphide ores that have a porous structure [96,97]. Heap bioleaching involves stacking ores on an impermeable surface and applying leaching agents such as acidified solutions for Cu or cyanide solutions for Au over the top of the heap. The leaching occurs through microbial activity and mineral oxidation within the heap, and metal-rich solution is collected at the base and directed to a pond to recover metals [98]. Despite its technical maturity and widespread industrial application, it has drawbacks, such as yielding low leaching rates within a long processing time. Extending the leaching time increases the demand for manpower and material resources, which restricts further development of this technology [96]. Table 7 provides a summary of commercial-scale bioleaching operations for the recovery of Au and Cu from refractory gold concentrates, copper ores, and sulphidic ores across various global mining sites. The table shows details about the mine location, operator, year of operation commencement, production amount, and type of bioleaching technology employed.

Table 7.
Commercial-scale bioleaching operations for refractory gold concentrate, copper ores, or sulphide ores [99,100].
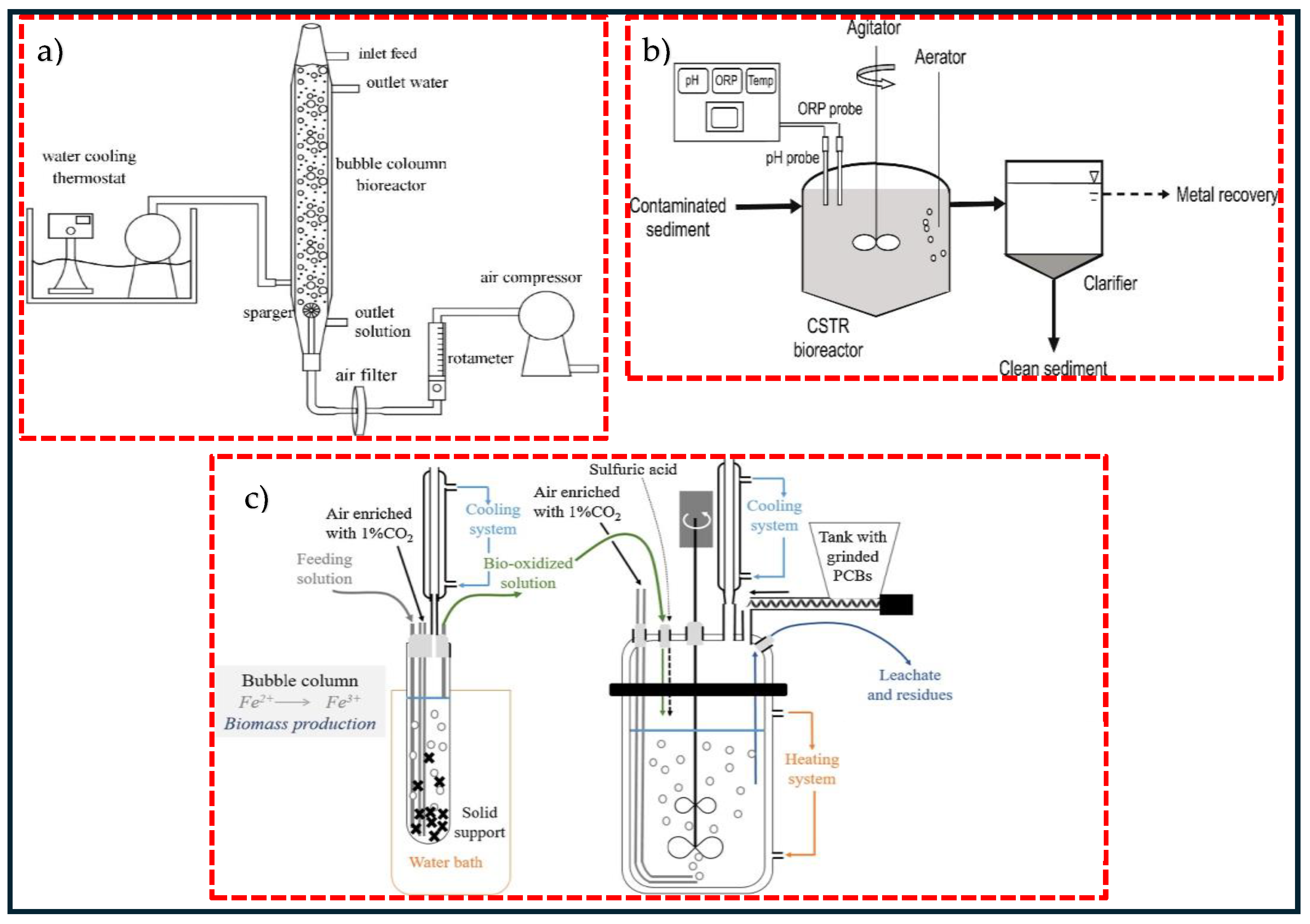
To enhance ore leaching efficiency, the process is initially performed in laboratory flasks prior to expanding to larger bioreactors. These bioreactors play a key role in process scale-up and are commonly operated in either batch or continuous modes. The development of an adapted bioreactor is the first step to move from an experimental scale to industrially relevant operating conditions. Bubble column reactors (BCRs) and continuous stirred-tank reactors (CSTRs) have been the widely used bioreactors in the bioleaching process. One of the advantages of these bioreactors compared to others is the ability to control the concentration of product and substrate during the process and to maintain the environmental operating conditions [22,30]. Ref. [101] investigated the extraction of heavy metals from a petroleum spent catalyst using A. thiooxidans in a bubble column reactor, and results showed 87% % Mo, 37% Ni, and 15% Al extraction with a pulp density of 0.9%, particle size of 60.7 μm, and aeration rate of 209 mL/min after 7 days. In another study, ref. [30] employed adapted A. ferrooxidans in a bubble column and leached approximately 54% Cu, 75% Ni, and 55% Fe with 2% waste PCBs in 9 days. Figure 2 illustrates a schematic diagram of two types of reactors employed in the bioleaching process. As seen in Figure 2a, the stirring in a BCR is conducted by air bubbling, and the aeration happens via air injection through a sparger, operating at high airflow. A water cooling thermostat is fitted to maintain the temperature. Figure 2b shows continuous bioleaching in a 50 L STR. The mixture of sediment slurry and deionised water is pumped to a reactor filled with sulphur-oxidising microorganisms and supplied with aerator [102]. Figure 2c represents a combination of BCR and STR in the bioleaching of metals by an acidophilic consortium. A continuous cultivation experiment of microorganisms carried out in a BCR and bioleaching in batch and continuous modes was performed in an STR [6,103]. CSTRs are more advantageous in comparison with BCRs because of the presence of high mass transfer