Effect of Carrier Film Phase Conversion Time on Polyacrylate Polymer Electrolyte Properties in All-Solid-State LIBs
Abstract
:1. Introduction
2. Experiment
2.1. Materials
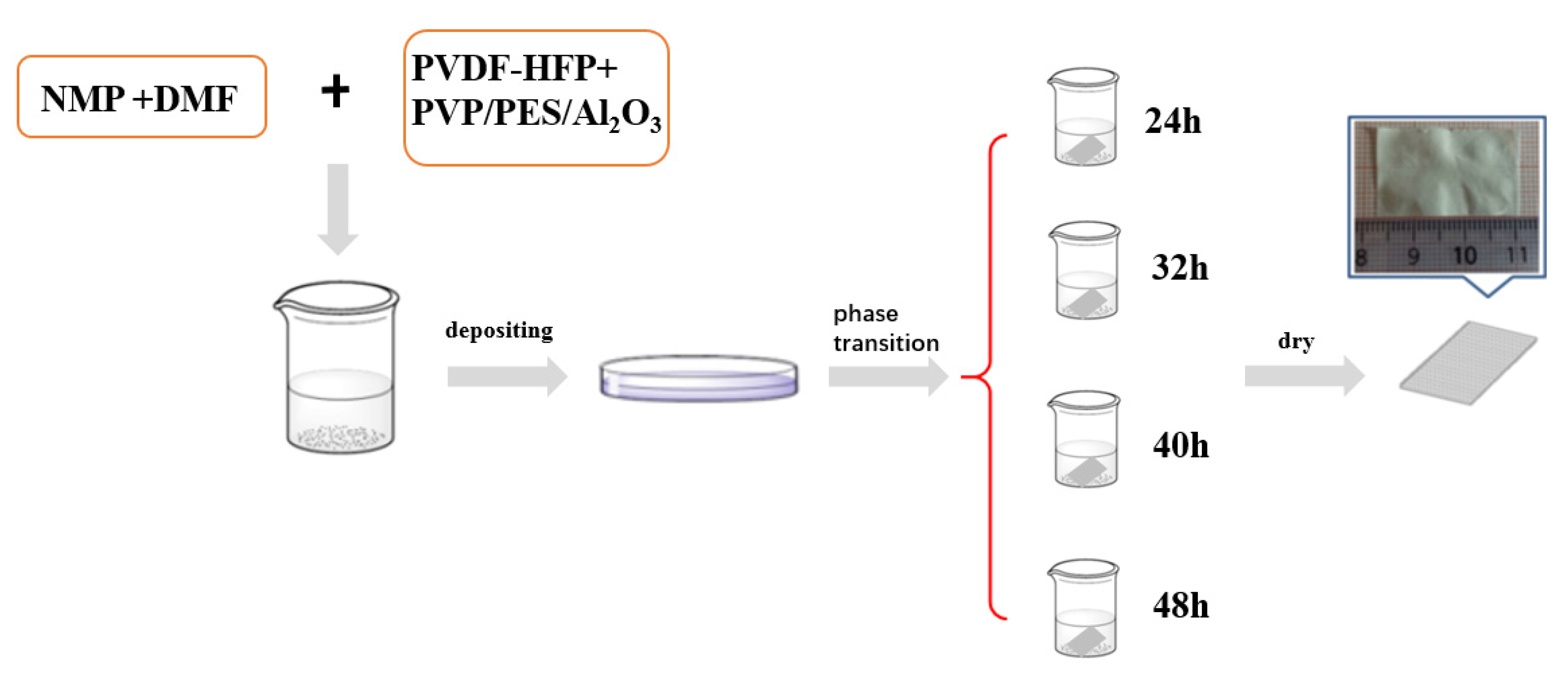
2.2. Preparation of Carrier Film and Polymer Electrolyte with Different Phase Transition Times
2.3. Preparation Method of Polymer Electrolyte
2.4. Material Characterization Method
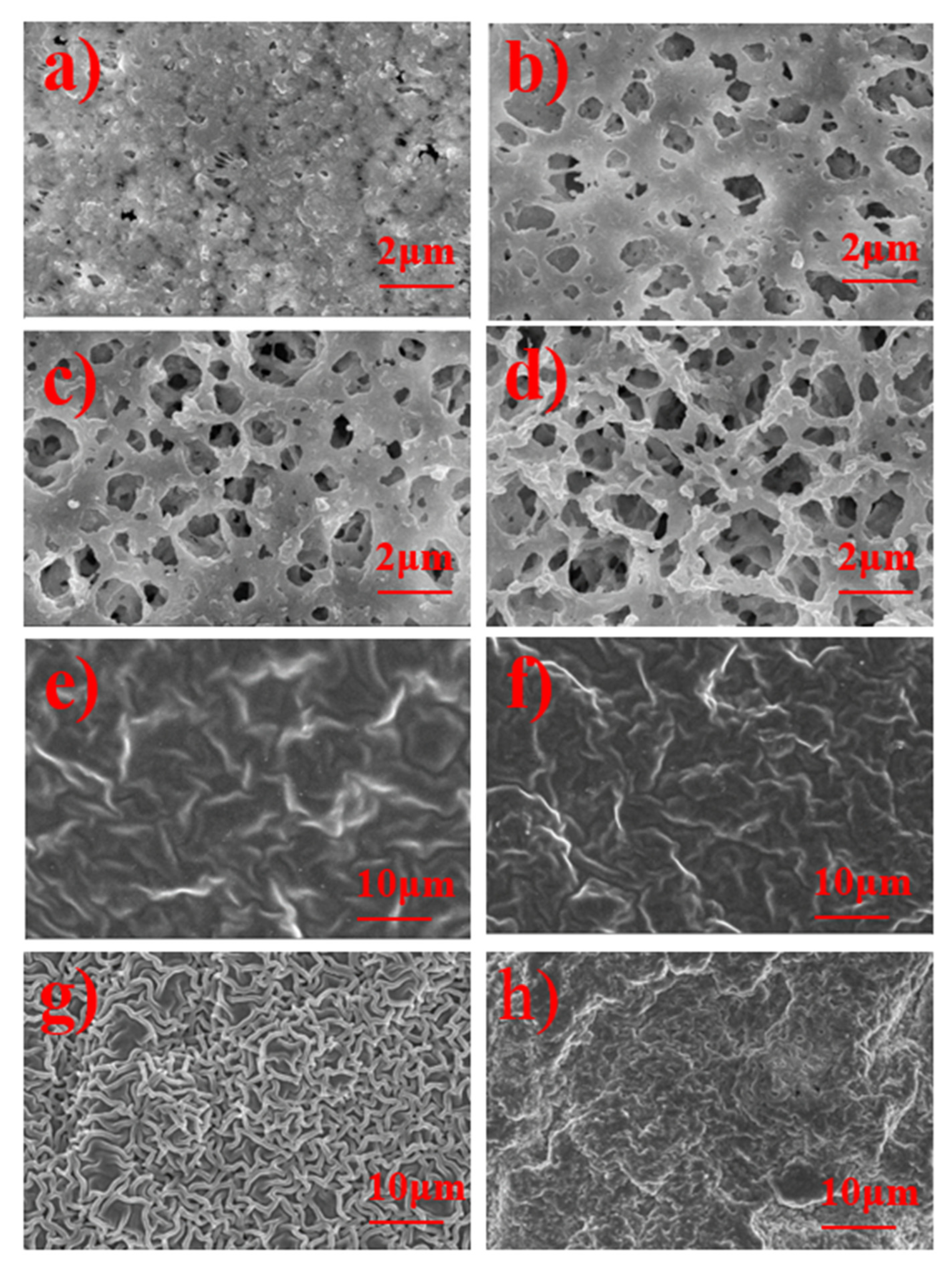
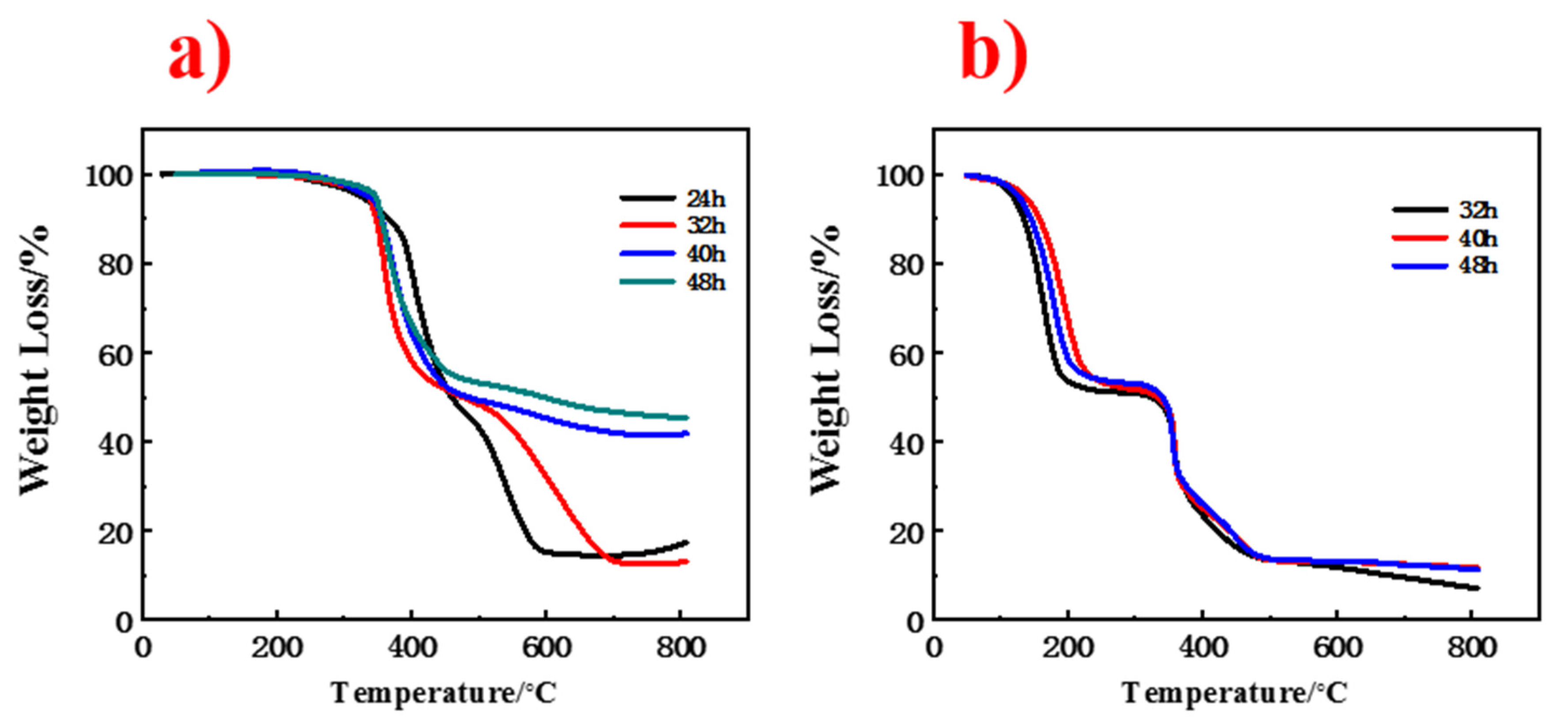
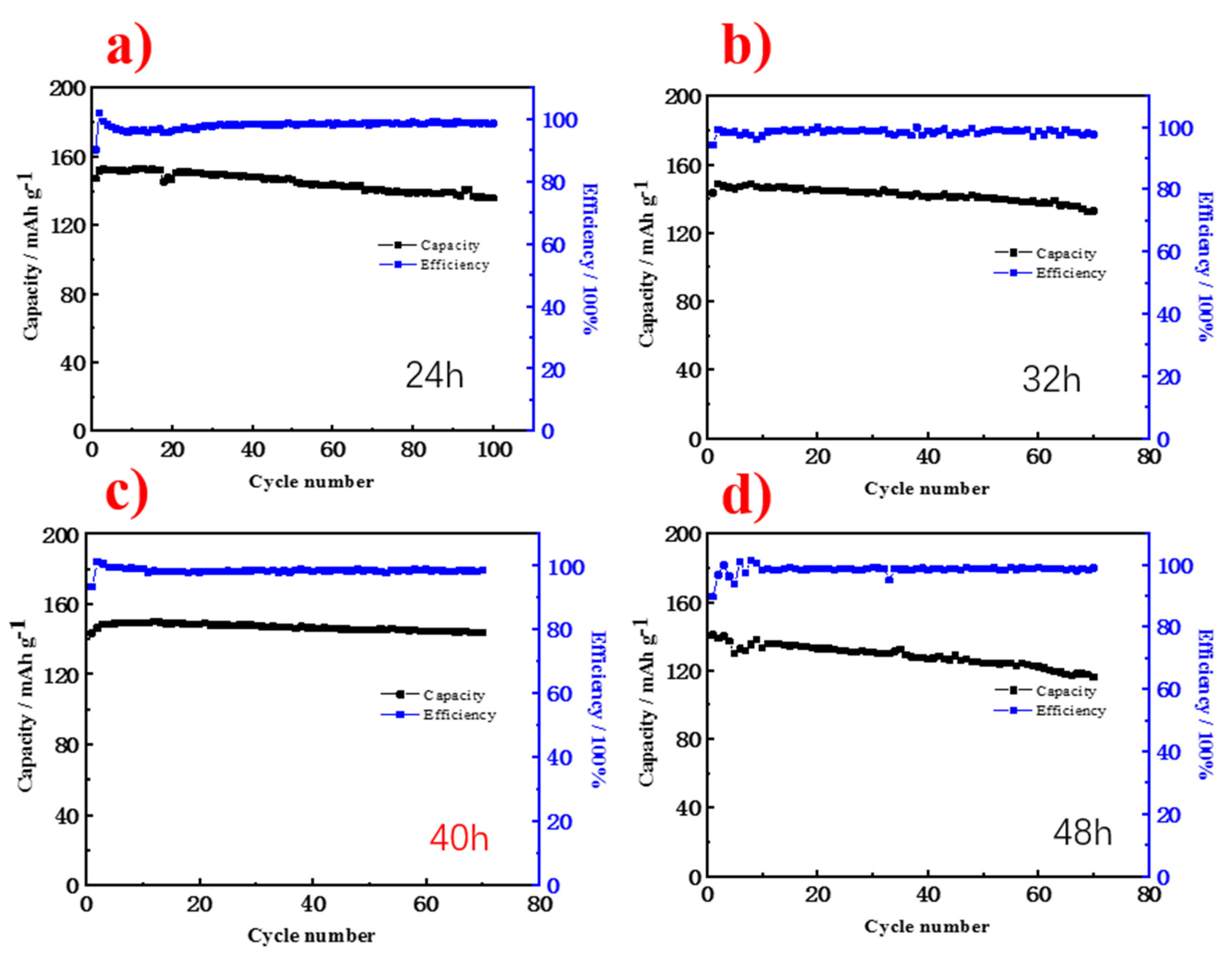
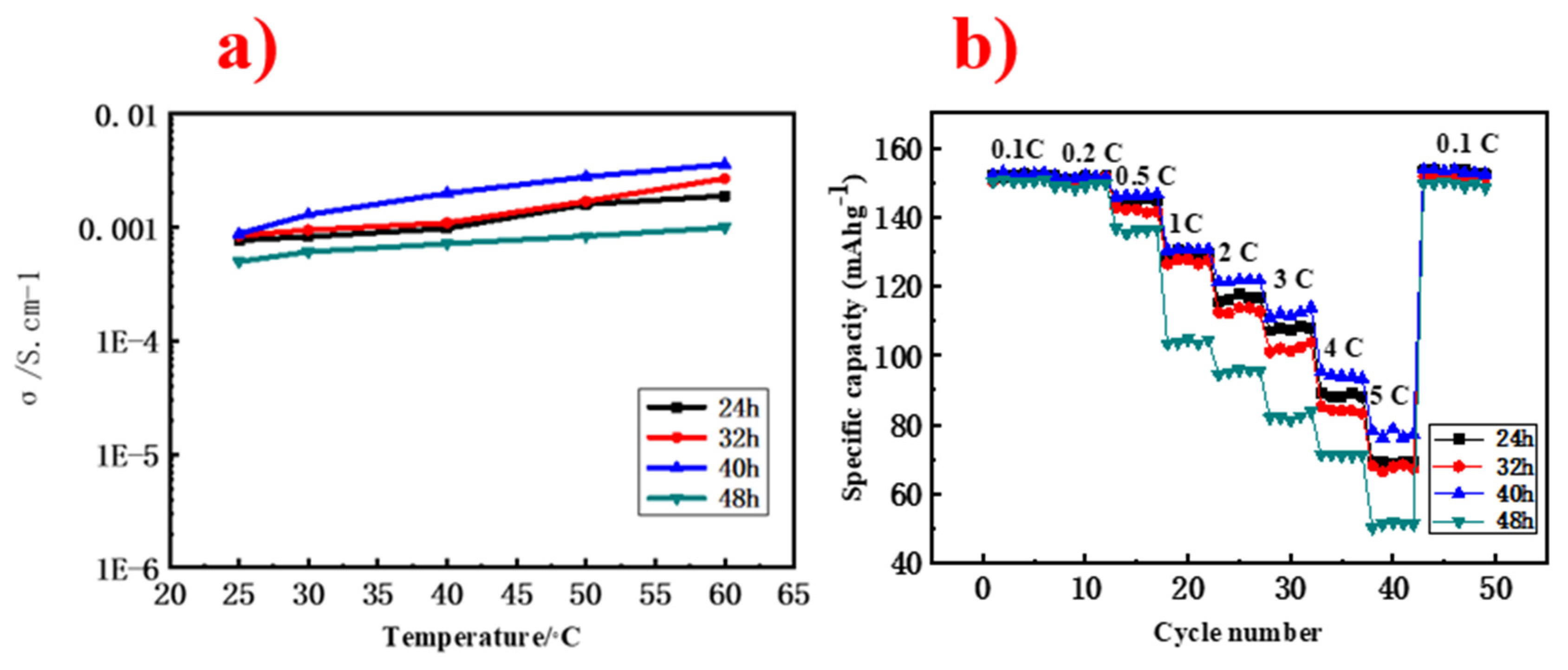
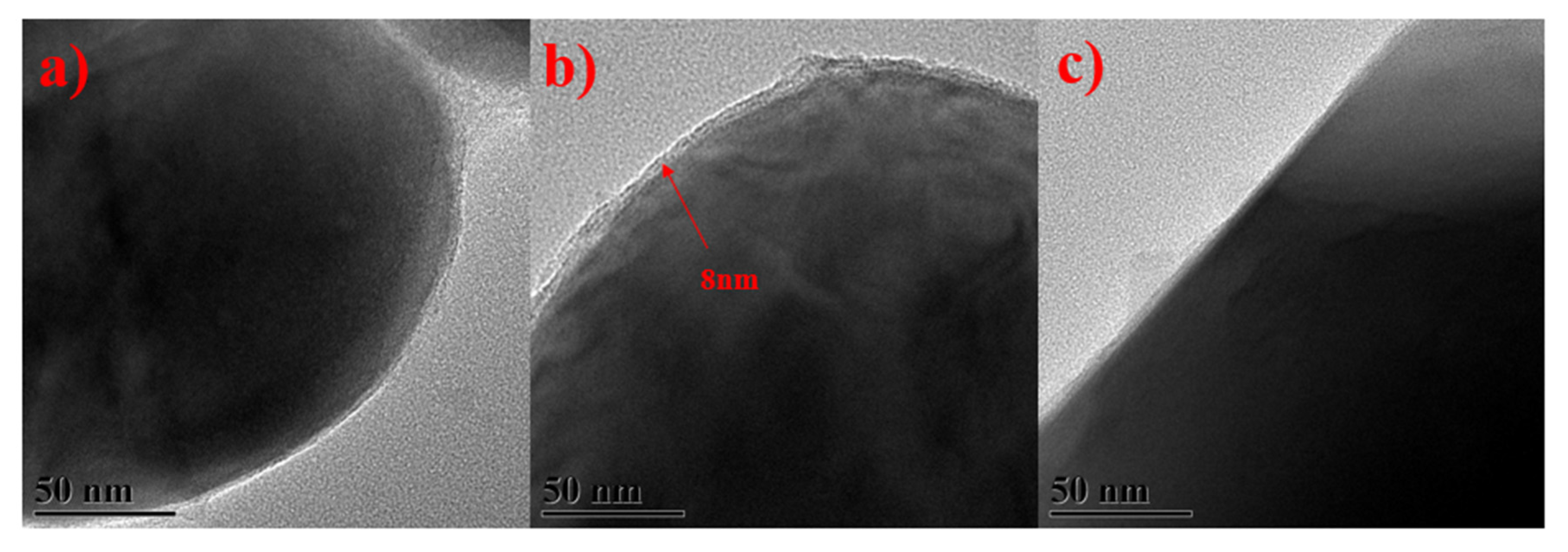
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Antonio, R.G.; Choy, K.L. Boron nitride enhanced polymer/salt hybrid electrolytes for all-solid-state lithium ion batteries. J. Power Sources 2019, 435, 226736. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Hu, W.W.; Liu, Q.L.; Zhu, A.M. Chitosan/polyvinylpyrrolidone-silica hybrid membranes for pervaporation separation of methanol/ethylene glycol azeotrope. J. Appl. Polym. Sci. 2013, 129, 3178–3184. [Google Scholar] [CrossRef]
- Liu, L.; Mo, J.; Li, J.; Liu, J.; Yan, H.; Lyu, J.; Jiang, B.; Chu, L.; Li, M. Comprehensively-modified polymer electrolyte membranes with multifunctional PMIA for highly-stable all-solid-state lithium-ion batteries. J. Energy Chem. 2020, 48, 334–343. [Google Scholar] [CrossRef]
