Characterization and Property Evaluation of Glasses Made from Mine Tailings, Glass Waste, and Fluxes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glass Synthesis
2.3. Analytical Methods
3. Results and Discussion
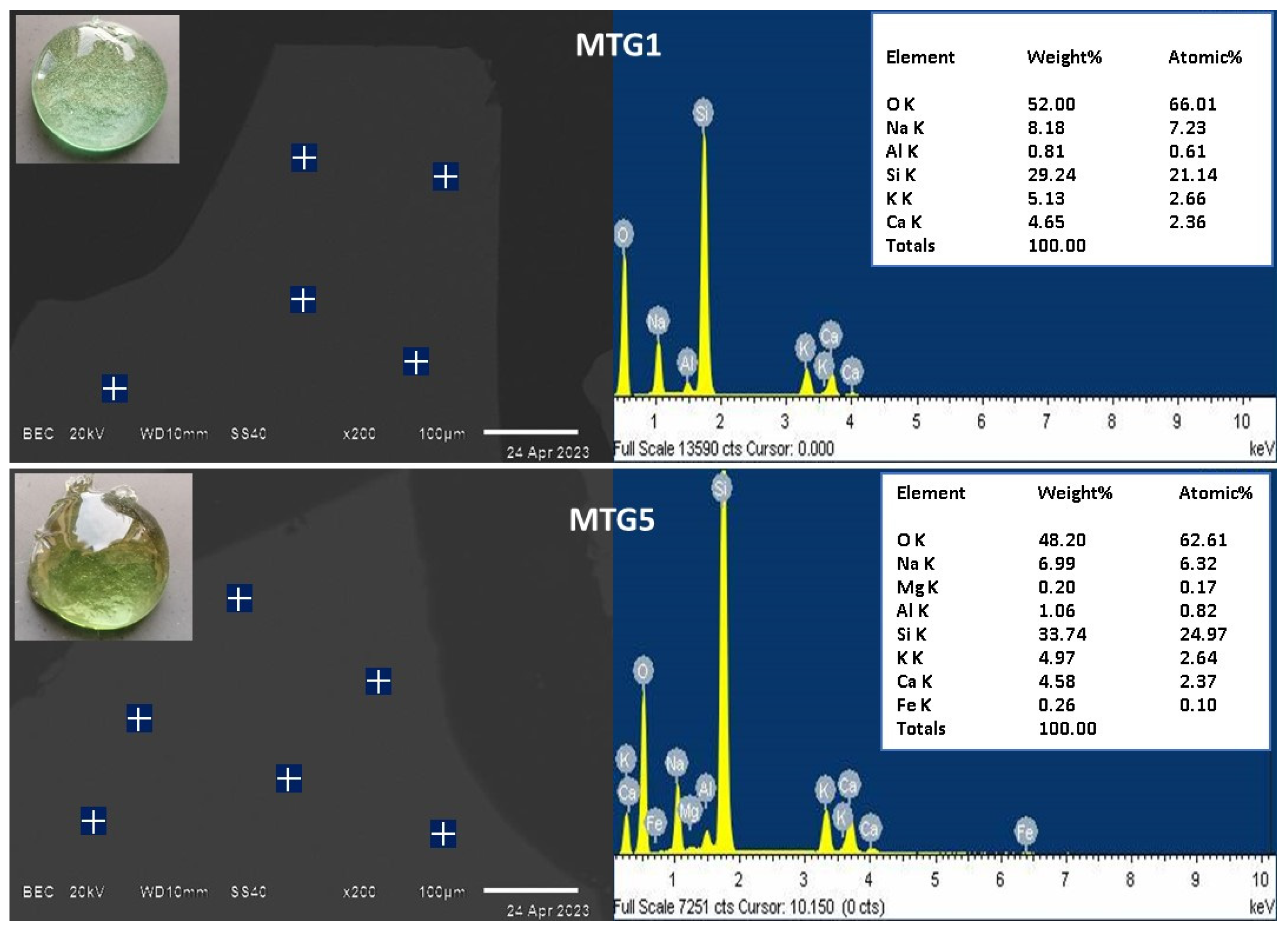
3.1. Glass Characterization
3.2. Properties Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armendáriz-Villegas, E.J.; Covarrubias-García, M.A.; Troyo-Diéguez, E.; Lagunes, E.; Arreola-Lizárraga, A.; Nieto-Garibay, A.; Beltrán-Morales, L.F.; Ortega-Rubio, A. Metal mining and natural protected areas in Mexico: Geographic overlaps and environmental implications. Environ. Sci. Policy 2015, 48, 9–19. [Google Scholar] [CrossRef]
- Alfonso, P.; Tomasa, O.; Garcia-Valles, M.; Tarragó, M.; Martínez, S. Glass-ceramic crystallization from tailings of the Morille tungsten deposit, Spain. Mater. Lett. 2022, 312, 131694. [Google Scholar] [CrossRef]
- De Lima Camelo, D.; Da Silva Filho, L.A.; De Arruda, D.L.; Cyrino, L.M.; Fonseca Barroso, G.; Metri Correa, M.; Sanches Barbeira, P.J.; Brandao Mendes, D.; Duarte Pasa, V.M.; Profet, D. Mineralogical fingerprint and human health risk from potentially toxic elements of Fe mining tailings from the Fundao dam. Sci. Total Environ. 2024, 912, 169328. [Google Scholar] [CrossRef]
- Sosa-Rodríguez, F.S.; Vazquez-Arenas, J.; Ponce-Pena, P.; Aragon-Piña, A.; Mallet, M.; Trejo-Cordova, G.; Núnez-Ramirez, D.M.; Escobedo-Bretado, M.A.; Lara, R.H. Sphalerite oxidation simulating acidic, circumneutral and alkaline conditions to account for weathering behavior and Zn release. J. Geochem. Explor. 2023, 247, 107163. [Google Scholar] [CrossRef]
