Potential of Natural Sheep Casings Waste as a Sole Nitrogen Source for the Marine Microalga Scenedesmus rubescens MDP19 Growth and Lipid Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of S. rubescens MDP19
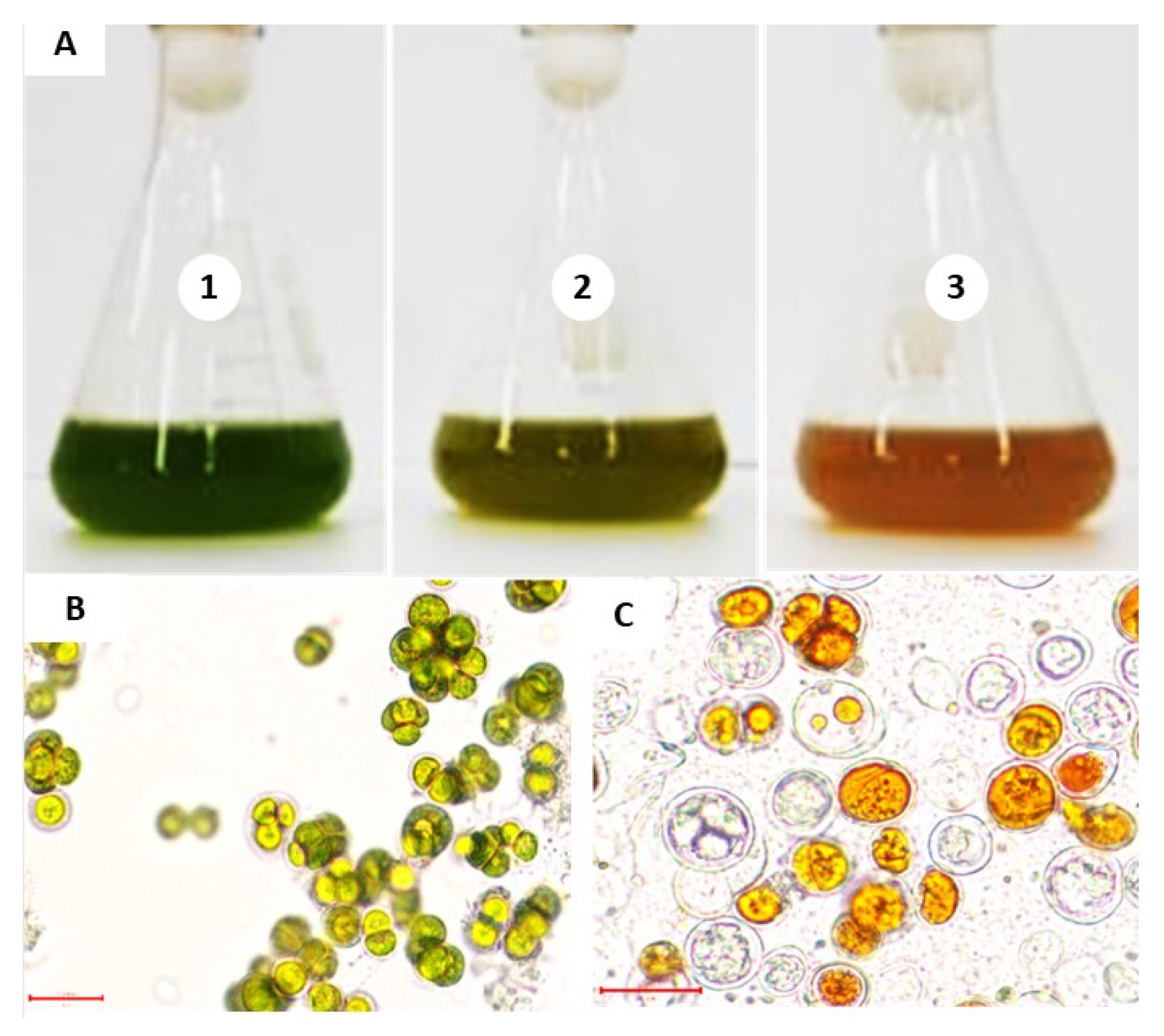
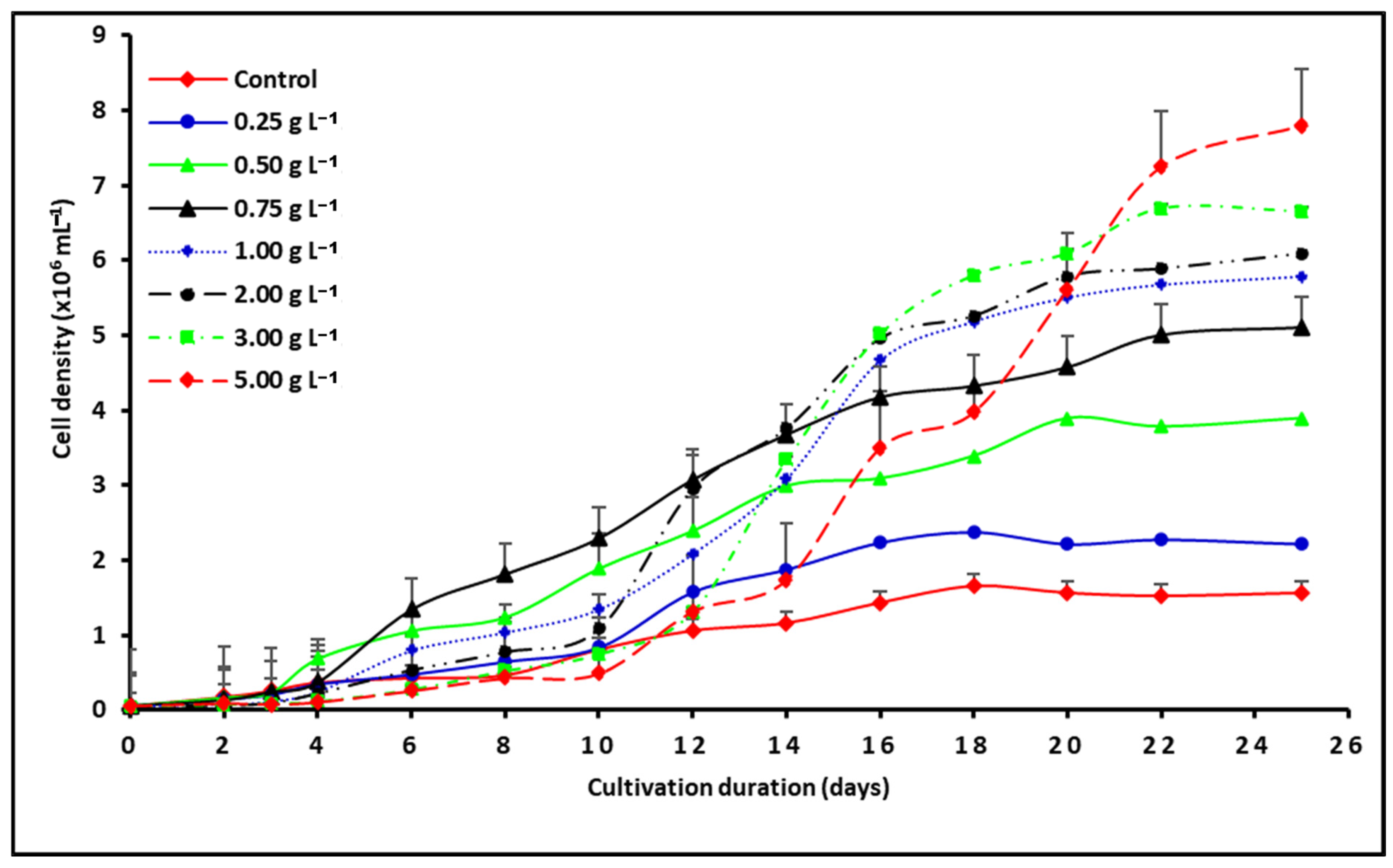
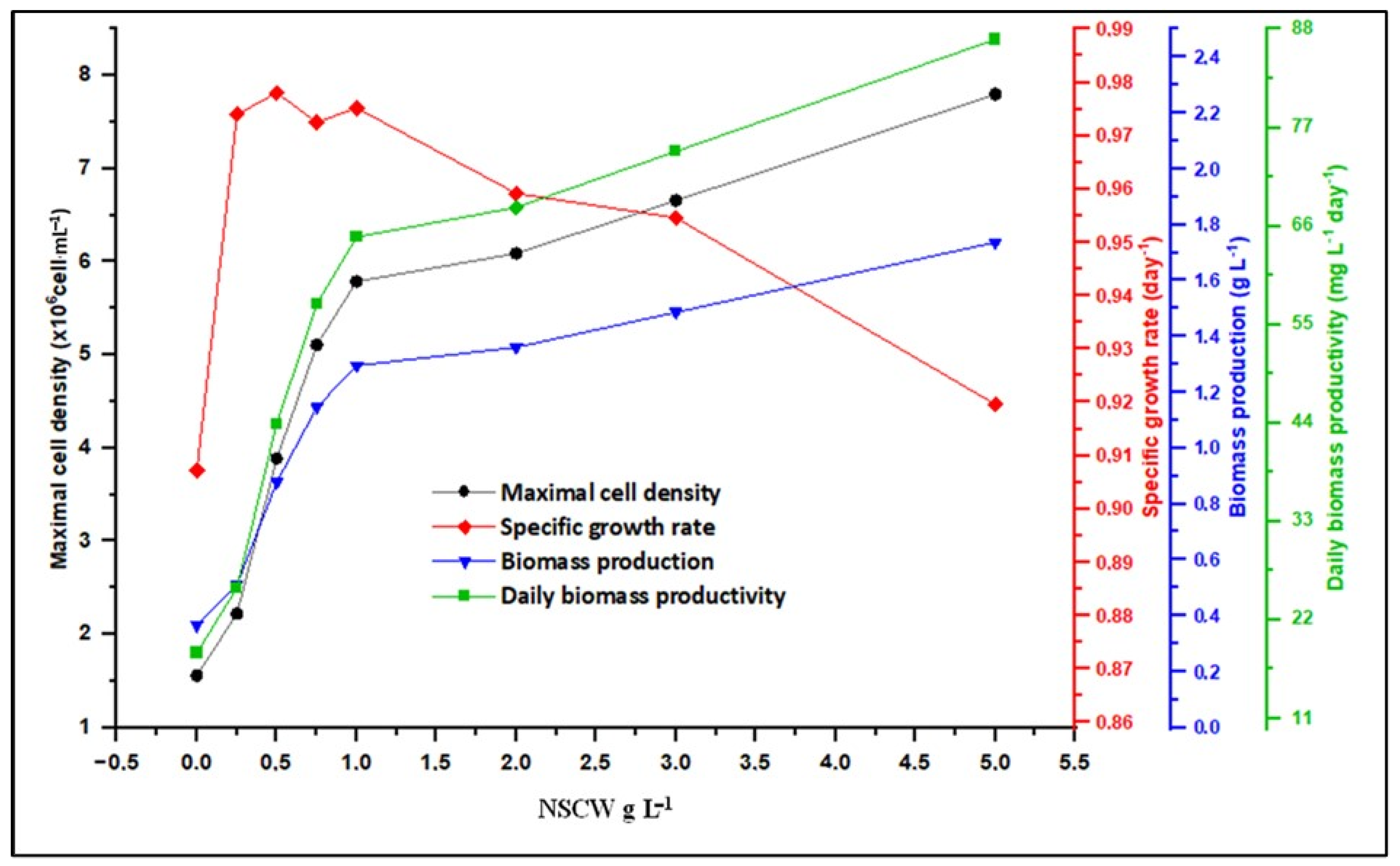
2.2. Effect of NSCW on S. rubescens MDP19 Growth and Biomass Production
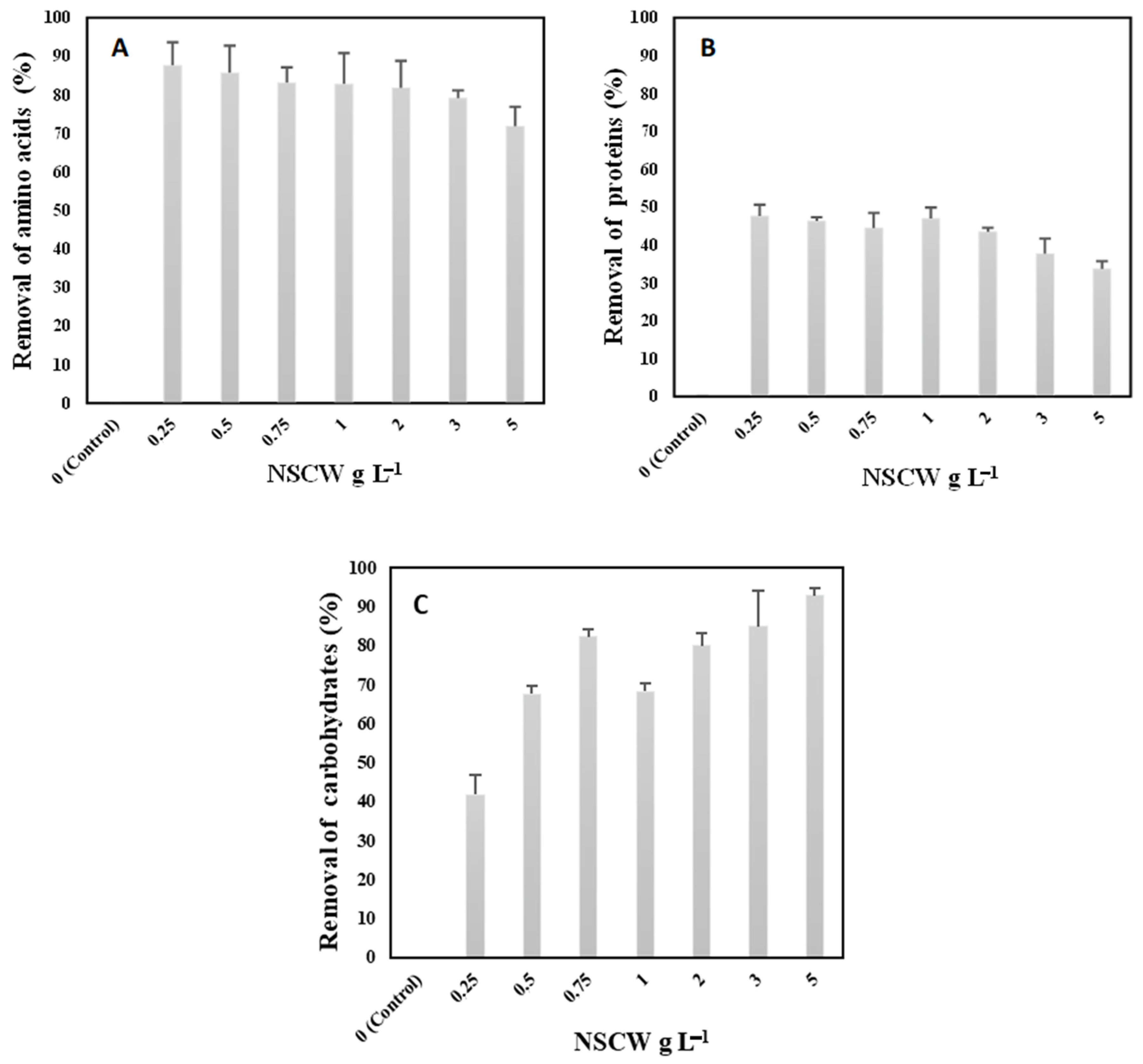
2.3. Utilization of Protein, Amino Acids, and Carbohydrates from NSCW by S. rubescens MDP19
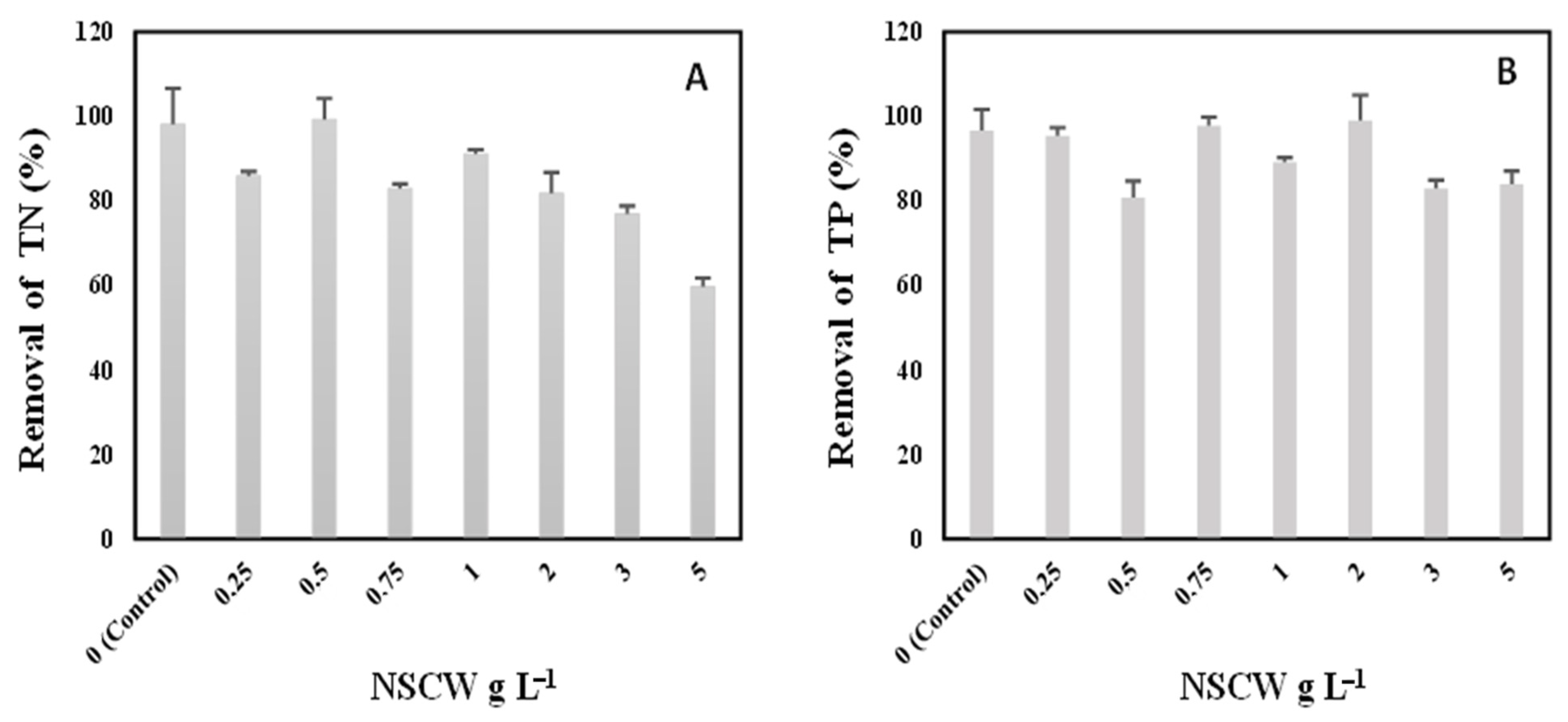
2.4. Removal Efficiency of Total Nitrogen and Total Phosphorus from NSCW by S. rubescens MDP19
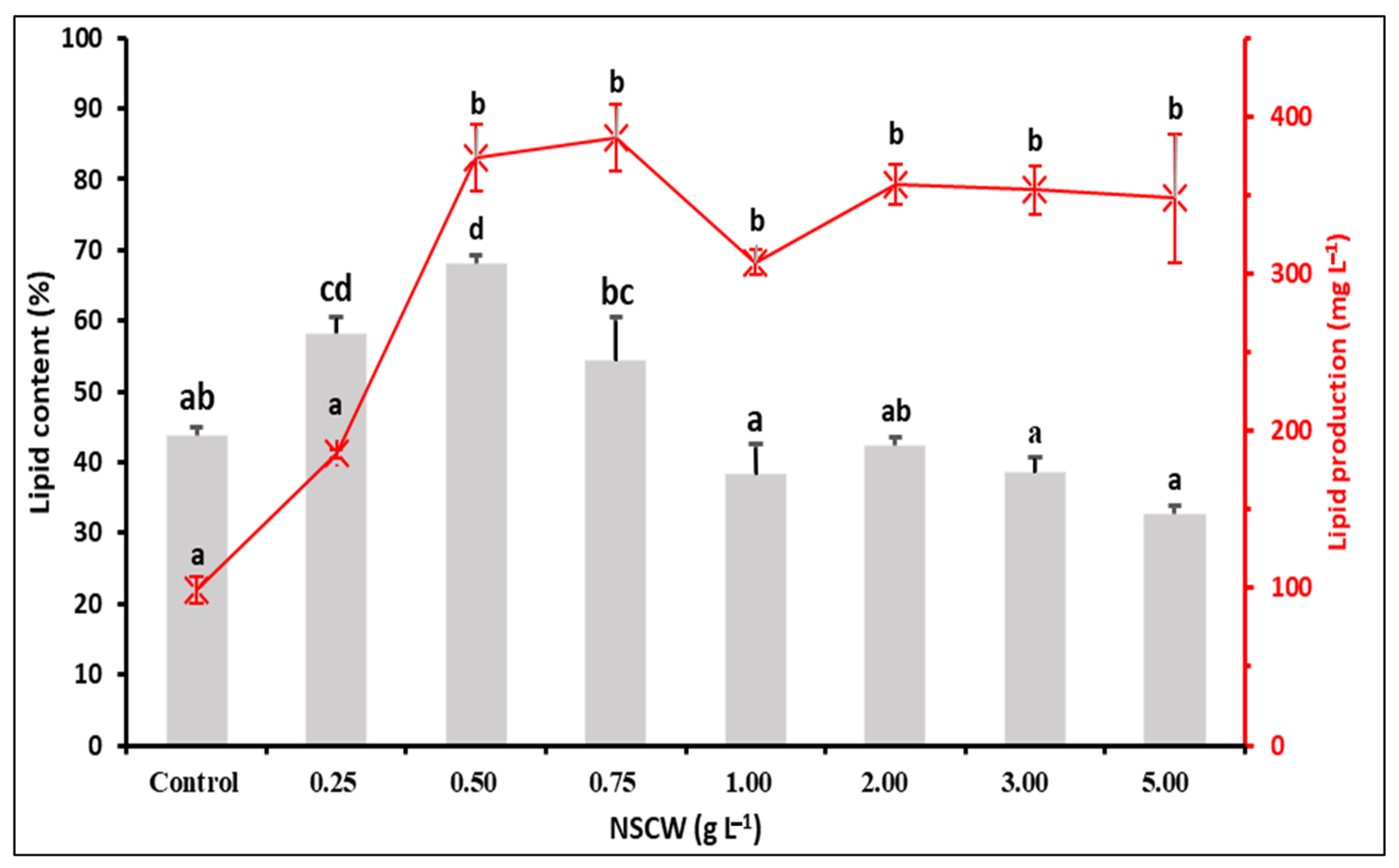
2.5. Effect of NSCW on Lipid Content and Production
2.6. Effect of NSCW on Chlorophyll Content and Total Carotenoid Content
2.7. Lipid Analysis by HPLC-APCI-MS
2.8. Pigment Analysis by HPLC-APCI-MS
3. Materials and Methods
3.1. Isolation and Identification of the Microalgal Strain
3.2. Waste Characterization
3.3. Testing the Effect of NSCW on Microalgae Growth and Biomass Production
3.3.1. Cultivation Experiments
3.3.2. Microalgal Growth, Biomass Production and Productivity
3.4. Testing the NSCW Utilization Capacity and Nutrient Removal Efficiency of the Microalgal Strain
3.4.1. Total Nitrogen (TN) and Total Phosphorus (TP)
3.4.2. Total Proteins
3.4.3. Total Amino Acids
3.4.4. Total Carbohydrates
3.5. Testing the Effect of NSCW on Lipid and Pigment Content
3.5.1. Lipid Extraction and Quantification
3.5.2. Pigments Extraction and Quantification
3.6. Analysis of Lipids and Pigments by HPLC-APCI-MS
3.6.1. Lipid Extraction
3.6.2. Pigments Extraction
3.6.3. HPLC-APCI-MS Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradley, R. Report on the safety of sheep intestine and natural casings derived therefrom in regard to risks from animal TSE and BSE in particular. In Report Prepared for the Tse/Bse Ad Hoc Group of the Scientific Steering Committee; European Commission: Brussels, Belgium, 2002; pp. 1–19. [Google Scholar]