- Pan, Q.; Barbash, D.; Smith, D.M.; Qi, H.; Gleeson, S.E.; Li, C.Y. Correlating Electrode-Electrolyte Interface and Battery Performance in Hybrid Solid Polymer Electrolyte-Based Lithium Metal Batteries. Adv. Energy Mater. 2017, 7, 1701231. [Google Scholar] [CrossRef]
- Kongthong, T.; Tuantranont, S.; Primpray, V.; Sriprachuabwong, C.; Poochai, C. Rechargeable lithium-ion dual carbon batteries utilising a quasi-solid-state anion co-intercalation electrolyte and palm kernel shell-derived hard carbon. Diam. Relat. Mater. 2023, 132, 109680. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, K.-H.; Cho, S.-J.; Yoo, J.; Lee, S.-S.; Lee, S.-Y. Flexible/shape-versatile, bipolar all-solid-state lithium-ion batteries prepared by multistage printing. Energy Environ. Sci. 2018, 11, 321–330. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, R.; He, Y.-B.; Li, F.; Liu, M.; Li, B.; Yang, Q.-H.; Cai, Q.; Kang, F. SiO2Hollow Nanosphere-Based Composite Solid Electrolyte for Lithium Metal Batteries to Suppress Lithium Dendrite Growth and Enhance Cycle Life. Adv. Energy Mater. 2016, 6, 1502214. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, H.; Goodenough, J.B. Low-Cost Hollow Mesoporous Polymer Spheres and All-Solid-State Lithium, Sodium Batteries. Adv. Energy Mater. 2016, 6, 1501802. [Google Scholar] [CrossRef]
