Fe3O4 Nanoparticle-Decorated Bimodal Porous Carbon Nanocomposite Anode for High-Performance Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Fe3O4@C9 Nanocomposite
2.2. Fabrication of LIBs
2.3. Characterization Methods
3. Results and Discussion
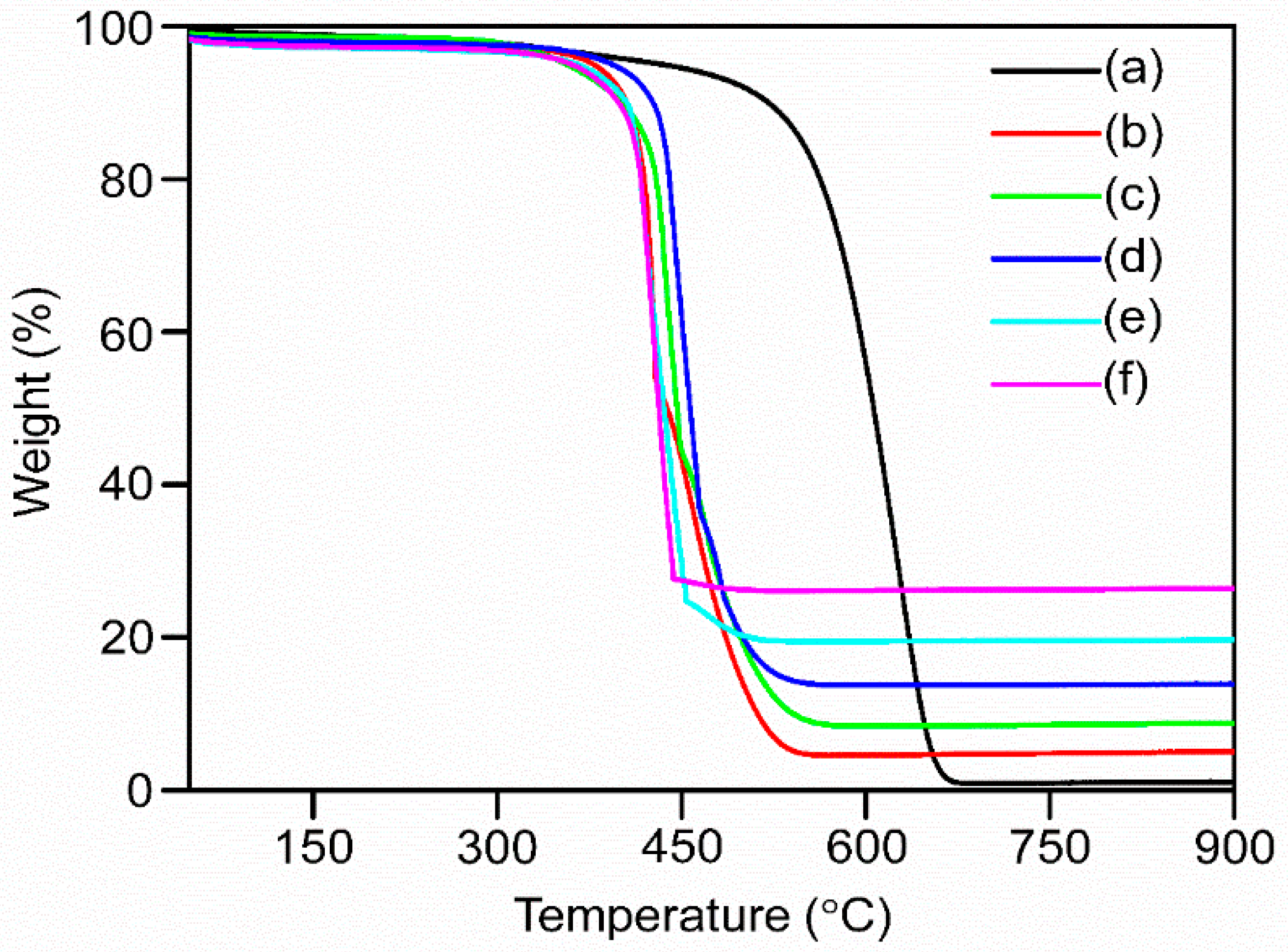
3.1. Structural, Surface Property and Thermal Behavior Investigation of the Nanocomposites
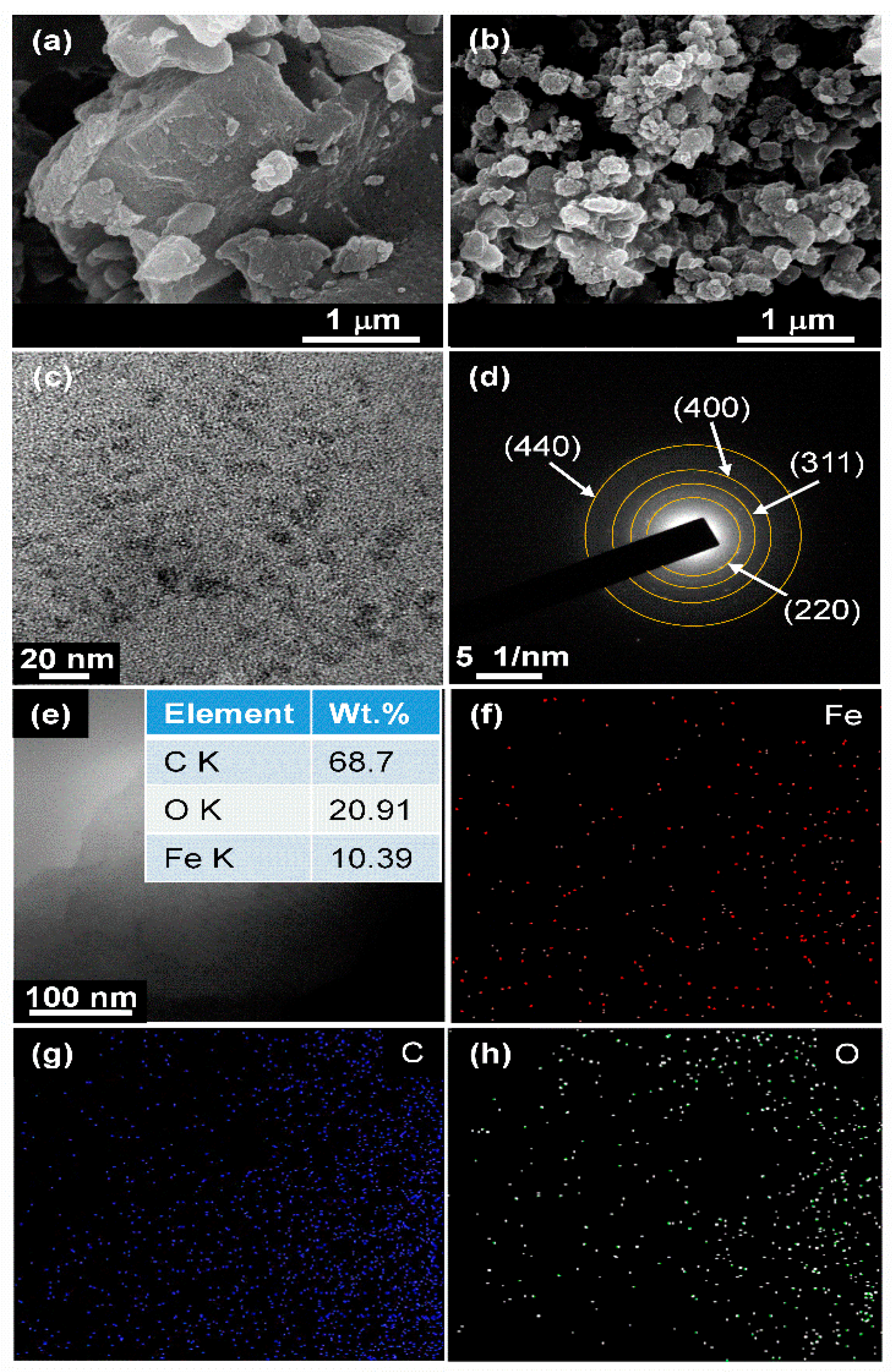
3.2. Morphological and Microstructural Features of the Nanocomposite
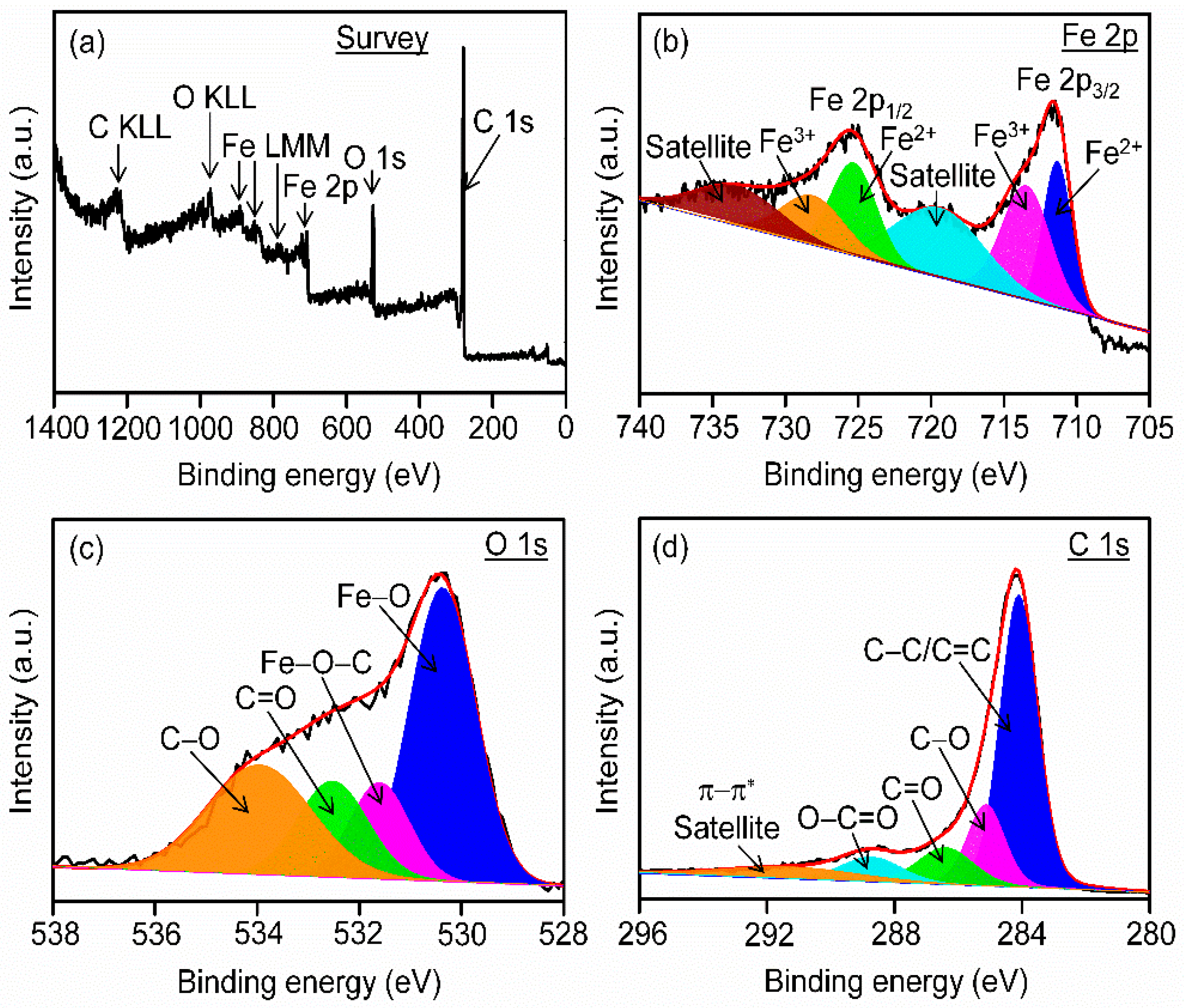
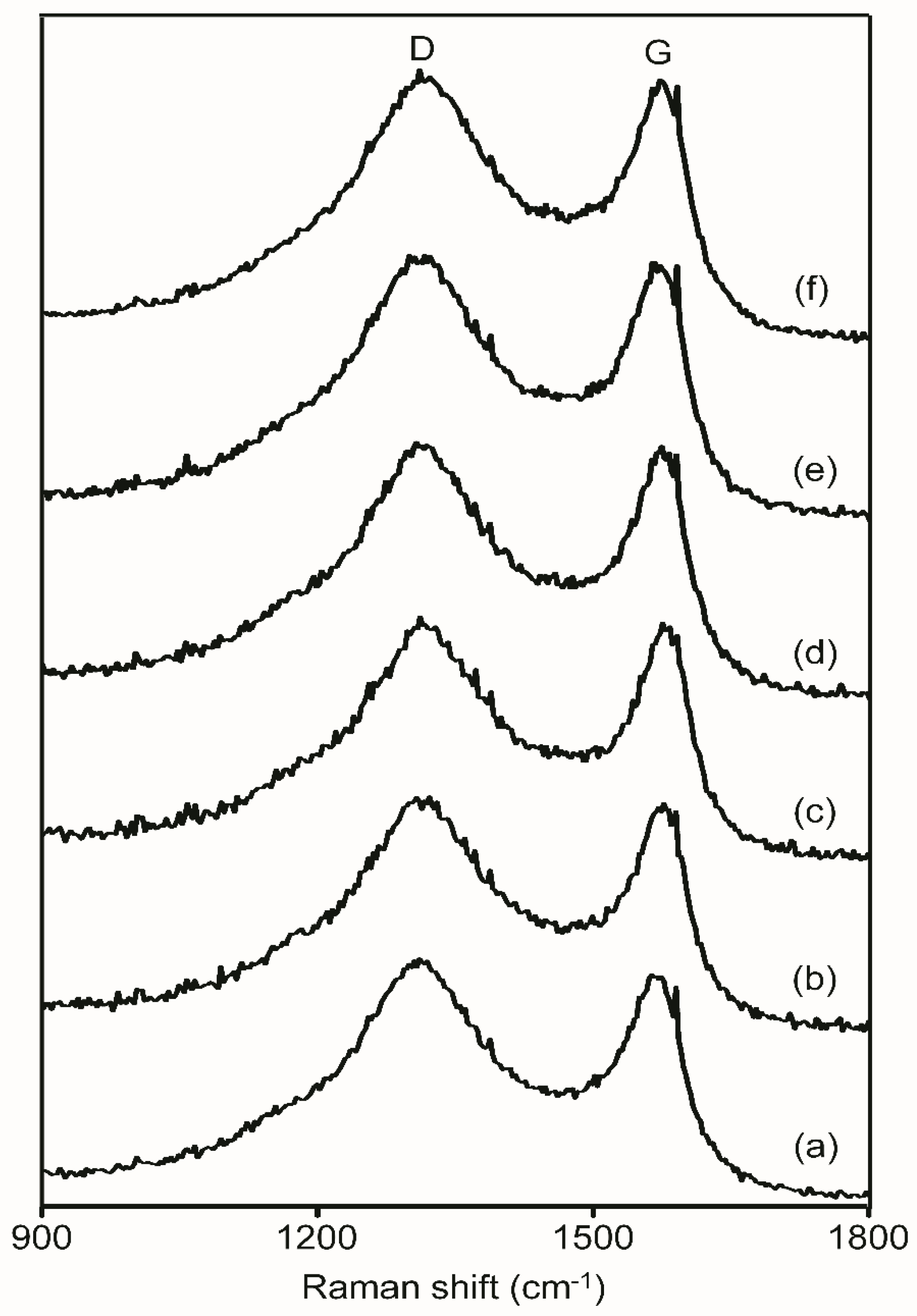
3.3. Analysis of Surface States and Degree of Graphitization
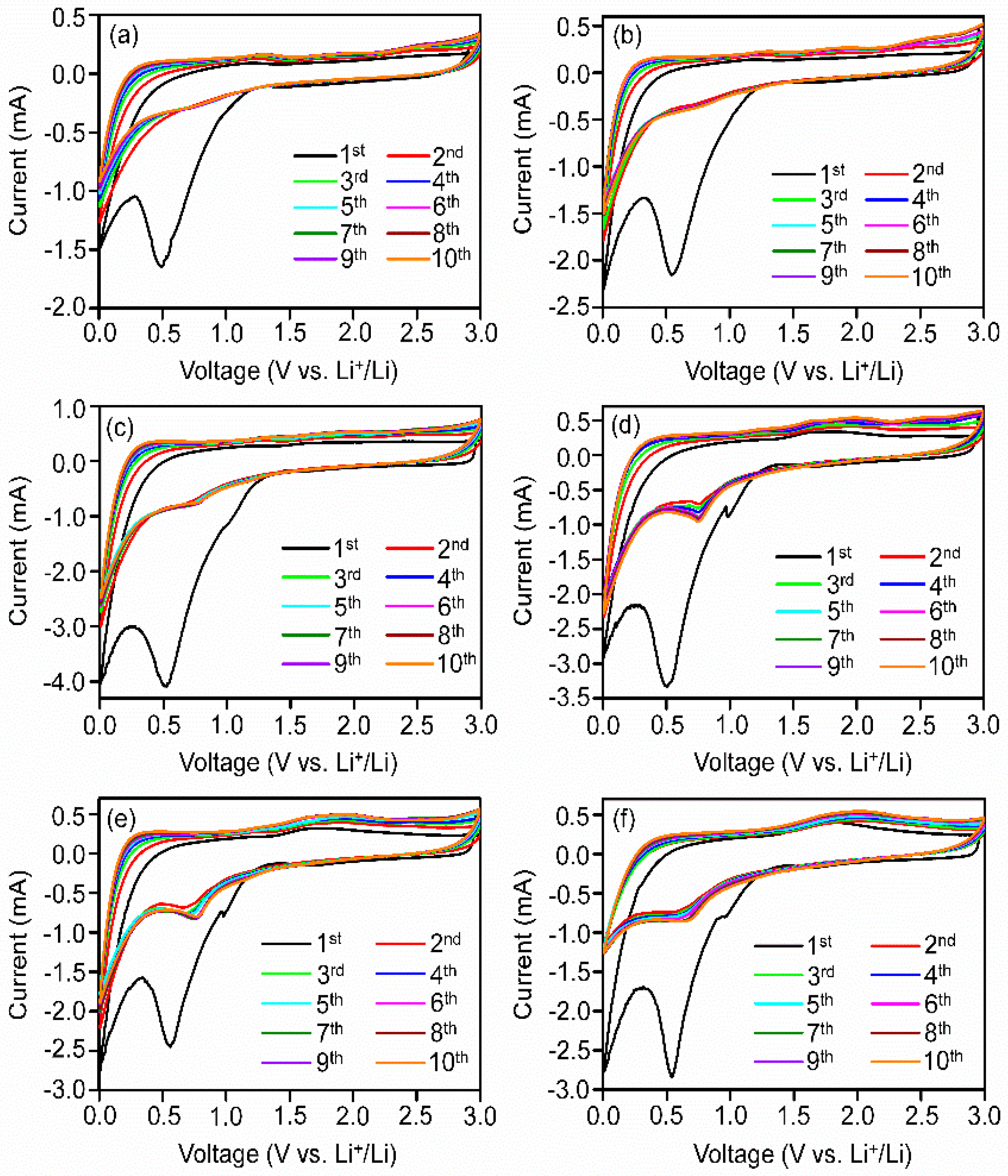
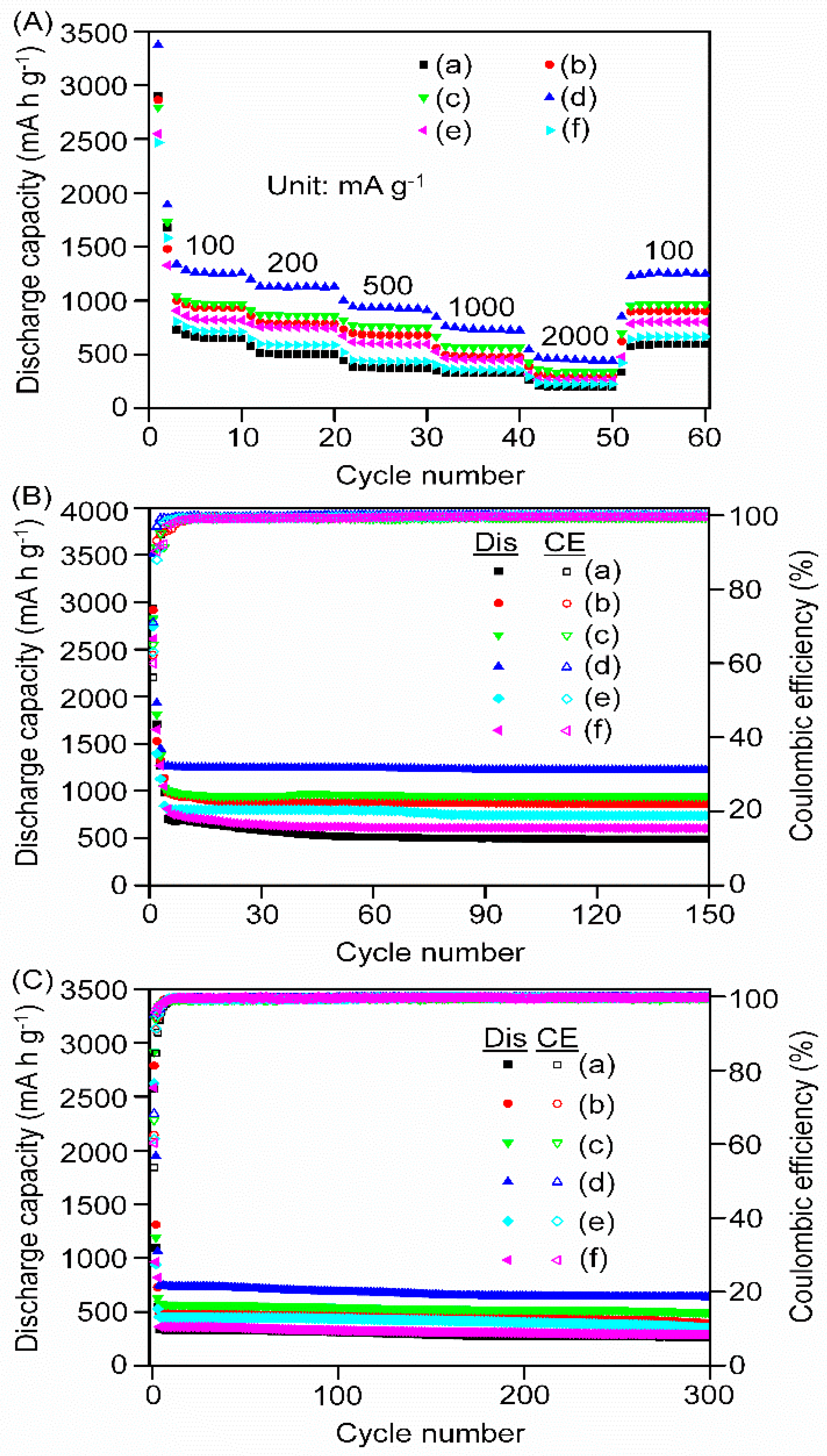
3.4. Electrochemical Performances of the Nanocomposites
3.5. Post-Cycle Evaluation of the Nanocomposite Anode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Link, S.; Neef, C.; Wicke, T. Trends in automotive battery cell design: A statistical analysis of empirical data. Batteries 2023, 9, 261. [Google Scholar] [CrossRef]
- Choi, D.; Shamim, N.; Crawford, A.; Huang, Q.; Vartanian, C.K.; Viswanathan, V.V.; Paiss, M.D.; Alam, M.J.; Reed, D.M.; Sprenkle, V.L. Li-ion battery technology for grid application. J. Power Sources 2021, 511, 230419. [Google Scholar] [CrossRef]
- He, L.; Xu, C.; Ma, P.; Wang, Q. Synthesis and characterization of Mn2O3 nanorods as anode materials for lithium ion batteries. Int. J. Electrochem. Sci. 2020, 15, 5908–5915. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Liu, Y.; Yuan, X.; Guo, S. Tailoring nanostructured MnO2 as anodes for lithium ion batteries with high reversible capacity and initial coulombic efficiency. J. Power Sources 2018, 379, 68–73. [Google Scholar] [CrossRef]
- Dai, L.; Zhong, X.; Zou, J.; Fu, B.; Su, Y.; Ren, C.; Wang, J.; Zhong, G. Highly ordered SnO2 nanopillar array as binder-free anodes for long-life and high-rate Li-ion batteries. Nanomaterials 2021, 11, 1307. [Google Scholar] [CrossRef]
