Two-Dimensional Molecular Brush-Based Ultrahigh Edge-Nitrogen-Doped Carbon Nanosheets for Ultrafast Potassium-Ion Storage
Abstract
1. Introduction
2. Materials and Methods
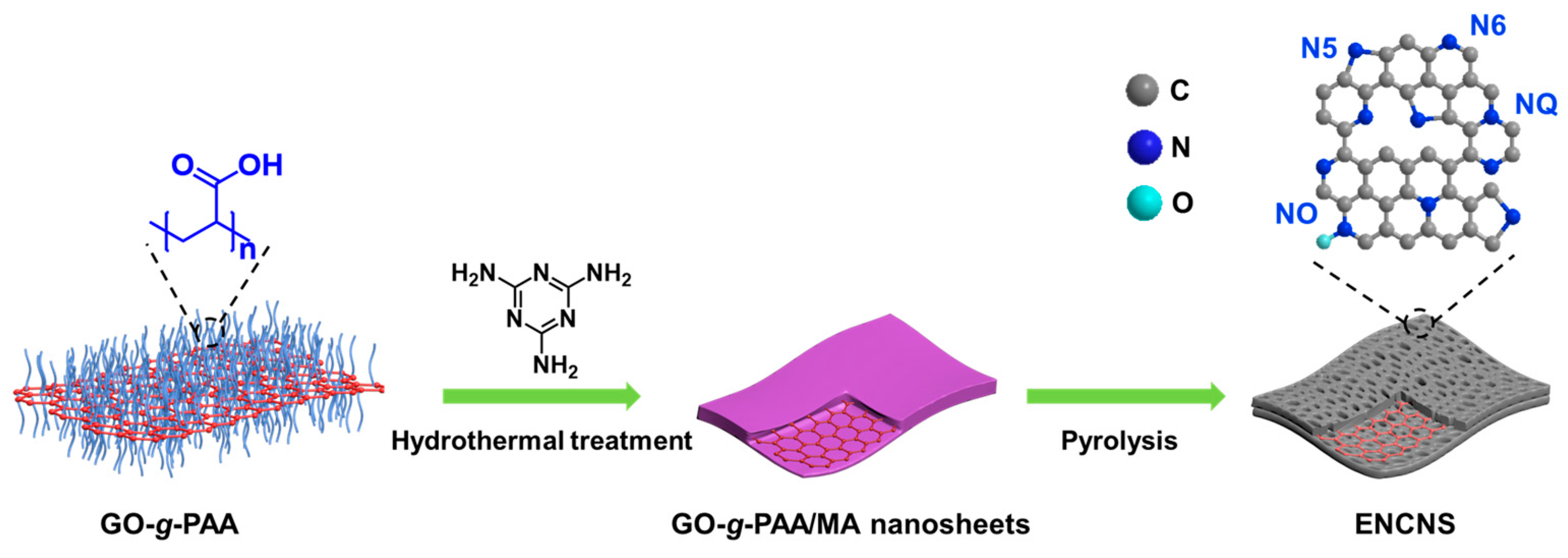
2.1. Synthesis of ENCNS, ENCNP, and CNS
2.2. Material Characterization
2.3. Electrochemical Measurements
2.4. Calculation of K-Ion Diffusion Coefficient ()
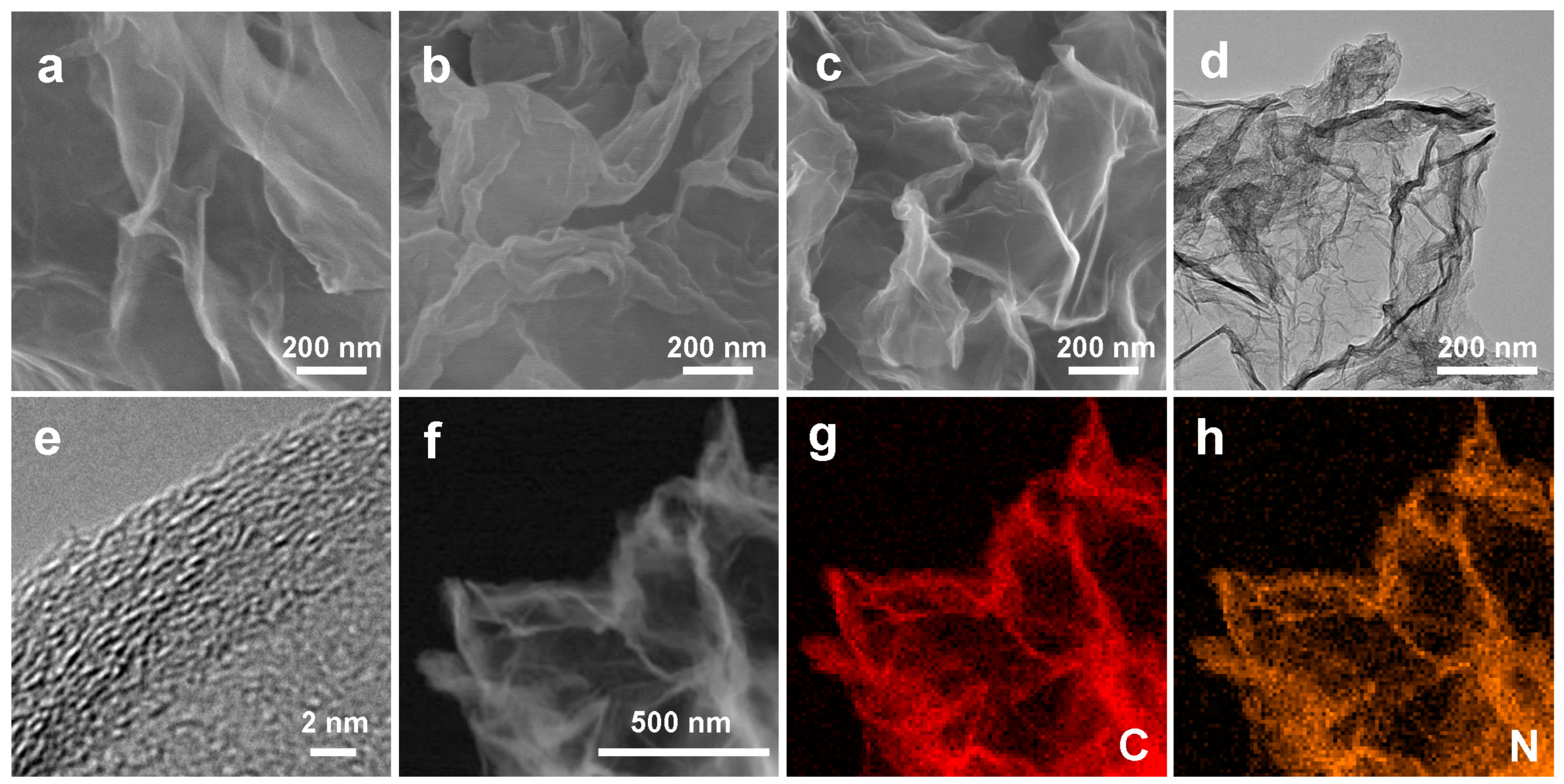
3. Results and Discussion
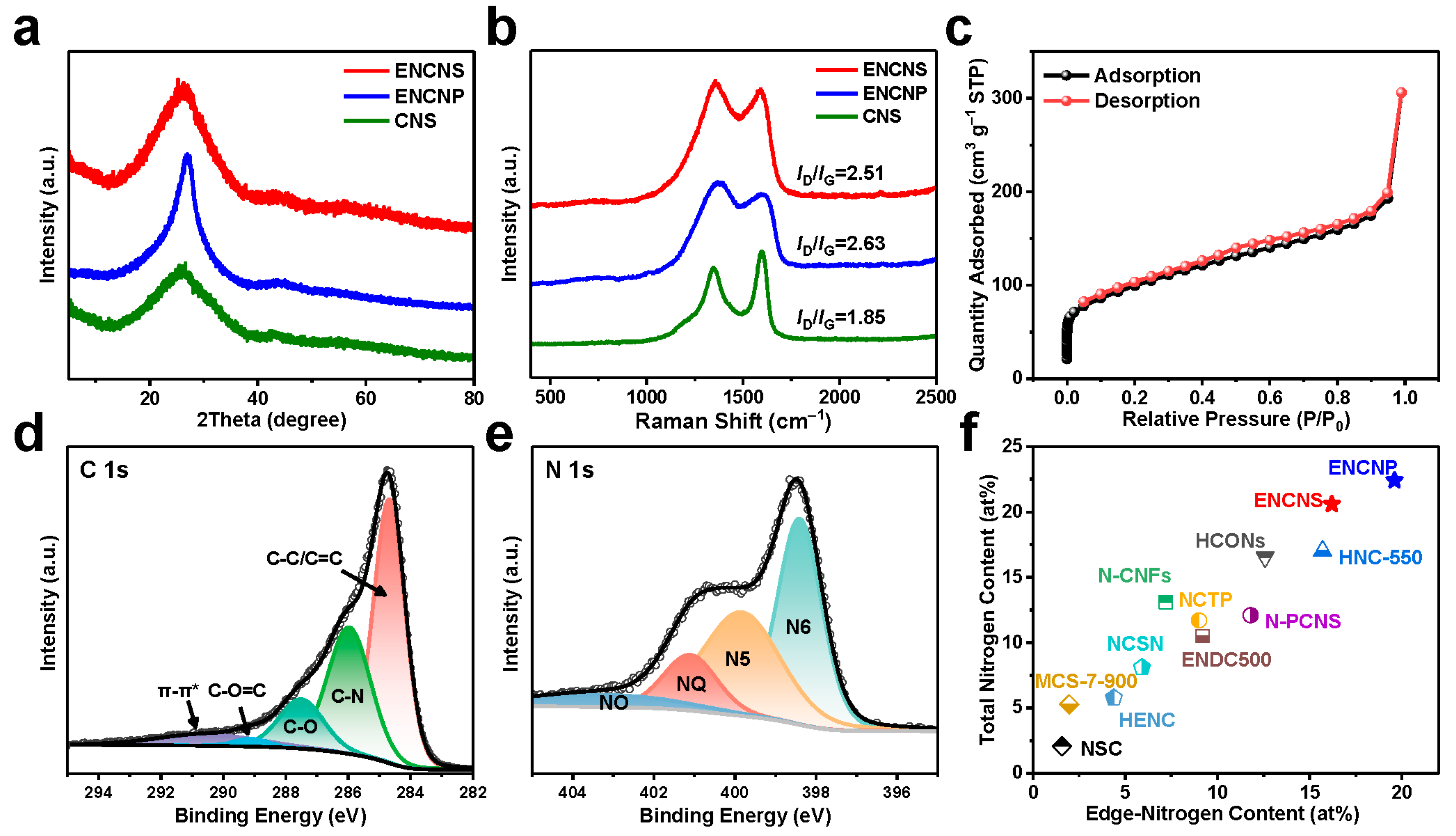
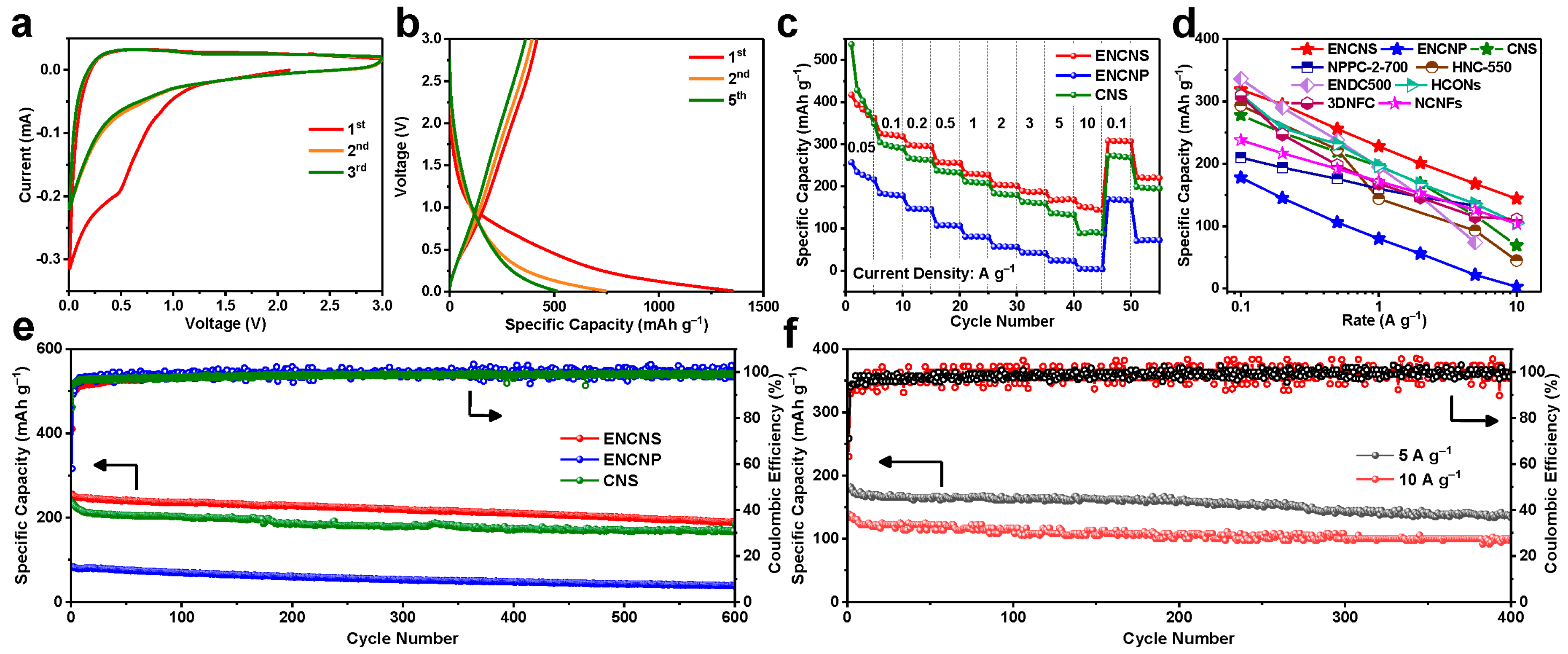
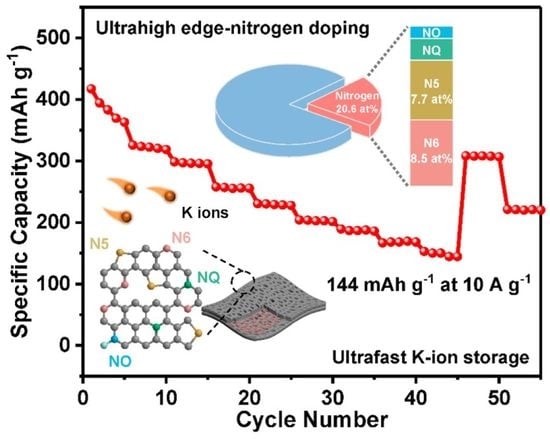
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hosein, I.D. The promise of calcium batteries: Open perspectives and fair comparisons. ACS Energy Lett. 2021, 6, 1560–1565. [Google Scholar] [CrossRef]
- Zhang, T.S.; Tang, Y.; Guo, S.; Cao, X.X.; Pan, A.Q.; Fang, G.Z.; Zhou, J.; Liang, S.Q. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Vincent, M.; Avvaru, V.S.; Rodriguez, M.C.; Haranczyk, M.; Etacheri, V. High-rate and ultralong-life Mg-Li hybrid batteries based on highly pseudocapacitive dual-phase TiO2 nanosheet cathodes. J. Power Sources 2021, 506, 230118. [Google Scholar] [CrossRef]
- Tu, J.W.; Tong, H.G.; Zeng, X.H.; Chen, S.; Wang, C.L.; Zheng, W.; Wang, H.; Chen, Q.W. Modification of porous N-doped carbon with sulfonic acid toward high-ICE/capacity anode material for potassium-ion batteries. Adv. Funct. Mater. 2022, 32, 2204991. [Google Scholar] [CrossRef]
- Wang, B.; Gu, L.; Yuan, F.; Zhang, D.; Sun, H.L.; Wang, J.; Wang, Q.J.; Wang, H.; Li, Z.J. Edge-enrich N-doped graphitic carbon: Boosting rate capability and cyclability for potassium ion battery. Chem. Eng. J. 2022, 432, 134321. [Google Scholar] [CrossRef]
- Xu, F.; Zhai, Y.X.; Zhang, E.; Liu, Q.H.; Jiang, G.S.; Xu, X.S.; Qiu, Y.Q.; Liu, X.M.; Wang, H.Q.; Kaskel, S. Ultrastable surface-dominated pseudocapacitive potassium storage enabled by edge-enriched N-doped porous carbon nanosheets. Angew. Chem. Int. Ed. 2020, 59, 19460–19467. [Google Scholar] [CrossRef]
