Hydrothermal Extraction of Cellulose from Sugarcane Bagasse for Production of Biodegradable Food Containers
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Ability
2.2. Cellulose Characterization
2.3. Food Container Production
3. Materials and Methods
3.1. Material
3.2. Cellulose Extraction
3.2.1. Chemical Method
3.2.2. Hydrothermal Method
3.3. Food Container Molding
3.4. Characterization
3.4.1. Chemical Composition
- The determination of hemicelluloses:
- The determination of lignin:
- The determination of cellulose:
3.4.2. Functional Group
3.4.3. Thermal Stability
3.4.4. Crystal Structure
3.4.5. Morphology
3.4.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, L.J.R.; Causer, T.P.; Ciolkosz, D. Biomass for energy: A review on supply chain management models. Renew. Sustain. Energy Rev. 2020, 120, 109658. [Google Scholar] [CrossRef]
- Husin, M.; Rahim, N.; Ahmad, M.R.; Romli, A.Z.; Ilham, Z. Hydrolysis of microcrystalline cellulose isolated from waste seeds of Leucaena leucocephala for glucose production. Malays. J. Fundam. Appl. Sci. 2019, 15, 200–205. [Google Scholar] [CrossRef]
- Chopra, L.; Manikanika. Extraction of cellulosic fibers from the natural resources: A short review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Trisanti, P.N.; Rifan, M.; Akbar, P.; Gunardi, I.; Sumarno, S. Isolation of cellulose from teak wood using hydrothermal method. AIP Conf. Proc. 2021, 2349, 020047. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, K.; Wang, M.; Xing, T.; Chen, C.; Wang, Q. Microwave-assisted Formic Acid/Cold Caustic Extraction for Separation of Cellulose and Hemicellulose from Biomass. BioResources 2023, 18, 6896–6912. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Zhao, H.; Sun, R.C. Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- Khumla, N.; Sakuanrungsirikul, S.; Punpee, P.; Hamarn, T.; Chaisan, T.; Soulard, L.; Songsri, P. Sugarcane Breeding, Germplasm Development and Supporting Genetics Research in Thailand. Sugar Tech 2022, 24, 193–209. [Google Scholar] [CrossRef]
- Amores, I.; Ballesteros, I.; Manzanares, P.; Sáez, F.; Michelena, G.; Ballesteros, M. Ethanol Production from Sugarcane Bagasse Pretreated by Steam Explosion. Electron. J. Energy Environ. 2013, 1, 25–36. [Google Scholar] [CrossRef]