- Chang, J.H.; Cheong, J.Y.; Shim, Y.; Park, J.Y.; Kim, S.J.; Lee, J.; Lee, H.J.; Lim, H.; Liu, W.; Zhang, Q.; et al. Unravelling high volumetric capacity of Co3O4 nanograin-interconnected secondary particles for lithium-ion battery anodes. J. Mater. Chem. A 2021, 9, 6242–6251. [Google Scholar] [CrossRef]
- Chang, L.; Wang, K.; Huang, L.; He, Z.; Shao, H.; Wang, J. Hierarchically porous CoO microsphere films with enhanced lithium/sodium storage properties. J. Alloys Compd. 2017, 725, 824–834. [Google Scholar] [CrossRef]
- Hwang, J.; Yadav, D.; Yang, H.; Jeon, I.; Yang, D.; Seo, J.-W.; Kang, M.; Jeong, S.-Y.; Cho, C.-R. In situ electrochemical impedance measurements of α-Fe2O3 nanofibers: Unravelling the Li-ion conduction mechanism in Li-ion batteries. Batteries 2022, 8, 44. [Google Scholar] [CrossRef]
- Maroni, F.; Gabrielli, S.; Palmieri, A.; Marcantoni, E.; Croce, F.; Nobili, F. High cycling stability of anodes for lithium-ion batteries based on Fe3O4 nanoparticles and poly(acrylic acid) binder. J. Power Sources 2016, 332, 79–87. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Cao, G.; Zhang, S.; Mu, Y.; Ming, H.; Qiu, J. Fe3O4-based anodes with high conductivity and fast ion diffusivity designed for high-energy lithium-ion batteries. Energy Fuels 2021, 35, 1810–1819. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ren, Y.; Gu, S.; Wang, Q.; Li, H.; Yue, K.; Gao, T.; Zhou, G. NiFe2V2O8@N-doped carbon yolk-double shell spheres for efficient lithium storage. Chem. Eng. J. 2023, 454, 140045. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Wang, Y.; Gong, Q.; Gu, S.; Gao, T.; Sun, X.; Zhou, G. Self-template formation of porous yolk-shell structure Mo-doped NiCo2O4 toward enhanced lithium storage performance as anode material. J. Mater. Sci. Technol. 2022, 102, 186–194. [Google Scholar] [CrossRef]
- Maroni, F.; Bruni, P.; Suzuki, N.; Aihara, Y.; Gabrielli, S.; Carbonari, G.; Agostini, M.; Branchi, M.; Ferrari, S.; Navarra, M.A.; et al. Highly stable Fe3O4/C composite: A candidate material for all solid-state lithium-ion batteries. J. Electrochem. Soc. 2020, 167, 070556. [Google Scholar] [CrossRef]
