Packed-Bed Pyrolysis of Alkali Lignin for Value-Added Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Measurements
2.3. Pelletization
2.4. Packed-Bed Reactor
2.5. Product Characterization
3. Results
3.1. Thermogravimetric Curves
3.2. Packed-Bed Pyrolysis of Alkali Lignin
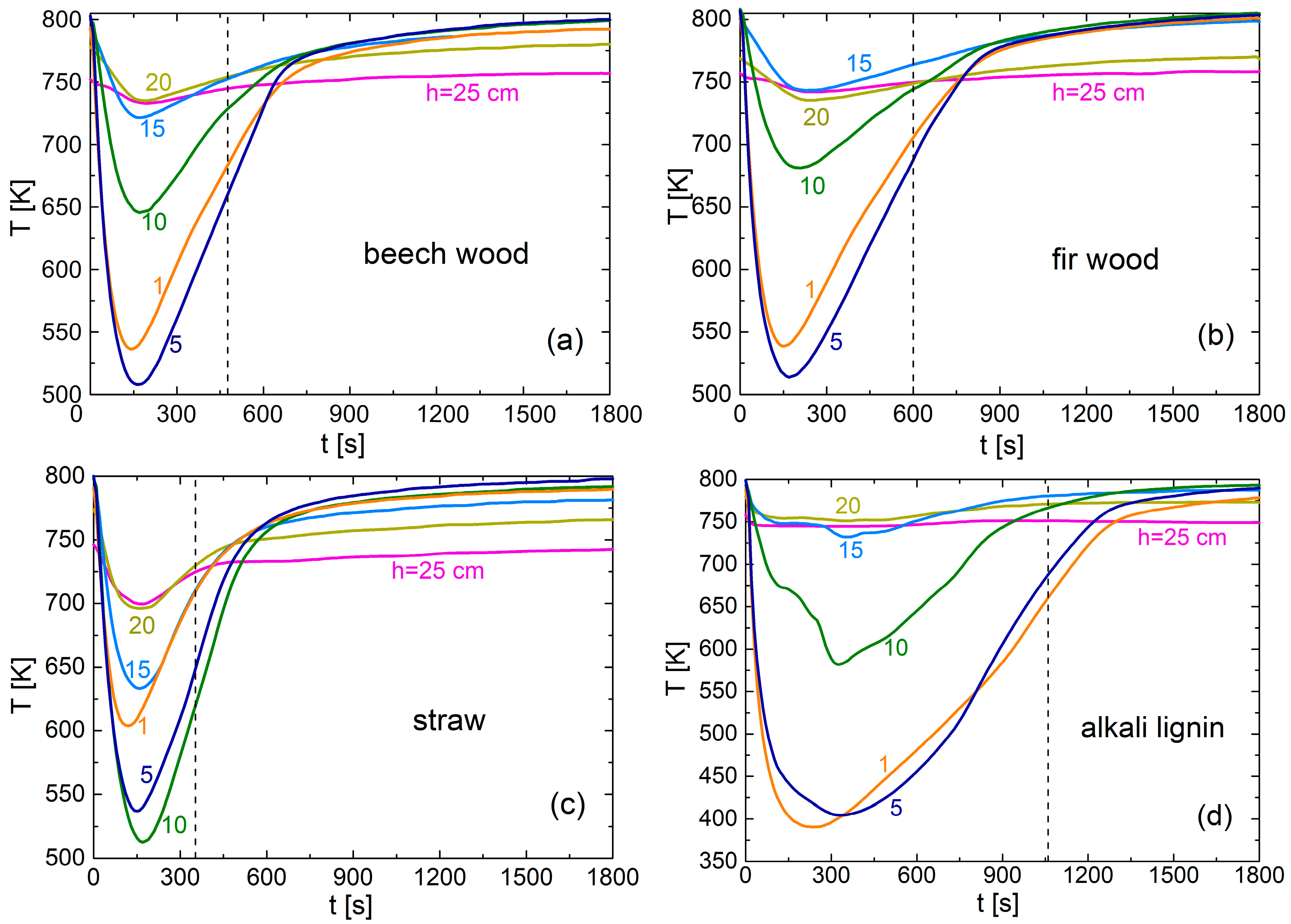
3.3. Comparison of Packed-Bed Pyrolysis of Alkali Lignin and Lignocellulosic Materials
3.4. Effects of Lignin Melting
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sa, L.C.R.; Silva, H.F.C. Thermochemical conversion of olive oil industry waste: Circular economy through energy recovery. Recycling 2020, 5, 12. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagard, B.M.; Wud, K.C.-W.; Tsang, D.C.W. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef]
- Nandal, P.; Arora, A.; Virmani, S. An appraisal on valorization of lignin: A byproduct from biorefineries and paper industries. Biomass Bioenergy 2021, 155, 106295. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef]
- Karthäuser, J.; Biziks, V.; Frauendorf, H.; Mai, C.; Militz, H. Vacuum low-temperature microwave-assisted pyrolysis of technical lignins. Polymers 2022, 14, 3383. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, W.; Chang, J. Study on the product characteristics of pyrolysis lignin with calcium salt additives. Materials 2019, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Zevallos Torres, L.A.; Woiciechowski, A.L.; Oliveira de Andrade Tanobe, V.; Karp, S.G.; Guimaraes Lorenci, L.C.