AlCl3-NaCl-ZnCl2 Secondary Electrolyte in Next-Generation ZEBRA (Na-ZnCl2) Battery
Abstract
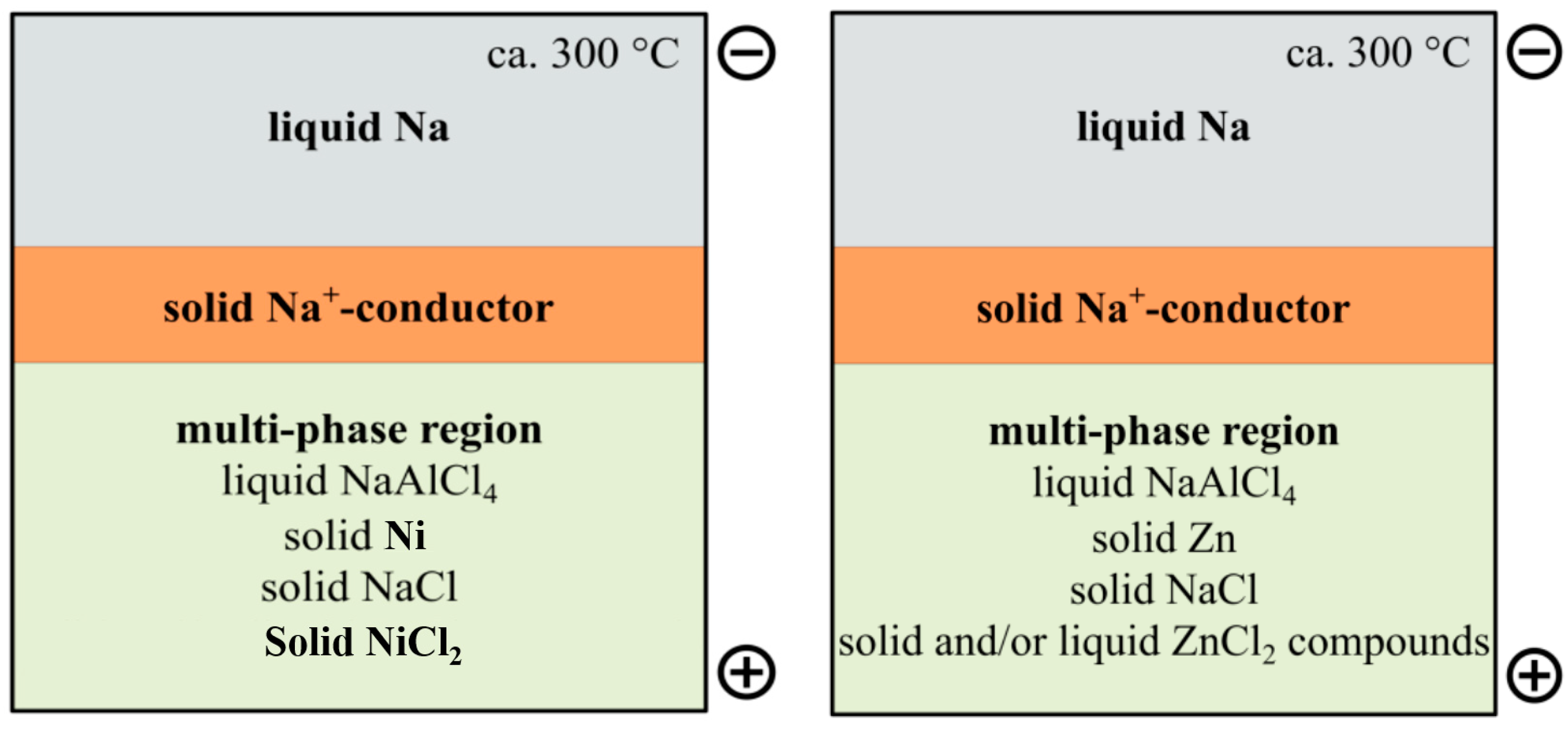
1. Introduction
2. Materials and Methods
2.1. FactSageTM Thermodynamic Simulation
2.2. Sample Preparation
2.3. Differential Scanning Calorimetry (DSC) for Thermal Analysis
2.4. Melting Point Measurement Apparatus (OptiMeltTM)
3. Results and Discussion
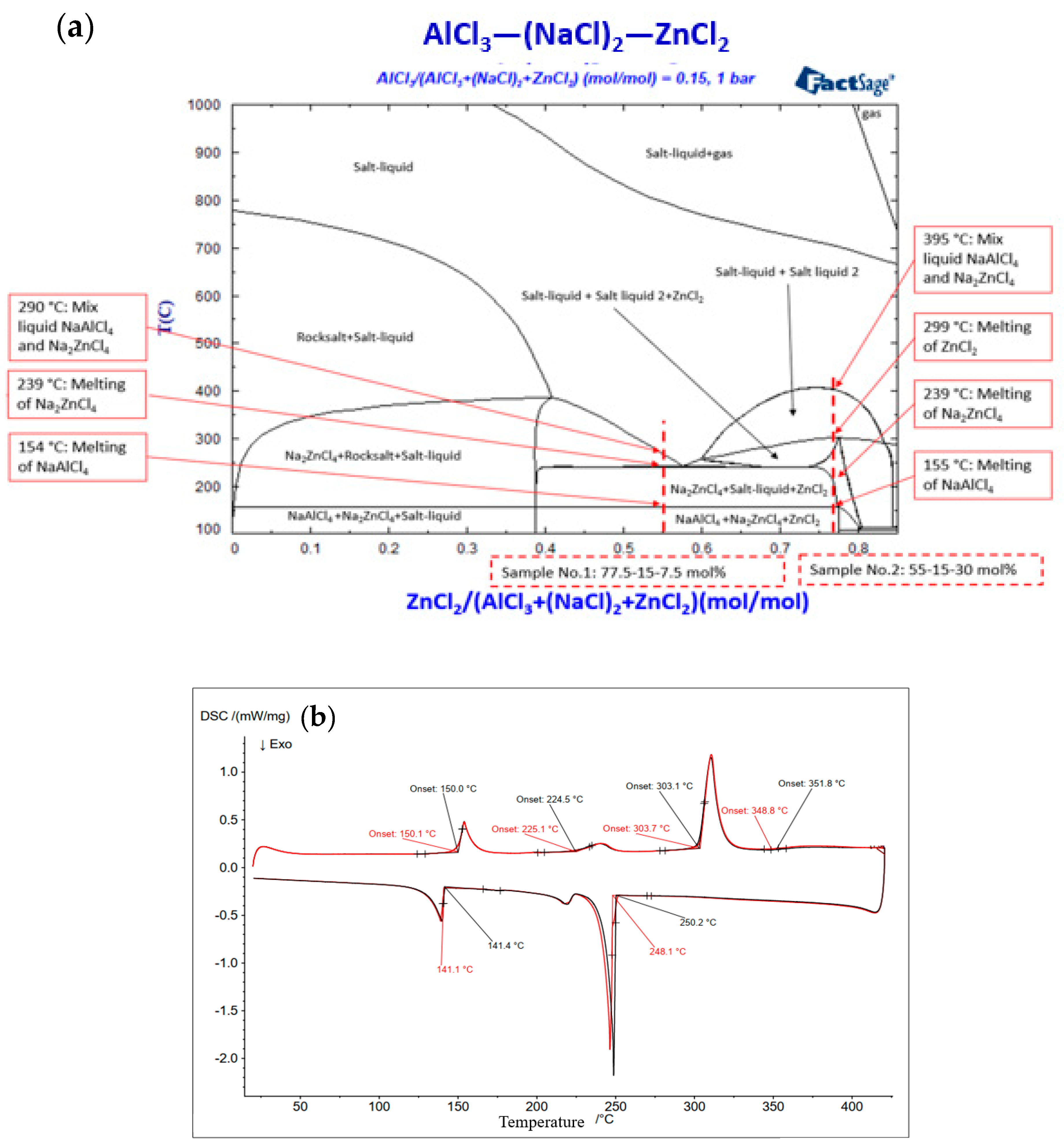
3.1. Simulated Phase Diagrams
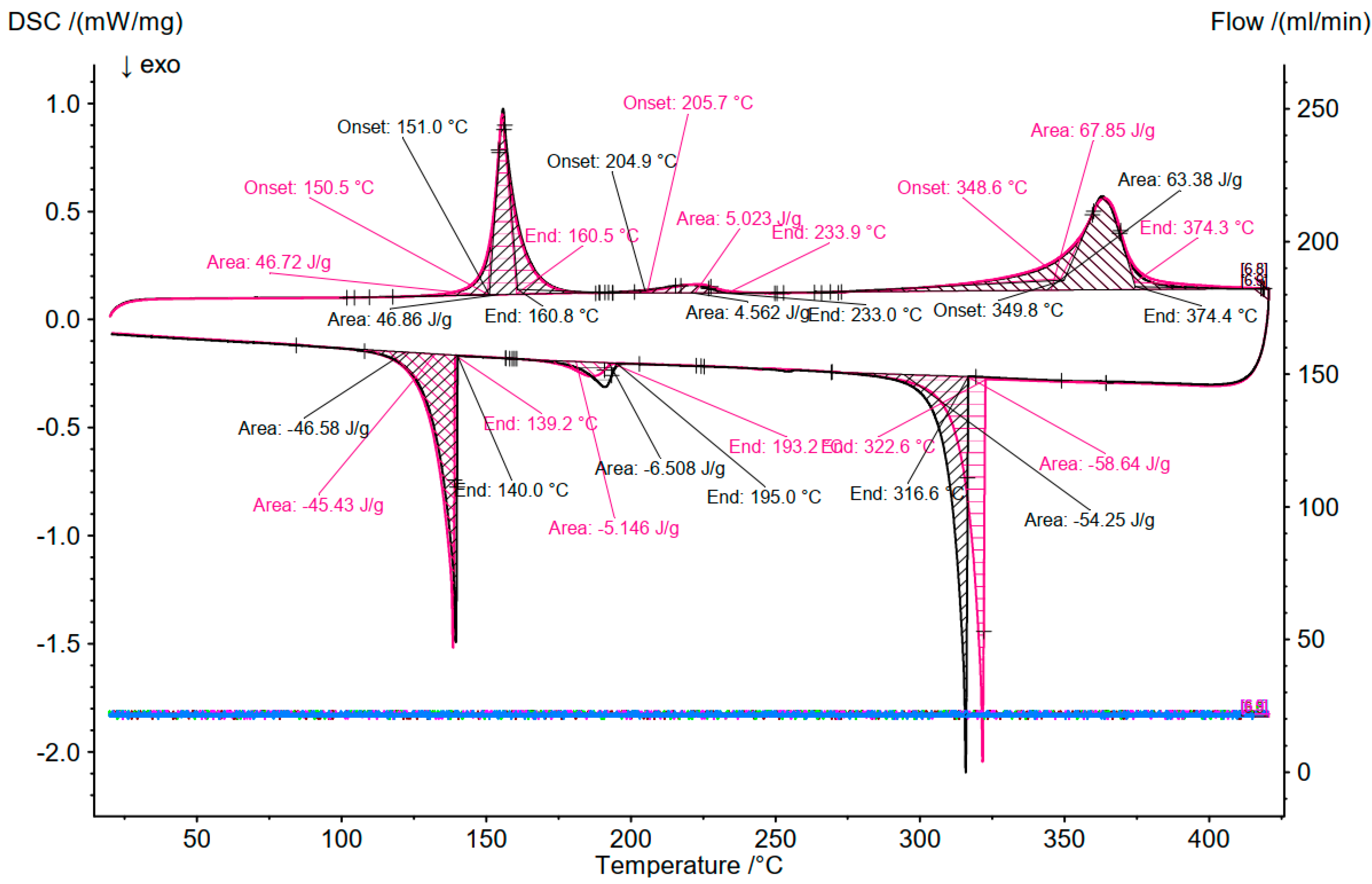
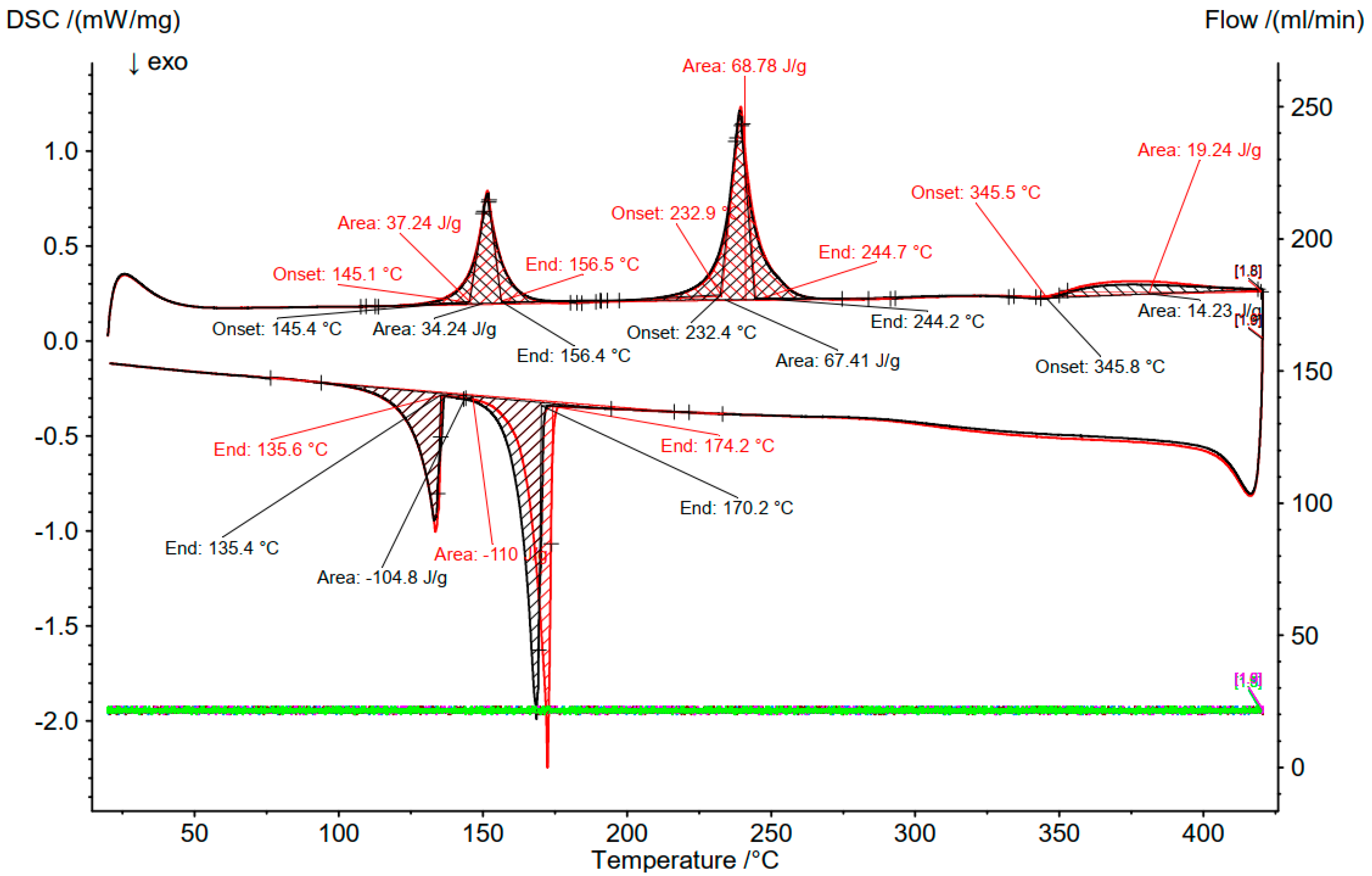
