Abstract
The widespread implementation of anaerobic digestion (AD) systems for organic waste treatment is increasingly challenged by emerging contaminants, including microplastics (MPs), antibiotics, and heavy metals (HMs), which exhibit environmental persistence and pose risks to ecological and human health. This review critically examines the sources, transformation pathways, and advanced mitigation strategies for these contaminants within AD systems. MPs, primarily derived from fragmented plastics and personal care products, accumulate in digestates and act as vectors for adsorbing toxic additives and pathogens. Antibiotics, introduced via livestock manure and wastewater, exert selective pressures that propagate antibiotic resistance genes (ARGs) while disrupting methanogenic consortia. HMs, originating from industrial and agricultural activities, impair microbial activity through bioaccumulation and enzymatic interference, with their bioavailability modulated by speciation shifts during digestion. To combat these challenges, promising mitigation approaches include the following: (1) bioaugmentation with specialized microbial consortia to enhance contaminant degradation and stabilize HMs; (2) thermal hydrolysis pretreatment to break down MPs and antibiotic residues; (3) chemical passivation using biochar or sulfides to immobilize HMs. Co-digestion practices inadvertently concentrate these contaminants, with MPs and HMs predominantly partitioning into solid phases, while antibiotics persist in both liquid and solid fractions. These findings highlight the urgency of optimizing mitigation strategies to minimize contaminant mobility and toxicity. However, critical knowledge gaps persist regarding the long-term impacts of biodegradable MPs, antibiotic transformation byproducts, and standardized regulatory thresholds for contaminant residues in digestate. This synthesis underscores the necessity for integrated engineering solutions and policy frameworks to ensure the safe resource recovery from AD systems, balancing energy production with environmental sustainability.
1. Introduction
Anaerobic digestion (AD) has emerged as a pivotal biotechnology for the sustainable management of organic wastes (e.g., livestock manure, crop residues, and food waste) and high-strength wastewater streams (e.g., swine effluent) through energy recovery [1,2]. Driven by renewable energy incentives, climate policies, and escalating waste disposal demands, global deployment of AD systems has expanded exponentially, with over 30 million commercial and household-scale digesters operational across Europe and Asia [3,4,5]. The process relies on synergistic microbial consortia to degrade organic substrates into methane-rich biogas under optimized operational parameters [6].
Despite its merits, AD faces critical challenges in digestate quality control due to co-introduced contaminants [7]. Co-digestion practices—integrating food waste with livestock manure or waste-activated sludge (WAS)—enhance methane yields but inadvertently introduce emerging pollutants with environmental persistence and bioaccumulative risks [8]. Three contaminant classes warrant urgent attention (Figure 1): (1) microplastics (MPs), derived from fragmented plastics (global production: 1.4 million tons in 1950 to 360 million tons in 2018) and personal care products, accumulate in WAS with >99% retention efficiency during wastewater treatment [9,10]; (2) antibiotics (e.g., ciprofloxacin, tetracyclines), extensively used in healthcare and intensive agriculture, enter AD systems via wastewater (30–90% excretion rates) and propagate antibiotic resistance genes (ARGs) through selective pressure [11,12]; (3) heavy metals (Cd, Zn, Cr), characterized by non-biodegradability and trophic transfer risks, exhibit speciation-dependent bioavailability that dictates their ecotoxicological impacts [13,14].
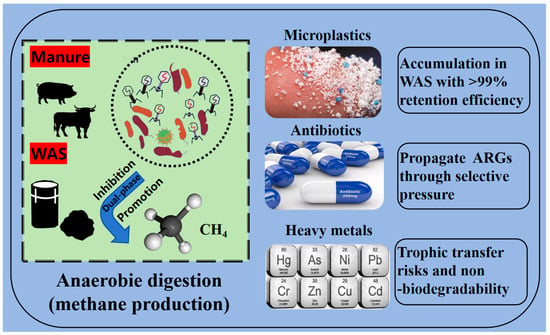
Figure 1.
Schematic diagram of the effect of three contaminant classes (microplastics, antibiotics, heavy metals) on anaerobic digestion.
Notably, MPs act as vectors for adsorbing antibiotics and metal ions, creating compounded toxicity that disrupts methanogenic pathways [15,16]. Current knowledge gaps persist regarding the fate of microplastics (MPs), antibiotics, and heavy metals (HMs) in anaerobic digestion (AD), particularly the fragmentation pathways of biodegradable MPs, dissemination mechanisms of antibiotic resistance genes, and synergistic microbial inhibition by metal–organic complexes. This review critically examines their sources, transformation behaviors, and toxicity risks to methanogenesis and microbial consortia. Advanced remediation strategies such as biochar adsorption, microbial augmentation, and bioreactors are proposed to enable safer organic waste valorization via multi-level synergies.
To ensure a comprehensive and unbiased analysis, this review employed a systematic literature search across Scopus, Web of Science, and PubMed databases (2010–2024) using an expanded set of keywords for the search query as follows:
“anaerobic digestion” AND “microplastics”, “anaerobic digestion” AND “antibiotics”, “anaerobic
digestion” AND “heavy metals”, “anaerobic digestion” AND “methane”, “anaerobic digestion” AND
“biochar”, “anaerobic digestion” AND “heavy metals” AND “antibiotics”, “anaerobic digestion” AND
“heavy metals” AND “microplastics”, “anaerobic digestion” AND “antibiotics” AND “microplastics”,
“removl” OR “microplastics” OR “antibiotics” OR “heavy metals”
digestion” AND “heavy metals”, “anaerobic digestion” AND “methane”, “anaerobic digestion” AND
“biochar”, “anaerobic digestion” AND “heavy metals” AND “antibiotics”, “anaerobic digestion” AND
“heavy metals” AND “microplastics”, “anaerobic digestion” AND “antibiotics” AND “microplastics”,
“removl” OR “microplastics” OR “antibiotics” OR “heavy metals”
The initial screening of over 500 articles applied three key inclusion criteria: (1) peer-reviewed studies providing empirical data on contaminant behavior in AD systems, with particular attention to studies employing advanced analytical techniques like LC-MS/MS and ICP-MS; (2) papers that quantitatively assessed impacts on methane production kinetics or microbial community shifts using molecular methods; and (3) research proposing innovative mitigation strategies with demonstrable efficacy through controlled experiments. Exclusion criteria were carefully designed to remove non-English articles, purely theoretical models lacking experimental validation, and studies without proper control comparisons to ensure data reliability. The final selection of 156 references underwent a rigorous three-stage vetting process involving title/abstract screening, full-text evaluation, and cross-verification by multiple authors. Special priority was given to longitudinal studies (>6 months duration, 30 papers selected) tracking contaminant fate over time, investigations examining multi-contaminant interactions (12 papers included), and large-scale (pilot/industrial) experiments (20 papers retained) that could demonstrate practical engineering applicability. The review structure was organized to first establish the fundamental mechanisms of contaminant impacts (sources, transformation pathways), then systematically evaluate their effects on AD performance at different operational scales, and finally critically assess mitigation technologies with emphasis on their implementation feasibility and cost-effectiveness. This methodological approach ensured both comprehensive coverage of contaminant dynamics and practical emphasis on engineering solutions that could be readily implemented in real-world AD systems.
2. Sources and Pathways of Contaminants Transport to AD System
2.1. Microplastics
Microplastics is the subject of the contamination studies of the 14 papers we consider for this critical review. The pervasive use of plastics—global production reached 360 million metric tons in 2018—has led to irreversible environmental fragmentation, with an estimated 12 billion tons of plastic waste projected to accumulate in landfills and ecosystems by 2050 [17,18]. Secondary microplastics (MPs, 1 μm–5 mm) predominantly originate from the degradation of microplastics through mechanical abrasion, UV weathering, and microbial action, while primary MPs are intentionally manufactured for industrial applications (e.g., electronics coatings, personal care products) [19,20]. China alone discharges approximately 306.9 million tons of plastic microbeads annually into aquatic environments, exemplifying the scale of anthropogenic MP emissions [21].
Wastewater treatment plants (WWTPs) act as critical interception points, retaining >90% of influent microplastics (MPs) in sewage sludge through sedimentation and filtration processes [22,23]. However, this retention inadvertently transforms sludge into a concentrated MP reservoir, with digestates containing 5-fold higher MP concentrations than raw waste-activated sludge (WAS) [24]. Globally, PS, PVC, PP, PET, and PE dominate sludge-associated MPs, exhibiting morphological diversity (spheres, fibers, fragments) and size heterogeneity (0.1–5 mm) [25,26]. Regional studies reveal substantial variations: Italian WWTPs report PA and PET concentrations of 29.3–1470 μg/g in sludge [27], while Canadian facilities detect 50–150 μg/L polystyrene nanoplastics (PsNPs) in anaerobically digested sludge [28].