- Ferreira-Leitão, V.; Perrone, C.C.; Rodrigues, J.; Franke, A.P.M.; Macrelli, S.; Zacchi, G. An approach to the utilisation of CO2 as impregnating agent in steam pretreatment of sugar cane bagasse and leaves for ethanol production. Biotechnol. Biofuels 2010, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Armijos, J.; Lapo, B.; Beltrán, C.; Sigüenza, J.; Madrid, B.; Chérrez, E.; Bravo, V.; Sanmartín, D. Effect of Alkaline and Hydrothermal Pretreatments in Sugars and Ethanol Production from Rice Husk Waste. Resources 2024, 13, 128. [Google Scholar] [CrossRef]
- Akatwijuka, O.; Gepreel, M.A.H.; Abdel-Mawgood, A.; Yamamoto, M.; Saito, Y.; Hassanin, A.H. Green hydrothermal extraction of banana cellulosic fibers by seawater-assisted media as an alternative to freshwater: Physico-chemical, morphological and mechanical properties. Cellulose 2023, 30, 9989–10008. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Ling, C.; Hou, W.; Yan, Z. Extraction and characterization of microcrystalline cellulose from waste cotton fabrics via hydrothermal method. Waste Manag. 2018, 82, 139–146. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Combined pretreatment using alkaline hydrothermal and ball milling to enhance enzymatic hydrolysis of oil palm mesocarp fiber. Bioresour. Technol. 2014, 169, 236–243. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono; Trisanti, P.N.; Setyawan, H.; Madhania, S.; Sambodho, K.; Winardi, S.; Adschiri, T.; Goto, M. Hydrothermal alkaline treatment of lignocellulosic biomass for microcrystalline cellulose generation at subcritical water. S. Afr. J. Chem. Eng. 2025, 51, 45–56. [Google Scholar] [CrossRef]
- Saelee, K.; Yingkamhaeng, N.; Nimchua, T.; Sukyai, P. Extraction and characterization of cellulose from sugarcane bagasse by using environmental friendly method. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Chiang Rai, Thailand, 26–29 November 2014; pp. 162–168. [Google Scholar]