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Lin, X.; Sui, S.; Tan, S.; Pittman, C.U.; Sun, J.; Zhang, Z. Fast pyrolysis of four lignins from different isolation processes using Py-GC/MS. Energies 2015, 8, 5107–5121. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Q.; Ye, J.; Yao, Q.; Zhao, C. Study on the thermal degradation behaviors and kinetics of alkali lignin for production of phenolic-rich bio-oil using TGA–FTIR andPy–GC/MS. J. Anal. Appl. Pyrolysis 2016, 117, 116–124. [Google Scholar] [CrossRef]
- Supriyanto, U.D.O.; Ylitervo, P.; Dou, J.; Sipponen, M.H.; Richards, T. Identifying the primary reactions and products of fast pyrolysis of alkali lignin. J. Anal. Appl. Pyrolysis 2020, 151, 104917. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Hu, J.; Zhong, W.; Hu, Z.; Wang, H. Comprehensive study of alkali lignin pyrolysis catalyzed by composite metal-modified molecular sieves for the preparation of hydrocarbon liquid fuels. J. Anal. Appl. Pyrolysis 2024, 181, 106608. [Google Scholar] [CrossRef]
- Wang, W.-L.; Ren, X.-Y.; Chang, J.-M.; Cai, L.-P.; Shi, S.Q. Characterization of bio-oils and bio-chars obtained from the catalytic pyrolysis of alkali lignin with metal chlorides. Fuel Proc. Technol. 2015, 138, 605–611. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, R.; Kumar, J.; Khan, A.A.; Krishna, B.B.; Bhaskar, T. Slow pyrolysis of prot, alkali and dealkaline lignins for production of chemicals. Bioresour. Technol. 2016, 213, 319–326. [Google Scholar] [CrossRef]
