The Characterization of Black Mass from Spent Lithium-Ion Scooter Batteries Using Multi-Analytical Techniques
Abstract
1. Introduction
2. Results and Discussion
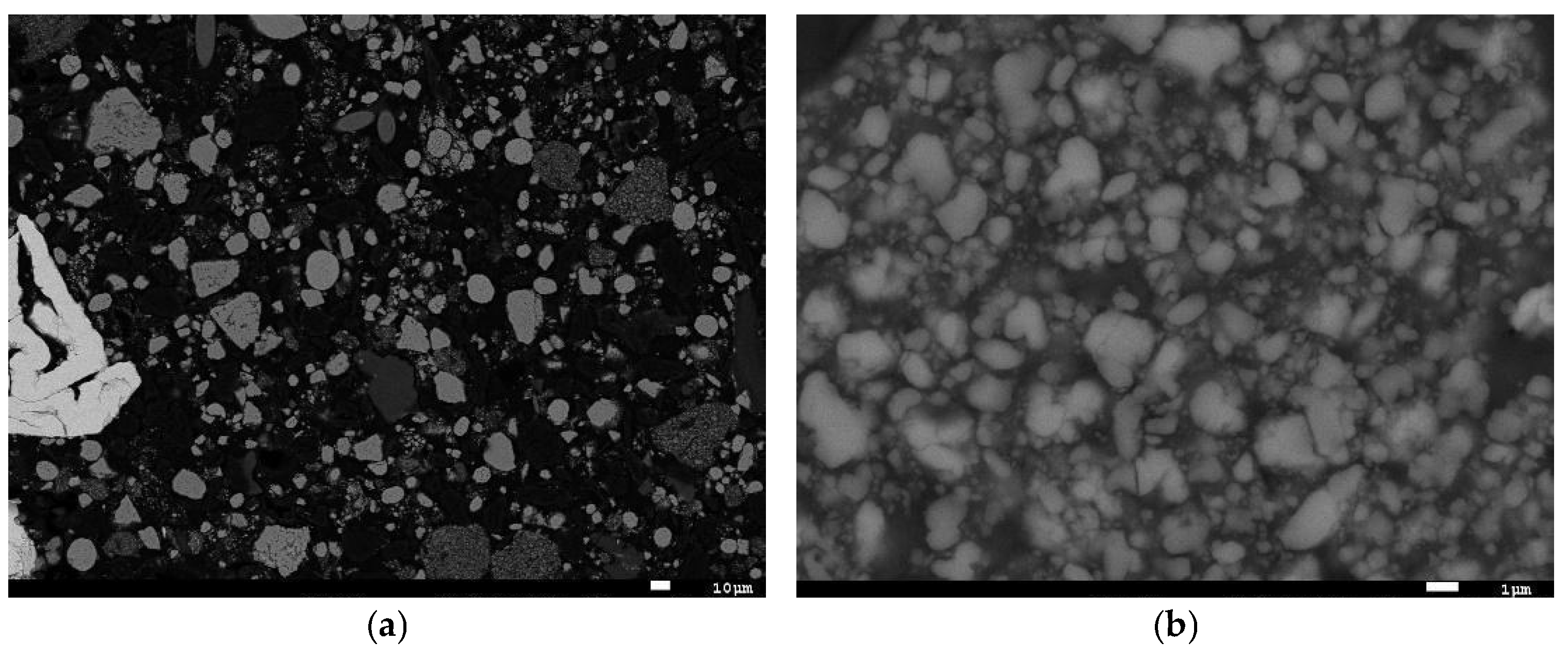
2.1. Particle Size Distribution (PSD)
2.2. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
2.3. X-Ray Fluorescence Analysis (XRF)
2.4. Compound Phases
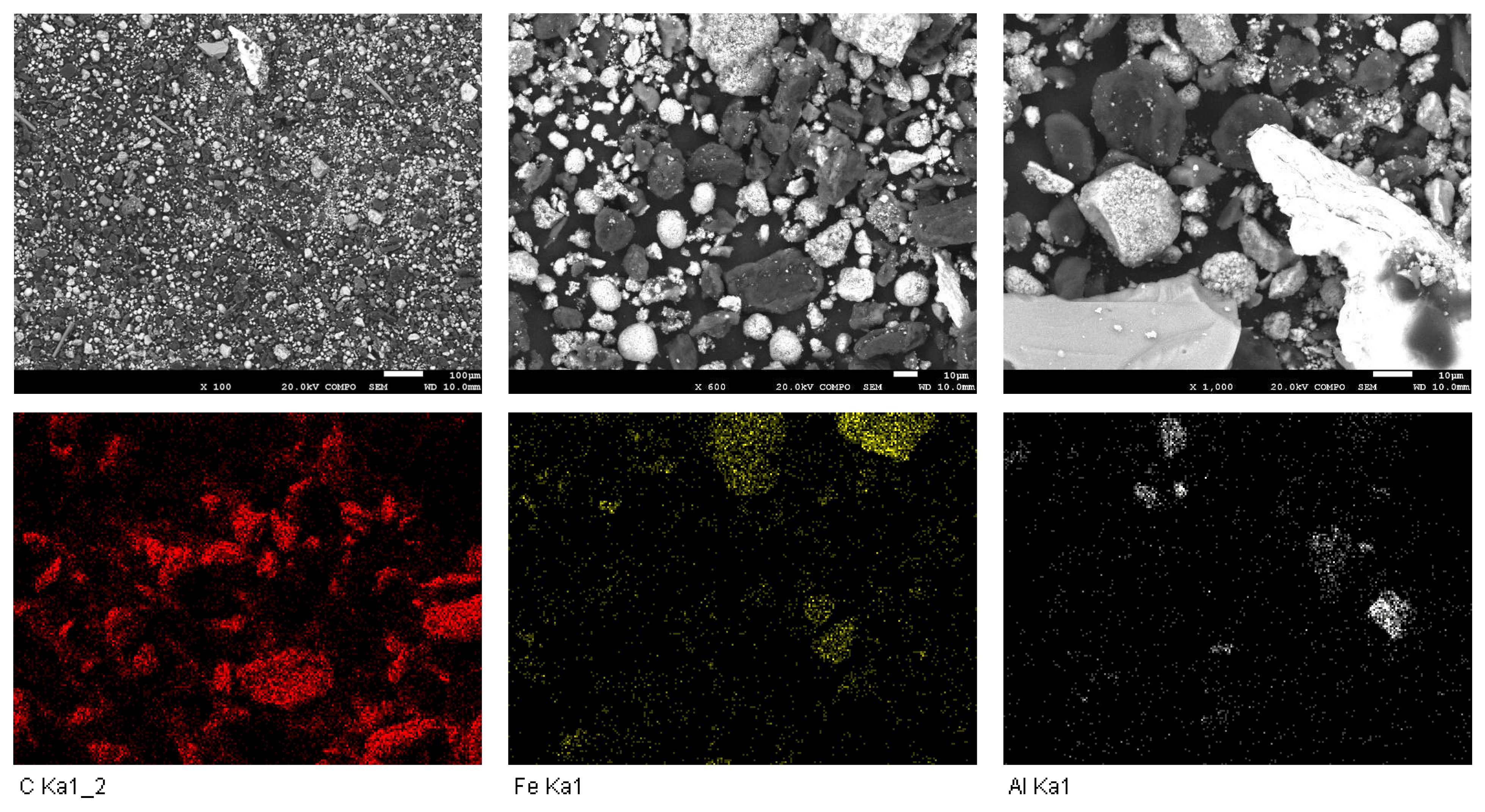
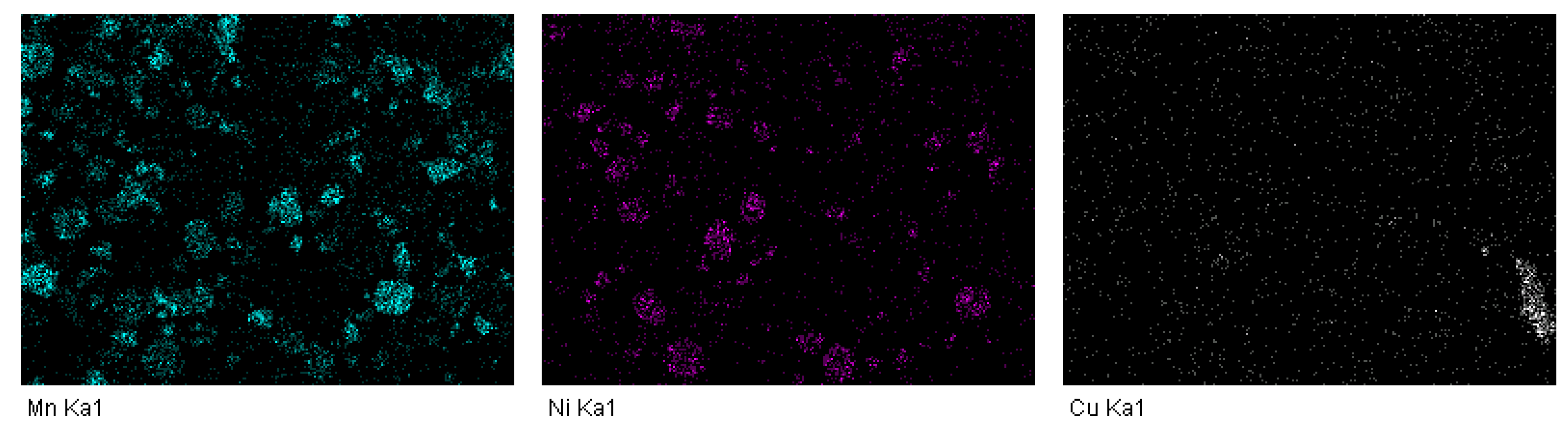
2.5. Scanning Electron Microscopy with Energy-Dispersive Spectroscopy (SEM-EDS)
2.6. Laser-Induced Breakdown Spectroscopy
2.7. Thermogravimetry–Differential Thermal Analysis (TG/DTA)
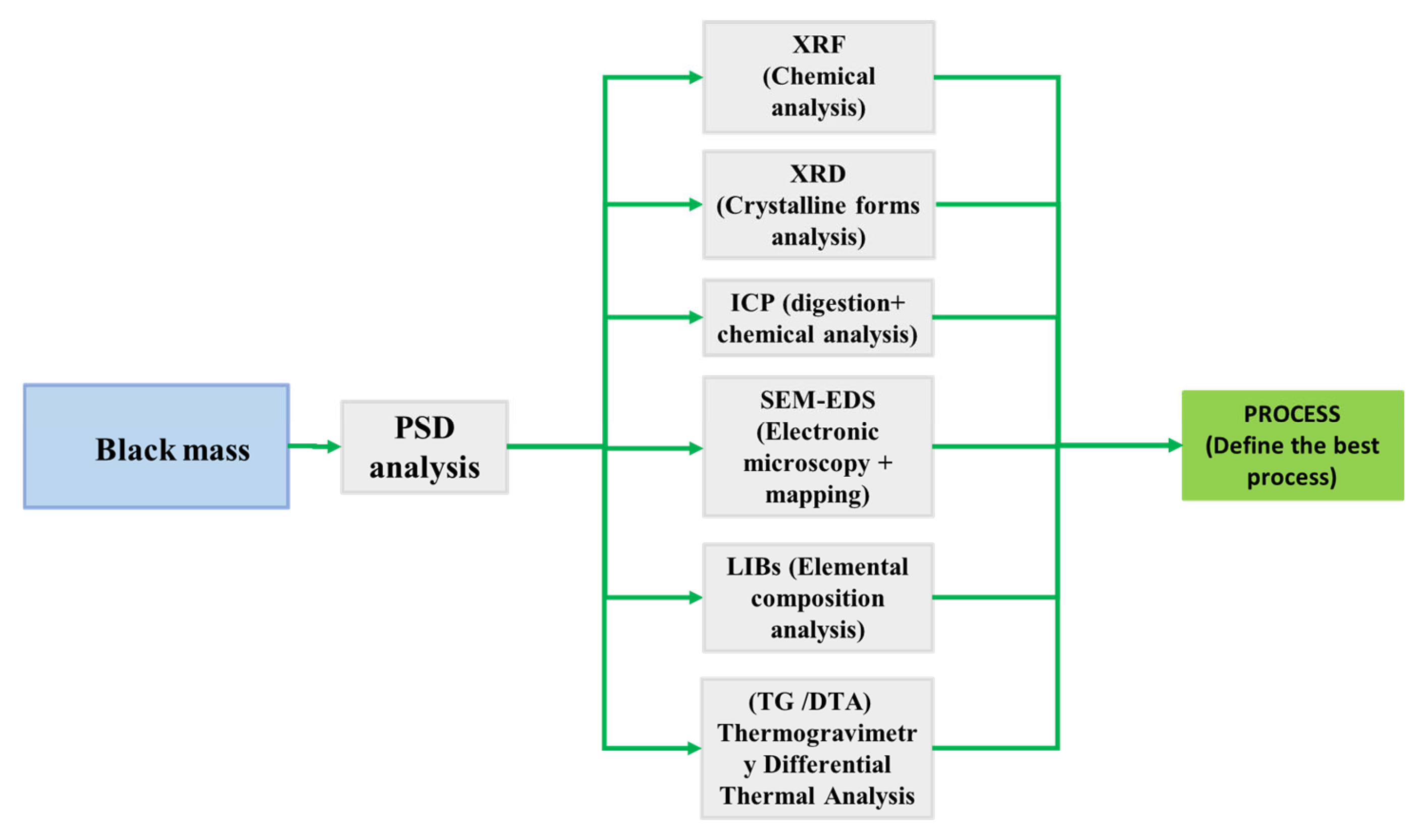
2.8. Beneficiation Flowsheet
3. Materials and Methods
3.1. Sample Preparation
3.2. Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Yurramendi, Y.; Hidalgo, J.; Siriwardana, A. A Sustainable Process for the Recovery of Valuable Metals from Spent Lithium Ion Batteries by Deep Eutectic Solvents Leaching. Mater. Proc. 2021, 5, 100. [Google Scholar] [CrossRef]