- Sosa-Rodríguez, F.S.; Vazquez-Arenas, J.; Ponce-Pena, P.; Escobedo-Bretado, M.A.; Castellanos-Juárez, F.X.; Labastida, I.; Lara, R.H. Spatial distribution, mobility and potential health risks of arsenic and lead concentrations in semiarid fine top-soils of Durango City, Mexico. Catena 2020, 190, 104540. [Google Scholar] [CrossRef]
- Nava-Reyna, E.; Medrano-Macías, J. Arsenic occurrence in the environment: Current situation of the Comarca Lagunera in northern Mexico and bioremediation approaches. J. Agric. Food Res. 2022, 10, 100379. [Google Scholar] [CrossRef]
- Shi, J.; He, F.; Ye, C.; Hu, L.; Xie, J.; Yang, H.; Liu, X. Preparation and characterization of CaO-Al2O3-SiO2 glass-ceramics from molybdenum tailings. Mater. Chem. Phys. 2017, 197, 57–64. [Google Scholar] [CrossRef]
- Garcia-Troncoso, N.; Baykara, H.; Cornejo, M.H.; Riofrio, A.; Tinoco-Hidalgo, M.; Flores-Rada, J. Comparative mechanical properties of conventional concrete mixture and concrete incorporating mining tailings sands. Case Stud. Constr. Mater. 2022, 16, e01031. [Google Scholar] [CrossRef]
- El-Sayed Seleman, M.M.; El-Kheshen, A.A.; Raslan, M.F.; Shoeir, R.H. Glass production using tailing of upgraded rare metal mineralization, Abu Rusheid area—Egypt for nuclear waste immobilization. Ceram. Int. 2022, 48, 569–577. [Google Scholar] [CrossRef]
- Cetin, S.; Marangoni, M.; Bernardo, E. Lightweight glass–ceramic tiles from the sintering of mining tailings. Ceram. Int. 2015, 41, 5294–5300. [Google Scholar] [CrossRef]
- Karhu, M.; Lagerbom, J.; Honkanen, M.; Huttunen-Saarivirta, E.; Kiilakoski, J.; Vuoristo, P.; Solismaa, S.; Kivikytö-Reponen, P. Mining tailings as a raw material for glass-bonded thermally sprayed ceramic coatings: Microstructure and properties. J. Eur. Ceram. Soc. 2020, 40, 4111–4114. [Google Scholar] [CrossRef]
