Polymer Recycling: A Comprehensive Overview and Future Outlook
Abstract
1. General Definitions and Aim of This Work
2. Recycling of Current Commercially Available Polymers
2.1. Pre-Treatment
2.2. Material Recycling
2.3. Chemical Recycling
3. Future Outlook
3.1. Design for Recycling
3.2. General Considerations
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ABS | Poly(acrylonitrile-butadiene-styrene) |
| CAN | Covalent Adaptable Network |
| CFD | Computational Fluid Dynamic |
| EPDM | Ethylene Propylene Diene Monomer |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GMA | Glycidyl methacrylate |
| GPC | Gel Permeation Chromatography |
| HDPE | High Density Polyethylene |
| HIPS | High Impact Poly(styrene) |
| i | Isotactic |
| LDPE | Low Density Polyethylene |
| LMW | Low Molecular Weight |
| MAH | Maleic anhydride |
| PAA | Poly(acrylic acid) |
| PC | Poly(carbonate) |
| PE | Poly(ethylene) |
| PET | Poly(ethylene terephthalate) |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | Poly(lactic acid) |
| PMMA | Poly(methyl methacrylate) |
| PP | Poly(propylene) |
| PS | Poly(styrene) |
| PU | Poly(urethane) |
| PVC | Poly(vinyl chloride) |
| SAN | Styrene-acrylonitrile resin |
| SAP | Super Adsorbant Polymer |
| r | Recycled |
| TRL | Technology Readiness Level |
References
- Gubanova, E.; Kupinets, L.; Deforzh, H.; Koval, V.; Gaska, K. Recycling of polymer waste in the context of developing circular economy. Archit. Civ. Eng. Environ. 2019, 12, 99–108. [Google Scholar] [CrossRef]
- Flizikowski, J.; Kruszelnicka, W.; Macko, M. The Development of Efficient Contaminated Polymer Materials Shredding in Recycling Processes. Polymers 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J. Polymer waste management—Biodegradation, incineration, and recycling. J. Macromol. Sci. Pure Appl. Chem. 1995, 32, 593–597. [Google Scholar] [CrossRef]
- Hu, H.L.; Chen, Y.A.; You, F.; Yao, J.L.; Yang, H.; Jiang, X.L.; Xia, B.Y. Recycling and Upgrading Utilization of Polymer Plastics. Chin. J. Chem. 2023, 41, 469–480. [Google Scholar] [CrossRef]
- Somers, M.J.; Alfaro, J.F.; Lewis, G.M. Feasibility of superabsorbent polymer recycling and reuse in disposable absorbent hygiene products. J. Clean. Prod. 2021, 313, 127686. [Google Scholar] [CrossRef]
- Russo, S.; Valero, A.; Valero, A.; Iglesias-Émbil, M. Exergy-Based Assessment of Polymers Production and Recycling: An Application to the Automotive Sector. Energies 2021, 14, 363. [Google Scholar] [CrossRef]
- Mekhzoum, M.; Benzeid, H.; Rodrigue, D.; Qaiss, A.; Bouhfid, R. Recent Advances in Polymer Recycling: A Short Review. Curr. Org. Synth. 2017, 14, 171–185. [Google Scholar] [CrossRef]
- Spinacé, M.A.D.; De Paoli, M.A. The technology of polymer recycling. Quim. Nova 2005, 28, 65–72. [Google Scholar] [CrossRef]
- Stein, R.S. Polymer recycling—Opportunities and limitations. Proc. Natl. Acad. Sci. USA 1992, 89, 835–838. [Google Scholar] [CrossRef]
- Bonardi, A.; Cilloni, R.; Paganuzzi, V.; Grizzuti, N. Effects of degree of recycling on the rheology and processability of thermoplastic polymers. J. Polym. Eng. 2003, 23, 79–94. [Google Scholar] [CrossRef]
- Fraïsse, F.; Verney, V.; Commereuc, S.; Obadal, M. Recycling of poly(ethylene terephthalate)/polycarbonate blends. Polym. Degrad. Stab. 2005, 90, 250–255. [Google Scholar] [CrossRef]
- Albitres, G.A.V.; Mendes, L.C.; Cestari, S.P. Polymer blends of polyamide-6/Spandex fabric scraps and recycled poly(ethylene terephthalate). J. Therm. Anal. Calorim. 2017, 129, 1505–1515. [Google Scholar] [CrossRef]
- Sutanto, P.; Picchioni, F.; Janssen, L.P.B.M. The use of experimental design to study the responses of continuous devulcanization processes. J. Appl. Polym. Sci. 2006, 102, 5028–5038. [Google Scholar] [CrossRef]
- Sutanto, P.; Picchioni, F.; Janssen, L. Modelling a continuous devulcanization in an extruder. Chem. Eng. Sci. 2006, 61, 7077–7086. [Google Scholar] [CrossRef]
- Sutanto, P.; Picchioni, F.; Janssen, L.P.B.M.; Dijkhuis, K.A.J.; Dierkes, W.K.; Noordermeer, J.W.M. EPDM rubber reclaim from devulcanized EPDM. J. Appl. Polym. Sci. 2006, 102, 5948–5957. [Google Scholar] [CrossRef]
- Sutanto, P.; Laksmana, F.; Picchioni, F.; Janssen, L. Modeling on the kinetics of an EPDM devulcanization in an internal batch mixer using an amine as the devulcanizing agent. Chem. Eng. Sci. 2006, 61, 6442–6453. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, J.W.; Ko, J.W.; Jo, K.; Park, H.J.; Chung, I. Effects of Recycled Polymer on Melt Viscosity and Crystallization Temperature of Polyester Elastomer Blends. Materials 2023, 16, 6067. [Google Scholar] [CrossRef] [PubMed]
- Mormann, W.; Frank, P. (Supercritical) ammonia for recycling of thermoset polymers. Macromol. Symp. 2006, 242, 165–173. [Google Scholar] [CrossRef]
- Vogt, B.D.; Stokes, K.K.; Kumar, S.K. Why is Recycling of Postconsumer Plastics so Challenging? Acs Appl. Polym. Mater. 2021, 3, 4325–4346. [Google Scholar] [CrossRef]
- dos Santos, W.N.; Agnelli, J.A.M.; Mummery, P.; Wallwork, A. Effect of recycling on the thermal properties of polymers. Polym. Test. 2007, 26, 216–221. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Jiun, Y.L.; Tze, C.T.; Moosa, U.; Tawawneh, M.A. Effects of Recycling Cycle on Used Thermoplastic Polymer and Thermoplastic Elastomer Polymer. Polym. Polym. Compos. 2016, 24, 735–739. [Google Scholar] [CrossRef]
- La Mantia, F.P. Mechanical properties of recycled polymers. Macromol. Symp. 1999, 147, 167–172. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Dintcheva, N.T. Time carbonyl groups equivalence in photo-oxidative aging of virgin/recycled polymer blends. Plast. Rubber Compos. 2004, 33, 184–186. [Google Scholar] [CrossRef]
- Momanyi, J.; Herzog, M.; Muchiri, P. Analysis of Thermomechanical Properties of Selected Class of Recycled Thermoplastic Materials Based on Their Applications. Recycling 2019, 4, 33. [Google Scholar] [CrossRef]
- Zhou, J.F.; Hsu, T.G.; Wang, J.P. Mechanochemical Degradation and Recycling of Synthetic Polymers. Angew. Chem.-Int. Ed. 2023, 135, e202300768. [Google Scholar] [CrossRef]
- Nelson, M.A.; Siwakoti, M.; Cardon, R.; Triggs, E.; Mailen, R.W. Effects of recycling on polystyrene shape memory polymers for in-situ resource utilization. Smart Mater. Struct. 2023, 32, 095037. [Google Scholar] [CrossRef]
- Fakirov, S. A new approach to plastic recycling via the concept of microfibrillar composites. Adv. Ind. Eng. Polym. Res. 2021, 4, 187–198. [Google Scholar] [CrossRef]
- Garforth, A.A.; Ali, S.; Hernández-Martínez, J.; Akah, A. Feedstock recycling of polymer wastes. Curr. Opin. Solid State Mater. Sci. 2004, 8, 419–425. [Google Scholar] [CrossRef]
- Karlsson, S. Recycled polyolefins. Material properties and means for quality determination. In Long-Term Properties of Polyolefins; Albertsson, A.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 201–229. [Google Scholar]
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent trends in recycling and reusing techniques of different plastic polymers and their composite materials. Sustain. Mater. Technol. 2022, 31, e00382. [Google Scholar] [CrossRef]
- Qureshi, J. A Review of Recycling Methods for Fibre Reinforced Polymer Composites. Sustainability 2022, 14, 16855. [Google Scholar] [CrossRef]
- Sasse, F.; Emig, G. Chemical recycling of polymer materials. Chem. Eng. Technol. 1998, 21, 777–789. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Beke, B.V. Recycling of Polymers: A Review. Chemsuschem 2014, 7, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Beigbeder, J.; Perrin, D.; Mascaro, J.-F.; Lopez-Cuesta, J.-M. Study of the physico-chemical properties of recycled polymers from waste electrical and electronic equipment (WEEE) sorted by high resolution near infrared devices. Resour. Conserv. Recycl. 2013, 78, 105–114. [Google Scholar] [CrossRef]
- Florestan, J.; Lachambre, A.; Mermilliod, N.; Boulou, J.; Marfisi, C. Recycling of plastics—Automatic identification of polymers by spectroscopic methods. Resour. Conserv. Recycl. 1994, 10, 67–74. [Google Scholar] [CrossRef]
- Bifulco, A.; Chen, J.; Sekar, A.; Klingler, W.W.; Gooneie, A.; Gaan, S. Recycling of flame retardant polymers: Current technologies and future perspectives. J. Mater. Sci. Technol. 2024, 199, 156–183. [Google Scholar] [CrossRef]
- da Silva, D.J.; Wiebeck, H. Current options for characterizing, sorting, and recycling polymeric waste. Prog. Rubber Plast. Recycl. Technol. 2020, 36, 284–303. [Google Scholar] [CrossRef]
- Boekhoff, S.; Zetzener, H.; Kwade, A. Comminution of Metal-Polymer Composites for Recycling. Chem. Ing. Tech. 2024, 96, 941–949. [Google Scholar] [CrossRef]
- Harmsen, P.; Scheffer, M.; Bos, H. Textiles for Circular Fashion: The Logic behind Recycling Options. Sustainability 2021, 13, 9714. [Google Scholar] [CrossRef]
- Bianchi, S.; Bartoli, F.; Bruni, C.; Fernandez-Avila, C.; Rodriguez-Turienzo, L.; Mellado-Carretero, J.; Spinelli, D.; Coltelli, M.-B. Opportunities and Limitations in Recycling Fossil Polymers from Textiles. Macromol 2023, 3, 120–148. [Google Scholar] [CrossRef]
- Boborodea, A.; O’Donohue, S.; Brookes, A. A new GPC/TREF/LinELSD instrument to determine the molecular weight distribution and chemical composition: Application to recycled polymers. Int. J. Polym. Anal. Charact. 2021, 26, 721–734. [Google Scholar] [CrossRef]
- Brasileiro, L.L.; Moreno-Navarro, F.; Martínez, R.T.; del Sol-Sánchez, M.; Matos, J.M.E.; Rubio-Gámez, M.d.C. Study of the feasability of producing modified asphalt bitumens using flakes made from recycled polymers. Constr. Build. Mater. 2019, 208, 269–282. [Google Scholar] [CrossRef]
- Buczyński, P.; Iwański, M. The Influence of a Polymer Powder on the Properties of a Cold-Recycled Mixture with Foamed Bitumen. Materials 2019, 12, 4244. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.; McNally, C.; Gibney, A.; Gilchrist, M.D. Development of a recycled polymer modified binder for use in stone mastic asphalt. Resour. Conserv. Recycl. 2008, 52, 1167–1174. [Google Scholar] [CrossRef]
- dos Reis, J.M.L.; Jurumenha, M.A.G. Experimental Investigation on the Effects of Recycled Aggregate on Fracture Behavior of Polymer Concrete. Mater. Res. Ibero Am. J. Mater. 2011, 14, 326–330. [Google Scholar]
- dos Reis, J.M.L.; Nunes, L.C.S.; Triques, A.L.C.; Valente, L.C.G.; Bragaa, A.M.B. Mechanical Characterization Using Optical Fiber Sensors of Polyester Polymer Concrete Made with Recycled Aggregates. Mater. Res. Ibero Am. J. Mater. 2009, 12, 269–271. [Google Scholar]
- Eui-Hwan, H.; Ko, Y.S.; Jeon, J.K. Effect of polymer cement modifiers on mechanical and physical properties of polymer-modified mortar using recycled waste concrete fine aggregate. J. Ind. Eng. Chem. 2007, 13, 387–394. [Google Scholar]
- Figueiredo, F.P.; Huang, S.-S.; Angelakopoulos, H.; Pilakoutas, K.; Burgess, I. Effects of Recycled Steel and Polymer Fibres on Explosive Fire Spalling of Concrete. Fire Technol. 2019, 55, 1495–1516. [Google Scholar] [CrossRef]