Notably, MPs’ high surface-area-to-volume ratio and hydrophobicity enable them to act as vectors for heavy metals, antibiotics, and pathogens through adsorption–desorption dynamics, amplifying contaminant mobility in AD systems [29,30]. Land application of MP-laden digestates introduces these composites into agricultural soils, posing long-term risks to food security—a critical nexus requiring regulatory intervention [31].
2.2. Antibiotics
Antibiotics is the subject of the contamination studies of 18 papers we consider for this critical review. Antibiotics, widely used as growth promoters in livestock and additives in personal care products, have experienced escalating environmental release due to surging global consumption [32]. Between 2000 and 2015, global antibiotic usage increased by 65%, with 30% allocated to livestock farming, while medical and aquaculture sectors accounted for 55% and 15%, respectively [33]. Approximately 17–90% of veterinary antibiotics are excreted as parent compounds or active metabolites, resulting in concentrations ranging from 100 μg/L to 500 mg/L in livestock manure [34]. To the best of our knowledge, there are four types of antibiotics including tetracyclines, sulfonamides, quinolones, and macrolides that were reportedly detected in the FW and related AD systems, as shown in Table 1. In China’s intensive farming systems, tetracyclines (TCs), sulfonamides (SAs), and fluoroquinolones (QNs) constitute >75% of the antibiotic load in swine manure, while enrofloxacin concentrations in poultry manure exceed those in swine/cattle manure by three orders of magnitude [35,36].

Table 1.
Antibiotic contents found in manure and digestates.
Antibiotics enter anaerobic digestion systems via two primary pathways: (1) co-digestion of livestock manure (e.g., TCs (tetracyclines): 21.4–152.0 μg/g dry weight) with municipal waste-activated sludge (WAS), directly suppressing methanogen metabolic activity [44,45]; (2) WAS-retained antibiotics (e.g., sulfamethoxazole: 3–9.4 μg/g) influencing microbial community function through extracellular polymeric substance-mediated adsorption–desorption dynamics [46]. Antibiotics with low MIC50 values (e.g., enrofloxacin MIC50 <0.06 μg/mL) inhibit key enzymatic activities via ribosomal targeting, reducing CH4 yield by 30–50% [47]. Concurrently, antibiotic-induced selective pressure drives horizontal transfer of antibiotic resistance genes (ARGs, e.g., sulfonamide resistance genes sul1 and sul2), increasing multidrug-resistant bacteria abundance by 2–3 orders of magnitude in AD sludge [48].
Despite the predominance of TCs and SAs (>75% detection rate in Chinese swine manure), global thresholds for antibiotic residues in agricultural digestate remain undefined [39]. Drawing from Germany’s regulatory model, integrated strategies should include source reduction (e.g., restricting high-risk antibiotics with MIC50 <1 μg/mL), process intervention (10% biochar addition achieves >90% sulfonamide adsorption), and legislative controls (ecotoxicology-based MRLs) to mitigate risks [49,50].
2.3. Heavy Metals
Heavy metals is the subject of the contamination studies of the 17 papers we consider for this critical review. The valorization of municipal sludge and livestock manure as organic fertilizers is hindered by heavy metal (HM) contamination, despite their rich nutrient content [51,52]. China generates over 60 million tons of sludge annually, with notable HM concentrations (e.g., Cu, Zn, Cd, Cr), where Fe, Mn, Cu, and Zn constitute >80% of total HMs in swine manure [53,54]. Table 2 shows the content of HMs in slurry. Total HM concentrations alone inadequately reflect ecological risks, as bioavailability and toxicity depend critically on chemical speciation—acid-soluble (F1) and reducible fractions (F2) exhibit high mobility, while oxidizable (F3) and residual fractions (F4) remain stable [55,56].

Table 2.
HMs contents in the slurry (mg/L).
Within anaerobic digestion (AD) systems, HMs undergo dynamic speciation transformations. During initial hydrolysis-acidogenesis, organic acids lower pH to 5.5–6.0, enhancing dissolution of HM ions (e.g., Cu2+, Zn2+) [63]. As methanogenesis consumes volatile fatty acids, pH 7.5–8.5, establishing a reductive environment that facilitates HM stabilization through: (1) sulfide precipitation (e.g., CuS/ZnS) mediated by sulfate-reducing bacteria [13]; (2) humic acid (HA) complexation, with HA-Cu/HA-Zn exhibiting 2–3× higher stability than fulvic acid-bound forms [64]; (3) extracellular polymeric substance (EPS) adsorption, achieving 60–85% binding efficiency [65]. Ultimately, >90% of HMs accumulate in digestate solids, with 3–25 μm particles carrying >70% of the HM load [66,67].
Toxicity rankings follow Hg > Cd > Cr(III), where Cd poses the highest risk due to its bioaccumulation factor (BCF > 5) [14]. Although passivation strategies (e.g., composite additives) reduce Cu bioavailability by 40–60%, global standards for HM thresholds in agricultural digestate remain absent [68]. Future efforts must establish speciation-based risk assessment frameworks and develop microbial enhancement technologies targeting EPS synthesis or sulfur metabolism to achieve controlled HM risks in AD systems [66].
3. Conversion and Influence of Contaminants on AD System
Methane production, the core economic product of anaerobic digestion (AD), is a sensitive indicator for assessing the stability of pollutant-disturbed systems. Environmentally persistent, bioaccumulative, and ecotoxic microplastics, antibiotics, and heavy metals can reduce AD efficiency through a dual inhibitory mechanism: the direct inhibition of methane-producing archaeal activity and the disruption of microbial polymorphic structures. Polymeric particles (e.g., PE, PVC) impede mass transfer through organic matter adsorption; anti-microbial agents (e.g., tetracyclines TC/OTC, sulfonamides SDZ) induce resistance genes interfering with metabolism; and metal ions (e.g., Cu2+, Zn2+) deactivate key enzymes of methanogenesis. This review gathers as many studies as possible on the effects of these three pollutants on the AD system. In this critical review, 14 papers among those identified deal with microplastics, 18 papers deal with antibiotics, and 17 papers deal with heavy metals.
3.1. Conversion and Influence of Microplastics on AD System
Five types of most frequently detected MPs including polystyrene (PS), polycarbonate (PC), polyvinyl chloride (PVC), polyamide (PA 6), and polypropylene (PP) with different size ranges were summarized in Table 3.

Table 3.
Effects of MPs on anaerobic digestion.
Micro(nano)plastics (MNPs, defined as plastic particles ranging from 1 nm to 5 mm in size, derived from environmental degradation or industrial production) exert multidimensional toxicity mechanisms in anaerobic digestion (AD) systems. Concentration-effect studies reveal polystyrene nanoplastics (PSNPs) induce methane yield inhibition (r2 = 0.93) at 0.05–0.2 g/L through dual pathways: acidogenesis blockage (35–45% reduction in volatile fatty acids) and F420 coenzyme inactivation (60–75% activity loss) [29]. Particle size governs toxicity intensity—50 nm PS NPs generate 3.8-fold higher reactive oxygen species (ROS) than 1 μm counterparts due to their larger specific surface area (2.5 × 103 m2/g vs. 1.2 × 102 m2/g), causing mitochondrial damage via endocytosis [71,77].
Polymer-specific leaching effects demonstrate chemical toxicity divergence. Polycarbonate (PC) microplastics at 30 particles/g TS enhance methane yield by 24.7% via bisphenol A (BPA, 0.8–1.2 mg/L)-mediated superoxide dismutase activation (+25%), whereas high concentrations (200 particles/g TS) trigger ROS bursts (3.5-fold H2O2 increase) and 48% acetate kinase inhibition [72]. Polyvinyl chloride (PVC) >20 particles/g TS releases 1.5–2.0 mg/L BPA, reducing hydrolysis coefficient (k) by 0.12–0.15 d−1 through thiol group oxidation (−40% protease activity) and F420 conformational changes [78,79].
Microbial community restructuring underpins MNP-induced AD dysfunction. PS NPs decrease Candidatus Methanofastidiosum abundance by 2–3 orders of magnitude while enriching Mariniphaga (+450%) and Candidatus Microthrix (+320%), redirecting carbon flux from acetoclastic to hydrogenotrophic pathways [80]. Polyethylene (PE) microplastics increase Atopostipes (hydrolytic bacteria) and Psychrobacter (acid-tolerant genera) by 2.1- and 3.7-fold, respectively, but reduce Methanosaeta (acetoclastic methanogens) by 85%, decreasing VFA/CH4 conversion efficiency by 22–35% [81]. Crucially, MNPs promote antibiotic resistance gene (ARG) dissemination via horizontal transfer, with sul1 and tetB abundance showing linear correlation with PS NP concentration (R = 0.86), elevating environmental dissemination risks by 3–5-fold. When combined with antibiotics, microplastics exacerbate anaerobic digestion pollution through synergistic effects. Co-exposure enhances ARG propagation by disrupting microbial communities and enriching resistant strains, while also impeding methane production by 20–30% due to toxicity accumulation. The adsorption of antibiotics onto microplastics further prolongs their persistence, creating hotspots for resistance development. This dual contamination amplifies ecological risks, with ARG transfer efficiency increasing by 1.8–2.2 times compared to single pollutants, necessitating urgent mitigation strategies [82].