- Damayanti, D.; Wu, H.S. Pyrolysis kinetic of alkaline and dealkaline lignin using catalyst. J. Polym. Res. 2018, 25, 7. [Google Scholar] [CrossRef]
- Ma, Z.; Custodis, V.; van Bokhoven, J.A. Selective deoxygenation of lignin during catalytic fast pyrolysis. Catal. Sci. Technol. 2014, 4, 766–772. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis procresses—An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Ryms, M.; Kosakowski, W. Thermal biomass conversion: A review. Processes 2020, 8, 516. [Google Scholar] [CrossRef]
- Ciesielski, P.N.; Pecha, M.B.; Lattanzi, A.M.; Bharadwaj, V.S.; Crowley, M.F.; Bu, L.; Vermaas, J.V.; Steirer, K.X.; Crowley, M.F. Advances in multiscale modeling of lignocellulosic biomass. ACS Sustain. Chem. Eng. 2020, 8, 3512–3531. [Google Scholar] [CrossRef]
- Tan, Z.; Li, Y.; Chen, F.; Liu, J.; Zhong, J.; Guo, L.; Zhang, R.; Chen, R. Challenges and perspectives of the conversion of lignin waste to high-value chemicals by pyrolysis. Processes 2024, 12, 589. [Google Scholar] [CrossRef]
- Becidan, M.; Skreiberg, O.; Hustad, J.E. Products distribution and gas release in pyrolysis of thermally thick biomass residues samples. J. Anal. Appl. Pyrolysis 2007, 78, 207–213. [Google Scholar] [CrossRef]
- Manya, J.J.; Ruiz, J.; Arauzo, J. Some peculiarities of conventional pyrolysis of several agricultural residues in a packed bed reactor. Ind. Eng. Chem. Res. 2007, 46, 9061–9070. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A. Biomass screening for the production of furfural via thermal decomposition. Ind. Eng. Chem. Res. 2010, 49, 2658–2671. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C.; Galgano, A. Experimental analysis about the exploitation of industrial hemp (Cannabis sativa) in pyrolysis. Fuel Proc. Technol. 2017, 162, 20–29. [Google Scholar] [CrossRef]
- Ábrego, J.; Plaza, D.; Luño, F.; Atienza-Martínez, M.; Gea, G. Pyrolysis of cashew nutshells: Characterization of products and energy balance. Energy 2018, 158, 72–80. [Google Scholar] [CrossRef]
- Bouzarour, A.; Pozzobon, V.; Perré, P.; Salvador, S. Experimental study of torrefied wood fixed bed: Thermal analysis and source term identification. Fuel 2018, 234, 247–255. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Sarnataro, F.E.; Gallo, A. Thermal runaway in the pyrolysis of some lignocellulosic biomasses. Energy Fuels 2014, 28, 2684–2696. [Google Scholar] [CrossRef]
- Branca, C.; Galgano, A.; Di Blasi, C. Dynamics and products of potato crop residue conversion under a pyrolytic runaway regime—Influences of feedstock variability. Energy 2023, 276, 127507. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental advances in biomass autothermal/oxidative pyrolysis: A review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Graham, S.; Jones, J.M.; Dekker, M. An integrated laboratory and industrial scale study of autothermal torrefaction of hardwood, softwood and Miscanthus. Biomass Bioenergy 2025, 195, 107723. [Google Scholar] [CrossRef]
