High-Performance High-Nickel Multi-Element Cathode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
2. History and Structural Characteristics of High-Nickel Multi-Element Cathode Materials
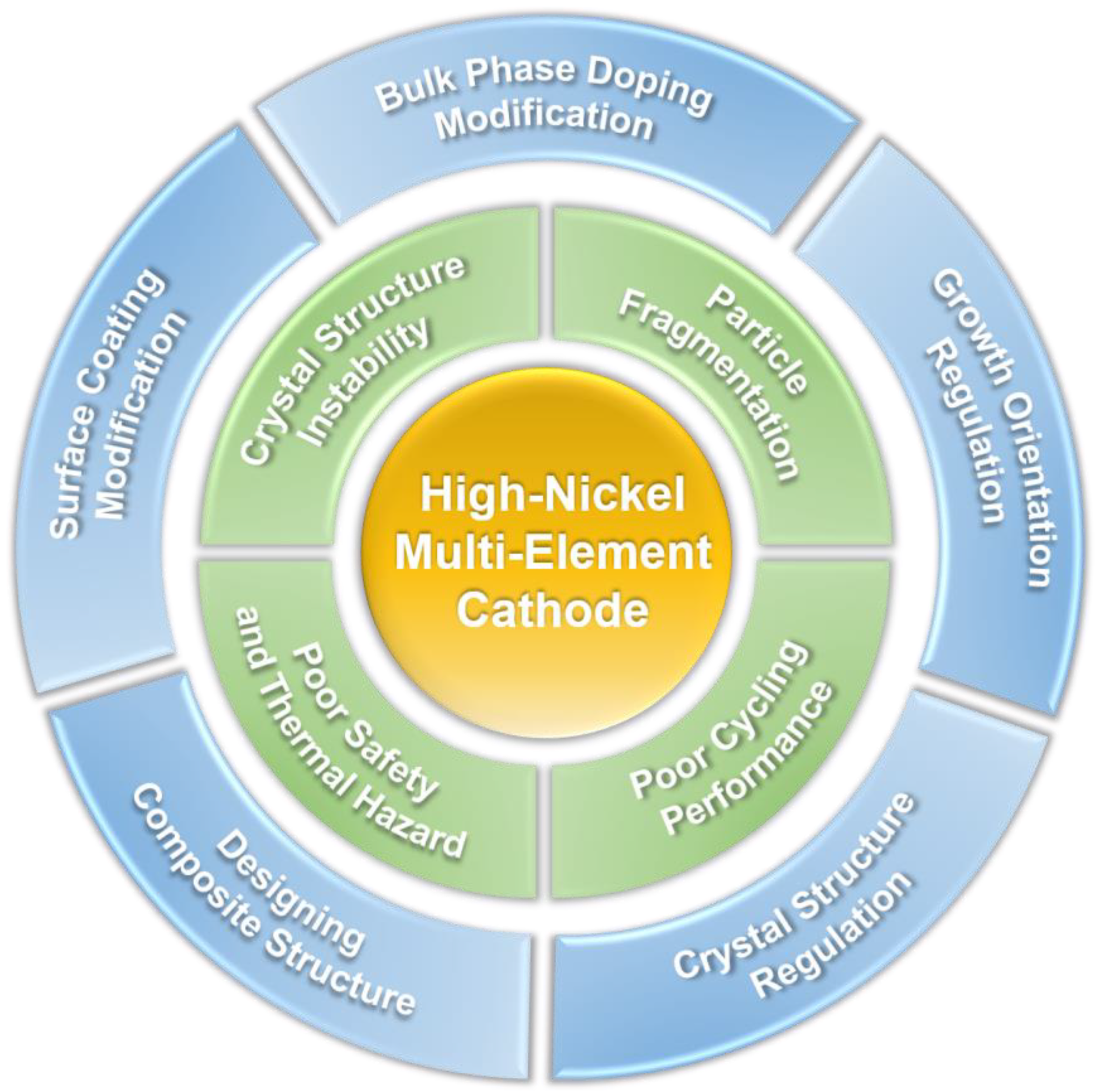
3. Current Issues of High-Nickel Multi-Element Cathode Materials
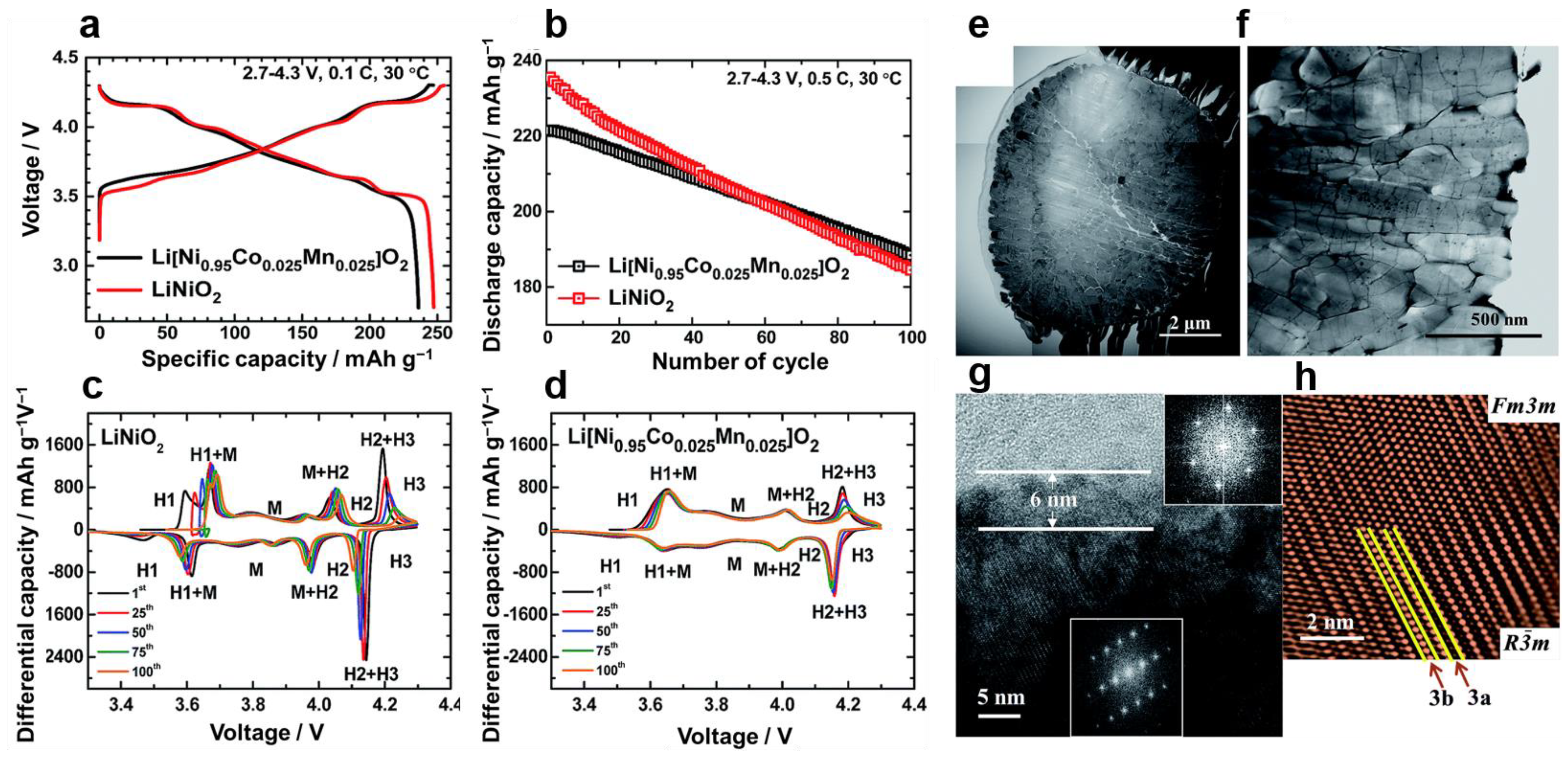
3.1. Poor Stability of the Crystal Structure
3.2. Microcracking and Particle Fragmentation
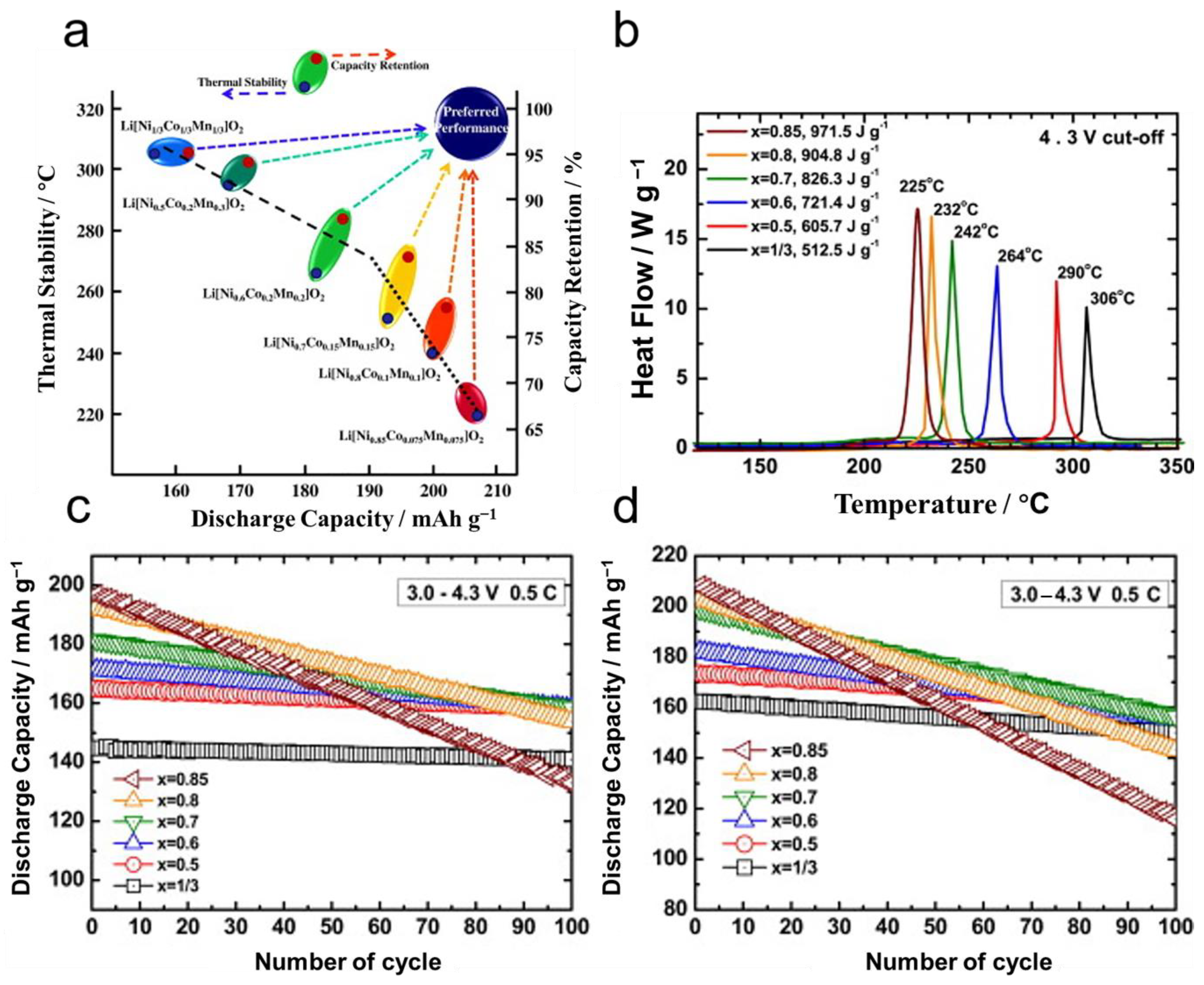
3.3. Poor Thermal Stability and Safety
3.4. Poor Cycling Life Performance
4. Performance Optimization of High-Nickel Multi-Element Cathode Materials
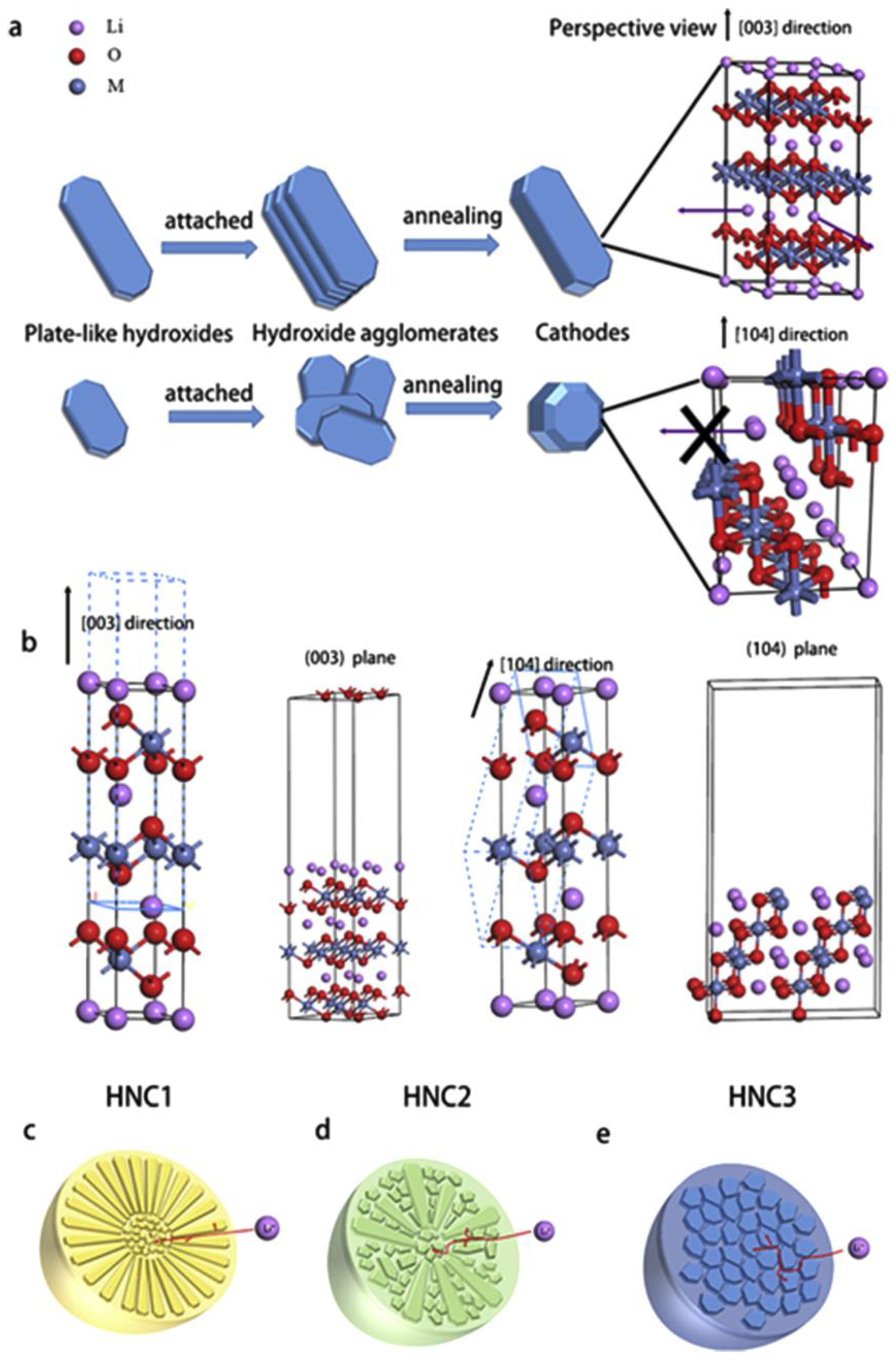
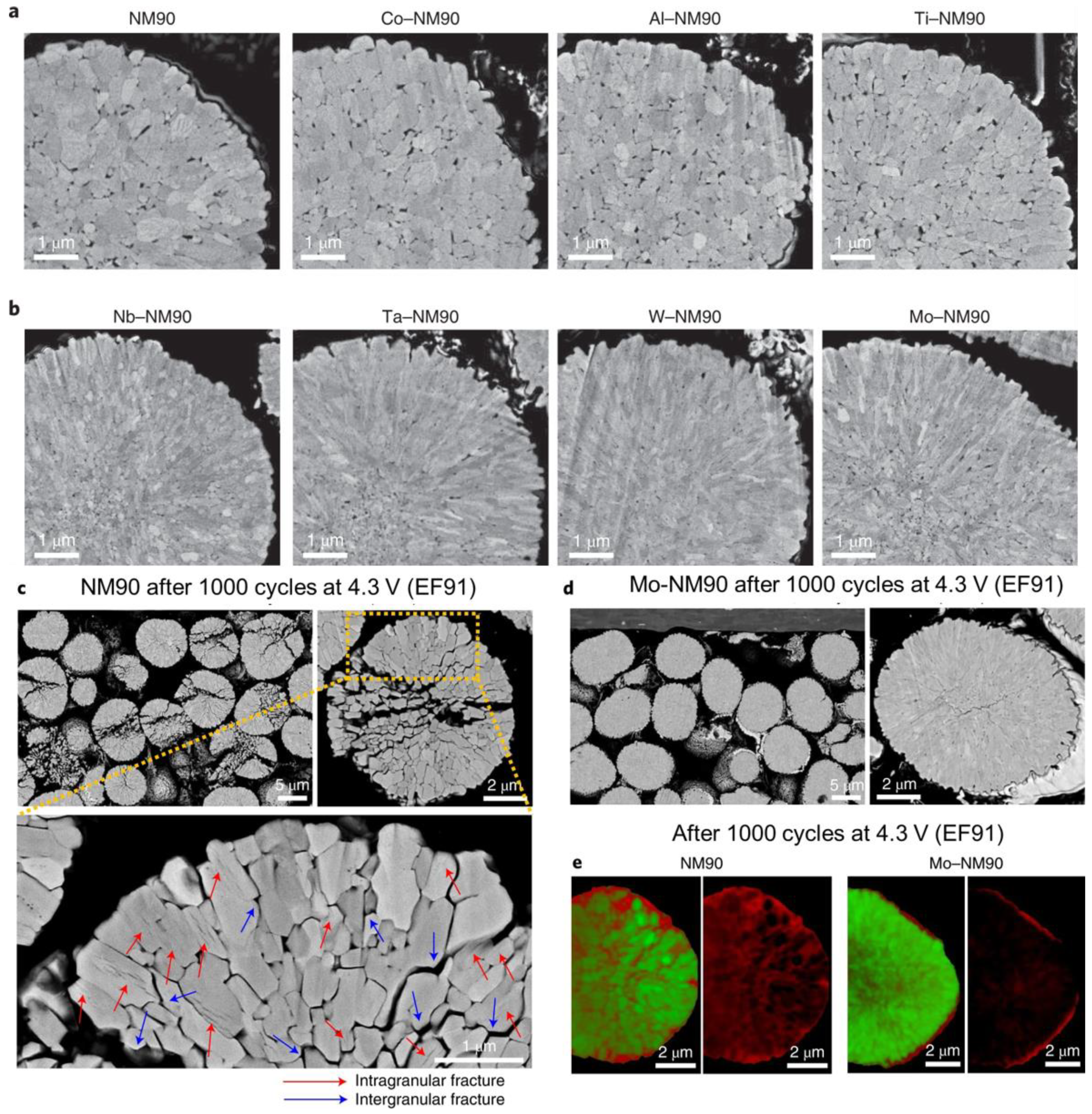
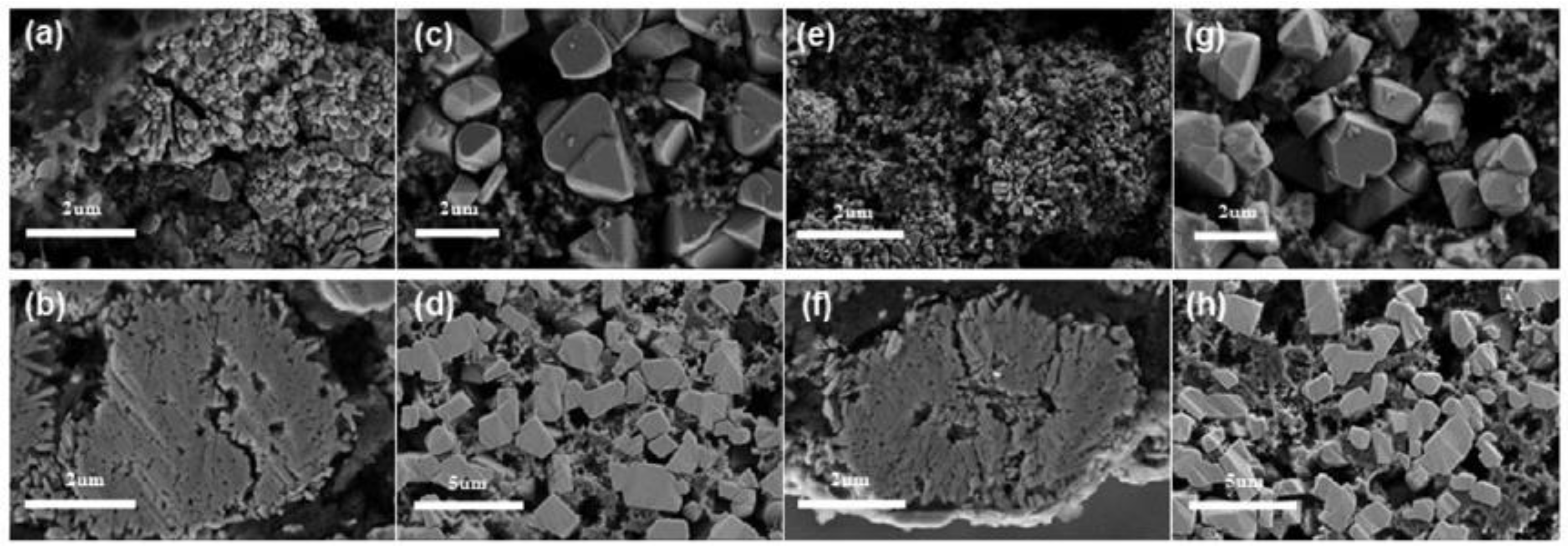
4.1. Regulation of Growth Orientation of Precursor
4.2. Structural Modification by Bulk Phase Doping
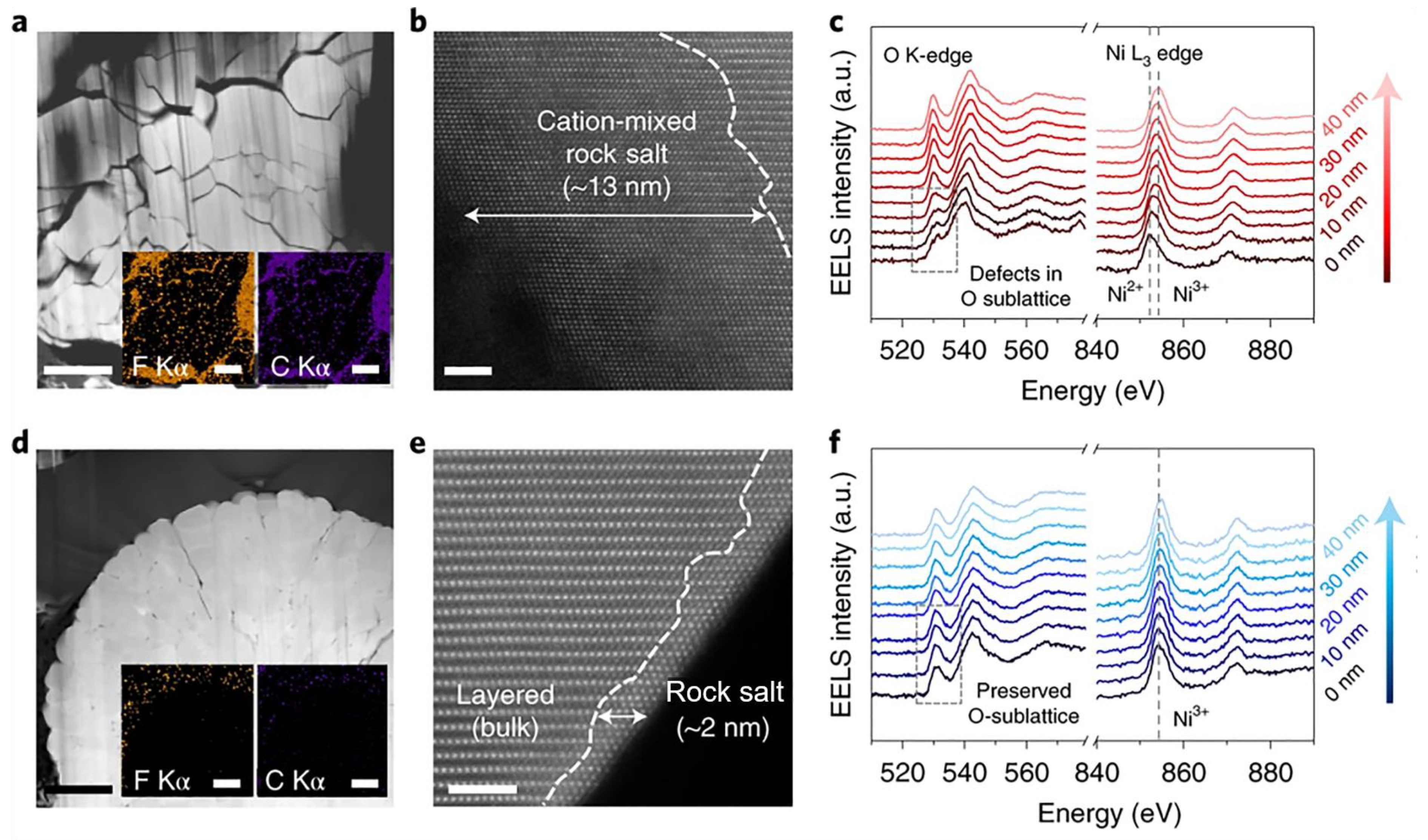
4.3. Interfacial Modification by Surface Coating
4.4. Optimal Regulation of Crystal Structure
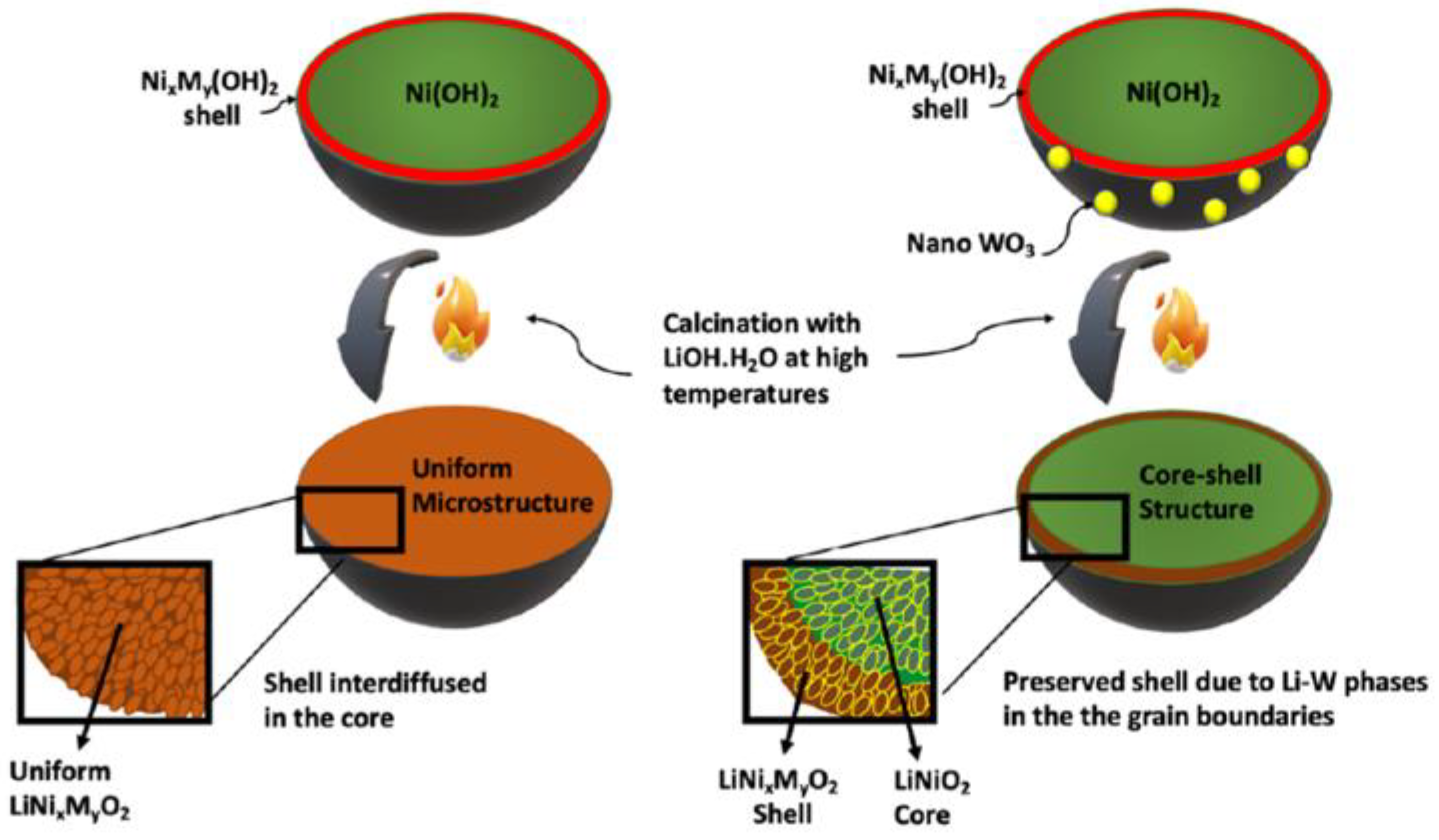
4.5. Designing Composite Structures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, M.; Li, H.; Xu, L.; Xue, Q.; Wang, X.; Bai, Y.; Wu, C. Lithium metal batteries for high energy density: Fundamental electrochemistry and challenges. J. Energy Chem. 2021, 59, 666–687. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, F.; Zhang, K.; Weng, S.; Wang, X.; Gao, M.; Sun, Y.; Cao, D.; Bai, Y.; Xu, H.; et al. Chlorinated dual-protective layers as interfacial stabilizer for dendrite-free lithium metal anode. Energy Storage Mater. 2021, 41, 485–494. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, K.; Wu, F.; Wang, X.; Guo, R.; Zhang, K.; Yuan, Y.; Bai, Y.; Wu, C. Moisture-assistant chlorinated separator with dual-protective interface for ultralong-life and high-rate lithium metal batteries. Chem. Eng. J. 2023, 453, 139348. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, K.; Liu, Y.; Gao, H.; Bai, Y.; Wang, X.; Wu, C. Polymer electrolytes and interfaces toward solid-state batteries: Recent advances and prospects. Energy Storage Mater. 2020, 33, 26–54. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, F.; Wang, X.; Zheng, L.; Yang, X.; Zhao, H.; Sun, Y.; Zhao, W.; Bai, Y.; Wu, C. An Ion-dipole-reinforced polyether electrolyte with ion-solvation cages enabling high–voltage-tolerant and ion-conductive solid-state lithium metal batteries. Adv. Funct. Mater. 2022, 32, 2107764. [Google Scholar] [CrossRef]
- Guo, R.; Wu, F.; Wang, X.; Bai1, Y.; Wu, C. Multi-electron reaction-boosted high energy density batteries: Material and system innovation. J. Electrochem. 2022, 28, 2219011. [Google Scholar]
- Liu, Z.; Yu, A.; Lee, J.Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 1999, 81–82, 416–419. [Google Scholar] [CrossRef]
- Tsutomu, O.; Yoshinari, M. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar]
- Zhuang, G.V.; Chen, G.Y.; Shim, J.P.; Song, X.Y.; Ross, P.N. Thomas J Richardson. Li2CO3 in LiNi0.8Co0.15Al0.05O2 cathodes and its effects on capacity and power. J. Power Sources 2004, 134, 293–297. [Google Scholar] [CrossRef]
- Noh, M.-J.; Lee, Y.; Cho, J. Water adsorption and storage characteristics of optimized LiCoO2 and LiNi1/3CO1/3Mn1/3O2 composite cathode material for Li-ion cells. J. Electrochem. Soc. 2006, 153, A935–A940. [Google Scholar]
- Shizuka, K.; Kiyohara, C.; Shima, K.; Takeda, Y. Effect of CO2 on layered Li1+z Ni1-x-yCoxMyO2 (M = Al, Mn) cathode materials. J. Power Sources 2007, 166, 233–238. [Google Scholar] [CrossRef]
- Xiong, X.-H.; Wang, Z.-X.; Yue, P.; Guo, H.-J.; Wu, F.-X.; Wang, J.-X.; Li, X.-H. Washing effects on electrochemical performance and storage characteristics of LiNi0.8Co0.1Mn0.1O2 as cathode material for lithium-ion batteries. J. Power Sources 2013, 222, 318–325. [Google Scholar] [CrossRef]
- Xiong, X.-H.; Ding, D.; Bu, Y.; Wang, H.-X.; Huang, B.; Guo, H.-J.; Li, X.-H. Enhanced electrochemical properties of a LiNiO2-based cathode material by removing lithium residues with (NH4)2HPO4. J. Mater. Chem. A 2014, 2, 11691–11696. [Google Scholar] [CrossRef]
- Wu, F.; Tian, J.; Su, Y.-F.; Wang, J.; Zhang, C.-Z.; Bao, L.-Y.; He, T.; Li, J.-H.; Chen, S. Effect of Ni2+ content on lithium/nickel disorder for Ni-rich cathode materials. ACS Appl. Mater. Inter. 2015, 7, 7702–7708. [Google Scholar] [CrossRef]
- Xiao, P.; Lv, T.-J.; Chen, X.-P.; Chang, C.-K. LiNi0.8Co0.15Al0.05O2: Enhanced electrochemical performance from reduced cationic disordering in Li slab. Sci. Rep. 2017, 7, 1408–1415. [Google Scholar] [CrossRef]
- Liang, M.; Song, D.-W.; Zhang, H.-Z.; Shi, X.-X.; Wang, Q.; Zhang, L.-Q. Improved performances of LiNi0.8Co0.15Al0.05O2 material employing NaAlO2 as a new aluminum source. ACS Appl. Mater. Inter. 2017, 9, 38567–38574. [Google Scholar] [CrossRef]
- Li, X.; Ge, W.-J.; Wang, H.; Yan, X.-X.; Deng, B.-W.; Chen, T.; Qu, M.-Z. Enhancing cycle stability and storage property of LiNi0.8Co0.15Al0.05O2 by using fast cooling method. Electrochim. Acta 2017, 227, 225–234. [Google Scholar] [CrossRef]
- Trease, N.-M.; Seymour, I.-D.; Radin, M.-D.; Liu, H.-D.; Liu, H.; Hy, S.; Chernova, N.; Parikh, P.; Devaraj, A.; Wiaderek, K.-M.; et al. Identifying the distribution of Al3+ in LiNi0.8Co0.15A10.05O2. Chem. Mater. 2016, 28, 8170–8180. [Google Scholar] [CrossRef]
- Purwanto, A.; Yudha, C.-S.; Ubaidillah, U.; Widiyandari, H.; Ogi, T.; Haerudin, H. NCA cathode material: Synthesis methods and performance enhancement efforts. Mater. Res. Express 2018, 5, 122001. [Google Scholar] [CrossRef]
- Lee, K.-K.; Kim, K.-B. Electrochemical and Structural Characterization of Li Ni1-yCoyO2 (0 ≤ y ≤ 0.2) Positive Electrodes during Initial Cycling. J. Electrochem. Soc. 2000, 147, 1709–1717. [Google Scholar] [CrossRef]
- Cheng, C.-X.; Tan, L.-T.; Liu, H.-W.; Huang, X.-T. High Rate Performances of the Cathode Material LiNi1/3Co1/3Mn1/3O2 Synthesized Using Low Temperature Hydroxide Precipitation. Mater. Res. Bull. 2011, 46, 2032–2035. [Google Scholar] [CrossRef]
- Tang, Z.-F.; Wang, S.; Liao, J.-Y.; Wang, S.; He, X.-D.; Pan, B.-C.; He, H.-Y.; Chen, C.-H. Facilitating lithium-ion diffusion in layered cathode materials by introducing Li+/Ni2+ antisite defects for high-rate Li-ion batteries. Research 2019, 2019, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.-W.; Zhu, Y.-M.; Teng, X.-G. Effect of pre-thermal treatment on the lithium storage performance of LiNi0.8Co0.15Al0.05O2. J. Mater. Sci. 2016, 51, 1400–1408. [Google Scholar] [CrossRef]
- Vu, D.-L.; Choi, J.-Y.; Kim, W.-B.; Lee, J.-W. Layered LiNi0.8Co0.1Mn0.1O2 prepared through calcination in air with preoxidized precursor. J. Electrochem. Soc. 2017, 164, A2670–A2676. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Pan, Q.-L.; Wei, H.-X.; Huang, Y.-D.; Tang, L.-B.; Wang, Z.-Y.; He, Z.-J.; Yan, C.; Mao, J.; Dai, K.-H.; et al. Towards Ni-rich layered oxides cathodes with low Li/Ni intermixing by mild molten-salt ion exchange for lithium-ion batteries. Nano Energy 2022, 102, 107626. [Google Scholar] [CrossRef]
- Xiao, P.; Li, W.-H.; Chen, S.; Li, G.; Dai, Z.-J.; Feng, M.-D.; Chen, X.; Yang, W.-S. Effects of Oxygen pressurization on Li+/Ni2+ cation mixing and the oxygen vacancies of LiNi0.8Co0.15Al0.05O2 vathode materials. ACS Appl. Mater. Inter. 2022, 14, 31851–31861. [Google Scholar] [CrossRef]
- Miller, D.-J.; Proff, C.; Wen, J.-G.; Daniel, P.-A.; Javier, B. Observation of microstructural evolution in Li battery cathode oxide particles by in situ electron microscopy. Adv. Energy Mater. 2013, 3, 1098–1103. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fade of LiAlyNi1-x-yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests surface analysis of LiAlyNi1-x-yCoxO2 cathode after cycle tests in restricted depth of discharge ranges. J. Power Source 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Eunmi, J.; Sooyeon, H.; Seung, M.-K.; Chang, W.-Y. Investigating the kinetic effect on structural evolution of LixNi0.8Co0.15Al0.05O2 cathode materials during the initial charge/discharge. Chem. Mater. 2017, 29, 2708–2716. [Google Scholar]