3.2. Conversion and Influence of Antibiotics on AD System
Antibiotics exhibit stage-specific and concentration-dependent inhibitory effects in anaerobic digestion (AD) systems. Table 4 shows the effects of several different antibiotics on anaerobic digestion. During the rate-limiting hydrolysis phase, chlorotetracycline (CTC) enhances hydrolysis rates by 30–40% through acid-forming bacterial activation (pH decrease 0.5–1.2 units), whereas azithromycin (AZM) increases hydrolytic community abundance by 25% [83,84]. In acidogenesis, sulfamethoxazole (SMX) ≥250 mg/L completely inhibits acetate synthesis and reduces butyrate production by 60–70%, attributable to irreversible inhibition of pyruvate dehydrogenase complexes [85]. Roxithromycin demonstrates dose-dependent effects (10–1000 μg/L), decreasing glucose degradation rates by 26.5–34.5% via 50S ribosomal subunit binding interference [86].

Table 4.
Effects of antibiotics on anaerobic digestion.
Methanogenesis exhibits heightened vulnerability through dual mechanisms: (1) suppression of acetoclastic pathway enzymes (e.g., 40–50% reduction in acetate kinase activity), diverting metabolism toward hydrogenotrophic routes; (2) selective inhibition of methanogens (2–3 orders of magnitude decrease in Methanosaeta abundance) coupled with proliferation of antibiotic-resistant genera (e.g., Clostridium spp.) [101,103]. Tetracycline displays threshold effects at 5.7–8.5 mg/L: low concentrations (1.65 mg/L) reduce methane yield by 10–20% while maintaining COD removal, whereas high concentrations (8.5 mg/L) inactivate propionate degraders and induce VFA accumulation [91,104]. Notably, long-term exposure triggers microbial adaptation—methanogenic function recovers to 80–90% baseline within 15–20 days post-cessation, indicating horizontal transfer of resistance genes (tetM, tetW).
3.3. Conversion and Influence of Heavy Metals on AD System
Seven types of the most frequently detected heavy metals including Cd, Fe, Zn, Hg, Cu, Ni, and Cr(III) with different concentrations were summarized in Table 5.

Table 5.
Effects of heavy metals on anaerobic digestion.
Heavy metals (HMs) exhibit biphasic concentration effects in anaerobic digestion (AD) systems, governed by well-defined threshold mechanisms. Trace levels of Cu2+ (0–100 mg/L), Fe2+ (50–4000 mg/L), Ni2+ (0.8–50 mg/L), and Zn2+ (0–5 mg/kg) enhance methanogenesis through activating key metabolic enzymes (e.g., 15–20% increase in cellulase activity) and stabilizing electron transport chains (30–50% upregulation of ferredoxin expression) [109,110]. Notably, Fe2+ demonstrates a pronounced dose–response relationship within 0–4000 mg/L, achieving peak biogas yield (+16.2%) at 1000 mg/L via sulfide chelation stabilization and cytochrome c oxidase activation [106,111].
Exceeding threshold concentrations triggers multidimensional inhibition. At Cu2+ > 300 mg/kg, cellulase activity declines by 5.86% with 40–60% reduction in microbial α-diversity, attributed to irreversible sulfhydryl group binding in enzyme active sites and ribosomal RNA structural damage [112,113]. Ni2+ exhibits a critical inhibitory threshold at 30 mg/L, beyond which methyl-coenzyme M reductase activity decreases by 25–35%, blocking the aceticlastic pathway [114]. Cd2+ displays the strongest biotoxicity, completely inhibiting F420 coenzyme synthesis at 1 mg/L and reducing system methane potential by 55% at 20 mg/L [105,115].
The methanogenic phase demonstrates heightened sensitivity to HM stress. Additionally, 5 mg/L Cu2+ increases Methanosarcina abundance by 2.5-fold, whereas 300 mg/kg reduces its relative abundance below detection limits [113]. Ni2+ dose–response curves reveal 2% biogas yield enhancement at 0.8 mg/L, but the methane content plummets from 60% to 35% beyond 30 mg/L [114]. This phase transition correlates with HM-induced microbial community restructuring—metal-resistant bacteria (e.g., Firmicutes) become selectively enriched, while methanogens (Methanothrix, Methanosarcina) decline by 50–80% [116,117].
4. Removal and Mitigation Strategies
The degradation of diverse substrates in anaerobic digestion (AD) systems involves distinct mechanisms, with pollutant concentrations and types (e.g., microplastics, antibiotics, heavy metals) largely determined by substrate sources [118]. Therefore, to effectively address the inhibitory effects of pollutants on AD systems, it is critical to develop universally applicable methods or tailored solutions for specific contaminants. This paper examines advanced treatment strategies, including biological pretreatment, bioreactors, and physicochemical treatment technologies for the removal and mitigation of contaminants.
4.1. Biological Pretreatment Technologies
Bioremediation technologies have emerged as the most promising strategy for removing contaminants in anaerobic digestion (AD) systems due to their cost-effectiveness and environmental compatibility [119]. These technologies rely on biosorption mechanisms involving ion exchange, surface complexation, and chemical precipitation, where interfacial interactions between non-viable biosorbents (e.g., agricultural wastes) and contaminants (heavy metals, antibiotics) play a dominant role. For instance, lignocellulosic biosorbents like rice husks exhibit ion exchange capacities of 2.1–3.8 mmol/g for Cd2+/Pb2+ under acidic conditions (pH 4–6), driven by sulfonic (-SO3H) and carboxyl (-COOH) functional groups. Surface complexation mechanisms are particularly effective for antibiotic removal, with tetracycline adsorption capacities exceeding 90% on cellulose-rich corncob biochar due to hydrogen bonding and π-π interactions [120,121]. Microbial pretreatment combined with physical grinding significantly enhances substrate accessibility; for instance, fungal-mechanical co-pretreatment increases methane yield from rice straw by 25–40% [122]. This process optimizes metabolic synergy between acidogens and methanogens, accelerating substrate decomposition during hydrolysis–acidogenesis and thereby boosting methane production rates at early AD stages [123].
Temperature-phased anaerobic digestion (TPAD) overcomes efficiency limitations of single-stage systems through thermophilic (50–70 °C) and mesophilic (35–40 °C) phase synergy. Akgul et al. [124] demonstrated that TPAD at 70 °C improves volatile solid (VS) degradation by 30–50% compared to single-stage thermophilic/mesophilic operations. The thermophilic phase achieves 85–90% lignin depolymerization via thermostable Caldicellulosiruptor spp., generating soluble aromatics that enhance acetoclastic methanogenesis in the mesophilic phase [124]. Kim et al. further verified that TPAD achieves 55% and 43% vs. degradation for primary and secondary biosolids, attributed to thermophilic-phase lignin depolymerization and mesophilic-phase acetogen enrichment [125]. In contrast, aerobic pretreatment depends on obligate microbial consortia for mineralizing complex organics into CO2, H2O, and nitrates under oxic conditions [126]. Although this method shortens methane stabilization periods in AD systems by 15–20 days through partial lignin removal (30–35%), its efficiency is constrained by biosolid properties (e.g., C/N ratio, particle size distribution). For high-C/N substrates (C/N > 30), aerobic pretreatment reduces nitrogen immobilization by 25%, but for fine particles (<0.5 mm), excessive mineralization lowers bioavailable carbon by 40%, negating methane yield improvements [127].
4.2. Use of Bioreactors in AD
Bioreactor technology significantly enhances the operational stability and pollutant degradation efficiency of anaerobic digestion (AD) systems through biomass enrichment, immobilization of functional microbial consortia, improved operational flexibility, and optimized treatment performance. Nevertheless, current mainstream anaerobic reactor systems for landfill leachate treatment—including Upflow Anaerobic Sludge Blanket (UASB) [128], Internal Circulation reactor (IC) [129], Moving Bed Biofilm Reactor (MBBR) [130], and Expanded Granular Sludge Bed (EGSB) [131]—still face critical challenges such as low microbial proliferation rates [132]. These systems frequently exhibit technical limitations including constrained treatment capacity, significant fluctuations in effluent pollutant concentrations, and inadequate sludge retention capabilities during practical operation.
