Model-Based Analysis and Optimization of Acidic Tin–Iron Flow Batteries
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Electrolyte Preparations
2.3. Cell Performance Tests
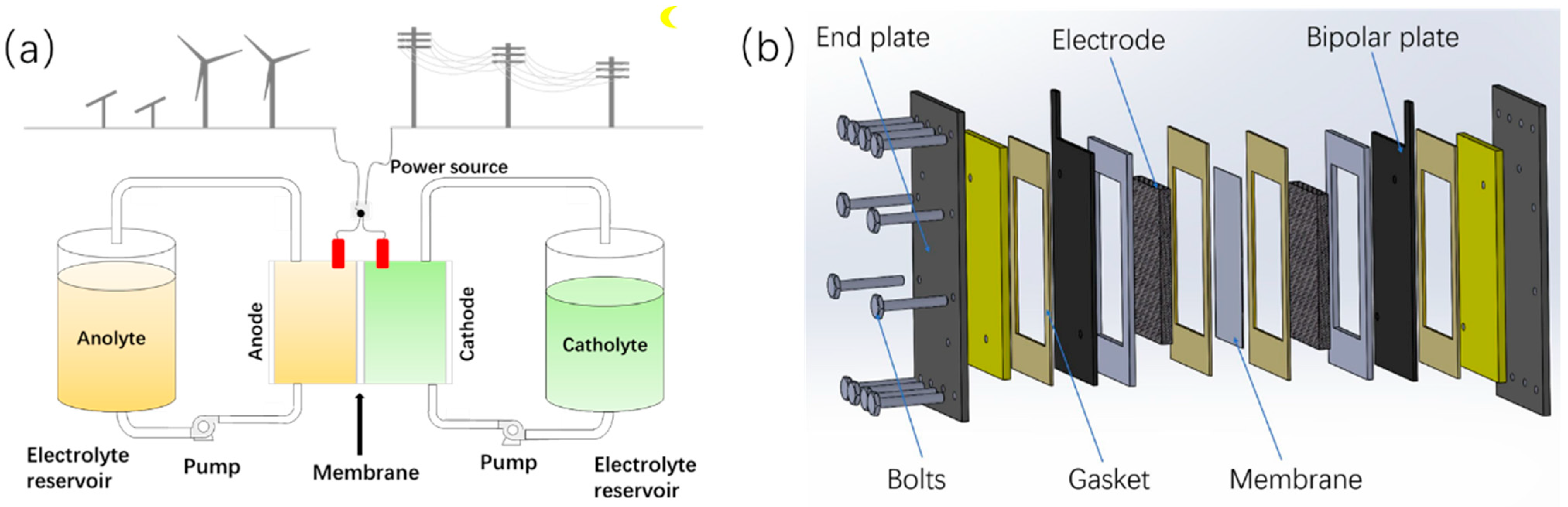
2.4. Dynamic Modeling
- (1)
- The electrolyte concentrations are uniform in the cell and reservoirs;
- (2)
- Gassing side reactions are negligible;
- (3)
- The temperature in the reservoirs and the cell are constant during operation;
- (4)
- Contact resistances between electrode and bipolar plate are minimized;
- (5)
- The variations of electrolyte volume in the cell and reservoirs can be neglected.
2.4.1. Electrochemical Model
- is the cell’s formal potential;
- R denotes the molar gas constant;
- T is temperature;
- z refers to the unit activity coefficient;
- F is the Faraday constant;
- i is current density;
- , , and are the concentrations of Sn2+, Fe2+, and Fe3+ in TIFB cell, respectively;
- and are the concentrations of reactant in negative and positive half-cell, respectively;
- km denotes the local mass transfer coefficient as expressed in Equation (10), where A1 is the cross-sectional area of the electrode and Q is the flow rate.
2.4.2. Mass Balance
- for Fe3+ ion:
- for Fe2+ ion:
- for Sn2+ ion:
- denote the concentrations of Fe2+, Fe3+, and Sn2+ in the reservoirs at time t, respectively;
- θ denotes the membrane thickness;
- V and V′ denote the electrolyte volume in the flow cell and reservoirs, respectively;
- A2 denotes the cross-sectional area of the membrane;
- “+” and “−”denote the charging and discharging processes, respectively;
- denote the diffusion coefficients of Fe3+, Fe2+, and Sn2+, respectively.
2.5. Cell Pressure, Pump Loss, and System Efficiency
2.5.1. Pressure Loss in Flow Cell
- is the total pressure loss;
- is the pressure loss through the pipe;
- is the pressure loss through the porous electrode, which can be determined according to Darcy’s law, as shown in Equations (21) and (22).