- Melesse, G.T.; Hone, F.G.; Mekonnen, M.A. Extraction of Cellulose from Sugarcane Bagasse Optimization and Characterization. Adv. Mater. Sci. Eng. 2022, 2022, 1712207. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Mafa, M.S.; Malgas, S.; Bhattacharya, A.; Rashamuse, K.; Pletschke, B.I. The Effects of Alkaline Pretreatment on Agricultural Biomasses (Corn Cob and Sweet Sorghum Bagasse) and Their Hydrolysis by a Termite-Derived Enzyme Cocktail. Agronomy 2020, 10, 1211. [Google Scholar] [CrossRef]
- Kumneadklang, S.; O-Thong, S.; Larpkiattaworn, S. Characterization of cellulose fiber isolated from oil palm frond biomass. Mater. Today Proc. 2019, 17, 1995–2001. [Google Scholar] [CrossRef]
- Rattanaporn, K.; Tantayotai, P.; Phusantisampan, T.; Pornwongthong, P.; Sriariyanun, M. Organic acid pretreatment of oil palm trunk: Effect on enzymatic saccharification and ethanol production. Bioprocess Biosyst. Eng. 2018, 41, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Xiong, B.; Ma, S.; Chen, B.; Feng, Y.; Peng, Z.; Tang, X.; Yang, S.; Sun, Y.; Lin, L.; Zeng, X.; et al. Formic acid-facilitated hydrothermal pretreatment of raw biomass for co-producing xylo-oligosaccharides, glucose, and lignin. Ind. Crops Prod. 2023, 193, 116195. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 846592. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; Liu, Y.; Chen, L.; Zhang, H.; Ma, L. Identification of the Soluble Byproducts Formed during the Hydrothermal Conversion of Cellulose Catalyzed by Solid Tungstated Alumina. ACS Omega 2020, 5, 19140–19150. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S. Valorization of sugarcane bagasse by chemical pretreatment and enzyme mediated deconstruction. Sci. Rep. 2019, 9, 15904. [Google Scholar] [CrossRef]
- Choquecahua Mamani, D.; Otero Nole, K.S.; Chaparro Montoya, E.E.; Mayta Huiza, D.A.; Pastrana Alta, R.Y.; Aguilar Vitorino, H. Minimizing Organic Waste Generated by Pineapple Crown: A Simple Process to Obtain Cellulose for the Preparation of Recyclable Containers. Recycling 2020, 5, 24. [Google Scholar] [CrossRef]
- Romruen, O.; Karbowiak, T.; Tongdeesoontorn, W.; Shiekh, K.A.; Rawdkuen, S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers 2022, 14, 1830. [Google Scholar] [CrossRef]
- Chen, Q.; Endo, T.; Wang, Q. Characterization of microcrystalline cellulose after pretreatment with low concentrations of ionic liquid-H2O for a pyrolysis process. BioResources 2015, 11, 159–173. [Google Scholar] [CrossRef][Green Version]
