Multi-Layer TiO2−x-PEDOT-Decorated Industrial Fe2O3 Composites as Anode Materials for Cycle-Performance-Enhanced Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe2O3/TiO2−x/PEDOT Composites
2.2. Characterization
2.3. Electrochemical Measurement
3. Results
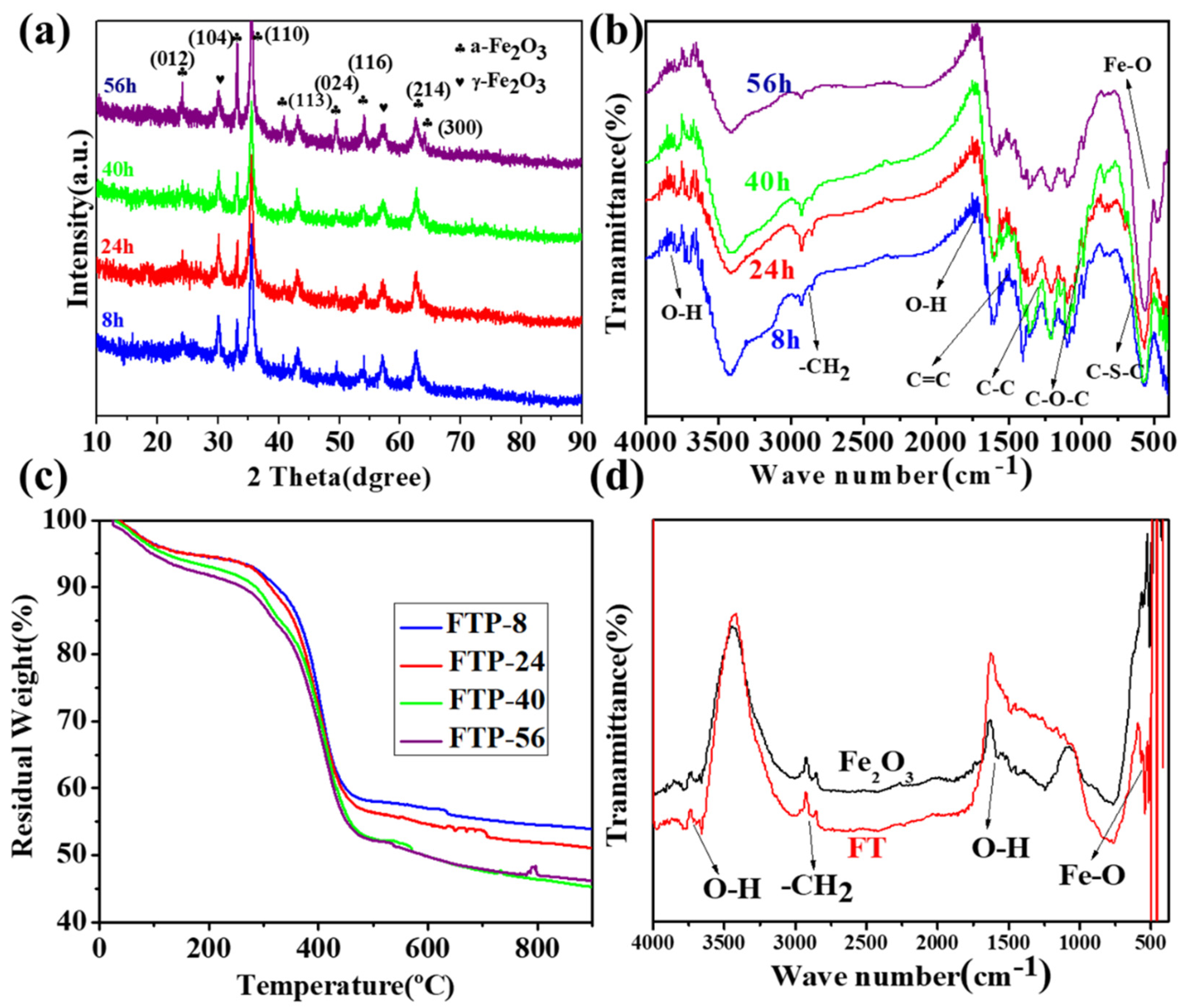
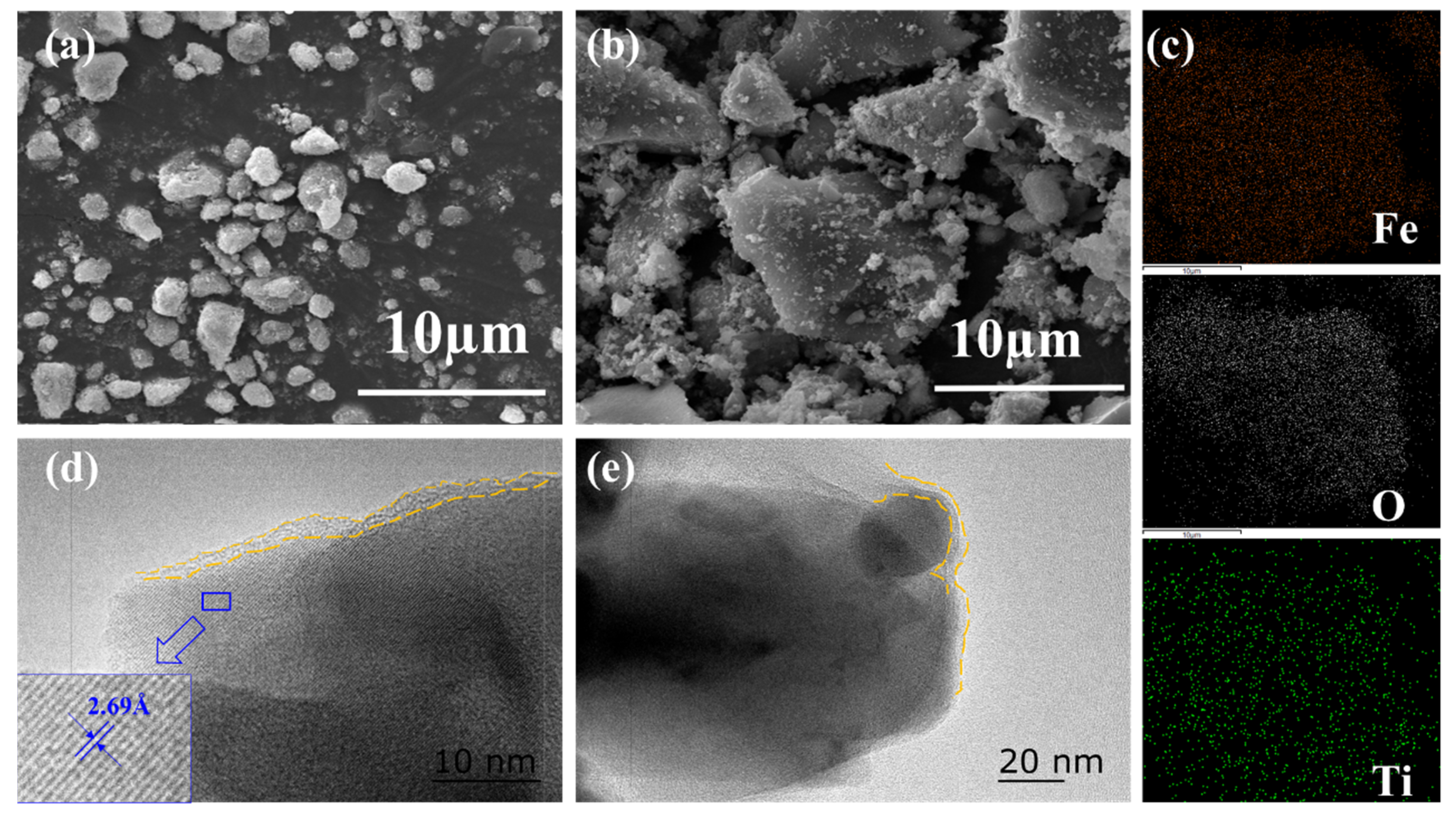
3.1. Structural and Physicochemical Analysis of the FTP Powders
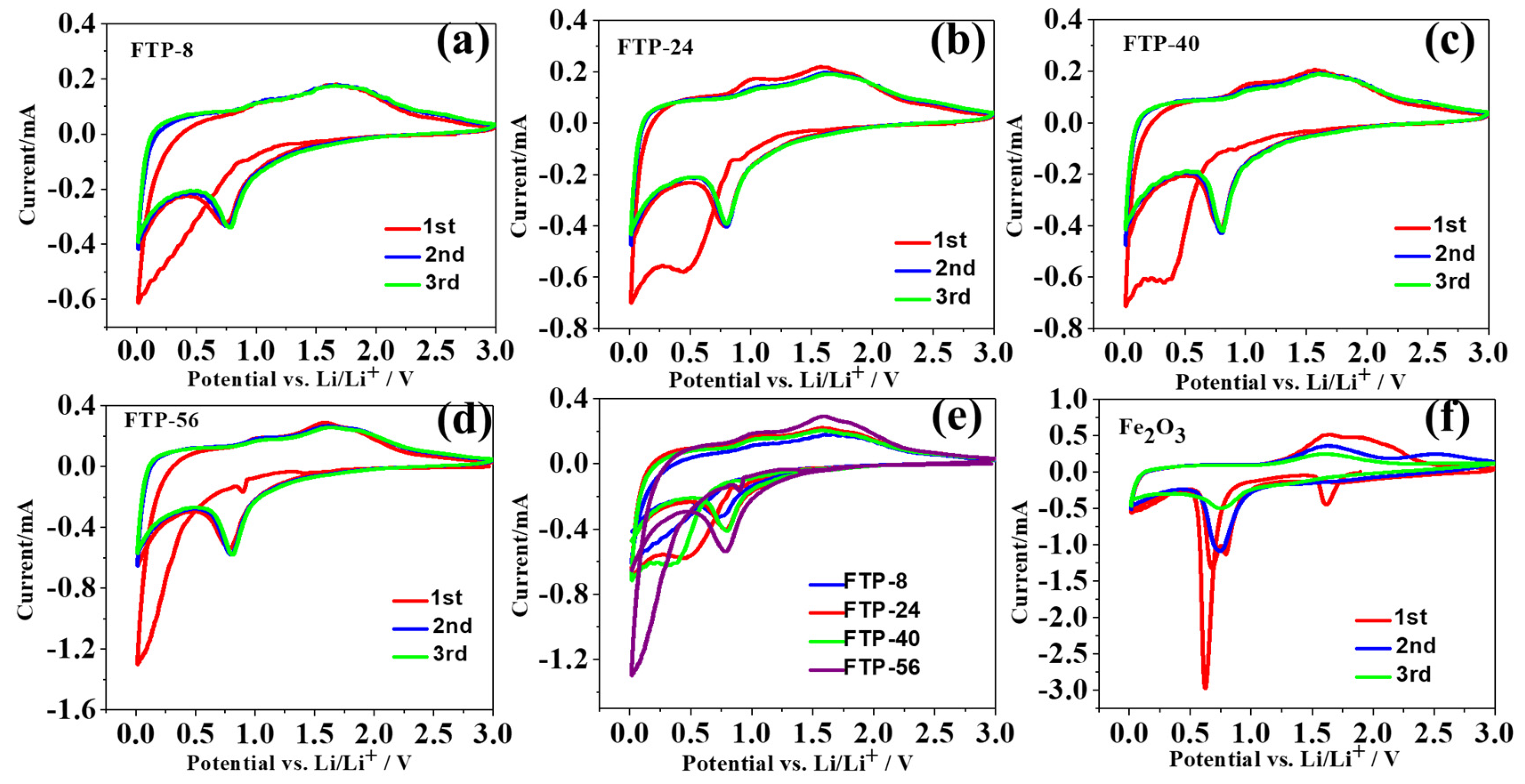
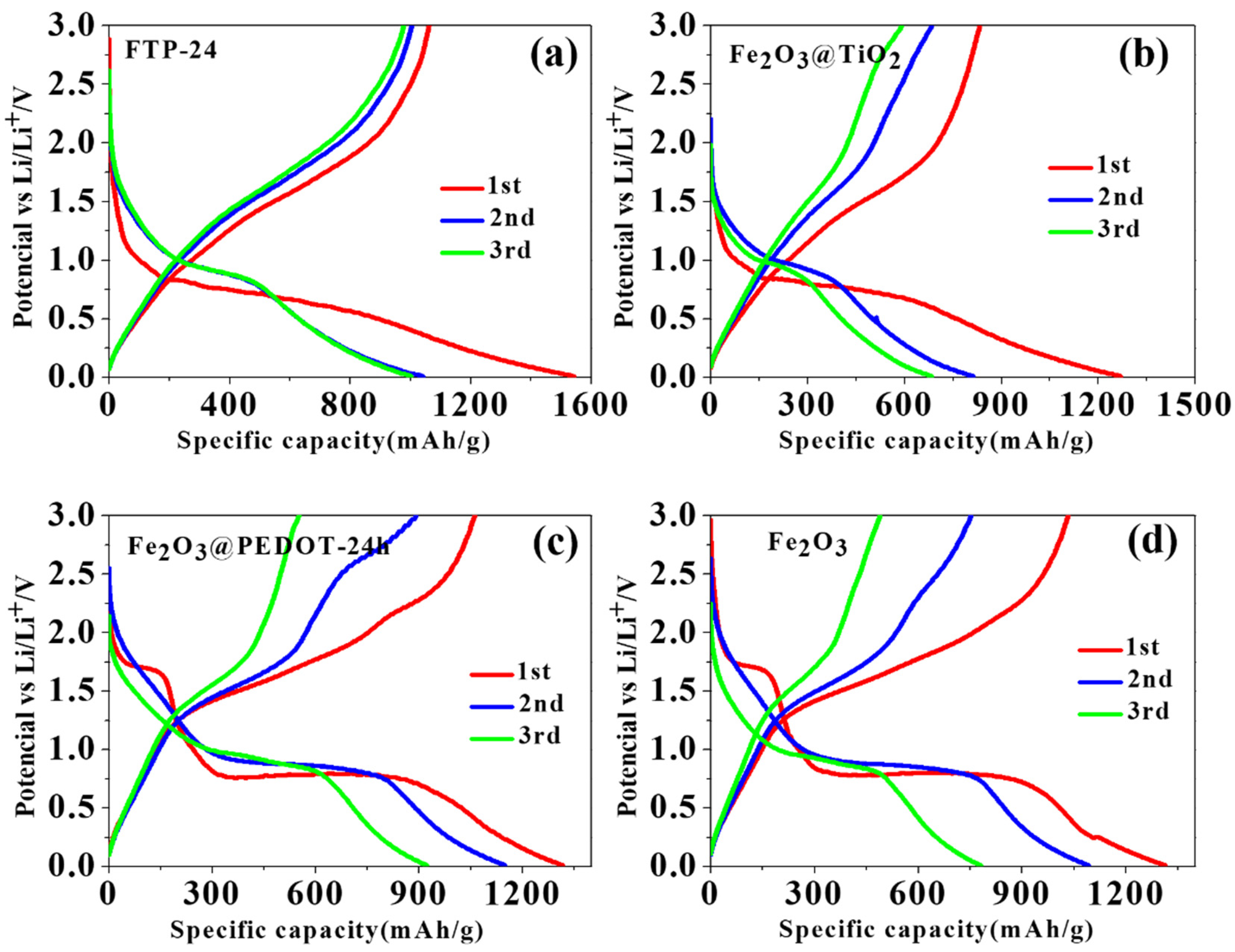
3.2. Electrochemical Performance of the Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, B.; Ding, M.; Ma, Y.; Song, G.; Li, Z.; Ge, J.; Yang, W.; Wen, C. Effect of MgO on electrochemical properties of silicon-based anode composite material. Solid State Sci. 2022, 131, 106940. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, N.; Ji, Y.; Zhang, J.; Zhu, Y.; Yi, T. Nanosized zinc oxides-based materials for electrochemical energy storage and conversion: Batteries and supercapacitors. Chin. Chem. Lett. 2022, 33, 714–729. [Google Scholar] [CrossRef]
- Xu, L.; Chon, M.J.; Mills, B.; Thompson, C.V. Mechanical stress and morphology evolution in RuO2 thin film electrodes during lithiation and delithiation. J. Power Sources 2022, 552, 232260. [Google Scholar] [CrossRef]
- Xu, L.; Thompson, C.V. Mechanisms of the cyclic (de)lithiation of RuO2. J. Mater. Chem. A 2020, 8, 21872–21881. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Q.; Wang, S.; Cai, Z.; Ma, Y.; Song, G.; Yang, W.; Wen, C.; Xie, Y. Enhanced electrochemical performance of silicon anode materials with titanium hydride treatment. J. Electroanal. Chem. 2023, 933, 117292. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Heng, Y.L.; Guo, J.Z.; Cao, J.M.; Wang, X.T.; Zhao, X.X.; Sun, Z.H.; Zheng, S.H.; Liang, H.J.; Li, B.; et al. Nano self-assembly of fluorophosphate cathode induced by surface energy evolution towards high-rate and stable sodium-ion batteries. Nano Res. 2023, 16, 439–448. [Google Scholar] [CrossRef]