- is the length of the porous electrode;
- denotes the viscosity of electrolyte;
- is the cross-sectional area of the porous electrode;
- is the permeability of the porous electrode;
- is the fiber diameter of the porous electrode;
- is the permeability of the porous electrode.
- denotes Darcy friction factor;
- denotes pipe diameter;
- denotes velocity of the flow;
- denotes pipe length.
2.5.2. Pump Loss and System Efficiency
3. Results and Discussion
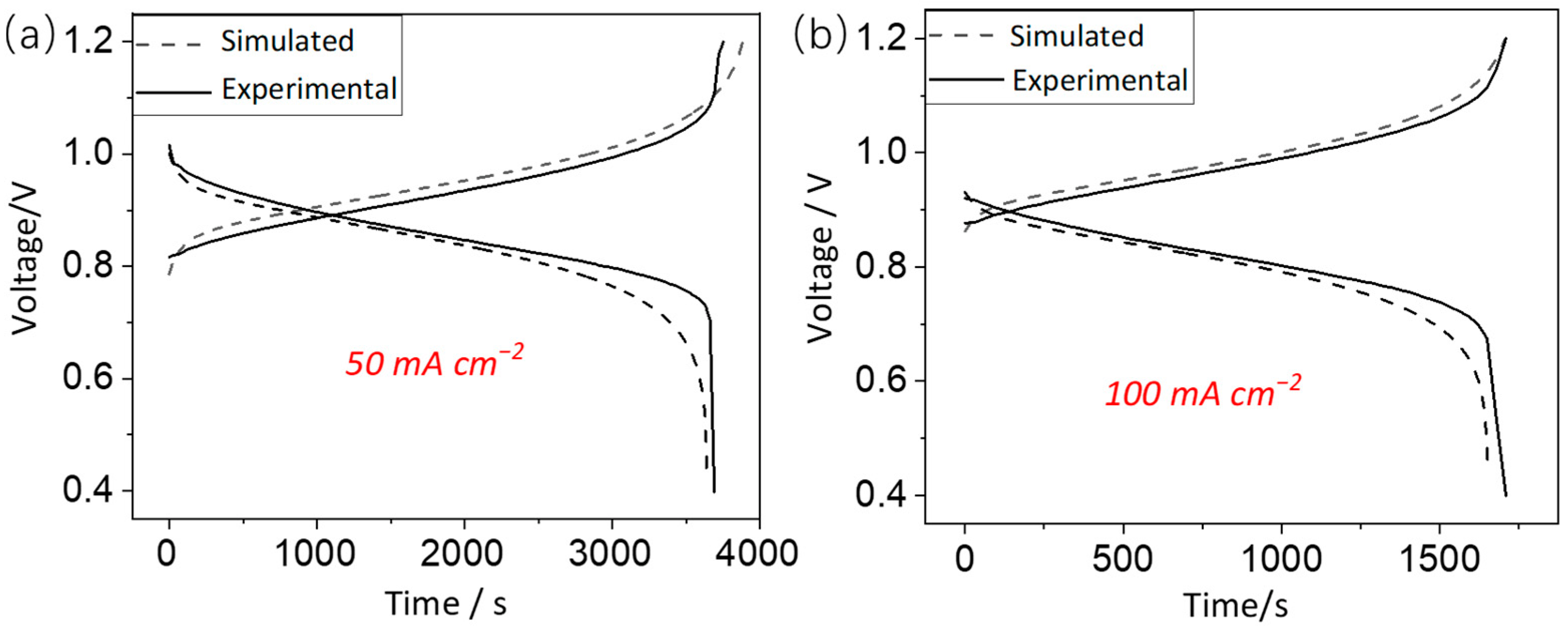
3.1. Model Validation
3.2. Key Operational Factors
3.2.1. Effects of Flow Rate
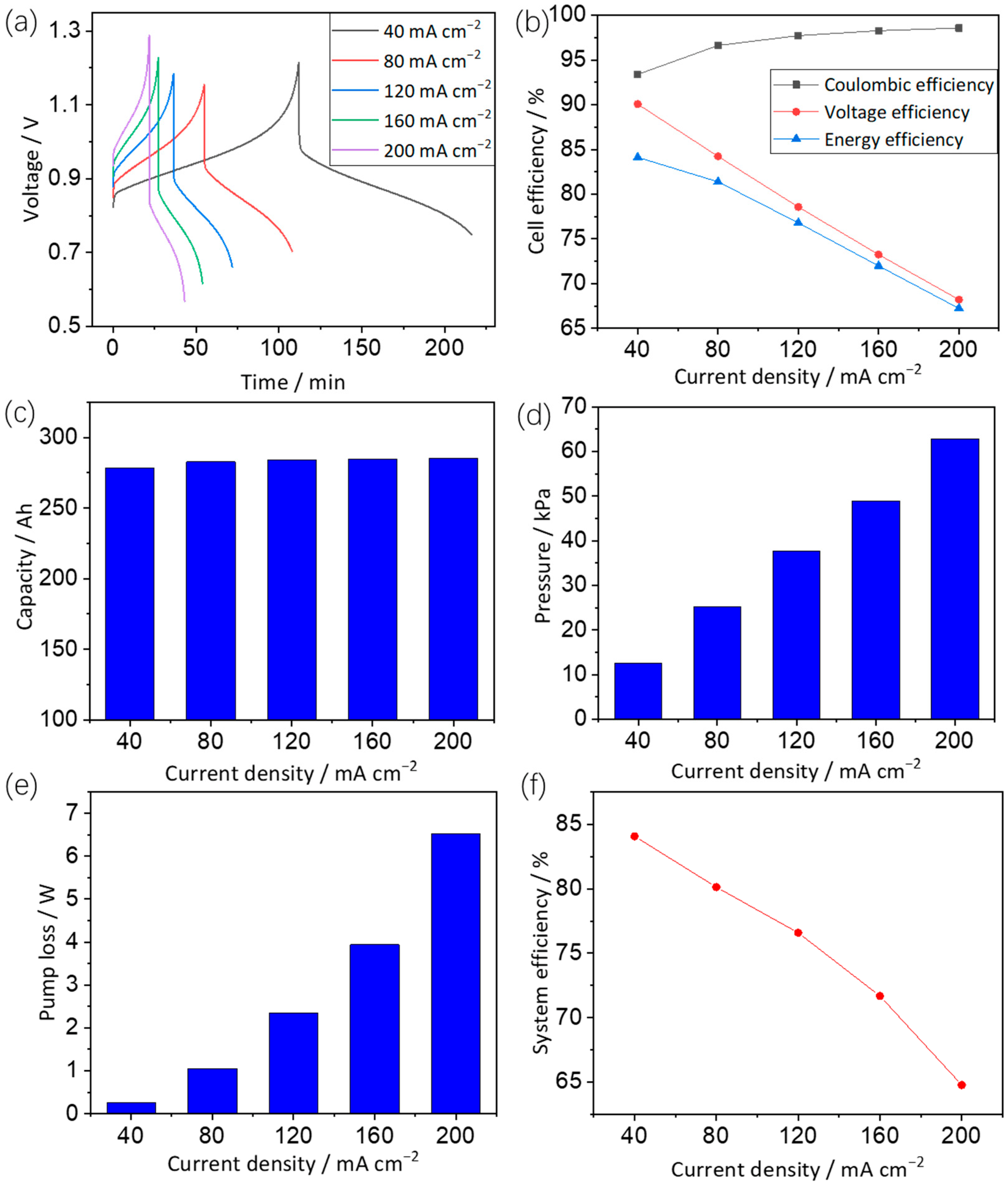
3.2.2. Effects of Current Density
3.3. Electrode Parameters
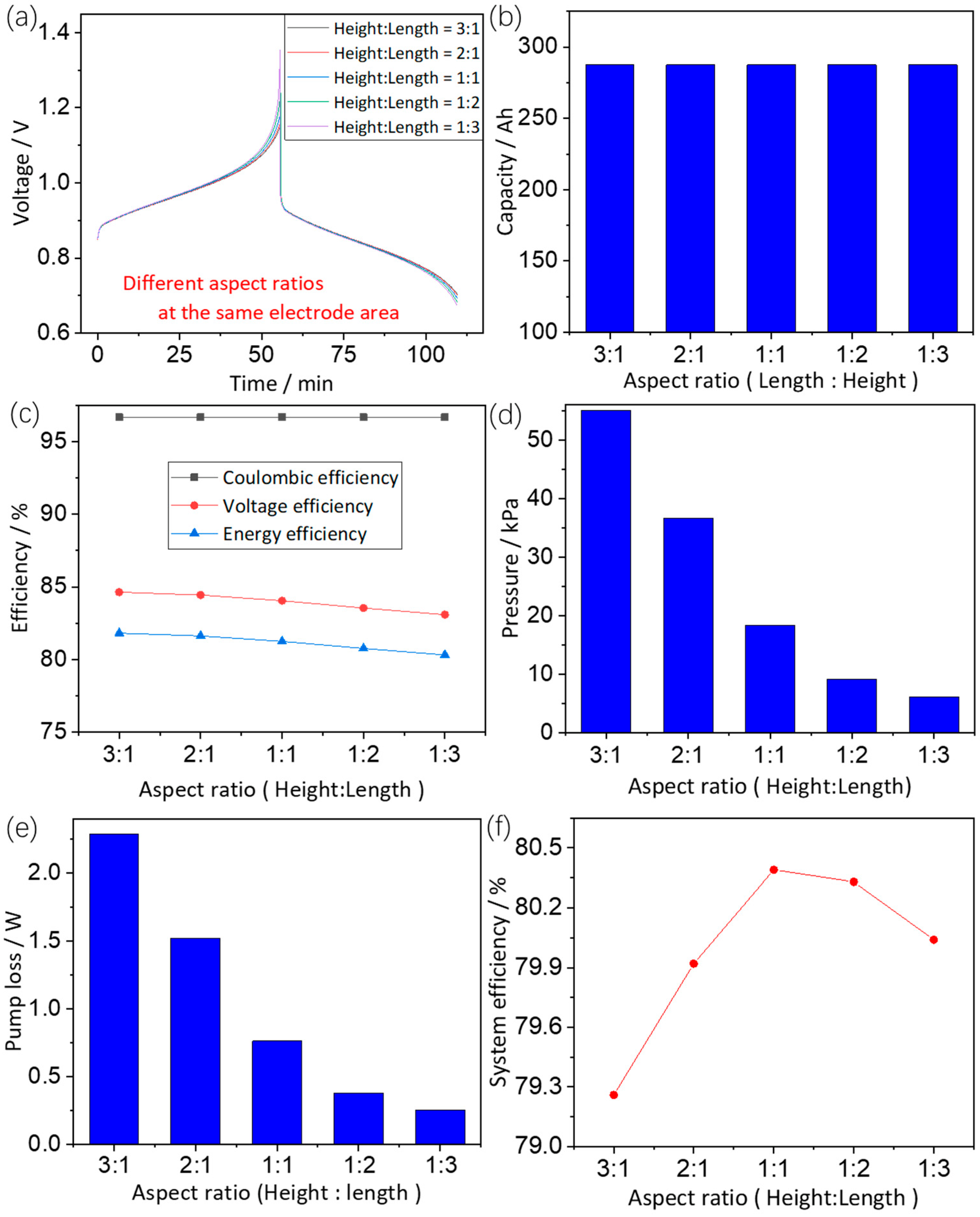
3.3.1. Effects of Electrode Dimensions
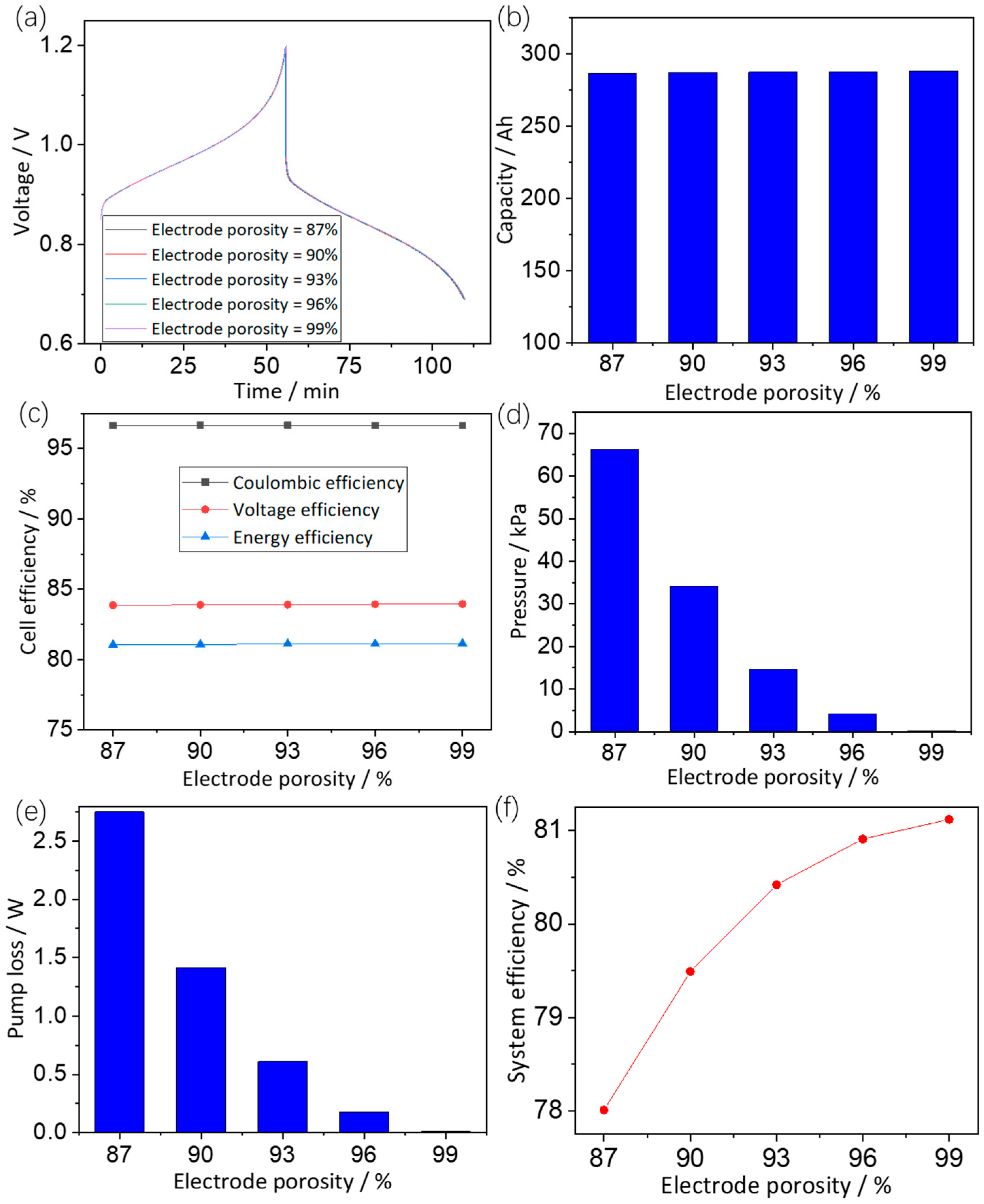
3.3.2. Effects of Electrode Porosity
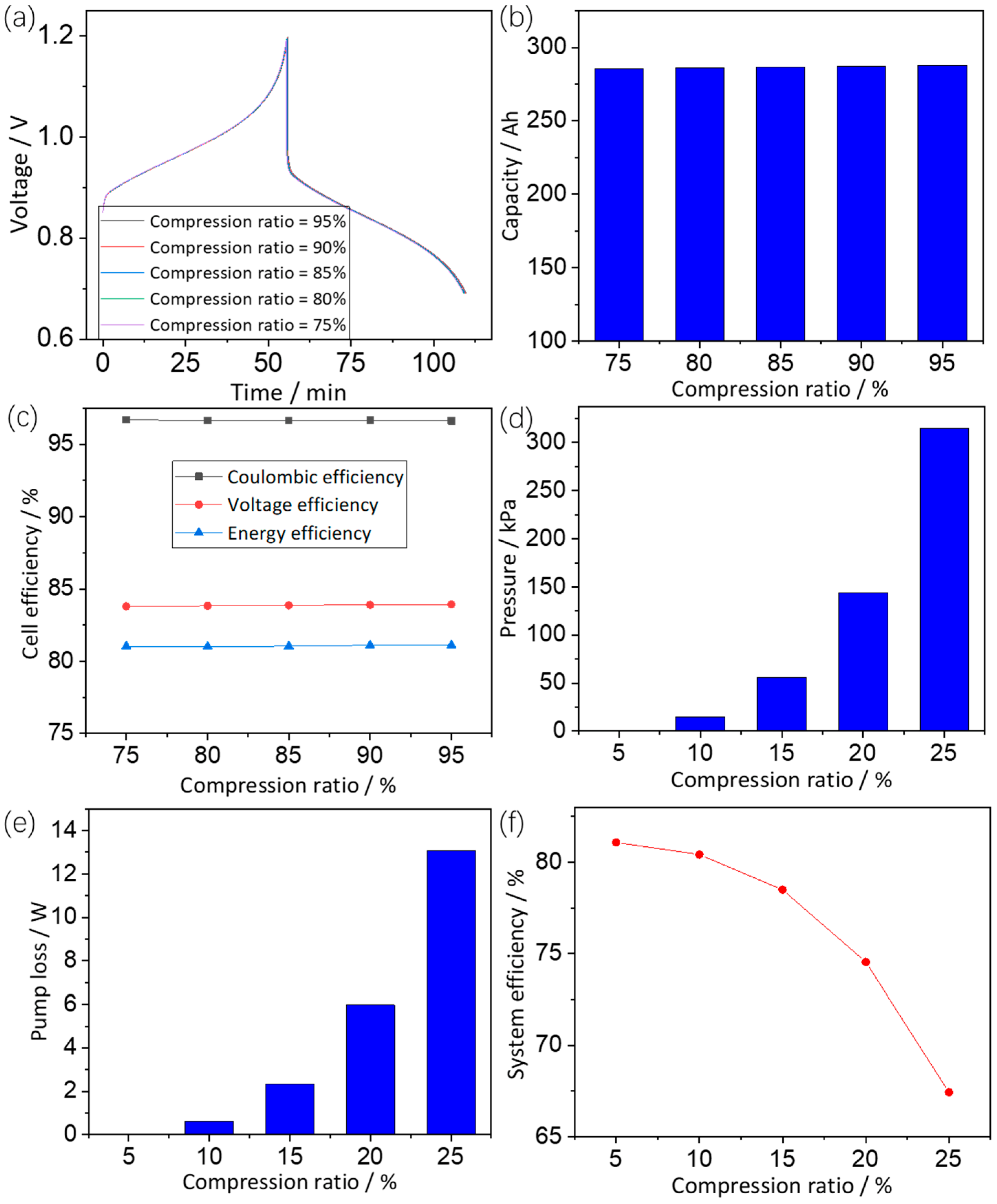
3.3.3. Effects of Compression Ratio
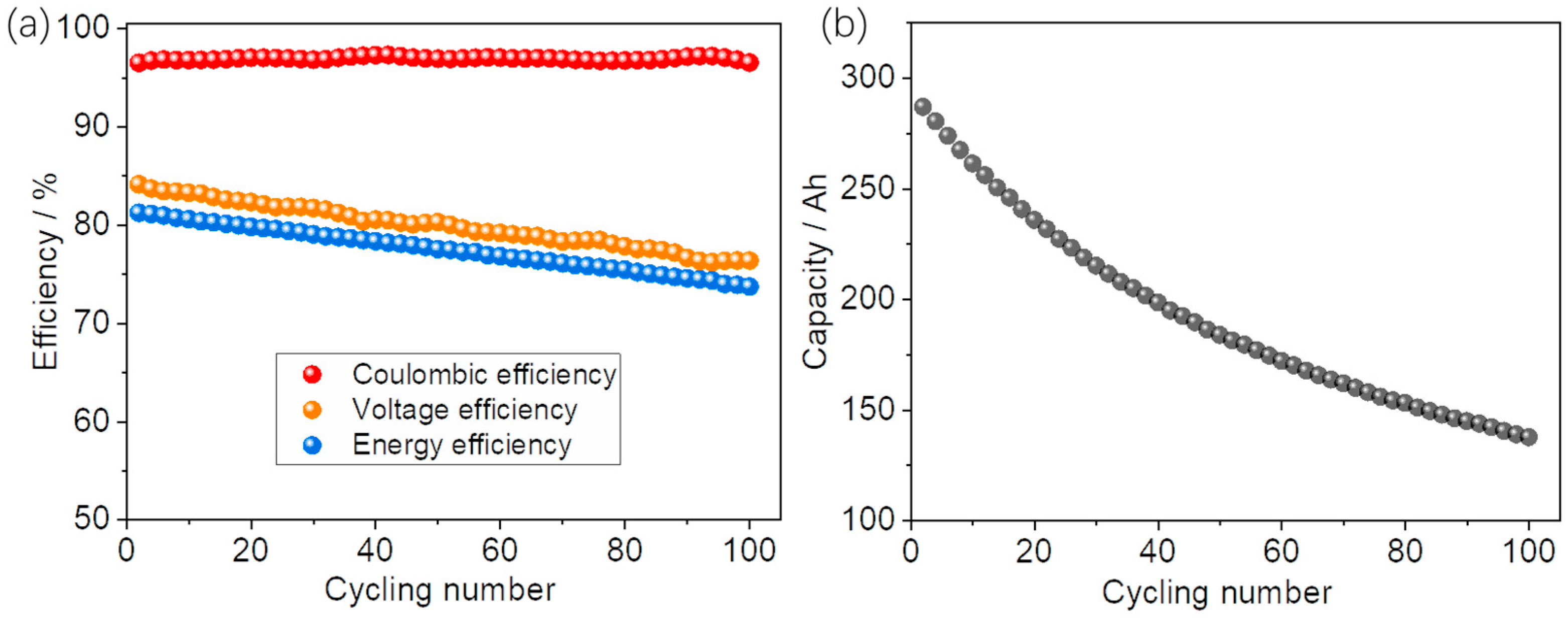
3.4. Stability Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Zou, H.; Zhu, X.; Ding, M.; Jia, C. Recent progress in zinc-based redox flow batteries: A review. J. Phys. D-Appl. Phys. 2022, 55, 163001. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, M.; Feng, X.; Chen, Y.; Chen, F.; Xu, J. Analysis and optimization for multi-stack vanadium flow battery module incorporating electrode permeability. J. Power Sources 2021, 515, 230606. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Lv, Y.; Tang, A.; Dai, L.; Wang, L.; He, Z. Perovskite enables high performance vanadium redox flow battery. Chem. Eng. J. 2022, 443, 136341. [Google Scholar] [CrossRef]
- Hu, Z.; Miao, Z.; Xu, Z.; Zhu, X.; Zhong, F.; Ding, M.; Wang, J.; Xie, X.; Jia, C.; Liu, J. Carbon felt electrode modified by lotus seed shells for high-performance vanadium redox flow battery. Chem. Eng. J. 2022, 450, 138377–138384. [Google Scholar] [CrossRef]
- Schroder, P.; Aguilo-Aguayo, N.; Obendorf, D.; Bechtold, T. Near to neutral pH all-iron redox flow battery based on environmentally compatible coordination compounds. Electrochim. Acta 2022, 430, 141042. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Yuan, Z.; Li, X. Low-cost all-iron flow battery with high performance towards long-duration energy storage. J. Energy Chem. 2022, 73, 445–451. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, K.; Li, X.; Yan, C.; Liu, Q.; Tang, A. Tuning the ferrous coordination structure enables a highly reversible Fe anode for long-life all-iron flow batteries. J. Mater. Chem. A 2021, 9, 26354–26361. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, X. Perspective of alkaline zinc-based flow batteries. Sci. China Chem. 2022, 65, 1–16. [Google Scholar] [CrossRef]