- Alcaraz, L.; Díaz-Guerra, C.; Calbet, J.; López, M.L.; López, F.L. Obtaining and Characterization of Highly Crystalline Recycled Graphites from Different Types of Spent Batteries. Materials 2022, 15, 3246. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ge, P.; Wang, L.; Sun, W.; Bu, Y.; Sun, M.; Yang, Y. The Recycling of Spent Lithium-Ion Batteries: Crucial Flotation for the Separation of Cathode and Anode Materials. Molecules 2023, 28, 4081. [Google Scholar] [CrossRef] [PubMed]
- Vanderbruggen, A.; Gugala, E.; Blannin, R.; Bachmann, K.; Serna-Guerrero, R.; Rudolph, M. Automated mineralogy as a novel approach for the compositional and textural characterization of spent lithium-ion batteries. Miner. Eng. 2021, 169, 106924. [Google Scholar] [CrossRef]
- Bhar, M.; Ghosh, S.; Krishnamurthy, S.; Kaliprasad, Y.; Martha, S.K. A review on spent lithium-ion battery recycling: From collection to black mass recovery. R. Soc. Chem. Sustain. 2023, 1, 1150–1167. [Google Scholar] [CrossRef]
- Zielinski, M.; Cassayre, L.; Floquet, P.; Macouin, M.; Destrac, P.; Coppey, N.; Foulet, C.; Biscans, B. A multi-analytical methodology for the characterization of industrial samples of spent Ni-MH battery powders. Waste Manag. 2020, 118, 677–687. [Google Scholar] [CrossRef]
- Donnelly, L.; Pirrie, D.; Power, M.; Corfe, I.; Kuva, J.; Lukkari, S.; Lahaye, Y.; Liu, X.; Dehaine, Q.; Jolis, E.M.; et al. The Recycling of End-of-Life Lithium-Ion Batteries and the Phase Characterisation of Black Mass. Recycling 2023, 8, 59. [Google Scholar] [CrossRef]
- Broussely, M.; Pistoia, G.E. Chapter 14: Battery Collection and Recycling. In Industrial Applications of Batteries. From Cars to Aerospace and Energy Storage; Elsevier B.V.: Amsterdam, The Netherlands, 2007; Available online: https://www-sciencedirect-com.recursos.biblioteca.upc.edu/book/9780444521606/industrial-applications-of-batteries (accessed on 13 February 2007).