- Du, K.D.; Meng, Y.F.; Zhao, X.X.; Wang, X.T.; Luo, X.X.; Zhang, W.; Wu, X.L. A unique co-recovery strategy of cathode and anode from spent LiFePO4 battery. Sci. China Mater. 2022, 65, 637–645. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Gu, Z.Y.; Ang, E.H.; Guo, J.Z.; Wang, X.T.; Wang, Y.; Wu, X.L. Advanced polyanionic electrode materials for potassium-ion batteries: Progresses, challenges and application prospects. Mater. Today 2022, 54, 189–201. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, D.; Ji, S.; Huo, Y.; Liu, J. Facile construction of CoSn/Co3Sn2@C nanocages as anode for superior lithium-/sodium-ion storage. Carbon Neutralization 2023, 2, 54–62. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Qu, X.; Huang, H.; Wan, T.; Hu, L.; Yu, Z.; Liu, Y.; Dou, A.; Zhou, Y.; Su, M.; Peng, X.; et al. An integrated surface coating strategy to enhance the electrochemical performance of nickel-rich layered cathodes. Nano Energy 2022, 91, 106665. [Google Scholar] [CrossRef]
- Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries. Nano Energy 2019, 58, 786–796. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Zhu, H.; Tang, X.; Ma, Y.; Yu, D.Y.W.; Zhang, S.; Song, G.; Yang, W.; Xu, Y.; et al. Improvement of stability and capacity of Co-free, Li-rich layered oxide Li1.2Ni0.2Mn0.6O2 cathode material through defect control. J. Colloid Interface Sci. 2023, 630, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chu, X.; Wang, Z.; Yang, H.; Yang, Z.; Ma, J.; Zhou, B.; Yang, T.; Chen, L. Rosa roxburghii-like hierarchical hollow sandwich-structure C@Fe2O3@C microspheres as second nanomaterialsfor superior lithium storage. J. Alloys Compd. 2021, 855, 157518. [Google Scholar] [CrossRef]