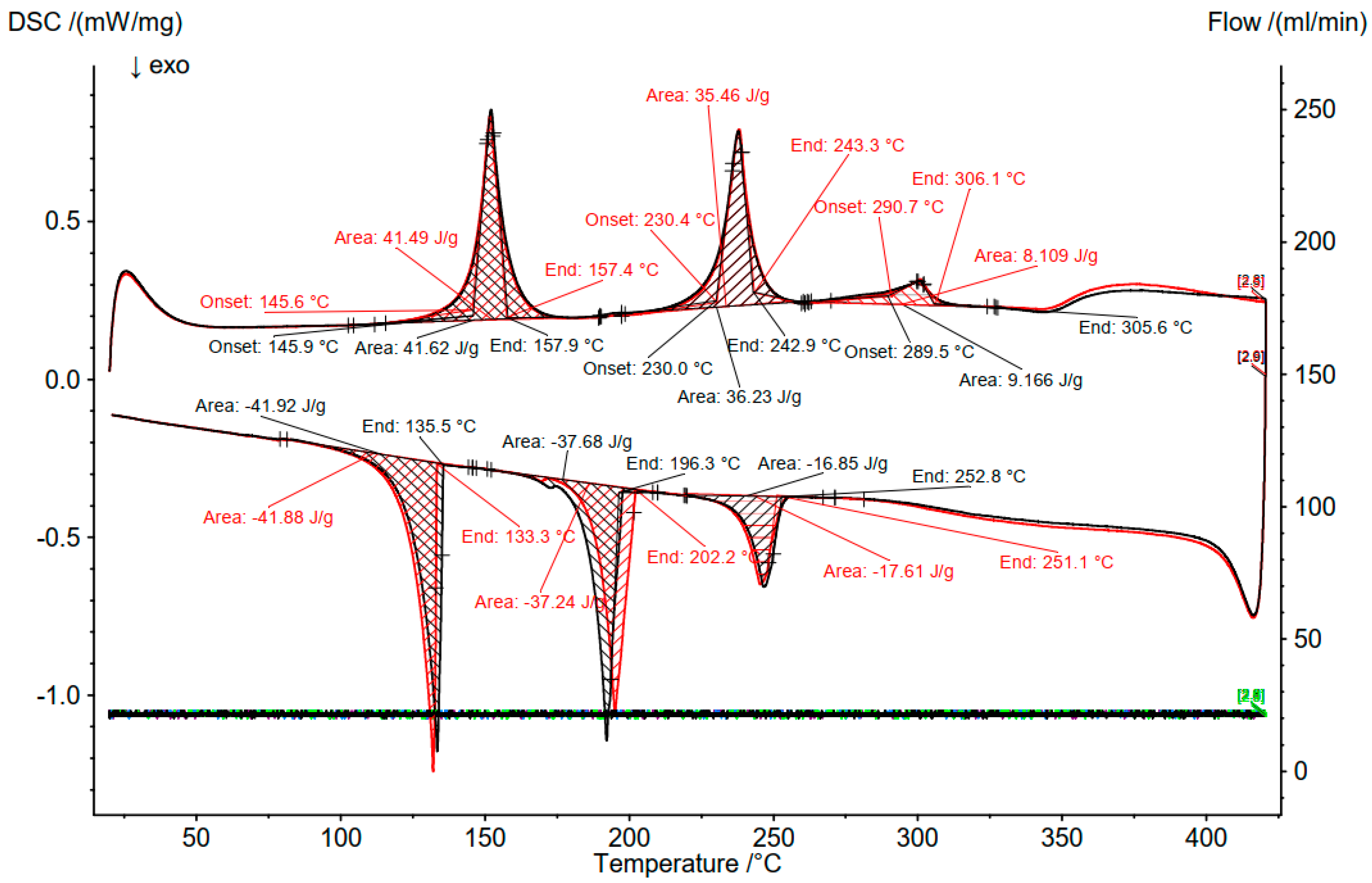
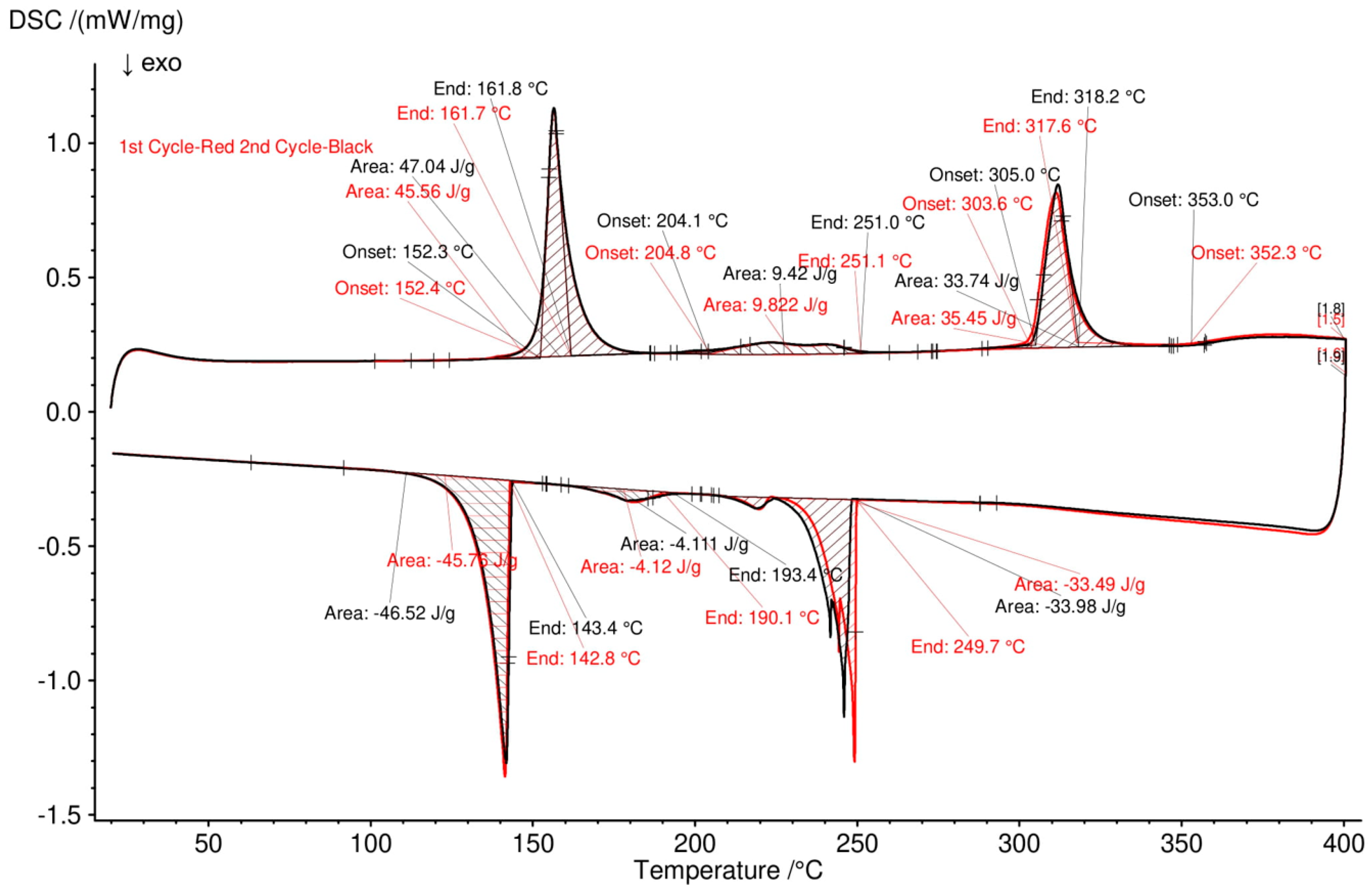
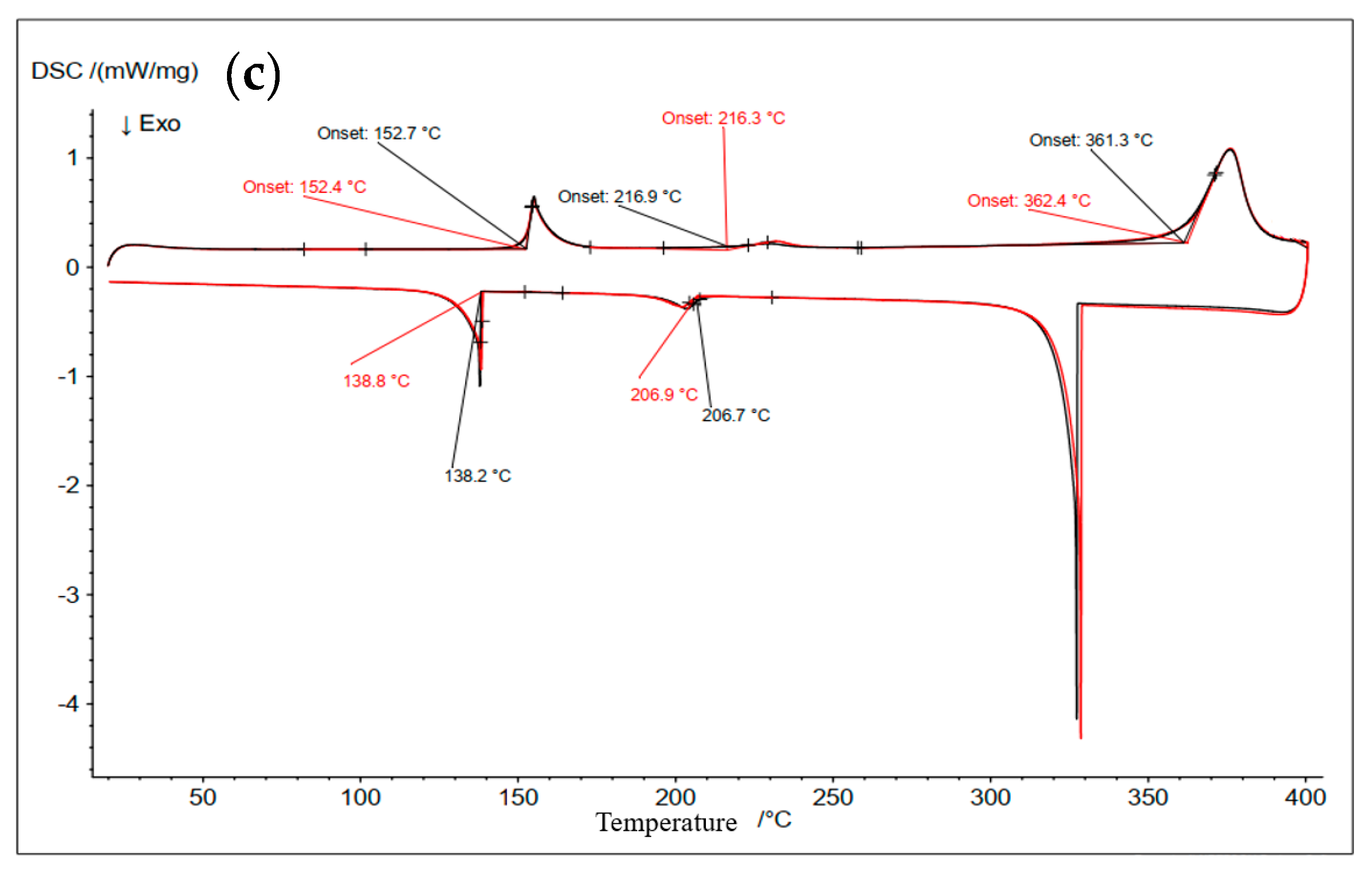
3.2. Differential Scanning Calorimetry (DSC)
3.3. Comparison of DSC and OptiMeltTM
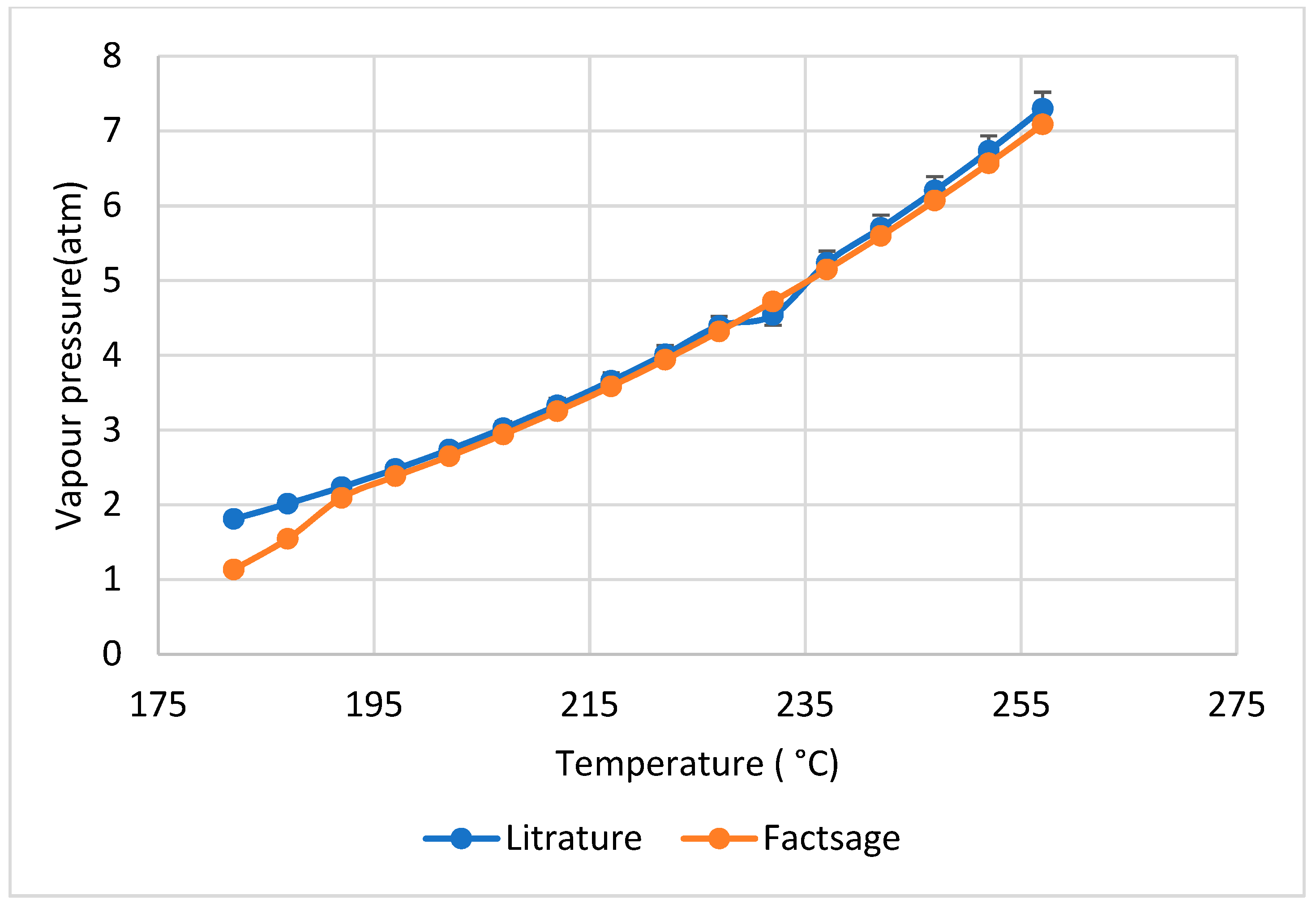
3.4. Vapor Pressure Simulation
4. Conclusions
- The simulation results (e.g., phase diagrams and vapor pressures of salt mixtures) via FactSageTM are in good agreement with the experimental results from DSC and OptiMeltTM in this work and from the literature. The deviation of the