- Donnelly, L.; Pirrie, D.; Power, M.; Corfe, I.J.; Kuva, J.; Lukkari, S.; Lahaye, Y.; Liu, X.; Butcherda, A.R. The sampling and phase characterization of black mass. IMPublications Open 2022, 11, 397–409. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-Guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Hayagan, N.; Bachmann, K.; Ferreira, A.; Werner, D.; Horn, D.; Peuker, U.; Serna-Guerrero, R.; Rudolph, M. Lithium-Ion Battery Recycling Influence of Recycling Processes on Component Liberation and Flotation Separation Efficiency. ACS EST Eng. 2022, 2, 2130–2141. [Google Scholar] [CrossRef]
- Dadé, M.; Wallmach, T.; Laugier, O. Detailed Microparticle Analyses Providing Process Relevant Chemical and Microtextural Insights into the Black Mass. Minerals 2022, 12, 119. [Google Scholar] [CrossRef]
- Passerini, S.; Barelli, L.; Baumann, M.; Peters, J.F.; Weil, M. Emerging Battery Technologies to Boost the Clean Energy Transition: Cost, Sustainability, and Performance Analysis; The Materials Research Society Series; Springer: Gewerbestrasse, Switzerland, 2024; ISSN 2730-7360. ISBN 978-3-031-48358-5. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Cláudia Santos, A.; et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability 2023, 15, 15. [Google Scholar] [CrossRef]
- Arturo Gomez-Moreno, L.; Klemettinen, A.; Serna-Guerrero, R. A simple methodology for the quantification of graphite in end-of-life lithium-ion batteries using thermogravimetric analysis. Science 2023, 26, 107782. [Google Scholar] [CrossRef]
- Traore, N.; Kelebek, S. Characteristics of Spent Lithium Ion Batteries and Their Recycling Potential Using Flotation Separation: A Review. Miner. Process. Extr. Metall. Rev. 2023, 44, 231–259. [Google Scholar] [CrossRef]
- Renier, O.; Pellini, A.; Spooren, J. Advances in the Separation of Graphite from Lithium Iron Phosphate from End-of-Life Batteries Shredded Fine Fraction Using Simple Froth Flotation. Batteries 2023, 9, 589. [Google Scholar] [CrossRef]