- Rehman, W.; Jiang, Z.; Qu, Z.; Wang, X.; Du, X.; Ghani, A.; Kabir, F.; Xu, Y. Porous carbon derived from cherry blossom leaves treatment with Fe3O4 enhanced the electrochemical performance of a lithium storage anode. Electrochim. Acta 2023, 455, 142426. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Wei, D.; Anitowska, E.; Bellani, V.; Ortolani, L.; Morandi, V.; Gazzano, M.; Zanelli, A.; Borini, S.; Palermo, V. Electrochemically exfoliated graphene oxide/iron oxide composite foams for lithium storage, produced by simultaneous graphene reduction and Fe(OH)3 condensation. Carbon 2015, 84, 254–262. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Lin, C.-W.; Lai, Y.-H.; Zeng, Y.-H.; Chen, P.-H.; Kao, H.-M.; Yang, Y.-C. Insight into the superior lithium storage properties of ultrafine CoO nanoparticles confined in a 3D bimodal ordered mesoporous carbon CMK-9 anode. ChemSusChem 2020, 13, 2952–2965. [Google Scholar] [CrossRef]
- Ban, C.; Wu, Z.; Gillaspie, D.T.; Chen, L.; Yan, Y.; Blackburn, J.L.; Dillon, A.C. Nanostructured Fe3O4/SWNT electrode: Binder-free and high-rate Li-ion anode. Adv. Mater. 2010, 22, E145–E149. [Google Scholar] [CrossRef]
- Velásquez, C.A.; Vásquez, F.A.; Alvarez-Láinez, M.; Zapata-González, A.; Calderón, J.A. Carbon nanofibers impregnated with Fe3O4 nanoparticles as a flexible and high capacity negative electrode for lithium-ion batteries. J. Alloys Compd. 2021, 862, 158045. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, Y.; Zhao, Y.; Tian, Y.; Kurmanbayeva, I.; Bakenov, Z. Hierarchical sandwiched Fe3O4@C/graphene composite as anode material for lithium-ion batteries. J. Electroanal Chem. 2019, 847, 113240. [Google Scholar] [CrossRef]
