In Situ Solidification by γ−ray Irradiation Process for Integrated Solid−State Lithium Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
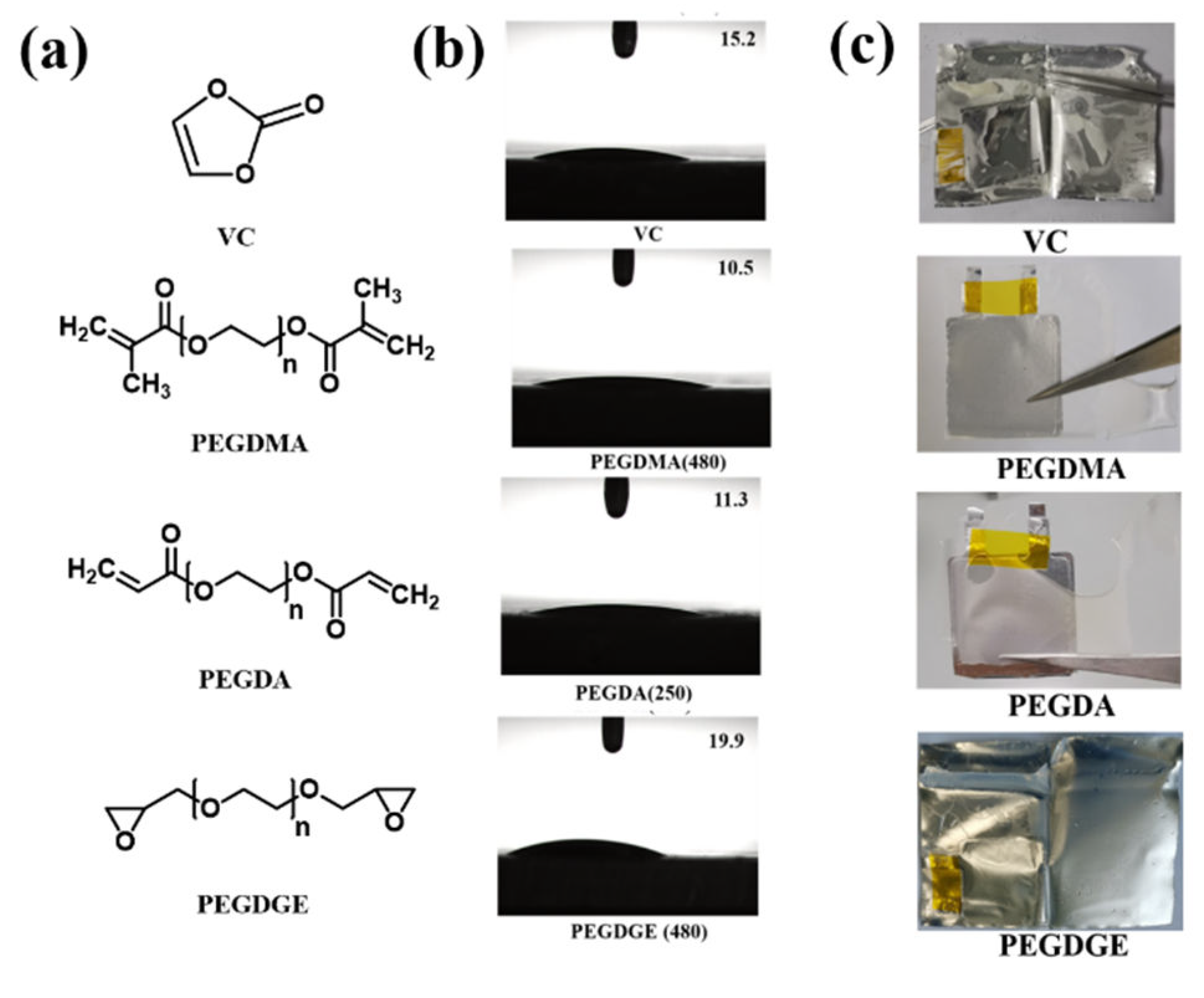
2.2. Preparation of Polymer–Lithium Salt Solutions
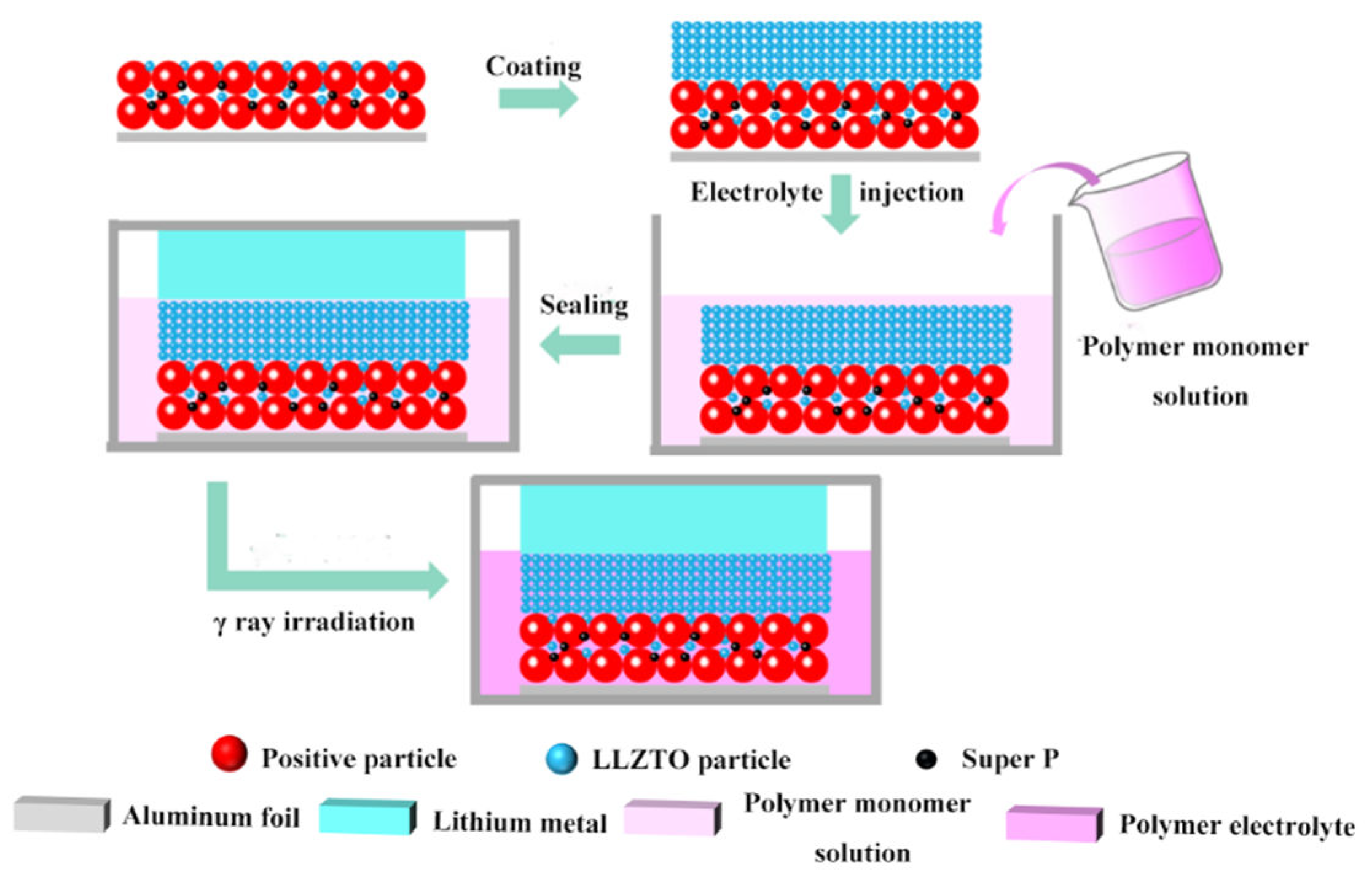
2.3. Preparation of In Situ Solidification Batteries
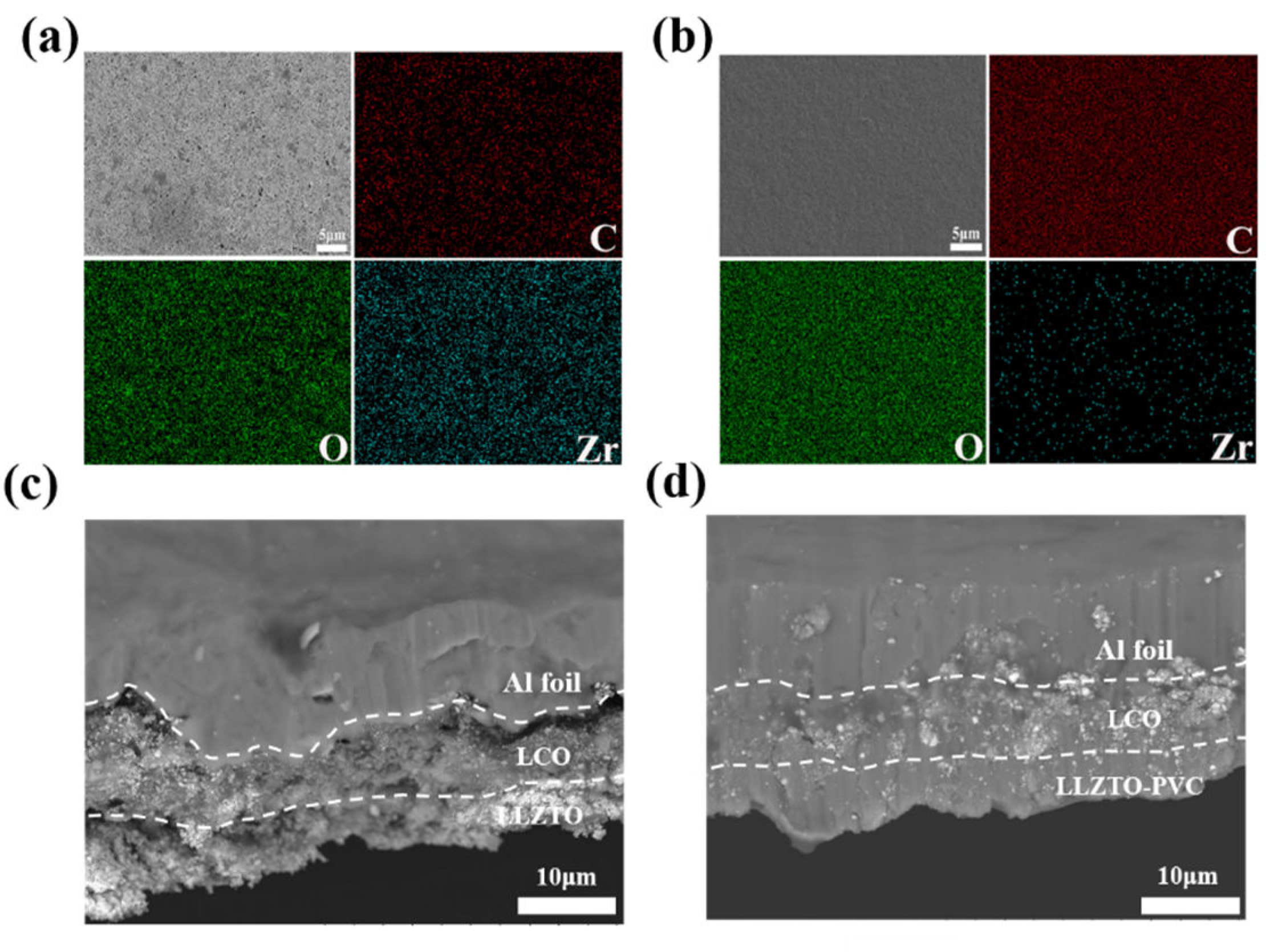
2.4. Material Characterization
2.4.1. Physical Characterization
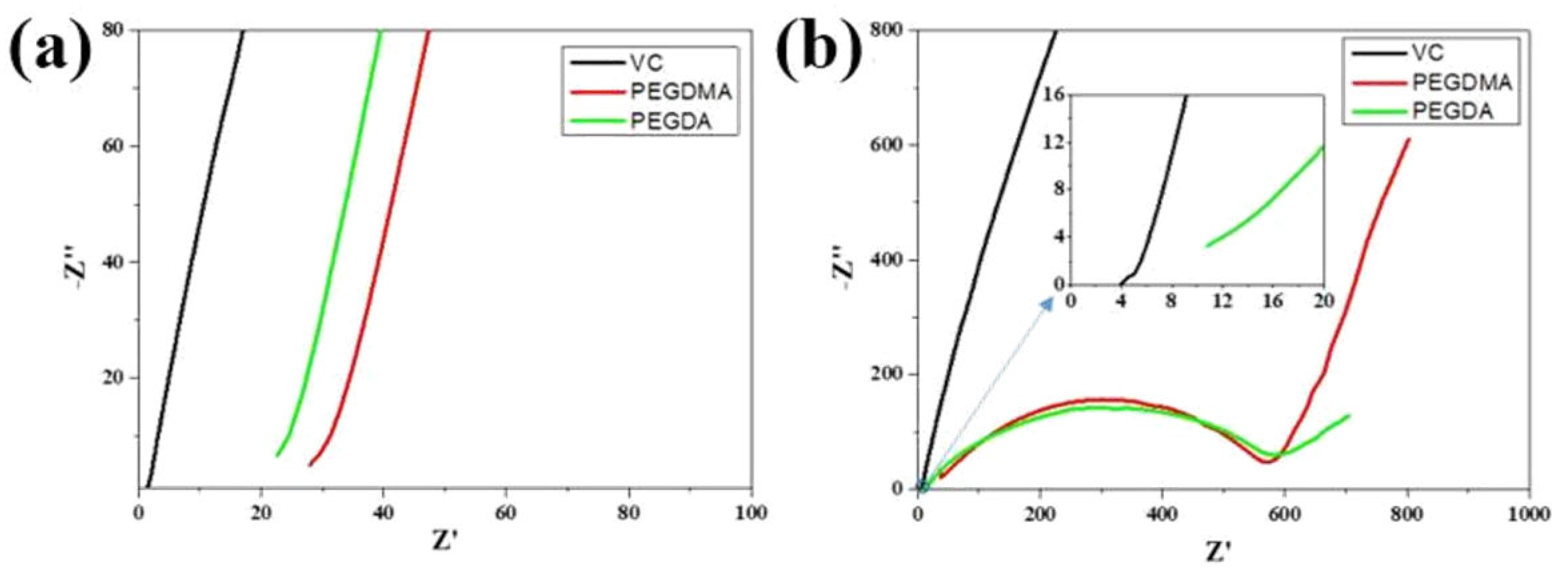
2.4.2. Electrochemical Characterization
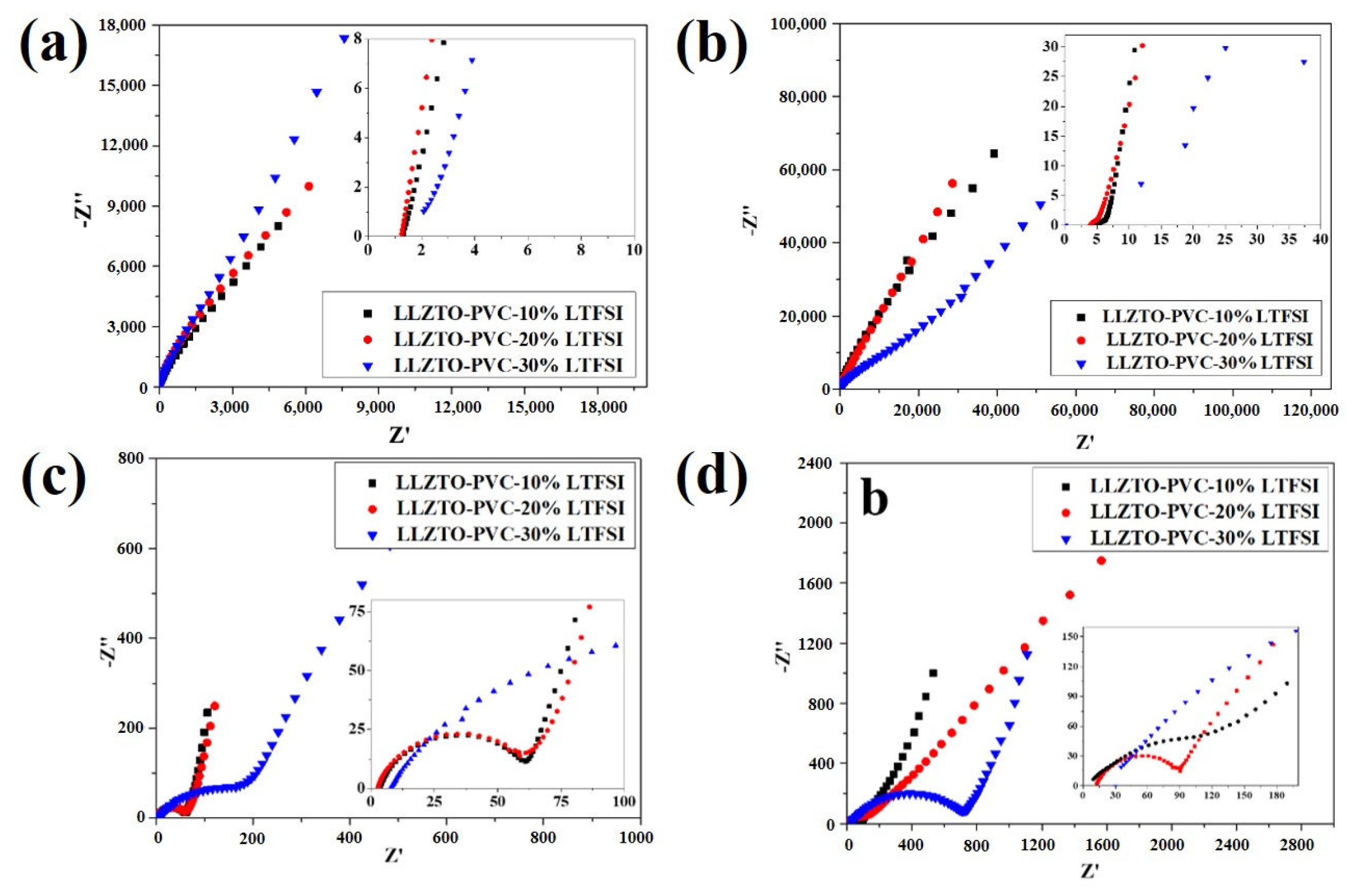
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J. Changing our lives and building the foundation of society—The dramatic competition for R&D behind the Nobel Prize. J. Electrochem. 2019, 25, 616–620. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical challenges and future perspectives of all-solid-state lithium-metal batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Tan, S.-J.; Zeng, X.-X.; Ma, Q.; Wu, X.-W.; Guo, Y.-G. Recent advancements in polymer-based composite electrolytes for Rechargeable Lithium Batteries. Electrochem. Energy Rev. 2018, 1, 113–138. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, L.; Zhang, W.; Li, Z.; Xie, X.; Huang, Y. Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries. Energy Environ. Sci. 2021, 14, 12–36. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Borstel, G. Towards a practical rechargeable 5 V Li ion battery. Phys. Stat. Sol. A 2005, 202, R13–R15. [Google Scholar] [CrossRef]
- Eglitis, R.I. Theoretical prediction of the 5 V rechargeable Li ion battery using Li2CoMn3O8 as a cathode. Phys. Scr. 2015, 90, 094012. [Google Scholar] [CrossRef]
- York, M.; Larson, K.; Harris, K.C.; Carmona, E.; Albertus, P.; Sharma, R.; Noked, M.; Strauss, E.; Ragones, H.; Golodnitsky, D. Recent advances in solid-state beyond lithium batteries. J. Solid State Electrochem. 2022, 26, 1851–1869. [Google Scholar] [CrossRef]
- Guo, B.; Fu, Y.; Wang, J.; Gong, Y.; Zhao, Y.; Yang, K.; Zhou, S.; Liu, L.; Yang, S.; Liu, X.; et al. Strategies and characterization methods for achieving high performance PEO-based solid-state lithium-ion batteries. Chem. Commun. 2022, 58, 8182–8193. [Google Scholar] [CrossRef]