- Fuentes-Audén, C.; Sandoval, J.A.; Jerez, A.; Navarro, F.J.; Martínez-Boza, F.J.; Partal, P.; Gallegos, C. Evaluation of thermal and mechanical properties of recycled polyethylene modified bitumen. Polym. Test. 2008, 27, 1005–1012. [Google Scholar] [CrossRef]
- Asdollah-Tabar, M.; Heidari-Rarani, M.; Aliha, M.R.M. The effect of recycled PET bottles on the fracture toughness of polymer concrete. Compos. Commun. 2021, 25, 100684. [Google Scholar] [CrossRef]
- Hwang, Y.-T.; Ahn, S.; Koh, H.-I.; Park, J.; Kim, H.-S. Evaluation of mechanical/dynamic properties of polyetherimide recycled polymer concrete for reducing rail slab noise. Funct. Compos. Struct. 2019, 1, 025002. [Google Scholar] [CrossRef]
- Jo, B.W.; Park, S.K.; Park, J.C. Mechanical properties of polymer concrete made with recycled PET and recycled concrete aggregates. Constr. Build. Mater. 2008, 22, 2281–2291. [Google Scholar] [CrossRef]
- Jo, B.W.; Park, S.K.; Kim, C.H. Mechanical properties of polyester polymer concrete using recycled polyethylene terephthalate. Aci Struct. J. 2006, 103, 219–225. [Google Scholar]
- Martínez-Barrera, G.; del Coz-Díaz, J.J.; Martínez-Cruz, E.; Martínez-López, M.; Ribeiro, M.C.; Velasco-Santos, C.; Lobland, H.E.H.; Brostow, W. Modified recycled tire fibers by gamma radiation and their use on the improvement of polymer concrete. Constr. Build. Mater. 2019, 204, 327–334. [Google Scholar] [CrossRef]
- Martuscelli, C.C.; dos Santos, J.C.; Oliveira, P.R.; Panzera, T.H.; Aguilar, M.T.P.; Garcia, C.T. Polymer-cementitious composites containing recycled rubber particles. Constr. Build. Mater. 2018, 170, 446–454. [Google Scholar] [CrossRef]
- Mortazavi, S.B.; Rasoulzadeh, Y.; Yousefi, A.A.; Khavanin, A. Properties of Modified Bitumen Obtained from Vacuum Bottom by Adding Recycled Waste Polymers and Natural Bitumen. Iran. Polym. J. 2010, 19, 197–205. [Google Scholar]
- Navarro, F.; Partal, P.; García-Morales, M.; Martín-Alfonso, M.; Martínez-Boza, F.; Gallegos, C.; Bordado, J.; Diogo, A. Bitumen modification with reactive and non-reactive (virgin and recycled) polymers: A comparative analysis. J. Ind. Eng. Chem. 2009, 15, 458–464. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Boom, Y.J.; Giustozzi, F. Sustainable Polymers from Recycled Waste Plastics and Their Virgin Counterparts as Bitumen Modifiers: A Comprehensive Review. Polymers 2021, 13, 3242. [Google Scholar] [CrossRef] [PubMed]
- Rebeiz, K.S. Precast use of polymer concrete using unsaturated polyester resin based on recycled PET waste. Constr. Build. Mater. 1996, 10, 215–220. [Google Scholar] [CrossRef]
- Rebeiz, K.S.; Fowler, D.W. Flexural strength of reinforced polymer concrete made with recycled plastic waste. Aci Struct. J. 1996, 93, 524–530. [Google Scholar]
- Rebeiz, K.S.; Yang, S.; Fowler, D.W. Polymer mortar composites made with recycled plastics. Aci Mater. J. 1994, 91, 313–319. [Google Scholar]
- Rebeiz, K.S.; Fowler, D.W.; Paul, D.R. Mechanical-properties of polymer concrete systems made with recycled plastic. Aci Mater. J. 1994, 91, 40–45. [Google Scholar]
- Reis, J.M.L.; Chianelli-Junior, R.; Cardoso, J.L.; Marinho, F.J.V. Effect of recycled PET in the fracture mechanics of polymer mortar. Constr. Build. Mater. 2011, 25, 2799–2804. [Google Scholar] [CrossRef]
- Suresh, M.; Pal, M.; Sarkar, D. Performance of polymer-reinforced bituminous mixes using recycled coarse aggregate. Proc. Inst. Civ. Eng.-Transp. 2021, 174, 142–156. [Google Scholar] [CrossRef]
- Tao, Y.; Hadigheh, S.A.; Wei, Y. Recycling of glass fibre reinforced polymer (GFRP) composite wastes in concrete: A critical review and cost benefit analysis. Structures 2023, 53, 1540–1556. [Google Scholar] [CrossRef]
- Viscione, N.; Presti, D.L.; Veropalumbo, R.; Oreto, C.; Biancardo, S.A.; Russo, F. Performance-based characterization of recycled polymer modified asphalt mixture. Constr. Build. Mater. 2021, 310, 125243. [Google Scholar] [CrossRef]
- Wieser, M.; Schaur, A.; Unterberger, S.H.; Lackner, R. On the Effect of Recycled Polyolefins on the Thermorheological Performance of Polymer-Modified Bitumen Used for Roofing-Applications. Sustainability 2021, 13, 3284. [Google Scholar] [CrossRef]
- Xiong, C.; Lan, T.; Li, Q.; Li, H.; Long, W. Study of Mechanical Properties of an Eco-Friendly Concrete Containing Recycled Carbon Fiber Reinforced Polymer and Recycled Aggregate. Materials 2020, 13, 4592. [Google Scholar] [CrossRef]
- Yu, Z.; Kong, W.; Ji, Z.; Wang, Y. Impact of virgin and recycled polymer fibers on the rheological properties of cemented paste backfill. Sci. Rep. 2024, 14, 17783. [Google Scholar] [CrossRef]
- Samchenko, S.V.; Larsen, O.A. Modifying the Sand Concrete with Recycled Tyre Polymer Fiber to Increase the Crack Resistance of Building Structures. Buildings 2023, 13, 897. [Google Scholar] [CrossRef]
- Assaad, J.J.; Issa, C.A. Effect of Recycled Acrylic-Based Polymers on Bond Stress-Slip Behavior in Reinforced Concrete Structures. J. Mater. Civ. Eng. 2017, 29, 04016173. [Google Scholar] [CrossRef]
- Baričević, A.; Rukavina, M.J.; Pezer, M.; Štirmer, N. Influence of recycled tire polymer fibers on concrete properties. Cem. Concr. Compos. 2018, 91, 29–41. [Google Scholar] [CrossRef]
- Gawdzinska, K.; Nabialek, M.; Sandu, A.V.; Bryll, K. The Choice of Recycling Methods for Single-Polymer Polyester Composites. Mater. Plast. 2018, 55, 658–665. [Google Scholar] [CrossRef]
- Kibert, C.J. Construction materials from recycled polymers. Proc. Inst. Civ. Eng.-Struct. Build. 1993, 99, 455–464. [Google Scholar] [CrossRef]
- Devasahayam, S.; Raman, R.K.S.; Chennakesavulu, K.; Bhattacharya, S. PlasticsVillain or Hero? Polymers and Recycled Polymers in Mineral and Metallurgical ProcessingA Review. Materials 2019, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Audén, C.; Martínez-Boza, F.J.; Navarro, F.J.; Partal, P.; Gallegos, C. Formulation of new synthetic properties of recycled binders: Thermo-mechanical polymer/oil blends. Polym. Test. 2007, 26, 323–332. [Google Scholar] [CrossRef]
- Duflou, J.; Boudewijn, A.; Cattrysse, D.; Wagner, F.; Accili, A.; Dimitrova, G.; Peeters, J. Product clustering as a strategy for enhanced plastics recycling from WEEE. Cirp Ann.-Manuf. Technol. 2020, 69, 29–32. [Google Scholar] [CrossRef]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H. Recycling WEEE: Polymer characterization and pyrolysis study for waste of crystalline silicon photovoltaic modules. Waste Manag. 2017, 60, 716–722. [Google Scholar] [CrossRef]
- Deshmukh, D.; Kulkarni, H.; Srivats, D.S.; Bhanushali, S.; More, A.P. Recycling of acrylonitrile butadiene styrene (ABS): A review. Polym. Bull. 2024, 81, 28–38. [Google Scholar] [CrossRef]
- Gabriel, A.P.; Santana, R.M.C.; Veit, H.M. Evaluation of Recycled Polymers From CRT Monitor Frames of Different Years of Manufacture. Prog. Rubber Plast. Recycl. Technol. 2014, 30, 55–66. [Google Scholar] [CrossRef]
- Achilias, D.; Antonakou, E.; Koutsokosta, E.; Lappas, A. Chemical Recycling of Polymers from Waste Electric and Electronic Equipment. J. Appl. Polym. Sci. 2009, 114, 212–221. [Google Scholar] [CrossRef]
- Achilias, D.S.; Giannoulis, A.; Papageorgiou, G.Z. Recycling of polymers from plastic packaging materials using the dissolution-reprecipitation technique. Polym. Bull. 2009, 63, 449–465. [Google Scholar] [CrossRef]
- Cecon, V.S.; Curtzwiler, G.W.; Vorst, K.L. A Study on Recycled Polymers Recovered from Multilayer Plastic Packaging Films by Solvent-Targeted Recovery and Precipitation (STRAP). Macromol. Mater. Eng. 2022, 307, 2200346. [Google Scholar] [CrossRef]
- Hadi, A.J.; Najmuldeen, G.F.; Yusoh, K.B. Dissolution/reprecipitation technique for waste polyolefin recycling using new pure and blend organic solvents. J. Polym. Eng. 2013, 33, 471–481. [Google Scholar] [CrossRef]
- Li, T.; Theodosopoulos, G.; Lovell, C.; Loukodimou, A.; Maniam, K.K.; Paul, S. Progress in Solvent-Based Recycling of Polymers from Multilayer Packaging. Polymers 2024, 16, 1670. [Google Scholar] [CrossRef]
- Denolf, R.; Hogie, J.; Figueira, F.L.; Mertens, I.; De Somer, T.; D’Hooge, D.R.; Hoogenboom, R.; De Meester, S. Constructing and validating ternary phase diagrams as basis for polymer dissolution recycling. J. Mol. Liq. 2023, 387, 122630. [Google Scholar] [CrossRef]
- Pappa, G.; Boukouvalas, C.; Giannaris, C.; Ntaras, N.; Zografos, V.; Magoulas, K.; Lygeros, A.; Tassios, D. The selective dissolution/precipitation technique for polymer recycling: A pilot unit application. Resour. Conserv. Recycl. 2001, 34, 33–44. [Google Scholar] [CrossRef]
- Li, B.; Ding, T.; Qu, C.; Liu, W. Modification of fresh and hardened properties of 3D-printed recycled mortar by superabsorbent polymers. J. Build. Eng. 2024, 95, 110189. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef]
- Bavasso, I.; Bracciale, M.P.; De Bellis, G.; Pantaleoni, A.; Tirillò, J.; Pastore, G.; Gabrielli, S.; Sarasini, F. Recycling of a commercial biodegradable polymer blend: Influence of reprocessing cycles on rheological and thermo-mechanical properties. Polym. Test. 2024, 134, 108418. [Google Scholar] [CrossRef]
- Paiva, R.; Aznar, M.; Wrona, M.; Batista, A.P.d.L.; Nerín, C.; Cruz, S.A. Biobased Polymer Recycling: A Comprehensive Dive into the Recycling Process of PLA and Its Decontamination Efficacy. ACS Appl. Polym. Mater. 2024, 6, 12154–12163. [Google Scholar] [CrossRef]
- Beltrán, F.; Barrio, I.; Lorenzo, V.; del Río, B.; Urreaga, J.M.; de la Orden, M. Valorization of poly(lactic acid) wastes via mechanical recycling: Improvement of the properties of the recycled polymer. Waste Manag. Res. 2019, 37, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. The effect of post-consumer recycled polyethylene (PCRPE) on the properties of polyethylene blends of different densities. Polym. Degrad. Stab. 2021, 190, 109627. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. Dataset of the properties of polyethylene (PE) blends of different densities mixed with post-consumer recycled polyethylene (PCRPE). Data Brief 2021, 38, 107452. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Liao, W.-H.; Hsieh, M.-W.; Chien, R.-D.; Lin, S.-H. Influence of Recycled ABS Added to Virgin Polymers on the Physical, Mechanical Properties and Molding Characteristics. Polym. Technol. Eng. 2011, 50, 306–311. [Google Scholar] [CrossRef]
- Titone, V.; Mistretta, M.C.; Botta, L.; La Mantia, F.P. Toward the Decarbonization of Plastic: Monopolymer Blend of Virgin and Recycled Bio-Based, Biodegradable Polymer. Polymers 2022, 14, 5362. [Google Scholar] [CrossRef]
- Picchioni, F.; Broekhuis, A.A. Material properties and processing in chemical product design. Curr. Opin. Chem. Eng. 2012, 1, 459–464. [Google Scholar] [CrossRef]
- Dorigato, A. Recycling of polymer blends. Adv. Ind. Eng. Polym. Res. 2021, 4, 53–69. [Google Scholar] [CrossRef]