- Mennik, F.; Ilkyaz Dinç, N.; Burat, B. Selective recovery of metals from spent mobile phone lithium-ion batteries through froth flotation followed by magnetic separation procedure. Results Eng. 2023, 17, 100868. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Chen, W.; Ho, H. Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods. Metals 2018, 8, 321. [Google Scholar] [CrossRef]
- Peschel, P.; Wickeren, S.V.; Preibisch, Y.; Naber, V.; Werner, D.; Frankenstein, L.; Horsthemke, F.; Peuker, U.; Winter, M.; Nowak, S. Comprehensive Characterization of Shredded Lithium-Ion Battery Recycling Material. Chem.-A Eur. J. 2022, 28, e202200485. [Google Scholar]
- Rinne, M.; Aromaa-Stubb, R.; Elomaa, H.; Porvali, A.; Lundström, M. Evaluation of hydrometallurgical black mass recycling with simulation-based life cycle assessment. Int. J. Life Cycle Assess. 2024, 29, 1582–1597. [Google Scholar] [CrossRef]
- Sommerfeld, M.; Vonderstein, C.; Dertmann, C.; Klimko, J.; Orác, D.; Miškufová, A.; Havlík, T.; Friedrich, B. A Combined Pyro- and Hydrometallurgical Approach to Recycle Pyrolyzed Lithium-Ion Battery Black Mass Part 1: Production of Lithium Concentrates in an Electric Arc Furnace. Metals 2020, 10, 1069. [Google Scholar] [CrossRef]
- Królicka, A.; Maj, A.; Lój, G. Application of Laser-Induced Breakdown Spectroscopy for Depth Profiling of Multilayer and Graded Materials. Materials 2023, 16, 6641. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sales, A.; Ferreira, A.; Rudolph, M.; Serna-Guerrero, R. Improving Separation Efficiency in End-of-Life Lithium-Ion Batteries Flotation Using Attrition Pre-Treatment. Minerals 2022, 12, 72. [Google Scholar] [CrossRef]
- Smyrek, P.; Proll, J.; Seifert, H.J.; Pfleging, W. Laser-Induced Breakdown Spectroscopy of Laser-Structured Li(NiMnCo)O2 Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, AA26. [Google Scholar]
- Imashuku, S.; Taguchi, H.; Fujieda, S.; Suzuki, S.; Wagatsuma, K. Three-dimensional lithium mapping of graphite anode using laser-induced breakdown spectroscopy. Electrochim. Acta 2019, 293, 78–83. [Google Scholar] [CrossRef]