- Hwang, S.; Chang, W.; Kim, S.-M.; Su, D.; Dong, H.-K.; Lee, J.-Y.; Chung, K.-Y.; Eric, A.S. Investigation of changes in the surface-structure of LiNi0.8Co0.15Al0.05O2 cathode materials induced by the initial charge. Chem. Mater. 2014, 26, 1084–1092. [Google Scholar] [CrossRef]
- Yano, A.; Aoyama, S.; Shikano, M.; Shikano, M.; Sakaebe, H.; Tatsumi, K.; Ogumi, Z. Surface structure and high-voltage charge/discharge characteristics of Al-oxide coated LiNi1/3Co1/3Mn1/3O2 cathodes. J. Electrochem. Soc. 2015, 162, A3137–A3144. [Google Scholar] [CrossRef]
- Sallis, S.; Pereira, N.; Mukherjee, P.; Quackenbush, N.-F.; Faenza, N.; Schlueter, C.; Lee, T.-L.; Yang, W.-L.; Cosandey, F.; Amatucci, G.-G.; et al. Surface degradation of Li1-xNi0.8Co0.15Al0.05O2 cathodes: Correlating charge transfer impedance with surface phase transformations. Appl. Phys. Lett. 2016, 108, 263902. [Google Scholar] [CrossRef]
- Chen, J.-N.; Yang, Y.; Tang, Y.-S.; Wang, Y.-F.; Li, H.; Xiao, X.-H.; Wang, S.-N.; Mariyam, S.D.D.; Etter, M.; Missyul, A.; et al. Constructing a thin disordered self-protective layer on the LiNiO2 primary particles against oxygen release. Adv. Funct. Mater. 2022, 33, 2211515. [Google Scholar] [CrossRef]
- Li, X.-H.; Wang, Q.; Guo, H.-Y.; Artrith, N.; Urban, A. Understanding the onset of surface degradation in LiNiO2 cathodes. ACS Appl. Energy Mater. 2022, 5, 5730–5741. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Yu, R.-N.; Xiao, Z.-T.; Zhu, Z.; Zhou, L.; Wu, J.-S. Co-gradient Li-rich cathode relieving the capacity decay in Lithium-ion batteries. Nano Energy 2022, 100, 107439. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Liu, M.; Ma, J.-B.; Yang, K.; Chen, Z.; Li, K.-K.; Zhang, C.; Wei, Y.-P.; Zhou, M.; Wang, P.; et al. Lithium hexamethyldisilazide as electrolyte additive for efficient cycling of high-voltage non-aqueous lithium metal batteries. Nat. Commun. 2022, 13, 6966. [Google Scholar] [CrossRef]
- Lin, W.-C.; Ye, Y.-K.; Chen, T.-W.; Jiang, Y.; Ouyang, C.-Y.; Pan, F.; Zheng, J.-X. Defect-mediated Jahn-Teller effect in layered LiNiO2. Sci. China Mater. 2022, 65, 1696–1700. [Google Scholar] [CrossRef]
- Li, S.; Yao, Z.-P.; Zheng, J.-M.; Fu, M.-S.; Cen, J.-J.; Hwang, S.; Jin, H.; Orlov, A.; Gu, L.; Wang, S.; et al. Direct observation of defect-aided structural evolution in a nickel-rich layered cathode. Angew. Chem. Int. Ed. Engl. 2020, 59, 22092–22099. [Google Scholar] [CrossRef]
- Zheng, J.-X.; Ye, Y.-K.; Liu, T.-C.; Xiao, Y.-G.; Wang, C.-M.; Wang, F.; Pan, F. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Li, W.-D.; Asl, H.-Y.; Xie, Q.; Manthiram, A. Collapse of LiNi1- x-yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 2019, 141, 5097–5101. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Geder, G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Foster, J.-M.; Offer, G.; Marinescu, M. A shrinking-core model for the degradation of high-nickel cathodes (NMC811) in Li-ion batteries: Passivation layer growth and oxygen evolution. J. Electrochem. Soc. 2021, 168, 020509. [Google Scholar] [CrossRef]
- Mikheenkova, A.; Gustafsson, O.; Misiewicz, C.; Brant, W.-R.; Hahlin, M.; Lacey, M.J. Resolving high potential structural deterioration in Ni-rich layered cathode materials for lithium-ion batteries operando. J. Energy Storage 2023, 57, 106211. [Google Scholar] [CrossRef]
- Vetter, J.; Nov, K.-P.; Wagner, M.-R.; Viet, C.; Möller, K.-C.; Besenhard, J.-O.; Winte, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in Lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Edström, K.; Gustafsson, T.; Thomas, J.-O. The cathode–electrolyte interface in the Li-ion battery. Electrochim. Acta 2004, 50, 397–403. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.-J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.-H.; Fenning, D.-P.; Lux, S.-F.; Paschos, O.; Bauer, C.; et al. Electrode-electrolyte interface in Li-ion batteries: Current understanding and new insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef]
- Zheng, S.-J.; Huang, R.; Makimura, Y.; Ukyo, Y.; Fisher, C.A.J.; Hirayama, T.; Ikuhara, Y. Microstructural changes in LiNi0.8Co0.15Al0.05O2 positive electrode material during the first cycle. J. Electrochem. Soc. 2011, 158, A357–A362. [Google Scholar] [CrossRef]
- Makimura, Y.; Zheng, S.-J.; Ikuhara, Y.; Ukyo, Y. Microstructural observation of Li Ni0.8Co0.15Al0.05O2 after charge and discharge by scanning transmission electron microscopy. J. Electrochem. Soc. 2012, 159, A1070–A1073. [Google Scholar] [CrossRef]
- Fu, C.-C.; Li, G.-S.; Luo, D.; Li, Q.; Fan, J.-M.; Li, L.-P. Nickel-rich layered microspheres cathodes: Lithium/nickel disordering and electrochemical performance. ACS Appl. Mater. Interfaces 2014, 6, 15822–15831. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.-N.; Yu, X.-Q.; Senanayake, S.-D.; Cho, S.-J.; Kim, K.-B.; Chung, K.-Y.; Yang, X.-Q.; Nam, K.-W. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Bak, S.-M.; Nam, K.-W.; Chang, W.-Y.; Yu, X.-Q.; Hu, E.-Y.; Hwang, S.; Stach, E.-A.; Kim, K.-B.; Chung, K.-Y.; Yang, X.-Q. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials. Chem. Mater. 2013, 25, 337–351. [Google Scholar] [CrossRef]
- Yoon, C.-S.; Ryu, H.-H.; Park, G.-T.; Kim, J.-H.; Kim, K.-H.; Sun, Y.-K. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathode for high-energy-density lithium-ion batteries. J. Mater. Chem. A 2018, 6, 4126–4132. [Google Scholar] [CrossRef]
- Miller, D.-J.; Proff, C.; Wen, J.-G.; Abraham, D.-P.; Bareño, J. Direct observation of microstructural evolution in Li battery cathode oxide particles during electrochemical cycling by in situ electron microscopy. Microsc. Microanal. 2012, 18, 1108–1109. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fading of LiAlyNi1−x−yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (effect of depth of discharge in charge—Discharge cycling on the suppression of the micro-crack generation of LiAlyNi1−x−yCoxO2 particle). J. Power Sources 2014, 260, 50–56. [Google Scholar]
- Ishidzu, K.; Oka, Y.; Nakamura, T. Lattice volume change during charge/discharge reaction and cycle performance of Li[NixCoyMnz]O2. Solid State Ion. 2016, 288, 176–179. [Google Scholar] [CrossRef]
- Palacín, M.-R.; Guibert, A.-D. Why do batteries fail. Science 2016, 351, 1253292. [Google Scholar] [CrossRef]
- Kondrakov, A.-O.; Schmidt, A.; Xu, J.; Gesswein, H.; Monig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic lattice strain and mechanical degradation of high-and low-nickel NCM cathode materials for Li-ion batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Mukai, K. Pseudo zero-strain insertion materials for Li-ion batteries: Cross-sectional observations of LiNi1/2Co1/2O2, LiNi1/3Co1/3Mn1/3O2, and LiNi0.8Co0.15Al0.05O2. Ionics 2018, 24, 2181–2186. [Google Scholar] [CrossRef]
- Lim, J.-M.; Hwang, T.; Kim, D.; Park, M.-S.; Cho, K.; Cho, M. Intrinsic origins of crack generation in Ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 2017, 7, 39669–39679. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, K.-J.; Yoon, C.-S.; Sun, Y.-K. Capacity Fading of Ni-Rich Li[NixCoyMn1−x−y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation. Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 2018, 8, 1702028. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.-W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Hua, W.-B.; Zhang, J.-L.; Wang, S.-N.; Cheng, Y.; Li, H.; Tseng, J.; Wu, Z.-H.; Shen, C.-H.; Dolotko, O.; Liu, H.; et al. Long-range cationic disordering induces two distinct degradation pathways in co-free Ni-rich layered cathodes. Angew. Chem. Int. Ed. 2022, 62, e202214880. [Google Scholar] [CrossRef]