- Rydzkowski, T. Properties of recycled polymer mixtures obtained in the screw-disc extrusion process. Polimery 2011, 56, 135–139. [Google Scholar] [CrossRef]
- Picchioni, F.; Aglietto, M.; Passaglia, E.; Ciardelli, F. Blends of syndiotactic polystyrene with SEBS triblock copolymers. Polymer 2002, 43, 3323–3329. [Google Scholar] [CrossRef]
- Picchioni, F.; Casentini, E.; Passaglia, E.; Ruggeri, G. Blends of SBS triblock copolymer with poly(2,6-dimethyl-1,4-phenylene oxide)/polystyrene mixture. J. Appl. Polym. Sci. 2003, 88, 2698–2705. [Google Scholar] [CrossRef]
- Picchioni, F.; Giorgi, I.; Passaglia, E. Blending of styrene-block-butadiene-block styrene copolymer with sulfonated vinyl aromatic polymers. Polym. Int. 2001, 50, 714–721. [Google Scholar] [CrossRef]
- Picchioni, F.; Goossens, J.; van Duin, M. Solid-state modification of polypropylene (PP): Grafting of styrene on atactic PP. Macromol. Symp. 2001, 176, 245–263. [Google Scholar] [CrossRef]
- Picchioni, F.; Goossens, J.G.P.; van Duin, M. Solid-state modification of isotactic polypropylene (iPP) via grafting of styrene. II. Morphology and melt processing. J. Appl. Polym. Sci. 2005, 97, 575–583. [Google Scholar] [CrossRef][Green Version]
- Picchioni, F.; Goossens, J.G.P.; van Duin, M. Solid-state modification of isotactic polypropylene (iPP) via grafting of styrene. I. Polymerization experiments. J. Appl. Polym. Sci. 2003, 89, 3279–3291. [Google Scholar] [CrossRef]
- Picchioni, F.; Passaglia, E.; Ruggeri, G.; Ciardelli, F. Blends of syndiotactic polystyrene with SBS triblock copolymers. Macromol. Chem. Phys. 2001, 202, 2142–2147. [Google Scholar] [CrossRef]
- Picchioni, F.; Passaglia, E.; Ruggeri, G.; Piccini, M.T.; Aglietto, M. Blends of styrene-butadiene-styrene triblock copolymer with random styrene-maleic anhydride copolymers. Macromol. Chem. Phys. 2002, 203, 1396–1402. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Hernández-Hernández, E.; Sifuentes-Nieves, I.; Lara-Sánchez, J.F.; Ledezma-Pérez, A.S.; Alvarado-Canché, C.N.; Ramírez-Vargas, E. Active Mono-Material Films from Natural and Post-consumer Recycled Polymers with Essential Oils for Food Packaging Applications. J. Polym. Environ. 2023, 31, 5198–5209. [Google Scholar] [CrossRef]
- Garcia, D.; Balart, R.; Crespo, J.E.; Lopez, J. Mechanical properties of recycled PVC blends with styrenic polymers. J. Appl. Polym. Sci. 2006, 101, 2464–2471. [Google Scholar] [CrossRef]
- Lepadatu, A.M.; Asaftei, S.; Vennemann, N. Recycling of EPDM Rubber Waste Powder by Activation with liquid Polymers. Kgk-Kautsch. Gummi Kunststoffe 2014, 67, 41–47. [Google Scholar]
- Möllnitz, S.; Feuchter, M.; Duretek, I.; Schmidt, G.; Pomberger, R.; Sarc, R. Processability of Different Polymer Fractions Recovered from Mixed Wastes and Determination of Material Properties for Recycling. Polymers 2021, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.P.; Gonçalves, J.V.R.V.; Robinson, L.C.; da Rosa, L.C.; Schneider, E.L. Recycling and Mechanical Characterization of Polymer Blends Present in Printers. Mater. Res.-Ibero-Am. J. Mater. 2017, 20, 202–208. [Google Scholar] [CrossRef][Green Version]
- Akkapeddi, M.K.; Van Buskirk, B.; Mason, C.D.; Chung, S.S.; Swamikannu, X. Performance blends based on recycled polymers. Polym. Eng. Sci. 1995, 35, 72–78. [Google Scholar] [CrossRef]
- Titone, V.; Gulino, E.F.; La Mantia, F.P. Recycling of Heterogeneous Mixed Waste Polymers through Reactive Mixing. Polymers 2023, 15, 1367. [Google Scholar] [CrossRef]
- Stein, R.S. Polymer recycling: Thermodynamics and economics. In Plastics, Rubber, and Paper Recycling: A Pragmatic Approach; Rader, C.P., Baldwin, S.D., Cornell, D.D., Sadler, G.D., Stockel, R.F., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 27–46. [Google Scholar]
- Stein, R.S. Polymer recycling: Thermodynamics and economics. Macromol. Symp. 1998, 135, 295–314. [Google Scholar] [CrossRef]
- Aldosari, S.M.; AlOtaibi, B.M.; Alblalaihid, K.S.; Aldoihi, S.A.; AlOgab, K.A.; Alsaleh, S.S.; Alshamary, D.O.; Alanazi, T.H.; Aldrees, S.D.; Alshammari, B.A. Mechanical Recycling of Carbon Fiber-Reinforced Polymer in a Circular Economy. Polymers 2024, 16, 1363. [Google Scholar] [CrossRef]
- Amberg, M.; Höhener, M.; Rupper, P.; Hanselmann, B.; Hufenus, R.; Lehner, S.; Perret, E.; Hegemann, D. Surface modification of recycled polymers in comparison to virgin polymers using Ar/O2 plasma etching. Plasma Process. Polym. 2022, 19, 2200068. [Google Scholar] [CrossRef]
- Ateeq, M.; Shafique, M.; Azam, A.; Rafiq, M. A review of 3D printing of the recycled carbon fiber reinforced polymer composites: Processing, potential, and perspectives. J. Mater. Res. Technol. 2023, 26, 2291–2309. [Google Scholar] [CrossRef]
- Czvikovszky, T.; Hargitai, H. Electron beam surface modifications in reinforcing and recycling of polymers. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 1997, 131, 300–304. [Google Scholar] [CrossRef]
- Ciardelli, F.; Aglietto, M.; Passaglia, E.; Picchioni, F. Controlled functionalization of olefin/styrene copolymers through free radical processes. Polym. Adv. Technol. 2000, 11, 371–376. [Google Scholar] [CrossRef]
- Passaglia, E.; Ghetti, S.; Picchioni, F.; Ruggeri, G. Grafting of diethyl maleate and maleic anhydride onto styrene-b-(ethylene-co-1-butene)-b-styrene triblock copolymer (SEBS). Polymer 2000, 41, 4389–4400. [Google Scholar] [CrossRef]
- Passaglia, E.; Aglietto, M.; Ruggeri, G. Formation and compatibilizing effect of the grafted copolymer in the reactive blending of 2-diethylsuccinate containing polyolefins with poly-epsilon-caprolactam (Nylon-6). Polym. Adv. Technol. 1998, 9, 273–281. [Google Scholar] [CrossRef]
- Passaglia, E.; Picchioni, F.; Aglietto, M. Features of graft copolymer formation through reaction of functionalized polyolefins with poly-epsilon-caprolatam. Turk. J. Chem. 1997, 21, 262–269. [Google Scholar]
- Suresh, S.S.; Mohanty, S.; Nayak, S.K. Epoxidized soybean oil toughened recycled blends: A new method for the toughening of recycled polymers employing renewable resources. Polym. Bull. 2020, 77, 6543–6562. [Google Scholar] [CrossRef]
- Capuano, R.; Bonadies, I.; Castaldo, R.; Cocca, M.; Gentile, G.; Protopapa, A.; Avolio, R.; Errico, M.E. Valorization and Mechanical Recycling of Heterogeneous Post-Consumer Polymer Waste through a Mechano-Chemical Process. Polymers 2021, 13, 2783. [Google Scholar] [CrossRef] [PubMed]
- Korol, J.; Hejna, A.; Wypiór, K.; Mijalski, K.; Chmielnicka, E. Wastes from Agricultural Silage Film Recycling Line as a Potential Polymer Materials. Polymers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Martikka, O.; Kärki, T. Promoting Recycling of Mixed Waste Polymers in Wood-Polymer Composites Using Compatibilizers. Recycling 2019, 4, 6. [Google Scholar] [CrossRef]
- Mengeloglu, F.; Karakus, K. Some Properties of Eucalyptus Wood Flour Filled Recycled High Density Polyethylene Polymer-Composites. Turk. J. Agric. For. 2008, 32, 537–546. [Google Scholar]
- Nukala, S.G.; Kong, I.; Kakarla, A.B.; Kong, W.; Kong, W. Development of Wood Polymer Composites from Recycled Wood and Plastic Waste: Thermal and Mechanical Properties. J. Compos. Sci. 2022, 6, 194. [Google Scholar] [CrossRef]
- Van Kets, K.; Delva, L.; Ragaert, K. Structural stabilizing effect of SEBSgMAH on a PP-PET blend for multiple mechanical recycling. Polym. Degrad. Stab. 2019, 166, 60–72. [Google Scholar] [CrossRef]
- Saikrishnan, S.; Jubinville, D.; Tzoganakis, C.; Mekonnen, T.H. Thermo-mechanical degradation of polypropylene (PP) and low-density polyethylene (LDPE) blends exposed to simulated recycling. Polym. Degrad. Stab. 2020, 182, 109390. [Google Scholar] [CrossRef]
- Lima, M.S.; Matias, A.; Costa, J.R.C.; Fonseca, A.C.; Coelho, J.F.J.; Serra, A.C. Glycidyl methacrylate-based copolymers as new compatibilizers for polypropylene/polyethylene terephthalate blends. J. Polym. Res. 2019, 26, 127. [Google Scholar] [CrossRef]
- Barhoumi, N.; Jaziri, M.; Massardier, V.; Cassagnau, P. Valorization of poly(butylene terephthalate) wastes by blending with virgin polypropylene: Effect of the composition and the compatibilization. Polym. Eng. Sci. 2008, 48, 1592–1599. [Google Scholar] [CrossRef]
- Borovanska, I.; Krastev, R.; Benavente, R.; Pradas, M.M.; Lluch, A.V.; Samichkov, V.; Iliev, M. Ageing effect on morphology, thermal and mechanical properties of impact modified LDPE/PP blends from virgin and recycled materials. J. Elastomers Plast. 2014, 46, 427–447. [Google Scholar] [CrossRef]
- Chikh, A.; Benhamida, A.; Kaci, M.; Bourmaud, A.; Bruzaud, S. Recyclability assessment of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(butylene succinate) blends: Combined influence of sepiolite and compatibilizer. Polym. Degrad. Stab. 2017, 142, 234–243. [Google Scholar] [CrossRef]
- Ciesielska, D.; Liu, P. An investigation of recycled expanded polystyrene/polybutadiene blends. Cell. Polym. 1999, 18, 237–250. [Google Scholar]
- de Oliveira, T.A.; Barbosa, R.; Mesquita, A.B.S.; Ferreira, J.H.L.; de Carvalho, L.H.; Alves, T.S. Fungal degradation of reprocessed PP/PBAT/thermoplastic starch blends. J. Mater. Res. Technol. 2020, 9, 2338–2349. [Google Scholar] [CrossRef]
- de Oliveira, R.R.; de Oliveira, T.A.; da Silva, L.R.C.; Barbosa, R.; Alves, T.S.; de Carvalho, L.H.; Rodrigues, D.T. Effect of Reprocessing Cycles on the Morphology and Mechanical Properties of a Poly(Propylene)/Poly(Hydroxybutyrate) Blend and its Nanocomposite. Mater. Res.-Ibero-Am. J. Mater. 2021, 24, e20200372. [Google Scholar] [CrossRef]
- Collar, E.P.; García-Martínez, J.M. A Dynamic Mechanical Analysis on the Compatibilization Effect of Two Different Polymer Waste-Based Compatibilizers in the Fifty/Fifty Polypropylene/Polyamide 6 Blend. Polymers 2024, 16, 2523. [Google Scholar] [CrossRef] [PubMed]
- Kuram, E.; Ozcelik, B.; Yilmaz, F. The effects of recycling process on thermal, chemical, rheological, and mechanical properties of PC/ABS binary and PA6/PC/ABS ternary blends. J. Elastomers Plast. 2016, 48, 164–181. [Google Scholar] [CrossRef]
- Karlsson, S.; Albertsson, A.C. Recycling of cheap packaging waste versus expensive engineering materials. Macromol. Symp. 1998, 135, 1–5. [Google Scholar] [CrossRef]
- Gere, D.; Czigany, T. Future trends of plastic bottle recycling: Compatibilization of PET and PLA. Polym. Test. 2020, 81, 106160. [Google Scholar] [CrossRef]
- Hirayama, D.; Nunnenkamp, L.A.; Braga, F.H.G.; Saron, C. Enhanced mechanical properties of recycled blends acrylonitrile-butadiene-styrene/high-impact polystyrene from waste electrical and electronic equipment using compatibilizers and virgin polymers. J. Appl. Polym. Sci. 2022, 139, 51873. [Google Scholar] [CrossRef]
- He, H.Z.; Zhan, Z.M.; Zhu, Z.W.; Xue, B.; Li, J.Q.; Chen, M.; Wang, G.Z. Microscopic morphology, rheological behavior, and mechanical properties of polymers: Recycled acrylonitrile-butadiene-styrene/polybutylene terephthalate blends. J. Appl. Polym. Sci. 2020, 137, 48310. [Google Scholar] [CrossRef]