- Ha, H.-J.; Kil, E.-H.; Kwon, Y.H.; Kim, J.Y.; Lee, C.K.; Lee, S.-Y. UV-curable semi-interpenetrating polymer network-integrated, highly bendable plastic crystal composite electrolytes for shape-conformable all-solid-state lithium ion batteries. Energy Environ. Sci. 2012, 5, 6491. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhang, P.; Zhu, W.Q.; Zhang, D.; Li, Z.J.; Wang, H.; Sun, H.; Wang, B.; Fan, L.-Z. A two-step strategy for constructing stable gel polymer electrolyte interfaces for long-life cycle lithium metal batteries. J. Mater. 2022, 8, 1048–1057. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Xu, J.; Jing, M.; Yao, S.; Shen, X.; Li, S.; Tu, F. Boosting the performance of poly (ethylene oxide)-based solid polymer electrolytes by blending with poly (vinylidene fluoride-co-hexafluoropropylene) for solid-state lithium-ion batteries. Int. J. Energy Res. 2020, 44, 7831–7840. [Google Scholar] [CrossRef]
- Sha, Y.; Yu, T.; Dong, T.; Wu, X.; Tao, H.; Zhang, H. In Situ Network Electrolyte Based on a Functional Polymerized Ionic Liquid with High Conductivity toward Lithium Metal Batteries. ACS Appl. Energy Mater. 2021, 4, 14755–14765. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; He, K.; Meng, X.; Que, L.; Wang, Z. Effect of UV light polymerization time on the properties of plastic crystal composite polyacrylate polymer electrolyte for all solid-state lithium-ion batteries. J. Appl. Polym. Sci. 2022, 139, e52001. [Google Scholar] [CrossRef]
- Doyle, E.S.; Ford, H.O.; Webster, D.N.; Giannini, P.J.; Tighe, M.E.; Bartsch, R.; Peaslee, G.F.; Schaefer, J.L. Influence of Inorganic Glass Ceramic Particles on Ion States and Ion Transport in Composite Single-Ion Conducting Gel Polymer Electrolytes with Varying Chain Chemistry. ACS Appl. Polym. Sci. 2022, 4, 1095–1109. [Google Scholar] [CrossRef]
- Deng, L.; Yu, F.-D.; Xia, Y.; Jiang, Y.-S.; Sui, X.-L.; Zhao, L.; Meng, X.-H.; Que, L.-F.; Wang, Z.-B. Stabilizing fluorine to achieve high-voltage and ultra-stable Na3V2(PO4)(2)F-3 cathode for sodium ion batteries. Nano Energy 2021, 82, 105659. [Google Scholar] [CrossRef]
- Wang, D.L.; Li, K.; Teo, W.K. Porous PVDF asymmetric hollow fiber membranes prepared with the use of small molecular additives. J. Membr. Sci. 2000, 178, 13–23. [Google Scholar] [CrossRef]
- Jeon, H.G.; Ito, Y.; Sunohara, Y.; Ichikawa, M. Dependence of photocurrent generation on the crystalline phase of titanyl phthalocyanine film in heterojunction photovoltaic cells. Jpn. J. Appl. Phys. 2015, 54, 091601. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F. A developed asymmetric PVDF hollow fiber membrane structure for CO2 absorption. Int. J. Greenh. Gas Control 2011, 5, 374–380. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Jiang, J.; Li, M.; Shen, Y. Ultrathin Li7La3Zr2O12@PAN composite polymer electrolyte with high conductivity for all-solid-state lithium-ion battery. Solid State Ion. 2020, 347, 115227. [Google Scholar] [CrossRef]
- Wang, L.Y.; Yong, W.F.; Yu, L.E.; Chung, T.S. Design of high efficiency PVDF-PEG hollow fibers for air filtration of ultrafine particles. J. Membr. Sci. 2017, 535, 342–349. [Google Scholar] [CrossRef]
- Liu, S.J.; Liu, M.H.; Xu, Q.; Zeng, G.F. Lithium Ion Conduction in Covalent Organic Frameworks. Chin. J. Struct. Chem. 2022, 41, 2211003-17. [Google Scholar]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.; Yang, Q. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Y.; Li, Z.; Zhang, N.; Zeng, D.; Xu, G.; Zhang, Y.; Sun, Y.; Ke, H.; Cheng, H. An AB alternating diblock single ion conducting polymer electrolyte membrane for all-solid-state lithium metal secondary batteries. J. Membr. Sci. 2018, 566, 181–189. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, J.; Lu, W.; Wang, J.; Nuli, Y. Advanced semi-interpenetrating polymer network gel electrolyte for rechargeable lithium batteries. Electrochim. Acta 2015, 152, 489–495. [Google Scholar] [CrossRef]