- Makan, A. Windrow co-composting of natural casings waste with sheep manure and dead leaves. Waste Manag. 2015, 42, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Nandal, M.; Khosla, B. Microbes as vital additives for solid waste composting. Heliyon 2020, 6, e03343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, J.; Ma, S.; Zhang, Y.; Wang, Y.; Xu, J. Comprehensive evaluation of growth characteristics, nitrogen removal capacity, and nutritional properties of three diet microalgae. Front. Mar. Sci. 2023, 10, 1117043. [Google Scholar] [CrossRef]
- Waqas, M.; Hashim, S.; Humphries, U.W.; Ahmad, S.; Noor, R.; Shoaib, M.; Naseem, A.; Hlaing, P.T.; Lin, H.A. Composting processes for agricultural waste management: A comprehensive review. Processes 2023, 11, 731. [Google Scholar] [CrossRef]
- Arias, J.Z.; Reuter, T.; Sabir, A.; Gilroyed, B.H. Ambient alkaline hydrolysis and anaerobic digestion as a mortality management strategy for whole poultry carcasses. Waste Manag. 2018, 81, 71–77. [Google Scholar] [CrossRef]
- Patnaik, R.; Mallick, N. Microalgal biodiesel production: Realizing the sustainability index. Front. Bioeng. Biotechnol. 2021, 9, 620777. [Google Scholar] [CrossRef]
- Chhandama, M.V.L.; Satyan, K.B.; Changmai, B.; Vanlalveni, C.; Rokhum, S.L. Microalgae as a feedstock for the production of biodiesel: A review. Bioresour. Technol. Rep. 2021, 15, 100771. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Kiyani, D.A.; Maryam, S.; Amina, S.J.; Ahmad, A.; Chattha, M.W.A.; Janjua, H.A. Lipid extraction and analysis of microalgae strain pectinodesmus PHM3 for biodiesel production. BMC Biotechnol. 2023, 23, 20. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Biotechnol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- Al Jabri, H.; Taleb, A.; Touchard, R.; Saadaoui, I.; Goetz, V.; Pruvost, J. Cultivating microalgae in desert conditions: Evaluation of the effect of light-temperature summer conditions on the growth and metabolism of Nannochloropsis QU130. Appl. Sci. 2021, 11, 3799. [Google Scholar] [CrossRef]
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; Thomas-Hall, S.R.; Schenk, P.M. Biodiversity impacts of bioenergy production: Microalgae vs. first generation biofuels. Renew. Sustain. Energy Rev. 2017, 74, 1131–1146. [Google Scholar] [CrossRef]