- Flores, I.; Etxeberria, A.; Irusta, L.; Calafel, I.; Vega, J.F.; Martínez-Salazar, J.; Sardon, H.; Müller, A.J. PET-ran-PLA Partially Degradable Random Copolymers Prepared by Organocatalysis: Effect of Poly(L-lactic acid) Incorporation on Crystallization and Morphology. Acs Sustain. Chem. Eng. 2019, 7, 8647–8659. [Google Scholar] [CrossRef]
- Gere, D.; Czigany, T. Rheological and mechanical properties of recycled polyethylene films contaminated by biopolymer. Waste Manag. 2018, 76, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Moini, N.; Kabiri, K. Organosilane compounds for tunable recycling of waste superabsorbent polymer fine particles. Polym. Bull. 2022, 79, 10229–10249. [Google Scholar] [CrossRef]
- Ropota, I.; Bratu, M.; Dragnea, D.; Dumitrescu, O.; Muntean, O.; Muntean, M. Recycling Solid Wastes As Polymer Composites. Stud. Univ. Babes-Bolyai Chem. 2013, 58, 213–225. [Google Scholar]
- Wiśniewska, P.; Wójcik, N.A.; Ryl, J.; Bogdanowicz, R.; Vahabi, H.; Formela, K.; Saeb, M.R. Rubber wastes recycling for developing advanced polymer composites: A warm handshake with sustainability. J. Clean. Prod. 2023, 427, 139010. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Kurcok, M. Effects of compatibilizers and electron radiation on thermomechanical properties of composites consisting of five recycled polymers. Polym. Test. 2008, 27, 420–427. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Dzwonkowski, J. Effects of electron radiation and compatibilizers on impact strength of composites of recycled polymers. Polym. Test. 2007, 26, 903–907. [Google Scholar] [CrossRef]
- Niño, C.G.; Vidal, J.; Del Cerro, M.; Royo-Pascual, L.; Murillo-Ciordia, G.; Castell, P. Effect of Gamma Radiation on the Processability of New and Recycled PA-6 Polymers. Polymers 2023, 15, 613. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, F.P. Re-stabilization of recycled polymers. Macromol. Symp. 2000, 152, 201–210. [Google Scholar] [CrossRef]
- Bek, M.; Aulova, A.; Črešnar, K.P.; Matkovič, S.; Kalin, M.; Perše, L.S. Long-Term Creep Compliance of Wood Polymer Composites: Using Untreated Wood Fibers as a Filler in Recycled and Neat Polypropylene Matrix. Polymers 2022, 14, 2539. [Google Scholar] [CrossRef]
- Begley, T.H.; Hollifield, H.C. Food packaging made form recycled polymers—Functional barrier considerations. In Plastics, Rubber, and Paper Recycling: A Pragmatic Approach; Rader, C.P., Baldwin, S.D., Cornell, D.D., Sadler, G.D., Stockel, R.F., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 445–457. [Google Scholar]
- Cecon, V.S.; Da Silva, P.F.; Curtzwiler, G.W.; Vorst, K.L. The challenges in recycling post-consumer polyolefins for food contact applications: A review. Resour. Conserv. Recycl. 2021, 167, 105422. [Google Scholar] [CrossRef]
- Sadler, G.D. Recycling of polymers for food use: A current perspective. In Plastics, Rubber, and Paper Recycling: A Pragmatic Approach; Rader, C.P., Baldwin, S.D., Cornell, D.D., Sadler, G.D., Stockel, R.F., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 380–388. [Google Scholar]
- Cruz, S.A.; Oliveira, E.C.; de Oliveira, F.C.S.; Garcia, P.S.; Kaneko, M. Recycled Polymers for Food Contact. Polim. Cienc. E Tecnol. 2011, 21, 340–345. [Google Scholar] [CrossRef]
- Kolek, Z. Recycled polymers from food packaging in relation to environmental protection. Pol. J. Environ. Stud. 2001, 10, 73–76. [Google Scholar]
- Vergnaud, J.M. Problems encountered for food safety with polymer packages: Chemical exchange, recycling. Adv. Colloid Interface Sci. 1998, 78, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Perou, A.L.; Vergnaud, J.M. Contaminant transfer during the processing of thick three-layer food packages with a recycled polymer between two virgin polymer layers. Int. J. Numer. Methods Heat Fluid Flow 1998, 8, 841–852. [Google Scholar] [CrossRef]
- Perou, A.L.; Vergnaud, J.M. Contaminant transfer during the co-extrusion of food packages made of recycled polymer and virgin polymer layers. Polymer 1997, 38, A1–A6. [Google Scholar] [CrossRef]
- Perou, A.L.; Vergnaud, J.M. Contaminant transfer during the coextrusion of thin three-layer food packages with a recycled polymer between two virgin polymer layers. J. Polym. Eng. 1997, 17, 349–361. [Google Scholar] [CrossRef]
- Feigenbaum, A.; Laoubi, S.; Vergnaud, J.M. Kinetics of diffusion of a pollutant from a recycled polymer through a functional barrier: Recycling plastics for food packaging. J. Appl. Polym. Sci. 1997, 66, 597–607. [Google Scholar] [CrossRef]
- Bhat, G.; Narayanan, V.; Wadsworth, L.; Dever, M. Conversion of recycled polymers fibers into melt-blown nonwovens. Polym.-Plast. Technol. Eng. 1999, 38, 499–511. [Google Scholar] [CrossRef]
- Evstatiev, M.; Fakirov, S.; Krasteva, B.; Friedrich, K.; Covas, J.A.; Cunha, A.M. Recycling of poly(ethylene terephthalate) as polymer-polymer composites. Polym. Eng. Sci. 2002, 42, 826–835. [Google Scholar] [CrossRef]
- Daren, S. Homomicronization—A route for polymer recycling. The Newplast process. Polimery 1998, 43, 379–383. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Ahmed, W.; Arshad, H.; Ullah, S. Recycling of the glass/carbon fibre reinforced polymer composites: A step towards the circular economy. Polym.-Plast. Technol. Mater. 2022, 61, 761–788. [Google Scholar] [CrossRef]
- Deng, J.; Xu, L.; Liu, J.; Peng, J.; Han, Z.; Shen, Z.; Guo, S. Efficient method of recycling carbon fiber from the waste of carbon fiber reinforced polymer composites. Polym. Degrad. Stab. 2020, 182, 109419. [Google Scholar] [CrossRef]
- May, D.; Goergen, C.; Friedrich, K. Multifunctionality of polymer composites based on recycled carbon fibers: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 70–81. [Google Scholar] [CrossRef]
- Vega-Leal, C.; Zárate-Pérez, C.; Gomez-Culebro, V.A.; Burelo, M.; Franco-Urquiza, E.A.; Treviño-Quintanilla, C.D. Mechanical recycling of carbon fibre reinforced polymers. Part 1: Influence of cutting speed on recycled particles and composites properties. Int. J. Sustain. Eng. 2024, 17, 159–168. [Google Scholar] [CrossRef]
- Ateeq, M. A state of art review on recycling and remanufacturing of the carbon fiber from carbon fiber polymer composite. Compos. Part C Open Access 2023, 12, 100412. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Seidlitz, H.; Goracy, K.; Urbaniak, M.; Rösch, J.J. Recycling of Carbon Fiber Reinforced Composite Polymers-Review-Part 1: Volume of Production, Recycling Technologies, Legislative Aspects. Polymers 2021, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Bledzki, A.K.; Seidlitz, H.; Krenz, J.; Goracy, K.; Urbaniak, M.; Rösch, J.J. Recycling of Carbon Fiber Reinforced Composite Polymers-Review-Part 2: Recovery and Application of Recycled Carbon Fibers. Polymers 2020, 12, 3003. [Google Scholar] [CrossRef]
- Butenegro, J.A.; Bahrami, M.; Abenojar, J.; Martínez, M. Recent Progress in Carbon Fiber Reinforced Polymers Recycling: A Review of Recycling Methods and Reuse of Carbon Fibers. Materials 2021, 14, 6401. [Google Scholar] [CrossRef]
- Unterweger, C.; Mayrhofer, T.; Piana, F.; Duchoslav, J.; Stifter, D.; Poitzsch, C.; Fürst, C. Impact of fiber length and fiber content on the mechanical properties and electrical conductivity of short carbon fiber reinforced polypropylene composites. Compos. Sci. Technol. 2020, 188, 107998. [Google Scholar] [CrossRef]
- Sawada, T.; Kusaka, T. Strength predictions by applied effective volume theory in short glass-fibre-reinforced plastics. Polym. Test. 2017, 62, 143–153. [Google Scholar] [CrossRef]
- Khanam, P.N.; AlMaadeed, M.A. Improvement of ternary recycled polymer blend reinforced with date palm fibre. Mater. Des. 2014, 60, 532–539. [Google Scholar] [CrossRef]
- Zadeh, K.M.; Ponnamma, D.; Al-Maadeed, M.A. Date palm fibre filled recycled ternary polymer blend composites with enhanced flame retardancy. Polym. Test. 2017, 61, 341–348. [Google Scholar] [CrossRef]
- Taşdemır, M.; Koçak, D.; Usta, I.; Akalin, M.; Merdan, N. Properties of Recycled Polycarbonate/Waste Silk and Cotton Fiber Polymer Composites. Int. J. Polym. Mater. 2008, 57, 797–805. [Google Scholar] [CrossRef]
- Rodríguez-Castellanos, W.; Flores-Ruiz, F.J.; Martínez-Bustos, F.; Chiñas-Castillo, F.; Espinoza-Beltrán, F.J. Nanomechanical properties and thermal stability of recycled cellulose reinforced starch-gelatin polymer composite. J. Appl. Polym. Sci. 2015, 132, 41787. [Google Scholar] [CrossRef]
- Dib, A.; Fantuzzi, N.; Agnelli, J.; Paleari, L.; Bragaglia, M.; Nanni, F.; Pierattini, A. Parametric characterization of recycled polymers with nanofillers. Polym. Compos. 2023. [Google Scholar] [CrossRef]
- Zare, Y. Recent progress on preparation and properties of nanocomposites from recycled polymers: A review. Waste Manag. 2013, 33, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Zare, Y.; Daraei, A.; Vatani, M.; Aghasafari, P. An analysis of interfacial adhesion in nanocomposites from recycled polymers. Comput. Mater. Sci. 2014, 81, 612–616. [Google Scholar] [CrossRef]
- Zdiri, K.; Elamri, A.; Hamdaoui, M.; Harzallah, O.; Khenoussi, N.; Brendlé, J. Reinforcement of recycled PP polymers by nanoparticles incorporation. Green Chem. Lett. Rev. 2018, 11, 296–311. [Google Scholar] [CrossRef]
- Djoumaliisky, S.; Zipper, P. Modification of recycled polymer blends with activated natural zeolite. Macromol. Symp. 2004, 217, 391–400. [Google Scholar] [CrossRef]
- Hong, B.; Byun, H. Preparation of Polymer-modified Mortars with Recycled PET and Their Sound Absorption Characteristics. Polymer 2010, 34, 410–414. [Google Scholar]
- Singh, N.; Singh, R.; Ahuja, I.P.S. Recycling of polymer waste with SiC/Al2O3 reinforcement for rapid tooling applications. Mater. Today Commun. 2018, 15, 124–127. [Google Scholar] [CrossRef]
- Singh, R.; Singh, N.; Fabbrocino, F.; Fraternali, F.; Ahuja, I. Waste management by recycling of polymers with reinforcement of metal powder. Compos. Part B-Eng. 2016, 105, 23–29. [Google Scholar] [CrossRef]
- Keskisaari, A.; Kärki, T.; Vuorinen, T. Mechanical Properties of Recycled Polymer Composites Made from Side-Stream Materials from Different Industries. Sustainability 2019, 11, 6054. [Google Scholar] [CrossRef]
- Toncheva, A.; Brison, L.; Dubois, P.; Laoutid, F. Recycled Tire Rubber in Additive Manufacturing: Selective Laser Sintering for Polymer-Ground Rubber Composites. Appl. Sci. 2021, 11, 8778. [Google Scholar] [CrossRef]
- Tong, D.J.Y.; Koay, S.C.; Chan, M.Y.; Tshai, K.Y.; Ong, T.K.; Buys, Y.F. Conductive Polymer Composites Made from Polypropylene and Recycled Graphite Scrap. J. Phys. Sci. 2021, 32, 31–44. [Google Scholar] [CrossRef]
- Watanabe, T.; Minato, H.; Sasaki, Y.; Hiroshige, S.; Suzuki, H.; Matsuki, N.; Sano, K.; Wakiya, T.; Nishizawa, Y.; Uchihashi, T.; et al. Closed-loop recycling of microparticle-based polymers. Green Chem. 2023, 25, 3418–3424. [Google Scholar] [CrossRef]