- Zhang, W.C.; Liu, Y.J.; Guo, Z.P. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef]
- Chu, K.N.; Zhang, X.J.; Yang, Y.; Li, Z.Q.; Wei, L.Z.; Yao, G.; Zheng, F.C.; Chen, Q.W. Edge-nitrogen enriched carbon nanosheets for potassium-ion battery anodes with an ultrastable cycling stability. Carbon 2021, 184, 277–286. [Google Scholar] [CrossRef]
- Han, Y.; Li, T.Q.; Li, Y.; Tian, J.; Yi, Z.; Lin, N.; Qian, Y.T. Stabilizing antimony nanocrystals within ultrathin carbon nanosheets for high-performance K-ion storage. Energy Storage Mater. 2019, 20, 46–54. [Google Scholar] [CrossRef]
- Sultana, I.; Ramireddy, T.; Rahman, M.M.; Chen, Y.; Glushenkov, A.M. Tin-based composite anodes for potassium-ion batteries. Chem. Commun. 2016, 52, 9279–9282. [Google Scholar] [CrossRef]
- Cui, R.C.; Zhou, H.Y.; Li, J.C.; Yang, C.C.; Jiang, Q. Ball-cactus-like Bi embedded in N-riched carbon nanonetworks enables the best potassium storage performance. Adv. Funct. Mater. 2021, 31, 2103067. [Google Scholar] [CrossRef]
- Shi, X.L.; Gan, Y.M.; Zhang, Q.X.; Wang, C.Y.; Zhao, Y.; Guan, L.H.; Huang, W. A partial sulfuration strategy derived multi-yolk-shell structure for ultra-stable K/Na/Li-ion storage. Adv. Mater. 2021, 33, 2100837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Ling, F.X.; Wang, L.F.; Xu, R.; Ma, M.Z.; Cheng, X.L.; Bai, R.L.; Shao, Y.; Huang, H.J.; Li, D.J.; et al. An open-ended Ni3S2-Co9S8 heterostructures nanocage anode with enhanced reaction kinetics for superior potassium-ion batteries. Adv. Mater. 2022, 34, 2201420. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fang, M.J.; Liu, J.W.; Yang, D.X.; Liang, Y.H.; Zhong, J.S.; Yuan, Y.J.; Zhang, Y.N.; Liu, X.L.; Zheng, R.K.; et al. Ultranarrow bandgap Se- deficient bimetallic selenides for high performance alkali metal-ion batteries. Adv. Funct. Mater. 2022, 32, 2205880. [Google Scholar] [CrossRef]