- Yadav, G.; Panda, S.P.; Sen, R. Strategies for the effective solid, liquid and gaseous waste valorization by microalgae: A circular bioeconomy perspective. J. Environ. Chem. Eng. 2020, 8, 104518. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Feng, S.; Yang, T.; Xu, L.; Shen, B.; Bi, Y.; Lyu, H. Cultivation of microalgae–bacteria consortium by waste gas–waste water to achieve CO2 fixation, wastewater purification and bioproducts production. Biotechnol. Biofuels Bioprod. 2024, 17, 26. [Google Scholar] [CrossRef]
- Chekanov, K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Mar. Drugs. 2023, 21, 108. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Chiang, A.; Herojit, N.; Arumugam, M. Sustainable microalgal cultivation in poultry slaughterhouse wastewater for biorefinery products and pollutant removal. Bioresour. Technol. 2023, 374, 128790. [Google Scholar] [CrossRef]
- Sohrabi, D.; Jazini, M.; Mobasheri, S.; Tohidi, M.; Shariati, M. Waste Gastro-intestinal Wall of Sheep as an Alternative Nutrition Source for Cultivation of Dunaliella salina. Appl. Biochem. Biotechnol. 2022, 194, 1178–1192. [Google Scholar] [CrossRef]
- Shanthi, G.; Premalatha, M.; Anantharaman, N. Potential utilization of fish waste for the sustainable production of microalgae rich in renewable protein and phycocyanin-Arthrospira platensis/Spirulina. J. Clean. Prod. 2021, 294, 126106. [Google Scholar] [CrossRef]
- Trost, J.T.; Chisholm, D.A.; Jordan, D.B.; Diner, B.A. The D1 C-terminal Processing Protease of Photosystem II from Scenedesmus obliquus: Protein purification and gene characterization in wild type and processing mutants. J. Biol. Chem. 1997, 272, 20348–20356. [Google Scholar] [CrossRef] [PubMed]
- Xupeng, C.; Song, X.; Xuran, F. Amino acid changes during energy storage compounds accumulation of microalgae under the nitrogen depletion. In Amino Acid-New Insights and Roles in Plant and Animal, 1st ed.; Asao, T., Asaduzzaman, M.D., Eds.; IntechOpen Limited: London, UK, 2017; Volume 9, pp. 197–208. [Google Scholar]