- Luzi, F.; Puglia, D.; Sarasini, F.; Tirillò, J.; Maffei, G.; Zuorro, A.; Lavecchia, R.; Kenny, J.M.; Torre, L. Valorization and extraction of cellulose nanocrystals from North African grass: Ampelodesmos mauritanicus (Diss). Carbohydr. Polym. 2019, 209, 328–337. [Google Scholar] [CrossRef]
- Cheng, G.; Varanasi, P.; Li, C.; Liu, H.; Melnichenko, Y.B.; Simmons, B.A.; Kent, M.S.; Singh, S. Transition of Cellulose Crystalline Structure and Surface Morphology of Biomass as a Function of Ionic Liquid Pretreatment and Its Relation to Enzymatic Hydrolysis. Biomacromolecules 2011, 12, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xia, Q.; Lin, Z.; Zhan, P.; Qing, Y.; Wang, H.; Shao, L.; Liu, N.; He, J.; Liu, J. Differentiated Fractionation of Various Biomass Resources by p-Toluenesulfonic Acid at Mild Conditions. ACS Omega 2023, 8, 24247–24255. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Mustapa Kamal, S.; Radiah, D. Comparison of sodium hydroxide and sodium bicarbonate pretreatment methods for characteristic and enzymatic hydrolysis of sago palm bark. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 46, 7274–7284. [Google Scholar] [CrossRef]
- Mo, W.; Chen, K.; Yang, X.; Kong, F.; Liu, J.; Li, B. Elucidating the hornification mechanism of cellulosic fibers during the process of thermal drying. Carbohydr. Polym. 2022, 289, 119434. [Google Scholar] [CrossRef]
- Mensah, I.; Ahiekpor, J.C.; Herold, N.; Bensah, E.C.; Pfriem, A.; Antwi, E.; Amponsem, B. Biomass and plastic co-pyrolysis for syngas production: Characterisation of Celtis mildbraedii sawdust as a potential feedstock. Sci. Afr. 2022, 16, e01208. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- ASTM D828-16e1; Standard Test Method for Tensile Properties of Paper and Paperboard Using Constant-Rate-of-Elongation Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D1938-14; Standard Test Method for Tear-Propagation Resistance (Trouser Tear) of Plastic Film and Thin Sheeting by a Single-Tear Method. ASTM International: West Conshohocken, PA, USA, 2014.
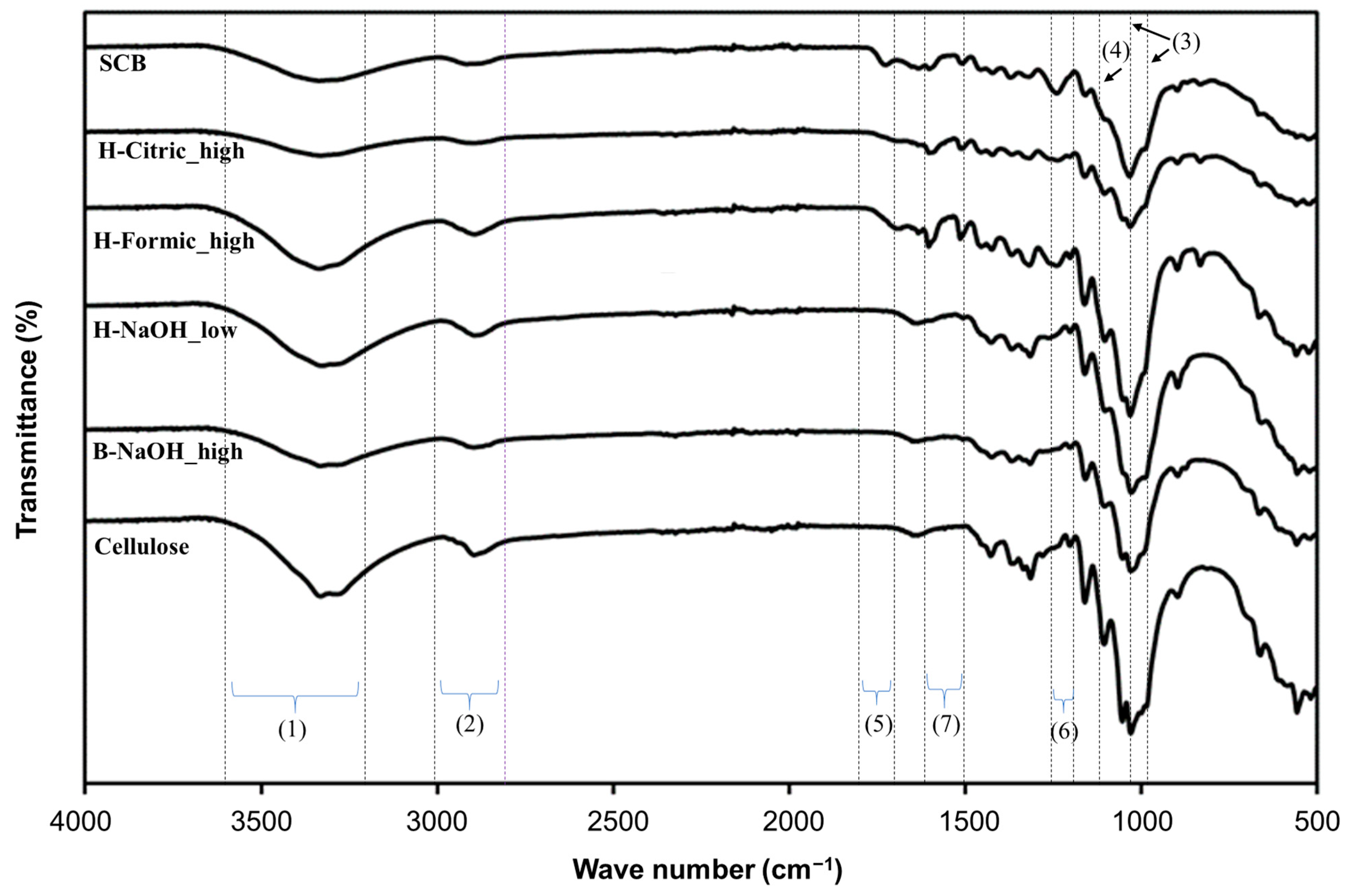

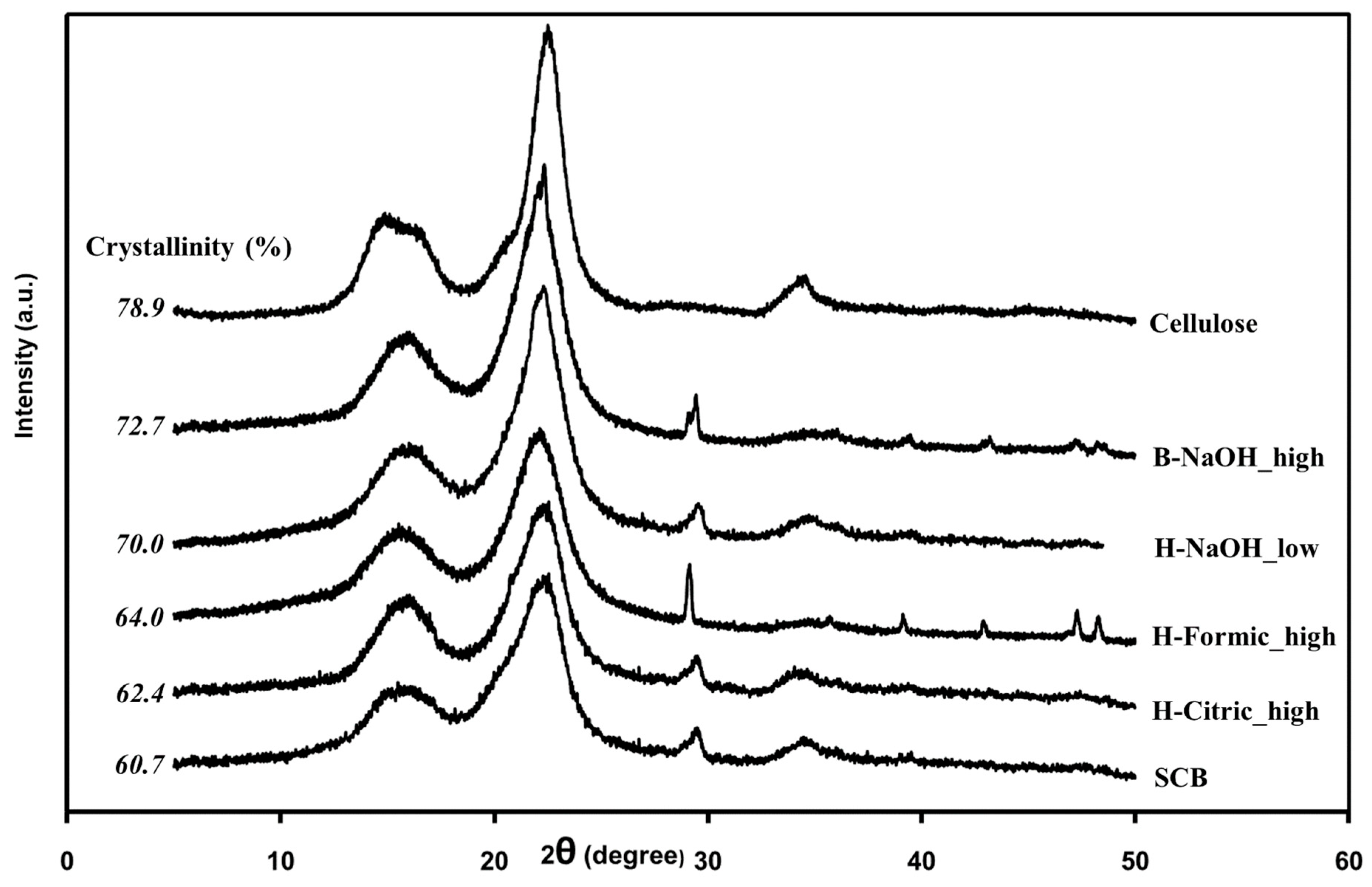

| Sample | Extraction | Composition (wt%) | ||||