- Hussain, N.; Li, M.X.; Tian, B.B.; Wang, H.H. Co3Se4 quantum dots as an ultrastable host material for potassium-ion intercalation. Adv. Mater. 2021, 33, 2102164. [Google Scholar] [CrossRef]
- Huang, H.J.; Xu, R.; Feng, Y.Z.; Zeng, S.F.; Jiang, Y.; Wang, H.J.; Luo, W.; Yu, Y. Sodium/potassium-ion batteries: Boosting the rate capability and cycle life by combining morphology, defect and structure engineering. Adv. Mater. 2020, 32, 1904320. [Google Scholar] [CrossRef]
- Lu, Y.H.; Tang, Y.C.; Tang, K.H.; Wu, D.C.; Ma, Q. Controllable fabrication of superhierarchical carbon nanonetworks from 2D molecular brushes and their use in electrodes of flexible supercapacitors. New Carbon Mater. 2022, 37, 978–987. [Google Scholar] [CrossRef]
- Mahmood, A.; Li, S.; Ali, Z.; Tabassum, H.; Zhu, B.J.; Liang, Z.B.; Meng, W.; Aftab, W.; Guo, W.H.; Zhang, H.; et al. Ultrafast sodium/potassium-ion intercalation into hierarchically porous thin carbon shells. Adv. Mater. 2019, 31, 1805430. [Google Scholar] [CrossRef]
- Feng, W.C.; Wang, H.; Jiang, Y.L.; Zhang, H.Z.; Luo, W.; Chen, W.; Shen, C.L.; Wang, C.X.; Wu, J.S.; Mai, L.Q. A strain-relaxation red phosphorus free-standing anode for non-aqueous potassium ion batteries. Adv. Energy Mater. 2022, 12, 2103343. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.X.; Xu, Y.S.; Meng, Q.S.; Gao, J.C.; Sun, Y.G.; Hu, Y.S.; Chang, B.B.; Liu, C.T.; Cao, A.M. Pitch-derived soft carbon as stable anode material for potassium ion batteries. Adv. Mater. 2020, 32, 2000505. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, F.Q.; Shi, C.R.; Wang, Z.Y.; Fan, X.H.; Wang, L.; Zhao, J.F.; Jiang, L.L.; Li, Y.H.; Chen, C.; et al. Fast peel-off ultrathin, transparent, and free-standing films assembled from low-dimensional materials using MXene sacrificial layers and produced bubbles. Small Methods 2022, 6, 2101388. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.L.; Luo, W.; Ji, X.L. Carbon electrodes for K-ion batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Tang, Y.C.; Liu, R.L.; Li, C.F.; Liu, S.H.; Zhu, Y.L.; Wu, D.C. Multifunctional Templating Strategy for Fabrication of Fe, N-Codoped Hierarchical Porous Carbon Nanosheets. Chin. J. Polym. Sci. 2022, 40, 2–6. [Google Scholar] [CrossRef]