- Smyrek, P.; Bergfeldt, T.; Jürgen Seifert, H.; Pfleging, W. Laser-induced breakdown spectroscopy for the quantitative measurement of lithium concentration profiles in structured and unstructured electrodes. J. Mater. Chem. A 2019, 7, 5656–5665. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ye, G. Effect of Graphite on the Recovery of Valuable Metals from Spent Li-Ion Batteries in Baths of Hot Metal and Steel. Recycling 2022, 7, 5. [Google Scholar] [CrossRef]
- Kandioller, W.; Theiner, J.; Kepplera, B.K.; Kowol, C.R. Elemental analysis: An important purity control but prone to manipulations. Inorg. Chem. Front. 2022, 9, 412–416. [Google Scholar] [CrossRef]
- Gazulla, M.F.; Ventura, M.G.; Andreu, C.; Gilabert, J.; Orduña, M.; Rodrigo, M. Praseodymium oxides. Complete characterization by determining oxygen content. Microchem. J. 2019, 148, 291–298. [Google Scholar] [CrossRef]
- Hammesa, K.; Smernik, R.J.; Skjemstad, J.O.; Schmid, M.W.I. Characterisation and evaluation of reference materials for black carbon analysis using elemental composition, colour, BET surface area and 13C NMR spectroscopy. Appl. Geochem. 2008, 23, 2113–2122. [Google Scholar] [CrossRef]
- Panitz, J.-C.; Joho, F.; Novák, P. In Situ Characterization of a Graphite Electrode in a Secondary Lithium-Ion Battery Using Raman Microscopy. Appl. Spectrosc. 1999, 53, 1188–1199. [Google Scholar] [CrossRef]
- Sandmann, D.; Haser, S.; Gutzmer, J. Characterisation of graphite by automated mineral liberation analysis. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2014, 123, 184–189. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, A.M. Flotation; McGRAW-HILL Book Company, Inc.: New York, NY, USA; London, UK, 1922. [Google Scholar]
- Gupta, A.; Yan, D.S. Mineral Processing Design and Operations: An Introduction; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Badenhorst, C.; Kuzniarska-Biernacka, I.; Guedes, A.; Mousa, E.; Ramos, V.; Rollinson, G.; Ye, G.; Valentim, B. Recovery of Graphite from Spent Lithium-Ion Batteries. Recycling 2023, 8, 79. [Google Scholar] [CrossRef]
- Vanderbruggen, A. Lithium-Ion Batteries Recycling with Froth Flotation—A Study on Characterization and Liberation Strategies. Doctoral Thesis, Aalto University, Espoo, Finland, 2022. [Google Scholar] [CrossRef]