- Calatrava, V.; Hom, E.F.; Llamas, Á.; Fernández, E.; Galvan, A. Nitrogen scavenging from amino acids and peptides in the model alga Chlamydomonas reinhardtii. The role of extracellular l-amino oxidase. Algal Res. 2019, 38, 101395. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, F.; Yu, C.; Li, C.; Huang, T.; Hu, H. Amino acid catabolism during nitrogen limitation in Phaeodactylum tricornutum. Front. Plant Sci. 2020, 11, 589026. [Google Scholar] [CrossRef]
- Gu, H.; Nagle, N.; Pienkos, P.T.; Posewitz, M.C. Nitrogen recycling from fuel-extracted algal biomass: Residuals as the sole nitrogen source for culturing Scenedesmus acutus. Bioresour. Technol. 2015, 184, 153–160. [Google Scholar] [CrossRef]
- Santo, G.E.; Barros, A.; Costa, M.; Pereira, H.; Trovão, M.; Cardoso, H.; Carvalho, B.; Soares, M.; Correia, N.; Silva, J.T.; et al. Scenedesmus rubescens Heterotrophic Production Strategies for Added Value Biomass. Mar. Drugs. 2023, 21, 411. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Dolganyuk, V.; Michaud, P.; Ivanova, S. Influence of carbohydrate additives on the growth rate of microalgae biomass with an increased carbohydrate content. Mar. Drugs. 2021, 19, 381. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Isiramen, O.E.; Bahri, P.A.; Moheimani, N.R. Effect of recycling treated effluent on the cultivation of Scenedesmus sp. in anaerobically digested abattoir effluent (ADAE). Algal Res. 2024, 77, 103367. [Google Scholar] [CrossRef]
- Medrano-Barboza, J.; Herrera-Rengifo, K.; Aguirre-Bravo, A.; Ramírez-Iglesias, J.R.; Rodríguez, R.; Morales, V. Pig slaughterhouse wastewater: Medium culture for microalgae biomass generation as raw material in biofuel industries. Water 2022, 14, 3016. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Liu, T.; Yuan, M.; Liu, G.; Fang, J.; Yang, B. Exploration of microalgal species for nutrient removal from anaerobically digested swine wastewater and potential lipids production. Microorganisms 2021, 9, 2469. [Google Scholar] [CrossRef]
- Khanzada, Z.T. Phosphorus removal from landfill leachate by microalgae. Biotechnol. Rep. 2020, 25, e00419. [Google Scholar] [CrossRef]
