Abstract
Currently, residual frying oil has three purposes: it is used again in the preparation of fried foods, mixed with new vegetable oil, which can cause cardiovascular disease in the consumer; it is collected by government institutions, without having an exclusive use; or it is thrown into the drain, causing serious pollution problems to water resources. An alternative is to transform it into biodiesel, through transesterification with methanol, to be used in internal combustion engines, biodiesel-diesel mixtures of 10:90, 15:85 and 20:80 (v/v), according to international regulations in such a way that, in the combustion process, less CO2 and greenhouse gas emissions are generated. Residual frying oil served as raw material, which was collected, mixed and homogenized to evaluate physicochemical properties before transformation. The biodiesel generated had a density of 0.886 g L−1, an acidity of 0.516%, a viscosity of 7.535 mm2 s−1, a flash point of 166.8 °C and an oxidative stability of 49 days at 25 °C. Additionally, the content of functional groups characteristic of biodiesel formation was evaluated by Infrared Spectroscopy. The Biodiesel obtained is of good quality for use in internal combustion engines and agricultural machinery, thus validating its continuous production and complying with the standard values of international regulations.
1. Introduction
Within the 2030 Agenda for Sustainable Development [1], of the 17 Goals, number seven on “Affordable and non-polluting energy” talks about guaranteeing access to clean and accessible energy, which can be key to the development of agriculture, businesses, communications, education, health and transportation, by leaving behind fuels that pollute and generate toxic gases that contribute to climate change and that come from non-renewable sources such as oil [2,3]. Without a doubt, the transition towards accessible, safe and sustainable energy systems is in process, however, it is slow: research in the laboratory and the field is required, as well as the in-situ application of the biofuel generated, which takes years to complete.
Biofuels such as biodiesel and bioethanol have been used as alternatives to fossil liquid fuels [4,5,6]. According to the American Society for Testing and Materials Standard (ASTM) D6751-20a [7], biodiesel is a liquid formed by monoalkyl esters of fatty acids from vegetable oils and animal fats.
Trying to obtain new sources of biodiesel production is not something new; however, alternative resources that involve residue or waste must be considered to give them added value for other uses.
Generally, biofuels can be produced from different organic waste from agricultural processes (straw) [8], forestry (wood sawdust) [9,10], agroindustry (bagasse) [11], agricultural products (excrement, animal fats) [12], for urban use (residual frying oils) [5,13] and even plastics [14].
Residual frying oil is the oil (usually soybean, canola, palm or sunflower) used to fry foods and that can no longer be used for that purpose, because during frying chemical transformations of the oil (rancidity) occur at high temperatures (160–200 °C), favoring the hydrolysis of triacylglicerides and the release of fatty acids, mono and diacylglicerides and glycerin; they reduce the nutritional value of the food and produce volatile compounds that impart unpleasant odors and flavors [15].
Using residual frying oils for biodiesel production is an alternative that can mitigate water pollution. According to Gonzalez and Gonzalez [16], as well as SEDEMA [17], one liter of oil is enough to pollute up to a thousand liters of water and 40,000 L of drinking water. When used oil is poured into sinks or toilets, it reaches different aquifers, where, in addition to the pollution it causes because it is not miscible with water, it creates a layer on the water surface that prevents the oxygen exchange, causing death to the wildlife that lives there.
On the other hand, residual frying oil does not compete with raw materials used in the generation of biodiesel that are also used as food, such as linseed, tobacco, and jojoba, among others [18], and that require large areas of arable land to obtain biofuel. By treating residual frying oil as a waste, biodiesel production costs are reduced by up to 40%, as is the better treatment and disposal of this oil [19]. For this reason, residual frying oil is classified as a hazardous waste according to the General Law on Prevention and Comprehensive Waste Management [20], which is applicable in Mexico and is attached to the Sustainable Development Goals [1,13].
Although indeed, the management of residual frying oil for the manufacture of biofuels, is not the only alternative, it is also used to make soaps, waxes, candles, and organic fertilizers in the form of composting. There are also collection centers for this oil through an initiative of the Ministry of Health of the Government of the Mexican Republic, in which, in Mexico City, at least in 2021, 11.123 L of oil were collected, a vegetable used for cooking [17], a quantity that is increasing, due to population growth and the ease of obtaining and consuming fried products.