melting temperatures of the main species such as NaAlCl4 and Na2ZnCl4 between simulations and experiments is smaller than 10 °C.
- From the phase diagrams it is concluded that AlCl3 is needed to be present in the secondary salt electrolyte to ensure enough liquid salt existing during charging/discharging for low resistance of the battery, since the high resistance due to the secondary salt electrolyte leads to bad battery performance.
- With increasing concentration of ZnCl2 at higher SOC, the melting temperature of the secondary salt electrolyte decreases significantly, since Na2ZnCl4 (about 240 °C) has much lower melting temperature than NiCl2. As a result of replacement of Ni with Zn, active materials (ZnCl2 and NaCl) should be present in the liquid state over a wider SOC range during battery cell cycling compared to classical Na-NiCl2 ZEBRA cells.
- Increasing molar fraction of AlCl3 has both positive and negative effect on the salt electrolyte. On one hand it can decrease the melting point and increase the ionic conductivity at lower temperatures, which could improve the battery performance, but on other hand it can increase the vapor pressure and could affect the battery costs, life span and safety (e.g., mechanical stability of the BASE electrolyte, cell pressure management). Moreover, increasing AlCl3 can lead to more challenges such as corrosion of sealing.
- It is suggested in future work to determine the optimal concentration of AlCl3 in the secondary salt electrolyte regarding the performance and storage cost of the battery, based on phase diagrams, vapor pressure, charging/discharging range, compatibility with other materials in the battery, etc.
- The salt vapor pressures vs. temperature and mol% AlCl3 in AlCl3-ZnCl2-NaCl were simulated. Considering the rapid increase in salt vapor pressure at high temperatures and AlCl3 content, it is strongly recommended to consider the impact of salt vapor pressure on battery safety and pressure management. The maximum allowable temperature is suggested to 400 °C for safe operation of the cell with the secondary salt electrolyte (AlCl3 < 40 mol%).
