Efficient Recovery of Gadolinium from Contaminated Waters Using Manganese Ferrite Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Characterization of Manganese Ferrite Nanoparticles
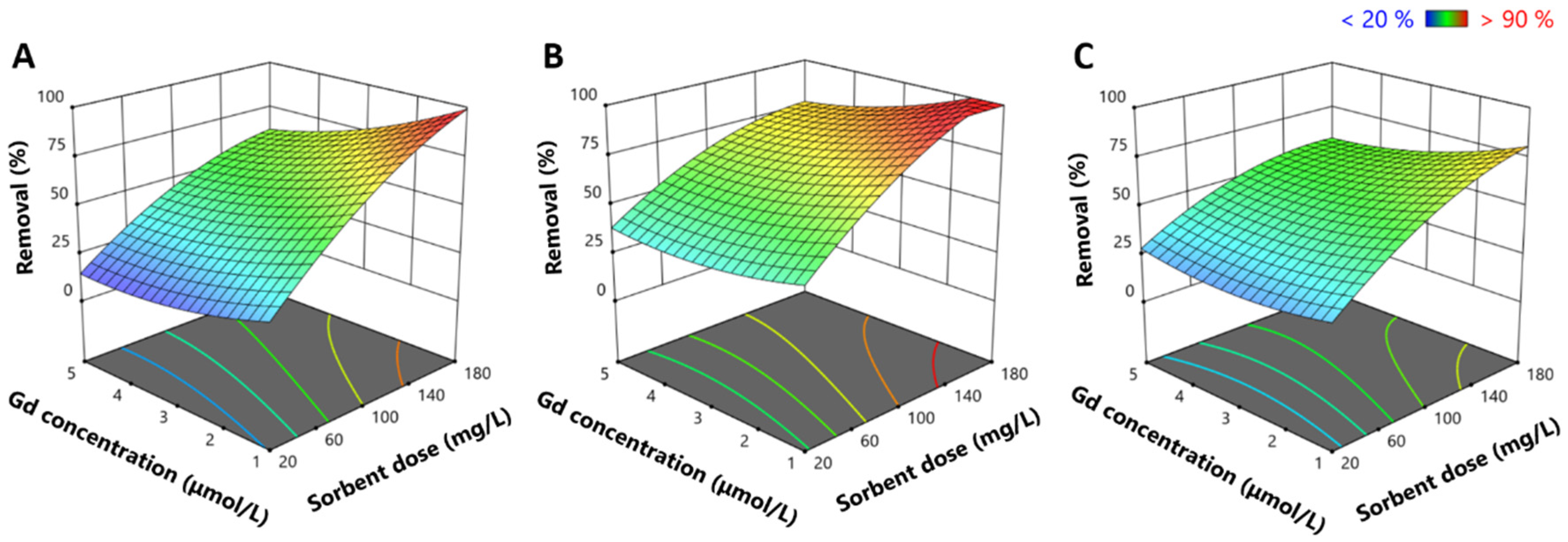
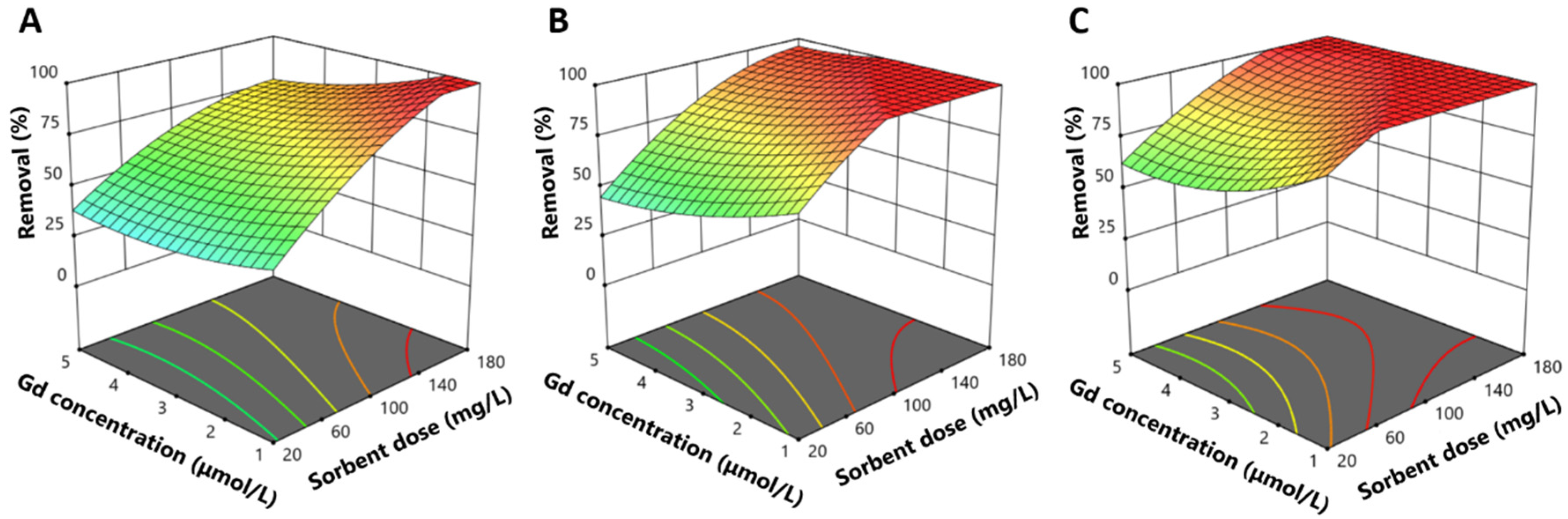
2.2. Response Surface Experiments: Evaluation of the Influence of Salinity, Gadolinium Concentration, and Sorbent Dose
2.2.1. Influence of Ionic Strength
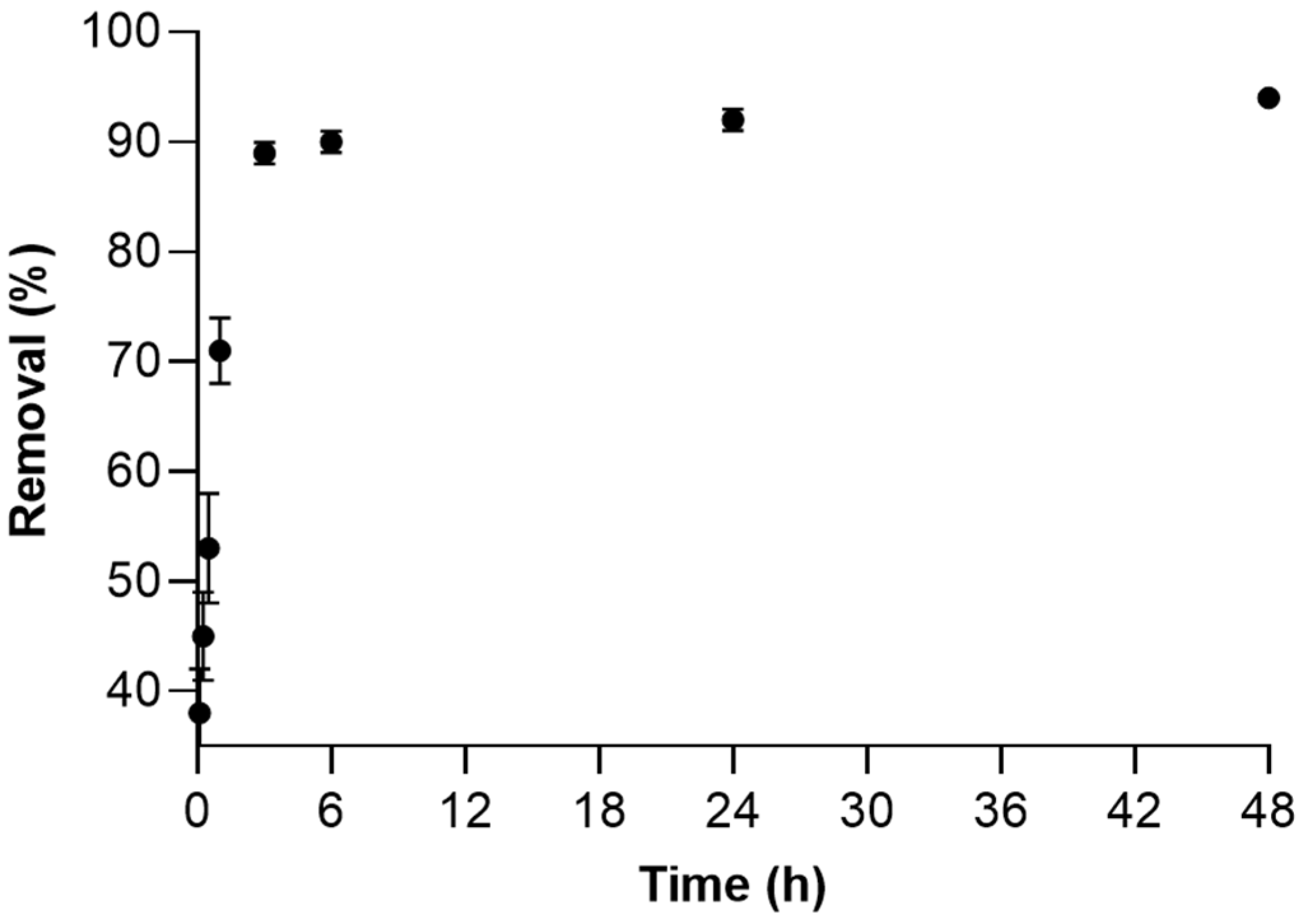
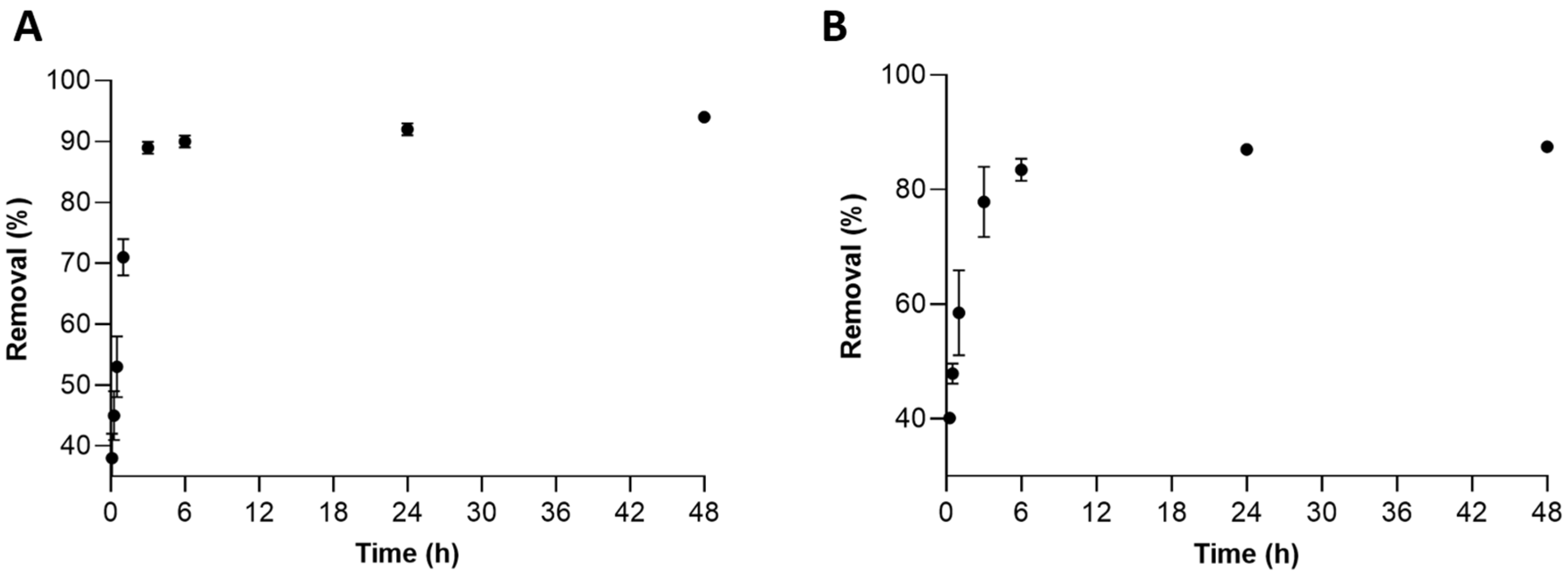
2.2.2. Influence of Contact Time
2.2.3. Sorption Kinetics Studies
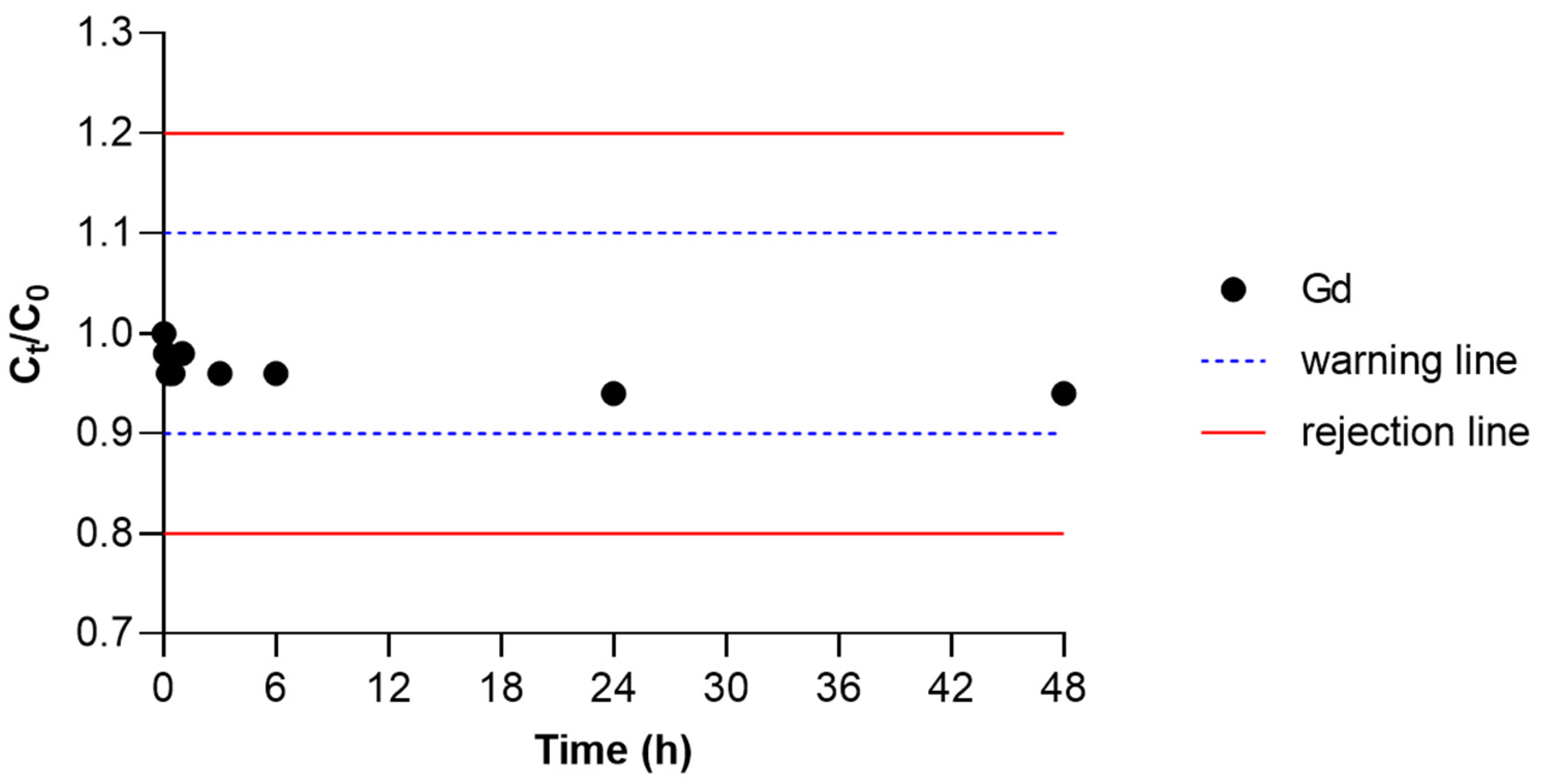
2.2.4. Influence of Matrix Complexity
3. Discussion
3.1. Gd Removal Efficiency by MnFe2O4
3.2. Analysis of Sorption Mechanisms
3.3. Solution in Complex Matrices
3.4. Comparison with Other Sorbents and Environmental Applications
3.5. Limitations and Future Directions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Synthesis and Characterization of MnFe2O4 Nanoparticles
4.3. Response Surface Experiments: Evaluation of the Influence of Salinity, Gadolinium Concentration, and Sorbent Dose
Sorption Kinetics Studies
4.4. Comparison Between Mono-Elemental and Multi-Elemental Sorption of REEs in Different Matrices by MnFe2O4
4.5. Gadolinium Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migaszewski, Z.M.; Gałuszka, A. The Use of Gadolinium and Europium Concentrations as Contaminant Tracers in the Nida River Watershed in South-Central Poland. Geol. Q. 2016, 60, 67–76. [Google Scholar] [CrossRef]
- Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolska, L. Gadolinium as a New Emerging Contaminant of Aquatic Environments. Environ. Toxicol. Chem. 2018, 37, 1523–1534. [Google Scholar] [CrossRef]
- Telgmann, L.; Sperling, M.; Karst, U. Determination of Gadolinium-Based MRI Contrast Agents in Biological and Environmental Samples: A Review. Anal. Chim. Acta 2013, 764, 1–16. [Google Scholar]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Laczovics, A.; Csige, I.; Szabó, S.; Tóth, A.; Kálmán, F.K.; Tóth, I.; Fülöp, Z.; Berényi, E.; Braun, M. Relationship between Gadolinium-Based MRI Contrast Agent Consumption and Anthropogenic Gadolinium in the Influent of a Wastewater Treatment Plant. Sci. Total Environ. 2023, 877, 162844. [Google Scholar] [CrossRef]
- Swan, S.K.; Lambrecht, L.J.; Townsend, R.; Davies, B.E.; McCloud, S.; Parker, J.R.; Bensel, K.; LaFrance, N.D. Safety and Pharmacokinetic Profile of Gadobenate Dimeglumine in Subjects with Renal Impairment. Investig. Radiol. 1999, 34, 443–448. [Google Scholar] [CrossRef]
- de Campos, F.F.; Enzweiler, J. Anthropogenic Gadolinium Anomalies and Rare Earth Elements in the Water of Atibaia River and Anhumas Creek, Southeast Brazil. Environ. Monit. Assess. 2016, 188, 281. [Google Scholar] [CrossRef]
- Pedreira, R.M.A.; Pahnke, K.; Böning, P.; Hatje, V. Tracking Hospital Effluent-Derived Gadolinium in Atlantic Coastal Waters off Brazil. Water Res. 2018, 145, 62–72. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Cyanobacteria Arthospira Platensis as an Effective Tool for Gadolinium Removal from Wastewater. Clean. Technol. 2023, 5, 638–651. [Google Scholar] [CrossRef]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on Gadolinium Chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [PubMed]