On the other hand, and alarmingly, residual frying oil cannot be incorporated back into the supply chain and used again as cooking oil, since heating it and raising its temperature by more than 185 °C produces unwanted toxic compounds. The Federal Consumer Protection Agency (PROFECO) [21] surveyed Mexico’s reuse of used cooking oil and indicated that 77.5% reuse edible oil once; 18.7% reuse it twice; 1.2% three times and 2.5% up to four times. This is alarming and the focus of attention for cardiovascular diseases due to the consumption of oxidized oils [5,22].
In Mexico, the situation regarding biofuel generation is encouraging, since there are already established companies for its production and marketing [23,24,25]. In Chiapas, few companies are dedicated to producing biodiesel; some of them had their peak from 2010 to 2015 [26], making use of Jatropha curcas, which is a non-edible plant that produces a quality oil to make biodiesel; It is from the Euphorbiaceae family, also known as coquillo, pine nut, tempate, among others; It grows wild and is also planted as a living fence [27]; having a 19% proportion of biodiesel production [28].
In addition, the high cost of fossil fuels in Latin America in recent years has opened the door for biofuels, such as biodiesel, to gain greater acceptance, demand, and use.
According to the Spanish Standard UNE-EN 14214 [29], internal combustion vehicles can use a part of biodiesel as fuel in a maximum proportion of 15%, however, there are tests on diesel engines, in which it was evaluated up to 30% biodiesel and 70% diesel, giving high engine power and efficiency results.
However, the benefits obtained from the use of biodiesel in internal combustion engines are better performance in terms of power, torque, and thermal stability in a proportion of 20% biodiesel, and 80% biodiesel [30]; it minimizes engine wear due to its lubricating power and for the environment, the main benefit is reducing greenhouse gas emissions (CO2 and CO) [31].
For this research, the biodiesel generated is part of the National Strategic Projects (ProNacEs) “Incorporation of liquid biofuels into the local and regional consumption chain of the Central and Frailesca Regions of the State of Chiapas”, funded by the Secretary of Science, Humanities, Technology and Innovation (SECIHTI, for its acronym in Spanish, formerly CONAHCYT), within of the Energy and Climate Change Strategy related to Research and Advocacy Projects to transition to a socially and environmentally sustainable energy system, whose objective is to optimize the processes of the productive sector and agro-industrial transformation, minimize the environmental impact and integrate society into a productive value chain, in such a way that it replaces the use of fossil fuels for the development of efficient, reliable and economically viable systems for the processing of food products with biofuels, which minimize losses due to poor conservation of products that increase their added value when processed and are healthy, without burning fossil fuels.
This alternative use of residual frying oil can serve as a model for a social and solidarity economy in rural communities worldwide. Disposing of these oils in an environmentally friendly manner will generate more jobs through their collection and subsequent processing while mitigating greenhouse gases by reducing fossil fuel consumption.
Thus, the objective of this study was to evaluate the feasibility of using residual frying oil in Biodiesel through a chemical transformation, whose application will be in combination with diesel as fuel in different agricultural implements and use in agro-industries, partially replacing fossil fuels.
2. Materials and Methods
2.1. Residual Frying Oil
The residual frying oil was obtained from different businesses and cafeterias where foods such as potatoes, bananas, and chicken, among others, were fried in the municipality of Suchiapa, Chiapas, Mexico (16°35′00′′ N 93°08′00′′ W), which is a mixture that contains palm oil, soybean oil, and canola oil, among others, in different proportions. The oils were received in 4 and 20 L bottles, filtered, and homogenized in a stainless-steel kettle. It was characterized by measuring density [29,32], acidity index [33], free fatty acids [34] and viscosity [35,36].
2.2. Process of Obtaining Biodiesel
The biodiesel was generated in the pilot plant of the Agroindustrial Engineering Workshop of the Polytechnic University of Chiapas, located in Suchiapa, Chiapas, México (16°36′56′′ N, 93°05′22′′ W). It was obtained by transesterification of residual frying oil mixed with methanol (Merck®, Naucalpan de Juarez, Mexico) in a 6:1 ratio, i.e., 166 mL of methanol was used for each liter of residual frying oil; 1% NaOH (Meyer®, Benito Juárez, Mexico) was added as a catalyst; the mixture was heated to 60 °C, with a stirring speed of 600 rpm and a reaction time of 50 min.
After the reaction time, the mixture was placed in a separatory funnel for 24 h, where the mixture was separated into biodiesel and glycerin. The biodiesel was purified with distilled water washes, stirring constantly, separating the water, and repeating the process until the pH value was close to neutrality. Completing the separation process, the product was heated at 110 °C for 10 min to evaporate traces of water, allowed to cool, and stored in closed containers and in a dark room at 25–28 °C.