- Zhao, P.; Jiang, L.; Li, P.; Xiong, B.; Zhou, N.; Liu, C.; Jia, J.; Ma, G.; Zhang, M. Tailored engineering of Fe3O4 and reduced graphene oxide coupled architecture to realize the full potential as electrode materials for lithium-ion batteries. J. Colloid Interface Sci. 2023, 634, 737–746. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, X.; Li, Y.; Song, J.; Tian, Q.; Yang, L.; Sui, Z. Dispersive Fe3O4 encapsulated in porous carbon for high capacity and long life anode of lithium-ion batteries. J. Alloys Compd. 2022, 899, 163342. [Google Scholar] [CrossRef]
- Hwang, J.-K.; Lim, H.-S.; Sun, Y.-K.; Suh, K.-D. Monodispersed hollow carbon/Fe3O4 composite microspheres for high performance anode materials in lithium-ion batteries. J. Power Sources 2013, 244, 538–543. [Google Scholar] [CrossRef]
- Jia, P.; Sun, J.; Jiang, Z.; Wang, W.; Song, Z.; Mao, Y.; Zhao, X. Construction of N-doped porous carbon-coated Fe3O4 with efficient ion transfer performance for enhanced-performance lithium storage. Electrochim. Acta 2022, 428, 140935. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fan, Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front. 2017, 1, 1001–1027. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.Q.; Shi, Y.F.; Yang, H.F.; Li, Z.; Yu, C.Z.; Tu, B.; Zhao, D.Y. Ordered mesoporous polymers and homologous carbon frameworks: Amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 2005, 44, 7053–7059. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.Q.; Shi, Y.F.; Cheng, L.; Feng, D.; Wu, Z.X.; Chen, Z.X.; Wan, Y.; Stein, A.; et al. A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Lu, A.-H.; Schüth, F. Nanocasting: A versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 2136–2137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, C.; Dai, S. Nano/microporous materials: Mesoporous and surface-functionalized mesoporous carbon. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–18. [Google Scholar] [CrossRef]