- Mao, Y.-W.; Wang, X.-L.; Xia, S.-H.; Zhang, K.; Wei, C.-X.; Bak, S.; Shadike, Z.; Liu, X.-J.; Yang, Y.; Xu, R.; et al. High-voltage charging-induced strain, heterogeneity, and micro-cracks in secondary particles of a nickel-rich layered cathode material. Adv. Funct. Mater. 2019, 29, 1900247. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, H.; Park, J.; Roh, Y.; Byun, S.; Lim, J.; Jung, S.; Kim, N.; Lee, K.-T.; Lee, Y.-M. A microcrack propagation-based life prediction model for lithium-ion batteries with Ni-rich cathode materials. J. Energy Storage 2023, 58, 106420. [Google Scholar] [CrossRef]
- Quilty, C.-D.; West, P.-J.; Li, W.-Z.; Dunkin, M.-R.; Wheeler, G.-P.; Ehrlich, S.; Ma, L.; Jaye, C.; Fischer, D.-A.; Takeuchi, E.-S.; et al. Multimodal electrochemistry coupled microcalorimetric and X-ray probing of the capacity fade mechanisms of Nickel rich NMC–progress and outlook. Phys. Chem. Chem. Phys. 2022, 24, 11471. [Google Scholar] [CrossRef]
- Wu, L.-M.; Xiao, X.-H.; Wen, Y.-H.; Zhang, J. Three-dimensional finite element study on stress generation in synchrotron X-ray tomography reconstructed nickel-manganese cobalt based half cell. J. Power Sources 2016, 336, 8–18. [Google Scholar] [CrossRef]
- Nam, G.-W.; Park, N.-Y.; Park, K.-J.; Yang, J.; Liu, J.; Yoon, C.-S.; Sun, Y.-K. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent. ACS Energy Lett. 2019, 4, 2995–3001. [Google Scholar] [CrossRef]
- Park, N.-Y.; Park, G.-T.; Kim, S.-B.; Jung, W.; Park, B.-C.; Sun, Y.-K. Degradation mechanism of Ni-rich cathode materials: Focusing on particle interior. ACS Energy Lett. 2022, 7, 2362–2369. [Google Scholar] [CrossRef]
- Yoon, W.-S.; Balasubramanian, M.; Yang, X.-Q.; Mcbreen, J.; Hanson, J. Time-resolved XRD study on the thermal decomposition of Li1-xNi0.8Co0.15Al0.05O2 cathode materials for Li-ion batteries. Electrochem. Solid. St. 2005, 8, A83–A86. [Google Scholar] [CrossRef]
- Yoon, W.-S.; Hanson, J.; Mcbreen, J.; Yang, X.-Q. A study on the newly observed intermediate structures during the thermal decomposition of nickel-based layered cathode materials using time-resolved XRD. Electrochem. Commun. 2006, 8, 859–862. [Google Scholar] [CrossRef]
- Yoon, W.-S.; Chung, K.-Y.; Balasubramanian, M.; Hanson, J.; Mcbreen, J.; Yang, X.-Q. Time-resolved XRD study on the thermal decomposition of nickel-based layered cathode materials for Li-ion batteries. J. Power Sources 2006, 163, 219–222. [Google Scholar] [CrossRef]
- Wu, L.; Nam, K.-W.; Wang, X.; Zhou, Y.; Zheng, J.-C.; Yang, X.-Q.; Zhu, Y. Structural origin of overcharge-induced thermal instability of Ni-containing layered-cathodes for high-energy-density lithium batteries. Chem. Mater. 2011, 23, 3953–3960. [Google Scholar] [CrossRef]
- Nam, K.-W.; Bak, S.-M.; Hu, E.; Yu, X.-Q.; Zhou, Y.; Wang, X.-J.; Wu, L.-J.; Zhu, Y.; Chung, K.-Y.; Yang, X.-Q. Combining in situ synchrotron X-Ray diffraction and absorption techniques with transmission electron microscopy to study the origin of thermal instability in overcharged cathode materials for lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 1047–1063. [Google Scholar] [CrossRef]
- Ma, L.; Nie, M.-Y.; Xia, J.; Dahn, J.R. A systematic study on the reactivity of different grades of charged Li[NixMnyCoz]O2 with electrolyte at elevated temperatures using accelerating rate calorimetry. J. Power Sources 2016, 327, 145–150. [Google Scholar] [CrossRef]
- Walker, W.-Q.; Darst, J.-J.; Finegan, D.-P.; Bayles, G.-A.; Johnson, K.L.; Darcy, E.-C.; Rickman, S.-L. Decoupling of heat generated from ejected and non-ejected contents of 18650-format lithium-ion cells using statistical methods. J. Power Sources 2019, 415, 207–218. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.-J.; Yoon, C.-S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8, 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Gong, J.-Q.; Wang, Q.-S.; Sun, J.-H. Thermal analysis of nickel cobalt lithium manganese with varying nickel content used for lithium-ion batteries. Thermochim. Acta 2017, 655, 176–180. [Google Scholar] [CrossRef]
- Shi, C.-G.; Peng, X.-X.; Dai, P.; Xiao, P.H.; Zheng, W.-C.; Li, H.-Y.; Li, H.; Indris, S.; Mangold, S.; Hong, Y.-H.; et al. Investigation and suppression of oxygen release by LiNi0.8Co0.1Mn0.1O2 cathode under overcharge conditions. Adv. Energy Mater. 2022, 12, 2200569. [Google Scholar] [CrossRef]
- Hayashi, T.; Okada, J.; Toda, E.; Kuzuo, R.; Oshimura, N.; Kuwata, N.; Kawamura, J. Degradation mechanism of LiNi0.82Co0.15Al0.03O2 positive electrodes of a lithium-ion battery by a long-term cycling test. J. Electrochem. Soc. 2014, 161, A1007–A1011. [Google Scholar] [CrossRef]
- Sun, H.-H.; Choi, W.; Lee, J.-K.; Oh, I.-H.; Jung, H.-G. Control of electrochemical properties of nickel-rich layered cathode materials for lithium-ion batteries by variation of the manganese to cobalt ratio. J. Power Sources 2015, 275, 877–883. [Google Scholar] [CrossRef]
- Kim, H.-R.; Woo, S.-G.; Kim, J.-H.; Cho, W.; Kim, Y.-J. Capacity fading behavior of Ni-Rich layered cathode materials in li-ion full cells. J. Electroanal. Chem. 2016, 782, 168–173. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.-N.; Cui, Y.; Dufek, E.-J.; Goodenough, J.-B.; Khalifah, P.; Li, Q.-Y.; Liaw, B.-Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Kim, K.; Hwang, D.; Kim, S.; Park, S.-O.; Cha, Y.; Lee, Y.-S.; Cho, J.; Kwak, S.-K.; Choi, N.-S. Cyclic aminosilane-based additive ensuring stable electrode–electrolyte interfaces in Li-ion batteries. Adv. Energy Mater. 2020, 10, 2000012. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.-M.; Xie, M.; He, Y.-H.; Huang, G.-Y.; Yang, Y.-C. Growth mechanisms for spherical mixed hydroxide agglomerates prepared by co-precipitation method: A case of Ni1/3Co1/3Mn1/3(OH)2. J. Alloy. Compd. 2015, 619, 846–853. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.-X.; Li, X.-H.; Guo, H.-J.; Wang, J.-X. Synthesis of Ni0.8Co0.1Mn0.1(OH)2 precursor and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium batteries. Trans. Nonferrous Met. Soc. China 2015, 25, 2253–2259. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D. Synthesis of high-density nickel cobalt aluminum hydroxide by continuous coprecipitation method. ACS Appl. Mater. Interfaces 2012, 4, 586–589. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwon, K.-Y.; Choi, B.-K.; Yoo, H.-D. A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for Lithium-ion batteries. J. Electroanal. Chem. 2022, 924, 116828. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.-M.; Zhang, C.-M.; Zhang, L.; Wen, J.-W.; Wang, C.-X.; Huang, G.-Y. Synthesis of high-nickel and high-performance ternary cathode materials through spent lithium-ion batteries recycling system. Sustain. Chem. Pharm. 2023, 31, 100959. [Google Scholar] [CrossRef]
- Yang, C.-K.; Qi, L.-Y.; Zuo, Z.-C.; Wang, R.-N.; Ye, M.; Lu, J.; Zhou, H.-H. Insights into the inner structure of high-nickel agglomerate as high-performance lithium-ion cathodes. J. Power Sources 2016, 331, 487–494. [Google Scholar] [CrossRef]
- Duan, D.-G.; Wu, C.; Cao, Y.-B.; Huang, D.-H.; Du, K.; Peng, Z.D.; Hu, G.-R. Enhanced compacting density and cycling performance of Ni-riched electrode via building mono dispersed micron scaled morphology. J. Alloy. Compd. 2017, 695, 91–99. [Google Scholar] [CrossRef]
- Park, G.-T.; Park, N.-Y.; Noh, T.-C.; Namkoong, B.; Ryu, H.-H.; Shin, J.-Y.; Beierling, T.; Yoon, C.-S.; Sun, Y.-K. High-performance Ni-rich Li[Ni0.9-xCo0.1Alx]O2 cathodes via multi-stage microstructural tailoring from hydroxide precursor to the lithiated oxide. Energy Environ. Sci. 2021, 14, 5084–5095. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Zhou, Y.; Hai, C.-X.; Zeng, J.-B.; Ren, X.-F.; Sun, Y.-X.; Shen, Y.; Li, X.; Dong, S.-D.; Zhang, G.-T. Improving electrochemical performance of NCM811 cathodes for lithium-ion batteries via consistently arranging the hexagonal nanosheets with exposed {104} facets. Ceram. Int. 2022, 48, 17279–17288. [Google Scholar] [CrossRef]