- Abdelhadi, K.; Latifa, O.; BKhadija, B.; Lahcen, B. Valorization of mining waste and tailings through paste backfilling solution, Imiter operation, Morocco. Int. J. Min. Sci. Technol. 2016, 26, 511–516. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Zhang, H.; Luo, L.; Ding, L.; Shi, W.; Jiang, W.; Wang, H. Recycling of arsenic residue and waste soda-lime silicate glass via vitrification. J. Non-Cryst. Solids 2023, 609, 122300. [Google Scholar] [CrossRef]
- Terry Lay, G.F.; Rockwell, M.C.; Wiltshire, J.C.; Ketata, C. Characteristics of silicate glasses derived from vitrification of manganese crust tailings. Ceram. Int. 2009, 35, 1961–1967. [Google Scholar] [CrossRef]
- Alfonso, P.; Tomasa, O.; Garcia-Valles, M.; Tarragó, M.; Martínez, S.; Esteves, H. Potential of tungsten tailings as glass raw materials. Mater. Lett. 2018, 228, 456–458. [Google Scholar] [CrossRef]
- Alfonso, P.; Castro, D.; Garcia-Valles, M.; Tarragó, M.; Tomasa, O.; Martínez, S. Recycling of tailings from the Barruecopardo tungsten deposit for the production of glass. J. Therm. Anal. Calorim. 2016, 125, 681–687. [Google Scholar] [CrossRef]
- Khan, S.; Allu, A.R.; Gaddam, A.; Fernandes, H.R.; Dutta, S.; Kongar, P.S.; Tarafder, A.; Ferreira, J.M.F.; Annapurna, K. Use of colemanite and borax penta-hydrate in soda lime silicate glass melting—A strategy to reduce energy consumption and improve glass properties. Ceram. Int. 2022, 48, 1181–1190. [Google Scholar] [CrossRef]
- Baccarin, L.I.P.; Bragança, S.R. Thermodynamic simulations for optimizing the firing of traditional ceramic formulations using waste glass as a flux. Mater. Today Commun. 2024, 38, 107913. [Google Scholar] [CrossRef]
- Divina, R.; Sathiyapriya, G.; Marimuthu, K.; Askin, A.; Sayyed, M.I. Structural, elastic, optical and γ-ray shielding behavior of Dy3+ ions doped heavy metal incorporated borate glasses. J. Non-Cryst. Solids 2020, 545, 120269. [Google Scholar] [CrossRef]