- Singh-Morgan, A.; Puente-Urbina, A.; van Bokhoven, J.A. Technology overview of fast pyrolysis of lignin: Current state and potential for scale-up. ChemSusChem 2022, 15, e202200343. [Google Scholar] [CrossRef]
- Ghalibaf, M.; Alen, R.; Hita, I.; Deuss, P.J.; Jan Heeres, H.; de Wild, P. Valorization potential of technical lignins from Norway spruce (Picea abies) via pyrolysis. J. Anal. Appl. Pyrolysis 2022, 165, 105549. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. Multi-step devolatilization kinetics of Klason lignin isolated from beech wood and agro-industrial wastes. Fuel 2024, 374, 132469. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Lyczko, N.; Pham Minh, D. The catalytic effect of inherent and adsorbed metals on the fast/flash pyrolysis of biomass: A review. Energy 2019, 170, 326–337. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. A systematic study of the kinetics of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Lin, H.; Sun, W.; Luo, Z. Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Biores. Technol. 2015, 182, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; E, J.Q.; Xue, Y. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Kristanto, J.; Daniyal, A.F.; Pratama, D.Y.; Bening, I.N.M.; Setiawan, L.; Azis, M.M.; Purwono, S. Kinetic study on the slow pyrolysis of isolated cellulose and lignin from teak sawdust. Thermochim. Acta 2022, 711, 179202. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The complexity of lignin thermal degradation in the isothermal context. Processes 2021, 9, 1154. [Google Scholar] [CrossRef]
