Calorimetric Studies on Chemically Delithiated LiNi0.4Mn0.4Co0.2O2: Investigation of Phase Transition, Gas Evolution and Enthalpy of Formation
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. ICP-OES and CGHE Results for Pristine and Chemically Delithiated Samples
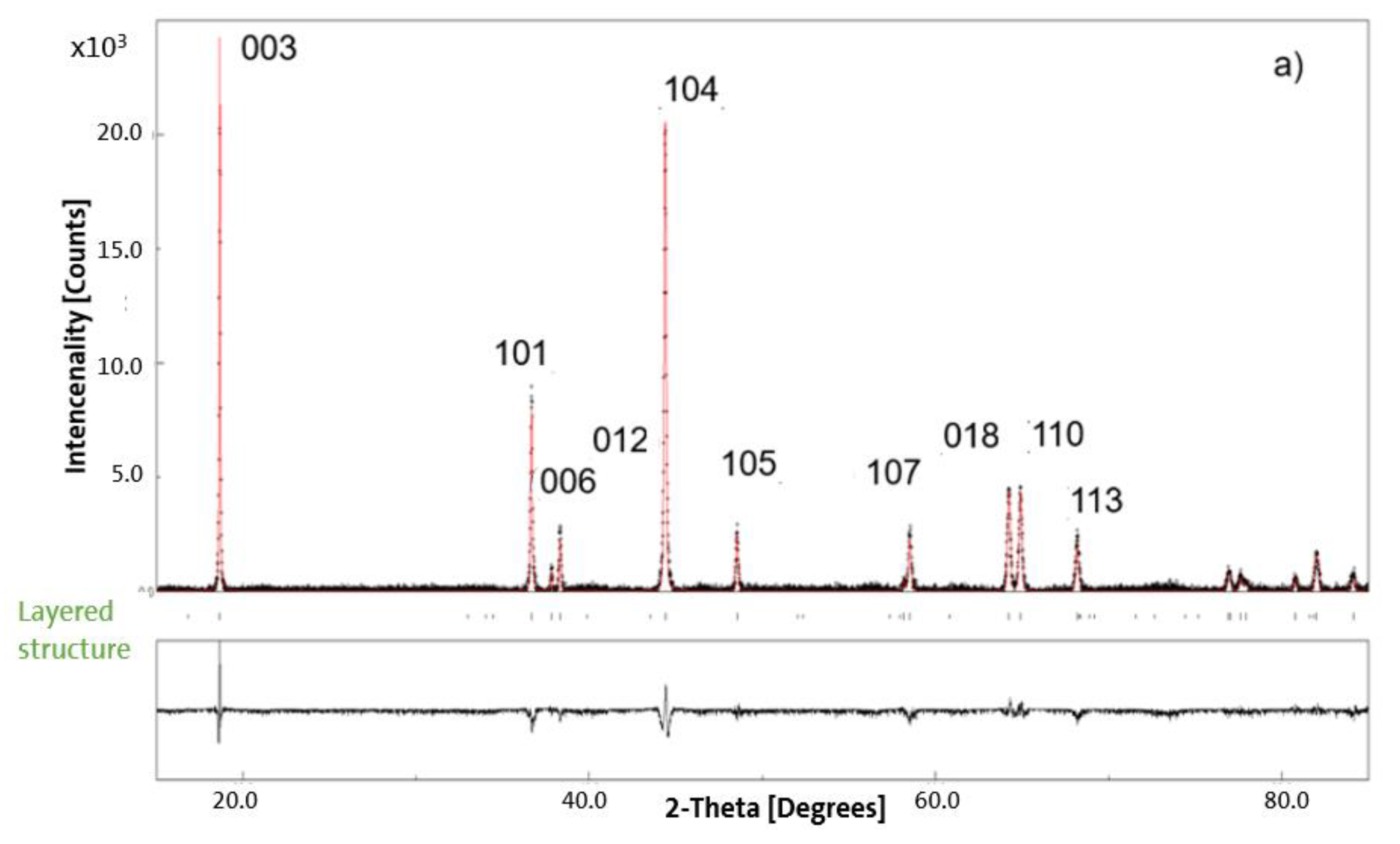
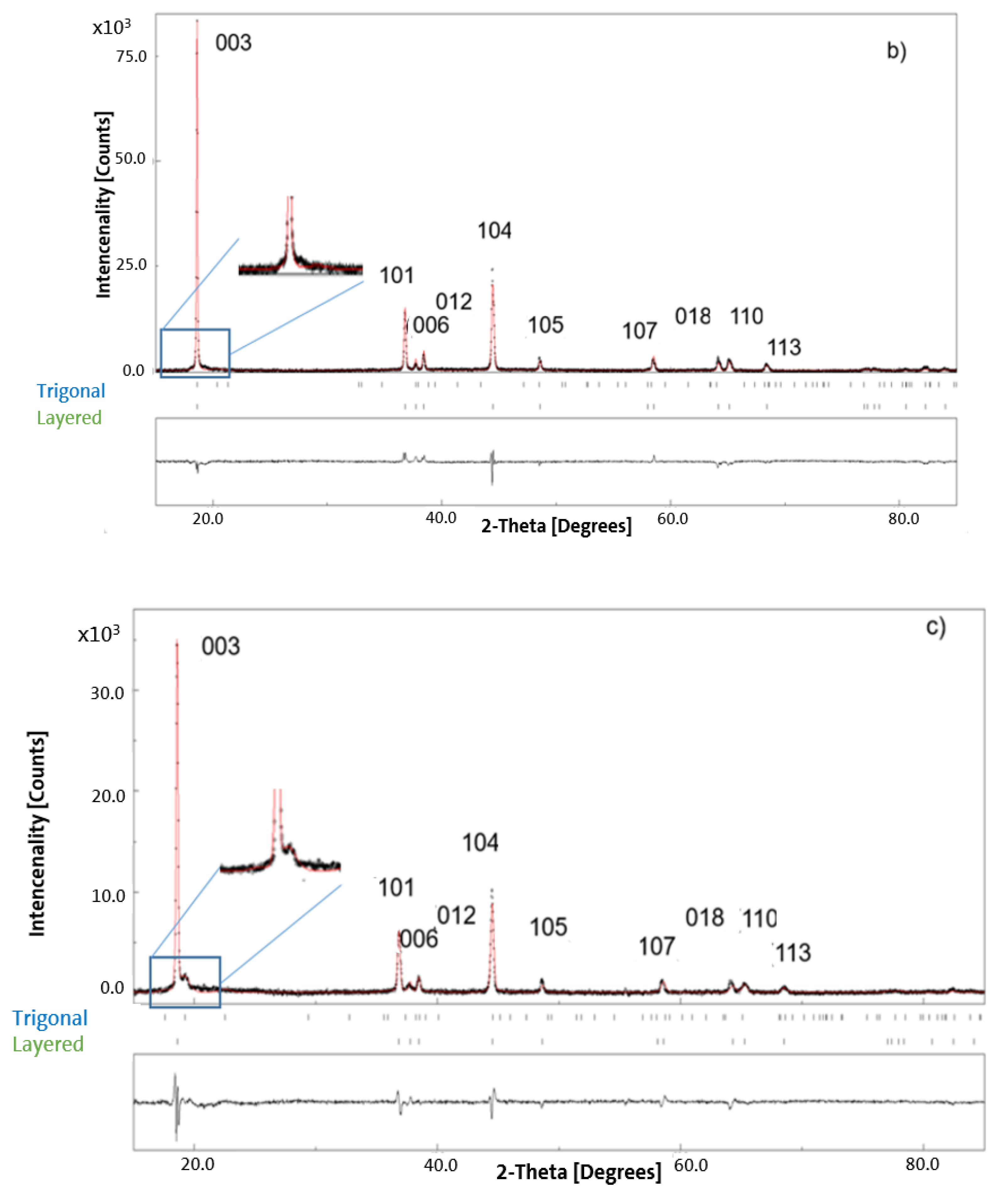
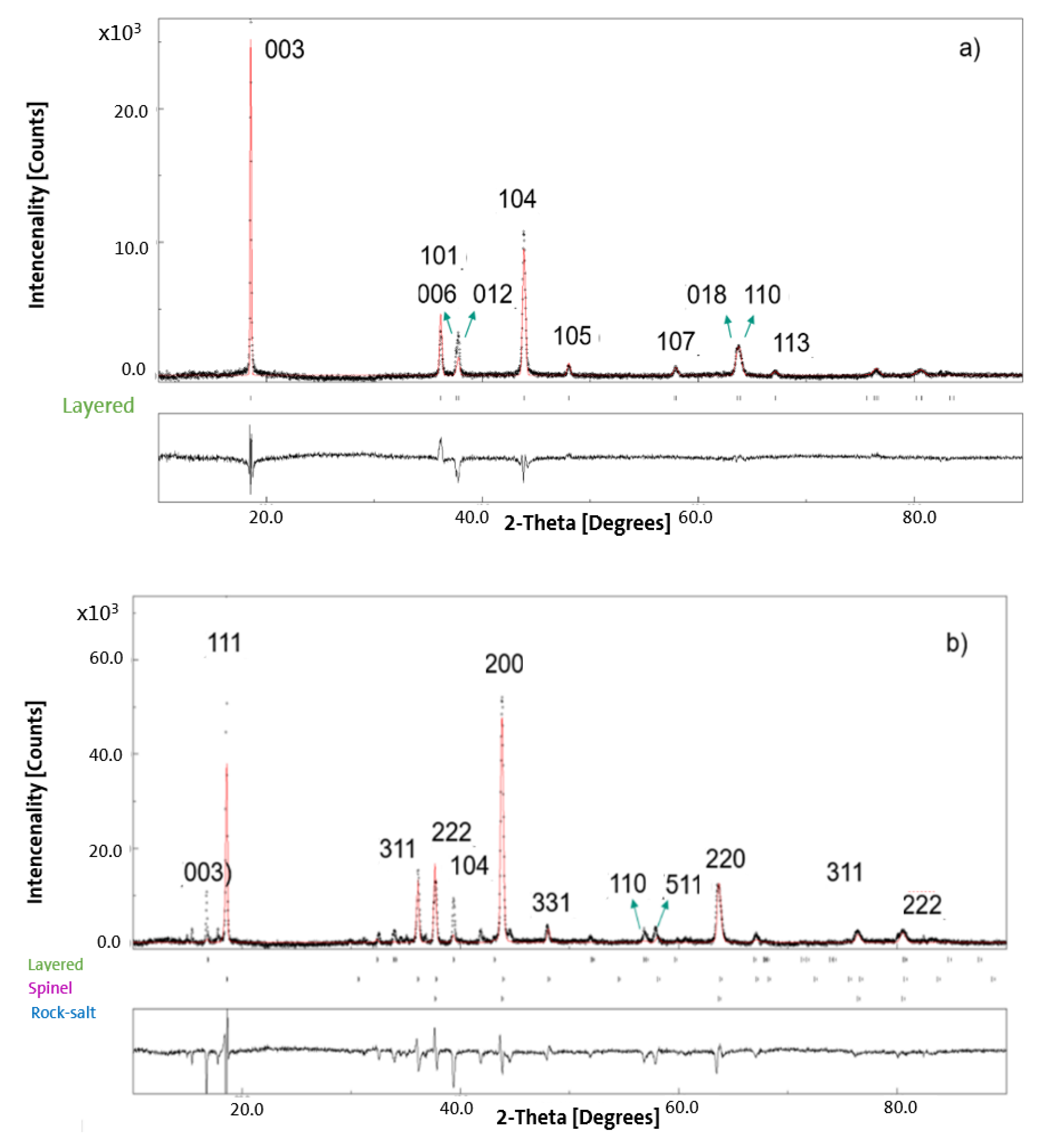
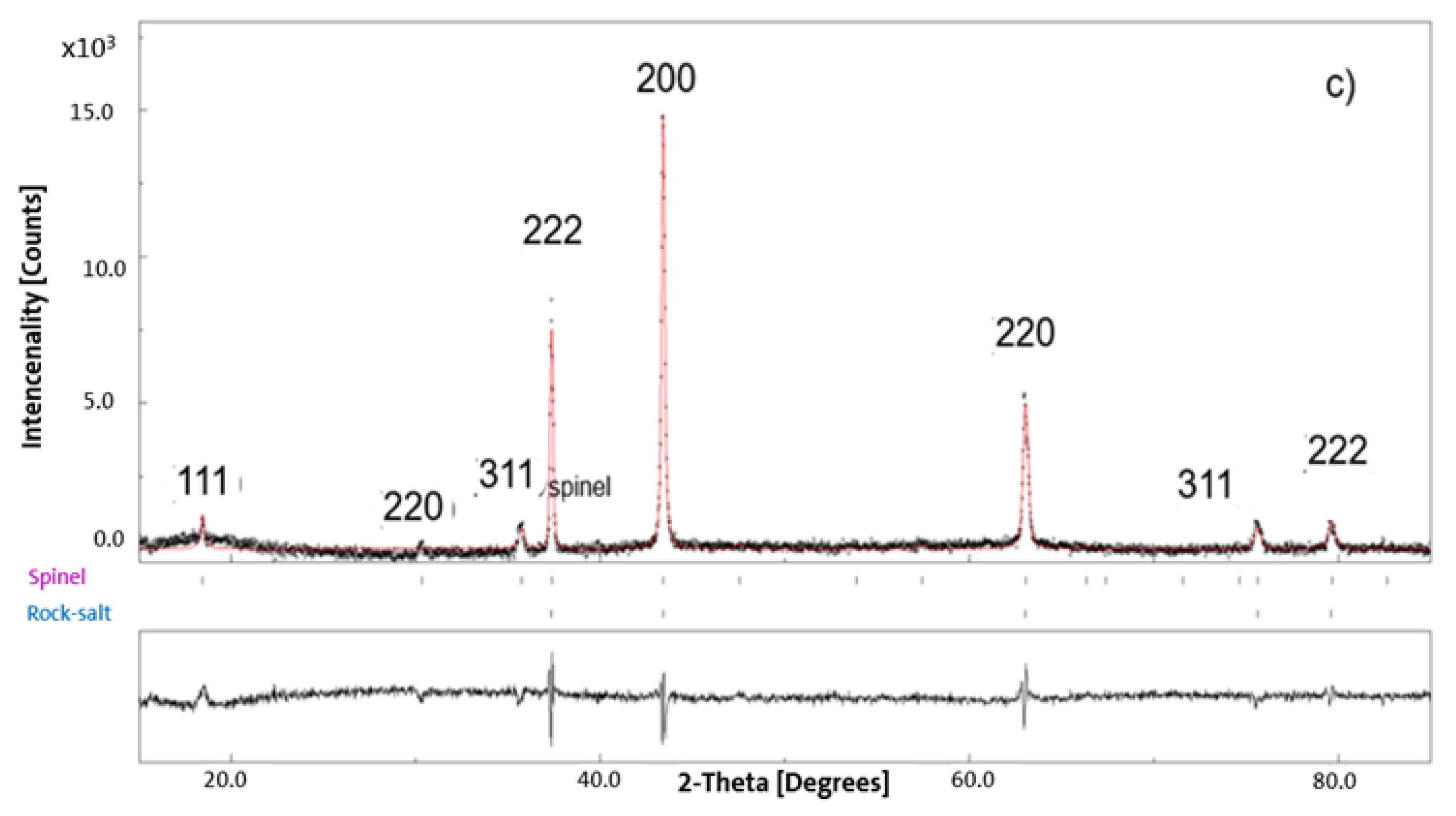
3.2. XRD Results for Pristine and Chemically Delithiated Specimens
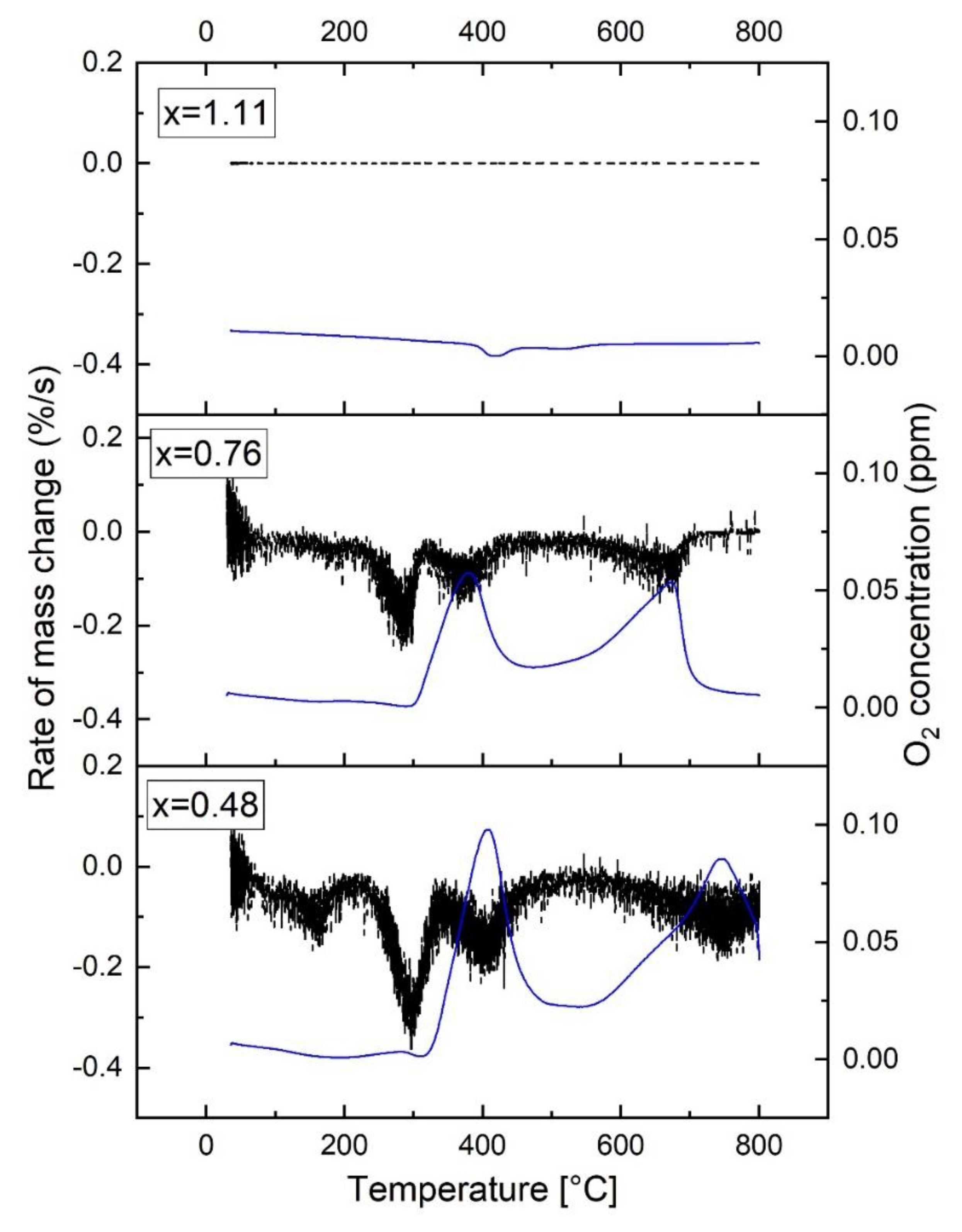
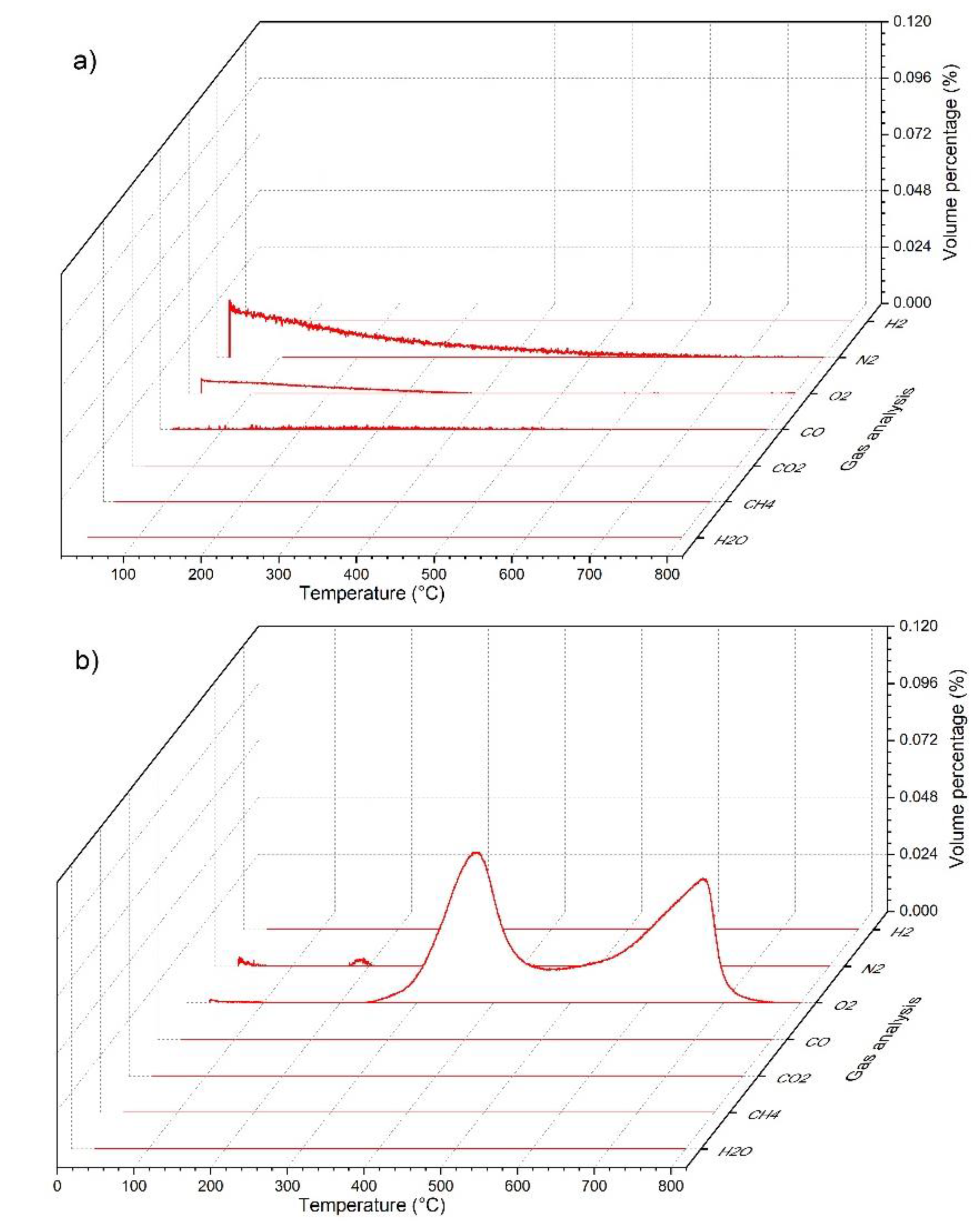
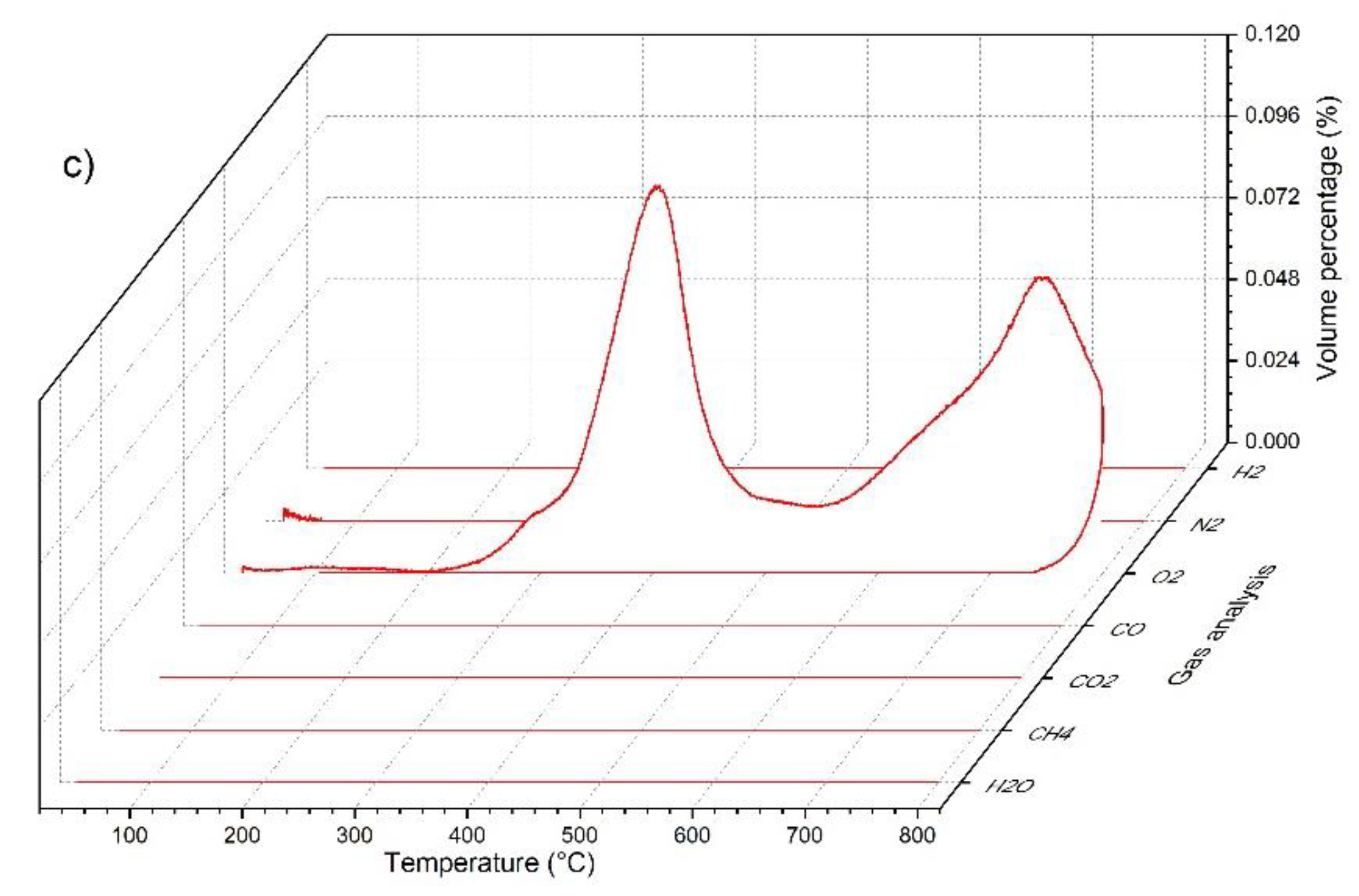
3.3. Simultaneous Thermal Analysis (STA) and Gas Evolution Study
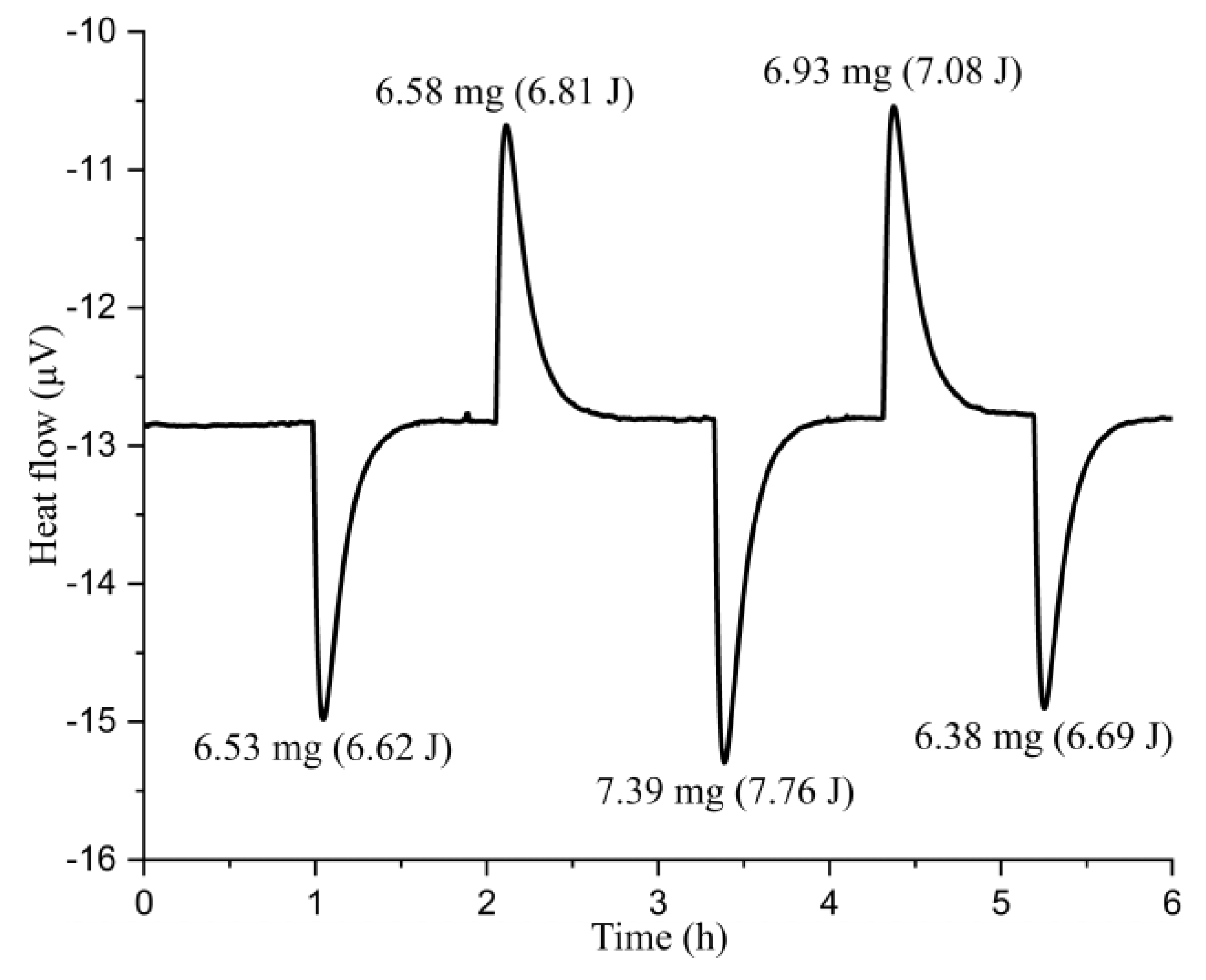
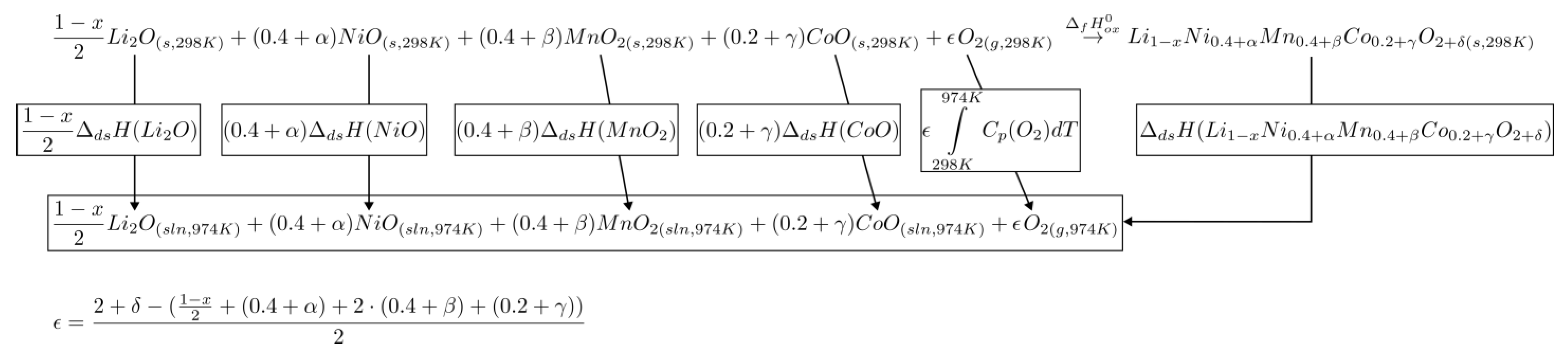
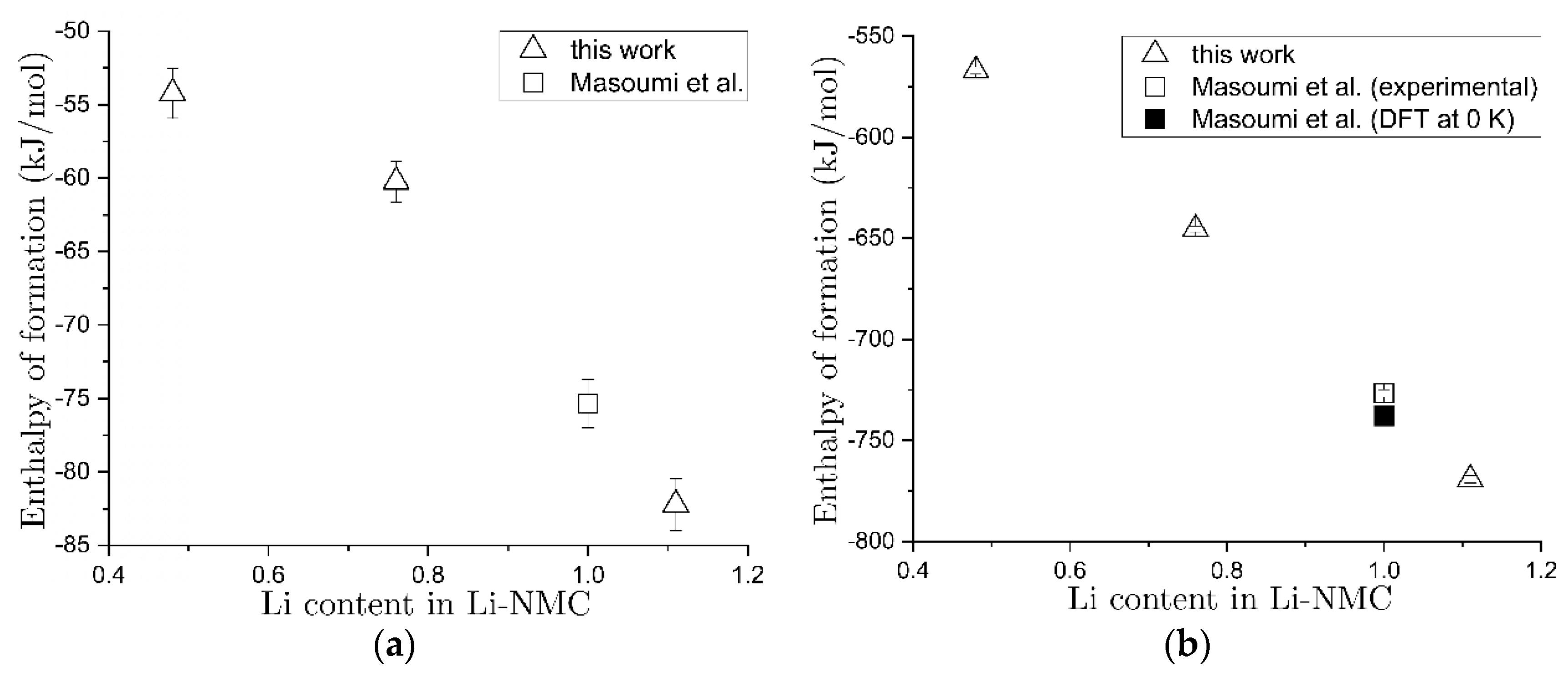
3.4. Enthalpy Analysis for Pristine and Chemically Delithiated Samples
| Nominal Stoichiometry | Enthalpy of Drop Solution (kJ/mol) | Enthalpy of Formation from Oxides (kJ/mol) | Enthalpy of Formation from Elements (kJ/(mol of Formula) |
|---|---|---|---|