- Yang, M.X.; Zhao, B.Q.; Li, J.Y.; Li, S.M.; Zhang, G.; Liu, S.Q.; Cui, Y.; Liu, H. Modified Poly(vinylidene fluoride-co-hexafluoropropylene) Polymer Electrolyte for Enhanced Stability and Polymer Degradation Inhibition toward the Li Metal Anode. ACS Appl. Energy Mater. 2022, 5, 9049–9057. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Q.; Qi, F.; Zhang, J.; Liu, K.; Li, J.; Chen, W.; Du, Y.; Jin, Y.; Liang, Y.; et al. Epoxy containing solid polymer electrolyte for lithium ion battery. Electrochim. Acta 2019, 318, 302–313. [Google Scholar] [CrossRef]
- Tao, C.; Gao, M.-H.; Yin, B.-H.; Li, B.; Huang, Y.-P.; Xu, G.; Bao, J.-J. A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Electrochim. Acta 2017, 257, 31–39. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, R.; Suresh, M.; Das, M.K.; Kar, K.K. Structural and ion transport properties of lithium triflate/poly (vinylidene fluoride-co-hexafluoropropylene)-based polymer electrolytes: Effect of lithium salt concentration. J. Elastom. Plast. 2017, 49, 513–526. [Google Scholar] [CrossRef]
- Zhou, D.; He, Y.-B.; Liu, R.; Liu, M.; Du, H.; Li, B.; Cai, Q.; Yang, Q.-H.; Kang, F. In Situ Synthesis of a Hierarchical All-Solid-State Electrolyte Based on Nitrile Materials for High-Performance Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1500353. [Google Scholar] [CrossRef]