- Zhang, W.L.; Yin, J.; Sun, M.L.; Wang, W.X.; Chen, C.L.; Altunkaya, M.; Emwas, A.H.; Han, Y.; Schwingenschlogl, U.; Alshareef, H.N. Direct pyrolysis of supermolecules: An ultrahigh edge-nitrogen doping strategy of carbon anodes for potassium-ion batteries. Adv. Mater. 2020, 32, 2000732. [Google Scholar] [CrossRef]
- Ma, X.Q.; Xiao, N.; Xiao, J.; Song, X.D.; Guo, H.D.; Wang, Y.T.; Zhao, S.J.; Zhong, Y.P.; Qiu, J.S. Nitrogen and phosphorus dual-doped porous carbons for high-rate potassium ion batteries. Carbon 2021, 179, 33–41. [Google Scholar] [CrossRef]
- Share, K.; Cohn, A.P.; Carter, R.; Rogers, B.; Pint, C.L. Role of nitrogen-doped graphene for improved high-capacity potassium ion battery anodes. ACS Nano 2016, 10, 9738–9744. [Google Scholar] [CrossRef]
- Zhang, D.M.; Chen, Z.W.; Bai, J.; Yang, C.C.; Jiang, Q. Highly nitrogen-doped porous carbon nanosheets as high-performance anode for potassium-ion batteries. Batter. Supercaps 2020, 3, 185–193. [Google Scholar] [CrossRef]
- Li, Z.J.; Wu, X.F.; Luo, W.; Wang, C.X.; Feng, W.C.; Hong, X.F.; Mai, L.Q. Dual sulfur-doped sites boost potassium storage in carbon nanosheets derived from low-cost sulfonate. Chem. Eng. J. 2022, 431, 134207. [Google Scholar] [CrossRef]
- Liu, Q.D.; Han, F.; Zhou, J.F.; Li, Y.; Chen, L.; Zhang, F.Q.; Zhou, D.W.; Ye, C.; Yang, J.X.; Wu, X.; et al. Boosting the potassium-ion storage performance in soft carbon anodes by the synergistic effect of optimized molten salt medium and N/S dual-doping. ACS Appl. Mater. Inter 2020, 12, 20838–20848. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, X.P.; Li, Z.Q.; Wei, L.Z.; Yao, G.; Niu, H.L.; Yang, Y.; Zheng, F.C.; Chen, Q.W. Boosting the K+-adsorption capacity in edge-nitrogen doped hierarchically porous carbon spheres for ultrastable potassium ion battery anodes. Nanoscale 2021, 13, 19634–19641. [Google Scholar] [CrossRef]