- Hugo, A.-M.; Scelsi, L.; Hodzic, A.; Jones, F.R.; Dwyer-Joyce, R. Development of recycled polymer composites for structural applications. Plast. Rubber Compos. 2011, 40, 317–323. [Google Scholar] [CrossRef]
- Grillo, C.C.; Saron, C. Accelerated aging of polymer composite from Pennisetum purpureum fibers with recycled low-density polyethylene. J. Compos. Mater. 2023, 57, 3231–3242. [Google Scholar] [CrossRef]
- Paraye, P.; Sarviya, R.M. Advances in polymer composites, manufacturing, recycling, and sustainable practices. Polym.-Plast. Technol. Mater. 2024, 63, 1474–1497. [Google Scholar] [CrossRef]
- Vieira, D.R.; Vieira, R.K.; Chain, M.C. Strategy and management for the recycling of carbon fiber-reinforced polymers (CFRPs) in the aircraft industry: A critical review. Int. J. Sustain. Dev. World Ecol. 2017, 24, 214–223. [Google Scholar] [CrossRef]
- Scaffaro, R.; Di Bartolo, A.; Dintcheva, N.T. Matrix and Filler Recycling of Carbon and Glass Fiber-Reinforced Polymer Composites: A Review. Polymers 2021, 13, 3817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jiang, X.; Wang, S.; Li, Z.; Guo, Q.; Li, Y.; Jiang, J. Efficient and sustainable strategy for recycling of carbon fiber-reinforced thermoset composite waste at ambient pressure. Polymer 2024, 297, 126838. [Google Scholar] [CrossRef]
- Zhao, X.; Copenhaver, K.; Wang, L.; Korey, M.; Gardner, D.J.; Li, K.; Lamm, M.E.; Kishore, V.; Bhagia, S.; Tajvidi, M.; et al. Recycling of natural fiber composites: Challenges and opportunities. Resour. Conserv. Recycl. 2022, 177, 105962. [Google Scholar] [CrossRef]
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.-H.; Xing, F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method. Compos. Part A-Appl. Sci. Manuf. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Feng, H.; Xu, X.; Wang, B.; Su, Y.; Liu, Y.; Zhang, C.; Zhu, J.; Ma, S. Facile preparation, closed-loop recycling of multifunctional carbon fiber reinforced polymer composites. Compos. Part B-Eng. 2023, 257, 110677. [Google Scholar] [CrossRef]
- Gao, C.; Huang, L.; Yan, L.; Kasal, B.; Li, W.; Jin, R.; Wang, Y.; Li, Y.; Deng, P. Compressive performance of fiber reinforced polymer encased recycled concrete with nanoparticles. J. Mater. Res. Technol. 2021, 14, 2727–2738. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; Deng, Y.; Ye, F.; Zhang, C.; Hu, X.; Liu, Y.; Zhang, D. Recycling and Reutilizing Polymer Waste via Electrospun Micro/Nanofibers: A Review. Nanomaterials 2022, 12, 1663. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, G.S. Single Polymer Composites by Partially Melting Recycled Polyamide 6 Fibers: Preparation and Characterization. J. Appl. Polym. Sci. 2010, 118, 3357–3363. [Google Scholar] [CrossRef]
- Gregor-Svetec, D.; Tisler-Korljan, B.; Leskovsek, M.; Sluga, F. Monofilaments produced by blending virgin with recycled polypropylene. Tekst. Ve Konfeksiyon 2009, 19, 181–188. [Google Scholar]
- Jurumenha, M.A.G.; Reis, J.M.L.D. Fracture Mechanics of Polymer Mortar Made with Recycled Raw Materials. Mater. Res.-Ibero-Am. J. Mater. 2010, 13, 475–478. [Google Scholar] [CrossRef]
- Castro, A.M.; Ribeiro, M.; Santos, J.; Meixedo, J.; Silva, F.; Fiúza, A.; Dinis, M.; Alvim, M. Sustainable waste recycling solution for the glass fibre reinforced polymer composite materials industry. Constr. Build. Mater. 2013, 45, 87–94. [Google Scholar] [CrossRef]
- Clark, E.; Bleszynski, M.; Valdez, F.; Kumosa, M. Recycling carbon and glass fiber polymer matrix composite waste into cementitious materials. Resour. Conserv. Recycl. 2020, 155, 104659. [Google Scholar] [CrossRef]
- Dehghan, A.; Peterson, K.; Shvarzman, A. Recycled glass fiber reinforced polymer additions to Portland cement concrete. Constr. Build. Mater. 2017, 146, 238–250. [Google Scholar] [CrossRef]
- Dertinger, S.C.; Gallup, N.; Tanikella, N.G.; Grasso, M.; Vahid, S.; Foot, P.J.S.; Pearce, J.M. Technical pathways for distributed recycling of polymer composites for distributed manufacturing: Windshield wiper blades. Resour. Conserv. Recycl. 2020, 157, 104810. [Google Scholar] [CrossRef]
- Cruz Sanchez, F.A.; Boudaoud, H.; Hoppe, S.; Camargo, M. Polymer recycling in an open-source additive manufacturing context: Mechanical issues. Addit. Manuf. 2017, 17, 87–105. [Google Scholar] [CrossRef]
- Yousaf, A.; Al Rashid, A.; Polat, R.; Koç, M. Potential and challenges of recycled polymer plastics and natural waste materials for additive manufacturing. Sustain. Mater. Technol. 2024, 41, e01103. [Google Scholar] [CrossRef]
- Fico, D.; Rizzo, D.; De Carolis, V.; Montagna, F.; Corcione, C.E. Sustainable Polymer Composites Manufacturing through 3D Printing Technologies by Using Recycled Polymer and Filler. Polymers 2022, 14, 3756. [Google Scholar] [CrossRef]
- Habiba, R.D.; Malça, C.; Branco, R. Exploring the Potential of Recycled Polymers for 3D Printing Applications: A Review. Materials 2024, 17, 2915. [Google Scholar] [CrossRef] [PubMed]
- Maraveas, C.; Kyrtopoulos, I.V.; Arvanitis, K.G. Evaluation of the Viability of 3D Printing in Recycling Polymers. Polymers 2024, 16, 1104. [Google Scholar] [CrossRef]
- Pernica, J.; Vodák, M.; Šarocký, R.; Šustr, M.; Dostál, P.; Černý, M.; Dobrocký, D. Mechanical Properties of Recycled Polymer Materials in Additive Manufacturing. Manuf. Technol. 2022, 22, 200–203. [Google Scholar] [CrossRef]
- Pinho, A.C.; Amaro, A.M.; Piedade, A.P. 3D printing goes greener: Study of the properties of post-consumer recycled polymers for the manufacturing of engineering components. Waste Manag. 2020, 118, 426–434. [Google Scholar] [CrossRef]
- Flores, J.D.S.; Augusto, T.d.A.; Cunha, D.A.L.V.; Beatrice, C.A.G.; Backes, E.H.; Costa, L.C. Sustainable polymer reclamation: Recycling poly(ethylene terephthalate) glycol (PETG) for 3D printing applications. J. Mater. Sci.-Mater. Eng. 2024, 19, 16. [Google Scholar] [CrossRef]
- Olawumi, M.A.; Oladapo, B.I.; Olugbade, T.O. Evaluating the impact of recycling on polymer of 3D printing for energy and material sustainability. Resour. Conserv. Recycl. 2024, 209, 107769. [Google Scholar] [CrossRef]
- Mantelli, A.; Romani, A.; Suriano, R.; Diani, M.; Colledani, M.; Sarlin, E.; Turri, S.; Levi, M. UV-Assisted 3D Printing of Polymer Composites from Thermally and Mechanically Recycled Carbon Fibers. Polymers 2021, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Alsewailem, F.D.; Aljlil, S.A. Recycled polymer/clay composites for heavy-metals adsorption. Mater. Tehnol. 2013, 47, 525–529. [Google Scholar]
- Hunt, E.J.; Zhang, C.; Anzalone, N.; Pearce, J.M. Polymer recycling codes for distributed manufacturing with 3-D printers. Resour. Conserv. Recycl. 2015, 97, 24–30. [Google Scholar] [CrossRef]
- Achilias, D.S.; Megalokonomos, P.; Karayannidis, G.P. Current trends in chemical recycling of polyolefins. J. Environ. Prot. Ecol. 2006, 7, 407–413. [Google Scholar]
- Datta, J.; Kopczynska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Achilias, D.S. Polymer packaging waste recycling: Study of the pyrolysis of two blends via TGA. J. Therm. Anal. Calorim. 2020, 142, 1891–1895. [Google Scholar] [CrossRef]
- Jiang, H.B.; Zhang, X.H.; Liu, W.L.; Wang, S.H.; Zhang, L.D.; Jiang, C.; Qiao, J.L. Advances in Microwave-Assisted Polymer Recycling. Acta Polym. Sin. 2022, 53, 1032–1040. [Google Scholar]
- Vichare, P.; Engler, A.; Schwartz, J.; Kohl, P.A. Chemical recycling of polymer composites induced by selective variable frequency microwave heating. J. Appl. Polym. Sci. 2024, 141, e54811. [Google Scholar] [CrossRef]
- Adam, M.; Hjalmarsson, N.; Lee, C.S.; Irvine, D.J.; Robinson, J.; Binner, E. Understanding microwave interactions with polymers to enable advanced plastic chemical recycling. Polym. Test. 2024, 137, 108483. [Google Scholar] [CrossRef]
- Ali, S.; Garforth, A.A.; Harris, D.H.; Rawlence, D.J.; Uemichi, Y. Polymer waste recycling over “used” catalysts. Catal. Today 2002, 75, 247–255. [Google Scholar] [CrossRef]
- Chanda, M. Chemical aspects of polymer recycling. Adv. Ind. Eng. Polym. Res. 2021, 4, 133–150. [Google Scholar] [CrossRef]
- Gobin, K.; Manos, G. Polymer degradation to fuels over microporous catalysts as a novel tertiary plastic recycling method. Polym. Degrad. Stab. 2004, 83, 267–279. [Google Scholar] [CrossRef]
- Braido, R.S.; Borges, L.E.P.; Pinto, J.C. Chemical recycling of crosslinked poly(methyl methacrylate) and characterization of polymers produced with the recycled monomer. J. Anal. Appl. Pyrolysis 2018, 132, 47–55. [Google Scholar] [CrossRef]
- Kaminsky, W.; Predel, M.; Sadiki, A. Feedstock recycling of polymers by pyrolysis in a fluidised bed. Polym. Degrad. Stab. 2004, 85, 1045–1050. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Ma, Y.W. Chemical Recycling of Step-Growth Polymers Guided by Le Chatelier’s Principle. ACS Eng. Au 2024, 4, 432–449. [Google Scholar] [CrossRef]
- Tsuneizumi, Y.; Kuwahara, M.; Okamoto, K.; Matsumura, S. Chemical recycling of poly(lactic acid)-based polymer blends using environmentally benign catalysts. Polym. Degrad. Stab. 2010, 95, 1387–1393. [Google Scholar] [CrossRef]
- Ichiura, H.; Nakaoka, H.; Konishi, T. Recycling disposable diaper waste pulp after dehydrating the superabsorbent polymer through oxidation using ozone. J. Clean. Prod. 2020, 276, 123350. [Google Scholar] [CrossRef]
- Utekar, S.; Suriya, V.K.; More, N.; Rao, A. Comprehensive study of recycling of thermosetting polymer composites—Driving force, challenges and methods. Compos. Part B-Eng. 2021, 207, 108596. [Google Scholar] [CrossRef]
- Sachin, E.K.; Muthu, N.; Tiwari, P. Hybrid recycling methods for fiber reinforced polymer composites: A review. J. Reinf. Plast. Compos. 2024. [Google Scholar] [CrossRef]
- Shi, J.; Bao, L.M.; Kobayashi, R.; Kato, J.; Kemmochi, K. Reusing recycled fibers in high-value fiber-reinforced polymer composites: Improving bending strength by surface cleaning. Compos. Sci. Technol. 2012, 72, 1298–1303. [Google Scholar] [CrossRef]
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W.M.; Aymonier, C.; Horn, S. Semi-continuous flow recycling method for carbon fibre reinforced thermoset polymers by near- and supercritical solvolysis. Polym. Degrad. Stab. 2016, 133, 264–274. [Google Scholar] [CrossRef]
- Huang, H.H.; Liu, W.H.; Liu, Z.F. An additive manufacturing-based approach for carbon fiber reinforced polymer recycling. Cirp Ann.-Manuf. Technol. 2020, 69, 33–36. [Google Scholar] [CrossRef]
- Shetty, S.; Pinkard, B.R.; Novosselov, I.V. Recycling of carbon fiber reinforced polymers in a subcritical acetic acid solution. Heliyon 2022, 8, e12242. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Kim, D.-K.; Han, W.; Kim, B.-J. Comparison of the Characteristics of Recycled Carbon Fibers/Polymer Composites by Different Recycling Techniques. Molecules 2022, 27, 5663. [Google Scholar] [CrossRef]