rates, homogeneous mixing, and better control over parameters such as pH, temperature, and aeration [22]. A typical design of CSTRs involves a circuit of continuously flowing aerated tanks set up in series, parallel, or a combination of both, equipped with agitated impellers that keep the grounded minerals suspended in the solution and ensure an effective transfer of oxygen, which is necessary for both the dissolution of the metals and the growth of aerobic microorganisms. In CSTRs, the feed is added to the first tank and overflows from tank to tank in co-current flow with the microorganisms. When microorganisms are injected at the start-up of an operation, a batch culture is maintained until the microorganisms reach a certain stage close to the middle of the logarithmic phase, at which point fresh substrate is supplied continuously from the feed tank to the reactor. The continuous flow of the substrate through the tanks is provided to ensure the optimal growth of microorganisms for a high metal dissolution rate at the steady-state phase [104]. Since its introduction in 1986, CSTR-related technologies have undergone significant advancement, such as high-temperature performance in bioleaching. During the first decade of this century, the Beaconsfield plant in Australia and the Laizhou plant in China, utilising Mintek BacTech technology, have conducted bioleaching operations at moderate to high temperature, ranging between 45–55 °C. The BioCop™ process, pioneered by the BHP Billiton biotechnology group based in Johannesburg in South Africa, employs thermophilic bacteria thriving at a temperature between 70–80 °C to leach chalcopyrite [105].
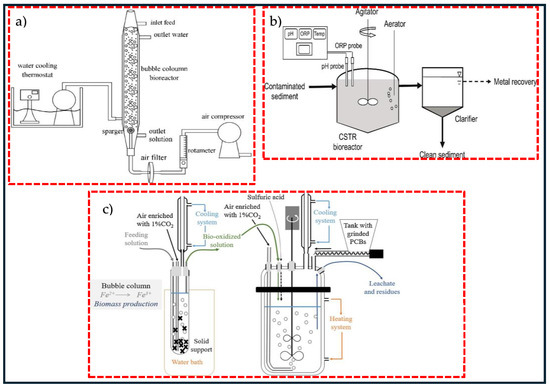
Figure 2.
A schematic diagram of two types of reactors in bioleaching process (a) bubble column reactor (BCR), (b) continuous stirred tank reactor (CSTR) and (c) a combination of bubble column and stirred tank reactors. Copyright © 2020, Elsevier (a), copyright © 2021, Elsevier (b), copyright © 2022, Elsevier (c) [6,30,102].
In a study conducted by [95], bioleaching with thermophilic bacteria, L. ferriphilum and A. caldus, was employed at a high pulp density of 8% PCBs using a 3 L stirred-tank reactor, resulting in a recovery of 85.23% Zn, 76.59% Cu, and 70.16% Al in 7 days. Ref. [106] employed a 12 L aerated rotating-drum reactor operated at 50 °C for the bioleaching of 76% Cu with a pulp density of 2.5% by the thermophilic bacterium S. thermosulfidooxidans and Fe2+ concentration of 5 g/l in 8 days. In another study, ref. [107] achieved a 99% leaching rate of Cu within 72 h using a 3 L CSTR containing 5% of low-grade PCBs and mixed acidophilic culture supplemented with 7.8 g/L of Fe2+. However, large-scale bioleaching has not been sufficiently investigated owing to factors such as reaction conditions, the complexity of the mechanism, and weakly efficient mass transfer rates [108]. More comprehensive investigation is crucial, as the findings are found to be not linearly scalable when the size of equipment is increased. While controlling conditions for a complete reaction at the laboratory scale is straightforward and flexible, maintaining microbial populations along with the aeration rate is challenging at the industrial scale, which affects the steady-state operations over long times of streams [24].