2.3. Physicochemical Characterization
The biodiesel generated was evaluated for density [29,32], acidity index [33], viscosity [35], and flash point [37]; these properties are basic information for the characterization of biodiesel. However, to complement the study, the characteristics of functional groups were also evaluated by Fourier Transform Infrared Spectroscopy (FT-IR) and the oxidative stability at different temperatures.
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
Fourier Transform Infrared Spectroscopy (Nicolet™ Is™10, Thermo Scientific, Waltham, MA, USA) with a spectral window of 400 to 4000 cm−1 was used to monitor the signals of the characteristic products of the transesterification reaction, the methyl esters trained [38] and using the OMNIC 32 6.0 software for spectral interpretation. Determinations were done in triplicate.
2.3.2. Oxidative Stability Determination
Oxidative stability was determined according to European Standard UNE-EN 14112 [39]. The equipment used in the test was Biodiesel Rancimat® 873 (Metrohm, Herisau, Switzerland) at different temperatures: 90, 110, and 130 °C with an airflow of 10 L h−1, 3 g of sample and using different diesel–biodiesel proportions (90:10, 85:15 and 80:20), as an exploratory analysis. Analyses were carried out in triplicate. The main objective of measuring the three temperatures was to estimate, through mathematical extrapolation, the oxidative capacity of biodiesel at different temperatures, even different from those integrated in the model, and thus determine its useful life [40,41].
3. Results
3.1. Characterization of Residual Frying Oils
Table 1 shows the characteristics measured for the collected residual frying oils. These characteristics reflect the main properties an oil requires to convert to biodiesel.

Table 1.
Characteristics of residual frying oils.
3.2. Obtaining Biodiesel
The biodiesel generated had a yellow-brown color, similar to #9e6b05 [42], without suspended solids, adequate fluidity, and a characteristic oil smell. It had a yield of 75% which indicates an adequate yield for the transesterification of vegetable oils used in cooking for biodiesel production, compared to other similar studies [4,5].
3.3. Physicochemical Characterization of Biodiesel
The biodiesel obtained had adequate values within the limits of density, acidity, and viscosity according to the “Guideline that establishes the quality and characteristics specifications for anhydrous ethanol (bioethanol), biodiesel, and pure bioturbosine” [43], which are shown in Table 2, along with the results of other studies where transforming residual frying oil into biodiesel

Table 2.
Basic properties of biodiesel (100).
The biodiesel formed from residual frying oil complies with national and international regulations for pure biodiesel, being considered of good quality, considering the triglycerides used as raw material have three reaction points with methanol to form glycerin and alkyl esters, however, there are also mono- and diglycerides that did not react and are part of the biodiesel formed, which can increase or decrease some basic characteristics of biodiesel such as viscosity.
Under the conditions used, 95 to 98% of triglycerides are converted to methyl esters, and 2 to 5% of free mono-, di-, and triglycerides are still present in the biodiesel [5]. To demonstrate that the transesterification reaction was properly formed, the biodiesel was evaluated by infrared spectroscopy.
3.4. FT—IR
In the evaluation by Fourier Transform Infrared Spectroscopy (FT—IR), the sample of mixtures of residual frying oils with prior filtration, the biodiesel formed, and the commercial diesel were evaluated, to compare the absorption signals (cm−1) in the spectra, which can be seen in Figure 1, Figure 2 and Figure 3. Signals or absorption bands of the functional groups corresponding to triglycerides, derived from residual frying oils, methyl esters formed in biodiesel, and aliphatic chains formed in diesel, as seen in Table 3.
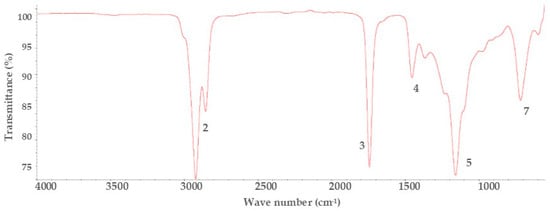
Figure 1.
Infrared spectroscopy spectra of residual frying oils.
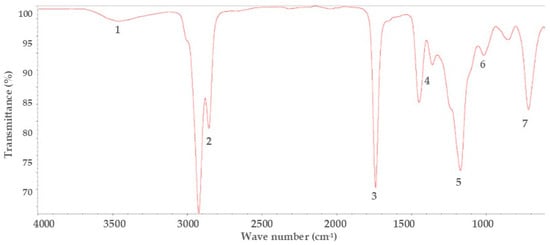
Figure 2.
Infrared spectroscopy spectra of biodiesel.