- Kim, U.-H.; Myung, S.-T.; Yoon, C.-S.; Sun, Y.-G. Extending the battery life using an Al-doped Li[Ni0.76Co0.09Mn0.15]O2 cathode with concentration gradients for lithium ion batteries. ACS Energy Lett. 2017, 2, 1848–1854. [Google Scholar] [CrossRef]
- Kim, U.-H.; Lee, S.-B.; Ryu, J.-H.; Yoon, C.-S.; Sun, Y.-G. Optimization of Ni-rich Li[Ni0.92−xCo0.04Mn0.04Alx]O2 cathodes for high energy density lithium-ion batteries. J. Power Sources 2023, 564, 232850. [Google Scholar] [CrossRef]
- You, Y.; Celio, H.; Li, J.; Dolocan, A.; Manthiram, A. Modified high-nickel cathodes with stable surface chemistry against ambient air for lithium-ion batteries. Angew. Chem. Int. Ed. 2018, 57, 6480–6485. [Google Scholar] [CrossRef]
- Kim, U.-H.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. Quaternary layered Ni-rich NCMA cathode for lithium-ion batteries. ACS Energy Lett. 2019, 4, 576–582. [Google Scholar] [CrossRef]
- Yu, H.-F.; Cao, Y.-Q.; Chen, L.; Hu, Y.-J.; Duan, X.-Z.; Dai, S.; Li, C.-Z.; Jiang, H. Surface enrichment and diffusion enabling gradient-doping and coating of Ni-rich cathode toward Li-ion batteries. Nat. Commun. 2021, 12, 4564. [Google Scholar] [CrossRef]
- Li, W.-D.; Lee, S.; Manthiram, A. High-Nickel NMA: A cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef]
- Xi, R.-H.; Zhang, J.-N.; Lan, Z.-W.; Yuan, Y.-X.; Kang, J.-L.; Li, Y.-Y.; Wang, J.-T.; Zhang, C.-H.; Hou, X.-Y. High-nickel and cobalt-free layered LiNi0.90Mn0.06Al0.04O2 cathode for lithium-ion batteries. Ceram. Int. 2022, 48, 36690–36696. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Z.; Sun, J.-Y.; Xu, R.-M.; Li, X.-W.; Yu, X.; Liu, Y. Facile synthesis of crack-free single-crystalline Al-doped Co-free Ni-rich cathode material for lithium-ion batteries. Electrochim. Acta 2023, 437, 141473. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.-H.; Zheng, J.-C.; Zhang, D.-W.; Yang, J.-C.; Chen, Y.-X.; Guo, J.; Zhu, J.; Xiong, Y.; Li, W. Dual-modification of Gd2O3 on the high-voltage electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode materials via the solid-state method. J. Solid State Electrochem. 2020, 24, 863–872. [Google Scholar] [CrossRef]
- Schipper, F.; Dixit, M.; Kovacheva, D.; Talianker, M.; Haik, O.; Grinblat, J.; Erickson, E.; Ghanty, C.; Major, D.; Markovsky, B.; et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: Zirconium-doped LiNi0.6Co0.2Mn0.2O2. J. Mater. 2016, 4, 16073–16084. [Google Scholar] [CrossRef]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.-M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Che, W.; Wan, X.-W.; Zhang, D.-Y.; Chang, C.-K. Stabilized performance of LiNi0.90Co0.07Al0.03O2 cathodes via Zr4+ doping upon 4.5 V application due to the suppression of H2-H3 phase transitions. ACS Sustain. Chem. Eng. 2021, 9, 5536–5545. [Google Scholar] [CrossRef]
- Ni, J.-X.; Tan, Y.-Y.; Xu, K.; Jiang, Y.-J.; Chang, W.-Y.; Lai, C.-Y.; Liu, H. Cobalt-free nickel-rich layered LiNi0.9Al0.1-xZrxO2 cathode for high energy density and stable lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2022, 136, 104421. [Google Scholar] [CrossRef]
- Du, R.; Bi, Y.-J.; Yang, W.-C.; Peng, Z.; Liu, M.; Liu, Y.; Wu, B.-M.; Yang, B.-C.; Ding, F.; Wang, D.-Y. Improved cyclic stability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut-off potential of 4.5V. Ceram. Int. 2015, 41, 7133–7139. [Google Scholar] [CrossRef]
- Jiang, Y.; Bi, Y.-J.; Liu, M.; Peng, Z.; Huai, L.-Y.; Dong, P.; Duan, J.-G.; Chen, Z.-L.; Li, X.; Wang, D.-Y. Improved stability of Ni-rich cathode by the substitutive cations with stronger bonds. Electrochim. Acta 2018, 268, 41–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, B.; Yi, M.-Y.; Fan, X.-M.; Zhang, Z.; Huang, X.-B.; Lai, Y.-Q. In situ co-doping strategy for achieving long-term cycle stability of single-crystal Ni-rich cathodes at high voltage. Chem. Eng. J. 2022, 445, 136825. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Wang, Z.-H.; Zhu, Y.-H.; Jiang, H.; Li, C.-Z. Enhancing surface-to-bulk stability of layered Co-free Ni-rich cathodes for long-life Li-ion batteries. Battery Energy 2022, 2, 20220048. [Google Scholar] [CrossRef]
- Kaneda, H.; Koshika, Y.; Mori, K. Improving the cycling performance and thermal stability of LiNi0.6Co0.2Mn0.2O2 cathode materials by Nb-doping and surface modification. Int. J. Electrochem. Sc. 2017, 12, 4640–4653. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Chen, X.; Zhao, J.-Y.; Su, J.-M.; Zhang, C.-C.; Huang, T.; Wu, J.-H.; Yu, A.-S. Uncovering the role of Nb modification in improving the structure stability and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode charged at higher voltage of 4.5 V. J. Power Sources 2018, 374, 149–157. [Google Scholar] [CrossRef]
- Liu, L.-H.; Li, M.-C.; Chu, L.-H.; Jiang, B.; Lin, R.-X.; Zhu, X.-P.; Cao, G.-Z. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar] [CrossRef]
- Kim, U.-H.; Lee, S.-B.; Park, N.-Y.; Kim, S.-J.; Yoon, C.-S.; Sun, Y.-K. High-energy-density Li-ion battery reaching full charge in 12 min. ACS Energy Lett. 2022, 7, 3880–3888. [Google Scholar] [CrossRef]
- Wang, J.-L.; Yi, Z.-C.; Liu, C.-J.; He, M.-Y.; Miao, C.; Li, J.-Q.; Xu, G.-L.; Xiao, W. Revealing the effect of Nb5+ on the electrochemical performance of nickel-rich layered LiNi0.83Co0.11Mn0.06O2 oxide cathode for lithium-ion batteries. J. Colloid Interface Sci. 2023, 635, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Weigel, T.; Schipper, F.; Erickson, E.-M.; Susai, F.-A.; Markovsky, B.; Aurbach, D. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 2019, 4, 508–516. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, G.-T.; Son, B.-K.; Nam, G.-W.; Liu, J.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge. Nat. Energy 2020, 5, 860–869. [Google Scholar] [CrossRef]
- Sun, H.-H.; Kim, U.-H.; Park, J.-H.; Park, S.-W.; Seo, D.-H.; Heller, A.; Mullins, C.-B.; Yoon, C.-S.; Sun, Y.-K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 6552. [Google Scholar] [CrossRef]
- Kimura, N.; Seki, E.; Konishi, H.; Hirano, T.; Takahashi, S.; Ueda, A.; Horiba, T. Cycle deterioration analysis of 0.6 Ah-class lithium-ion cells with cell chemistry of LiNi0.6Co0.2Mn0.2O2-based/graphite. J. Power Sources 2016, 332, 187–192. [Google Scholar] [CrossRef]
- Xue, L.-L.; Li, Y.-J.; Xu, B.; Chen, Y.-X.; Cao, G.-L.; Li, J.-G.; Deng, S.-Y.; Chen, Y.-J.; Chen, J. Effect of Mo doping on the structure and electrochemical performances of LiNi0.6Co0.2Mn0.2O2cathode material at high cut-off voltage. J. Alloy. Compd. 2018, 748, 561–568. [Google Scholar] [CrossRef]
- Sattar, T.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Influence of Mo addition on the structural and electrochemical performance of Ni-rich cathode material for lithium-ion batteries. Sci. Rep. 2020, 10, 8562. [Google Scholar] [CrossRef]
- Park, G.-T.; Namkoong, B.; Kim, S.-B.; Liu, J.; Yoon, C.-S.; Sun, Y.-K. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat. Energy 2022, 7, 946–954. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, G.-T.; Yoon, C.-S.; Sun, Y.-K. Suppressing detrimental phase transitions via tungsten doping of LiNiO2 cathode for next generation lithium-ion batteries. J. Mater. Chem. A 2019, 7, 18580–18588. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, K.-J.; Yoon, D.-R.; Aishova, A.; Yoon, C.-S.; Sun, Y.-K. Li[Ni0.9Co0.09W0.01]O2: A new type of layered oxide cathode with high cycling stability. Adv. Energy Mater. 2019, 9, 1902698. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, N.-Y.; Park, G.-T.; Kim, H.; Yoon, C.-S.; Sun, Y.-K. High-energy W-doped Li[Ni0.95Co0.04Al0.01]O2 cathodes for next-generation electric vehicles. Energy Stor. Mater. 2020, 33, 399–407. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, C.-J.; Wang, Q.; Xu, G.-L.; Miao, C.; Xu, M.-B.; Wang, C.-J.; Xiao, W. Investigation of W6+-doped in high-nickel LiNi0.83Co0.11Mn0.06O2 cathode materials for high-performance lithium-ion batteries. J. Colloid Interface Sci. 2022, 628, 338–349. [Google Scholar] [CrossRef]