In recent years, several projects in Europe, such as BIORECOVER, RAWMINA, RUBICON, and BIOCriticalMetals, have employed bioleaching for the recovery of various metals from e-wastes. Among the industrial contributors, the UK-based IT lifecycle services provider N2S is applying bioleaching for the extraction of precious metals from printed circuit boards and aligning with the UK’s environmental and cybersecurity regulations [109]. In Estonia, BiotaTec (formerly BiotaP), which initially focused on environmental monitoring using metagenomics, has since expanded their activities to the development of microorganism-driven bioleaching and biomining solutions for the recovery of metals from low-grade ores and various waste streams. Another notable company is Ekolive, a Slovak start-up which offers an EU/ETV-certified eco-innovative bioleaching method (InnoBioTech®) for processing minerals and waste using heterotrophic bacteria. Their large-scale pilot project, established in Slovenia in 2019, demonstrated the market maturity of this approach for industrial mineral recovery from mining waste [110].
6. Process Economics
6.1. Cost Estimation
Estimating both capital expenditure (CAPEX) and operational expenditure (OPEX) is essential to assess the economic feasibility of the bioleaching process and give insight into the opportunities to improve, optimise, and conduct further research if needed. CAPEX includes the cost of equipment, installation, construction, piping, electrical systems, service facilities, and land, and OPEX covers ongoing costs like electricity, water, materials, labour, and maintenance [111]. Estimating capital costs helps identify the key factors that affect the economic viability of the process. For instance, while lower pulp densities often yield higher metal leaching rates, they are associated with higher capital costs. In contrast, higher pulp densities may reduce leaching efficiency but result in lower CAPEX, making the process more economically viable. In one study, leaching of base metals from PCBs at 1% pulp density led to high operating costs and an annual loss of AUD 1007 per tonne of PCBs. However, increasing the pulp density to 5% significantly lowered the costs, resulting in an annual profit of AUD 2749 per tonne. Although the raw material costs can change over time, the process would be profitable. Even with a 40% increase in raw material costs, the study showed that an annual profit of AUD 2463 per tonne of PCBs was still achievable [42]. Labour costs for the preprocessing of e-waste, such as collecting, dismantling, and sorting, are the highest operating expenses, making it difficult to expand e-waste recycling in regions such as Australia, New Zealand, and other parts of Oceania. A techno-economic study assessed the use of biooxidation and cyanidation to extract gold from refractory ore. Over a 25-year mine life, processing 1200 tonnes of ore daily, the project required an AUD 220 million investment and had operating costs of AUD 58.27 million annually. With an expected annual revenue of AUD 78.48 million and a net present value (NPV) of AUD 34.4 million at a 7% interest rate, the process was found to be economically viable [112]. Another study compared two bioleaching technologies, including an aerated bioreactor and an aerated and stirred bioreactor, for Cu recovery from goethite using A. ferrooxidans. The aerated and stirred bioreactor proved to be financially viable at a pulp density of 10%, achieving an NPV of AUD 1.275 billion and an internal rate of return (IRR) of 65% over 20 years. With a CAPEX of AUD 119.8 million and annual OPEX of AUD 5.9 million, the plant is expected to become profitable within one year [39].
6.2. Cost Minimisation Strategies
In the bioleaching process, the initial investment required for setting up a reactor plant may seem to be high, but the ongoing operational expenses can be reduced through the optimisation of process parameters and the utilisation of cost-effective substances. For example, the use of commercial iron, sulphur, and specific additives contributes to the overall cost [28] but can be reduced by using low-grade pyrite as an alternative source [113]. In the case of heterotrophic bacteria, glucose is the main nutrient carbon source for bacterial growth and accounts for approximately 44% of the total initial costs. Using alternative affordable substrates such as molasses is an option to reduce the cost of the process [24]. Ref. [114] reported that using potato wastewater reduced the cost by 17% compared to glucose, and replacing corn stover with potato wastewater reduced the costs by 22% in comparison with potato wastewater. The formation of iron hydroxides and jarosite precipitations during bioleaching, which leads to a decrease in diffusion rates of oxidants to the surface of PCBs and results in non-economic losses in metal yields, can be avoided by maintaining low pH and using effective sulphur agents that oxidise sulphur to sulphuric acid. The costs of the preprocessing of e-waste (dismantling, separation, and grinding) and operation (labour, raw materials, and utilities) can render the bioleaching technique economically impractical without careful management of the design and process automation. Cost reduction in the grinding step can be achieved by maintaining a particle size that does not significantly impact both the mass transfer rate and the bioleaching yield [28,108]. For example, a study reported that using PCB chips instead of powdered PCBs resulted in high metal leaching efficiency and significantly lowered operational costs, as it avoided the energy-intensive milling process and reduced the risk of metal loss during preprocessing [115].