- Torkaman, N.F.; Bremser, W.; Wilhelm, R. Catalytic Recycling of Thermoset Carbon Fiber-Reinforced Polymers. Acs Sustain. Chem. Eng. 2024, 12, 7668–7682. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Kusenberg, M.; Shirazi, H.M.; Goshayeshi, B.; Van Geem, K.M. Towards full recyclability of end-of-life tires: Challenges and opportunities. J. Clean. Prod. 2022, 374, 134036. [Google Scholar] [CrossRef]
- Accetta, A.; Vergnaud, J.M. Rubber recycling—upgrading of scrap rubber powder by vulcanization. II. Rubber Chem. Technol. 1982, 55, 961–966. [Google Scholar] [CrossRef]
- Adesina, A. Overview of the influence of waste materials on the thermal conductivity of cementitious composites. Clean. Eng. Technol. 2021, 2, 100046. [Google Scholar] [CrossRef]
- Adhikari, B.; De, D.; Maiti, S. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Afash, H.; Ozarisoy, B.; Altan, H.; Budayan, C. Recycling of Tire Waste Using Pyrolysis: An Environmental Perspective. Sustainability 2023, 15, 14178. [Google Scholar] [CrossRef]
- Akhtar, A.Y.; Tsang, H.H. Dynamic leaching assessment of recycled polyurethane-coated tire rubber for sustainable engineering applications. Chem. Eng. J. 2024, 495, 153351. [Google Scholar] [CrossRef]
- Azunna, S.U.; Aziz, F.N.; Rashid, R.S.; Bakar, N.B. Review on the characteristic properties of crumb rubber concrete. Clean. Mater. 2024, 12, 100237. [Google Scholar] [CrossRef]
- Chittella, H.; Yoon, L.W.; Ramarad, S.; Lai, Z.-W. Rubber waste management: A review on methods, mechanism, and prospects. Polym. Degrad. Stab. 2021, 194, 109761. [Google Scholar] [CrossRef]
- Bu, C.; Zhu, D.; Lu, X.; Liu, L.; Sun, Y.; Yu, L.; Xiao, T.; Zhang, W. Modification of Rubberized Concrete: A Review. Buildings 2022, 12, 999. [Google Scholar] [CrossRef]
- Bilema, M.; Yuen, C.W.; Alharthai, M.; Al-Saffar, Z.H.; Al-Sabaeei, A.; Yusoff, N.I.M. A Review of Rubberised Asphalt for Flexible Pavement Applications: Production, Content, Performance, Motivations and Future Directions. Sustainability 2023, 15, 14481. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste Rubber Recycling: A Review on the Evolution and Properties of Thermoplastic Elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Grammelis, P.; Margaritis, N.; Dallas, P.; Rakopoulos, D.; Mavrias, G. A Review on Management of End of Life Tires (ELTs) and Alternative Uses of Textile Fibers. Energies 2021, 14, 571. [Google Scholar] [CrossRef]
- Han, W.W.; Han, D.S.; Chen, H.B. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef]
- Kazemi, M.; Zarmehr, S.P.; Yazdani, H.; Fini, E. Review and Perspectives of End-of-Life Tires Applications for Fuel and Products. Energy Fuels 2023, 37, 10758–10774. [Google Scholar] [CrossRef]
- Li, Y.; Chai, J.; Wang, R.; Zhou, Y.; Tong, X. A Review of the Durability-Related Features of Waste Tyre Rubber as a Partial Substitute for Natural Aggregate in Concrete. Buildings 2022, 12, 1975. [Google Scholar] [CrossRef]
- Milad, A.; Ali, A.S.B.; Yusoff, N.I.M. A Review of the Utilisation of Recycled Waste Material as an Alternative Modifier in Asphalt Mixtures. Civ. Eng. J. 2020, 6, 42–60. [Google Scholar] [CrossRef]
- Milad, A.; Ahmeda, A.G.F.; Taib, A.M.; Rahmad, S.; Solla, M.; Yusoff, N.I.M. A review of the feasibility of using crumb rubber derived from end-of-life tire as asphalt binder modifier. J. Rubber Res. 2020, 23, 203–216. [Google Scholar] [CrossRef]
- Ramarad, S.; Khalid, M.; Ratnam, C.; Chuah, A.L.; Rashmi, W. Waste tire rubber in polymer blends: A review on the evolution, properties and future. Prog. Mater. Sci. 2015, 72, 100–140. [Google Scholar] [CrossRef]
- Movahed, S.O.; Ansarifar, A.; Estagy, S. Review of the reclaiming of rubber waste and recent work on the recycling of ethylene-propylene-diene rubber waste. Rubber Chem. Technol. 2016, 89, 54–78. [Google Scholar] [CrossRef]
- Murtland, W.O. Reviews of recent rubber recycling literature, worldwide. Elastomerics 1983, 115, 13–36. [Google Scholar]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A Review on the Potential and Limitations of Recyclable Thermosets for Structural Applications. Polym. Rev. 2020, 60, 359–388. [Google Scholar] [CrossRef]
- Ren, F.; Mo, J.; Wang, Q.; Ho, J.C.M. Crumb rubber as partial replacement for fine aggregate in concrete: An overview. Constr. Build. Mater. 2022, 343, 128049. [Google Scholar] [CrossRef]
- Rowhani, A.; Rainey, T.J. Scrap Tyre Management Pathways and Their Use as a Fuel-A Review. Energies 2016, 9, 888. [Google Scholar] [CrossRef]
- Valentini, F.; Pegoretti, A. End-of-life options of tyres. A review. Adv. Ind. Eng. Polym. Res. 2022, 5, 203–213. [Google Scholar] [CrossRef]
- Ramos, G.; Alguacil, F.J.; López, F.A. The recycling of end-of-life tyres. Technol. Rev. Rev. Metal. 2011, 47, 273–284. [Google Scholar]
- Rashid, M.A.; Liu, W.; Wei, Y.; Jiang, Q. Review of intrinsically recyclable biobased epoxy thermosets enabled by dynamic chemical bonds. Polym.-Plast. Technol. Mater. 2022, 61, 1740–1782. [Google Scholar] [CrossRef]
- Roychand, R.; Gravina, R.J.; Zhuge, Y.; Ma, X.; Youssf, O.; Mills, J.E. A comprehensive review on the mechanical properties of waste tire rubber concrete. Constr. Build. Mater. 2020, 237, 117651. [Google Scholar] [CrossRef]
- Surehali, S.; Singh, A.; Biligiri, K.P. A state-of-the-art review on recycling rubber in concrete: Sustainability aspects, specialty mixtures, and treatment methods. Dev. Built Environ. 2023, 14, 100171. [Google Scholar] [CrossRef]
- Torretta, V.; Rada, E.C.; Ragazzi, M.; Trulli, E.; Istrate, I.A.; Cioca, L.I. Treatment and disposal of tyres: Two EU approaches. A review. Waste Manag. 2015, 45, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, S.-Y.; Zhang, Z.-Y.; Wang, Q.-Z.; Xiao, C.-Z. A technology review of recycling methods for fiber-reinforced thermosets. J. Reinf. Plast. Compos. 2022, 41, 459–480. [Google Scholar] [CrossRef]
- Chen, G.; Zhuo, K.-X.; Luo, R.-H.; Lai, H.-M.; Cai, Y.-J.; Xie, B.-X.; Lin, J.-X. Fracture behavior of environmentally friendly high-strength concrete using recycled rubber powder and steel fibers: Experiment and modeling. Case Stud. Constr. Mater. 2024, 21, e03501. [Google Scholar] [CrossRef]
- Abbassi, F.; Ahmad, F. Behavior analysis of concrete with recycled tire rubber as aggregate using 3D-digital image correlation. J. Clean. Prod. 2020, 274, 123074. [Google Scholar] [CrossRef]
- Abdelaleem, A.; Moawad, M.; El-Emam, H.; Salim, H.; Sallam, H. Long term behavior of rubberized concrete under static and dynamic loads. Case Stud. Constr. Mater. 2024, 20, e03087. [Google Scholar] [CrossRef]
- Abdelmonem, A.; El-Feky, M.; Nasr, E.-S.A.; Kohail, M. Performance of high strength concrete containing recycled rubber. Constr. Build. Mater. 2019, 227, 116660. [Google Scholar] [CrossRef]
- Abou-Chakra, A.; Blanc, G.; Turatsinze, A.; Escadeillas, G. Prediction of diffusion properties of cement-based materials incorporating recycled rubber aggregates: Effect of microstructure on macro diffusion properties. Mater. Today Commun. 2023, 35, 105750. [Google Scholar] [CrossRef]
- Aghamohammadi, O.; Mostofinejad, D.; Mostafaei, H.; Abtahi, S.M. Mechanical Properties and Impact Resistance of Concrete Pavement Containing Crumb Rubber. Int. J. Geomech. 2024, 24, 04023242. [Google Scholar] [CrossRef]
- Agrawal, D.; Waghe, U.; Ansari, K.; Amran, M.; Gamil, Y.; Alluqmani, A.E.; Thakare, N. Optimization of eco-friendly concrete with recycled coarse aggregates and rubber particles as sustainable industrial byproducts for construction practices. Heliyon 2024, 10, e25923. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.I.; Tobbala, D.E. Rubbered light concrete containing recycled PET fiber compared to macro-polypropylene fiber in terms of SEM, mechanical, thermal conductivity and electrochemical resistance. Constr. Build. Mater. 2024, 415, 135010. [Google Scholar] [CrossRef]
- Albidah, A.S.; Alsaif, A.S. Flexural Response of Functionally Graded Rubberized Concrete Beams. Materials 2024, 17, 1931. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, D.L.; Georgescu, L.P.; Carp, G.B.; Ghisman, V. Advanced Recycling of Modified EDPM Rubber in Bituminous Asphalt Paving. Buildings 2024, 14, 1618. [Google Scholar] [CrossRef]
- Abdalla, A.; Faheem, A.F.; Walters, E. Life cycle assessment of eco-friendly asphalt pavement involving multi-recycled materials: A comparative study. J. Clean. Prod. 2022, 362, 132471. [Google Scholar] [CrossRef]
- Abualia, A.; Akentuna, M.; Mohammad, L.N.; Cooper, S.B.; Cooper, S.B. Improving Asphalt Binder Durability Using Sustainable Materials: A Rheological and Chemical Analysis of Polymer-, Rubber-, and Epoxy-Modified Asphalt Binders. Sustainability 2024, 16, 5379. [Google Scholar] [CrossRef]
- Aldagari, S.; Karam, J.; Kazemi, M.; Kaloush, K.; Fini, E.H. Comparing the critical aging point of rubber-modified bitumen and plastic-modified bitumen. J. Clean. Prod. 2024, 437, 140540. [Google Scholar] [CrossRef]
- Apaza, F.R.; Vázquez, V.F.; Paje, S.E.; Gulisano, F.; Gagliardi, V.; Rodríguez, L.S.; Medina, J.G. Towards Sustainable Road Pavements: Sound Absorption in Rubber-Modified Asphalt Mixtures. Infrastructures 2024, 9, 65. [Google Scholar] [CrossRef]
- Borinelli, J.B.; Blom, J.; Vuye, C.; Hernando, D. Does the wet addition of crumb rubber and emission reduction agents impair the rheological performance of bitumen? Constr. Build. Mater. 2024, 417, 135351. [Google Scholar] [CrossRef]
- Bazoobandi, P.; Mousavi, S.R.; Karimi, F.; Karimi, H.R.; Ghasri, M.; Aliha, M. Cracking resistance of crumb rubber modified green asphalt mixtures, using calcium carbonate nanoparticles and two by-product wax-based warm mix additives. Constr. Build. Mater. 2024, 424, 135848. [Google Scholar] [CrossRef]
- Abdelkader, M.M.; Abou-Laila, M.T.; El-Deeb, M.S.S.; Taha, E.O.; El-Deeb, A.S. Structural, radiation shielding, thermal and dynamic mechanical analysis for waste rubber/EPDM rubber composite loaded with Fe2O3 for green environment. Sci. Rep. 2024, 14, 12440. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, J.I.; Ali, A.H.; Kara, I.H.; Mahan, H.M.; Konovalov, S.V.; Al-Nedawi, N.M. Preparing eco-friendly composite from end-life tires and epoxy resin and examining its mechanical, and acoustic insulation properties. Int. J. Nanoelectron. Mater. 2024, 17, 1–6. [Google Scholar] [CrossRef]
- Abdullah, Z.T. Waste tire remanufacturing: Quantitative-aided qualitative sustainability analysis. Environ. Qual. Manag. 2023, 33, 285–296. [Google Scholar] [CrossRef]
- Abdullah, Z.T. Remanufactured waste tire by-product valorization: Quantitative-qualitative sustainability-based assessment. Results Eng. 2024, 22, 102229. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Abdulkareem, S.A.; Amoloye, M.A.; Emenike, E.C.; Ezzat, A.O.; Iwuozor, K.O.; Al-Lohedan, H.A.; Aransiola, F.T.; Oyekunle, I.P. Balancing strength and sustainability: Incorporating recycled tyres and pawpaw fibre in polystyrene composites. Asia-Pac. J. Chem. Eng. 2024, 19, e3037. [Google Scholar] [CrossRef]