- Grobner, T.; Prischl, F.C. Gadolinium and Nephrogenic Systemic Fibrosis. Kidney Int. 2007, 72, 260–264. [Google Scholar] [PubMed]
- Mendichovszky, I.A.; Marks, S.D.; Simcock, C.M.; Olsen, Ø.E. Gadolinium and Nephrogenic Systemic Fibrosis: Time to Tighten Practice. Pediatr. Radiol. 2008, 38, 489–496. [Google Scholar] [PubMed]
- Piarulli, S.; Riedel, J.A.; Fossum, F.N.; Kermen, F.; Hansen, B.H.; Kvæstad, B.; Olsvik, P.A.; Farkas, J. Effects of Gadolinium (Gd) and a Gd-Based Contrast Agent (GBCA) on Early Life Stages of Zebrafish (Danio rerio). Chemosphere 2024, 350, 140950. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Raynal, I.; Schaefer, M.; Le Greneur, S.; Corot, C. Clinical and Biological Consequences of Transmetallation Induced by Contrast Agents for Magnetic Resonance Imaging: A Review. Fundam. Clin. Pharmacol. 2006, 20, 563–576. [Google Scholar]
- Le Goff, S.; Barrat, J.A.; Chauvaud, L.; Paulet, Y.M.; Gueguen, B.; Ben Salem, D. Compound-Specific Recording of Gadolinium Pollution in Coastal Waters by Great Scallops. Sci. Rep. 2019, 9, 8015. [Google Scholar] [CrossRef]
- Trapasso, G.; Coppola, F.; Queirós, V.; Henriques, B.; Soares, A.M.V.M.; Pereira, E.; Chiesa, S.; Freitas, R. How Ulva Lactuca Can Influence the Impacts Induced by the Rare Earth Element Gadolinium in Mytilus Galloprovincialis? The Role of Macroalgae in Water Safety towards Marine Wildlife. Ecotoxicol. Environ. Saf. 2021, 215, 112101. [Google Scholar] [CrossRef]