|---|---|---|---|---|---|---|
| Method | Substance | Concentration (M) | Cellulose | Hemicelluloses | Lignin | |
| SCB | - | - | - | 47.8 | 26.4 | 18.1 |
| B-NaOH_low | Boiling | NaOH | 0.25 | 60.4 | 16.1 | 11.0 |
| B-NaOH_high | NaOH | 2 | 70.5 | 13.1 | 8.3 | |
| H-NaOH_low | Hydrothermal | NaOH | 0.25 | 67.7 | 17.3 | 11.2 |
| H-NaOH_high | NaOH | 2 | 74.0 | 14.8 | 8.0 | |
| H-Citric_low | Citric acid | 0.25 | 54.2 | 20.2 | 13.4 | |
| H-Citric_high | Citric acid | 2 | 57.3 | 18.2 | 12.9 | |
| H-Formic_low | Formic acid | 0.25 | 52.5 | 19.3 | 13.2 | |
| H-Formic_high | Formic acid | 2 | 57.1 | 18.5 | 12.7 | |
| H-Water | Water | - | 50.1 | 20.5 | 14.3 | |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Tear Strength (N) |
|---|---|---|---|
| B-NaOH_high | 16.3 ± 2.0 | 4.0 ± 0.7 | 21.4 ± 2.6 |
| H-NaOH_low | 22.1 ± 2.4 | 4.1± 1.0 | 26.3 ± 2.1 |
| Food Container | Tensile Strength (MPa) | Elongation at Break (%) | Tear Strength (N) |
|---|---|---|---|
| Commercial 1 | 31.7 ± 2.3 | 3.5 ± 0.4 | 18.1 ± 4.6 |
| Commercial 2 | 8.3 ± 1.2 | 1.00 ± 0.2 | 9.0 ± 1.1 |
| Produced plate (H-NaOH_low) | 23.1 ± 3.3 | 4.0 ± 1.1 | 26.8 ± 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaturapiree, A.; Saowapark, T.; Sukrat, K.; Chaichana, E. Hydrothermal Extraction of Cellulose from Sugarcane Bagasse for Production of Biodegradable Food Containers. Recycling 2025, 10, 110. https://doi.org/10.3390/recycling10030110
Jaturapiree A, Saowapark T, Sukrat K, Chaichana E. Hydrothermal Extraction of Cellulose from Sugarcane Bagasse for Production of Biodegradable Food Containers. Recycling. 2025; 10(3):110. https://doi.org/10.3390/recycling10030110
Chicago/Turabian StyleJaturapiree, Adisak, Thanunya Saowapark, Kanjarat Sukrat, and Ekrachan Chaichana. 2025. "Hydrothermal Extraction of Cellulose from Sugarcane Bagasse for Production of Biodegradable Food Containers" Recycling 10, no. 3: 110. https://doi.org/10.3390/recycling10030110
APA StyleJaturapiree, A., Saowapark, T., Sukrat, K., & Chaichana, E. (2025). Hydrothermal Extraction of Cellulose from Sugarcane Bagasse for Production of Biodegradable Food Containers. Recycling, 10(3), 110. https://doi.org/10.3390/recycling10030110