Anaerobic membrane bioreactors (AnMBRs) integrate membrane separation with anaerobic digestion, achieving 95% chemical oxygen demand (COD) removal efficiency in high-suspended solids (SS) wastewater treatment [133]. Compared to conventional reactors (e.g., UASB, IC), AnMBR’s design with short hydraulic retention time (HRT) and long sludge retention time (SRT) enables deep organic mineralization while reducing sludge production by 40–60% [134], effectively addressing sludge disposal challenges in traditional systems [135]. The technology demonstrates unique advantages in treating swine wastewater and pharmaceutical effluents, where membrane modules (0.03–0.4 μm pore size) selectively retain functional microorganisms to prevent biomass loss and maintain system stability [136].
However, AnMBR’s large-scale application is hindered by membrane fouling—organic/inorganic pollutant accumulation reduces flux by 30–50% and increases transmembrane pressure (TMP) to 1.5–3.0 bar [137]. Innovative designs like dynamic membrane bioreactors (DMBRs) and electrochemical MBRs (eMBR) mitigate fouling rates by 60–80% through shear force optimization and electric field application (0.5–2.0 V/cm) [138,139]. For instance, eMBR coupling anodic oxidation with biodegradation achieves 98% removal efficiency for refractory pharmaceuticals like diclofenac [140].
For ammonia-inhibited substrates such as chicken manure, integrated strategies combining in situ ammonia stripping and hyper-thermophilic pretreatment (70–90 °C) enhance AnMBR methane yield by 25–35% while maintaining free ammonia concentrations below inhibitory thresholds (<200 mg/L) [141]. Future research must focus on synergistic optimization of antifouling membranes (e.g., graphene-coated membranes) and bioaugmentation techniques (e.g., electrogenic bacteria enrichment) to overcome AnMBR’s engineering limitations in complex matrices [142].
4.3. Physicochemical Treatment Technologies
Physicochemical technologies provide irreplaceable solutions for recalcitrant pollutants (e.g., microplastics) and complex toxicants (e.g., heavy metal complexes) through targeted molecular destruction or interfacial modulation. Fenton oxidation and photocatalysis achieve 70–85% mineralization of microplastics by breaking surface functional groups (-C=O, -OH) under pH 2.5–4.0 [143]. Ion-exchange resins exhibit selective adsorption capacities of 200–350 mg/g for Mn2+ and Cd2+, with coordination mechanisms effectively inhibiting metal re-dissolution [144,145]. Unlike biotreatment limited by enzymatic activity, these technologies demonstrate superior tolerance to extreme conditions (salinity > 5%, pH < 3), making them pivotal in emergency pollution control.
Composite passivators enhance heavy metal immobilization via multi-mechanistic synergy. Biochar (300–600 m2/g surface area) and humic acid form stable complexes with Cu2+/Zn2+ through carboxyl/phenolic groups, reducing acid-extractable metal fractions from 35–50% to 10–15% [63,146]. Simultaneously, passivators increase humification degree by 40–60%, further immobilizing metals via chelation.
Electrochemical pretreatment (EP) employs direct and indirect oxidation mechanisms. Direct mechanisms utilize high-voltage fields (5–15 kV) to induce cell lysis and polymer cleavage, while indirect mechanisms rely on electrogenerated radicals (·OH, ClO−) for non-selective degradation [147,148]. EP combined with sodium hypochlorite enhances sludge hydrolysis by 50–70% and methane yield by 25–35% [149].
However, scaling up physicochemical technologies requires balancing efficiency and cost. Microplastic (MP) enrichment in sludge reaches 75.7%, where source control (e.g., influent pretreatment) costs USD 0.2–0.5 per ton, and for end-of-life treatment, only 1/3–1/5 of end-of-pipe treatment [150,151]. Future efforts should focus on low-energy catalysts (e.g., non-noble metals) and smart process control systems to synergize pollutant removal with resource recovery.
5. Critical Analysis and Future Perspectives
Critical knowledge gaps persist regarding multiphase interactions of pollutants in anaerobic digestion (AD) systems. For microplastics (MNPs), systematic investigation is urgently needed on the synergistic effects of diverse MNP types during co-digestion of primary and waste-activated sludge, particularly focusing on fibrous MNPs (accounting for 60–75% of total sludge MNP load) and their interfacial behaviors. Standardized detection methods based on mass concentration (g/kg TS) rather than particle counts should be established to address data comparability issues caused by size heterogeneity [152]. Environmental fate studies of MNPs in biosolids must prioritize their role as vectors for antibiotic resistance genes (ARGs), especially the horizontal transfer efficiency of sul1 and tetW genes within soil–plant systems [153].
Breakthroughs in antibiotic removal technologies require deep mechanistic insights. Integrating metagenomics with metabolic flux analysis can elucidate electron transfer enhancement mechanisms at β-lactamase active sites by conductive materials like magnetite nanoparticles [154]. Hybrid AnMBR-UASB systems should be developed to optimize interphase mass transfer between biofilms and suspended sludge, achieving >95% removal efficiency for sulfonamide antibiotics [155]. Real-time biosensing systems using zebrafish embryo models are crucial for assessing the ecological toxicity of degradation intermediates [154].
Heavy metal (HM) bioremediation demands the directed development of resistant microbial resources. Genome editing of Methanosarcina spp. via CRISPR-Cas9 to introduce metallothionein expression modules could maintain >80% methanogenic activity under Cu2+ > 500 mg/L. A novel pyrolysis-AD cascade process (300–500 °C) could immobilize Cd/Pb in biochar matrices while enhancing methane yield by 30–45% [156]. Blockchain-based digestate traceability systems should be implemented for intelligent HM risk classification and land-use matching.
6. Conclusions
Anaerobic digestion (AD) systems confront a critical paradox: while achieving resource recovery from organic waste, they must simultaneously address escalating contamination challenges posed by microplastics (MPs), antibiotics, and heavy metals (HMs). Although AD systems function as biorefineries for methane recovery, they inadvertently concentrate persistent pollutants that threaten system stability. Polyethylene- and polypropylene-dominated MPs, coupled with tetracycline- and sulfonamide-class antibiotics, not only propagate antibiotic resistance genes but also suppress methanogenic archaeal activity. Furthermore, copper and zinc HMs compromise microbial vitality through bioaccumulation. Consequently, integrating AD with optimized reactor configurations, advanced materials, bioaugmentation strategies, and physicochemical technologies may present a promising multi-barrier approach for treating contaminated biowastes. This synergistic paradigm could potentially reconcile the dual objectives of waste valorization and contaminant mitigation in circular bioeconomy frameworks.
Author Contributions
Writing—review and editing, conceptualization, methodology, resources, supervision, project administration, funding acquisition, H.L.; writing—original draft preparation, X.Y.; data curation, visualization, Y.Y., L.Y. and J.Z.; writing—review and editing, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Projects of International Cooperation Shanghai grant number [STCSM 23230711300].