- Xiang, J.-F.; Chang, C.-X.; Zhang, F.; Sun, J.-T. Effects of Mg doping on the electrochemical properties of LiNi0.8Co0.2O2 cathode material. J. Alloy. Compd. 2009, 475, 483–487. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J.; Xiong, X.-H. Synthesis of Mg-doped LiNi0.8Co0.15Al0.05O2 oxide and its electrochemical behavior in high-voltage lithium-ion batteries. Ceram. Int. 2014, 40, 13223–13230. [Google Scholar] [CrossRef]
- Laskar, M.-R.; Jackson, D.-H.-K.; Xu, S.-Z.; Hamers, R.-J.; Morgan, D.; Kuech, T.-F. Atomic layer deposited MgO: A lower overpotential coating for LiNi0.5Mn0.3Co0.202 cathode. ACS Appl. Mater. Interfaces 2017, 9, 11231–11239. [Google Scholar] [CrossRef]
- Yang, G.-J.; Kim, Y. Electrochemical properties of Mg-added lithium nickel cobalt oxide induced by structural characteristics depending on the synthetic process. Ceram. Int. 2018, 44, 2198–2203. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.-Y.; Zhao, L.-M.; Zhou, Y.; Zhang, F.; He, D.-N. Improving the electrochemical performance of lithium-rich oxide layer material with Mg and La co-doping. J. Alloy. Compd. 2019, 782, 451–460. [Google Scholar] [CrossRef]
- Weber, R.; Li, H.-Y.; Chen, W.-F.; Kim, C.-Y.; Plucknett, K.; Dahn, J.-R. In situ XRD studies during synthesis of single-crystal LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 cathode materials. J. Electrochem. Soc. 2020, 167, 100501. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of co-free Ni-rich single crystal positive electrode materials for lithium-ion batteries: Part I. two-step lithiation method for Al or Mg-Doped LiNiO2. J. Electrochem. Soc. 2021, 168, 040531. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.-E.; Arab, P.; Li, H.-Y.; Dahn, J.-R. Synthesis of Co-Free Ni-Rich Single Crystal Positive Electrode Materials for Lithium Ion Batteries: Part II. One-Step Lithiation Method of Mg-Doped LiNiO2. J. Electrochem. Soc. 2021, 168, 050506. [Google Scholar] [CrossRef]
- Yu, H.-F.; Zhu, H.-W.; Yang, Z.-F.; Liu, M.-M.; Jiang, H.; Li, C.-Z. Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-life for Ni-rich layered cathodes. Chem. Eng. J. 2021, 412, 128625. [Google Scholar] [CrossRef]
- Chen, M.-M.; Zhao, E.-Y.; Chen, D.-F.; Wu, M.-M.; Han, S.-B.; Huang, Q.-Z.; Yang, L.-M.; Xiao, X.-L.; Hu, Z.-B. Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-Rich LiNi0.8Co0.1Mn0.1O2 by Ca doping. Inorg. Chem. 2017, 56, 8355–8362. [Google Scholar] [CrossRef]
- Wang, L.-C.; Chu, Y.-Q.; Nong, Y.-T.; Zheng, F.-H.; Li, Y.; Huang, Y.-Z.; Li, Y.-H.; Pan, Q.-C.; Wang, H.-Q.; Li, Q.-Y. Sr-Based Sub/Surface Integrated Layer and Bulk Doping to Enhance High-Voltage Cycling of a Ni-Rich Cathode Material. ACS Sustain. Chem. Eng. 2022, 10, 7883–7895. [Google Scholar] [CrossRef]
- Cui, Z.-H.; Xie, Q.; Manthiram, A. Zinc-Doped High-Nickel, Low-Cobalt Layered Oxide Cathodes for High-Energy-Density Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 15324–15332. [Google Scholar] [CrossRef]
- Kim, Y. Lithium nickel cobalt manganese oxide synthesized using alkali chloride flux: Morphology and performance as a cathode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 2329–2333. [Google Scholar] [CrossRef]
- Huang, Z.-J.; Wang, Z.-X.; Jing, Q.; Guo, H.-J.; Li, X.-H.; Yang, Z.-H. Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material. Electrochim. Acta. 2016, 192, 120–126. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Sun, Y.-Y.; Liu, S.; Li, G.-R.; Gao, X.-P. Na-doped LiNi0.8Co0.15Al0.05O2 with excellent stability of both capacity and potential as cathode materials for Li-ion batteries. ACS Appl. Energy Mater. 2018, 1, 3881–3889. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.; Wang, H.; Yan, X.-X.; Wang, L.; Deng, B.-W.; Ge, W.-J.; Qu, M.-Z. The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. J. Power Sources 2018, 374, 1–11. [Google Scholar] [CrossRef]
- Park, K.-J.; Jung, H.-G.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries. Adv. Energy Mater. 2018, 8, 1801202. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Seo, J.-H.; Yu, Y.-S.; Sharma, M.; Mucke, R.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries. Mater. Today 2020, 36, 73–82. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.-M.; Luo, B.; Zhao, Z.-W.; Shen, J.-X.; Liu, Z.-H.; Xiao, Z.-M.; Zhang, B.; Zhang, J.-F.; Ming, L. Understanding the enhancement effect of boron doping on the electrochemical performance of single-crystalline Ni-rich cathode materials. J. Colloid Interface Sci. 2021, 604, 776–784. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.-X.; Guo, H.-J.; Xiong, X.-H.; Li, X.-H. A low temperature fluorine substitution on the electrochemical performance of layered LiNi0.8Co0.1Mn0.1O2-zFz cathode materials. Electrochim. Acta 2013, 92, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Xie, Z.-W.; Liu, W.-J.; Ge, W.-J.; Wang, H.; Qu, M.-Z. Effects of fluorine doping on structure, surface chemistry, and electrochemical performance of LiNi0.8Co0.15Al0.05O2. Electrochim. Acta 2015, 174, 1122–1130. [Google Scholar] [CrossRef]
- Kong, F.-T.; Liang, C.-P.; Longo, R.-C.; Yeon, D.-H.; Zheng, Y.-P.; Park, J.-H.; Doo, S.-G.; Cho, K. Conflicting roles of anion doping on the electrochemical performance of Li-ion battery cathode materials. Chem. Mater. 2016, 28, 6942–6952. [Google Scholar] [CrossRef]
- Binder, J.-O.; Culver, S.-P.; Pinedo, R.; Weber, D.-A.; Friedrich, M.-S.; Gries, K.-I.; Volz, K.; Zeier, W.-G.; Janek, J. Investigation of fluorine and nitrogen as anionic dopants in nickel-rich cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 44452–44462. [Google Scholar] [CrossRef]
- Li, C.-L.; Kan, W.-H.; Xie, H.-L.; Jiang, Y.; Zhao, Z.-K.; Zhu, C.-Y.; Xia, Y.-H.; Zhang, J.; Xu, K.; Mu, D.-B.; et al. Inducing favorable cation antisite by doping halogen in Ni-rich layered cathode with ultrahigh stability. Adv. Sci. 2018, 6, 1801406. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sendek, A.-D.; Cubuk, E.-D.; Zhang, X.-K.; Lu, Z.-Y.; Gong, Y.-J.; Wu, T.; Shi, F.-F.; Liu, W.; Reed, E.-J. Atomic layer deposition of stable LiAlF4 lithium-ion conductive interfacial layer for stable cathode cycling. ACS Nano 2017, 11, 7019–7027. [Google Scholar] [CrossRef] [PubMed]
- Vanaphuti, P.; Chen, J.-J.; Cao, J.-Y.; Bigham, K.; Chen, B.; Yang, L.-F.; Chen, H.-L.; Wang, Y. Enhanced electrochemical performance of the lithium-manganese-rich cathode for Li-ion batteries with Na and F codoping. ACS Appl. Mater. Interfaces 2019, 11, 37842–37849. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Kim, U.-H.; Sun, Y.-K.; Yoon, C.-S. Multi-Doped (Ga,B) Li[Ni0.885Co0.100Al0.015]O2 Cathode. J. Electrochem. Soc. 2020, 167, 100557. [Google Scholar] [CrossRef]
- Feng, Z.; Rajagopalan, R.; Zhang, S.; Sun, D.; Tang, Y.-G.; Ren, Y.; Wang, H.-Y. A three in one strategy to achieve zirconium doping, boron doping, and interfacial coating for stable LiNi0.8Co0.1Mn0.1O2 cathode. Adv. Sci. 2020, 8, 2001809. [Google Scholar] [CrossRef]
- Choi, C.-M.; Park, J.-H.; Sun, Y.-K.; Yoon, C.-S. Ultra-stable cycling of multi-doped (Zr,B) Li[Ni0.885Co0.100Al0.015] O2 cathode. J. Power Sources 2021, 513, 230548. [Google Scholar] [CrossRef]
- Darjazi, H.; Gonzalo, E.; Acebedo, B.; Cid, R.; Zarrabeitia, M.; Bonilla, F. Improving high-voltage cycling performance of Nickel-rich NMC layered oxide cathodes for rechargeable lithiumeion batteries by Mg and Zr co-doping. Mater. Today 2022, 20, 100236. [Google Scholar]
- Ryu, H.-H.; Lim, H.-W.; Kang, G.-C.; Park, N.-Y.; Sun, Y.-K. Long-Lasting Ni-Rich NCMA Cathodes via Simultaneous Microstructural Refinement and surface Modification. ACS Energy Lett. 2023, 8, 1354–1361. [Google Scholar] [CrossRef]