- Allouch, M.; Kamoun, M.; Mars, J.; Wali, M.; Dammak, F. Mechanical behaviour of composite materials including waste rubber chips: Experimental and numerical investigations. Adv. Mater. Process. Technol. 2024, 10, 3804–3824. [Google Scholar] [CrossRef]
- Abdel-Rahman, H.A.; Younes, M.M.; Khattab, M.M. Recycling of polyurethane foam waste in the production of lightweight cement pastes and its irradiated polymer impregnated composites. J. Vinyl Addit. Technol. 2019, 25, 328–338. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.E.S.; Abdel-Raheim, A.R.M.; El-Saeed, S.M. Thermo-Catalytic Versus Thermo-Chemical Recycling of Polystyrene Waste. Waste Biomass Valorization 2013, 4, 37–46. [Google Scholar] [CrossRef]
- Ahmad; Ahmad, N.; Ahmed, U.; Jameel, A.G.A.; Amjad, U.E.S.; Hussain, M.; Arif, M.M. Production of fuel oil from elastomer rubber waste via methanothermal liquefaction. Fuel 2023, 338, 127330. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Alajmi, A.F.; Al-Salem, S.M. Investigation of Chemical, Physical, and Tribological Properties of Pyrolysis Oil Derived from End-of-Life Tires (ELTs) against Conventional Engine Oil. Lubricants 2024, 12, 188. [Google Scholar] [CrossRef]
- Abdulfattah, O.; Alsurakji, I.H.; El-Qanni, A.; Samaaneh, M.; Najjar, M.; Abdallah, R.; Assaf, I. Experimental evaluation of using pyrolyzed carbon black derived from waste tires as additive towards sustainable concrete. Case Stud. Constr. Mater. 2022, 16, e00938. [Google Scholar] [CrossRef]
- Abdulrahman, A.S.; Jabrail, F.H. Treatment of Scrap Tire for Rubber and Carbon Black Recovery. Recycling 2022, 7, 27. [Google Scholar] [CrossRef]
- Billotte, C.; Romana, L.; Flory, A.; Kaliaguine, S.; Ruiz, E. Recycled from waste tires carbon black/high-density polyethylene composite: Multi-scale mechanical properties and polymer aging. Polym. Compos. 2024, 45, 11605–11618. [Google Scholar] [CrossRef]
- Nishida, H. Development of materials and technologies for control of polymer recycling. Polym. J. 2011, 43, 435–447. [Google Scholar] [CrossRef]
- Zhang, H.C.; Cui, J.J.; Hu, G.; Zhang, B.A. Recycling strategies for vitrimers. Int. J. Smart Nano Mater. 2022, 13, 367–390. [Google Scholar] [CrossRef]
- Liguori, A.; Hakkarainen, M. Designed from Biobased Materials for Recycling: Imine-Based Covalent Adaptable Networks. Macromol. Rapid Commun. 2022, 43, e2100816. [Google Scholar] [CrossRef]
- Guo, J.; Picchioni, F.; Bose, R.K. Electrically and thermally healable nanocomposites via one-step Diels-Alder reaction on carbon nanotubes. Polymer 2023, 283, 126260. [Google Scholar] [CrossRef]
- Orozco, F.; Li, J.; Ezekiel, U.; Niyazov, Z.; Floyd, L.; Lima, G.M.; Winkelman, J.G.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Diels-Alder-based thermo-reversibly crosslinked polymers: Interplay of crosslinking density, network mobility, kinetics and stereoisomerism. Eur. Polym. J. 2020, 135, 109882. [Google Scholar] [CrossRef]
- Iqbal, M.; Knigge, R.A.; Heeres, H.J.; Broekhuis, A.A.; Picchioni, F. Diels(-)Alder-Crosslinked Polymers Derived from Jatropha Oil. Polymers 2018, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Yuliati, F.; Hong, J.; Indriadi, K.S.; Picchioni, F.; Bose, R.K. Thermally Reversible Polymeric Networks from Vegetable Oils. Polymers 2020, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Polgar, L.; Kingma, A.; Roelfs, M.; van Essen, M.; van Duin, M.; Picchioni, F. Kinetics of cross-linking and de-cross-linking of EPM rubber with thermoreversible Diels-Alder chemistry. Eur. Polym. J. 2017, 90, 150–161. [Google Scholar] [CrossRef]
- Polgar, L.M.; van Duin, M.; Broekhuis, A.A.; Picchioni, F. Use of Diels–Alder Chemistry for Thermoreversible Cross-Linking of Rubbers: The Next Step toward Recycling of Rubber Products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- van den Tempel, P.; Picchioni, F.; Bose, R.K. Designing End-of-Life Recyclable Polymers via Diels-Alder Chemistry: A Review on the Kinetics of Reversible Reactions. Macromol Rapid Commun 2022, 43, e2200023. [Google Scholar] [CrossRef] [PubMed]
- Flory, P. Molecular Size Distribution in Three Dimensional Polymers. J. Am. Chem. Soc. 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Stockmayer, W.H. Theory of Molecular Size Distribution and Gel Formation in Branched-Chain Polymers. J. Chem. Phys. 1943, 11, 45–55. [Google Scholar] [CrossRef]
- Cuvellier, A.; Verhelle, R.; Brancart, J.; Vanderborght, B.; Van Assche, G.; Rahier, H. The influence of stereochemistry on the reactivity of the Diels–Alder cycloaddition and the implications for reversible network polymerization. Polym. Chem. 2019, 10, 473–485. [Google Scholar] [CrossRef]
- Terryn, S.; Brancart, J.; Roels, E.; Verhelle, R.; Safaei, A.; Cuvellier, A.; Vanderborght, B.; Van Assche, G. Structure–Property Relationships of Self-Healing Polymer Networks Based on Reversible Diels–Alder Chemistry. Macromolecules 2022, 55, 5497–5513. [Google Scholar] [CrossRef]
- Safaei, A.; Terryn, S.; Vanderborght, B.; Van Assche, G.; Brancart, J. The Influence of the Furan and Maleimide Stoichiometry on the Thermoreversible Diels-Alder Network Polymerization. Polymers 2021, 13, 2522. [Google Scholar] [CrossRef] [PubMed]
- Tempel, P.v.D.; Wang, Y.; Duong, T.M.; Winkelman, J.G.; Picchioni, F.; Giuntoli, A.; Bose, R.K. Looping and gelation kinetics in reversible networks based on furan and maleimide. Mater. Today Chem. 2024, 42, 102361. [Google Scholar] [CrossRef]
- McReynolds, B.T.; Mojtabai, K.D.; Penners, N.; Kim, G.; Lindholm, S.; Lee, Y.; McCoy, J.D.; Chowdhury, S. Understanding the Effect of Side Reactions on the Recyclability of Furan-Maleimide Resins Based on Thermoreversible Diels-Alder Network. Polymers 2023, 15, 1106. [Google Scholar] [CrossRef]
- Orozco; Niyazov, Z.; Garnier, T.; Migliore, N.; Zdvizhkov, A.T.; Raffa, P.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Maleimide Self-Reaction in Furan/Maleimide-Based Reversibly Crosslinked Polyketones: Processing Limitation or Potential Advantage? Molecules 2021, 26, 2230. [Google Scholar] [CrossRef]
- Tempel, P.v.D.; van der Boon, E.O.; Winkelman, J.G.; Krasnikova, A.V.; Parisi, D.; Deuss, P.J.; Picchioni, F.; Bose, R.K. Beyond Diels-Alder: Domino reactions in furan-maleimide click networks. Polymer 2023, 274, 125884. [Google Scholar] [CrossRef]
- Beljaars, M.; Heeres, H.J.; Broekhuis, A.A.; Picchioni, F. Bio-Based Aromatic Polyesters Reversibly Crosslinked via the Diels–Alder Reaction. Appl. Sci. 2022, 12, 2461. [Google Scholar] [CrossRef]
- Briou, B.; Ameduri, B.; Boutevin, B. Trends in the Diels-Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Tesoro, G.C.; Sastri, V.R. Synthesis of Siloxane-Containing Bis(furans) and Polymerization with Bis(maleimides). Ind. Eng. Chem. Prod. Res. Dev. 1986, 25, 444–448. [Google Scholar] [CrossRef]
- Mackinnon, D.J.; Drain, B.; Becer, C.R. Exploring Polymeric Diene-Dienophile Pairs for Thermoreversible Diels-Alder Reactions. Macromolecules 2024, 57, 6024–6034. [Google Scholar] [CrossRef]
- Raut, S.K.; Sarkar, S.; Mondal, P.; Meldrum, A.; Singha, N.K. Covalent adaptable network in an anthracenyl functionalised non-olefinic elastomer; a new class of self-healing elastomer coupled with fluorescence switching. Chem. Eng. J. 2023, 453, 139641. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, S.-R.; Lee, D.-S. Self-healing of cross-linked PU via dual-dynamic covalent bonds of a Schiff base from cystine and vanillin. Mater. Des. 2019, 172, 107774. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Yuan, W.; Wang, B.; Zhu, J. Robust, Fire-Safe, Monomer-Recovery, Highly Malleable Thermosets from Renewable Bioresources. Macromolecules 2018, 51, 8001–8012. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Tibbitt, M.W. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Caillaud, K.; Ladavière, C. Water-Soluble (Poly)acylhydrazones: Syntheses and Applications. Macromol. Chem. Phys. 2022, 223, 2200064. [Google Scholar] [CrossRef]
- Cornellà, A.C.; Tabrizian, S.K.; Ferrentino, P.; Roels, E.; Terryn, S.; Vanderborght, B.; Van Assche, G.; Brancart, J. Self-Healing, Recyclable, and Degradable Castor Oil-Based Elastomers for Sustainable Soft Robotics. ACS Sustain. Chem. Eng. 2023, 11, 3437–3450. [Google Scholar] [CrossRef]
- Toncelli, C.; De Reus, D.C.; Picchioni, F.; Broekhuis, A.A. Properties of Reversible Diels–Alder Furan/Maleimide Polymer Networks as Function of Crosslink Density. Macromol. Chem. Phys. 2012, 213, 157–165. [Google Scholar] [CrossRef]
- Geng, H.; Wang, Y.; Yu, Q.; Gu, S.; Zhou, Y.; Xu, W.; Zhang, X.; Ye, D.-Z. Vanillin-Based Polyschiff Vitrimers: Reprocessability and Chemical Recyclability. ACS Sustain. Chem. Eng. 2018, 6, 15463–15470. [Google Scholar] [CrossRef]
- Lei, Y.; Fu, X.; Jiang, L.; Liu, Z.; Lei, J. Oxime-Urethane Structure-Based Dynamically Crosslinked Polyurethane with Robust Reprocessing Properties. Macromol. Rapid Commun. 2022, 43, e2100781. [Google Scholar] [CrossRef]
- Van Lijsebetten, F.; Debsharma, T.; Winne, J.M.; Du Prez, F.E. A Highly Dynamic Covalent Polymer Network without Creep: Mission Impossible? Angew. Chem. Int. Ed. Engl. 2022, 61, e202210405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Connal, L.A. Biobased Transesterification Vitrimers. Macromol. Rapid Commun. 2023, 44, 21. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar]
- Taplan, C.; Guerre, M.; Winne, J.M.; Du Prez, F.E. Fast processing of highly crosslinked, low-viscosity vitrimers. Mater. Horiz. 2020, 7, 104–110. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Bi, L.; Jiang, J.; Zhang, H.; Chen, Y. Catalyst-Free Self-Healing Bio-Based Polymers: Robust Mechanical Properties, Shape Memory, and Recyclability. J. Agric. Food Chem. 2021, 69, 9338–9349. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Zhang, Y.; Zhou, J.; Wang, B.; Wang, Z. Recyclable and Mechanically Robust Palm Oil-Derived Epoxy Resins with Reconfigurable Shape-Memory Properties. ACS Sustain. Chem. Eng. 2020, 8, 5296–5304. [Google Scholar] [CrossRef]
- Memon, H.; Wei, Y. Welding and reprocessing of disulfide-containing thermoset epoxy resin exhibiting behavior reminiscent of a thermoplastic. J. Appl. Polym. Sci. 2020, 137, 49541. [Google Scholar] [CrossRef]
- Croitoru, C.; Patachia, S.; Baltes, L.; Tierean, M. Colors distribution in polymer wastes and color prediction of recycled polymers. Environ. Eng. Manag. J. 2019, 18, 1039–1048. [Google Scholar] [CrossRef]
- Signoret, C.; Caro-Bretelle, A.-S.; Lopez-Cuesta, J.-M.; Ienny, P.; Perrin, D. MIR spectral characterization of plastic to enable discrimination in an industrial recycling context: I. Specific case of styrenic polymers. Waste Manag. 2019, 95, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Pavon; Aldas, M.; Ferri, J.M.; Bertomeu, D.; Pawlak, F.; Samper, M.D. Identification of biodegradable polymers as contaminants in the thermoplastic recycling process. Dyna 2021, 96, 415–421. [Google Scholar] [CrossRef]