- Jin, S.Y.; Liang, P.; Jiang, Y.T.; Min, H.H.; Niu, M.M.; Yang, H.; Zhang, R.G.; Yan, J.X.; Shen, X.D.; Wang, J. Preferentially engineering edge-nitrogen sites in porous hollow spheres for ultra-fast and reversible potassium storage. Chem. Eng. J. 2022, 435, 134821. [Google Scholar] [CrossRef]
- Tang, Y.C.; Liu, R.L.; Liu, S.H.; Zheng, B.N.; Lu, Y.H.; Fu, R.W.; Wu, D.C.; Zhang, M.Q.; Rong, M.Z. Cobalt and nitrogen codoped ultrathin porous carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. Carbon 2019, 141, 704–711. [Google Scholar] [CrossRef]
- Chang, X.Q.; Zhou, X.L.; Ou, X.W.; Lee, C.S.; Zhou, J.W.; Tang, Y.B. Ultrahigh nitrogen doping of carbon nanosheets for high capacity and long cycling potassium ion storage. Adv. Energy Mater. 2019, 9, 1902672. [Google Scholar] [CrossRef]
- Zhang, W.L.; Sun, M.L.; Yin, J.; Lu, K.; Schwingenschlogl, U.; Qiu, X.Q.; Alshareef, H.N. Accordion-like carbon with high nitrogen doping for fast and stable K ion storage. Adv. Energy Mater. 2021, 11, 2101928. [Google Scholar] [CrossRef]
- Wu, X.F.; Li, Z.J.; Liu, J.X.; Luo, W.; Gaumet, J.J.; Mai, L.Q. Defect engineering of hierarchical porous carbon microspheres for potassium-ion storage. Rare Met. 2022, 41, 3446–3455. [Google Scholar] [CrossRef]
- Wang, P.; Qi, X.H.; Zhao, W.; Qian, M.; Bi, H.; Huang, F.Q. Nitrogen-doped hierarchical few-layered porous carbon for efficient electrochemical energy storage. Carbon Energy 2021, 3, 349–359. [Google Scholar] [CrossRef]
- Xu, J.J.; Xu, F.; Qian, M.; Xu, F.F.; Hong, Z.L.; Huang, F.Q. Conductive carbon nitride for excellent energy storage. Adv. Mater. 2017, 29, 1701674. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.Y.; Xu, Z.; Gao, C. General avenue to individually dispersed graphene oxide-based two-dimensional molecular brushes by free radical polymerization. Macromolecules 2011, 44, 444–452. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Lei, L.L.; Kang, X.Y.; Su, X.Q.; Wang, F.C.; Wang, C.; Zhao, J.H.; Chen, Z.J. Preparation of the crosslinked GO/PAA aerogel and its adsorption properties for Pb(II) ions. Mater. Res. Express 2020, 7, 025514. [Google Scholar] [CrossRef]
- Olar, R.; Scaeteanu, G.V.; Danila, G.M.; Daniliuc, C.G.; Korosec, R.C.; Korosin, N.C.; Badea, M. Synthesis and characterization of cobalt acrylate-melamine co-crystals. J. Therm. Anal. Calorim. 2019, 135, 2257–2264. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, J.; Guo, S.J.; Pinna, N. Graphene/N-doped carbon sandwiched nanosheets with ultrahigh nitrogen doping for boosting lithium-ion batteries. J. Mater. Chem. A 2016, 4, 1423–1431. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Huang, J.L.; Chen, Z.R.; Shi, C.G.; Yang, H.Z.; Tang, Y.C.; Cen, Z.H.; Liu, S.H.; Fu, R.W.; Wu, D.C. Molecular engineering toward high-crystallinity yet high-surface-area porous carbon nanosheets for enhanced electrocatalytic oxygen reduction. Adv. Sci. 2022, 9, 2103477. [Google Scholar] [CrossRef]
- Huang, R.; Cao, Y.P.; Qin, S.Y.; Ren, Y.X.; Lan, R.C.; Zhang, L.Y.; Yu, Z.; Yang, H. Ultra-high N-doped open hollow carbon nano-cage with excellent Na+ and K+ storage performances. Mater. Today Nano 2022, 18, 100217. [Google Scholar] [CrossRef]
- Gong, J.; Zhao, G.Q.; Feng, J.K.; Wang, G.L.; Shi, Z.L.; An, Y.L.; Zhang, L.; Li, B. Control of the structure and composition of nitrogen-doped carbon nanofoams derived from CO2 foamed polyacrylonitrile as anodes for high-performance potassium-ion batteries. Electrochim Acta 2021, 388, 138630. [Google Scholar] [CrossRef]