- Nevolina, L.A.; Shtenberg, M.V.; Zherebtsov, D.A.; Koroleva, O.N. Structure and crystallizability of K2O-B2O3-SiO2 and K2O-B2O3-GeO2 glasses: Effect of composition and heat treatment mode. Ceram. Int. 2023, 49, 37228–37237. [Google Scholar] [CrossRef]
- Fernández Navarro, J.M. El Vidrio, Constitución, Fabricación, Propiedades, 3rd ed.; CSIC: Madrid, Spain, 2003. [Google Scholar]
- Pernice, P.; Esposito, S.; Aronne, A.; Sigaev, V.N. Structure and crystallization behavior of glasses in the BaO–B2O3–Al2O3 system. J. Non-Cryst. Solids 1999, 258, 1–10. [Google Scholar] [CrossRef]
- GOST 10134-82; Standard [Russian National Standard]. Standards of the USSR: Moscow, Russia, 1982.
- Heiskanen, J. Comparison of three methods for determining the particle density of soil with liquid pycnometers. Commun. Soil Sci. Plant Anal. 1992, 23, 841–846. [Google Scholar] [CrossRef]
- Doyle, P.J. Glass-Making Today an Introduction to Current Practice in Glass Manufacture, 1st ed.; Portcullis Press Ltd.: Sheffield, UK, 1979. [Google Scholar]
- Russell, J.H. Soda-Lime-Silica Glasses. In Encyclopedia of Materials Technical Ceramics and Glasses, 1st ed.; Pomeroy, M., Cambier, F., Galassi, C., Hampshire, S., Leriche, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 483–495. [Google Scholar]
- Hansen, E.; Perret, D.; Bardez-Giboire, I.; Diliberto, S.; Rapin, C. Iron enriched peraluminous glasses: Incorporation limit and effect of iron on glass transition temperature and viscosity. J. Non-Cryst. Solids 2022, 584, 121523. [Google Scholar] [CrossRef]