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chan, P.C.; de Toledo, R.A.; Iu, H.I.; Shim, H. Effect of Zinc Supplementation on Biogas Production and Short/Long Chain Fatty Acids Accumulation During Anaerobic Co-digestion of Food Waste and Domestic Wastewater. Waste Biomass Valoriz. 2019, 10, 3885–3895. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Kasama, T.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Effect of metal oxide based TiO2 nanoparticles on anaerobic digestion process of lignocellulosic substrate. Energy 2020, 191, 116580. [Google Scholar] [CrossRef]
- Zglobisz, N.; Castillo-Castillo, A.; Grimes, S.; Jones, P. Influence of UK energy policy on the deployment of anaerobic digestion. Energy Policy 2010, 38, 5988–5999. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Sweeney, S.; Feng, Y. Household biogas use in rural China: A study of opportunities and constraints. Renew. Sustain. Energy Rev. 2010, 14, 545–549. [Google Scholar] [CrossRef]
- Ferrer, I.; Garfí, M.; Uggetti, E.; Ferrer-Martí, L.; Calderon, A.; Velo, E. Biogas production in low-cost household digesters at the Peruvian Andes. Biomass Bioenergy 2011, 35, 1668–1674. [Google Scholar] [CrossRef]
- Dechrugsa, S.; Kantachote, D.; Chaiprapat, S. Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure. Bioresour. Technol. 2013, 146, 101–108. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Donner, E.; McGrath, S.P.; Tscharke, B.J.; O’Brien, J.W.; O’Brien, S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; et al. Plastics in biosolids from 1950 to 2016: A function of global plastic production and consumption. Water Res. 2021, 201, 117367. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wei, W.; Huang, Q.-S.; Wang, C.; Wang, Y.; Ni, B.-J. Insights into the microbial response of anaerobic granular sludge during long-term exposure to polyethylene terephthalate microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef]
- Stapleton, M.J.; Ansari, A.J.; Hai, F.I. Antibiotic sorption onto microplastics in water: A critical review of the factors, mechanisms and implications. Water Res. 2023, 233, 119790. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Dou, Y.; Ji, B.; Miao, M.; Li, Y.; Hao, T. Chlorination-improved adsorption capacity of microplastics for antibiotics: A combined experimental and molecular mechanism investigation. J. Hazard. Mater. 2024, 467, 133734. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Zhao, Q.; Wei, L.; Yang, Q. Heavy metal concentration and speciation of seven representative municipal sludges from wastewater treatment plants in Northeast China. Environ. Monit. Assess. 2012, 184, 1645–1655. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, H.; Zeng, G.; Li, H.; Wang, J.; Zhou, C.; Zhu, H.; Pei, X.; Liu, Z.; Liu, Z. Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge. Bioresour. Technol. 2011, 102, 4104–4110. [Google Scholar] [CrossRef]
- Zhou, W.; Tuersun, N.; Zhang, Y.; Wang, Y.; Cheng, C.; Chen, X. Optimization and system energy balance analysis of anaerobic co-digestion process of pretreated textile dyeing sludge and food waste. J. Environ. Chem. Eng. 2021, 9, 106855. [Google Scholar] [CrossRef]
- Bartrons, M.; Peñuelas, J. Pharmaceuticals and Personal-Care Products in Plants. Trends Plant Sci. 2017, 22, 194–203. [Google Scholar] [CrossRef]
- Ahmed, A.S.S.; Billah, M.M.; Ali, M.M.; Bhuiyan, M.K.A.; Guo, L.; Mohinuzzaman, M.; Hossain, M.B.; Rahman, M.S.; Islam, M.S.; Yan, M.; et al. Microplastics in aquatic environments: A comprehensive review of toxicity, removal, and remediation strategies. Sci. Total Environ. 2023, 876, 162414. [Google Scholar] [CrossRef]
- Piyawardhana, N.; Weerathunga, V.; Chen, H.-S.; Guo, L.; Huang, P.-J.; Ranatunga, R.R.M.K.P.; Hung, C.-C. Occurrence of microplastics in commercial marine dried fish in Asian countries. J. Hazard. Mater. 2022, 423, 127093. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Z.; Zhang, Y.; Zhu, P.; Jiang, R.; Wang, M.; Wang, Y.; Lu, G. Co-exposure of microplastics and sulfamethoxazole propagated antibiotic resistance genes in sediments by regulating the microbial carbon metabolism. J. Hazard. Mater. 2024, 463, 132951. [Google Scholar] [CrossRef]
- Repinc, S.K.; Bizjan, B.; Budhiraja, V.; Dular, M.; Gostiša, J.; Brajer Humar, B.; Kaurin, A.; Kržan, A.; Levstek, M.; Arteaga, J.F.M.; et al. Integral analysis of hydrodynamic cavitation effects on waste activated sludge characteristics, potentially toxic metals, microorganisms and identification of microplastics. Sci. Total Environ. 2022, 806, 151414. [Google Scholar] [CrossRef]
- Dong, S.; Gao, P.; Li, B.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Occurrence and migration of microplastics and plasticizers in different wastewater and sludge treatment units in municipal wastewater treatment plant. Front. Environ. Sci. Eng. 2022, 16, 142. [Google Scholar] [CrossRef]
- Xiao, Y.; Qin, Y.; Jiang, X.; Gao, P. Effects of polypropylene microplastics on digestion performance, microbial community, and antibiotic resistance during microbial anaerobic digestion. Bioresour. Technol. 2024, 411, 131358. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q.-S.; Sun, J.; Dai, X.; Ni, B.-J. Revealing the Mechanisms of Polyethylene Microplastics Affecting Anaerobic Digestion of Waste Activated Sludge. Environ. Sci. Technol. 2019, 53, 9604–9613. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Ceccarini, A.; Petri, A.; Vinciguerra, V. Nylon 6 and nylon 6,6 micro- and nanoplastics: A first example of their accurate quantification, along with polyester (PET), in wastewater treatment plant sludges. J. Hazard. Mater. 2021, 407, 124364. [Google Scholar] [CrossRef]
- Azizi, S.M.M.; Haffiez, N.; Zakaria, B.S.; Dhar, B.R. Thermal Hydrolysis of Sludge Counteracts Polystyrene Nanoplastics-Induced Stress during Anaerobic Digestion. ACS EST Eng. 2022, 2, 1306–1315. [Google Scholar] [CrossRef]
- Fu, S.-F.; Ding, J.-N.; Zhang, Y.; Li, Y.-F.; Zhu, R.; Yuan, X.-Z.; Zou, H. Exposure to polystyrene nanoplastic leads to inhibition of anaerobic digestion system. Sci. Total Environ. 2018, 625, 64–70. [Google Scholar] [CrossRef]
- Mohammad Mirsoleimani Azizi, S.; Hai, F.I.; Lu, W.; Al-Mamun, A.; Ranjan Dhar, B. A review of mechanisms underlying the impacts of (nano)microplastics on anaerobic digestion. Bioresour. Technol. 2021, 329, 124894. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Han, W.; Jiao, J.; Ren, W.; Jia, G.; Huang, C.; Yang, Q. Migration and transformation modes of microplastics in reclaimed wastewater treatment plant and sludge treatment center with thermal hydrolysis and anaerobic digestion. Bioresour. Technol. 2024, 400, 130649. [Google Scholar] [CrossRef]
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef]
- Bao, L.-J.; Mai, L.; Liu, L.-Y.; Sun, X.-F.; Zeng, E.Y. Microplastics on the Planet: Current Knowledge and Challenges. Environ. Sci. Technol. Lett. 2024, 11, 1262–1271. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Ahmed Siddique, M.B.; Kamal, A.; Coyne, M.S. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 704–720. [Google Scholar] [CrossRef]
- Li, Y.-X.; Zhang, X.-L.; Li, W.; Lu, X.-F.; Liu, B.; Wang, J. The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ. Monit. Assess. 2013, 185, 2211–2220. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 2012, 235–236, 178–185. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Ying, G.-G.; Liu, S.; Zhang, R.-Q.; Lai, H.-J.; Chen, Z.-F.; Pan, C.-G. Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci. Total Environ. 2013, 444, 183–195. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A.M. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Van den Meersche, T.; Van Pamel, E.; Van Poucke, C.; Herman, L.; Heyndrickx, M.; Rasschaert, G.; Daeseleire, E. Development, validation and application of an ultra high performance liquid chromatographic-tandem mass spectrometric method for the simultaneous detection and quantification of five different classes of veterinary antibiotics in swine manure. J. Chromatogr. A 2016, 1429, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Widyasari-Mehta, A.; Hartung, S.; Kreuzig, R. From the application of antibiotics to antibiotic residues in liquid manures and digestates: A screening study in one European center of conventional pig husbandry. J. Environ. Manag. 2016, 177, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, J.; Bai, Y.; Wang, F.; Xie, B. Incomplete degradation of aromatic–aliphatic copolymer leads to proliferation of microplastics and antibiotic resistance genes. Environ. Int. 2023, 181, 108291. [Google Scholar] [CrossRef]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Fels, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef]