- Kaczor, Z.; Bulinski, Z.; Werle, S. Modelling approaches to waste biomass pyrolysis: A review Renew. Energy 2020, 159, 427–443. [Google Scholar] [CrossRef]
- Basile, L.; Tugnoli, A.; Cozzani, V. Influence of macrocomponents on the pyrolysis heat demand of lignocellulosic biomass. Ind. Eng. Chem. Res. 2017, 56, 6432–6440. [Google Scholar] [CrossRef]
- Chhiti, Y.; Salvador, S.; Commandré, J.-M.; Broust, F. Thermal decomposition of bio-oil: Focus on the products yields under different pyrolysis conditions. Fuel 2012, 102, 274–281. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. Combustion kinetics of secondary biomass chars in the kinetic regime. Energy Fuels 2010, 24, 5741–5750. [Google Scholar] [CrossRef]
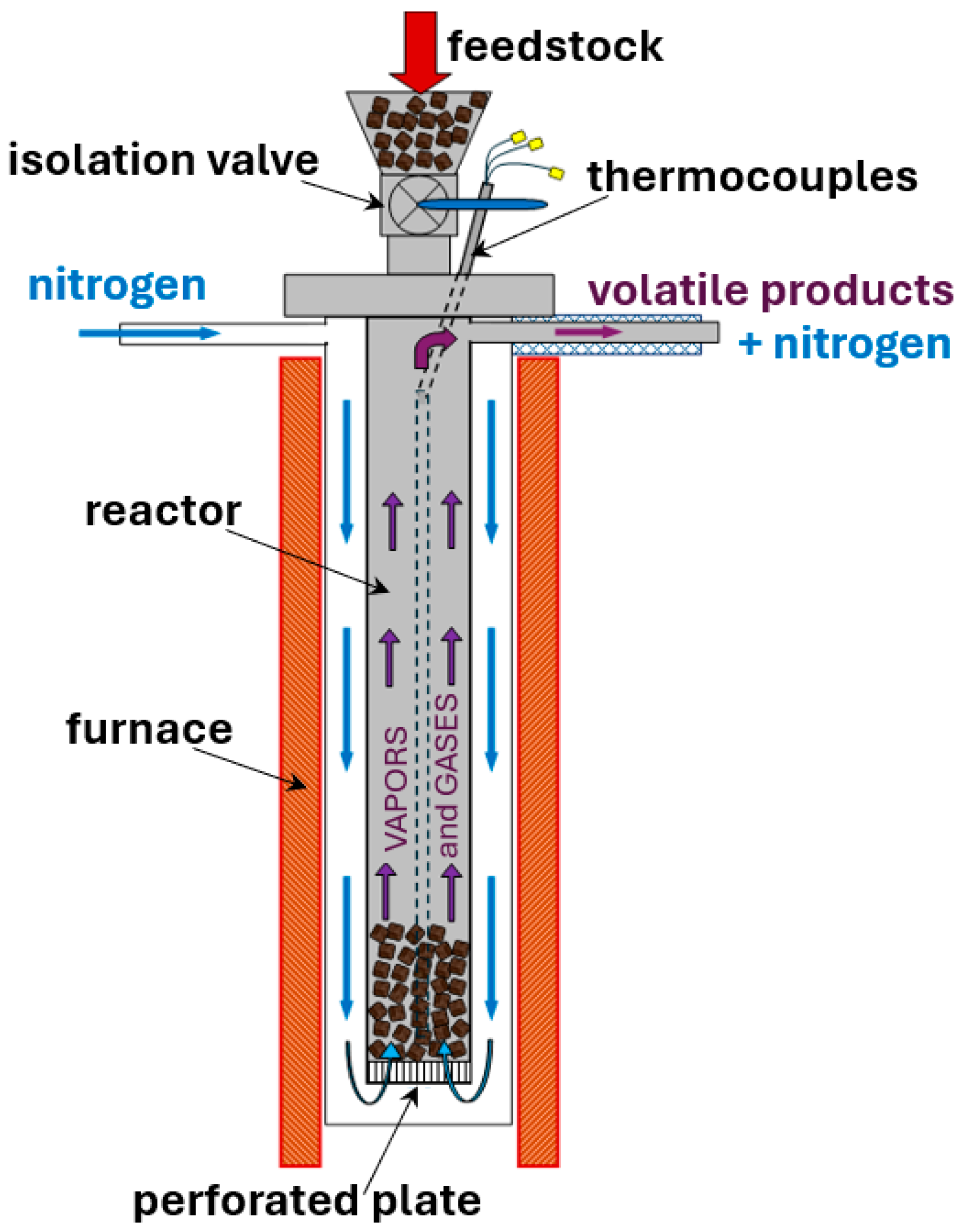

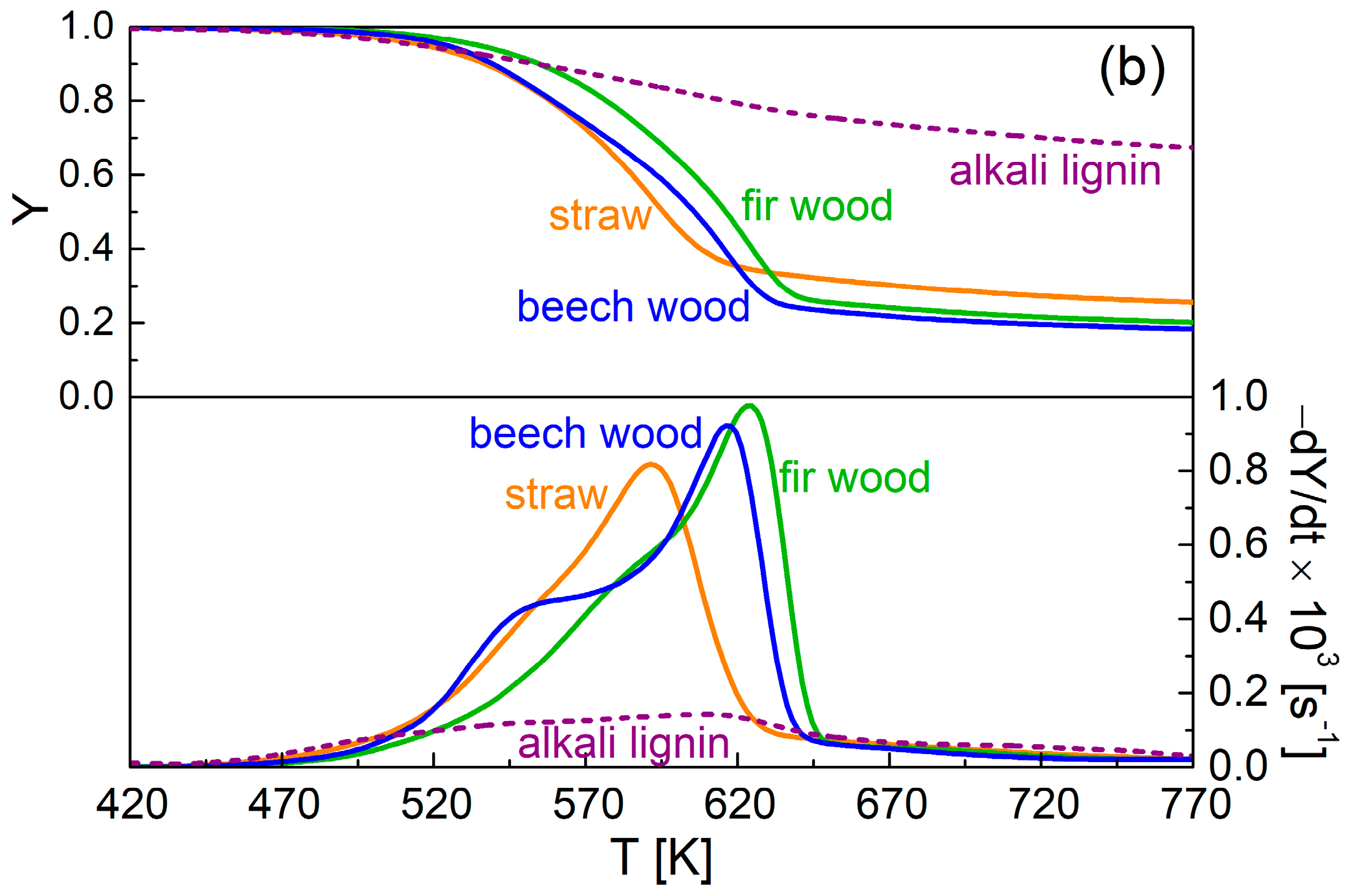
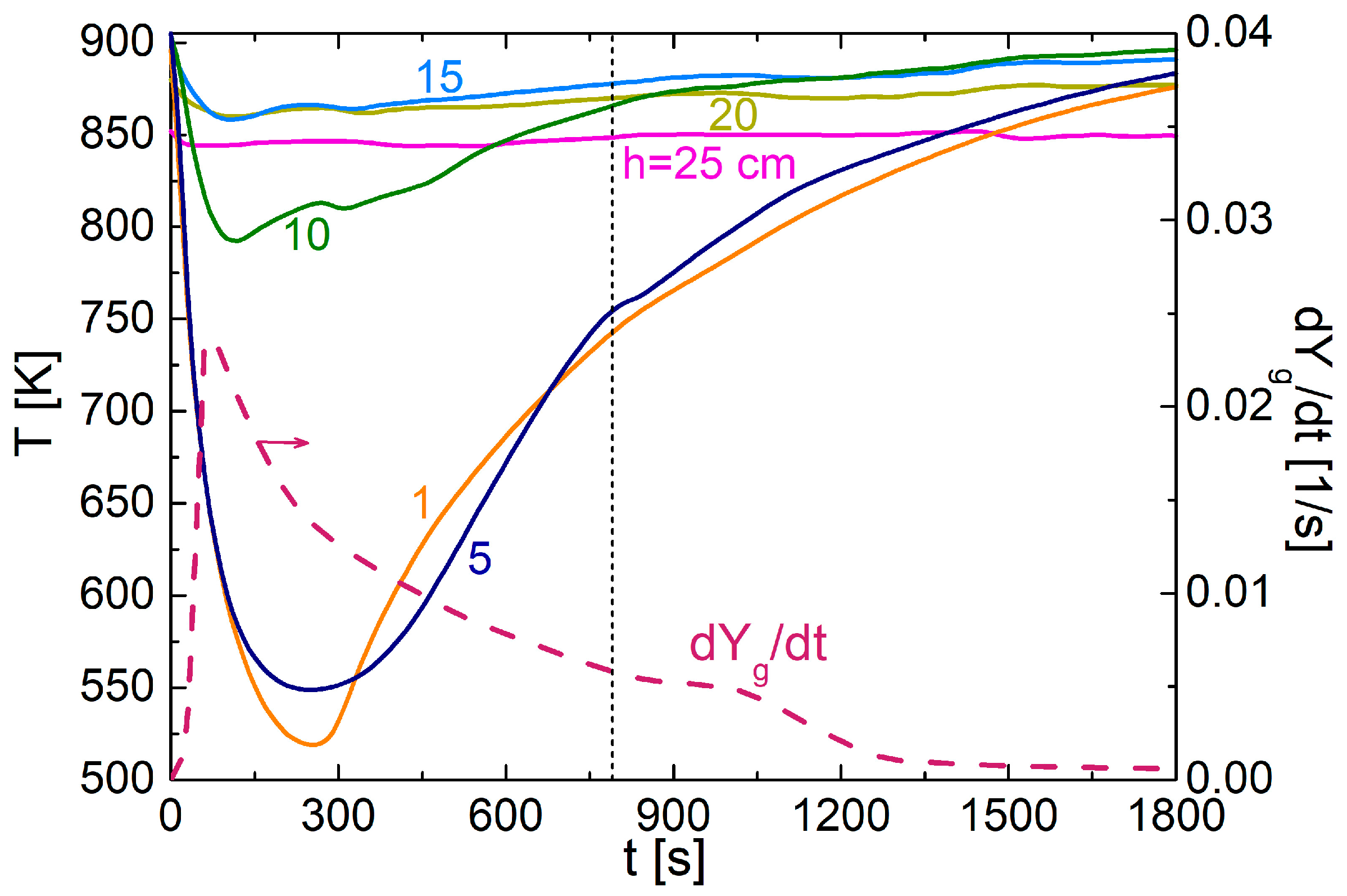
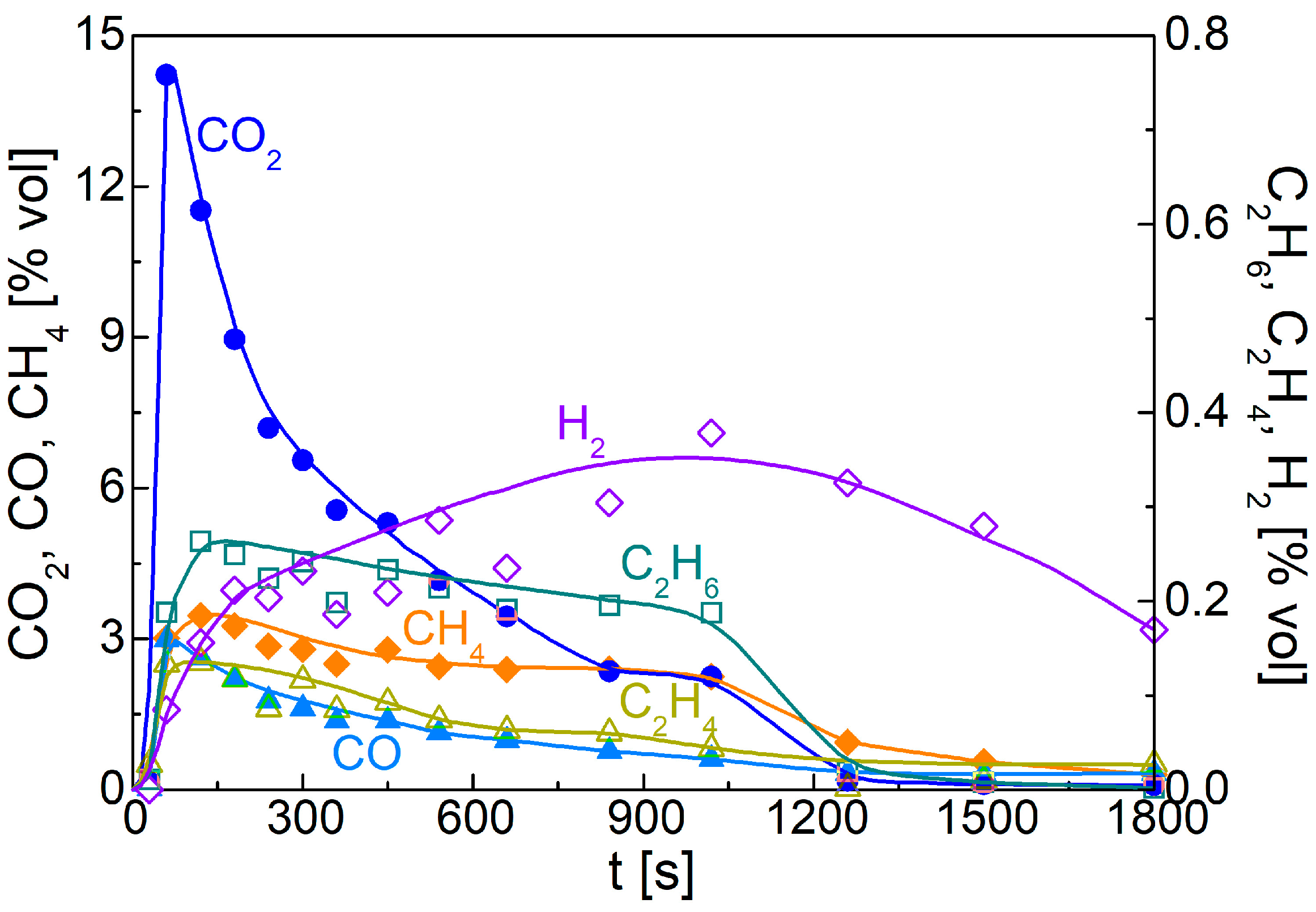



| Ref. | Experimental Device | Sample Mass | Thermal Conditions | Product Characterization | Heat and Mass Transfer Data |
|---|---|---|---|---|---|
| Lin et al. (2015) [8] | micro-Py-GC-MS | 0.5 mg | 623–923 K | semi-quantitative analysis of volatiles | no |
| Ma et al. (2016) [9] | micro-Py-GC-MS | 0.5 mg | 673–973 K | semi-quantitative analysis of volatiles | no |
| Supriyanto et al. (2020) [10] | micro-Py-GC-MS/FID | 0.5 mg | 673–873 K | semi-quantitative analysis of volatiles | no |
| Liu et al. (2024) [11] | Fixed bed + Py-GC-MS | 10 g | 673–1073 K | lumped product yields + semi-quantitative analysis of volatiles | no |