- Gradmann, R.; Berthold, C.; Schüssler, I. Composition and colouring agents of historical Islamic glazes measured with EMPA and μ-XRD2. Eur. J. Miner. 2015, 27, 325–335. [Google Scholar] [CrossRef]
- Bidegaray, A.I.; Nys, K.; Silvestri, A.; Cosyns, P.; Meulebroeck, W.; Terryn, H.; Godet, S.; Ceglia, A. 50 shades of colour: How thickness, iron redox and manganese/antimony contents influence perceived and intrinsic colour in Roman glass. Archeol. Anthr. Sci. 2020, 12, 109. [Google Scholar] [CrossRef]
- Reddy, A.A.; Goel, A.; Tulyaganov, D.U.; Kapoor, S.; Pradeesh, K.; Pascual, M.J.; Ferreira, J.M.F. Study of calcium magnesium aluminum silicate (CMAS) glass and glass-ceramic sealant for solid oxide fuel cells. J. Power Sourc. 2013, 231, 203–212. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Chen, J.; Liao, Q.; Zhu, H.; Zhou, J.; Qu, X.; Gong, Z.; Fu, X.; Zhu, Y. Effect of cerium oxide on phase composition, structure, thermal stability and aqueous durability of sodium-iron-boron-phosphate based glasses. J. Nucl. Mater. 2021, 556, 153199. [Google Scholar] [CrossRef]
- Almasri, K.A.; Aziz Sidek, H.A.; Matori, K.A.; Mohd Hafiz, M.Z. Effect of sintering temperature on physical, structural and optical properties of wollastonite based glass-ceramic derived from waste soda lime silica glasses. Results Phys. 2017, 7, 2242–2247. [Google Scholar] [CrossRef]
- Madheshiya, A.; Dey, K.K.; Ghosh, M.; Singh, J.; Gautam, C. Synthesis, structural, optical and solid state NMR study of lead bismuth titanate borosilicate glasses. J. Non-Cryst. Solids 2019, 503–504, 288–296. [Google Scholar] [CrossRef]