- Tan, Y.H.; Chai, M.K.; Na, J.Y.; Wong, L.S. Microalgal Growth and Nutrient Removal Efficiency in Non-Sterilised Primary Domestic Wastewater. Sustainability 2023, 15, 6601. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Long term outdoor microalgal phycoremediation of anaerobically digested abattoir effluent. J. Environ. Manag. 2022, 323, 116322. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.W.; Hong, J.W.; Do, J.M.; Na, H.; Kim, J.J.; Park, S.I.; Yoon, H.S. Nitrogen deficiency-dependent abiotic stress enhances carotenoid production in indigenous green microalga Scenedesmus rubescens KNUA042, for use as a potential resource of high value products. Sustainability 2020, 12, 5445. [Google Scholar] [CrossRef]
- Lv, J.; Guo, B.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Integration of wastewater treatment and flocculation for harvesting biomass for lipid production by a newly isolated self-flocculating microalga Scenedesmus rubescens SX. J. Clean. Prod. 2019, 240, 118211. [Google Scholar] [CrossRef]
- Aravantinou, A.F.; Theodorakopoulos, M.A.; Manariotis, I.D. Selection of microalgae for wastewater treatment and potential lipids production. Bioresour. Technol. 2013, 147, 130–134. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Liu, F.; Wang, C. The enhanced lipid accumulation in oleaginous microalga by the potential continuous nitrogen-limitation (CNL) strategy. Bioresour. Technol. 2016, 203, 150–159. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Osei-Wusu, D.; Wang, Y.; Liu, T. Development of nitrogen supply strategy for Scenedesmus rubescens attached cultivation toward growth and lipid accumulation. Bioprocess. Biosyst. Eng. 2018, 41, 435–442. [Google Scholar] [CrossRef]
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Cointet, E.; Wielgosz-Collin, G.; Bougaran, G.; Rabesaotra, V.; Gonçalves, O.; Méléder, V. Effects of light and nitrogen availability on photosynthetic efficiency and fatty acid content of three original benthic diatom strains. PLoS ONE 2019, 14, e0224701. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [PubMed]