| Wang et al. (2015) [12] | fixed bed + GC-MS | n.a. | 873 K | lumped product yields + semi-quantitative analysis of volatiles | no |
| Biswas et al. (2016) [13] | fixed bed + GC-MS | 10 g | 573–723 K | lumped product yields + semi-quantitative analysis of volatiles | no |
| Damayanti and Wu (2018) [14] | fixed bed + GC-MS | 100 g | 773 K | lumped product yields + semi-quantitative analysis of volatiles | no |
| Ma et al. (2014) [15] | pyroprobe | 1.5 g | 923 K | lumped product yields + semi-quantitative analysis of volatiles | no |
| This study | fixed bed + GC-TCD + GC-MS | 175–185 g | 800–900 K | lumped product yields + quantitative analysis of volatiles | yes |
| Specie | RT [min] | Yield [wt%] | Δσ [%] |
|---|---|---|---|
| Hydroxyacetaldehyde | 8.02 | 0.13 | 5.0 |
| Acetic acid | 9.20 | 0.01 | 6.5 |
| Hydroxypropanone | 10.82 | 0.01 | 5.9 |
| Propionic acid | 13.53 | 0.02 | 7.6 |
| 2-Methyl-2-cyclopentenone | 21.70 | 0.06 | 9.4 |
| 3-Methyl-2-cyclopentenone | 26.42 | 0.23 | 8.3 |
| Phenol | 31.30 | 1.37 | 7.3 |
| Guaiacol | 31.64 | 7.64 | 5.7 |
| Cresols | 35.00 | 1.31 | 6.6 |
| 4-Methylguiacol | 36.54 | 0.44 | 6.7 |
| 3,4-Dimethylphenol | 37.09 | 0.11 | 9.7 |
| 2,5-Dimethylphenol | 38.73 | 0.40 | 8.8 |
| 2-Ethhylphenol | 38.97 | 0.17 | 7.1 |
| 4-Ethylguiacol | 40.32 | 0.25 | 6.6 |
| Eugenol | 43.87 | 0.01 | 8.4 |
| 4-Propylguaiagol | 43.97 | 0.06 | 8.5 |
| Syringol | 44.90 | 0.02 | 8.1 |
| Isoeugenol | 46.21 | 0.15 | 8.5 |