7. Conclusions
Metal recycling from printed circuit boards (PCBs), as the major component of e-waste, is a beneficial option to protect the environment, conserve natural resources, and meet the need for metals, particularly the valuable ones that are critical for current industries. The bioleaching process as a green technology for metal extraction is typically conducted by direct (one-step and two-step) and indirect (spent-medium) methods, and relevant chemical mechanisms fundamentally include acidolysis, complexolysis, and redoxolysis. The latter is the primary mechanism associated with iron-oxidising bacteria. For over a century, Au extraction has relied on the cyanidation process, but environmental concerns limit its expansion, and the thiosulphate-based process is offered as a safe alternative for Au recovery. High thiosulphate consumption remains, however, one of the main challenges associated with this method, and various strategies such as the use of additives, control of operation conditions, and employment of biothiosulphate are options being considered. Upscaling bioleaching to an industrially feasible process in cost-effective operations remains a challenge, but the selection of a suitable microorganism and adapted design of bioreactor are contributing as closely as possible to the success of the process’s feasibility from economic and environmental perspectives. These studies indicate that the biological extraction of metals from PCBs presents a promising viable alternative to physico-chemical methods and that an improvement in handling large pulp density of PCBs within bioreactors is necessary for the practical achievement of a cost-effective and competitively upscaled process.
Author Contributions
Conceptualisation, Z.I. and F.A.; methodology, Z.I. and F.A.; formal analysis, Z.I. and F.A., investigation, Z.I.; resources, F.A.; data curation, Z.I.; writing—original draft preparation, Z.I.; writing—review and editing, Z.I. and F.A.; supervision, F.A.; project administration, F.A.; funding acquisition, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovate UK, KTP012187, and ICT Reverse Asset Management Ltd., A109952 for the financial supports of this work.
Acknowledgments
The authors acknowledge the support of Innovate UK and ICT Reverse Ltd. for financial supports of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vardanyan, A.; Vardanyan, N.; Abrahamyan, N.; Aatach, M.; Gaydardzhiev, S. Sequential biologically assisted extraction of Cu and Zn from printed circuit boards (PCB). Int. J. Environ. 2022, 81, 1756–1771. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Uddin, M.N.; Chowdhury, J.I.; Ahmed, S.F.; Uddin, M.N.; Mofijur, M.; Uddin, M.A. A review of the recent development, challenges, and opportunities of electronic waste (e-waste). Int. J. Environ. Sci. Technol. 2023, 20, 4513–4520. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.; Fei, W.; Liu, Z.; He, W.; Li, G. The reuse of electronic components from waste printed circuit boards: A critical review. Environ. Sci. Adv. 2023, 2, 196–214. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S. Advances in bioleaching of copper and nickel from electronic waste using Acidithiobacillus ferrooxidans: Evaluating daily pH adjustment. Chem. Pap. 2020, 74, 2211–2227. [Google Scholar] [CrossRef]
- Hubau, A.; Minier, M.; Chagnes, A.; Joulian, C.; Silvente, C.; Guezennec, A.G. Recovery of metals in a double-stage continuous bioreactor for acidic bioleaching of printed circuit boards (PCBs). Sep. Purif. Technol. 2020, 238, 116481. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Separ. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Baniasadi, M.; Graves, J.E.; Ray, D.A.; De Silva, A.L.; Renshaw, D.; Farnaud, S. Closed-loop recycling of copper from waste printed circuit boards using bioleaching and electrowinning processes. Waste Biomass Valorization 2021, 12, 3125–3136. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal recovery from waste printed circuit boards: A review for current status and perspectives. Resour. Conserv. Recycl. 2020, 157, 104787. [Google Scholar] [CrossRef]
- Deng, S.; Xiao, Z.; Zhang, W.; Noble, A.; Das, S.; Yih, Y.; Sutherland, J.W. Economic analysis of precious metal recovery from electronic waste through gas-assisted microflow extraction. Resour. Conserv. Recycl. 2023, 190, 106810. [Google Scholar] [CrossRef]
- Garg, H.; Nagar, N.; Ellamparuthy, G.; Angadi, S.I.; Gahan, C.S. Bench scale microbial catalysed leaching of mobile phone PCBs with an increasing pulp density. Heliyon 2019, 5, e02883. [Google Scholar] [CrossRef]
- Ahamed, A.; Ge, L.; Zhao, K.; Veksha, A.; Bobacka, J.; Lisak, G. Environmental footprint of voltammetric sensors based on screen-printed electrodes: An assessment towards “green” sensor manufacturing. Chemosphere 2021, 278, 130462. [Google Scholar] [CrossRef]
- Gui, Q.; Khan, M.I.; Wang, S.; Zhang, L. The ultrasound leaching kinetics of gold in the thiosulphate leaching process catalysed by cobalt ammonia. Hydrometallurgy 2020, 196, 105426. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115, 30–51. [Google Scholar] [CrossRef]