- Omanda, H.; Brousse, T.; Marhic, C.; Schleich, D.-M. Improvement of the thermal stability of LiNi0.8Co0.2O2 cathode by a SiOx protective coating. J. Electrochem. Soc. 2004, 151, A922–A929. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.-M.; Song, J.-H.; Yim, T.; Kim, J.-S.; Kim, Y.-J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Han, B.; Xu, S.; Zhao, S.; Lin, G.-X.; Feng, Y.-Z.; Chen, L.-B.; Ivey, G.-D.; Wang, P.; Wei, W.-F. Enhancing the structural stability of Ni-rich layered oxide cathodes with a preformed Zr-concentrated defective nanolayer. ACS Appl. Mater. Interfaces 2018, 10, 39599–39607. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J.; Huang, B. Structure and electrochemical performance of TiO2-coated LiNi0.80Co0.15Al0.05O2 cathode material. Mater. Lett. 2015, 143, 151–154. [Google Scholar] [CrossRef]
- Li, W.-W.; Zhang, X.-J.; Si, J.-J.; Yang, J.; Sun, X.-Y. TiO2-coated LiNi0.9Co0.08Al0.02O2 cathode materials with enhanced cycle performance for Li-ion batteries. Rare Met. 2021, 40, 1719–1726. [Google Scholar] [CrossRef]
- Wang, W.-C.; Lee, C.-H.; Yu, D.; Kondo, Y.; Miyahara, Y.; Abe, T.; Miyazaki, K. Effects of a solid solution outer layer of TiO2 on the surface and electrochemical properties of LiNi0.6Co0.2Mn0.2O2 cathodes for lithium-ion batteries through the use of thin-film electrodes. ACS Appl. Energy Mater. 2022, 5, 5117–5126. [Google Scholar] [CrossRef]
- Neudeck, S.; Strauss, F.; Garcia, G.; Wolf, H.; Janek, J.; Hartmann, P.; Brezesinski, T. Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material. Chem. Commun. 2019, 55, 2174–2177. [Google Scholar] [CrossRef]
- Mohanty, D.; Dahlberg, K.; King, D.-M.; David, L.-A.; Sefat, A.-S.; Wood, D.-L.; Daniel, C.; Dhar, S.; Mahajan, V.; Lee, M.; et al. Modification of Ni-rich FCG NMC and NCA cathodes by atomic layer deposition: Preventing surface phase transitions for high-voltage lithium-ion batteries. Sci. Rep. 2016, 6, 26532. [Google Scholar] [CrossRef]
- Lou, F.-H.; Xie, Q.-S.; Luo, X.-J.; Xie, Y.-T.; Wang, M.-Y.; Hao, H.-M.; Wang, Z.-Q.; Yang, L.-X.; Wang, G.; Chen, J.-Y.; et al. Preventing structural collapse and thermal runaway to improve the electrochemical performance and safety of LiNi0.8Co0.1Mn0.1O2 by a negative-thermal-expansion material of Al2(WO3)4. Ind. Eng. Chem. Res. 2022, 61, 4588–4600. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Qiu, Y.-G.; Cheng, F.-Y.; Wei, P.; Li, Y.-Y.; Liu, Y.; Sun, S.-X.; Xu, Y.; Li, Q.; Fang, C.; et al. Realization of a high-voltage and high-rate nickel-rich NCM cathode material for LIBs by Co and Ti dual modification. ACS Appl. Mater. Interfaces 2021, 13, 17707–17716. [Google Scholar] [CrossRef]
- Yoon, M.; Dong, Y.-H.; Hwang, J.; Sung, J.; Cha, H.; Ahn, K.; Huang, Y.; Kang, S.-J.; Cho, J. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 2021, 6, 362–371. [Google Scholar] [CrossRef]
- Xiao, P.; Cao, Y.; Li, W.-H.; Li, G.; Yu, Y.-L.; Dai, Z.-J.; Du, Z.-X.; Chen, X.; Sun, J.; Yang, W.-S. simple strategy for synthesizing LiNi0.8Co0.15Al0.05O2 using Co Al-LDH nanosheet-coated Ni(OH)2 as the orecursor: Dual effects of the buffer layer and synergistic diffusion. ACS Appl. Mater. Interfaces 2021, 13, 29714–29725. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, P.-P.; Zeng, T.-Y.; Yu, Z.-L.; Qu, X.-Y.; Peng, X.-Q.; Zhou, Y.; Duan, X.-G.; Dou, A.; Su, M.; et al. Improving the structure stability of LiNi0.8Co0.15Al0.05O2 by double modification of tantalum surface coating and doping. ACS Appl. Energy Mater. 2021, 4, 8641–8652. [Google Scholar] [CrossRef]
- Cai, M.-Z.; Dong, Y.-H.; Xie, M.; Dong, W.-J.; Dong, C.-L.; Dai, P.; Zhang, H.; Wang, X.; Sun, X.-Z.; Zhang, S.-N.; et al. Stalling oxygen evolution in high-voltage cathodes by lanthurization. Nat. Energy 2023, 8, 159–168. [Google Scholar] [CrossRef]
- Kim, H.-B.; Park, B.-C.; Myung, S.-T.; Amine, K.; Prakash, J.; Sun, Y.-K. Electrochemical and thermal characterization of AlF3-coated LiNi0.8Co0.15A10.05O2 cathode in lithium- ion cells. J. Power Sources 2008, 179, 347–350. [Google Scholar] [CrossRef]
- Park, B.-C.; Kim, H.-B.; Bang, H.-J.; Prakash, J. Improvement of electrochemical performance of Li[Ni0.8Co0.15Al0.05]O2 cathode materials by AlF3 coating at various temperatures. Ind. Eng. Chem. Res. 2008, 47, 3876–3882. [Google Scholar] [CrossRef]
- Xiong, X.-H.; Wang, Z.-X.; Yin, X.; Guo, H.-J.; Li, X.-H. A modified LiF coating process to enhance the electrochemical performance characteristics of LiNi0.8Co0.1Mn0.1O2 cathode materials. Mater. Lett. 2013, 110, 4–9. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J. Lithium-reactive Co3(PO4)2 nanoparticle coating on high-capacity LiNi0.8Co0.16A10.04O2 cathode material for lithium rechargeable batteries. J. Electrochem. Soc. 2007, 154, A495–A499. [Google Scholar] [CrossRef]
- Lee, D.-J.; Scrosati, B.; Sun, Y.-K. Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at 55 °C. J. Power Sources 2011, 196, 7742–7746. [Google Scholar] [CrossRef]
- Jo, M.; Oh, P.; Kim, J.; Choi, J.-H.; Kim, S.; Ha, S.; Son, Y. Electrochemical lithium storage performance at high voltage and temperature of LiNi0.6Co0.2Mn0.2O2 cathode for Lithium-ion batteries by facile Mn3(PO4)2 dry coating. Appl. Surf. Sci. 2013, 613, 156018. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J. A facile process for coating amorphous FePO4 onto LiNi0.8Co0.15Al0.05O2 and the effects on its electrochemical properties. Mater. Lett. 2014, 131, 210–213. [Google Scholar] [CrossRef]
- Qi, R.; Shi, J.-L.; Zhang, X.-D.; Zeng, X.-X. Improving the stability of LiNi0.80Co0.15Al0.05O2 by AlPO4 nanocoating for lithium-ion batteries. Sci. China Chem. 2017, 60, 1230–1235. [Google Scholar] [CrossRef]
- Cheng, W.-D.; Li, L.; Hao, S.-L.; Wu, Y.-X.; Huo, J.-S.; Ji, Y.-Y.; Liu, X.-Q. Face-lifting the surface of LiNi0.8Co0.15Al0.05O2 cathode via Y(PO3)3 to form an in situ triple composite Li-ion conductor coating layer with the enhanced electrochemical performance. Nanotechnology 2022, 33, 375701. [Google Scholar] [CrossRef]
- Peng, Z.-D.; Huang, M.; Wang, W.-G.; Du, K.; Guan, D.-C.; Hu, G.; Cao, Y.-B. Enhancing the structure and interface stability of LiNi0.83Co0.12Mn0.05O2 cathode material for Li-ion batteries via facile CeP2O7 coating. ACS Sustain. Chem. Eng. 2022, 10, 4881–4893. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Cheng, W.-D.; Hao, S.; Li, L.; Ran, Q.-W.; Liu, L.; Ji, Y.-Y.; Huo, J.-S.; Liu, X.-Q. Utilizing dual functions of LaPO4 to enhance the electrochemical performance of LiNi0.87Co0.09Al0.04O2 cathode material. Nanotechnology 2023, 34, 075706. [Google Scholar] [CrossRef]
- Liu, W.-M.; Hu, G.-R.; Du, K.; Peng, Z.-D.; Cao, Y.-B. Surface coating of LiNi0.8Co0.15Al0.05O2 with LiCoO2 by a molten salt method. Surf. Coat. Technol. 2013, 216, 267–272. [Google Scholar] [CrossRef]
- Yang, C.-K.; Shao, R.; Mi, Y.-Y.; Shen, L.-Y.; Zhao, B.-L.; Wang, Q.; Wu, K.; Lui, W.; Gao, P.; Zhou, H.-H. Stable interstitial layer to alleviate fatigue fracture of high nickel cathode for lithium-ion batteries. J. Power Sources 2018, 376, 200–206. [Google Scholar] [CrossRef]
- Ito, S.; Fujiki, S.; Yamada, T.; Aihara, Y.; Park, Y.; Kim, T.-Y.; Baek, S.-W.; Lee, J.-M.; Doo, S.; Machida, N. A rocking chair type all-solid-state lithium-ion battery adopting Li2O-ZrO2 coated LiNi0.8Co0.15Al0.05O2 and a sulfide based electrolyte. J. Power Sources 2014, 248, 943–950. [Google Scholar] [CrossRef]
- Song, B.-H.; Li, W.-D.; Oh, S.-M.; Manthiram, A. Long-life nickel-rich layered oxide cathodes with a uniform Li2ZrO3 surface coating for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 9718–9725. [Google Scholar] [CrossRef]
- Sun, X.-W.; Wang, L.-L.; Ma, J.; Yu, X.-R.; Zhang, S.; Zhou, X.-H.; Cui, G.-L. A Bifunctional Chemomechanics Strategy to Suppress Electrochemo-Mechanical Failure of Ni-Rich Cathodes for All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 17674–17681. [Google Scholar] [CrossRef]