- Ding, B.; Cai, Z.; Ahsan, Z.; Ma, Y.; Zhang, S.; Song, G.; Yuan, C.; Yang, W.; Wen, C. A Review of Metal Silicides for Lithium-Ion Battery Anode Application. Acta Metall. Sin. 2021, 34, 291–308. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Liu, W.; Yan, T.; Zhai, C.; Wu, P.; Zhou, Y. Double-Network Gel-Enabled Uniform Incorporation of Metallic Matrices with Silicon Anodes Realizing Enhanced Lithium Storage. ACS Appl. Energy Mater. 2019, 2, 2268–2275. [Google Scholar] [CrossRef]
- Fang, R.; Xiao, W.; Miao, C.; Mei, P.; Zhang, Y.; Yan, X.; Jiang, Y. Enhanced lithium storage performance of core-shell structural Si@TiO2/NC composite anode via facile sol-gel and in situ N-doped carbon coating processes. Electrochim. Acta 2019, 317, 575–582. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, W.; Wei, Y.; Yang, X.; Jiang, C.; Liu, H.; Zhang, W.; Liang, C.; Dai, L.; Xu, X. Facile Constructing Hierarchical Fe3O4@C Nanocomposites as Anode for Superior Lithium-Ion Storage. Batteries 2023, 9, 403. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, T.; Sow, C.H.; Shen, Z.X.; Lim, C.T.; Rao, G.V.S.; Chowdari, B.V.R. α-Fe2O3 nanoflakes as an anode material for li-ion batteries. Adv. Funct. Mater. 2007, 17, 2792–2799. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Li, Z.; Shi, Z.; Zhu, J.; Mei, H. Fe2O3/N doped rGO anode hybridized with NiCo LDH/Co(OH)2 cathode for battery-like supercapacitor. Chem. Eng. J. 2021, 403, 126325. [Google Scholar] [CrossRef]
- Hwang, J.; Yadav, D.; Yang, H.; Jeon, I.; Yang, D.; Seo, J.W.; Kang, M.; Jeong, S.Y.; Cho, C.R. In Situ Electrochemical Impedance Measurements of α-Fe2O3 Nanofibers: Unravelling the Li-Ion Conduction Mechanism in Li-Ion Batteries. Batteries 2022, 8, 44. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, J.; Chen, Y.; Liang, C.; Ni, J. Hierarchical-Structured Fe2O3 Anode with Exposed (001) Facet for Enhanced Lithium Storage Performance. Nanomaterials 2023, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, J.-Q.; Lin, Y.-Y.; Sun, Y.-H. Flower-like multi-shells MO/Fe2O3 (M = Cu, Mn and Ni) hollow microspheres for lithium ion battery with super cycle stability. Inorg. Chem. Commun. 2023, 154, 110947. [Google Scholar] [CrossRef]
- Li, J.; Yan, L. Synthesis of Fe2O3/rGO Based Composites as Anode material for Lithium ion batteries using sewage sludge as source of Iron (III) oxide. Int. J. Electrochem. Sci. 2022, 17, 221173. [Google Scholar] [CrossRef]
- Pan, Y.; Luo, C.; Yang, D.; Sun, P.; Chen, J.; Sui, Z.; Tian, Q. Ultrathin porous Fe2O3@C nanosheets: Novel preparation strategy and high lithium storage. Appl. Surf. Sci. 2023, 635, 157763. [Google Scholar] [CrossRef]
- Ge, Y.; Gao, W.; Yuan, Z.; Tao, S.; Kong, F.; Qian, B. Synergistic effect on the improved lithium ion storage performance in the porous Fe2O3@Fe3C@C composite. Mater. Res. Bull. 2023, 164, 112287. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, M.; Wu, S.; Chen, Y.; Li, L.; Zou, X.; Chen, L.; Yang, H.; Pang, H. Interfacial engineering of graphene aerogel encapsulated FeSe2-Fe2O3 heterojunction nanotubes for enhanced lithium storage. J. Alloys Compd. 2023, 934, 167939. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, W.; Qing, T.; Chen, G.; Zeng, S.; Huang, J. Constructing 3D sandwich-like carbon coated Fe2O3/helical carbon nanofibers composite as a superior lithium-ion batteries anode. J. Electroanal. Chem. 2023, 929, 117098. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, C.; Lu, Y.; Wan, L.; Yan, K.; Bai, Y.; Liu, Z.; Yang, X.; Su, Y.; Wu, F. Facile Synthesizing Yolk-Shelled Fe3O4@Carbon Nanocavities with Balanced Physiochemical Synergism as Efficient Hosts for High-Performance Lithium–Sulfur Batteries. Batteries 2023, 9, 295. [Google Scholar] [CrossRef]