- Wang, J.; Faraji, F.; Ramsay, J.; Ghahreman, A. A review of biocyanidation as a sustainable route for gold recovery from primary and secondary low-grade resources. J. Clean. Prod. 2021, 296, 126457. [Google Scholar] [CrossRef]
- Ilkhani, Z.; Vakilchap, F.; Sadeghi, N.; Mousavi, S.M. Base metals (Fe, Al, Ti) and rare earth elements (Ce, La, Pr) leaching from red mud through an efficient chemical-biological hybrid approach. Miner. Eng. 2024, 208, 108603. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W.; Liu, Y.; Jia, H.; Zhou, J.; Wei, P.; Zhou, H. Bioleaching of dewatered electroplating sludge for the extraction of base metals using an adapted microbial consortium: Process optimization and kinetics. Hydrometallurgy 2020, 191, 105227. [Google Scholar] [CrossRef]
- Dong, Y.; Zan, J.; Lin, H. Bioleaching of heavy metals from metal tailings utilizing bacteria and fungi: Mechanisms, strengthen measures, and development prospect. J. Environ. Manag. 2023, 344, 118511. [Google Scholar] [CrossRef]
- Diaz, M.A.; De Ranson, I.U.; Dorta, B.; Banat, I.M.; Blazquez, M.L.; Gonzalez, F.; Ballester, A. Metal removal from contaminated soils through bioleaching with oxidizing bacteria and rhamnolipid biosurfactants. Soil Sediment Contam. Int. J. 2015, 24, 16–29. [Google Scholar] [CrossRef]
- Williamson, A.J.; Folens, K.; Matthijs, S.; Cortes, Y.P.; Varia, J.; Du Laing, G.; Hennebel, T. Selective metal extraction by biologically produced siderophores during bioleaching from low-grade primary and secondary mineral resources. Miner. Eng. 2021, 163, 106774. [Google Scholar] [CrossRef]
- Sadeghi, N.; Vakilchap, F.; Ilkhani, Z.; Mousavi, S.M. Assessment of the visible light effect on one-step bacterial leaching of metals from spent lithium-ion batteries cathode pretreated by a bio-chemical lixiviant. J. Clean. Prod. 2024, 436, 140432. [Google Scholar] [CrossRef]
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.; Contamine, F.; d’Hugues, P. A review of sulphide minerals microbially assisted leaching in stirred tank reactors. Int. Biodeter. Biodegr. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Priyanka, B.; Kumar, P.S.; Karishma, S.; Jeevanantham, S.; Indraganti, S. A review on recent advancements in recovery of valuable and toxic metals from e-waste using bioleaching approach. Chemosphere 2022, 287, 132230. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Xie, H.; Liu, L. A novel dismantling process of waste printed circuit boards using water-soluble ionic liquid. Chemosphere 2013, 93, 1288–1294. [Google Scholar] [CrossRef]
- Li, J.; Duan, H.; Yu, K.; Liu, L.; Wang, S. Characteristic of low-temperature pyrolysis of printed circuit boards subjected to various atmosphere. Resour. Conserv. Recycl. 2010, 54, 810–815. [Google Scholar] [CrossRef]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste printed circuit boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Pathak, A.; Kothari, R.; Dastidar, M.G.; Sreekrishnan, T.R.; Kim, D.J. Comparison of bioleaching of heavy metals from municipal sludge using indigenous sulphur and iron-oxidizing microorganisms: Continuous stirred tank reactor studies. J. Environ. Sci. Health A 2014, 49, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Pourhossein, F.; Mousavi, S.M.; Yaghmaei, S. Green recovery of Cu-Ni-Fe from a mixture of spent PCBs using adapted A. ferrooxidans in a bubble column bioreactor. Sep. Purif. Technol. 2021, 272, 118701. [Google Scholar] [CrossRef]
- Naderi, A.; Vakilchap, F.; Motamedian, E.; Mousavi, S.M. Regulatory-systemic approach in Aspergillus niger for bioleaching improvement by controlling precipitation. Appl. Microbiol. Biotechnol. 2023, 107, 7331–7346. [Google Scholar] [CrossRef] [PubMed]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Vardanyan, A.; Vardanyan, N.; Aâtach, M.; Malavasi, P.; Gaydardzhiev, S. Bio-Assisted Leaching of Non-Ferrous Metals from Waste Printed Circuit Boards—Importance of Process Parameters. Metals 2022, 12, 2092. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Deng, X.; Bohu, T.; Zea, L.; Khaleque, H.N.; Gumulya, Y.; Cheng, K.Y. Prospective directions for biohydrometallurgy. Hydrometallurgy 2020, 195, 105376. [Google Scholar] [CrossRef]
- Dong, Y.; Zan, J.; Lin, H. Recovery of precious metals from waste printed circuit boards though bioleaching route: A review of the recent progress and perspective. J. Environ. Manag. 2023, 348, 119354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, Q.; Wei, Z.; Shah, K.; Efstathiadis, H.; Meng, X.; Liang, Y. Leaching of valuable metals from cathode active materials in spent lithium-ion batteries by levulinic acid and biological approaches. Heliyon 2023, 9, e15788. [Google Scholar] [CrossRef]