- Figueiredo, C.; Grilo, T.F.; Oliveira, R.; Ferreira, I.J.; Gil, F.; Lopes, C.; Brito, P.; Ré, P.; Caetano, M.; Diniz, M.; et al. Gadolinium Ecotoxicity Is Enhanced in a Warmer and Acidified Changing Ocean as Shown by the Surf Clam Spisula Solida through a Multibiomarker Approach. Aquat. Toxicol. 2022, 253, 106346. [Google Scholar] [CrossRef]
- Hanana, H.; Turcotte, P.; André, C.; Gagnon, C.; Gagné, F. Comparative Study of the Effects of Gadolinium Chloride and Gadolinium—Based Magnetic Resonance Imaging Contrast Agent on Freshwater Mussel, Dreissena Plymorpha. Chemosphere 2017, 181, 197–207. [Google Scholar] [CrossRef]
- Perrat, E.; Parant, M.; Py, J.S.; Rosin, C.; Cossu-Leguille, C. Bioaccumulation of Gadolinium in Freshwater Bivalves. Environ. Sci. Pollut. Res. 2017, 24, 12405–12415. [Google Scholar] [CrossRef]
- Martino, C.; Bonaventura, R.; Byrne, M.; Roccheri, M.; Matranga, V. Effects of Exposure to Gadolinium on the Development of Geographically and Phylogenetically Distant Sea Urchins Species. Mar. Environ. Res. 2017, 128, 98–106. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent Advances in Selective Separation Technologies of Rare Earth Elements: A Review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Ghobadi, M.; Gharabaghi, M.; Abdollahi, H.; Boroumand, Z.; Moradian, M. MnFe2O4-Graphene Oxide Magnetic Nanoparticles as a High-Performance Adsorbent for Rare Earth Elements: Synthesis, Isotherms, Kinetics, Thermodynamics and Desorption. J. Hazard. Mater. 