- Shi, W.C.; Shao, Y.R.; Shen, Y.; Li, Z.F.; Hu, T.L. Facile synthesis of porous iron oxides heterostructural nanosheets as an enhanced anode material for lithium ion battery. Mater. Lett. 2022, 321, 132454. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, L.; Hu, J.; Xue, C. Anchoring iron oxide nanoparticles on polypyrrole/rGO derived nitrogen-doped carbon as lithium-ion battery anode. J. Alloys Compd. 2017, 723, 729–735. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, P.; Huang, J.; Liu, H.; Zao, Y.; Hu, Z.; Zhang, L.; Chen, H.; Wang, M.S.; Peng, D.L.; et al. High performance columnar-like Fe2O3@carbon composite anode via yolk@shell structural design. J. Energy Chem. 2020, 41, 126–134. [Google Scholar] [CrossRef]
- Qin, G.; Zeng, M.; Wu, X.; Wen, J.; Li, J. Fabrication of Fe2O3@TiO2 core–shell nanospheres as anode materials for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 12944–12950. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L.; Cai, Z.; Huang, X.; Ding, B.; Ahsan, Z.; Song, G.; Xu, Y.; Yang, W.; Wen, C. Study of TiO2 -Coated α-Fe2O3 Composites and the Oxygen-Defects Effect on the Application as the Anode Materials of High-Performance Li-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 11666–11673. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Lu, B.; Wang, X.; Sun, S. Stem-like nano-heterostructural MWCNTs/α-Fe2O3@TiO2 composite with high lithium storage capability. J. Alloys Compd. 2016, 684, 419–427. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Wang, J.; Wang, J.; Bai, Y.; Du, X. Morphology controllable nano-sheet polypyrrole-graphene composites for high-rate supercapacitor. Phys. Chem. Chem. Phys. 2015, 17, 19885–19894. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xie, D.; Ai, S.; Huang, H.; Zheng, Z.; Xie, S.; Liu, P.; Wang, S.; Zhang, M.; Cheng, F. Facile fabrication of core-shell α-Fe2O3@PPy imbedded into porous biomass-derived carbon for enhanced lithium storage. J. Energy Storage 2023, 67, 107625. [Google Scholar] [CrossRef]
- Rasool, M.; Chiu, H.C.; Zank, B.; Zeng, Y.; Zhou, J.; Zaghib, K.; Perepichka, D.F.; Demopoulos, G.P. PEDOT Encapsulated and Mechanochemically Engineered Silicate Nanocrystals for High Energy Density Cathodes. Adv. Mater. Interfaces 2020, 7, 202000226. [Google Scholar] [CrossRef]
- da Silva, R.J.; Lima, R.M.A.P.; de Oliveira, M.C.A.; Alcaraz-Espinoza, J.J.; de Melo, C.P.; de Oliveira, H.P. Supercapacitors based on (carbon nanostructure)/PEDOT/(eggshell membrane) electrodes. J. Electroanal. Chem. 2020, 856, 113658. [Google Scholar] [CrossRef]
- Jeong, J.M.; Choi, B.G.; Lee, S.C.; Lee, K.G.; Chang, S.J.; Han, Y.K.; Lee, Y.B.; Lee, H.U.; Kwon, S.; Lee, G.; et al. Hierarchical hollow spheres of Fe2O3@polyaniline for lithium ion battery anodes. Adv. Mater. 2013, 25, 6250–6255. [Google Scholar] [CrossRef]
- Chen, H.W.; Li, C. PEDOT: Fundamentals and Its Nanocomposites for Energy Storage. Chin. J. Polym. Sci. 2020, 38, 435–448. [Google Scholar] [CrossRef]
- Wu, C.; Chen, J.; Liang, L.; Li, N. N-doped/oxygen-deficient Fe2O3−x @PEDOT cruciform nanosheet arrays: Boosting the performance of all-solid-state flexible supercapacitors. Chem. Phys. Lett. 2023, 823, 2–6. [Google Scholar] [CrossRef]
- Chaudhari, S.; Srinivasan, M. 1D hollow α-Fe2O3 electrospun nanofibers as high performance anode material for lithium ion batteries. J. Mater. Chem. 2012, 22, 23049–23056. [Google Scholar] [CrossRef]
- Zhu, G.; Gao, B.; Tu, G.; Liu, H.; Wang, M. Improving Effect of Graphene on Electrochemical Properties of Fe2O3 Anode Materials. Metals 2022, 12, 593. [Google Scholar] [CrossRef]