- Ebin, B.; Petranikova, M.; Ekberg, C. Physical separation, mechanical enrichment and recycling-oriented characterization of spent NiMH batteries. J. Mater. Cycles Waste Manag. 2018, 20, 2018–2027. [Google Scholar] [CrossRef]
- Sahivirta, H.; Wilson, B.P.; Lundstrom, M.; Serna-Guerrero, R. A study on recovery strategies of graphite from mixed lithium-ion battery chemistries using froth flotation. Waste Manag. 2024, 180, 96–105. [Google Scholar] [CrossRef]
- Hong, G.; Park, H.; Gomez-Flores, A.; Kim, H.; Lee, J.M.; Lee, J. Direct flotation separation of active materials from the black mass of lithium nickel cobalt manganese oxides-type spent lithium-ion batteries. Sep. Purif. Technol. 2024, 336, 126327. [Google Scholar] [CrossRef]
- Premathilake, D.S.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R.; Vaccari, M. Designing of a Decentralized Pretreatment Line for EOL-LIBs Based on Recent Literature of LIB Recycling for Black Mass. Metals 2023, 13, 374. [Google Scholar] [CrossRef]

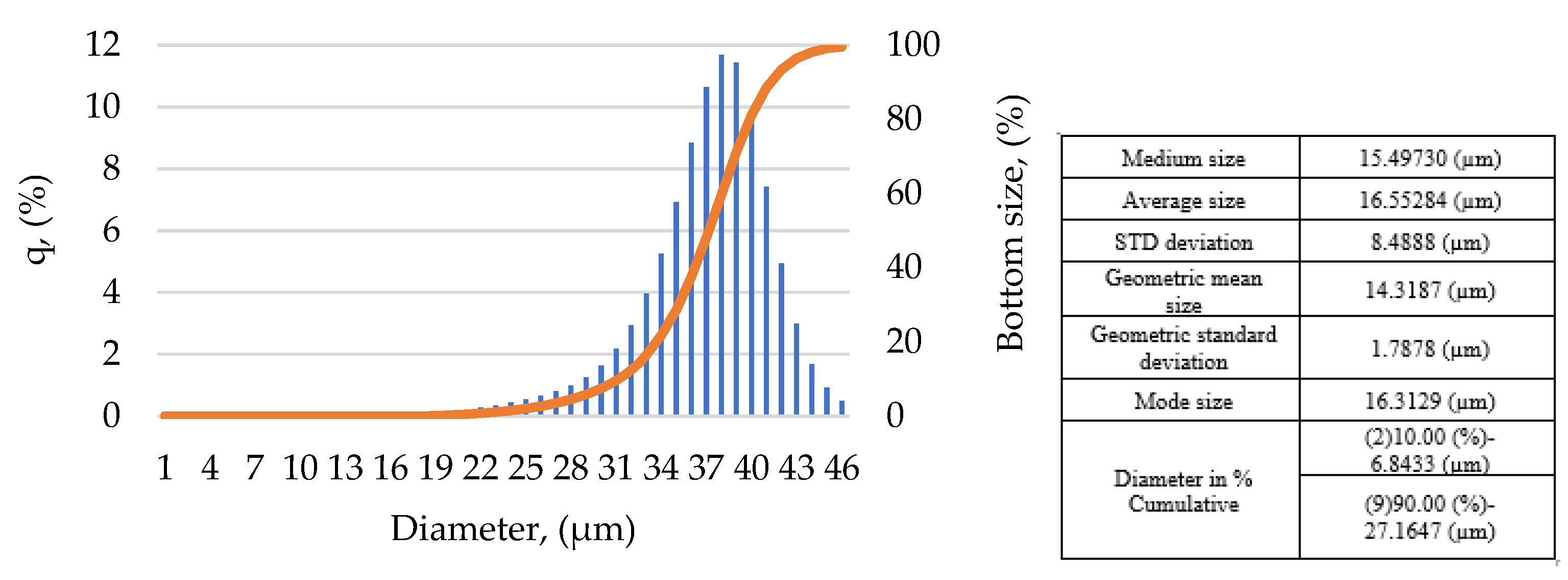

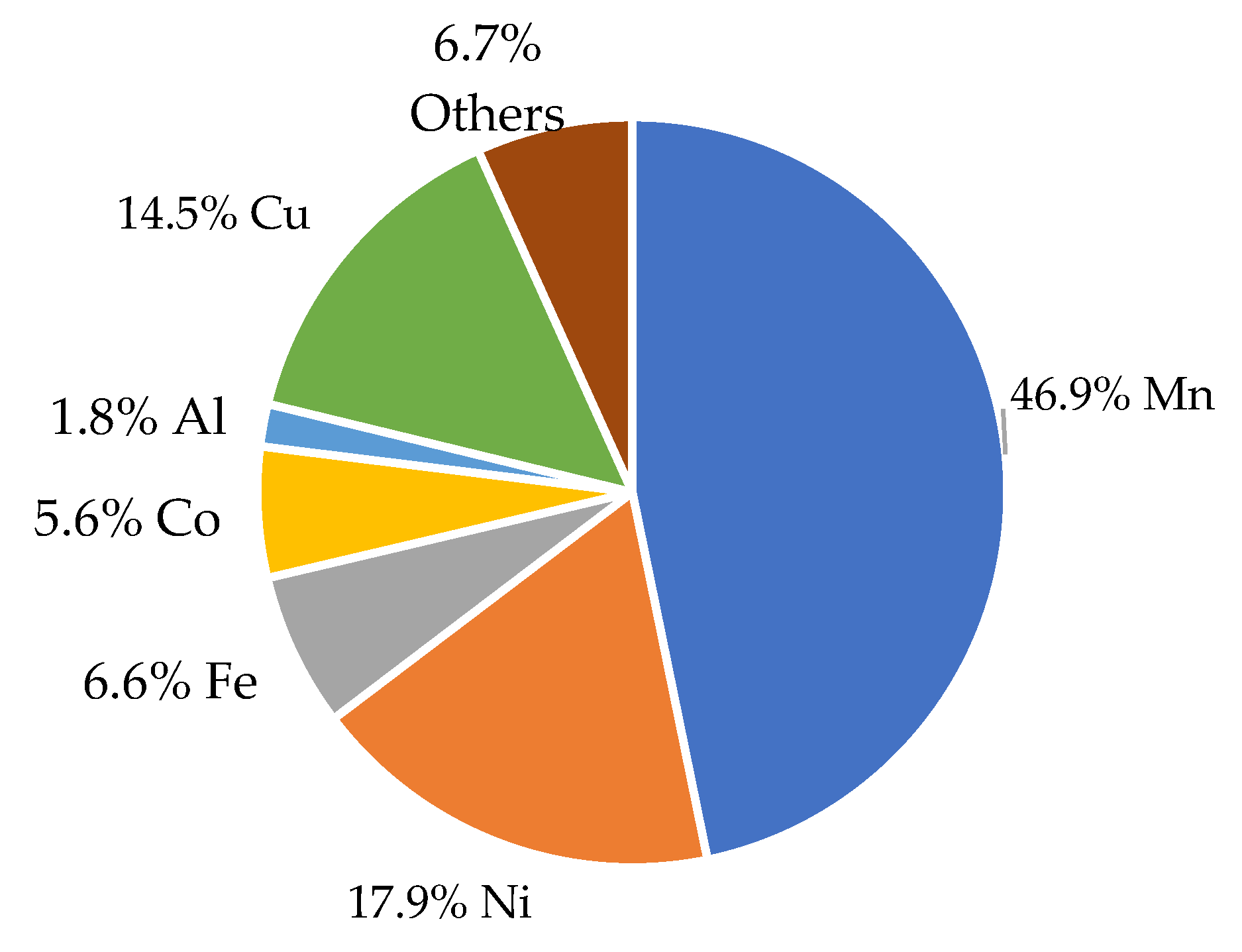

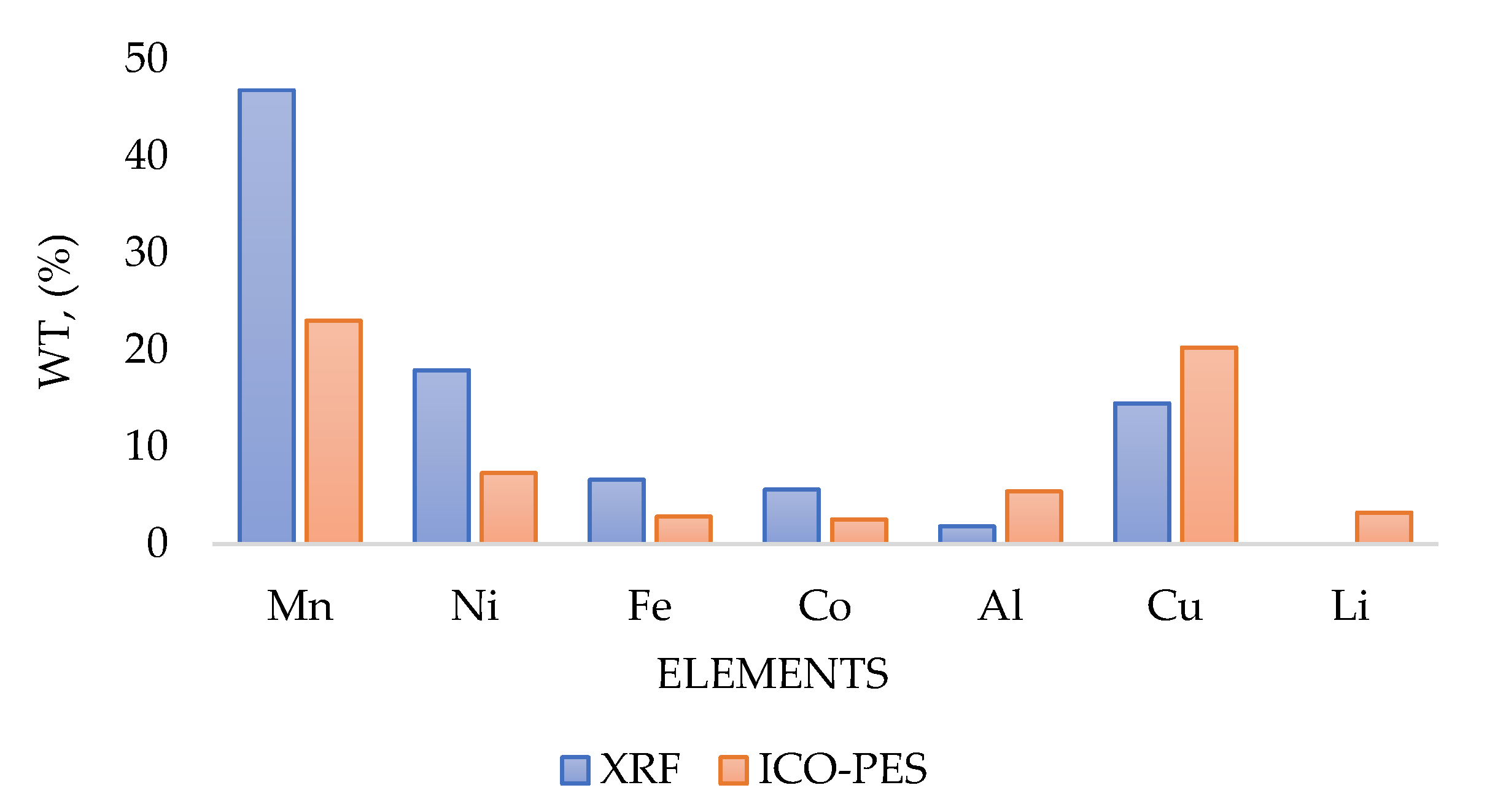




| Sieve (µm) | Sieving (µm) | Average Sieve (µm) | Differential Mass (%) | Cumulative Mass (%) | Retained Mass (%) | |
|---|---|---|---|---|---|---|
| +1000 | 1000 | 1000 | 0.0 | 100.0 | 0.0 | 28.9% |
| −1000 + 500 | 500 | 750 | 3.2 | 100.0 | 3.2 | |
| −500 + 200 | 200 | 350 | 16.3 | 96.8 | 19.5 | |
| −200 + 100 | 100 | 150 | 9.4 | 80.5 | 28.9 | |
| −100 + 53 | 53 | 77 | 8.3 | 71.1 | 37.2 | 71.1% |
| −53 | 1 | 0 | 62.8 | 62.8 | 100.0 | |
| Σ | 100.0 |
| Black Mass Size Ranges | (µm) | |||||
|---|---|---|---|---|---|---|
| Total | <53 | 53–100 | 100–200 | 200–500 | >500 | |
| Mn | 46.8 | 55.1 | 39.39 | 12.2 | 11.4 | 21.1 |
| Ni | 17.9 | 22.2 | 13.64 | 4.3 | 4.7 | 8.33 |
| Fe | 6.6 | 6.9 | 8.04 | 3.5 | 2.8 | 5.06 |
| Co | 5.6 | 7.1 | 4.26 | 1.2 | 1.3 | 2.44 |
| P | 2.9 | 2.5 | 2.86 | 2.2 | 2.6 | 2.95 |
| Al | 1.8 | 1.8 | 3.27 | 8.9 | 21.9 | 26.01 |
| Cu | 14.5 | 2.4 | 22.89 | 51.2 | 37.4 | 27.65 |
| Ca | 0.9 | 0.5 | 1.48 | 4.1 | 3.0 | 1.51 |
| Si | 0.7 | 0.4 | 1.41 | 5.1 | 5.1 | 1.75 |