- Jeyakumar, J.; Wu, Y.-S.; Wu, S.-H.; Jose, R.; Yang, C.-C. Surface-modified quaternary layered Ni-rich cathode materials by Li2ZrO3 for improved electrochemical performance for high-power Li-ion batteries. ACS Appl. Energy Mater. 2022, 5, 4796–4807. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Y.; Guo, F.-Q.; Zhu, C.-Q.; Qiu, L.; Zhou, J.-B.; Deng, Y.-T.; Zheng, Z.; Liu, Y.; Sun, Y.; et al. Effective and low-cost in situ surface engineering strategy to enhance the interface stability of an ultrahigh Ni-rich NCMA cathode. ACS Appl. Mater. Interfaces 2022, 14, 51835–51845. [Google Scholar]
- Qiao, Y.; Hao, R.-H.; Shi, X.-W.; Li, Y.-B.; Wang, Y.-L.; Zhang, Y.; Tang, C.; Li, G.-H.; Wang, G.-L.; Liu, J.-M.; et al. Improving the cycling stability of LiNi0.8Co0.1Mn0.1O2 by enhancing the structural integrity via synchronous Li2SiO3 coating. ACS Appl. Energy Mater. 2022, 5, 4885–4892. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, W.; Yao, J.; Bu, L.; Li, X.; Tian, K.; Lu, H.; Quan, C.; Xu, S.; Xu, K.; et al. Suppressing structural degradation of Ni-rich cathode materials towards improved cycling stability enabled by a Li2MnO3 coating. J. Mater. Chem. A 2020, 8, 17429–17441. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Strauss, F.; Bartsch, T.; Teo, J.-H.; Janek, J.; Brezesinski, T. Effect of surface carbonates on the cyclability of LiNbO3-coated NCM622 in all-solid-state batteries with lithium thiophosphate electrolytes. Sci. Rep. 2021, 11, 5367. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-X.; Zhao, X.-Y.; Xie, Y.-G.; Luo, S.; Wang, Z.Y.; Zhu, L.-Y.; Zhang, X. Insights into capacity fading mechanism and coating modification of high-nickel cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 55491–55502. [Google Scholar] [CrossRef]
- Chen, J.-H.; Zhu, L.; Jia, D.; Jiang, X.-B.; Wu, Y.-G.; Hao, O.-L.; Xia, X.-F.; Ouyang, Y.; Peng, L.; Tang, W.-P.; et al. LiNi0.8Co0.15Al0.05O2 cathodes exhibiting improved capacity retention and thermal stability due to a lithium iron phosphate coating. Electrochim 2019, 312, 179–187. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Lan, Z.W.; Xi, R.-H.; Li, Y.-Y.; Zhang, C.-H. The cycle stability and rate performance of LiNi0.8Mn0.1Co0.1O2 enhanced by Mg doping and LiFePO4 coating. ChemElectroChem 2022, 9, e202101654. [Google Scholar]
- Tang, Z.-F.; Wu, R.; Huang, P.-F.; Wang, Q.-S.; Chen, C.-H. Improving the electrochemical performance of Ni-rich cathode material LiNi0.815Co0.15Al0.035O2 by removing the lithium residues and forming Li3PO4 coating layer. J. Alloy. Compd. 2017, 693, 1157–1163. [Google Scholar] [CrossRef]
- Yan, P.-F.; Zheng, J.-M.; Liu, J.; Wang, B.-Q.; Cheng, X.-P.; Zhang, Y.-F.; Sun, C.-M.; Wang, X.-L.; Zhang, J.-G. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 2018, 3, 600–605. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.-J.; Jin, B.-S.; Kim, H.-S. Dual function Li-reactive coating from residual lithium-on Ni-rich NCM cathode material for Lithium-ion batteries. Sci. Rep. 2021, 11, 18590. [Google Scholar] [CrossRef]
- Cheng, W.-D.; Hao, S.; Ji, Y.-Y.; Li, L.; Liu, L.; Xiao, Y.; Wu, Y.-X.; Huo, J.-S.; Tang, F.; Liu, X.-Q. Optimizing surface residual alkali and enhancing electrochemical performance of LiNi0.8Co0.15Al0.05O2cathode by LiH2PO4. Nanotechnology 2022, 33, 045404. [Google Scholar] [CrossRef]
- Du, F.-H.; Sun, P.-P.; Zhou, Q.; Zeng, D.; Hu, D.; Fan, Z.-X.; Hao, Q.; Mei, C.-X.; Xu, T.; Zheng, J.-W. Interlinking primary grains with lithium boron oxide to enhance the stability of LiNi0.8Co0.15Al0.05O2. ACS Appl. Mater. Interfaces 2020, 12, 56963–56973. [Google Scholar] [CrossRef]
- Tan, X.-X.; Peng, W.-J.; Duan, H.; Wang, Z.-X.; Guo, H.-J.; Luo, G.; Yuan, R.-Z.; Li, X.-H.; Wang, J.-X. A scalable dry chemical method for lithium borate coating to improve the performance of LiNi0.90Co0.06Mn0.04O2 cathode material. Solid State Ion. 2022, 28, 2073–2082. [Google Scholar] [CrossRef]
- Yang, J.-C.; Li, Y.-J.; Xi, X.-M.; Zheng, J.-C.; Yu, J.; He, Z.-J. Suppressed internal intrinsic stress engineering in high-performance Ni-Rich cathode via multilayered in situ coating structure. Energy Environ. Mater. 2022, 24, 12574. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, A.-Q.; Wang, K.; Mao, Q.-Z.; Huang, H.; Zhang, J.; He, X.-P.; Gan, Y.-P.; Xiao, Z.; Zhang, W.-K. Industrial modification comparison of Ni-Rich cathode materials towards enhanced surface chemical stability against ambient air for advanced lithium-ion batteries. Chem. Eng. J. 2022, 450, 138382. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.-J.; Doo, S.-G.; Jin, B.-S.; Kim, H.-S. A synergetic modification approach toward high capacity Ni-rich cathode materials for next generation lithium-ion batteries. Solid State Ion. 2022, 387, 116053. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.-F.; Lu, S.-R.; Ding, W.-B.; Yu, X.-L.; Liang, G.-M.; Zou, J.-S.; Kang, F.-Y.; Zhang, J.-J.; Cao, Y.-D. B2O3/LiBO2 dual-modification layer stabilized Ni-rich cathode for lithium-ion battery. J. Power Sources 2022, 536, 231510. [Google Scholar] [CrossRef]
- Hee, S.; Kang, S.; Lee, Y.-S.; Shin, W.-K.; Kim, S.; Shin, K.; Kim, D.-W. Improvement of the Cycling Performance of LiNi0.6Co0.2Mn0.2O2 Cathode Active Materials by a Dual-Conductive Polymer Coating. ACS Appl. Mater. 2014, 6, 2546–2552. [Google Scholar]
- Wu, Y.-S.; Pham, Q.-T.; Yang, C.-C.; Chern, C.-S.; Babulal, L.-M.; Seenivasan, M.; Jeyakumar, J.; Mengesha, T.-H.; Placke, T.; Brunklaus, G.; et al. Coating of a novel lithium-containing hybrid oligomer additive on nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode materials for high-stability and high-safety lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 7394–7408. [Google Scholar] [CrossRef]
- Xu, X.; Huo, H.; Jian, J.-Y.; Wang, L.-G.; Zhu, H.; Xu, S.; He, X.-S.; Yin, G.-P.; Du, C.-Y.; Sun, X.-L. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1803963. [Google Scholar] [CrossRef]
- Wang, T.; Ren, K.-L.; He, M.; Dong, W.-H.; Xiao, W.; Pan, H.-Y.; Yang, J.; Yang, Y.; Liu, P.; Cao, Z.-J.; et al. Synthesis and Manipulation of Single-Crystalline Lithium Nickel Manganese Cobalt Oxide Cathodes: A Review of Growth Mechanism. Front. Chem. 2020, 8, 747. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Jessie, H.; Jeff, D. Microstructural Observations of “Single Crystal” Positive Electrode Materials Before and After Long Term Cycling by Cross-section Scanning Electron Microscopy. J. Electrochem. Soc. 2020, 167, 020512. [Google Scholar] [CrossRef]
- Kong, X.-B.; Zhang, Y.-G.; Peng, S.-Y.; Zeng, J.; Zhao, J.-B. Superiority of single crystal to polycrystalline LiNixCoyMn1-x-yO2 cathode materials in storage behaviors for lithium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 14938–14948. [Google Scholar] [CrossRef]
- Qian, G.-N.; Zhang, Y.-T.; Li, L.-S.; Zhang, R.-X.; Xu, J.-M.; Chen, Z.-J.; Xie, S.-J.; Wang, H.; Rao, Q.-L.; He, Y.-S.; et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Fan, X.-M.; Hu, G.-R.; Zhang, B.; Ou, X.; Zhang, J.-F.; Zhao, W.-G.; Jia, H.-P.; Zou, L.-F.; Li, P.; Yang, Y. Crack-free single crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano. Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Han, Y.-K.; Xu, J.-M.; Wang, W.; Long, F.; Qu, Q.; Wang, Y.; Zheng, H. Implanting an electrolyte additive on a single crystal Ni-rich cathode surface for improved cycle ability and safety. J. Mater. Chem. A 2020, 8, 24579–24589. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Fan, C.-L.; Han, S.-H. A highly stabilized single crystalline nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode through a novel surface spinel-phase modification. Electrochim. Acta 2020, 341, 136075. [Google Scholar] [CrossRef]
- Zheng, L.-T.; Bennett, J.-C.; Obrovac, M.-N. All-dry synthesis of single crystal NMC cathode materials for Li-ion batteries. J. Electrochem. Soc. 2020, 167, 130536. [Google Scholar] [CrossRef]