| Li1.11Ni0.42Mn0.41Co0.17O2.11 | 102.66 ± 1.15 (8) | −82.23 ± 1.78 | −769.20 ± 1.85 |
| LiNi0.4Mn0.4Co0.2O2 | 98.86 ± 1.09 (18) e | −75.35 ± 1.65 e | −726.59 ± 1.72 e |
| Li0.76Ni0.41Mn0.42Co0.17O2.10 | 99.57 ± 0.95 (12) | −60.24 ± 1.39 | −645.39 ± 1.47 |
| Li0.48Ni0.38Mn0.46Co0.16O2.07 | 111.27 ± 1.52 (11) | −54.23 ± 1.70 | −566.95 ± 1.77 |
| Li2O | −93.02 ± 2.24 a,b | −597.935 ± 0.334 c | |
| NiO | 35.73 ± 0.95 (8) a | −239.743 ± 0.543 c | |
| MnO2 | 128.92 ± 0.91 (11) d | −521.493 ± 0.836 c |
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Song, K.; Zhang, X.; Hu, N.; Li, L.; Li, W.; Zhang, L.; Zhang, H. Safety issues in Lithium ion batteries: Materials and cell design. Front. Energy Res. 2019, 7, 65. [Google Scholar] [CrossRef]
- Chombo, P.; Laoonal, Y. A review of safety strategies of a Li ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Rozier, P.; Tarascon, J.M. Review—Li-rich layered oxide cathodes for next-generation Li-ion batteries: Chances and challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.; Manthiram, A. High-Nickel layered oxide cathodes for Lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Tian, C.; Lin, F.; Doeff, M. Electrochemical characteristics of layered transition metal oxide cathode materials for lithium ion batteries: Surface, bulk behavior, and thermal properties. Acc. Chem. Res. 2018, 51, 89–96. [Google Scholar] [CrossRef]
- Kasnatscheev, J.; Röser, S.; Börner, M.; Winter, M. Do increased Ni contents in LiNixMnyCozO2 (NMC) electrodes decrease structural and thermal stability of Li ion batteries? A thorough look by consideration of the Li+ extraction ratio. ACS Appl. Energy Mater. 2019, 2, 7733–7737. [Google Scholar] [CrossRef]
- Bak, S.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.; Cho, S.; Kim, K.; Chung, K.; Yang, X.; Nam, K. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in-situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, T.; Hu, Z.; Wie, Y.; Song, X.; Ren, Y.; Wang, W.; Rao, M.; Lin, Y.; Chen, Z.; et al. Tuning of thermal stability in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2016, 138, 13326–13334. [Google Scholar] [CrossRef]
- Tian, C.; Xu, Y.; KaN, W.; Sokaras, D.; Nordlund, D.; Shen, H.; Chen, K.; Liu, Y.; Doeff, M. Distinct surface and bulk thermal behaviors of LiNi0.6Mn0.2Co0.2O2 cathode materials as a function of state of charge. ACS Appl. Mat. Interfaces 2020, 12, 11643–11656. [Google Scholar] [CrossRef]
- Ma, M.; Chernova, N.; Toby, B.; Zavalij, P.; Whittingham, M. Structural and electrochemical behavior of LiMn0.4Ni0.4Co0.2O2. J. Power Sources 2007, 165, 517–534. [Google Scholar] [CrossRef]
- Cupid, D.M.; Reif, A.; Seifert, H.J. Enthalpy of formation of Li1+xMn2−xO4 (0<x<0.1) spinel phases. Thermochim. Acta 2015, 599, 35–41. [Google Scholar]
- Ngala, K.; Chernova, N.A.; Ma, M.; Mamak, M.; Zavalija, P.Y.; Whittingham, M.S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem. 2004, 14, 214–220. [Google Scholar] [CrossRef]
- Yin, S.-C.; Rho, Y.-H.; Swainson, I.; Nazar, L. X-ray/Neutron diffraction and electrochemical studies of Lithium de-/re-intercalation in Li1-xCo1/3Ni1/3Mn1/3O2 (x=0–>1). Chem. Mater. 2006, 18, 1901–1910. [Google Scholar] [CrossRef]