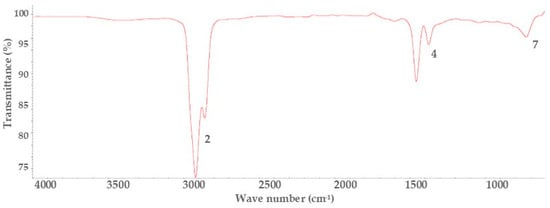
Figure 3.
Infrared spectroscopy spectra of commercial diesel.
The evaluation of functional groups using IR spectroscopy in the substances studied (residual frying oil, biodiesel, and diesel) reflects the importance of this technique for determining and comparing the chemical structure of the substances and interpreting the compounds present.
3.5. Oxidative Stability
The evaluation of oxidative stability, induction time, or induction period reflects the time and temperature at which the biodiesel has its optimal function and application and provides resistance to oxidation. Thus, it can be used without adding additives or antioxidants that delay its useful life [46].
The Rancimat method is an accelerated deterioration test. Air is guided through the sample by the constantly elevated temperature of the reaction vessel. This is how fatty acids are oxidized. At the end of the test, volatile secondary reaction products are generated, which are carried by the air stream into a measuring beaker, where they are absorbed by a measuring solution (distilled water). The continuously recorded electrical conductivity increases due to the absorption of the ionic reaction products. The time until secondary reaction products develop is called induction time [47].
The induction time or oxidative stability was evaluated in diesel, biodiesel and their mixtures at different proportions, as well as in the raw material for their production (residual frying oils) as seen in Table 4. According to the NFPA 110 [48] standard, diesel being a liquid hydrocarbon of crude oil, has an estimated useful life of 1.5 to 2 years under storage conditions of 20 a 25 °C.

Table 4.
Oxidative stability (h) of different substances at 110 °C.
Due to the maturity of the substance studied, biodiesel has an induction time at 110 °C of 2.22 h, while the European standard UNE-EN 14112 [39] reports it at a minimum of 3 h. Now, residual frying oils have additives such as antioxidants that slow down the decomposition time at high temperatures [49]. The use of these antioxidants in the vegetable oils used for cooking is the reason for the greater oxidative stability found in residual frying oil (2168 h = 3.01 months).
Now, the stability evaluation was expanded with biodiesel-diesel mixtures at different temperatures to extrapolate the data and predict cold, warm, or hot temperatures that could influence the conditions of use and storage of the biodiesel. The results are shown in Figure 4.
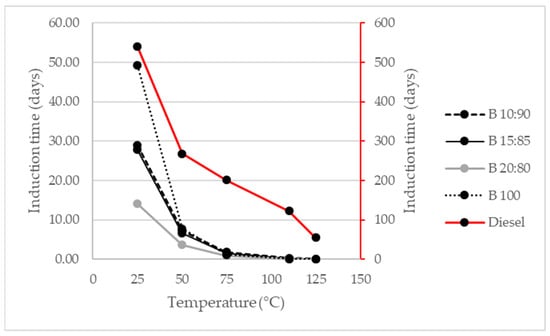
Figure 4.
Evaluation of oxidative stability in biodiesel-diesel mixtures.
It is generally observed that, as the temperature increases, the induction time decreases. This is due to the increase in the oxidation rate, which increases the level of oxygen present and changes the chemical structure of the substance, releasing free radicals that can affect the process and the useful life of the substance.
The Rancimat method produces exponential equations in which Biodiesel exposure temperatures can be simulated, as shown in Equation (1) for Biodiesel (100), Equation (2) for Biodiesel—diesel (10:90 v/v), Equation (3) for Biodiesel—diesel (15:85 v/v) and Equation (4) for Biodiesel—Diesel (20:80 v/v).
where t = induction time (h); T = desired temperature (°C)
t = 7476.636 x e^(−0.073 x T)
t = 2741.642 x e^(−0.054 x T)
t = 2779.142 x e^(−0.057 x T)
t = 130.967 x e^(−0.054 x T)
At 25 °C, Biodiesel (100) has an approximate useful life of 1200 h, that is, 50 d, according to Equation (1). This value decreases when the 20:80 ratio (20% Biodiesel and 80% diesel) is used. Diesel has an oxidative stability of approximately one and a half years at room temperature (25 °C), however, as the temperature increases, this effective use time decreases. It is important to mention that both biodiesels, its mixtures, and diesel reduce the induction time or oxidative stability, as the temperature increases, this is due to the destabilization of the chemical structures of the compounds, in such a way that, at temperatures greater than 125 °C, the substances studied, except diesel, have an average oxidative stability of 1.8 h.