- Wu, F.; Huang, R.; Mu, D.; Shen, X.; Wu, B. A novel composite with highly dispersed Fe3O4 nanocrystals on ordered mesoporous carbon as an anode for lithium ion batteries. J. Alloys Compd. 2014, 585, 783–789. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, X. Encapsulated Fe3O4 into tubular mesoporous carbon as a superior performance anode material for lithium-ion batteries. J. Alloys Compd. 2020, 815, 152542. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, H.; Jiang, Y.; Jiao, Z.; Wu, M.; Zhao, B. Ordered CoO/CMK-3 nanocomposites as the anode materials for lithium-ion batteries. J. Power Sources 2010, 195, 2950–2955. [Google Scholar] [CrossRef]
- Panja, T.; Bhattacharjya, D.; Yu, J.-S. Nitrogen and phosphorus co-doped cubic ordered mesoporous carbon as a supercapacitor electrode material with extraordinary cyclic stability. J. Mater. Chem. A 2015, 3, 18001–18009. [Google Scholar] [CrossRef]
- Zhang, P.; Qiu, J.; Zheng, Z.; Liu, G.; Ling, M.; Martens, W. Free-standing and bendable carbon nanotubes/TiO2 nanofibres composite electrodes for flexible lithium ion batteries. Electrochim. Acta 2013, 104, 41–47. [Google Scholar] [CrossRef][Green Version]
- Mao, J.Y.; Niu, D.C.; Zheng, N.; Jiang, G.Y.; Zhao, W.R.; Shi, J.L.; Li, Y.S. Fe3O4-embedded and N-doped hierarchically porous carbon nanospheres as high-performance lithium ion battery anodes. ACS Sustain. Chem. Eng. 2019, 7, 3424–3433. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Li, M.; Lu, L.; Xue, J. Fe3O4 nanoparticles embedded in uniform mesoporous carbon spheres for superior high-rate battery applications. Adv. Funct. Mater. 2014, 24, 319–326. [Google Scholar] [CrossRef]
- Cui, Y.P.; Feng, W.T.; Liu, W.; Li, J.J.; Zhang, Y.; Du, Y.X.; Li, M.Z.; Huang, W.; Wang, H.L.; Liu, S. Template-assisted loading of Fe3O4 nanoparticles inside hollow carbon “rooms” to achieve high volumetric lithium storage. Nanoscale 2020, 12, 10816–10826. [Google Scholar] [CrossRef]