- Utomo, J.C.; Kim, Y.M.; Cho, H.U.; Park, J.M. Evaluation of Scenedesmus rubescens for Lipid Production from Swine Wastewater Blended with Municipal Wastewater. Energies 2020, 13, 4895. [Google Scholar] [CrossRef]
- Kröger, M.; Klemm, M.; Nelles, M. Extraction behavior of different conditioned S. rubescens. Energies 2019, 12, 1336. [Google Scholar] [CrossRef]
- Li, P.; Lin, J. Effect of ultraviolet radiation on photosynthesis, biomass, and fatty acid content and profile of a Scenedesmus rubescens-like microalga. Bioresour. Technol. 2012, 111, 316–322. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V. Synthesis of Biodiesel by Interesterification of Triglycerides with Methyl Formate. Appl. Sci. 2022, 12, 9912. [Google Scholar] [CrossRef]
- Tobar, M.; Núñez, G.A. Supercritical transesterification of microalgae triglycerides for biodiesel production: Effect of alcohol type and co-solvent. J. Supercrit. Fluids. 2018, 137, 50–56. [Google Scholar] [CrossRef]
- Amaro, H.M.; Fernandes, F.; Valentão, P.; Andrade, P.B.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Effect of solvent system on extractability of lipidic components of Scenedesmus obliquus (M2-1) and Gloeothece sp. on antioxidant scavenging capacity thereof. Mar. Drugs. 2015, 13, 6453–6471. [Google Scholar] [CrossRef]
- Tukaj, Z.; Matusiak-Mikulin, K.; Lewandowska, J.; Szurkowski, J. Changes in the pigment patterns and the photosynthetic activity during a light-induced cell cycle of the green alga Scenedesmus armatus. Plant Physiol. Biochem. 2003, 41, 337–344. [Google Scholar] [CrossRef]
- Kotzabasis, K.; Senger, H. Evidence for the presence of chlorophyllide b in the green alga Scenedesmus obliquus In Vivo. Bot. Acta 1989, 102, 173–177. [Google Scholar] [CrossRef]
- Paper, M.; Glemser, M.; Haack, M.; Lorenzen, J.; Mehlmer, N.; Fuchs, T.; Schenk, G.; Garbe, D.; Weuster-Botz, D.; Eisenreich, W.; et al. Efficient green light acclimation of the green algae Picochlorum sp. triggering geranylgeranylated chlorophylls. Front. Bioeng. Biotechnol. 2022, 10, 885977. [Google Scholar] [CrossRef]
- Fernandes, A.S.; do Nascimento, T.C.; Pinheiro, P.N.; Jacob-Lopes, E.; Zepka, L.Q. Determination of profile of chlorophyll compounds in microalgae species. Braz. J. Dev. 2021, 7, 4381–4399. [Google Scholar] [CrossRef]