4. Discussion
The characteristics measured for residual frying oil after transformation into biodiesel are adequate density, acidity index, percentage of free fatty acids, and kinematic viscosity. According to Suzihaque et al. [19], the density of used oil mixtures can vary between 0.91 and 0.94 g mL−1, depending on the oil’s use.
Regarding the percentage of free fatty acids, values above 1% reflect more free fatty acids [50], which is undesirable, since they can react with the catalyst used to produce biodiesel and form soaps. Optimal values between 0.2 and 0.8% are expressed as grams of oleic acid per 100 g of substance [19].
The acidity index also reflects that the oil was used several times and prolonged heating, which increased free fatty acids. Various studies have reported acidity index values for oils that will be used to obtain biodiesel, ranging from 1.37 to 7.62% [19,44], so the acidity index obtained for biodiesel production is adequate.
Finally, the kinematic viscosity measured at 40 °C according to ASTM D445 [35], must be less than 50 mm2 s−1 and can be classified as ISO VG 32 viscosity grade (28–35 mm2 s−1), which reflects a viscosity media for use lubricants in pneumatic systems, reducing wear and friction [44]. High viscosities (7–10 mm2/s) have been reported in other studies where biodiesel derived from Jatropha curcas is obtained [51]; for biodiesel obtained from residual frying oil, viscosity values vary between 1.9–6.0 mm2/s and higher viscosities have not been reported, however, the use of this biodiesel is not 100%, since in the application biodiesel is used with a diesel mixture in a proportion of 15:85 and the mixture its viscosity changes to 5.5 mm2/s.
Only the mixture of used oils could be used as an alternative lubricant. However, since this mixture comes from vegetable oils, its useful life and oxidative stability are very short compared to lubricants from petroleum. That is why its alternative use is made by modifying its chemical structure through transesterification and conversion to biodiesel.
After the infrared spectroscopy evaluation of the biodiesel formed, the spectra analysis gave the functional groups of the expected components. According to De Menezes et al. [40], the characteristic bands for the formation of methyl esters in biodiesel are axial deformation (C=O (carbonyl), CO) that are found around 1750 and 1220 cm−1, respectively. However, in this study, they were found at 1740 and 1172 cm−1, along with the bands 1016 and 852 cm−1 found in Figure 2 which, according to Silverstein and Bassier [45] are characteristic functional groups of biodiesels made up of structures of ester (RCOOR) and methyl ester (CH3-RCOOH) respectively (Figure 5).

Figure 5.
Transesterification reaction.
In Figure 2, biodiesel spectrum, a characteristic band is also found at 3460 cm−1, which may be due to terminal hydroxyl groups (-OH) [45] and to unsaturations (H-C=) characteristic of fatty acids unsaturated [40], however, if it were unsaturated fatty acids, this band would also appear in Figure 1 of residual frying oils. Since this is not the case, this band can be attributed to a lack of evaporation of washing water in the formation of the biodiesel or a lack of separation of glycerin, remembering that the biodiesel formed is crude and has not been purified.
On the other hand, Figure 3 shows the characteristic bands of aliphatic chains of alkanes (-CH) between 2800–3000 cm−1, methylenes (-CH2) between 1400 and 1450 cm−1, and more than four methylenes together with a band in 729 cm−1, following Lafont et al. [52] for commercial diesel. Now, the characteristic spectra of biodiesel are present, which shows that waste cooking oils have good quality, combined with the physicochemical characterization test shown in Table 2.
Figure 5 shows the main chemical structures of the transesterification reaction for biodiesel production. For triacyl glyceride, the functional groups are esters that are formed from glycerol and long-chain carboxylic acids (fatty acids), which react with short-chain alcohol (methanol) in the presence of a catalyst (generally KOH or NaOH), to form methyl esters of fatty acids and glycerol [19]. The evaluation of functional groups using IR spectroscopy in the substances studied (residual frying oil, biodiesel, and diesel) reflects the importance of this technique in knowing and comparing the chemical structure of the substances and the interpretation of compounds present.
The evaluation of the oxidative stability of biodiesel in mixtures with diesel provides information for the wear and adequate temperature of use, effective at the flash point of biodiesel mentioned in Table 2, since this point is the temperature at which a substance will spontaneously burn at normal atmospheric pressure [19]. This means that, at storage temperature (25–28 °C) and in a dark room, biodiesel has an oxidative stability between 40 and 50 days.