| Mg | 0.2 | 0.2 | 0.58 | 1.4 | 1.0 | 0.55 |
| Cl | 0.6 | 0.1 | 0.66 | 3.3 | 5.2 | 0.37 |
| S | 0.4 | 0.1 | 0.11 | 0.2 | 0.2 | 0.24 |
| Nb | 0.2 | 0.2 | 0.16 | 0.0 | 0.1 | 0.10 |
| Sn | 0.3 | 0.2 | 0.55 | 0.5 | 0.2 | 0.35 |
| Cr | 0.1 | 0.1 | 0.08 | 0.0 | 0.0 | 0.04 |
| Ti | 0.1 | 0.0 | 0.08 | 0.2 | 0.7 | 0.11 |
| Pb | 0.1 | 0.1 | 0.07 | 0.1 | 0.0 | 0.11 |
| Zn | 0.1 | 0.0 | 0.16 | 0.3 | 0.1 | 0.29 |
| Br | 0.2 | 0.0 | 0.13 | 0.7 | 1.6 | 0.21 |
| Compound Name | Chemical Formula | |
|---|---|---|
| 1 | Copper | Cu |
| 2 | Triphylite, syn | LiFe(PO4) |
| 3 | Graphite | C |
| 4 | Lithium Nickel Manganese Oxide | LiNi0.18Mn1.82O4 |
| 5 | Lithium Nickel Cobalt Oxide | (Li0.973Ni0.027)(Ni0.8973Co0.1027)O2 |
| Element | Black Particles | Metallic Particles |
|---|---|---|
| Cu | 2% | 2% |
| Fe | - | 2% |
| Li | 100% | 100% |
| Mg | - | 2% |
| Mn | 58% | 16% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pourmohammad, M.; Moncunill, J.O.; Anticoi, H.; Sampaio, C.H.; Alfonso, P.; Valderrama, C.; Cortina Pallas, J.L. The Characterization of Black Mass from Spent Lithium-Ion Scooter Batteries Using Multi-Analytical Techniques. Recycling 2025, 10, 54. https://doi.org/10.3390/recycling10020054
Pourmohammad M, Moncunill JO, Anticoi H, Sampaio CH, Alfonso P, Valderrama C, Cortina Pallas JL. The Characterization of Black Mass from Spent Lithium-Ion Scooter Batteries Using Multi-Analytical Techniques. Recycling. 2025; 10(2):54. https://doi.org/10.3390/recycling10020054
Chicago/Turabian StylePourmohammad, Mahsa, Josep Oliva Moncunill, Hernan Anticoi, Carlos Hoffmann Sampaio, Pura Alfonso, César Valderrama, and Jose Luis Cortina Pallas. 2025. "The Characterization of Black Mass from Spent Lithium-Ion Scooter Batteries Using Multi-Analytical Techniques" Recycling 10, no. 2: 54. https://doi.org/10.3390/recycling10020054
APA StylePourmohammad, M., Moncunill, J. O., Anticoi, H., Sampaio, C. H., Alfonso, P., Valderrama, C., & Cortina Pallas, J. L. (2025). The Characterization of Black Mass from Spent Lithium-Ion Scooter Batteries Using Multi-Analytical Techniques. Recycling, 10(2), 54. https://doi.org/10.3390/recycling10020054