- Plank, C.O. Plant Analysis Reference Procedures for the Southern Region of the United States: Southern Cooperative Series Bulletin; Universidad of Georgia: Athens, GA, USA, 1992; p. 13. [Google Scholar]
- Rahutomo, S.; Kovar, J.L.; Thompson, M.L. Malachite green method for determining phosphorus concentration in diverse matrices. Commun. Soil. Sci. Plant Anal. 2019, 50, 1743–1752. [Google Scholar] [CrossRef]
- Harwood, J.E.; Kühn, A.L. A colorimetric method for ammonia in natural waters. Water Res. 1970, 4, 805–811. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- El-Yazal, M.A.S.; Rady, M.M. Exogenous onion extract hastens bud break, positively alters enzyme activity, hormone, amino acids and phenol contents, and improves fruit quality in ‘Anna’apple trees. Sci. Hortic. 2014, 169, 154–160. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1956; pp. 29–60. [Google Scholar]
- Ren, X.; Zhao, X.; Turcotte, F.; Deschênes, J.S.; Tremblay, R.; Jolicoeur, M. Current lipid extraction methods are significantly enhanced adding a water treatment step in Chlorella protothecoides. Microb. Cell Fact. 2017, 16, 26. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 1987, 131, 101–110. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]

| Compounds | [M-H]− | % |
|---|---|---|
| C18:3 | 277.3 | 8.5 ± 1.3 |
| C16:1 | 253.1 | 7.8 ± 0.9 |
| C18:2 | 279.0 | 7.2 ± 0.8 |
| C18:1 | 281.2 | 4.1 ± 0.4 |
| DGDG (36:6) | 935.6 | 3.4 ± 0.3 |
| SQDG (32:2) | 788.4 | 2.9 ± 0.5 |
| DGDG (34:1) | 937.5 | 9.5 ± 1.7 |
| PE (34:3) | 460.3 | 2.2 ± 0.2 |
| SQDG (32:1) | 790.5 | 1.9 ± 0.1 |
| SQDG (34:2) | 816.5 | 1.5 ± 0.1 |
| PG (36:2) | 792.5 | 5.8 ± 1.9 |
| PE (34:2) | 714.5 | 6.3 ± 2.1 |
| SQDG (34:1) | 818.5 | 7.1 ± 1.8 |
| DGDG (34:2) | 915.6 | 7.8 ± 1.9 |
| DG (32:5) | 581.5 | 8.2 ± 1.7 |
| TG (SOO/SSL/PLA) | 885.5 | 8.5 ± 1.9 |
| TG (OOMo/LnLn18:4) | 870.4 | 8.5 ± 1.8 |
| Compounds | [M-H]− | [M+H]+ | λmax (nm) | % (Carotenoids) | % (Chlorophylls) |
|---|---|---|---|---|---|
| Lutein | 568.4 * | 330–438–465 | 20.5 ± 2.1 | ||