- Lv, P.; Yang, J.; Liu, G.; Liu, H.; Li, S.; Tang, C.; Mei, J.; Li, Y.; Hui, D. Flexible solid electrolyte based on UV cured polyurethane acrylate/succinonitrile-lithium salt composite compatibilized by tetrahydrofuran. Compos. Part B Eng. 2017, 120, 35–41. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, C.; Liu, W.; Pei, H.; Zhan, B.; Zou, W.; Yu, S.; Guo, R.; Xie, J. Constructing Self-Protected Li and Non-Li Candidates for Advanced Lithium Ion and Lithium Metal Batteries. J. Phys. Chem. C 2019, 123, 13318–13323. [Google Scholar] [CrossRef]
- Zhang, S.J.; Lu, Y.; He, K.W.; Que, L.F.; Zhao, L.; Wang, Z.B. The plastic crystal composite polyacrylate polymer electrolyte with a semi-interpenetrating network structure for all-solid-state LIBs. New J. Chem. 2022, 46, 21640–21647. [Google Scholar] [CrossRef]
- Lu, Y.; He, K.-W.; Zhang, S.-J.; Zhou, Y.-X.; Wang, Z.-B. UV-curable-based plastic crystal polymer electrolyte for high-performance all-solid-state Li-ion batteries. Ionics 2018, 25, 1607–1615. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Li, F.; Zhao, J.; Ji, S.; Liu, J.; Huo, Y. Defects-Abundant Ga2O3 Nanobricks Enabled Multifunctional Solid Polymer Electrolyte for Superior Lithium-Metal Batteries. Chem. Eur. J. 2023, 29, e202204035. [Google Scholar] [CrossRef]