Considering the need to migrate to more conscious practices regarding the use of natural resources and address the problem of soil and water contamination by residual frying oilss, obtaining biodiesel is considered viable to give an alternative use to the oil. Its use in agricultural machinery promises to reduce processing costs in small and medium industries such as dairy.
5. Conclusions
The search for alternative uses of agroindustry waste is increasing, given population growth and the lack of awareness of recycling or reuse. Agroindustry waste forms a potential market to give added value to its derived products, generating a circular, social and solidarity economy.
An easily accessible waste, which causes contamination to water and soil and damage to human health if fried foods with reused and burned oil are consumed, is residual frying oil.
An alternative applicability of residual frying oil is the chemical transformation to biodiesel, which helps mitigate water and soil contamination problems due to the release of these oils into aquifers; having a good quality biofuel that meets the characteristics and values of national and international regulations; suitable for mechanical, industrial applications and agricultural machinery.
While it is true that the process of obtaining biodiesel is not a new technology and that it generates another waste such as crude glycerin, the important thing is the creation of structured networks with communities that are dedicated to the cultivation of various vegetables where they make use of agricultural machinery that uses diesel as fuel and that is replaced by a mixture composed of 15% biodiesel and 85% diesel, thus helping to minimize CO2 generation, farmer costs and serving as a model for other farming and producing communities worldwide.
Author Contributions
Conceptualization, Y.C.P.-L., P.T.V.-V. and R.B.-H.; Formal analysis, P.T.V.-V.; Investigation, Y.C.P.-L., P.T.V.-V. and E.G.G.-V. Methodology, R.B.-H. and M.A.C.-P.; Resources, R.B.-H.; Supervision, Y.C.P.-L. and R.B.-H.; Validation, Y.C.P.-L., Y.S.-R. and S.S.-T.; Writing—original draft, Y.C.P.-L. and P.T.V.-V.; Writing—review & editing, A.L.-G. and L.R.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Secretary of Science, Humanities, Technology and Innovation (SECIHTI, formerly CONAHCYT), grant number 000000000315293 and student grant for P.T.V.-V. 408688.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank Rocio Meza-Gordillo of the Technological Institute of Tuxtla Gutierrez for the facilities in the use of laboratory equipment.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bárcena, A.; Prado, A. Agenda 2030 and the Sustainable Development Goals; D-CEPAL: Mexico City, Mexico, 2017. [Google Scholar]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaye, A.H.; Jasim, D.J.; Ameen, H.F.M.; Al-Robai, H.A.; Al-Assal, J.R. The Impacts of Petroleum on the Environment. IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 032014. [Google Scholar] [CrossRef]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Beghetto, V. Waste cooking oils into high-value products: Where is the industry going? Polymers 2025, 17, 887. [Google Scholar] [CrossRef]
- Gui, C.; Wang, L.; Liu, G.; Ogunbiyi, A.T.; Li, W. The catalytic valorization of lignin from biomass for the production of liquid fuels. Energies 2025, 18, 1478. [Google Scholar] [CrossRef]
- ASTM D6751-20a; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. American Society for Testing and Materials: Conshohocken, PA, USA, 2023.
- Prado, E.R.L.; Rian, R.C. Advances in conversion technologies for biofuels from wheat and corn straws. Biocatal. Agric. Biotechnol. 2024, 63, 103481. [Google Scholar] [CrossRef]
- Puc-Blanco, N. Evaluation of Bioethanol Production from Lignocellulosic Waste of Tzalam Wood (Lysiloma latisiliquum). Bachelor’s Thesis, Quintana Roo University, Chetumal, Mexico, 2017. [Google Scholar]
- Ayala-Mendivil, N.; Sandoval, G. Bioenergy from forestry and Wood waste. Wood For. 2018, 24, e2401877. [Google Scholar] [CrossRef]
- Alvarado-Ludeña, G.R. Obtaining Bioethanol from Sugarcane Bagasse Through Enzymatic Hydrolysis. Bachelor’s Thesis, Salesian Polytechnic University, Cuenca, Ecuador, 2021. [Google Scholar]