- Wang, F.; Ge, M.-Y.; Wi, S.-G.; Liu, X.; Bai, J.-M.; Steven, E.; Lu, D.-Y.; Lee, W.-K.; Chen, Z.-H.; Wang, F. Kinetic limitations in single-crystal high-nickel cathodes. Angew. Chem. Int. Ed. 2021, 60, 17350–17355. [Google Scholar]
- Hu, J.-T.; Li, L.-Z.; Bi, Y.-J.; Tao, J.-H.; Lochala, J.; Liu, D.-Y.; Wu, B.-B.; Cao, X.; Chae, S.; Wang, C.-M.; et al. Locking Oxygen in Lattice: A Quantifiable Comparison of Gas Generation in Polycrystalline and Single Crystal Ni-Rich Cathodes. Energy Storage Mater. 2022, 47, 195–202. [Google Scholar] [CrossRef]
- Zhao, W.-G.; Zou, L.-F.; Zhang, L.-T.; Fan, X.-M.; Zhang, H.-H.; Pagani, F.; Brack, E.; Seidl, L.; Ou, X.; Egorov, K.; et al. Assessing long-term cycling stability of single-crystal versus polycrystalline nickel-rich NCM in pouch cells with 6 mAh · cm−2 Electrodes. Small 2022, 18, 2107357. [Google Scholar] [CrossRef]
- Han, G.-M.; Kim, Y.-S.; Ryu, H.-H.; Sun, Y.-K.; Yoon, C.-H. Structural stability of single-crystalline Ni-rich layered cathode upon delithiation. ACS Energy Lett. 2022, 7, 2919–2926. [Google Scholar] [CrossRef]
- Dai, P.-P.; Kong, X.-B.; Yang, H.-Y.; Li, J.-Y.; Zeng, J.; Zhao, J.-B. Single-crystal Ni-rich layered LiNi0.9Mn0.1O2 enables superior performance of co-Free cathodes for lithium-ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 4381–4390. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Cha, H.; Kostecki, R.; Chen, G.-Y. Composite cathode design for high-energy all-solid-state lithium batteries with long cycle life. ACS Energy Lett. 2023, 8, 521–528. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.-P.; Tian, H.-Y.; Yan, J.-Q.; Tong, J.-F.; Liu, X.-H.; Zhang, H.-X.; Huang, H.-Q.; Hao, S.-M.; Gao, J.; et al. Pulse high temperature sintering to prepare single-crystal high nickel oxide cathodes with enhanced electrochemical performance. Adv. Energy Mater. 2023, 13, 2203188. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Kim, M.-H.; Prakash, J.; Amine, K. Synthesis and characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the microscale core-shell structure as the positive electrode material for lithium batteries. J. Am. Chem. Soc. 2005, 127, 13411–13418. [Google Scholar] [CrossRef]
- Kim, M.-H.; Shin, H.-S.; Shin, D.; Sun, Y.-K. Synthesis and electrochemical properties of Li[Ni0.8Co0.1Mn0.1]O2 and Li[Ni0.8Co0.2]O2 via co-precipitation. J. Power Sources 2006, 159, 1328–1333. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Park, B.-C.; Amine, K. Synthesis of spherical nano-to microscale core-shell particles Li[(Ni0.8Co0.1Mn0.1)1-x(Ni0.5Mn0.5)x]O2 and their applications to lithium batteries. Chem. Mater. 2006, 18, 5159–5163. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Lee, B.-R.; Noh, H.-J.; Wu, H.; Myung, S.-T.; Amine, K. A novel concentration-gradient Li[Ni0.83Co0.07Mn0.10]O2 cathode material for high-energy lithium-ion batteries. J. Mater. Chem. 2011, 21, 10108–10112. [Google Scholar] [CrossRef]
- Myung, S.-T.; Noh, H.-J.; Yoon, S.-J.; Lee, E.-J.; Sun, Y.-K. Progress in High-Capacity Core-Shell Cathode Materials for Rechargeable Lithium Batteries. J. Phys. Chem. Lett. 2014, 5, 671–679. [Google Scholar] [CrossRef]
- Li, Q.; Dang, R.; Chen, M.; Lee, Y.; Hu, Z.-B.; Xiao, X.-L. A synthesis method for long cycle life lithium-ion cathode material: Ni-rich core-shell LiNi0.8Co0.1Mn0.1O2. ACS Appl. Mater. Interfaces 2018, 10, 17850–17860. [Google Scholar] [CrossRef]
- Chen, X.-L.; Jia, X.-B.; Qu, Y.-Y.; Li, D.; Chen, D.-M.; Chen, Y. High-voltage performance of concentration-gradient Li[Ni0.6Co0.2Mn0.2]O2 layered oxide cathode materials for lithium batteries. New J. Chem. 2018, 42, 5868. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Noh, T.-C.; Kang, G.-C.; Maglia, F.; Kim, S.-J.; Yoon, C.-S.; Sun, Y.-K. Microstrain alleviation in high-energy Ni-rich NCMA cathode for long battery life. ACS Energy Lett. 2021, 6, 216–223. [Google Scholar] [CrossRef]
- Zheng, J.-C.; Yang, Z.; Dai, A.; Tang, L.-B.; Wei, H.-X.; Li, Y.-J.; He, Z.-J.; Lu, J. Boosting Cell Performance of LiNi0.8 Co0.15 Al0.05 O2 via Surface Structure Design. Small 2019, 15, 1904854. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-J.; Liu, M.; Xiao, B.-W.; Jiang, Y.; Lin, H.; Zhang, Z.-G.; Chen, G.-X.; Sun, Q.; He, H.-Y.; Huang, F.; et al. Highly stable Ni-rich layered oxide cathode enabled by a thick protective layer with bio-tissue structure. Energy Storage Mater. 2020, 24, 291–296. [Google Scholar] [CrossRef]
- Park, G.-T.; Sun, H.-H.; Noh, T.-C.; Maglia, F.; Kim, S.-J.; Lamp, P.; Sun, Y.-K. Nanostructured co-free layered oxide cathode that affords fast-charging lithium-ion batteries for electric vehicles. Adv. Energy Mater. 2022, 12, 2202719. [Google Scholar] [CrossRef]
- Rathore, D.; Garayt, M.; Liu, Y.-L.; Geng, C.-X.; Johnson, M.; Dahn, J.-R.; Yang, C.-Y. Preventing interdiffusion during synthesis of Ni-rich core-shell cathode materials. ACS Energy Lett. 2022, 7, 2189–2195. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Park, B.-C.; Prakash, J.; Belharouak, L.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320–324. [Google Scholar] [CrossRef]
- Noh, H.-J.; Chen, Z.-H.; Yoon, C.-S.; Lu, J.; Amine, K.; Sun, Y.-K. Cathode Material with Nanorod Structure-An Application for Advanced High-Energy and Safe Lithiμm Batteries. Chem. Mater. 2013, 25, 2109–2115. [Google Scholar] [CrossRef]
- Yoon, C.-S.; Sun, Y.K.; Kim, U.-H.; Park, K.-J.; Ryu, H.-H.; Kim, H.-S.; Sun, Y.-K. Microstructure Evolution of Concentration Gradient Li[Ni0.75Co0.10Mn0.15]O2 Cathode for Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802090. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.-Y.; Li, Q.; Yang, Y.-Q.; Deng, X.; Dang, R.-B.; Wu, M.-M.; Wu, Z.-J.; Xiao, X.-L.; Yu, X.-Q. In situ synthesis of nickel concentration gradient structure promising superior electrochemical properties of Ni-rich LiNi0.8Co0.15Al0.05O2 at high cut-off voltage. Nanoscale 2020, 12, 11182–11191. [Google Scholar] [CrossRef]
- Park, N.-Y.; Ryu, H.-H.; Park, G.-T.; Noh, T.-C.; Sun, Y.-K. Optimized Ni-rich NCMA cathode for electric vehicle batteries. Adv. Energy Mater. 2021, 11, 2003767. [Google Scholar] [CrossRef]
- Shang, M.-W.; Chen, X.; Niu, J.-J. Nickel-rich layered LiNi0.8Mn0.1Co0.1O2 with dual gradients on both primary and secondary particles in lithium-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100767. [Google Scholar] [CrossRef]
| Materials | Abbreviation | Molar Fraction of Ni Element | Molar Fraction of Co Element | Molar Fraction of Mn Element | Molar Fraction of Al Element | Initial Discharge Capacity (mAh g−1) |
|---|---|---|---|---|---|---|
| LiNi1/3Co1/3Mn1/3O2 | NCM111 | 1/3 | 1/3 | 1/3 | / | ~153 |
| LiNi0.5Co0.2Mn0.3O2 | NCM523 | 0.5 | 0.2 | 0.3 | / | ~162 |
| LiNi0.6Co0.2Mn0.2O2 | NCM622 | 0.6 | 0.2 | 0.2 | / | ~169 |
| LiNi0.7Co0.2Mn0.1O2 | NCM721 | 0.7 | 0.2 | 0.1 | / | ~189 |
| LiNi0.8Co0.1Mn0.1O2 | NCM811 | 0.8 | 0.1 | 0.1 | / | ~198 |
| LiNi0.9Co0.05Mn0.05O2 | NCM90 | 0.9 | 0.05 | 0.05 | / | ~231 |
| LiNi0.8Co0.15Al0.05O2 | NCA | 0.8 | 0.15 | / | 0.05 | ~202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Guo, R.; Bai, Y.; Li, N.; Wang, X.; Wang, J.; Wu, C. High-Performance High-Nickel Multi-Element Cathode Materials for Lithium-Ion Batteries. Batteries 2023, 9, 319. https://doi.org/10.3390/batteries9060319
Tian X, Guo R, Bai Y, Li N, Wang X, Wang J, Wu C. High-Performance High-Nickel Multi-Element Cathode Materials for Lithium-Ion Batteries. Batteries. 2023; 9(6):319. https://doi.org/10.3390/batteries9060319
Chicago/Turabian StyleTian, Xinyong, Ruiqi Guo, Ying Bai, Ning Li, Xinran Wang, Jiantao Wang, and Chuan Wu. 2023. "High-Performance High-Nickel Multi-Element Cathode Materials for Lithium-Ion Batteries" Batteries 9, no. 6: 319. https://doi.org/10.3390/batteries9060319
APA StyleTian, X., Guo, R., Bai, Y., Li, N., Wang, X., Wang, J., & Wu, C. (2023). High-Performance High-Nickel Multi-Element Cathode Materials for Lithium-Ion Batteries. Batteries, 9(6), 319. https://doi.org/10.3390/batteries9060319