- Scott, G. ‘Green’ polymers. Polym. Degrad. Stab. 2000, 68, 1–7. [Google Scholar] [CrossRef]
- Samper, M.D.; Bertomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J. Interference of Biodegradable Plastics in the Polypropylene Recycling Process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef] [PubMed]
- Podara, C.; Termine, S.; Modestou, M.; Semitekolos, D.; Tsirogiannis, C.; Karamitrou, M.; Trompeta, A.-F.; Milickovic, T.K.; Charitidis, C. Recent Trends of Recycling and Upcycling of Polymers and Composites: A Comprehensive Review. Recycling 2024, 9, 37. [Google Scholar] [CrossRef]
- Jeong, D.; Kwon, D.; Won, G.; Kim, S.; Bang, J.; Shim, J. Toward Sustainable Polymer Materials for Rechargeable Batteries: Utilizing Natural Feedstocks and Recycling/Upcycling of Polymer Waste. Chemsuschem 2024, 17, e202401010. [Google Scholar] [CrossRef]
- Jeziórska, R.; Swierz-Motysia, B.; Szadkowska, A. Modifiers applied in polymer recycling: Processing, properties and application. Polimery 2010, 55, 748–756. [Google Scholar] [CrossRef]
- Versteeg, F.A.; Benita, B.B.; Jongstra, J.A.; Picchioni, F. Reactive Extrusion Grafting of Glycidyl Methacrylate onto Low-Density and Recycled Polyethylene Using Supercritical Carbon Dioxide. Appl. Sci. 2022, 12, 3022. [Google Scholar] [CrossRef]
- Lahtela, V.; Mielonen, K.; Parkar, P.; Kärki, T. The Effects of Bromine Additives on the Recyclability of Injection Molded Electronic Waste Polymers. Glob. Chall. 2023, 7, 2300157. [Google Scholar] [CrossRef]
- Schlummer, M.; Mäurer, A. Recycling of styrene polymers from shredded screen housings containing brominated flame retardants. J. Appl. Polym. Sci. 2006, 102, 1262–1273. [Google Scholar] [CrossRef]
- Riess, M.; Ernst, T.; Popp, R.; Müller, B.; Thoma, H.; Vierle, O.; Wolf, M.; van Eldik, R. Analysis of flame retarded polymers and recycling materials. Chemosphere 2000, 40, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.; Strömberg, E.; Karlsson, S. Comparison of extraction methods for sampling of low molecular compounds in polymers degraded during recycling. Eur. Polym. J. 2008, 44, 1583–1593. [Google Scholar] [CrossRef]
- Alfonso, J.M.; Valencia, C.; Sánchez, M.; Franco, J.; Gallegos, C. Influence of some processing variables on the rheological properties of lithium lubricating greases modified with recycled polymers. Int. J. Mater. Prod. Technol. 2012, 43, 184–200. [Google Scholar] [CrossRef]
- Yao, H.; Zuo, Y.; Song, Y.; Huang, W.; Jiang, Q.; Xue, X.; Jiang, L.; Xu, J.; Yang, H.; Jiang, B. Precisely Tailoring and Renewing Polymers: An Efficient Strategy for Polymer Recycling. Macromol. Chem. Phys. 2022, 223, 2200117. [Google Scholar] [CrossRef]
- Andraju, N.; Curtzwiler, G.W.; Ji, Y.; Kozliak, E.; Ranganathan, P. Machine-Learning-Based Predictions of Polymer and Postconsumer Recycled Polymer Properties: A Comprehensive Review. Acs Appl. Mater. Interfaces 2022, 14, 42771–42790. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Okamoto, H. Estimation method to achieve desired mechanical properties with minimum virgin polymer in plastics recycling. Resour. Conserv. Recycl. 2024, 211, 107856. [Google Scholar] [CrossRef]
- van Gisbergen, J.; den Doelder, J. Processability predictions for mechanically recycled blends of linear polymers. J. Polym. Eng. 2020, 40, 771–781. [Google Scholar] [CrossRef]
- Al Rashid, A.; Khan, S.A.; Koç, M. Life cycle assessment on fabrication and characterization techniques for additively manufactured polymers and polymer composites. Clean. Environ. Syst. 2024, 12, 100159. [Google Scholar] [CrossRef]
- Al Omar, S.; Abdelhadi, A. Comparative Life-Cycle Assessment of Steel and GFRP Rebars for Procurement Sustainability in the Construction Industry. Sustainability 2024, 16, 3899. [Google Scholar] [CrossRef]
- Alavi, Z.; Khalilpour, K.; Florin, N.; Hadigheh, A.; Hoadley, A. End-of-life wind turbine blade management across energy transition: A life cycle analysis. Resour. Conserv. Recycl. 2025, 213, 108008. [Google Scholar] [CrossRef]
- Arena, U.; Parrillo, F.; Ardolino, F. An LCA answer to the mixed plastics waste dilemma: Energy recovery or chemical recycling? Waste Manag. 2023, 171, 662–675. [Google Scholar] [CrossRef]
- Costa, L.P.; de Miranda, D.M.V.; Pinto, J.C. Critical Evaluation of Life Cycle Assessment Analyses of Plastic Waste Pyrolysis. Acs Sustain. Chem. Eng. 2022, 10, 3799–3807. [Google Scholar] [CrossRef]
- Backer, S.A.; Leal, L. Biodegradability as an Off-Ramp for the Circular Economy: Investigations into Biodegradable Polymers for Home and Personal Care. Acc. Chem. Res. 2022, 55, 2011–2018. [Google Scholar] [CrossRef]
- Deeney, P.; Nagle, A.J.; Gough, F.; Lemmertz, H.; Delaney, E.L.; McKinley, J.M.; Graham, C.; Leahy, P.G.; Dunphy, N.P.; Mullally, G. End-of-Life alternatives for wind turbine blades: Sustainability Indices based on the UN sustainable development goals. Resour. Conserv. Recycl. 2021, 171, 105642. [Google Scholar] [CrossRef]
- Ardolino, F.; Cardamone, G.F.; Arena, U. How to enhance the environmental sustainability of WEEE plastics management: An LCA study. Waste Manag. 2021, 135, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Diab, L.; Al-Qadi, I.L. Life Cycle Assessment for the Use of Waste Plastics in Asphalt Concrete Mixes. Transp. Res. Rec. 2024. [Google Scholar] [CrossRef]
- Witik, R.A.; Payet, J.; Michaud, V.; Ludwig, C.; Månson, J.-A.E. Assessing the life cycle costs and environmental performance of lightweight materials in automobile applications. Compos. Part A-Appl. Sci. Manuf. 2011, 42, 1694–1709. [Google Scholar] [CrossRef]
- Bac, U. The Role of Environmental Factors in the Investment Prioritization of Facilities Using Recycled PVC. Pol. J. Environ. Stud. 2021, 30, 2981–2993. [Google Scholar] [CrossRef]
- Bachmann, J.; Hidalgo, C.; Bricout, S. Environmental analysis of innovative sustainable composites with potential use in aviation sector-A life cycle assessment review. Sci. China-Technol. Sci. 2017, 60, 1301–1317. [Google Scholar] [CrossRef]
- Balaguera; Carvajal, G.I.; Albertí, J.; Fullana-i-Palmer, P. Life cycle assessment of road construction alternative materials: A literature review. Resour. Conserv. Recycl. 2018, 132, 37–48. [Google Scholar] [CrossRef]
- Balaguera, A.; Carvajal, G.I.; Albertí, J.; Fullana-I-Palmer, P. Identification of the environmental hotspots of a recycling process- Case study of a Pt PEMFC catalyst closed-loop recycling system evaluated via life cycle assessment methodology. Int. J. Hydrogen Energy 2024, 63, 396–410. [Google Scholar]
- Bourtsalas, A.C.; Yepes, I.M.; Tian, Y.X. US plastic waste exports: A state-by-state analysis pre- and post-China import ban. J. Environ. Manag. 2023, 344, 118604. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.R. Polymer recycling: Economic realities. In Plastics, Rubber, and Paper Recycling: A Pragmatic Approach; Rader, C.P., Baldwin, S.D., Cornell, D.D., Sadler, G.D., Stockel, R.F., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 62–69. [Google Scholar]
- Synyuk, O.; Musiał, J.; Zlotenko, B.; Kulik, T. Development of Equipment for Injection Molding of Polymer Products Filled with Recycled Polymer Waste. Polymers 2020, 12, 2725. [Google Scholar] [CrossRef]
- Erenkov, O.Y.; Yavorskiy, D.O. Modernized Design of a Polymer Recycling Unit. Chem. Pet. Eng. 2023, 58, 795–797. [Google Scholar] [CrossRef]
- Karuppaiah, C.; Vasiljevic, N.; Chen, Z. Circular Economy of Polymers—Electrochemical Recycling and Upcycling. Electrochem. Soc. Interface 2021, 30, 55–58. [Google Scholar] [CrossRef]
- Nguyen, B.K.; McNally, G.; Clarke, A. Automatic extruder for processing recycled polymers with ultrasound and temperature control system. Proc. Inst. Mech. Eng. Part E-J. Process Mech. Eng. 2016, 230, 355–370. [Google Scholar] [CrossRef]
- Nguyen, B.K.; McNally, G.; Clarke, A. Real time measurement and control of viscosity for extrusion processes using recycled materials. Polym. Degrad. Stab. 2014, 102, 212–221. [Google Scholar] [CrossRef]
- Pachner, S.; Aigner, M.; Miethlinger, J. A heuristic method for modeling the initial pressure drop in melt filtration using woven screens in polymer recycling. Polym. Eng. Sci. 2019, 59, 1105–1113. [Google Scholar] [CrossRef]
- Hussain, A.; Podgursky, V.; Viljus, M.; Awan, M.R. The role of paradigms and technical strategies for implementation of the circular economy in the polymer and composite recycling industries. Adv. Ind. Eng. Polym. Res. 2023, 6, 1–12. [Google Scholar] [CrossRef]
- Rejewski, P.; Kijenski, J. Waste polymers—An available and perspective raw materials for recycling processes. Polimery 2010, 55, 711–717. [Google Scholar] [CrossRef]
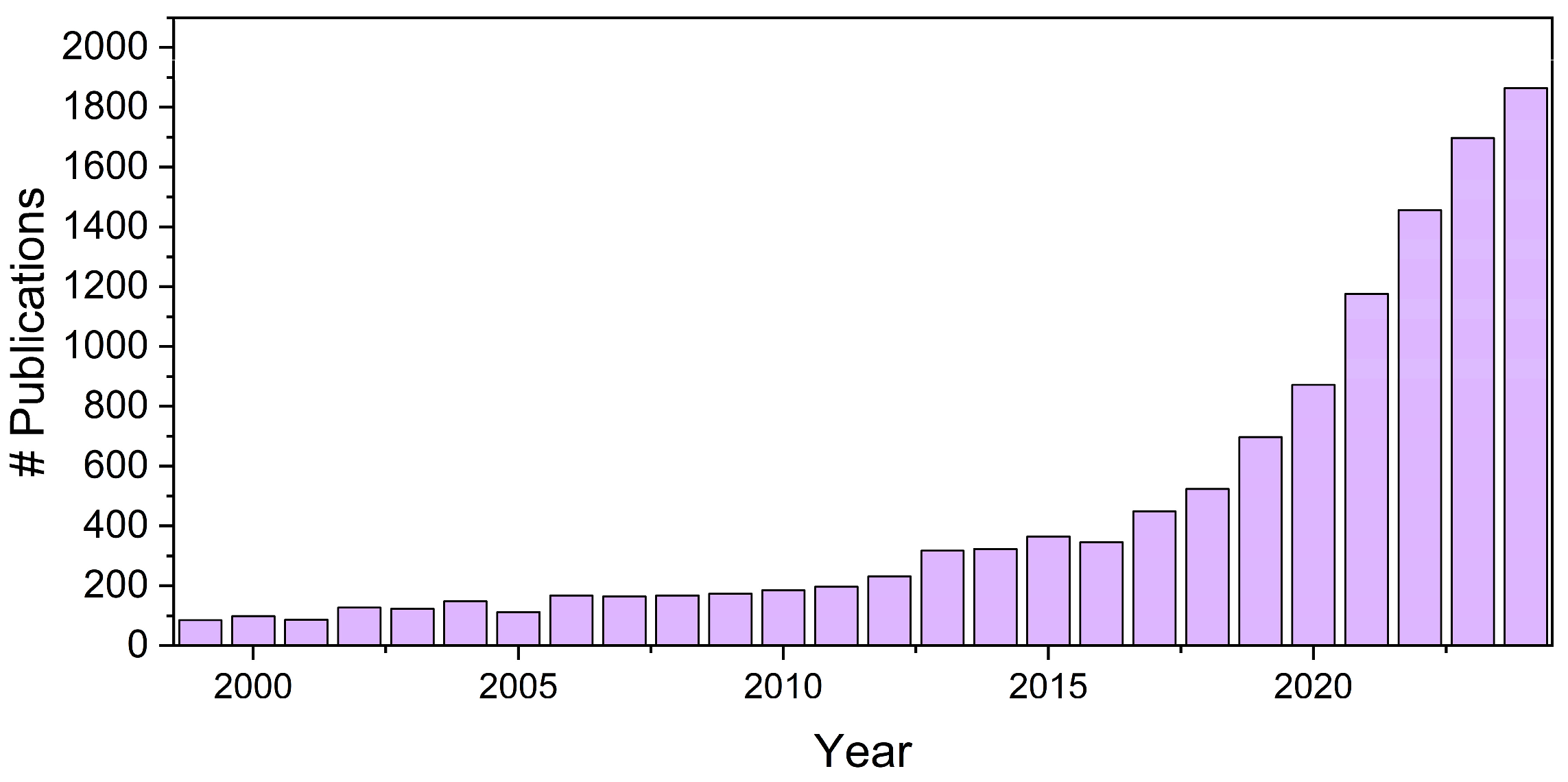
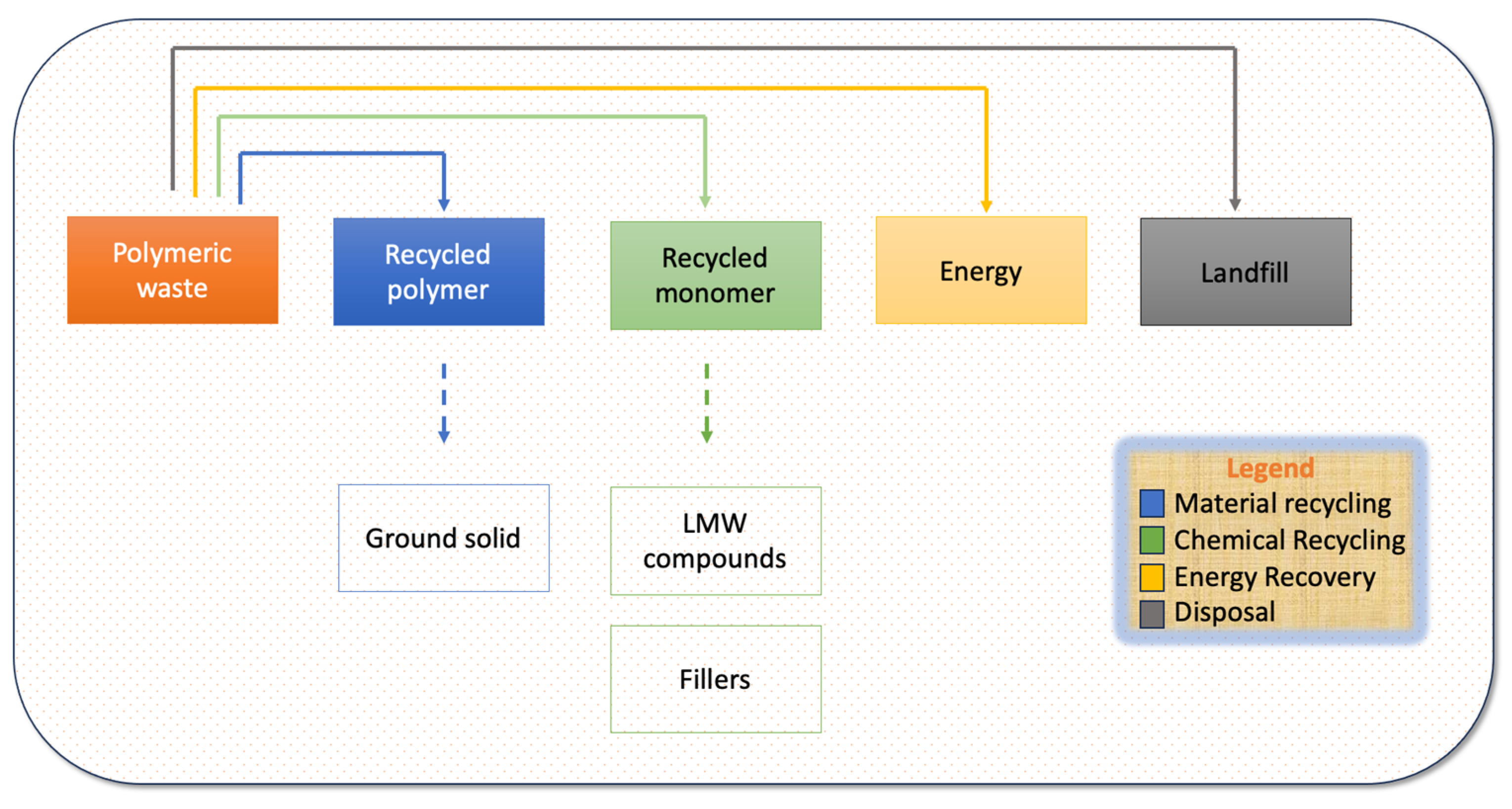

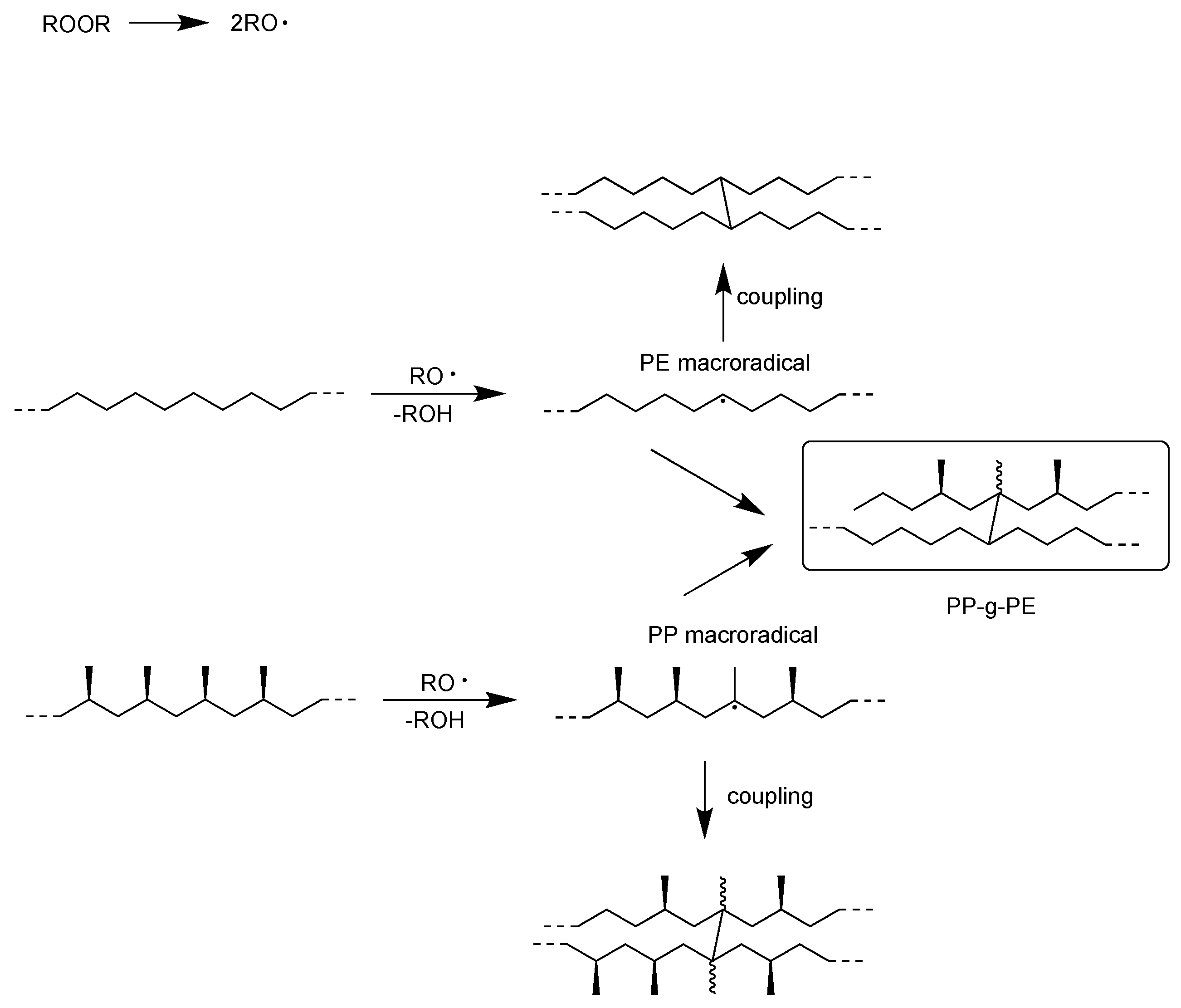

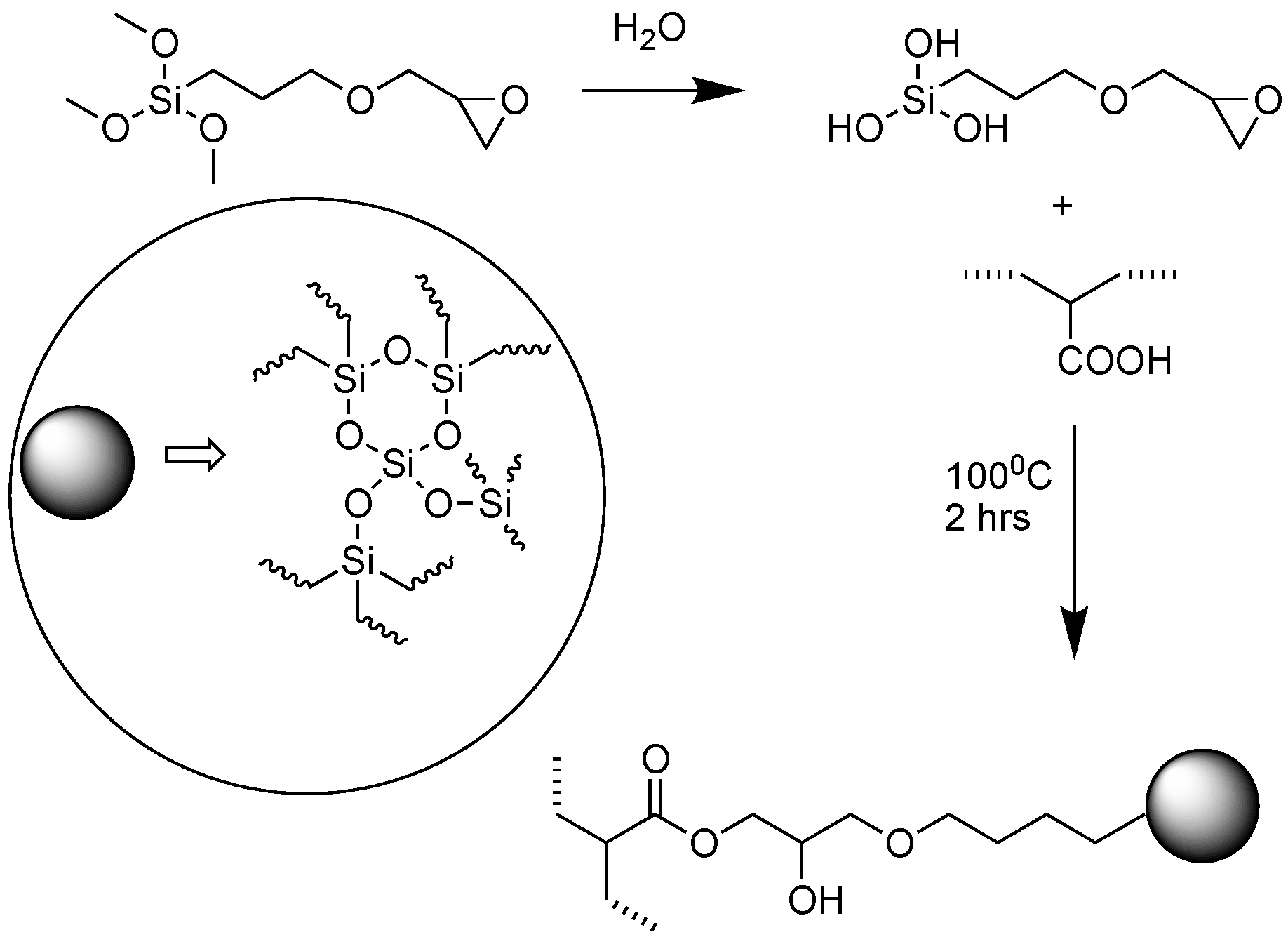



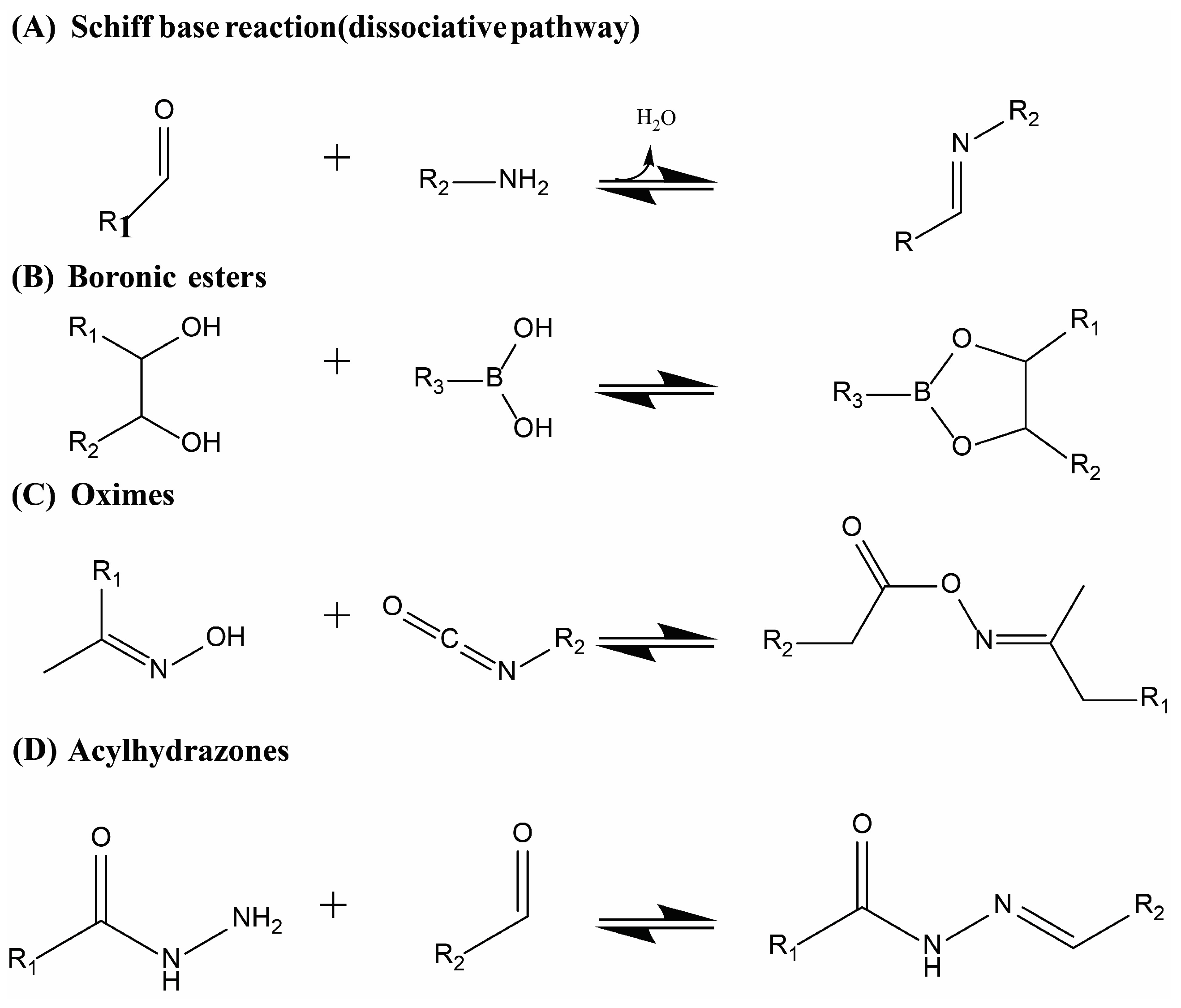


| Chemistry | T (°C) | Method | Comments | Reference |
|---|---|---|---|---|
| Diels–Alder: furan–maleimide crosslinked castor oil | 130 | Free flow into mold | three cycles | [337] |
| Diels–Alder: furan–maleimide crosslinked polyketones | 120–150 | Dynamic mechanical thermal analysis | seven cycles | [338] |
| Diels–Alder: furan–maleimide crosslinked EPDM rubber | 175 | Hot pressing | One cycle shown in tensile tests | [317] |
| Schiff base: vanillin based | 50 | Acid hydrolysis | Shown once with NMR | [339] |
| Schiff base: vanillin based | 170 | Hot pressing | three cycles | [333] |
| Oximes | 155 | Hot pressing | four cycles | [340] |
| Chemistry | Temperature | Method | Comments | Reference |
|---|---|---|---|---|
| Trans-esterification: fractionated lignin and sebacic acid | 160 °C | Hot pressing | Zn(acac)2 as catalyst | [345] |
| Trans-esterification: palm oil-based epoxy and citric acid | 170 °C | Hot pressing | Catalyst free | [346] |
| Di-sulfide metathesis | 180 °C | Hot pressing | Three cycles, welding performance tested | [347] |
| Polyurethane | 150 °C | Extrusion | Twin-screw extrusion, one cycle | [344] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Tempel, P.; Picchioni, F. Polymer Recycling: A Comprehensive Overview and Future Outlook. Recycling 2025, 10, 1. https://doi.org/10.3390/recycling10010001
van den Tempel P, Picchioni F. Polymer Recycling: A Comprehensive Overview and Future Outlook. Recycling. 2025; 10(1):1. https://doi.org/10.3390/recycling10010001
Chicago/Turabian Stylevan den Tempel, Paul, and Francesco Picchioni. 2025. "Polymer Recycling: A Comprehensive Overview and Future Outlook" Recycling 10, no. 1: 1. https://doi.org/10.3390/recycling10010001
APA Stylevan den Tempel, P., & Picchioni, F. (2025). Polymer Recycling: A Comprehensive Overview and Future Outlook. Recycling, 10(1), 1. https://doi.org/10.3390/recycling10010001