- Kopuklu, B.B.; Tasdemir, A.; Gursel, S.A.; Yurum, A. High stability graphene oxide aerogel supported ultrafine Fe3O4 particles with superior performance as a Li-ion battery anode. Carbon 2021, 174, 158–172. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Tan, Q.; Wan, Q.; Han, K.; Liu, Z.; Li, P.; An, F.; Qu, X. A synergetic strategy for an advanced electrode with Fe3O4 embedded in a 3D N-doped porous graphene framework and a strong adhesive binder for lithium/potassium ion batteries with an ultralong cycle lifespan. J. Mater. Chem. A 2019, 7, 19430–19441. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Lu, B.-J.; Chen, Y.-C.; Lian, J.-W.; Kao, H.-M.; Yang, Y.-C. Pinecone-derived biomass carbons as anodes for lithium and sodium-ion batteries by template-assisted and chemically activated approaches. J. Power Sources 2023, 580, 233329. [Google Scholar] [CrossRef]
- Tu, M.; Yang, C.; Zhang, R.; Kong, X.; Jia, R.; Yu, L.; Xu, B. One-step engineering carbon supported magnetite nanoparticles composite in a submicron pomegranate configuration for superior lithium-ion storage. Materials 2023, 16, 313. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Deka, J.R.; Zeng, Y.-H.; Hsu, H.-C.; Chen, Y.-C.; Kao, H.-M.; Yang, Y.-C. New insights into the outstanding performances of nickel doped manganese oxide nanoparticles embedded in a 3D carbon nanopipes framework as anode for lithium and sodium-ion batteries. Int. J. Energy Res. 2022, 46, 22454–22473. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Q.; Wen, M.; Wu, Q.; Li, J.; Cui, Y.; Pinna, N.; Fan, Y.; Wu, T. Sea-sponge like structure of nano-Fe3O4 on skeleton-C with long cycle life under high rate for li-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 19656–19663. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Kim, M.-S.; Kim, J.H.; Lim, S.; Yu, J.-S. Ordered multimodal porous carbon with hierarchical nanostructure for high Li storage capacity and good cycling performance. J. Mater. Chem. 2010, 20, 10253–10259. [Google Scholar] [CrossRef]