- Zheng, J.F.; Wu, Y.J.; Tong, Y.; Liu, X.; Sun, Y.J.; Li, H.Y.; Niu, L. High capacity and fast kinetics of potassium-ion batteries boosted by nitrogen-doped mesoporous carbon spheres. Nano-Micro Lett. 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.H.; He, X.J.; Yang, L.; Li, H.Q.; Jin, B.Y.; Qiu, J.S. Interconnected carbon nanocapsules with high N/S co-doping as stable and high-capacity potassium-ion battery anode. J. Energy Chem. 2022, 66, 195–204. [Google Scholar] [CrossRef]
- Zhang, W.L.; Cao, Z.; Wang, W.X.; Alhajji, E.; Emwas, A.-H.; Costa, P.M.F.J.; Cavallo, L.; Alshareef, H.N. A site-selective doping strategy of carbon anodes with remarkable K-ion storage capacity. Angew Chem. Int. Ed. 2020, 59, 4448–4455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Z.T.; Yi, Y.Y.; Lu, C.; Wang, M.L.; Xia, Z.; Lian, X.Y.; Liu, Z.F.; Sun, J.Y. Precise synthesis of N-doped graphitic carbon via chemical vapor deposition to unravel the dopant functions on potassium storage toward practical K-ion batteries. Nano Res. 2021, 14, 1413–1420. [Google Scholar] [CrossRef]
- Wang, K.; Li, N.N.; Sun, L.; Zhang, J.; Liu, X.H. Free-standing N-doped carbon nanotube films with tunable defects as a highcapacity anode for potassium-ion batteries. ACS Appl. Mater. Inter 2020, 12, 37506–37514. [Google Scholar] [CrossRef]
- Liu, S.T.; Yang, B.B.; Zhou, J.S.; Song, H.H. Nitrogen-rich carbon-onion-constructed nanosheets: An ultrafast and ultrastable dual anode material for sodium and potassium storage. J. Mater. Chem. A 2019, 7, 18499–18509. [Google Scholar] [CrossRef]
- Cheng, N.; Zhou, W.; Liu, J.L.; Liu, Z.G.; Lu, B.A. Reversible oxygen-rich functional groups grafted 3D honeycomb-like carbon anode for super-long potassium ion batteries. Nano-Micro Lett. 2022, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Yu, R.H.; Luo, W.; Feng, W.C.; Shen, Y.H.; Xu, N.; Mai, L.Q. Chemical cross-linking and mechanically reinforced carbon network constructed by graphene boosts potassium ion storage. Nano Res. 2022, 15, 9019–9025. [Google Scholar] [CrossRef]