- Marappa, N.; Ramachandran, L.; Dharumadurai, D.; Nooruddin, T. Recovery of gold and other precious metal resources from environmental polluted E-waste printed circuit board by bioleaching Frankia. Int. J. Environ. Res. 2020, 14, 165–176. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.P. Gold biorecovery from e-waste: An improved strategy through spent medium leaching with pH modification. Chemosphere 2015, 136, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Kara, I.T.; Wagland, S.T.; Coulon, F. Techno-economic assessment of bioleaching for metallurgical by-products. J. Environ. Manag. 2024, 358, 120904. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of metals from e-waste using microorganisms: A re-view. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Pradhan, J.K.; Kumar, S. Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag. Res. 2012, 30, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.; Kaksonen, A.H. Techno-economic analysis of an integrated bio-and hydrometallurgical process for base and precious metal recovery from waste printed circuit boards. Hydrometallurgy 2023, 222, 106193. [Google Scholar] [CrossRef]
- Kim, B.J.; Koh, Y.K.; Kwon, J.S. Bioleaching of pyrrhotite with bacterial adaptation and biological oxidation for iron recovery. Metals 2021, 11, 295. [Google Scholar] [CrossRef]
- Gu, T.; Rastegar, S.O.; Mousavi, S.M.; Li, M.; Zhou, M. Advances in bioleaching for recovery of metals and bioremediation of fuel ash and sewage sludge. Bioresour. Technol. 2018, 261, 428–440. [Google Scholar] [CrossRef]
- Li, X.; Feng, C.; Lei, M.; Luo, K.; Wang, L.; Liu, R.; Hu, Y. Bioremediation of organic/heavy metal contaminants by mixed cultures of microorganisms: A review. Open Chem. 2022, 20, 793–807. [Google Scholar] [CrossRef]
- Qiu, M.Q.; Xiong, S.Y.; Zhang, W.M.; Wang, G.X. A comparison of bioleaching of chalcopyrite using pure culture or a mixed culture. Miner. Eng. 2005, 18, 987–990. [Google Scholar] [CrossRef]
- Yachkula, A.; Rozova, O.; Abashina, T.; Vainshtein, M.; Grouzdev, D.; Bulaev, A. Attempts to stimulate leaching activity of Acidithiobacillus ferrooxidans strain TFBk. Minerals 2022, 12, 1051. [Google Scholar] [CrossRef]
- Tay, S.B.; Natarajan, G.; Rahim, M.N.B.A.; Tan, H.T.; Chung, M.C.M.; Ting, Y.P.; Yew, W.S. Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci. Rep. 2013, 3, 2236. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z. Precious metals recovery from waste printed circuit boards: A review for current status and perspective. Resour. Conserv. Recycl. 2016, 113, 28–39. [Google Scholar] [CrossRef]
- Panda, R.; Dinkar, O.S.; Jha, M.K.; Pathak, D.D. Recycling of gold from waste electronic components of devices. Korean. J. Chem. Eng. 2020, 37, 111–119. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. A novel rapid and selective microbially thiosulphate bioleaching of precious metals from discarded telecommunication printed circuited boards (TPCBs). Resour. Conserv. Recycl. 2022, 187, 106599. [Google Scholar] [CrossRef]
- Jorjani, E.; Sabzkoohi, H.A. Gold leaching from ores using biogenic lixiviants—A review. Curr. Res. Biotechnol. 2022, 4, 10–20. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). J. Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, C.; Ma, C. Bioleaching of gold from waste printed circuit boards by Chromobacterium violaceum. J. Mater. Cycles Waste Manag. 2015, 17, 529–539. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, H.S.; Kumar, S. Bioleaching of gold and silver from waste printed circuit boards by Pseudomonas balearica SAE1 isolated from an e-waste recycling facility. Curr. Microbiol. 2018, 75, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhu, X.; Qian, Y.; Hu, J. A new strain for recovering precious metals from waste printed circuit boards. Waste Manag. 2014, 34, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.D.; Lee, J.C.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.P. Pretreatment of e-waste and mutation of alkali-tolerant cyanogenic bacteria promote gold biorecovery. Bioresour. Technol. 2014, 152, 80–85. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Y.; Ai, F.; Lin, M.; Ruan, J. Biofilm for leaching precious metals from waste printed circuit boards using biocyanidation technology. J. Hazard. Mater. 2021, 403, 123586. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The role of microorganisms in gold processing and recovery—A review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A review of ammoniacal thiosulphate leaching of gold: An update useful for further research in non-cyanide gold lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.C.; Jeong, J.; Hai, H.T.; Jha, M.K. Thiosulphate leaching of gold from waste mobile phones. J. Hazard. Mater. 2010, 178, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Chen, J.N.; Wang, J.; Wang, W. Review of gold leaching in thiosulphate-based solutions. Trans. Nonferrous Met. Soc. China 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Zhao, H.F.; Yang, H.Y.; Chen, X.; Chen, G.B.; Tong, L.L.; Jin, Z.N. Effect of Triethanolamine as a New and Efficient Additive on Thiosulphate-Copper-Ammonia System Leaching of Gold. Jom 2020, 72, 946–952. [Google Scholar] [CrossRef]