- Ponce-Peña, P.; González-Lozano, M.A.; Escobedo-Bretado, M.A.; Núñez-Ramírez, D.M.; Rodríguez-Pulido, A.; Quiñones Jurado, Z.V.; Poisot, M.; Sulbarán-Rangel, B. Crystallization of Glasses Containing K2O, PbO, BaO, Al2O3, B2O3, and TiO2. Crystals 2022, 12, 574. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Tulyaganov, D.U.; Goel, A.; Ribeiro, M.J.; Pascual, M.J.; Ferreira, J.M.F. Effect of Al2O3 and K2O content on structure, properties and devitrification of glasses in the Li2O–SiO2 system. J. Eur. Ceram. Soc. 2010, 30, 2017–2030. [Google Scholar] [CrossRef]
- Paul, A. Chemistry of Glasses, 2nd ed.; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Melcher, M.; Schreiner, M. Statistical evaluation of potash-lime-silica glass weathering. Anal Bioanal. Chem. 2004, 379, 628–639. [Google Scholar] [CrossRef]
- Tournié, A.; Ricciardi, P.; Colomban, P. Glass corrosion mechanisms: A multiscale analysis. Solid State Ionics 2008, 179, 2142–2154. [Google Scholar] [CrossRef]
- Barbieri, L.; Karamanov, A.; Corradi, A.; Lancellotti, I.; Pelino, M.; Rincon, J.M. Structure, chemical durability and crystallization behavior of incinerator-based glassy systems. J. Non-Cryst. Solids 2008, 354, 521–528. [Google Scholar] [CrossRef]
- Fernández Navarro, J.M.; Mari, E.A. Influencia de la adición de ZrO2 y TiO2 sobre el comportamiento químico de vidrios de silicato y borosilicato sódico-cálcico. Bol. Soc. Esp. Ceram. Vidr. 1990, 29, 235–244. [Google Scholar]
- Fluegel, A. Global model for calculating room-temperature glass density from the composition. J. Am. Ceram. Soc. 2007, 90, 2622–2625. [Google Scholar] [CrossRef]
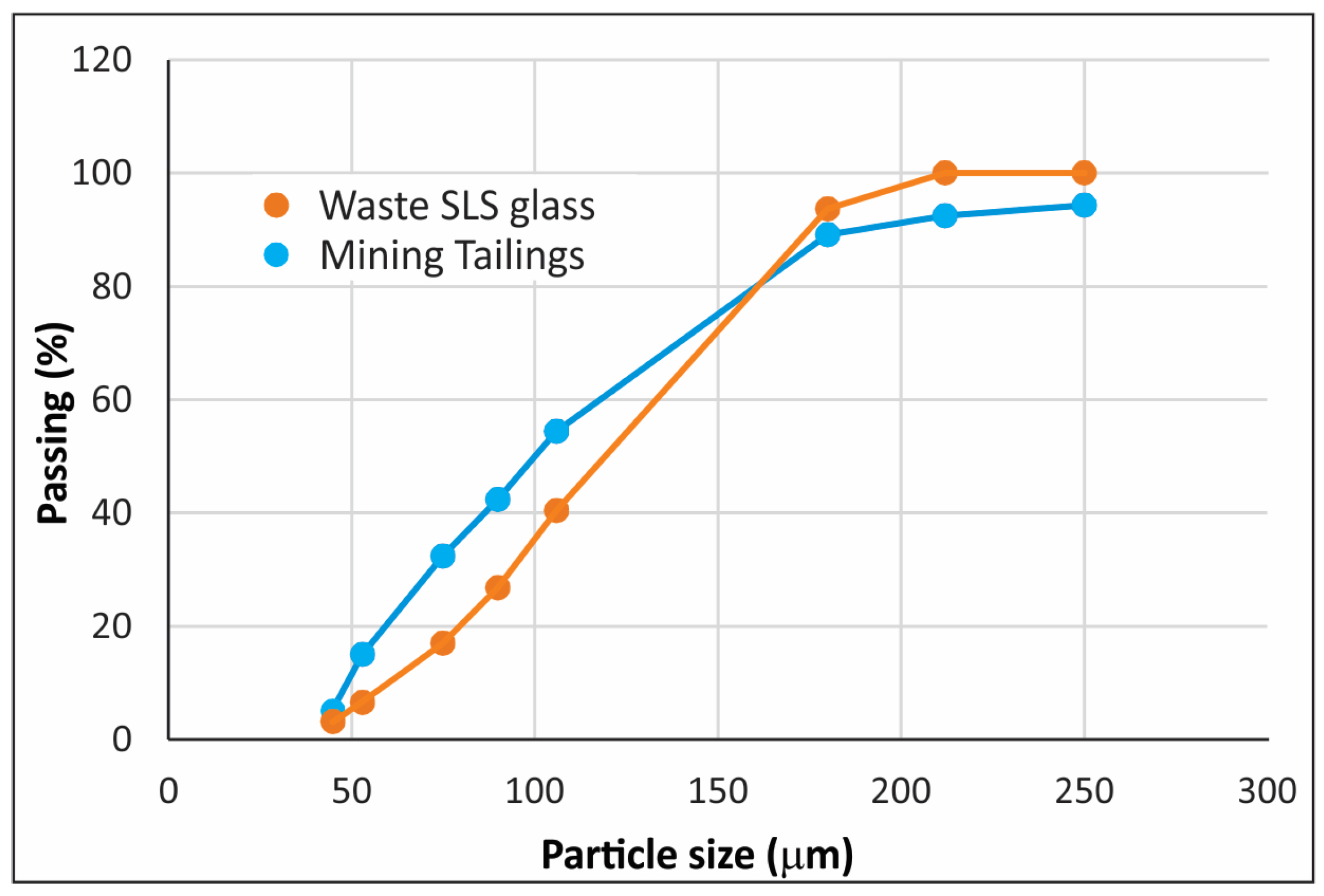
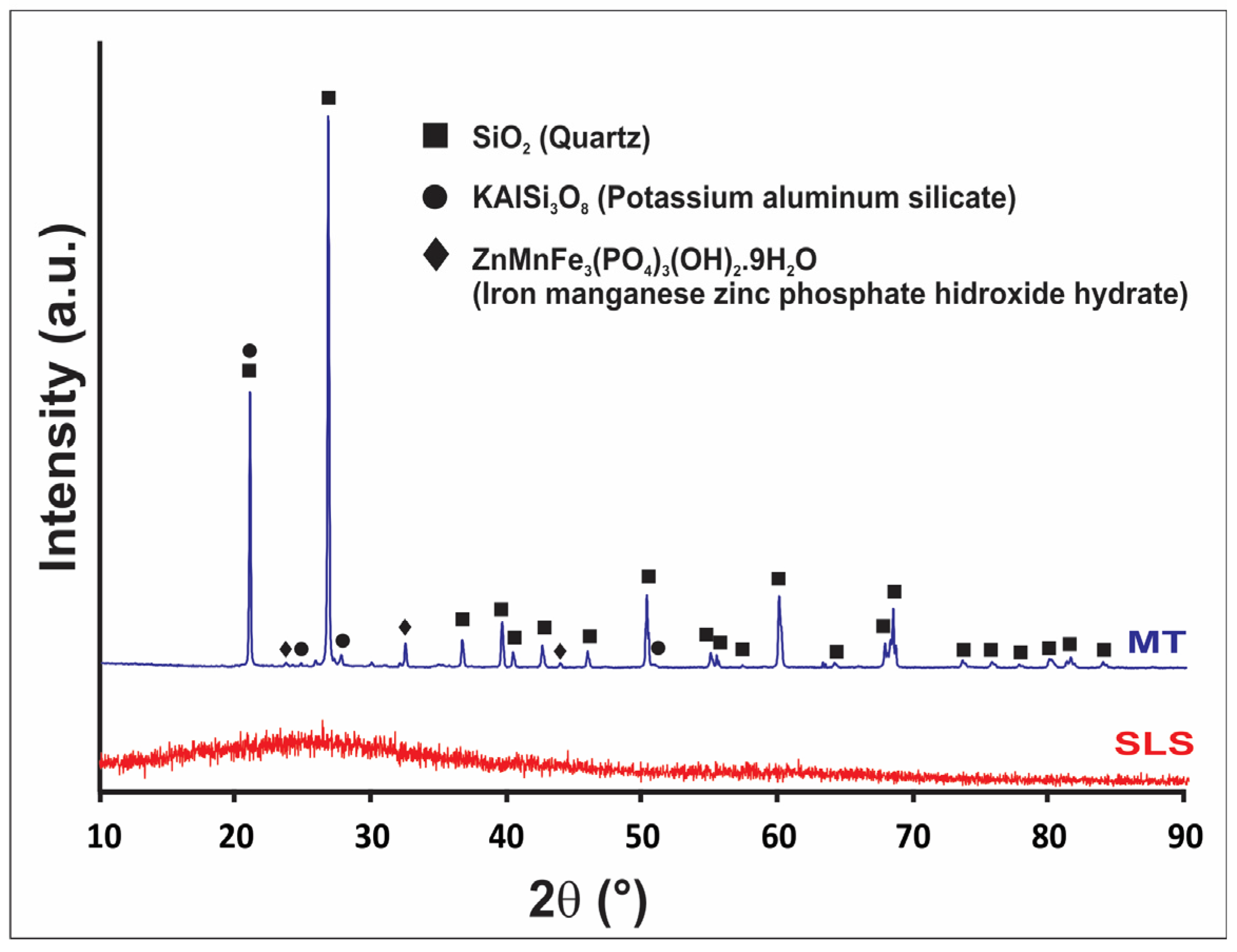
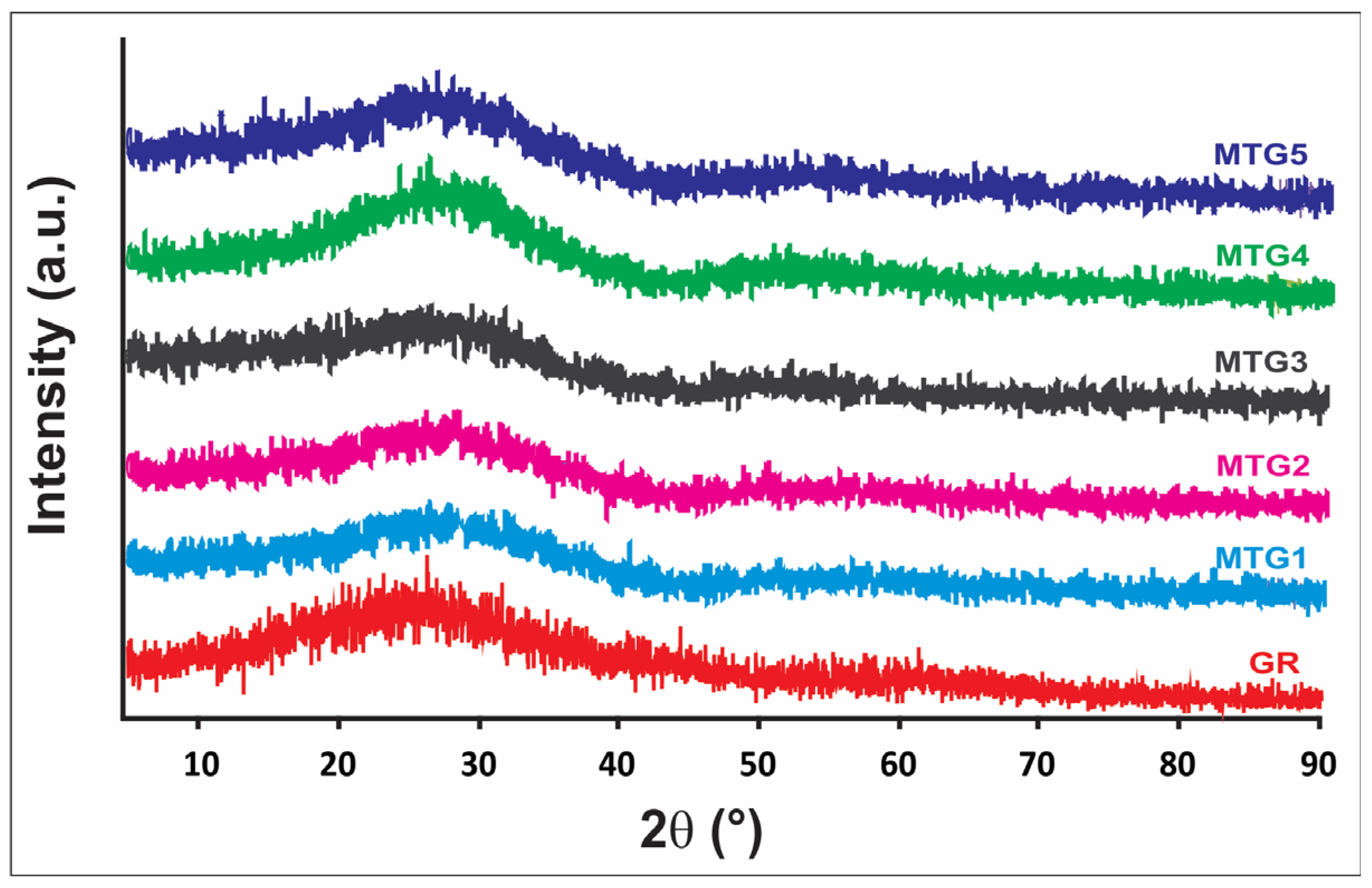
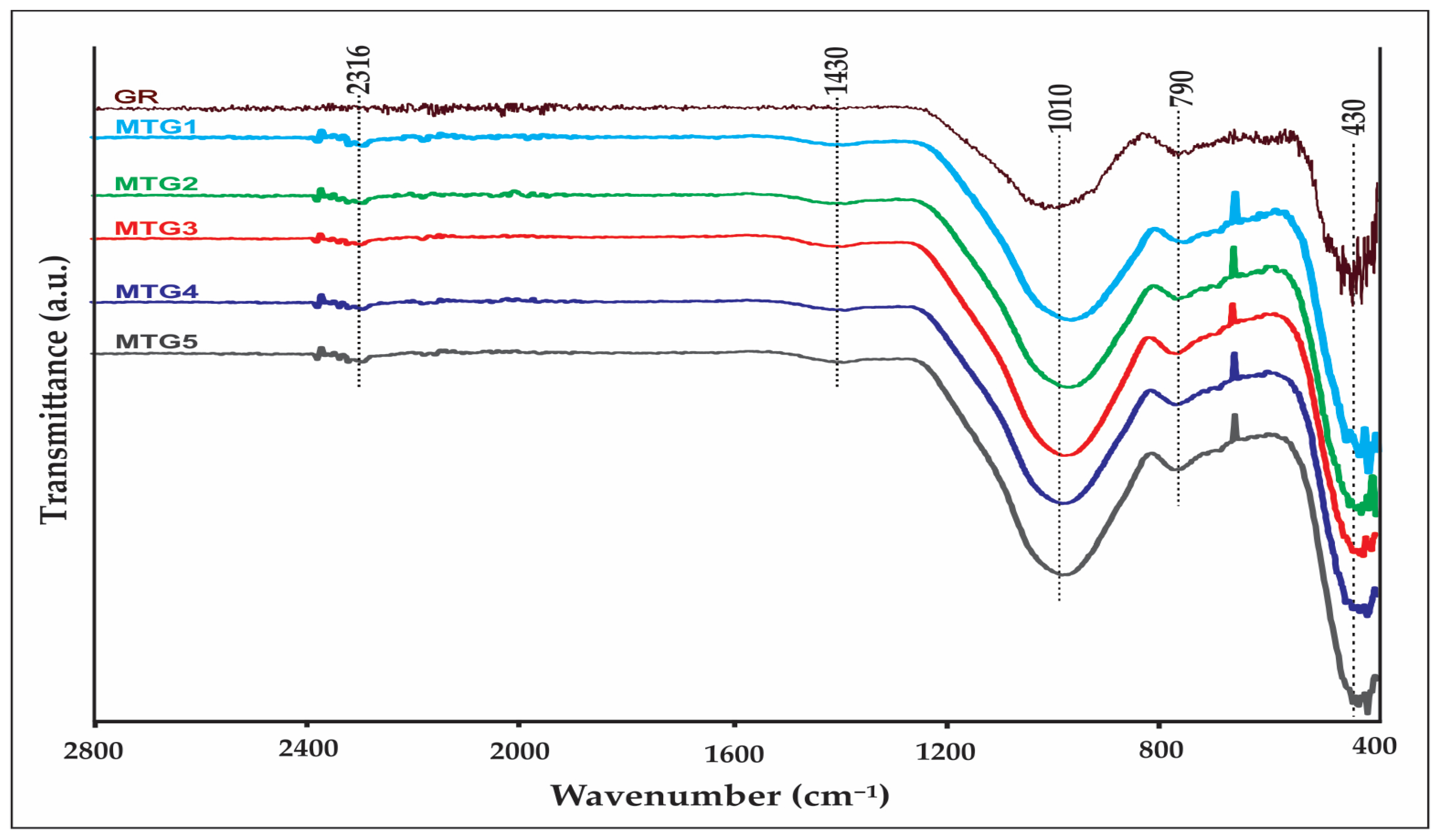


| Raw Materials | Oxide Content (Weight %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Na2O | K2O | Fe2O3 | TiO2 | MnO | P2O5 | ZnO | |
| MT | 90.13 | 4.06 | 0.075 | 0.07 | 0.176 | 2.23 | 1.64 | 0.08 | 0.012 | 0.052 | 0.021 |