- Saikia, D.; Wang, T.-H.; Chou, C.-J.; Fang, J.; Tsai, L.-D.; Kao, H.-M. A comparative study of ordered mesoporous carbons with different pore structures as anode materials for lithium-ion batteries. RSC Adv. 2015, 5, 42922–42930. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Chen, D.; Zhao, J. A facile electrophoretic deposition route to the Fe3O4/CNTs/rGO composite electrode as a binder-free anode for lithium ion battery. ACS Appl. Mater. Interfaces 2016, 8, 26730–26739. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhang, K.; Jia, K.; Liu, G.; He, X.; Liu, W.; Li, K.; Zhang, Z. Nitrogen-doped graphene nanosheet coated nanospherical Fe3O4 from zeolitic imidazolate frameworks template as anode of lithium ion batteries. Energy Fuels 2020, 34, 14986–14994. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, B.; Zheng, Z.; Yang, J.; Yang, Z.; Zhang, P.; Ren, W.; Yan, X. Facile preparation of one-dimensional wrapping structure: Graphene nanoscroll-wrapped of Fe3O4 nanoparticles and its application for lithium-ion battery. ACS Appl. Mater. Interfaces 2014, 6, 9890–9896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, D.; Zheng, K. Fe3O4/Fe/Carbon composite and its application as anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, K.; Zhu, S.; Huang, L.; Chen, M.; Guo, J.; Pei, S.; Shao, H.; Wang, J. MOF-derived hierarchical MnO-doped Fe3O4@C composite nanospheres with enhanced lithium storage. ACS Appl. Mater. Interfaces 2018, 10, 10974–10985. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Wang, S.; Zheng, Y.; Qiao, L.; He, D. Carbon-wrapped Fe3O4 nanoparticle films grown on nickel foam as binder-free anodes for high-rate and long-life lithium storage. ACS Appl. Mater. Interfaces 2014, 6, 648–654. [Google Scholar] [CrossRef]
- Staffolani, A.; Darjazi, H.; Carbonari, G.; Maroni, F.; Gabrielli, S.; Nobili, F. Fe3O4/graphene composite anode material for fast-charging Li-ion batteries. Molecules 2021, 26, 4316. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shen, X.; Chen, K.; Wu, Q.; Zhang, P.; Zhang, X.; Diao, G. Nitrogen-doped mesoporous carbon-encapsulation urchin-like Fe3O4 as anode materials for high performance Li-ions batteries. Electrochim. Acta 2016, 195, 94–105. [Google Scholar] [CrossRef]