- Wang, J.; Xie, F.; Wang, W.; Bai, Y.; Fu, Y.; Dreisinger, D. Eco-friendly leaching of gold from a carbonaceous gold concentrate in copper-citrate-thiosulphate solutions. Hydrometallurgy 2020, 191, 105204. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A review of thiosulphate leaching of gold: Focus on thiosulphate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Camelino, S.; Rao, J.; Padilla, R.L.; Lucci, R. Initial studies about gold leaching from printed circuit boards (PCB’s) of waste cell phones. Procedia Mater. Sci. 2015, 9, 105–112. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulphate leaching of pressure oxidized sulphide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Oraby, E.A. Gold leaching in Thiosulphate Solutions and Its Environmental Effects Compared with Cyanide. Doctoral Dissertation, Curtin University, Perth, Australia, 2009. [Google Scholar]
- Navarro, P.; Vargas, C.; Villarroel, A.; Alguacil, F.J. On the use of ammoniacal/ammonium thiosulphate for gold extraction from a concentrate. Hydrometallurgy 2002, 65, 37–42. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium thiosulphate extraction of gold from printed circuit boards (PCBs) of end-of-life mobile phones and its recovery from pregnant leach solution by cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Ficeriová, J.; Baláž, P.; Villachica, C.L. Thiosulphate leaching of silver, gold and bismuth from a complex sulphide concentrate. Hydrometallurgy 2005, 77, 35–39. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulphate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Petter, P.M.H.; Veit, H.M.; Bernardes, A.M. Evaluation of gold and silver leaching from printed circuit board of cellphones. J. Waste Manag. 2014, 34, 475–482. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Zhong, J.; Nie, Y.; Xiang, P. The copper–ethanediamine–thiosulphate leaching of gold ore containing limonite with cetyltrimethyl ammonium bromide as the synergist. Hydrometallurgy 2014, 150, 178–183. [Google Scholar] [CrossRef]
- Sitando, O.; Senanayake, G.; Dai, X.; Nikoloski, A.N.; Breuer, P. A review of factors affecting gold leaching in non-ammoniacal thiosulphate solutions including degradation and in-situ generation of thiosulphate. Hydrometallurgy 2018, 178, 151–175. [Google Scholar] [CrossRef]
- Breuer, P.L.; Jeffrey, M.I. The reduction of copper(II) and the oxidation of thiosulphate and oxysulphur anions in gold leaching solutions. Hydrometallurgy 2003, 70, 163–173. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of ethylenediaminetetraacetic acid (EDTA). Miner. Eng. 2010, 23, 143–150. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, L.; Chen, P.; Zhang, Q.; Chen, Y.; Zainiddinovich, N.Z.; Jia, F. Efficient and stable leaching of gold in a novel ethydiaminedhephen acetic-thiosulphate system. Miner. Eng. 2024, 209, 108639. [Google Scholar] [CrossRef]
- Chandra, I.; Jeffrey, M.I. An electrochemical study of the effect of additives and electrolyte on the dissolution of gold in thiosulphate solutions. Hydrometallurgy 2004, 73, 305–312. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M.; Tan, W. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulphur-oxidizing bacteria. Bioresour. Bioprocess 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Jiang, T.; Li, Q.; Zhang, X.; Wang, D. Improved thiosulphate leaching of a refractory gold concentrate calcine with additives. Hydrometallurgy. 2015, 152, 214–222. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.Q.; Liu, X.M.; Pang, X.; Zhang, C.J.; Yang, C.L.; Chen, L.X. Sulphur oxidation in the acidophilic autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 9, 3290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Kara, I.T.; Huntington, V.E.; Simmons, N.; Wagland, S.T.; Coulon, F. Extracting Metal Ions from Basic Oxygen Steelmaking Dust by using Bio-Hydrometallurgy. Heliyon 2024, 10, e32437. [Google Scholar] [CrossRef]
- McNeice, J.; Mahandra, H.; Ghahreman, A. Application of biogenic thiosulfate produced by methylophaga sulfidovorans for sustainable gold extraction. ACS Sustain. Chem. Eng. 2022, 10, 10034–10046. [Google Scholar] [CrossRef]
- De Wit, R.; van Gemerden, H. Oxidation of sulphide to thiosulphate by Microcoleus chtonoplastes. FEMS Microbiol. Ecol. 1987, 3, 7–13. [Google Scholar] [CrossRef]
- Hutt, L.P.; Huntemann, M.; Clum, A.; Pillay, M.; Palaniappan, K.; Varghese, N.; Boden, R. Permanent draft genome of Thiobacillus thioparus DSM 505 T, an obligately chemolithoautotrophic member of the Betaproteobacteria. Stand. Genom. Sci. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Kunert, J.; Stránský, Z. Thiosulphate production from cystine by the keratinolytic prokaryote Streptomyces fradiae. Arch. Microbiol. 1988, 150, 600–601. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, H.; Cui, F.; Liu, H.; Xun, L. Recombinant Escherichia coli with sulphide: Quinone oxidoreductase and persulphide dioxygenase rapidly oxidises sulphide to sulfite and thiosulphate via a new pathway. Environ. Microbiol. 2016, 18, 5123–5136. [Google Scholar] [CrossRef]