- Chakraborty, M.; Murcia-Lopez, S.; Morante, J.; Andreu, T. Structural influence of the anode materials towards efficient Zn deposition/dissolution in aqueous Zn-Iodide flow batteries. J. Electrochem. Soc. 2021, 168, 040532. [Google Scholar] [CrossRef]
- Wang, P.; Jia, T.; Wang, B. Review-Recent Advance in Self-Supported Electrocatalysts for Rechargeable Zinc-Air Batteries. J. Electrochem. Soc. 2020, 167, 110564. [Google Scholar] [CrossRef]
- Yao, S.; Huang, X.; Sun, X.; Zhou, R.; Cheng, J. Structural modification of negative electrode for Zinc—Nickel single-flow battery based on polarization analysis. J. Electrochem. Soc. 2021, 168, 070512. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, Q.; Li, Y.; Wang, J.; Lund, P. Review of zinc dendrite formation in zinc bromine redox flow battery. Renew. Sustain. Energy Rev. 2020, 127, 109838. [Google Scholar] [CrossRef]
- Liu, N.; Mohanapriya, K.; Pan, J.; Hu, Y.; Sun, Y.; Liu, X. A facile preparation of λ-MnO2 as Cathode material for high-performance zinc-manganese redox flow battery. J. Electrochem. Soc. 2020, 167, 040517. [Google Scholar] [CrossRef]