2018, 351, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Gupta, A.; Ramteke, P.; Sahoo, H.; Sengupta, A. Biosorption-a Green Method for the Preconcentration of Rare Earth Elements (REEs) from Waste Solutions: A Review. J. Mol. Liq. 2019, 274, 148–164. [Google Scholar] [CrossRef]
- Estrade, G.; Marquis, E.; Smith, M.; Goodenough, K.; Nason, P. REE Concentration Processes in Ion Adsorption Deposits: Evidence from the Ambohimirahavavy Alkaline Complex in Madagascar. Ore Geol. Rev. 2019, 112, 103027. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, W. Adsorption of Cerium (III) by Zeolites Synthesized from Kaolinite after Rare Earth Elements (REEs) Recovery. Chemosphere 2022, 303, 134941. [Google Scholar] [CrossRef]
- Xiao, J.; Li, B.; Qiang, R.; Qiu, H.; Chen, J. Highly Selective Adsorption of Rare Earth Elements by Honeycomb-Shaped Covalent Organic Frameworks Synthesized in Deep Eutectic Solvents. Environ. Res. 2022, 214, 113977. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Kołodyńska, D.; Kozioł, M.; Gorbyk, P.P. Gd-DTPA Adsorption on Chitosan/Magnetite Nanocomposites. Nanoscale Res. Lett. 2016, 11, 168. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of Rare Earth Metals from Wastewater by Nanomaterials: A Review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Pinto, J.; Fernandes, R.; Tavares, D.; Henriques, B.; Trindade, T.; Pereira, E. Removal of Rare Earth Elements from Complex Mixtures by Using Manganese Ferrite Nanoparticles: Optimization through Surface Response Methodology. J. Environ. Manag. 2024, 368, 122211. [Google Scholar] [CrossRef]