- Yang, B.J.; Chen, J.T.; Liu, L.Y.; Ma, P.J.; Liu, B.; Lang, J.W.; Tang, Y.; Yan, X.B. 3D nitrogen-doped framework carbon for high-performance potassium ion hybrid capacitor. Energy Storage Mater. 2019, 23, 522–529. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.L.; Zhou, M.; Fu, Q.; Zhao, C.X.; Wu, M.H.; Lei, Y. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat. Commun. 2018, 9, 1720. [Google Scholar] [CrossRef]
- Ben, L.B.; Zhou, J.; Ji, H.X.; Yu, H.L.; Zhao, W.W.; Huang, X.J. Si nanoparticles seeded in carbon-coated Sn nanowires as an anode for high-energy and high-rate lithium-ion batteries. Mater. Futur. 2022, 1, 015101. [Google Scholar] [CrossRef]
- Lu, Z.X.; Wang, J.; Feng, W.L.; Yin, X.P.; Feng, X.C.; Zhao, S.Y.; Li, C.X.; Wang, R.X.; Huang, Q.A.; Zhao, Y.F. Zinc Single-Atom-Regulated Hard Carbons for High-Rate and Low-Temperature Sodium-Ion Batteries. Adv. Mater. 2023, 35, 2211461. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, Z.; Tang, Y.; Huang, J.; Chen, Y.; Yang, H.; Miao, D.; Wu, D.; Liu, S. Two-Dimensional Molecular Brush-Based Ultrahigh Edge-Nitrogen-Doped Carbon Nanosheets for Ultrafast Potassium-Ion Storage. Batteries 2023, 9, 363. https://doi.org/10.3390/batteries9070363
Cen Z, Tang Y, Huang J, Chen Y, Yang H, Miao D, Wu D, Liu S. Two-Dimensional Molecular Brush-Based Ultrahigh Edge-Nitrogen-Doped Carbon Nanosheets for Ultrafast Potassium-Ion Storage. Batteries. 2023; 9(7):363. https://doi.org/10.3390/batteries9070363
Chicago/Turabian StyleCen, Zongheng, Youchen Tang, Junlong Huang, Yongqi Chen, Haozhen Yang, Dongtian Miao, Dingcai Wu, and Shaohong Liu. 2023. "Two-Dimensional Molecular Brush-Based Ultrahigh Edge-Nitrogen-Doped Carbon Nanosheets for Ultrafast Potassium-Ion Storage" Batteries 9, no. 7: 363. https://doi.org/10.3390/batteries9070363
APA StyleCen, Z., Tang, Y., Huang, J., Chen, Y., Yang, H., Miao, D., Wu, D., & Liu, S. (2023). Two-Dimensional Molecular Brush-Based Ultrahigh Edge-Nitrogen-Doped Carbon Nanosheets for Ultrafast Potassium-Ion Storage. Batteries, 9(7), 363. https://doi.org/10.3390/batteries9070363