- Luo, J.; Huang, W.; Zhang, Q.; Wu, Y.; Fang, F.; Cao, J.; Su, Y. Distinct effects of hypochlorite types on the reduction of antibiotic resistance genes during waste activated sludge fermentation: Insights of bacterial community, cellular activity, and genetic expression. J. Hazard. Mater. 2021, 403, 124010. [Google Scholar] [CrossRef]
- Zubair, M.; Li, Z.; Zhu, R.; Wang, J.; Liu, X.; Liu, X. The Antibiotics Degradation and Its Mechanisms during the Livestock Manure Anaerobic Digestion. Molecules 2023, 28, 4090. [Google Scholar] [CrossRef]
- Haffiez, N.; Chung, T.H.; Zakaria, B.S.; Shahidi, M.; Mezbahuddin, S.; Hai, F.I.; Dhar, B.R. A critical review of process parameters influencing the fate of antibiotic resistance genes in the anaerobic digestion of organic waste. Bioresour. Technol. 2022, 354, 127189. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, L.; Fu, H.; Zhang, M.; Meng, J.; Althakafy, J.T.; Abo-Dief, H.M.; El-Bahy, S.M.; Zhang, Y.; Wei, H.; et al. Effect of sulfamethazine on anaerobic digestion of manure mediated by biochar. Chemosphere 2022, 306, 135567. [Google Scholar] [CrossRef]
- Matter, D.; Rossano, A.; Limat, S.; Vorlet-Fawer, L.; Brodard, I.; Perreten, V. Antimicrobial resistance profile of Actinobacillus pleuropneumoniae and Actinobacillus porcitonsillarum. Vet. Microbiol. 2007, 122, 146–156. [Google Scholar] [CrossRef]
- Gao, P.; Tang, X.; Tong, Y.; Chen, Y. Application of sewage sludge compost on highway embankments. Waste Manag. 2008, 28, 1630–1636. [Google Scholar] [CrossRef]
- Roca-Pérez, L.; Martínez, C.; Marcilla, P.; Boluda, R. Composting rice straw with sewage sludge and compost effects on the soil–plant system. Chemosphere 2009, 75, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, Z.; Guo, T.; Wu, J.; Wang, B.; Wei, Z.; Jia, L.; Kang, K. Effects of heavy metals stress on chicken manures composting via the perspective of microbial community feedback. Environ. Pollut. 2022, 294, 118624. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Wu, K.; Liang, S. Micronutrients in Soils, Crops, and Livestock. Earth Sci. Front. 2008, 15, 110–125. [Google Scholar] [CrossRef]
- Hsu, J.-H.; Lo, S.-L. Effect of composting on characterization and leaching of copper, manganese, and zinc from swine manure. Environ. Pollut. 2001, 114, 119–127. [Google Scholar] [CrossRef]
- Amir, S.; Hafidi, M.; Merlina, G.; Revel, J.-C. Sequential extraction of heavy metals during composting of sewage sludge. Chemosphere 2005, 59, 801–810. [Google Scholar] [CrossRef]
- Jin, H.; Chang, Z. Distribution of Heavy Metal Contents and Chemical Fractions in Anaerobically Digested Manure Slurry. Appl. Biochem. Biotechnol. 2011, 164, 268–282. [Google Scholar] [CrossRef]
- Ni, P.; Lyu, T.; Sun, H.; Dong, R.; Wu, S. Liquid digestate recycled utilization in anaerobic digestion of pig manure: Effect on methane production, system stability and heavy metal mobilization. Energy 2017, 141, 1695–1704. [Google Scholar] [CrossRef]
- Luo, H.; Lv, T.; Shi, M.; Wu, S.; Carvalho, P.N.; Dong, R. Stabilization of Preliminary Anaerobically Digested Slurry in Post-Storage: Dynamics of Chemical Characteristics and Hygienic Quality. Water Air Soil Pollut. 2017, 228, 306. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Li, G.; Luo, W.; Sun, Y. Manure digestate storage under different conditions: Chemical characteristics and contaminant residuals. Sci. Total Environ. 2018, 639, 19–25. [Google Scholar] [CrossRef]
- Riaz, L.; Wang, Q.; Yang, Q.; Li, X.; Yuan, W. Potential of industrial composting and anaerobic digestion for the removal of antibiotics, antibiotic resistance genes and heavy metals from chicken manure. Sci. Total Environ. 2020, 718, 137414. [Google Scholar] [CrossRef] [PubMed]
- GB 5084-2021; Standard for Irrigation Water Quality. Ministry of Ecology and Environment of the Peoole’s Republic of China: Beijing, China, 2021.
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhuang, J.; Xue, J.; Peng, M.; Zhang, W.; Mao, L. Passivation mechanism of Cu and Zn with the introduction of composite passivators during anaerobic digestion of pig manure. Bioresour. Technol. 2023, 369, 128360. [Google Scholar] [CrossRef] [PubMed]
- Gutnick, D.L.; Bach, H. Engineering bacterial biopolymers for the biosorption of heavy metals; new products and novel formulations. Appl. Microbiol. Biotechnol. 2000, 54, 451–460. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Huang, J.; Du, Z.; Zhouyang, S.; Wang, Y.; Zheng, Y.; Li, Q.; Shen, X. The influence of variables on the bioavailability of heavy metals during the anaerobic digestion of swine manure. Ecotoxicol. Environ. Saf. 2020, 195, 110457. [Google Scholar] [CrossRef]
- Marcato, C.E.; Pinelli, E.; Pouech, P.; Winterton, P.; Guiresse, M. Particle size and metal distributions in anaerobically digested pig slurry. Bioresour. Technol. 2008, 99, 2340–2348. [Google Scholar] [CrossRef]
- Singh, R.; Agrawal, M. Use of sewage sludge as fertiliser supplement for Abelmoschus esculentus plants: Physiological, biochemical and growth responses. Int. J. Environ. Waste Manag. 2009, 3, 91. [Google Scholar] [CrossRef]
- Wang, C.; Wei, W.; Chen, Z.; Wang, Y.; Chen, X.; Ni, B.J. Polystyrene microplastics and nanoplastics distinctively affect anaerobic sludge treatment for hydrogen and methane production. Sci. Total Environ. 2022, 850, 158085. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Wei, W.; Chen, H.; Ni, B.-J. Removal of microplastics and nanoplastics from urban waters: Separation and degradation. Water Res. 2022, 221, 118820. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, K.; Zhu, Y.; Ou, B.; Yu, W.; Liang, S.; Hou, H.; Yuan, S.; Gan, F.; Mi, R.; et al. Deciphering the role of microplastic size on anaerobic sludge digestion: Changes of dissolved organic matter, leaching compounds and microbial community. Environ. Res. 2022, 214, 114032. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Z.; Tang, M.; Yang, X.; Tsang, Y.F. Polycarbonate microplastics induce oxidative stress in anaerobic digestion of waste activated sludge by leaching bisphenol A. J. Hazard. Mater. 2023, 443, 130158. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, B.; Fan, C.; Zeng, G.; Xiong, W.; Zhou, C.; Yang, Y.; Ren, X.; Li, X.; Luo, K. The presence of cationic polyacrylamide attenuated the toxicity of polyvinyl chloride microplastics to anaerobic digestion of waste activated sludge. Chem. Eng. J. 2022, 427, 131442. [Google Scholar] [CrossRef]
- Mortezaei, Y.; Gaballah, M.S.; Demirer, G.N.; Lammers, R.W.; Williams, M.R. From wastewater to sludge: The role of microplastics in shaping anaerobic digestion performance and antibiotic resistance gene dynamics. J. Hazard. Mater. 2025, 489, 137413. [Google Scholar] [CrossRef]
- Chen, H.; Tang, M.; Yang, X.; Tsang, Y.F.; Wu, Y.; Wang, D.; Zhou, Y. Polyamide 6 microplastics facilitate methane production during anaerobic digestion of waste activated sludge. Chem. Eng. J. 2021, 408, 127251. [Google Scholar] [CrossRef]
- Wang, J.; Ma, D.; Feng, K.; Lou, Y.; Zhou, H.; Liu, B.; Xie, G.; Ren, N.; Xing, D. Polystyrene nanoplastics shape microbiome and functional metabolism in anaerobic digestion. Water Res. 2022, 219, 118606. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, L.; Zhu, X.; Huang, W.; Wang, J. Size-dependent oxidative stress effect of nano/micro-scaled polystyrene on Karenia mikimotoi. Mar. Pollut. Bull. 2020, 154, 111074. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q.-S.; Sun, J.; Wang, J.-Y.; Wu, S.-L.; Ni, B.-J. Polyvinyl Chloride Microplastics Affect Methane Production from the Anaerobic Digestion of Waste Activated Sludge through Leaching Toxic Bisphenol-A. Environ. Sci. Technol. 2019, 53, 2509–2517. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Wang, F.; Zhou, H.; Zhang, L.; Xie, B. Anaerobic Degradation of Aromatic and Aliphatic Biodegradable Plastics: Potential Mechanisms and Pathways. Environ. Sci. Technol. 2024, 58, 19462–19474. [Google Scholar] [CrossRef]
- Li, L.; Geng, S.; Li, Z.; Song, K. Effect of microplastic on anaerobic digestion of wasted activated sludge. Chemosphere 2020, 247, 125874. [Google Scholar] [CrossRef]