- Noh, H.; Youn, S.; Yoon, C.; Sun, Y. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Koyama, Y.; Tanaka, I.; Adachi, H.; Makimura, Y.; Ohzuku, T. Crystal and electronic structures of superstructural Li1-x[Co1/3Ni1/3Mn1/3]O2 (0≤x≤1). J. Power Sources 2003, 119, 644–648. [Google Scholar] [CrossRef]
- Kim, J.M.; Chung, H.T. The first cycle characteristics of Li[Ni1/3Co1/3Mn1/3]O2 charged up to 4.7 V. Electrochim. Acta 2004, 49, 937–944. [Google Scholar] [CrossRef]
- Qiao, R.; Liu, J.; Kourtakis, K.; Roelofs, M.; Peterson, D.; Duff, J.; Deibler, D.; Wray, L.; Yang, W. Transition-metal redox evolution in LiNi0.5Mn0.3Co0.2O2 electrodes at high potentials. J. Power Sources 2017, 360, 294–300. [Google Scholar] [CrossRef]
- Masoumi, M.; Cupid, D.; Reichmann, T.; Music, D.; Schneider, J.; Seifert, H. Enthalpies of formation of layered LiNixMnxCo1-2xO2 compounds as promising Li-ion battery cathode materials. Int. J. Mater. Res. 2017, 108, 1–10. [Google Scholar] [CrossRef]
- Wang, M.; Navrotsky, A. Enthalpy of formation of LiNiO2, LiCoO2 and their solid solution, LiNi1−xCoxO2. Solid State Ion. 2004, 166, 167–173. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Wang, M. Enthalpy of formation of LixCoO2 (0.5≤x≤1.0). J. Electrochem. Soc. 2005, 152, J82–J84. [Google Scholar] [CrossRef]