| GR | 71.65 | 1.21 | 11.99 | 0.94 | 12.99 | 0.94 | 0.23 | - | - | - | - |
| Sample | Composition (Weight %) | |||
|---|---|---|---|---|
| Mining Tailings | Glass Waste | K2O | B2O3 | |
| MTG1 | 4.2 | 81.0 | 7.0 | 7.8 |
| MTG2 | 8.5 | 76.7 | 7.0 | 7.8 |
| MTG3 | 12.8 | 72.4 | 7.0 | 7.8 |
| MTG4 | 17.0 | 68.2 | 7.0 | 7.8 |
| MTG5 | 21.3 | 63.9 | 7.0 | 7.8 |
| MTG6 | 25.6 | 59.7 | 7.0 | 7.8 |
| GR | 0.0 | 100.0 | 0.0 | 0.0 |
| Element | Concentration (mg/L) |
|---|---|
| Iron | 1631.00 |
| Arsenic | 4.14 |
| Zinc | 0.29 |
| Lead | 0.24 |
| Cadmium | 0.19 |
| Manganese | 0.15 |
| Sample | Composition (Weight %) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Na2O | K2O | B2O3 | Fe2O3 | |
| MTG1 | 61.82 | 1.15 | 9.72 | 0.76 | 10.53 | 7.91 | 7.80 | 0.26 |
| MTG2 | 62.17 | 1.27 | 9.20 | 0.73 | 9.98 | 7.86 | 7.80 | 0.32 |
| MTG3 | 63.41 | 1.40 | 8.69 | 0.69 | 9.43 | 7.97 | 7.80 | 0.38 |
| MTG4 | 64.19 | 1.52 | 8.19 | 0.65 | 8.89 | 8.02 | 7.80 | 0.44 |
| MTG5 | 64.98 | 1.64 | 7.68 | 0.62 | 8.34 | 8.08 | 7.80 | 0.50 |
| MTG6 | 65.85 | 1.76 | 7.18 | 0.58 | 7.80 | 8.13 | 7.80 | 0.56 |
| Sample | Basic Medium | Acid Medium | Density | ||||
|---|---|---|---|---|---|---|---|
| Average (% Losses) | Standard Deviation (σ) | Average (% Losses) | Standard Deviation (σ) | Average (g/cm3) | Standard Deviation (σ) | Calculated from Table 4 | |
| MTG1 | −9.07 | 3.21 | 1.5 | 2.55 | 2.35 | 0.021 | 2.56 |
| MTG2 | −6.11 | 7.14 | 0.86 | 0.59 | 2.27 | 0.003 | 2.55 |
| MTG3 | −8.27 | 8.11 | 0.65 | 0.99 | 2.21 | 0.027 | 2.54 |
| MTG4 | −0.62 | 3.16 | 0.23 | 0.52 | 2.18 | 0.086 | 2.52 |
| MTG5 | −0.79 | 3.97 | 0.18 | 0.15 | 2.12 | 0.122 | 2.51 |
| GR | −3.41 | 3.96 | 0.18 | 0.1 | 2.11 | 0.075 | 2.50 |
| Analyte | Concentration (mg/L) | ||