- Amini, K.; Pritzker, M. A two-dimensional transient model for a zinc-cerium redox flow battery validated by extensive experimental data. J. Power Sources 2021, 506, 230237. [Google Scholar] [CrossRef]
- Selverston, S.; Savinell, R.; Wainright, J. Zinc-Iron flow batteries with common electrolyte. J. Electrochem. Soc. 2017, 164, A1069–A1075. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, G.; Zhi, L.; Zhang, H.; Yuan, Z.; Li, X. In Situ Defect-Free Vertically Aligned Layered Double Hydroxide Composite Membrane for High Areal Capacity and Long-Cycle Zinc-Based Flow Battery. Adv. Funct. Mater. 2021, 32, 1906803. [Google Scholar] [CrossRef]
- Kim, M.; Yun, D.; Jeon, J. Effect of a bromine complex agent on electrochemical performances of zinc electrodeposition and electrodissolution in Zinc—Bromide flow battery. J. Power Sources 2019, 438, 227020. [Google Scholar] [CrossRef]
- Guo, L.; Guo, H.; Huang, H.; Tao, S.; Cheng, Y. Inhibition of zinc dendrites in zinc-based flow batteries. Front. Chem. 2020, 8, 557. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, M. Toward Dendrite-Free Deposition in Zinc-Based Flow Batteries: Status and Prospects. Batteries 2022, 8, 117. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Z.; Li, Z.; Lu, Y. A dendrite-free tin anode for high-energy aqueous redox flow batteries. Adv. Mater. 2021, 33, 2008095. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Q.; Gao, W.; Liu, J.; Yan, C.; Liu, Q. Study on a high current density redox flow battery with tin (II)/tin as negative couple. J. Power Sources 2015, 280, 227–230. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Z.; Lu, F.; Xi, Y. A novel tin-bromine redox flow battery for large-scale energy storage. Appl. Energy 2019, 255, 113756. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, L.; Lv, Y.; Zhang, X.; Wu, Q. A Sn-Fe flow battery with excellent rate and cycle performance. J. Power Sources 2018, 404, 89–95. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, L.; Lv, Y.; Zhang, X.; Fan, L.; Wu, Q. Elucidating effects of component materials and flow fields on Sn-Fe hybrid flow battery. J. Power Sources 2020, 450, 227613. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Zhang, S.; Cheng, M.; Chen, F.; Xu, Y.; Luo, J. A low-cost neutral aqueous redox flow battery with dendrite-free tin Anode. J. Electrochem. Soc. 2021, 168, 110547. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, L.; Su, H.; Zhang, W.; Yang, W.; Xu, Q. Numerical investigation on the dispersion effect in vanadium redox flow battery. Chem. Eng. J. 2022, 393, 124753. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, W.; Liu, Y.; Zeng, Y.; He, Q.; Tan, P.; Ni, M. Mathematical modeling and numerical analysis of alkaline zinc-iron flow batteries for energy storage applications. Chem. Eng. J. 2021, 405, 126684. [Google Scholar] [CrossRef]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–167. [Google Scholar] [CrossRef]
- Li, Y.; Skyllas-Kazacos, M.; Bao, J. A dynamic plug flow reactor model for a vanadium redox flow battery cell. J. Power Sources 2016, 311, 57–67. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, M.; Liu, L.; Wang, Y.; Chen, F.; Ma, X.; Zhang, Q. Mathematical modeling and in-depth analysis of 10 kW-class iron-vanadium flow batteries. J. Power Sources 2023, 563, 232813. [Google Scholar] [CrossRef]


| Parameters | Value |
|---|---|
| Sn ion concentration | 0.5 mol L−1 |
| Fe ion concentration | 1 mol L−1 |
| Electrolyte volume | 60 mL |
| Flow rate | 40 mL min−1 |
| Electrode size | 4 cm × 7 cm × 4.2 mm |
| Voltage range | 0.4–1.2 V |
| Parameters | Value |
|---|---|
| Electrolyte volume | 25 L |
| Fe-ion concentration | 1 mol L−1 |
| Sn-ion concentration | 0.5 mol L−1 |
| Electrode size | 50 cm × 40 cm × 4.2 mm |