- Suo, Y.; Zhao, Q.Q.; Meng, J.K.; Li, J.; Zheng, X.C.; Guan, X.X.; Liu, Y.S.; Zhang, J.M. Fabrication and electrochemical properties of 3D Fe3O4@graphene aerogel composites as lithium-ion battery anodes. Mater. Lett. 2016, 174, 36–39. [Google Scholar] [CrossRef]
- Shen, L.; Wang, D.; Ali, K.; Li, M.; Shi, Z. Electrochemical Preparation of Nano-Sized Silicon as a Lithium-Ion Battery Anode Material. J. Electrochem. Soc. 2021, 168, 120509. [Google Scholar] [CrossRef]
- Jiang, J.; Ou, Y.; Jiang, Y.; Hu, X.; Xing, C.; Liu, S.; Liu, X.; Li, W.; Zhao, B. Preparation of SiOx–TiO2/Si/CNTs composite microspheres as novel anodes for lithium-ion battery with good cycle stability. J. Mater. Sci. Mater. Electron. 2022, 33, 11025–11037. [Google Scholar] [CrossRef]
- Ranjbar-Azad, M.; Behpour, M. Facile in situ co-precipitation synthesis of CuO–NiO/rGO nanocomposite for lithium-ion battery anodes. J. Mater. Sci. Mater. Electron. 2021, 32, 18043–18056. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X.; Wang, A.; Wang, Y. Carbon@SnS2 core-shell microspheres for lithium-ion battery anode materials. Ionics 2018, 24, 2915–2923. [Google Scholar] [CrossRef]


| Sample | Initial Charge Specific Capacity (mAh/g) | Initial Discharge Specific Capacity (mAh/g) | Initial Coulombic Efficiency (%) | Charge Capacity after 150 Cycles (mAh/g) | Discharge Capacity after 150 Cycles (mAh/g) |
|---|---|---|---|---|---|
| FTP-8 | 731.1 | 1107.0 | 66.04 | 513.6 | 518.4 |
| FTP-24 | 1062.1 | 1543.4 | 68.81 | 623.4 | 628.2 |
| FTP-40 | 761.4 | 1180.1 | 64.52 | 433.3 | 437.6 |
| FTP-56 | 659.7 | 986.7 | 66.86 | 358.6 | 356.9 |
| FT | 833.6 | 1269.3 | 65.67 | 230.0 | 228.6 |
| FP-24 | 1062.5 | 1318.2 | 80.60 | 97.1 | 97.5 |
| Fe2O3 | 1033.3 | 1313.8 | 78.65 | 75.87 | 76.0 |