|---|---|---|---|
| MTG4 (HCl) | MTG5 (HCl) | MTG5 (NaOH) | |
| As | 0.66 | 0.51 | 3.81 |
| Pb | 0.16 | 0.31 | Not detected |
| Zn | 0.19 | 0.18 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-Bretado, M.Á.; Ponce-Peña, P.; Poisot, M.; Rodríguez-Pulido, A.; Núñez-Ramírez, D.M.; Bretado-Aragón, L.A.; Lara, R.H.; Medina-Torres, L.; Quiñones-Jurado, Z.V.; Briones-Gallardo, R.; et al. Characterization and Property Evaluation of Glasses Made from Mine Tailings, Glass Waste, and Fluxes. Recycling 2025, 10, 39. https://doi.org/10.3390/recycling10020039
Escobedo-Bretado MÁ, Ponce-Peña P, Poisot M, Rodríguez-Pulido A, Núñez-Ramírez DM, Bretado-Aragón LA, Lara RH, Medina-Torres L, Quiñones-Jurado ZV, Briones-Gallardo R, et al. Characterization and Property Evaluation of Glasses Made from Mine Tailings, Glass Waste, and Fluxes. Recycling. 2025; 10(2):39. https://doi.org/10.3390/recycling10020039
Chicago/Turabian StyleEscobedo-Bretado, Miguel Ángel, Patricia Ponce-Peña, Martha Poisot, Alicia Rodríguez-Pulido, Diola Marina Núñez-Ramírez, Luis Alberto Bretado-Aragón, René H. Lara, Luis Medina-Torres, Zoe V. Quiñones-Jurado, Roberto Briones-Gallardo, and et al. 2025. "Characterization and Property Evaluation of Glasses Made from Mine Tailings, Glass Waste, and Fluxes" Recycling 10, no. 2: 39. https://doi.org/10.3390/recycling10020039
APA StyleEscobedo-Bretado, M. Á., Ponce-Peña, P., Poisot, M., Rodríguez-Pulido, A., Núñez-Ramírez, D. M., Bretado-Aragón, L. A., Lara, R. H., Medina-Torres, L., Quiñones-Jurado, Z. V., Briones-Gallardo, R., & González-Lozano, M. A. (2025). Characterization and Property Evaluation of Glasses Made from Mine Tailings, Glass Waste, and Fluxes. Recycling, 10(2), 39. https://doi.org/10.3390/recycling10020039