- Pinto, J.; Branco, D.; Carvalho, L.; Henriques, B.; Freitas, R.; Trindade, T.; Tavares, D.; Pereira, E. Influence of Experimental Parameters on the Sorption Behavior of Rare Earth Elements on Manganese Ferrite Nanoparticles. Environ. Technol. Innov. 2023, 32, 103432. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.R.; Sood, A.K. Graphene Oxide-MnFe2O4 Magnetic Nanohybrids for Efficient Removal of Lead and Arsenic from Water. ACS Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.S.; Lopes, C.B.; Almeida, J.C.; Vale, C.; Pereira, E.; Trindade, T. Spinel-Type Ferrite Nanoparticles for Removal of Arsenic(V) from Water. Environ. Sci. Pollut. Res. 2020, 27, 22523–22534. [Google Scholar] [CrossRef]
- Homayonfard, A.; Miralinaghi, M.; Seyed Mohammad Shirazi, R.H.; Moniri, E. Efficient Removal of Cadmium (II) Ions from Aqueous Solution by CoFe2O4/Chitosan and NiFe2O4/Chitosan Composites as Adsorbents. Water Sci. Technol. 2018, 78, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Viltres, H.; Gupta, N.K.; Paz, R.; Dhavale, R.P.; Park, H.H.; Leyva, C.; Srinivasan, S.; Rajabzadeh, A.R. Mercury Remediation from Wastewater through Its Spontaneous Adsorption on Non-Functionalized Inverse Spinel Magnetic Ferrite Nanoparticles. Environ. Technol. 2022, 45, 1155–1168. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Shyichuk, A.; Sojka, Z.; Gryboś, J.; Naushad, M.; Kotsyubynsky, V.; Kowalska, M.; Kwiatkowska-Marks, S.; Danyliuk, N. Green Synthesis, Structure, Cations Distribution and Bonding Characteristics of Superparamagnetic Cobalt-Zinc Ferrites Nanoparticles for Pb(II) Adsorption and Magnetic Hyperthermia Applications. J. Mol. Liq. 2021, 328, 115375. [Google Scholar] [CrossRef]