- Shi, J.; Dang, Q.; Zhang, C.; Zhao, X. Insight into effects of polyethylene microplastics in anaerobic digestion systems of waste activated sludge: Interactions of digestion performance, microbial communities and antibiotic resistance genes. Environ. Pollut. 2022, 310, 119859. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, D.; Jin, B.; Su, Y.; Zhang, Y. Deciphering the role of polyethylene microplastics on antibiotic resistance genes and mobile genetic elements fate in sludge thermophilic anaerobic digestion process. Chem. Eng. J. 2023, 452, 139520. [Google Scholar] [CrossRef]
- Stone, J.J.; Clay, S.A.; Zhu, Z.; Wong, K.L.; Porath, L.R.; Spellman, G.M. Effect of antimicrobial compounds tylosin and chlortetracycline during batch anaerobic swine manure digestion. Water Res. 2009, 43, 4740–4750. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, N.A.; Sakai, K.; Shirai, Y.; Maeda, T. Impact of different antibiotics on methane production using waste-activated sludge: Mechanisms and microbial community dynamics. Appl. Microbiol. Biotechnol. 2016, 100, 9355–9364. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Orhon, D. How do sulfamethoxazole and tetracycline affect the utilization of short chain fatty acids under anaerobic conditions? J. Environ. Chem. Eng. 2018, 6, 1305–1313. [Google Scholar] [CrossRef]
- Ni, B.-J.; Zeng, S.; Wei, W.; Dai, X.; Sun, J. Impact of roxithromycin on waste activated sludge anaerobic digestion: Methane production, carbon transformation and antibiotic resistance genes. Sci. Total Environ. 2020, 703, 134899. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Ince, B.; Orhon, D.; Ince, O. Anaerobic sulfamethoxazole degradation is driven by homoacetogenesis coupled with hydrogenotrophic methanogenesis. Water Res. 2016, 90, 79–89. [Google Scholar] [CrossRef]
- Gartiser, S.; Urich, E.; Alexy, R.; Kümmerer, K. Anaerobic inhibition and biodegradation of antibiotics in ISO test schemes. Chemosphere 2007, 66, 1839–1848. [Google Scholar] [CrossRef]
- Sponza, D.T.; Demirden, P. Treatability of sulfamerazine in sequential upflow anaerobic sludge blanket reactor (UASB)/completely stirred tank reactor (CSTR) processes. Sep. Purif. Technol. 2007, 56, 108–117. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J.; Frear, C. The effects of the antibiotics ampicillin, florfenicol, sulfamethazine, and tylosin on biogas production and their degradation efficiency during anaerobic digestion. Bioresour. Technol. 2013, 149, 244–252. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Ince, B.; Gros, M.; Rodriguez-Mozaz, S.; Barceló, D.; Orhon, D.; Ince, O. Chronic impact of tetracycline on the biodegradation of an organic substrate mixture under anaerobic conditions. Water Res. 2013, 47, 2959–2969. [Google Scholar] [CrossRef]
- Xiong, Y.; Harb, M.; Hong, P.-Y. Performance and microbial community variations of anaerobic digesters under increasing tetracycline concentrations. Appl. Microbiol. Biotechnol. 2017, 101, 5505–5517. [Google Scholar] [CrossRef] [PubMed]
- Massé, D.I.; Lu, D.; Masse, L.; Droste, R.L. Effect of antibiotics on psychrophilic anaerobic digestion of swine manure slurry in sequencing batch reactors. Bioresour. Technol. 2000, 75, 205–211. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Liu, J.; Yu, D.; Zhong, H.; Wang, Y.; Chen, M.; Tong, J.; Wei, Y. Effects of chlortetracycline, Cu and their combination on the performance and microbial community dynamics in swine manure anaerobic digestion. J. Environ. Sci. 2018, 67, 206–215. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodríguez, N.; Amils, R. The action of antibiotics on the anaerobic digestion process. Appl. Microbiol. Biotechnol. 1996, 46, 587–592. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Gu, J.; Qian, X.; Gao, H.; Qin, Q. Effects of oxytetracycline on archaeal community, and tetracycline resistance genes in the anaerobic co-digestion of pig manure and wheat straw. Environ. Technol. 2016, 37, 3177–3185. [Google Scholar] [CrossRef]
- Beneragama, N.; Lateef, S.A.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. The combined effect of cefazolin and oxytetracycline on biogas production from thermophilic anaerobic digestion of dairy manure. Bioresour. Technol. 2013, 133, 23–30. [Google Scholar] [CrossRef]
- Lallai, A.; Mura, G.; Onnis, N. The effects of certain antibiotics on biogas production in the anaerobic digestion of pig waste slurry. Bioresour. Technol. 2002, 82, 205–208. [Google Scholar] [CrossRef]
- Arikan, O.A.; Sikora, L.J.; Mulbry, W.; Khan, S.U.; Rice, C.; Foster, G.D. The fate and effect of oxytetracycline during the anaerobic digestion of manure from therapeutically treated calves. Process Biochem. 2006, 41, 1637–1643. [Google Scholar] [CrossRef]
- Álvarez, J.A.; Otero, L.; Lema, J.M.; Omil, F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour. Technol. 2010, 101, 8581–8586. [Google Scholar] [CrossRef]
- Aydin, S.; Ince, B.; Ince, O. Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Res. 2015, 76, 88–98. [Google Scholar] [CrossRef]
- Aydin, S.; Ince, B.; Cetecioglu, Z.; Arikan, O.; Ozbayram, E.G.; Shahi, A.; Ince, O. Combined effect of erythromycin, tetracycline and sulfamethoxazole on performance of anaerobic sequencing batch reactors. Bioresour. Technol. 2015, 186, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mai, D.T.; Stuckey, D.C.; Oh, S. Effect of ciprofloxacin on methane production and anaerobic microbial community. Bioresour. Technol. 2018, 261, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Hao, X.; Zhang, R.; Wang, J.; Liu, R.; Liu, C. Tetracycline removal and effect on the formation and degradation of extracellular polymeric substances and volatile fatty acids in the process of hydrogen fermentation. Bioresour. Technol. 2016, 212, 20–25. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Biogas production as affected by heavy metals in the anaerobic digestion of sludge. Egypt. J. Pet. 2014, 23, 409–417. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Shirai, T.; Yamashiro, T.; Yasui, S.; Iwasaki, M.; Ihara, I.; Nishida, T.; Tangtaweewipat, S.; Umetsu, K. Valorizing waste iron powder in biogas production: Hydrogen sulfide control and process performances. J. Environ. Manag. 2018, 208, 134–141. [Google Scholar] [CrossRef]
- Hao, H.; Tian, Y.; Zhang, H.; Chai, Y. Copper stressed anaerobic fermentation: Biogas properties, process stability, biodegradation and enzyme responses. Biodegradation 2017, 28, 369–381. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Chai, Y.; Wang, L.; Mi, X.; Zhang, L.; Ware, M.A. Biogas properties and enzymatic analysis during anaerobic fermentation of Phragmites australis straw and cow dung: Influence of nickel chloride supplement. Biodegradation 2017, 28, 15–25. [Google Scholar] [CrossRef]
- Facchin, V.; Cavinato, C.; Fatone, F.; Pavan, P.; Cecchi, F.; Bolzonella, D. Effect of trace element supplementation on the mesophilic anaerobic digestion of foodwaste in batch trials: The influence of inoculum origin. Biochem. Eng. J. 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef]
- Khatri, S.; Wu, S.; Kizito, S.; Zhang, W.; Li, J.; Dong, R. Synergistic effect of alkaline pretreatment and Fe dosing on batch anaerobic digestion of maize straw. Appl. Energy 2015, 158, 55–64. [Google Scholar] [CrossRef]
- Guo, X.; Gu, J.; Gao, H.; Qin, Q.; Chen, Z.; Shao, L.; Chen, L.; Li, H.; Zhang, W.; Chen, S.; et al. Effects of Cu on metabolisms and enzyme activities of microbial communities in the process of composting. Bioresour. Technol. 2012, 108, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, Z.; Tian, G. Inhibitory effects of Cu (II) on fermentative methane production using bamboo wastewater as substrate. J. Hazard. Mater. 2011, 195, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, X.; Tian, Y.; Li, Y.; Yang, K.; Hao, H.; Chai, Y.; Xu, X. Process analysis of anaerobic fermentation of Phragmites australis straw and cow dung exposing to elevated chromium (VI) concentrations. J. Environ. Manag. 2018, 224, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Miglani, P.; Gupta, R.K.; Bhattacharya, T.K. Impact of Ni(II), Zn(II) and Cd(II) on biogassification of potato waste. J. Environ. Biol. 2006, 27, 61–66. [Google Scholar]
- Mal, J.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Effect of heavy metal co-contaminants on selenite bioreduction by anaerobic granular sludge. Bioresour. Technol. 2016, 206, 1–8. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, Y.; Yang, G.; Qin, X.; Song, Z. Household biogas development in rural China: On policy support and other macro sustainable conditions. Renew. Sustain. Energy Rev. 2012, 16, 5617–5624. [Google Scholar] [CrossRef]