| Canthaxanthin | 564.4 * | 565.4 | 472 | 21.5 ± 2.3 | |
| Neoxanthin | 599.1 | 601.2 | 418–438–465 | 58.0 ± 1.5 | |
| Hydrochlorophyllide b | 643.1 | 645.1 | 470–660 | 8.5 ± 2.6 | |
| Chlorophyll a | 891.2 | 894.4 | 430–666 | 7.5 ± 1.9 | |
| Chlorophyllide b | - | 629.2 # | 469–654 | 12.5 ± 1.8 | |
| Geranylgeranyl-chlorophyll a | 886.4 | 887.4 # | 425–665 | 63.5 ± 2.5 | |
| Pheophytin a | 869.4 | 871.4 # | 406–665 | 8.0 ± 2.1 |
| Parameter | Unit | Amount |
|---|---|---|
| Total organic carbon (TOC) | (mg g−1 L−1) | 2960 |
| Total nitrogen (TN) | (mg g−1 L−1) | 480 |
| Total phosphorus (TP) | (mg g−1 L−1) | 80 |
| Ammonia | (mg g−1 L−1) | 0 |
| Total proteins | (%) | 57.0 |
| Total carbohydrates | (%) | 32.3 |
| Total amino acids | (%) | 52.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouzakar, S.; Skali Senhaji, N.; Rigano, F.; Cafarella, C.; Cacciola, F.; Mondello, L.; Abrini, J. Potential of Natural Sheep Casings Waste as a Sole Nitrogen Source for the Marine Microalga Scenedesmus rubescens MDP19 Growth and Lipid Production. Recycling 2025, 10, 109. https://doi.org/10.3390/recycling10030109
Ouzakar S, Skali Senhaji N, Rigano F, Cafarella C, Cacciola F, Mondello L, Abrini J. Potential of Natural Sheep Casings Waste as a Sole Nitrogen Source for the Marine Microalga Scenedesmus rubescens MDP19 Growth and Lipid Production. Recycling. 2025; 10(3):109. https://doi.org/10.3390/recycling10030109
Chicago/Turabian StyleOuzakar, Sanaa, Nadia Skali Senhaji, Francesca Rigano, Cinzia Cafarella, Francesco Cacciola, Luigi Mondello, and Jamal Abrini. 2025. "Potential of Natural Sheep Casings Waste as a Sole Nitrogen Source for the Marine Microalga Scenedesmus rubescens MDP19 Growth and Lipid Production" Recycling 10, no. 3: 109. https://doi.org/10.3390/recycling10030109
APA StyleOuzakar, S., Skali Senhaji, N., Rigano, F., Cafarella, C., Cacciola, F., Mondello, L., & Abrini, J. (2025). Potential of Natural Sheep Casings Waste as a Sole Nitrogen Source for the Marine Microalga Scenedesmus rubescens MDP19 Growth and Lipid Production. Recycling, 10(3), 109. https://doi.org/10.3390/recycling10030109