- Srinivasan, N.R.; Mitra, S.; Bandyopadhyaya, R. Improved electrochemical performance of SnO2–mesoporous carbon hybrid as a negative electrode for lithium ion battery applications. Phys. Chem. Chem. Phys. 2014, 16, 6630–6640. [Google Scholar] [CrossRef]
- Gao, C.W.; Jiang, Z.J.; Wang, P.X.; Jensen, L.R.; Zhang, Y.F.; Yue, Y.Z. Optimized assembling of MOF/SnO2/Graphene leads to superior anode for lithium ion batteries. Nano Energy 2020, 74, 104868. [Google Scholar] [CrossRef]

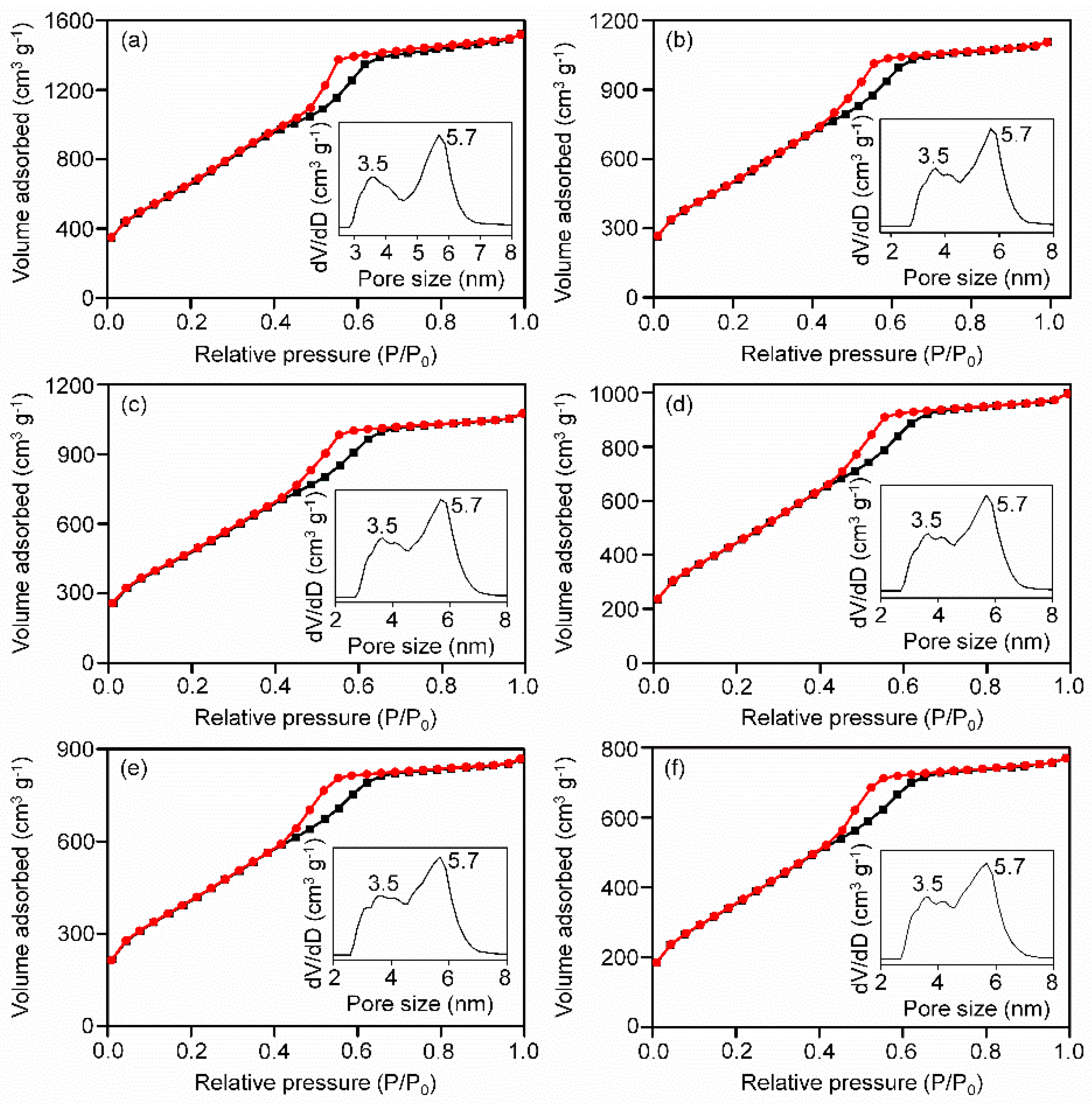



| Materials | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| Pristine CMK-9 | 2487 | 2.36 | 3.5, 5.7 |
| Fe3O4(4)@C9 | 1859 | 1.72 | 3.5, 5.7 |
| Fe3O4(8)@C9 | 1785 | 1.66 | 3.5, 5.7 |
| Fe3O4(13)@C9 | 1654 | 1.55 | 3.5, 5.7 |
| Fe3O4(18)@C9 | 1497 | 1.34 | 3.5, 5.7 |
| Fe3O4(25)@C9 | 1312 | 1.20 | 3.5, 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, J.R.; Saikia, D.; Lai, Y.-H.; Kao, H.-M.; Yang, Y.-C. Fe3O4 Nanoparticle-Decorated Bimodal Porous Carbon Nanocomposite Anode for High-Performance Lithium-Ion Batteries. Batteries 2023, 9, 482. https://doi.org/10.3390/batteries9100482
Deka JR, Saikia D, Lai Y-H, Kao H-M, Yang Y-C. Fe3O4 Nanoparticle-Decorated Bimodal Porous Carbon Nanocomposite Anode for High-Performance Lithium-Ion Batteries. Batteries. 2023; 9(10):482. https://doi.org/10.3390/batteries9100482
Chicago/Turabian StyleDeka, Juti Rani, Diganta Saikia, Yuan-Hung Lai, Hsien-Ming Kao, and Yung-Chin Yang. 2023. "Fe3O4 Nanoparticle-Decorated Bimodal Porous Carbon Nanocomposite Anode for High-Performance Lithium-Ion Batteries" Batteries 9, no. 10: 482. https://doi.org/10.3390/batteries9100482
APA StyleDeka, J. R., Saikia, D., Lai, Y.-H., Kao, H.-M., & Yang, Y.-C. (2023). Fe3O4 Nanoparticle-Decorated Bimodal Porous Carbon Nanocomposite Anode for High-Performance Lithium-Ion Batteries. Batteries, 9(10), 482. https://doi.org/10.3390/batteries9100482