- Glushko, V.P.; Medvedev, V.A. Thermal Constants of Substances; Hemisphere Publishing Company: New York, NY, USA, 1990. [Google Scholar]
- Wang, M.; Navrotsky, A. Thermochemistry of Li1+xMn2−xO4 (0≤x≤1/3) spinel. J. Solid State Chem. 2005, 178, 1182–1189. [Google Scholar] [CrossRef]

| Raw Material | Chemical Formula | Source | Initial Mass Fraction Purity |
|---|---|---|---|
| Lithium nickel manganese cobalt oxide | Li1.11Ni0.42Mn0.41Co0.17O2 | MTI | 0.9776 |
| Ammoniumpersulfat | (NH4)2S2O8 | Alfa Aesar | 0.98 |
| Analyzed Elements | Pristine Specimen | Medium Degree Delithiated Specimen | High Degree Delithiated Specimen | |
|---|---|---|---|---|
| ICP-OES and CGHE wt.% | Li | 7.68 ± 0.16 | 5.33 ± 0.11 | 3.40 ± 0.07 |
| Ni | 24.40 ± 0.37 | 24.37 ± 0.37 | 22.43 ± 0.34 | |
| Mn | 22.50 ± 0.41 | 23.30 ± 0.42 | 25.90 ± 0.47 | |
| Co | 10.10 ± 0.19 | 10.13 ± 0.19 | 9.75 ± 0.19 | |
| O | 33.60 ± 2.99 | 34 ± 3.0 | 33.7 ± 3.0 | |