| 4-Methylsyringol | 48.68 | 0.19 | 7.2 |
| Vanillin | 49.35 | 0.01 | 8.5 |
| Hydroquinone | 49.74 | 0.01 | 6.0 |
| 4-Acetonguiacol | 54.61 | 0.21 | 4.6 |
| Parameter | Beech Wood | Fir Wood | Wheat Straw | Alkali Lignin | Δσ [%] |
|---|---|---|---|---|---|
| tgm [s] | 195 | 285 | 120 | 70 | 17.6 |
| dYgm [1/s]; % gas | 0.028; 25.1 | 0.018; 25.6 | 0.057; 15.4 | 0.015; 4.7 | 5.1; 6.5 |
| tmin [s] | 160 | 170 | 143 | 323 | 6.8 |
| Tmin [K] | 508 | 514 | 537 | 403 | 0.3 |
| tc [s] | 476 | 600 | 353 | 1060 | 2.6 |
| Tc [K] | 663 | 690 | 654 | 757 | 1.8 |
| biochar [% wt] | 24.3 | 24.1 | 28.6 | 63.0 | 1.2 |
| gas [% wt] | 12.4 | 11.4 | 15.4 | 9.3 | 3.7 |
| bio-oil [% wt] | 63.3 (55.4) | 64.5 (56.2) | 56.0 (48.0) | 27.7 (19.7) | 4.9 |
| mass closure [% wt] | 92.1 | 92.7 | 92.0 | 92.0 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branca, C.; Di Blasi, C. Packed-Bed Pyrolysis of Alkali Lignin for Value-Added Products. Recycling 2025, 10, 66. https://doi.org/10.3390/recycling10020066
Branca C, Di Blasi C. Packed-Bed Pyrolysis of Alkali Lignin for Value-Added Products. Recycling. 2025; 10(2):66. https://doi.org/10.3390/recycling10020066
Chicago/Turabian StyleBranca, Carmen, and Colomba Di Blasi. 2025. "Packed-Bed Pyrolysis of Alkali Lignin for Value-Added Products" Recycling 10, no. 2: 66. https://doi.org/10.3390/recycling10020066
APA StyleBranca, C., & Di Blasi, C. (2025). Packed-Bed Pyrolysis of Alkali Lignin for Value-Added Products. Recycling, 10(2), 66. https://doi.org/10.3390/recycling10020066