- Seongsoo, P.; Rashama, C.; Sang, A.H.; Hamzen, Q.; Moon, J.; Park, M.-S.; Kim, J.H. Ionic conductivity and mechanical properties of the solid electrolyte interphase in lithium metal batteries. Energy Mater. 2023, 3, 300005. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhu, H.; Que, L.; Leng, X.; Zhao, L.; Wang, Z. Effect of Carrier Film Phase Conversion Time on Polyacrylate Polymer Electrolyte Properties in All-Solid-State LIBs. Batteries 2023, 9, 471. https://doi.org/10.3390/batteries9090471
Zhang S, Zhu H, Que L, Leng X, Zhao L, Wang Z. Effect of Carrier Film Phase Conversion Time on Polyacrylate Polymer Electrolyte Properties in All-Solid-State LIBs. Batteries. 2023; 9(9):471. https://doi.org/10.3390/batteries9090471
Chicago/Turabian StyleZhang, Shujian, Hongmo Zhu, Lanfang Que, Xuning Leng, Lei Zhao, and Zhenbo Wang. 2023. "Effect of Carrier Film Phase Conversion Time on Polyacrylate Polymer Electrolyte Properties in All-Solid-State LIBs" Batteries 9, no. 9: 471. https://doi.org/10.3390/batteries9090471
APA StyleZhang, S., Zhu, H., Que, L., Leng, X., Zhao, L., & Wang, Z. (2023). Effect of Carrier Film Phase Conversion Time on Polyacrylate Polymer Electrolyte Properties in All-Solid-State LIBs. Batteries, 9(9), 471. https://doi.org/10.3390/batteries9090471