- McNeice, J.; Mahandra, H.; Ghahreman, A. Biogenesis of thiosulfate in microorganisms and its applications for sustainable metal extraction. Rev. Environ. Sci. Biotechnol. 2022, 21, 993–1015. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. Improvement of gold bioleaching extraction from waste telecommunication printed circuit boards using biogenic thiosulphate by Acidithiobacillus thiooxidans. J. Hazard. Mater. 2023, 450, 131073. [Google Scholar] [CrossRef] [PubMed]
- McNeice, J.; Mahandra, H.; Ghahreman, A. Biogenic Production of Thiosulphate from Organic and Inorganic Sulphur Substrates for Application to Gold Leaching. Sustainability 2022, 14, 16666. [Google Scholar] [CrossRef]
- Xia, M.C.; Wang, Y.P.; Peng, T.J.; Shen, L.; Yu, R.L.; Liu, Y.D.; Zeng, W.M. Recycling of metals from pretreated waste printed circuit boards effectively in stirred tank reactor by a moderately thermophilic culture. J. Biosci. Bioeng. 2017, 123, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, H.; Tong, L.; Sand, W. Some aspects of industrial heap bioleaching technology: From basics to practice. Min. Proc. Ext. Meta. Rev. 2022, 43, 510–528. [Google Scholar] [CrossRef]
- Jia, Y.; Ruan, R.; Qu, J.; Tan, Q.; Sun, H.; Niu, X. Multi-Scale and Trans-Disciplinary Research and Technology Developments of Heap Bioleaching. Minerals 2024, 14, 808. [Google Scholar] [CrossRef]
- Devi, A.S.; Ganesh, S. Recent bioleaching approaches employed for the extraction of metals in mining fields for the purpose of utilization and creating the sustainable future. Environ. Qual. Manag. 2024, 33, 317–333. [Google Scholar] [CrossRef]
- Tezyapar Kara, I.; Kremser, K.; Wagland, S.T.; Coulon, F. Bioleaching metal-bearing wastes and by-products for resource recovery: A review. Environ. Chem. Lett. 2023, 21, 3329–3350. [Google Scholar] [CrossRef]
- Roberto, F.F.; Schippers, A. Progress in bioleaching: Part B, applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- Shahrabi-Farahani, M.; Yaghmaei, S.; Mousavi, S.M.; Amiri, F. Bioleaching of heavy metals from a petroleum spent catalyst using Acidithiobacillus thiooxidans in a slurry bubble column bioreactor. Sep. Purif. Technol. 2014, 132, 41–49. [Google Scholar] [CrossRef]
- Chen, S.Y.; Wu, J.Q.; Sung, S. Effects of sulfur dosage on continuous bioleaching of heavy metals from contaminated sediment. J. Hazard. Mater. 2022, 424, 127257. [Google Scholar] [CrossRef]
- Hubau, A.; Minier, M.; Chagnes, A.; Joulian, C.; Perez, C.; Guezennec, A.G. Continuous production of a biogenic ferric iron lixiviant for the bioleaching of printed circuit boards (PCBs). Hydrometallurgy 2018, 180, 180–191. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef]
- Batty, J.D.; Rorke, G.V. Development and commercial demonstration of the BioCOP™ thermophile process. Hydrometallurgy 2006, 83, 83–89. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Leão, V.A.; Gomes, O.; Lambert, F.; Bastin, D.; Gaydardzhiev, S. Copper extraction from coarsely ground printed circuit boards using moderate thermophilic bacteria in a rotating-drum reactor. J. Waste Manag. 2015, 41, 148–158. [Google Scholar] [CrossRef]
- Mäkinen, J.; Bachér, J.; Kaartinen, T.; Wahlström, M.; Salminen, J. The effect of flotation and parameters for bioleaching of printed circuit boards. Miner. Eng. 2015, 75, 26–31. [Google Scholar] [CrossRef]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Nikoloski, A.N.; Moheimani, N.; Valix, M.; Kaksonen, A.H. An integrated biohydrometallurgical approach for the extraction of base metals from printed circuit boards. Hydrometallurgy 2023, 216, 105998. [Google Scholar] [CrossRef]
- N2s. Available online: https://www.n2s.co.uk/ (accessed on 31 December 2022).
- Abdel Azim, A.; Bellini, R.; Vizzarro, A.; Bassani, I.; Pirri, C.F.; Menin, B. Highlighting the role of archaea in urban mine waste exploitation and valorisation. Recycling 2023, 8, 20. [Google Scholar] [CrossRef]
- Thompson, V.S.; Gupta, M.; Jin, H.; Vahidi, E.; Yim, M.; Jindra, M.A.; Reed, D.W. Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
- Andrianandraina, S.H.; Alamdari, H.; Blais, J.F. Mass balance and techno-economic study of a complete treatment chain of bio-oxidation for the extraction and recovery of precious metals from gold ore. Miner. Eng. 2023, 202, 108247. [Google Scholar] [CrossRef]
- Bryan, C.G.; Watkin, E.L.; McCredden, T.J.; Wong, Z.R.; Harrison, S.T.L.; Kaksonen, A.H. The use of pyrite as a source of lixiviant in the bioleaching of electronic waste. Hydrometallurgy 2015, 152, 33–43. [Google Scholar] [CrossRef]
- Jin, H.; Reed, D.W.; Thompson, V.S.; Fujita, Y.; Jiao, Y.; Crain-Zamora, M.; Fisher, J.; Scalzone, K.; Griffel, M.; Hartley, D.; et al. Sustainable bioleaching of rare earth elements from industrial waste materials using agricultural wastes. ACS Sustain. Chem. Eng. 2019, 7, 15311–15319. [Google Scholar] [CrossRef]
- Maluleke, M.D.; Kotsiopoulos, A.; Govender-Opitz, E.; Harrison, S.T. Bioleaching of printed circuit boards in a two-stage reactor system with enhanced ferric iron regeneration in a re-circulating packed-bed reactor from PCB leaching. Miner. Eng. 2024, 218, 109000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).