- Tu, Y.J.; You, C.F.; Chen, M.H.; Duan, Y.P. Efficient Removal/Recovery of Pb onto Environmentally Friendly Fabricated Copper Ferrite Nanoparticles. J. Taiwan Inst. Chem. Eng. 2017, 71, 197–205. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, P.; Gupta, C.; Goel, P.; Jain, A.; Anand, S.; Pundir, A. Engineered Magnetic Nanoparticles as Efficient Sorbents for Wastewater Treatment: A Review. Mater. Res. Innov. 2018, 22, 434–450. [Google Scholar] [CrossRef]
- Gupta, V.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Suhas Low-Cost Adsorbents: Growing Approach to Wastewater Treatmenta Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, G.; Li, X.; Lu, X. Removal of Rare Earth Elements by MnFe2O4 Based Mesoporous Adsorbents: Synthesis, Isotherms, Kinetics, Thermodynamics. J. Alloys Compd. 2021, 856, 158185. [Google Scholar] [CrossRef]
- Sousa, J.; Tavares, D.S.; Pinto, J.; Henriques, B.; Rocha, J.; Trindade, T.; Lapa, N.; Pereira, E. Neodymium Removal and Recovery from Simulated NdFeB Leachate Using Manganese Ferrite Nanoparticles. J. Water Process Eng. 2025, 71, 107200. [Google Scholar] [CrossRef]
- Cabral, G.H.; Estrada, A.C.; Santos, P.S.M. Removal of Zn(II), Cu(II) and Pb(II) from Rainwater by White Bean Peel: Optimization by Response Surface Methodology. Appl. Sci. 2025, 15, 627. [Google Scholar] [CrossRef]
- Água Das Caldas de Penacova. Available online: https://www.caldasdepenacova.pt/ (accessed on 30 March 2025).