| Molar ratio | Li/sum TM | 1.11 | 0.76 | 0.48 |
| Ni/sum TM | 0.42 | 0.41 | 0.38 | |
| Mn/sum TM | 0.41 | 0.42 | 0.46 | |
| Co/sum TM | 0.17 | 0.17 | 0.16 | |
| O/sum TM | 2.11 | 2.10 | 2.07 | |
| Nominal composition | Li1.11Ni0.42Mn0.41Co0.17O2.11 | Li0.76Ni0.41Mn0.42Co0.17O2.10 | Li0.48Ni0.38Mn0.46Co0.16O2.07 | |
| Li Content x | Space Group | Lattice Parameters | Phase wt.% | ||
|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | |||
| 1.11 | Rm | 2.8769 (7) | - | 14.2922 (0) | - |
| 0.76 | Rm | 2.8606 (8) | - | 14.2926 (7) | 84.5 |
| 0.48 | Rm | 2.8562 (2) | - | 14.2722 (5) | 86.4 |
| 1.11 | Rm | 2.861 | 14.238 | Ref. [11] | |
| 1.04 | Rm | 2.865 | 14.245 | ||
| 0.97 | Rm | 2.870 | 14.265 | ||
| 0.90 | Rm | 2.874 | 14.280 | ||
| 1 | Rm | 2.8694 (1) | 14.266 (1) | Ref. [12] | |
| 0.94 | Rm | 2.8616 (3) | 14.272 (2) | ||
| 0.5 | Rm | 2.8280 (5) | 14.456 (4) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Gebauer, J.; Bergfeldt, T.; Rohde, M.; Ziebert, C.; Du, Y.; Seifert, H.J. Calorimetric Studies on Chemically Delithiated LiNi0.4Mn0.4Co0.2O2: Investigation of Phase Transition, Gas Evolution and Enthalpy of Formation. Batteries 2023, 9, 275. https://doi.org/10.3390/batteries9050275
Zhao W, Gebauer J, Bergfeldt T, Rohde M, Ziebert C, Du Y, Seifert HJ. Calorimetric Studies on Chemically Delithiated LiNi0.4Mn0.4Co0.2O2: Investigation of Phase Transition, Gas Evolution and Enthalpy of Formation. Batteries. 2023; 9(5):275. https://doi.org/10.3390/batteries9050275
Chicago/Turabian StyleZhao, Wenjiao, Julian Gebauer, Thomas Bergfeldt, Magnus Rohde, Carlos Ziebert, Yong Du, and Hans J. Seifert. 2023. "Calorimetric Studies on Chemically Delithiated LiNi0.4Mn0.4Co0.2O2: Investigation of Phase Transition, Gas Evolution and Enthalpy of Formation" Batteries 9, no. 5: 275. https://doi.org/10.3390/batteries9050275
APA StyleZhao, W., Gebauer, J., Bergfeldt, T., Rohde, M., Ziebert, C., Du, Y., & Seifert, H. J. (2023). Calorimetric Studies on Chemically Delithiated LiNi0.4Mn0.4Co0.2O2: Investigation of Phase Transition, Gas Evolution and Enthalpy of Formation. Batteries, 9(5), 275. https://doi.org/10.3390/batteries9050275