- Garg, R.; Sabouni, R.; Ahmadipour, M. From waste to fuel: Challenging aspects in sustainable biodiesel production from lignocellulosic biomass feedstocks and role of metal organic framework as innovative heterogeneous catalysts. Ind. Crop. Prod. 2023, 206, 117554. [Google Scholar] [CrossRef]
- Sheinbaum, C.; Balam, M.V.; Robles, G.; Lelo de Larrea, S.; Mendoza, R. Biodiesel from waste cooking oil in Mexico City. Waste Manag. Res. 2015, 33, 730–739. [Google Scholar] [CrossRef]
- Sunil Kumar, K.; Babu, J.M.; Venu, H.; Muthuraja, A. Waste plastic as a source of biofuel for stationary diesel engine: A critical review. Int. J. Ambient Energy 2022, 43, 8577–8591. [Google Scholar] [CrossRef]
- Badui, S. Food Chemistry, 4th ed.; PEARSON Education: Mexico City, Mexico, 2006. [Google Scholar]
- González, C.I.; González, U.J.A. Used cooking oils. Environmental problems, incidents in sewage networks, and cost of treatment in wastewater treatment plants. Sewag. Info. 2015, 1–8. [Google Scholar]
- SEDEMA. Separate Used Oil and Take It to Collection Centers; Secretary of the Environment: Mexico City, Mexico, 2023. [Google Scholar]
- Sahoo, P.K.; Das, L.M. Process optimization for biodiesel production from Jatropha, Karanja and Polanga oils. Fuel 2009, 88, 1588–1594. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Alwi, H.; Ibrahim, U.K.; Abdullah, S.; Haron, N. Biodiesel production from waste cooking oil: A brief review. Mater. Today Proc. 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- General Law for the Prevention and Integral Management of Waste. 2023. Available online: https://www.diputados.gob.mx/LeyesBiblio/pdf/LGPGIR.pdf (accessed on 8 September 2024).
- PROFECO. Despite the Damage to Health, Mexicans Reuse Cooking Oil; Consumer Attorney General: Mexico City, Mexico, 2023. [Google Scholar]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption and cooking manners of vegetable oils on cardiovascular diseases critical review. Trends Food Sci. Technol. 2018, 71, 132–154. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Rosales-Quintero, A.; Torrestiana-Sánchez, B. Evaluation and characterization of waste cooking fats and oils for biodiesel production: A case study. Rev. Int. Contam. Ambient 2016, 32, 303–313. [Google Scholar] [CrossRef]
- Rosas-Barajas, A.; Aguilar-Ortega, A.; Cornejo-Corona, I.; Rizo-Fernández, Z.; Córdova, S.E.; Ramos-Frausto, L.G.; Esparza-Claudio, J.D.J. Analysis of bioethanol and biodiesel supply chains in Mexico: Case study. Nova Sci. 2018, 10, 13–29. [Google Scholar] [CrossRef][Green Version]
- Sosa-Rodríguez, F.S.; Vazquez-Arenas, J. The biodiesel market in Mexico: Challenges and perspectives to overcome in Latin-American countries. Energy Convers. Manag. 2021, 12, 100–149. [Google Scholar] [CrossRef]
- Padilla, V.J. Jatropha curcas for Biodiesel Production in Chiapas. Participating Farmers, Land Used and Crop Replacement. Ph.D. Dissertation, The College of the Southern Border, Lerma, Mexico, 2010. [Google Scholar]
- SADER. Jatropha curcas Gold and Green Promise; Secretary of Agriculture and Rural Development: Mexico City, Mexico, 2015. [Google Scholar]
- Avila-Soler, E.; García-Salazar, J.A.; Valtierra-Pacheco, E.; García-Mata, R.; Hoyos-Fernández, G. Jathopha derived biodiesel production: A competitiveness study in the state of Chiapas, Mexico. Rev. Fitotec. Mex. 2018, 41, 461–468. [Google Scholar] [CrossRef]
- UNE-EN 14214; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. Spanish Standardization Association: Madrid, Spain, 2018.
- Susa, D.A.H.; Samtaella, J.R.B.; Alvarez, C.E.C. Analysis of Power and Torque Performance of a Diesel Engine Operating with Palm Biodiesel Blends. Ingeniería 2020, 25, 250–263. [Google Scholar] [CrossRef]
- Caldera, M.; Martinez, R.A.; Stocchi, A. Effect of Biodiesel Use on Engine Component Materials. II Ibero-American Days the Thermal Engines and Lubrication. 2016, pp. 67–78. Available online: https://sedici.unlp.edu.ar/handle/10915/77352 (accessed on 15 February 2025).
- ASTM D4052; Standard Test Method for Density and Relative Density of Liquids by Digital Density Meter. American Society for Testing and Materials: Conshohocken, PA, USA, 2002.
- ASTM D664; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. American Society for Testing and Materials: Conshohocken, PA, USA, 2018.