- Ebrahimi, P.; Barbieri, M. Gadolinium as an Emerging Microcontaminant in Water Resources: Threats and Opportunities. Geosciences 2019, 9, 93. [Google Scholar] [CrossRef]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of Lanthanides on Smectite and Kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Aksu, Z.; Balibek, E. Chromium(VI) Biosorption by Dried Rhizopus Arrhizus: Effect of Salt (NaCl) Concentration on Equilibrium and Kinetic Parameters. J. Hazard. Mater. 2007, 145, 210–220. [Google Scholar] [CrossRef]
- Tu, Y.J.; Lo, S.C.; You, C.F. Selective and Fast Recovery of Neodymium from Seawater by Magnetic Iron Oxide Fe3O4. Chem. Eng. J. 2015, 262, 966–972. [Google Scholar] [CrossRef]
- Tu, Y.J.; Johnston, C.T. Rapid Recovery of Rare Earth Elements in Industrial Wastewater by CuFe2O4 Synthesized from Cu Sludge. J. Rare Earths 2018, 36, 513–520. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Gamal, R.; Helal, A.A.; Abo-El-Enein, S.A.; Helal, A.A. Kinetic Sorption Study of Cerium (IV) on Magnetite Nanoparticles. Part. Sci. Technol. 2017, 35, 643–652. [Google Scholar] [CrossRef]
- Durán, S.V.; Lapo, B.; Meneses, M.; Sastre, A.M. Recovery of Neodymium (III) from Aqueous Phase by Chitosan-Manganese-Ferrite Magnetic Beads. Nanomaterials 2020, 10, 1204. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of Second-Order Models for Adsorption Systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]

| Kinetic Models | Parameters | Modeled Values | R2 |
|---|---|---|---|
| PFO | qe | 2.886 | 0.8899 |
| k1 | 8.605 | ||
| Sx/y | 0.4012 | ||
| PSO | qe | 3.337 | 0.9350 |
| k2 | 3.148 | ||
| Sx/y | 0.3084 | ||
| Elovich | α | 96.68 | 0.9623 |
| β | 1.592 | ||
| Sx/y | 0.2347 |
| Sorbent | Element | Element Concentration (mg/L) | pH | Sorbent Massa (g/L) | Salinity (g/L) | Contact Time (min) | Type of Matrix | Maximum Sorption Capacity (mg/g) | Removal (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| MnFe2O4 | La3+ and Ce3+ | 500 | 7 | 0.30 | ---- | 20 | Deionized water | La3+: 785 Ce3+: 770 | ---- | Ghobadi et al. [22] |
| MnFe2O4-GO | La3+: 1001 Ce3+: 982 | ---- | ||||||||
| MnFe2O4 | La3+ and Ce3+ | 500 | 7 | 0.30 | ---- | 30 | Ultrapure water | La3+: 757 Ce3+: 751 | 76.2 | Liu et al. [39] |
| MnFe2O4- Al2O4 | La3+: 885 Ce3+: 879 | 94.2 | ||||||||
| MnFe2O4@ SiO2-Chitosan | La3+: 1030 Ce3+: 1020 | 99.3 | ||||||||
| MnFe2O4 | Gd3+ | 0.204 | 6 | 0.165 | 13.4 | 60 | Saline water | ---- | 100 | This study |
| 0.472 | 0.10 | ---- | 360 | Mineral bottled water | ---- | 83.5 |
| Experiment | Sorbent Dose (mg/L) | Salinity (g/L) | Gd Concentration (μmol/L) |
|---|---|---|---|
| 1 | 180 | 0 | 3 |
| 2 | 100 | 15 | 3 |
| 3 | 20 | 15 | 5 |
| 4 | 100 | 0 | 1 |
| 5 | 180 | 15 | 5 |
| 6 | 180 | 15 | 1 |
| 7 | 100 | 15 | 3 |
| 8 | 100 | 15 | 3 |
| 9 | 100 | 30 | 5 |
| 10 | 100 | 30 | 1 |
| 11 | 20 | 0 | 3 |
| 12 | 100 | 0 | 5 |
| 13 | 20 | 15 | 1 |
| 14 | 20 | 30 | 3 |
| 15 | 180 | 30 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, J.; Pinto, J.; Barbosa, H.; Tavares, D.S.; Freitas, R.; Trindade, T.; Rocha, J.; Pereira, E. Efficient Recovery of Gadolinium from Contaminated Waters Using Manganese Ferrite Nanoparticles. Recycling 2025, 10, 57. https://doi.org/10.3390/recycling10020057
Sousa J, Pinto J, Barbosa H, Tavares DS, Freitas R, Trindade T, Rocha J, Pereira E. Efficient Recovery of Gadolinium from Contaminated Waters Using Manganese Ferrite Nanoparticles. Recycling. 2025; 10(2):57. https://doi.org/10.3390/recycling10020057
Chicago/Turabian StyleSousa, Joana, João Pinto, Helena Barbosa, Daniela S. Tavares, Rosa Freitas, Tito Trindade, João Rocha, and Eduarda Pereira. 2025. "Efficient Recovery of Gadolinium from Contaminated Waters Using Manganese Ferrite Nanoparticles" Recycling 10, no. 2: 57. https://doi.org/10.3390/recycling10020057
APA StyleSousa, J., Pinto, J., Barbosa, H., Tavares, D. S., Freitas, R., Trindade, T., Rocha, J., & Pereira, E. (2025). Efficient Recovery of Gadolinium from Contaminated Waters Using Manganese Ferrite Nanoparticles. Recycling, 10(2), 57. https://doi.org/10.3390/recycling10020057