- The reliable simulation results in this study, including phase diagrams and vapor pressures, show promise in assisting battery cell design and enhancement. Tests on a Na-ZnCl2 test cell, based on the suggested electrolyte composition, reveal a three-step charging and discharging behavior consistent with our predicted phase diagram. Further insights into the Na-ZnCl2 battery system’s performance will be published in an upcoming paper.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- IEA. World Energy Outlook 2022; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/world-energy-outlook-2022 (accessed on 20 July 2021).
- Sudworth, J.L. High-temperature battery systems. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1996, 354, 1595–1612. [Google Scholar]
- Shamim, N.; Thomsen, E.C.; Viswanathan, V.V.; Reed, D.M.; Sprenkle, V.L.; Li, G. Evaluating ZEBRA Battery Module under the Peak-Shaving Duty Cycles. Materials 2021, 14, 2280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, D.; Palomares, V.; Shanmukaraj, D.; Sun, B.; Tang, X.; Wang, C.; Armand, M.; Rojo, T.; Wang, G. Revitalising sodium–sulfur batteries for non-high-temperature operation: A crucial review. Energy Environ. Sci. 2020, 13, 3848–3879. [Google Scholar] [CrossRef]
- Sudworth, J.L. The sodium/nickel chloride (ZEBRA) battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Böhm, H.; Beyermann, G. ZEBRA batteries, enhanced power by doping. J. Power Sources 1999, 84, 270–274. [Google Scholar] [CrossRef]
- 2023 Company Profile of FZSoNick. Available online: https://drive.google.com/file/d/1yrmnzXKIolegXySwIu9-E5thOUHtb72k/view (accessed on 15 June 2023).
- Van Zyl, A. Review of the zebra battery system development. Solid State Ion. 1996, 86, 883–889. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. A novel low-cost sodium–zinc chloride battery. Energy Environ. Sci. 2013, 6, 1837–1843. [Google Scholar] [CrossRef]
- Galloway, R.C.; Dustmann, C.H. ZEBRA battery-material cost availability and recycling. In Proceeding of the International Electric Vehicle Symposium (EVS-20), Long Beach, CA, USA, 15–19 November 2003. [Google Scholar]
- Shaw, S.J.; George, S.P. NaCl-ZnCl2 phase diagram. Thermochim. Acta 1990, 157, 329–333. [Google Scholar] [CrossRef]
- Landmann, D.; Svaluto-Ferro, E.; Heinz, M.V.; Schmutz, P.; Battaglia, C. Elucidating the rate-limiting processes in high-temperature sodium-metal chloride batteries. Adv. Sci. 2022, 9, 2201019. [Google Scholar] [CrossRef]
- Bones, R.J.; Teagle, D.A.; Brooker, S.D.; Cullen, F.L. Development of a Ni, NiCl2 positive electrode for a liquid sodium (ZEBRA) battery cell. J. Electrochem. Soc. 1989, 136, 1274. [Google Scholar] [CrossRef]
- Heinz, M.V.; Graeber, G.; Landmann, D.; Battaglia, C. Pressure management and cell design in solid-electrolyte batteries, at the example of a sodium-nickel chloride battery. J. Power Sources 2020, 465, 228268. [Google Scholar] [CrossRef]
- Lu, X.; Chang, H.J.; Bonnett, J.F.; Canfield, N.L.; Jung, K.; Sprenkle, V.L.; Li, G. An intermediate-temperature high-performance Na–ZnCl2 battery. ACS Omega 2018, 3, 15702–15708. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, H.-J.; Byun, D.-J.; Cho, K.-K.; Ahn, J.-H.; Kim, C.-S. Electrochemically activated Na–ZnCl2 battery using a carbon matrix in the cathode compartment. J. Power Sources 2019, 440, 227110. [Google Scholar] [CrossRef]
- Godinez-Brizuela, O.E.; Niblett, D.; Einarsrud, K.E. Microstructural Analysis of Effective Electrode Conductivity in Molten Salt, Na-ZnCl2 Batteries. J. Electrochem. Soc. 2022, 169, 090510. [Google Scholar] [CrossRef]
- Ding, W.; Gong, Q.; Liang, S.; Hoffmann, R.; Zhou, H.; Li, H.; Wang, K.; Zhang, T.; Weisenburger, A.; Müller, G.; et al. Multi-cationic molten salt electrolyte of high-performance sodium liquid metal battery for grid storage. J. Power Sources 2023, 553, 232254. [Google Scholar] [CrossRef]
- Li, C.J.; Li, P.; Wang, K.; Molina, E.E. Survey of properties of key single and mixture halide salts for potential application as high temperature heat transfer fluids for concentrated solar thermal power systems. AIMS Energy 2014, 2, 133–157. [Google Scholar] [CrossRef]