| Materials | Current Density (mA/h) | Initial Discharge Specific Capacity (mAh/g) | Reversible Capacity (mAh/g) | Capacity Retention (%) | Ref. |
|---|---|---|---|---|---|
| 0.2rGO/Fe2O3 −175 °C | 100 | 1372 | 435 (50 Cycles) | 31.7 | [44] |
| Fe3O4@graphene | 100 | 1625 | 849 (100 Cycles) | 52.2 | [45] |
| Silicon-Carbon | 100 | 1090 | 200 (100 Cycles) | 18.3 | [46] |
| 10%-SC | 100 | 1227 | 800 (100 Cycles) | 65.2 | [47] |
| CuO-NiO/rGO | 100 | 990 | 680 (50 Cycles) | 68.7 | [48] |
| Carbon@SnS2 core–shell microspheres | 100 | 1611 | 500 (50 Cycles) | 31.0 | [49] |
| FTP-24 | 100 | 1543 | 588.9 (360 Cycles) | 40.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, Q.; Li, H.; Cai, Z.; Wang, S.; Zhang, L.; Li, J.; Song, G.; Xu, Y.; Yi, T. Multi-Layer TiO2−x-PEDOT-Decorated Industrial Fe2O3 Composites as Anode Materials for Cycle-Performance-Enhanced Lithium-Ion Batteries. Batteries 2023, 9, 481. https://doi.org/10.3390/batteries9090481
Ma Y, Li Q, Li H, Cai Z, Wang S, Zhang L, Li J, Song G, Xu Y, Yi T. Multi-Layer TiO2−x-PEDOT-Decorated Industrial Fe2O3 Composites as Anode Materials for Cycle-Performance-Enhanced Lithium-Ion Batteries. Batteries. 2023; 9(9):481. https://doi.org/10.3390/batteries9090481
Chicago/Turabian StyleMa, Yangzhou, Qi Li, Haoduo Li, Zhenfei Cai, Shuai Wang, Li Zhang, Jian Li, Guangsheng Song, Youlong Xu, and Tingfeng Yi. 2023. "Multi-Layer TiO2−x-PEDOT-Decorated Industrial Fe2O3 Composites as Anode Materials for Cycle-Performance-Enhanced Lithium-Ion Batteries" Batteries 9, no. 9: 481. https://doi.org/10.3390/batteries9090481
APA StyleMa, Y., Li, Q., Li, H., Cai, Z., Wang, S., Zhang, L., Li, J., Song, G., Xu, Y., & Yi, T. (2023). Multi-Layer TiO2−x-PEDOT-Decorated Industrial Fe2O3 Composites as Anode Materials for Cycle-Performance-Enhanced Lithium-Ion Batteries. Batteries, 9(9), 481. https://doi.org/10.3390/batteries9090481