- Phan, H.V.; Wickham, R.; Xie, S.; McDonald, J.A.; Khan, S.J.; Ngo, H.H.; Guo, W.; Nghiem, L.D. The fate of trace organic contaminants during anaerobic digestion of primary sludge: A pilot scale study. Bioresour. Technol. 2018, 256, 384–390. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Giese, E.C. Biosorption as green technology for the recovery and separation of rare earth elements. World J. Microbiol. Biotechnol. 2020, 36, 52. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, C.; Ai, S.; Wang, H.; Gao, Y.; Yan, L.; Mei, Z.; Wang, W. Biological pretreatment enhances the activity of functional microorganisms and the ability of methanogenesis during anaerobic digestion. Bioresour. Technol. 2019, 290, 121660. [Google Scholar] [CrossRef] [PubMed]
- Akgul, D.; Cella, M.A.; Eskicioglu, C. Influences of low-energy input microwave and ultrasonic pretreatments on single-stage and temperature-phased anaerobic digestion (TPAD) of municipal wastewater sludge. Energy 2017, 123, 271–282. [Google Scholar] [CrossRef]
- Kim, H.-W.; Nam, J.-Y.; Shin, H.-S. A comparison study on the high-rate co-digestion of sewage sludge and food waste using a temperature-phased anaerobic sequencing batch reactor system. Bioresour. Technol. 2011, 102, 7272–7279. [Google Scholar] [CrossRef]
- Banu, J.R.; Do, K.-U.; Yeom, I.-T. Effect of ferrous sulphate on nitrification during simultaneous phosphorus removal from domestic wastewater using a laboratory scale anoxic/oxic reactor. World J. Microbiol. Biotechnol. 2008, 24, 2981–2986. [Google Scholar] [CrossRef]
- Uthirakrishnan, U.; Sharmila, V.G.; Merrylin, J.; Adish Kumar, S.; Dharmadhas, J.S.; Varjani, S.; Banu, J.R. Current advances and future outlook on pretreatment techniques to enhance biosolids disintegration and anaerobic digestion: A critical review. Chemosphere 2022, 288, 132553. [Google Scholar] [CrossRef]
- Ye, J.; Mu, Y.; Cheng, X.; Sun, D. Treatment of fresh leachate with high-strength organics and calcium from municipal solid waste incineration plant using UASB reactor. Bioresour. Technol. 2011, 102, 5498–5503. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Z.; Ruan, W.; Zhao, M.; Shao, Y.; Miao, H. Insights into sludge granulation during anaerobic treatment of high-strength leachate via a full-scale IC reactor with external circulation system. J. Environ. Sci. 2018, 64, 227–234. [Google Scholar] [CrossRef]
- Chen, S.; Sun, D.; Chung, J.-S. Simultaneous removal of COD and ammonium from landfill leachate using an anaerobic–aerobic moving-bed biofilm reactor system. Waste Manag. 2008, 28, 339–346. [Google Scholar] [CrossRef]
- Luo, J.; Lu, X.; Liu, J.; Qian, G.; Lu, Y. Biogas recirculation for simultaneous calcium removal and biogas purification within an expanded granular sludge bed system treating leachate. Bioresour. Technol. 2014, 173, 317–323. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zou, X.; Feng, J.; Wu, Z. Microbial communities in an anaerobic dynamic membrane bioreactor (AnDMBR) for municipal wastewater treatment: Comparison of bulk sludge and cake layer. Process Biochem. 2013, 48, 510–516. [Google Scholar] [CrossRef]
- Wei, C.-H.; Harb, M.; Amy, G.; Hong, P.-Y.; Leiknes, T. Sustainable organic loading rate and energy recovery potential of mesophilic anaerobic membrane bioreactor for municipal wastewater treatment. Bioresour. Technol. 2014, 166, 326–334. [Google Scholar] [CrossRef]
- Greses, S.; Gaby, J.C.; Aguado, D.; Ferrer, J.; Seco, A.; Horn, S.J. Microbial community characterization during anaerobic digestion of Scenedesmus spp. under mesophilic and thermophilic conditions. Algal Res. 2017, 27, 121–130. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Anaerobic membrane bioreactors (AnMBRs) for municipal wastewater treatment- potential benefits, constraints, and future perspectives: An updated review. Sci. Total Environ. 2022, 802, 149612. [Google Scholar] [CrossRef]
- Li, Y.; Wu, B.; Zhai, X.; Li, Q.; Fan, C.; Li, Y.-Y.; Sano, D.; Chen, R. Removal of RNA viruses from swine wastewater using anaerobic membrane bioreactor: Performance and mechanisms. J. Hazard. Mater. 2024, 471, 134296. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Saleem, M.; Lavagnolo, M.C.; Campanaro, S.; Squartini, A. Dynamic membrane bioreactor (DMBR) for the treatment of landfill leachate; bioreactor’s performance and metagenomic insights into microbial community evolution. Environ. Pollut. 2018, 243, 326–335. [Google Scholar] [CrossRef]
- Asif, M.B.; Maqbool, T.; Zhang, Z. Electrochemical membrane bioreactors: State-of-the-art and future prospects. Sci. Total Environ. 2020, 741, 140233. [Google Scholar] [CrossRef]
- Chen, M.; Ren, L.; Qi, K.; Li, Q.; Lai, M.; Li, Y.; Li, X.; Wang, Z. Enhanced removal of pharmaceuticals and personal care products from real municipal wastewater using an electrochemical membrane bioreactor. Bioresour. Technol. 2020, 311, 123579. [Google Scholar] [CrossRef]
- Yin, D.-M.; Taherzadeh, M.J.; Lin, M.; Jiang, M.-M.; Qiao, W.; Dong, R.-J. Upgrading the anaerobic membrane bioreactor treatment of chicken manure by introducing in-situ ammonia stripping and hyper-thermophilic pretreatment. Bioresour. Technol. 2020, 310, 123470. [Google Scholar] [CrossRef]
- El Kik, O.; Lesage, G.; Zaviska, F.; Sauvêtre, A.; Heran, M.; Lestremau, F. Synergistic approach for enhanced wastewater treatment: Harnessing the potential of bioelectrochemical systems in integration with anaerobic membrane bioreactors. J. Environ. Chem. Eng. 2024, 12, 113162. [Google Scholar] [CrossRef]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C.; de Pedro, Z.M.; Casas, J.A. Insights into the degradation of microplastics by Fenton oxidation: From surface modification to mineralization. Chemosphere 2022, 309, 136809. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yang, L.; Zhou, Y.; Wang, X.; Xu, G.; Sun, K.; Yang, Y.; Wang, S.; Liu, T. Adsorptive photocatalytic removal of heavy metal Mn by organic photovoltaic materials. Sep. Purif. Technol. 2025, 368, 133066. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Chen, Y.; Zhuang, Z.; Chen, F.-F.; Zhu, Y.-J.; Yu, Y. Recycling heavy metals from wastewater for photocatalytic CO2 reduction. Chem. Eng. J. 2020, 402, 125922. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, B.; Peng, M.; Shen, R.; Mao, L.; Zhang, W. Effects of Inorganic Passivators on Gas Production and Heavy Metal Passivation Performance during Anaerobic Digestion of Pig Manure and Corn Straw. Int. J. Environ. Res. Public Health 2022, 19, 14094. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Zheng, L.; Wang, Z.; Dai, X. Perspective on enhancing the anaerobic digestion of waste activated sludge. J. Hazard. Mater. 2020, 389, 121847. [Google Scholar] [CrossRef]
- Ivanenko, A.A.; Laikova, A.A.; Zhuravleva, E.A.; Shekhurdina, S.V.; Loiko, N.G.; Kotova, I.B.; Kovalev, A.A.; Kovalev, D.A.; Panchenko, V.A.; Mamedov, S.E.; et al. Effect of indirect electrochemical pretreatment on the anaerobic digestion of swine manure. Int. J. Hydrog. Energy 2024, 95, 278–289. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, B.; Cheng, P.; Zhu, N.; Yin, C.; Ying, L. Pilot-scale study of enhanced anaerobic digestion of waste activated sludge by electrochemical and sodium hypochlorite combination pretreatment. Int. Biodeterior. Biodegrad. 2016, 110, 227–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Hou, L.; Kumar, D.; Yoo, C.G.; Gitsov, I.; Majumder, E.L.W. Conversion and removal strategies for microplastics in wastewater treatment plants and landfills. Chem. Eng. J. 2021, 406, 126715. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 16–22. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, D.; Su, Y.; Xie, B. Occurrence, influence and removal strategies of mycotoxins, antibiotics and microplastics in anaerobic digestion treating food waste and co-digestive biosolids: A critical review. Bioresour. Technol. 2021, 330, 124987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Meshref, M.N.A.; Dhar, B.R. Optimization of thermal hydrolysis process for enhancing anaerobic digestion in a wastewater treatment plant with existing primary sludge fermentation. Bioresour. Technol. 2021, 321, 124498. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).