- Zhou, H.; Li, H.; Gong, Q.; Yan, S.; Zhou, X.; Liang, S.; Ding, W.; He, Y.; Jiang, K.; Wang, K. A sodium liquid metal battery based on the multi-cationic electrolyte for grid energy storage. Energy Storage Mater. 2022, 50, 572–579. [Google Scholar] [CrossRef]
- Gong, Q.; Ding, W.; Bonk, A.; Li, H.; Wang, K.; Jianu, A.; Weisenburger, A.; Bund, A.; Bauer, T. Molten iodide salt electrolyte for low-temperature low-cost sodium-based liquid metal battery. J. Power Sources 2020, 475, 228674. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Molten chloride salts for next generation CSP plants: Selection of promising chloride salts & study on corrosion of alloys in molten chloride salts. AIP Conf. Proc. 2019, 2126, 200014. [Google Scholar]
- Ding, W.; Bonk, A.; Bauer, T. Corrosion behavior of metallic alloys in molten chloride salts for thermal energy storage in concentrated solar power plants: A review. Front. Chem. Sci. Eng. 2018, 12, 564–576. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Wang, K.; Molina, E.; Li, P.; Gervasio, D.; Kannan, A. Vapor pressure and corrosivity of ternary metal-chloride molten-salt based heat transfer fluids for use in concentrating solar power systems. Appl. Energy 2015, 159, 206–213. [Google Scholar] [CrossRef]





| Sample No. | SOC (%) | AlCl3-ZnCl2-(NaCl)2 (mol%) |
|---|---|---|
| 1 | 100 | 15:77.5:7.5 |
| 2 | 71 | 15:55:30 |
| 3 | 100 | 25:62.5:12.5 |
| 4 | 48 | 25:30:45 |
| 5 | 100 | 35:47.5:17.5 |
| 6 | 42 | 35:20:45 |
| 7 | 0 | 34.9:0:65.1 |
| 8 | 50 | 34.9:23.8:41.3 |
| 9 | 76.5 | 34.9:36.5:28.6 |
| 10 | 87.6 | 34.9:41.8:23.4 |
| 11 | 100 | 34.9:47.7:17.4 |
| Sample No. | AlCl3 | ZnCl2 | (NaCl)2 | FactsageTM | DSC | OptiMelt |
|---|---|---|---|---|---|---|
| mol% | mol% | mol% | TTransition (°C) | TOnset (°C) | TStart (°C) | |
| 1 | 15 | 77.5 | 7.5 | 155 (Solidus), melting of NaAlCl4 | 152 | about 150 |
| 239 (Transition), melting of Na2ZnCl4 | 225 | about 230 | ||||
| 299 (Transition), melting of ZnCl2 | 303 | - | ||||
| 395 (liquidus), mixing of NaAlCl4 and Na2ZnCl4 | 349 | - | ||||
| 2 | 15 | 55 | 30 | 154 (Solidus) | 152 | about 150 |
| 239 (Transition) | 216 | Continue melting | ||||
| 290 (liquidus) | 362 | |||||
| 3 | 25 | 62.5 | 12.5 | 155 (Solidus) | 151 | about 150 |
| 200 (Transition) | 200 | about 230 | ||||
| 260 (Transition) | - | Cont. melting | ||||
| 300 (liquidus) | 303 | |||||
| 4 | 25 | 30 | 45 | 154 (Solidus) | 153 | about 152 |
| 379 (liquidus) | 360 | Cont. melting | ||||
| 5 | 35 | 47.5 | 17.5 | 155 (Solidus) | 152 | about 150 |
| 200–260 (Transition) | 204 | about 230 | ||||
| 300 (liquidus) | 303.7 | Cont. melting | ||||
| 6 | 35 | 20 | 45 | 154 (Solidus) | 153 | about 152 |
| 379 (liquidus) | 341.5 | Cont. melting | ||||
| 7 (SOC 0%) | 34.89 | 0 | 65.11 | 155 (Solidus) | 152.6 | Not measured |
| 8 (SOC 50%) | 34.89 | 23.83 | 41.28 | 155 (Solidus) | 150.7 | |
| 230 (Transition) | 204.5 | |||||
| 345 (liquidus) | 349.5 | |||||
| 9 (SOC 76.5%) | 34.89 | 36.47 | 28.65 | 155 (Solidus) | 145.3 | |
| 230–245 (Transition) | 232.5 | |||||
| 345 (liquidus) | 349.5 | |||||
| 10 (SOC 87.6%) | 34.89 | 41.76 | 23.35 | 155 (Solidus) | 145.8 | |
| 235–260 (Transition) | 230.2 | |||||
| 295 (liquidus) | 290.1 | |||||
| 11 (SOC 100%) | 34.89 | 47.67 | 17.44 | 155 (Solidus) | 152 | |
| 200 (Transition) | 204 | |||||
| 300 (liquidus) | 303.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ding, W.; Hoffmann, R.; Sieuw, L.; Heinz, M.V.F.; Weber, N.; Bonk, A. AlCl3-NaCl-ZnCl2 Secondary Electrolyte in Next-Generation ZEBRA (Na-ZnCl2) Battery. Batteries 2023, 9, 401. https://doi.org/10.3390/batteries9080401
Kumar S, Ding W, Hoffmann R, Sieuw L, Heinz MVF, Weber N, Bonk A. AlCl3-NaCl-ZnCl2 Secondary Electrolyte in Next-Generation ZEBRA (Na-ZnCl2) Battery. Batteries. 2023; 9(8):401. https://doi.org/10.3390/batteries9080401
Chicago/Turabian StyleKumar, Sumit, Wenjin Ding, Ralf Hoffmann, Louis Sieuw, Meike V. F. Heinz, Norbert Weber, and Alexander Bonk. 2023. "AlCl3-NaCl-ZnCl2 Secondary Electrolyte in Next-Generation ZEBRA (Na-ZnCl2) Battery" Batteries 9, no. 8: 401. https://doi.org/10.3390/batteries9080401
APA StyleKumar, S., Ding, W., Hoffmann, R., Sieuw, L., Heinz, M. V. F., Weber, N., & Bonk, A. (2023). AlCl3-NaCl-ZnCl2 Secondary Electrolyte in Next-Generation ZEBRA (Na-ZnCl2) Battery. Batteries, 9(8), 401. https://doi.org/10.3390/batteries9080401