- NMX-F-101-SCFI; Food—Vegetable or Animal Oils and Fats—Determination of Free Fatty Acids—Test Method. Mexican Standard: Mexico City, Mexico, 2012.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids. American Society for Testing and Materials: Conshohocken, PA, USA, 2024.
- NOM-116-SCFI; Automotive Industry—Lubricating Oils for Gasoline and Diesel Engines—Specifications, Test Methods and Commercial Information. Mexican Official Standard: Mexico City, Mexico 2018.
- ASTM D93; Standard Test Methods for Flash Point by Pensky–Martens Closed Cup Tester. American Society for Testing and Materials: Conshohocken, PA, USA, 2020.
- Ortiz-Tapia, M.D.C.; García-Alamilla, P.; Lagunes-Gálvez, L.M.; Arregoitia-Quezada, M.I.; García-Alamilla, R.; León-Chávez, M.A. Obtaining biodiesel from crude palm oil (Elaeis guineensis Jacq.). Application of the ascending path method. Acta Univ. 2016, 26, 3–10. [Google Scholar] [CrossRef]
- UNE-EN 14112; Derivatives of Oils and Fats. Fatty Acid Methyl Esters (FAME). Determination of Stability Against Oxidation (Accelerated Oxidation Test). Spanish Standardization Association: Madrid, Spain, 2021.
- De Menezes, L.C.; de Sousa, E.R.; da Silva, G.S.; Marques, A.L.B.; Viegas, H.D.C.; Dos Santos, M.J.C. Investigations on Storage and Oxidative Stability of Biodiesel from Different Feedstocks Using the Rancimat Method, Infrared Spectroscopy, and Chemometry. ACS Omega 2022, 7, 30746–30755. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Villanueva, E.; Glorio, P.; Baquerizo, M. Oxidative stability and shelf life estimation of sacha inchi oil (Plukenetia volubilis L.). Sci. Agropecu. 2015, 6, 155–163. [Google Scholar] [CrossRef]
- PANTONE PMS 146. 2024. Available online: https://www.logorapid.com/pantone (accessed on 3 May 2024).
- DOF. Guidelines Establishing the Quality Specifications and Characteristics for Pure Anhydrous Ethanol (Bioethanol), Biodiesel and Bioturbosine; Official Journal of the Federation: Mexico City, Mexico, 2018. [Google Scholar]
- García-Muentes, S.A.; Lafargue-Pérez, F.; Labrada-Vázquez, B.; Díaz-Velázquez, M.; Sánchez-Lafita, A.E. Physicochemical properties of oil and biodiesel produced from Jatropha curcas L. in the province of Manabí, Ecuador. Rev. Cuba Quím. 2018, 30, 142–158. [Google Scholar]
- Silverstein, R.M.; Bassier, G.C. Spectrometric identification of organic compounds. J. Chem. Edu. 2005, 39, 546. [Google Scholar] [CrossRef]
- Longanesi, L.; Pereira, A.P.; Johnston, N.; Chuck, C.J. Oxidative stability of biodiesel: Recent insights. Biofuels Bioprod. Bioref. 2022, 16, 265–289. [Google Scholar] [CrossRef]
- Catalogue, M.C. Oxidation stability of oils and fats–Rancimat method. Appl. Bull. 2022, 204, 1–4. [Google Scholar]
- Bennon, M.; Wilson, D. NEPA Litigation over Large Energy and Transport Infrastructure Projects. Environ. Law Report. 2023, 53, 10836. [Google Scholar]
- Santamaría-Juárez, J.D.; Juárez-Vidal, O.; Sánchez-Cantú, M.; Santamaría-Juárez, G.; Varela-Caselis, J.; Castañeda-Antonio, M.D.; López-Morales, S. Oxidative stability index as a reference for quality control in biodiesel production. Ingeniería 2022, 25, 44–56. [Google Scholar]
- Salguero, H.S.; Morejón, F.B.; Carranza, C.C. Components present in used frying oil and determinants before its conversion into biodiesel. J. Res. Inst. Fac. Geol. Min. Metall. Geogr. Eng. 2019, 22, 33–38. [Google Scholar]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Atabani, A.E.; Chong, W.T. A global comparative review of biodiesel production from Jatropha curcas using different homogeneous acid and alkaline catalysts: Study of physical and chemical properties. Renew Sustain. Energy Rev. 2013, 24, 514–533. [Google Scholar] [CrossRef]
- Lafont, J.J.; Páez, M.S.; Torres, Y.C. Chemical analysis of biodiesel mixtures of used cooking oil and diesel by infrared spectroscopy. Technol. Inf. 2011, 22, 35–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).