| Electrode porosity | 93% |
| Temperature | 298.15 K |
| Cell formal potential | 0.9 V |
| Area resistance of cell | 0.7 Ω cm2 |
| Diffusion coefficient of Fe3+ | 4.0 × 10−12 |
| Diffusion coefficient of Fe2+ | 4.8 × 10−12 |
| Diffusion coefficient of Sn2+ | 5.0 × 10−12 |
| Cut-off for discharging | SOC = 10% |
| Cut-off for charging | SOC = 90% |
| Pipe length | 1 m |
| Pipe radius | 2.5 cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Wang, Y.; Shi, Y.; Chen, H.; Ma, X.; Zhang, Q. Model-Based Analysis and Optimization of Acidic Tin–Iron Flow Batteries. Batteries 2023, 9, 278. https://doi.org/10.3390/batteries9050278
Chen F, Wang Y, Shi Y, Chen H, Ma X, Zhang Q. Model-Based Analysis and Optimization of Acidic Tin–Iron Flow Batteries. Batteries. 2023; 9(5):278. https://doi.org/10.3390/batteries9050278
Chicago/Turabian StyleChen, Fuyu, Ying Wang, Ying Shi, Hui Chen, Xinzhi Ma, and Qinfang Zhang. 2023. "Model-Based Analysis and Optimization of Acidic Tin–Iron Flow Batteries" Batteries 9, no. 5: 278. https://doi.org/10.3390/batteries9050278
APA StyleChen, F., Wang, Y., Shi, Y., Chen, H., Ma, X., & Zhang, Q. (2023). Model-Based Analysis and Optimization of Acidic Tin–Iron Flow Batteries. Batteries, 9(5), 278. https://doi.org/10.3390/batteries9050278