- Wu, B.; Chen, C.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. All-solid-state thin film Li-ion batteries: New challenges, new materials, and new designs. Batteries 2023, 9, 186. [Google Scholar] [CrossRef]
- Wang, X.; Hua, H.; Xie, X.; Zhang, P.; Zhao, J. Hydroxyl on the filler surface promotes Li+ conduction in PEO all-solid-state electrolyte. Solid State Ionics 2021, 372, 115768. [Google Scholar] [CrossRef]
- Bai, L.; Li, E.; Du, Z.; Yuan, S. Structural changes of PMMA substrates with different electrolyte solutions: A molecular dynamics study. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 51–57. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, S.; Huang, X.; Xu, B.; Lin, Y.; Xu, B.; Li, L.; Nan, C.-W.; Shen, Y. Synergistic Coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li +-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, J.; Dong, S.; Li, X.; Cui, G. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv. Mater. 2019, 31, e1902029. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew.Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Reddy, I.N.; Akkinepally, B.; Reddy, C.V.; Sreedhar, A.; Ko, T.J.; Shim, J. A systematic study of annealing environment and Al dopant effect on NASICON-type LiZr2(PO4)3 solid electrolyte. Ionics 2020, 26, 4287–4298. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Kanno, R. Li10GeP2S12-type superionic conductors: Synthesis, structure, and ionic transportation. Adv. Energy Mater. 2020, 10, 2002153. [Google Scholar] [CrossRef]
- Akkinepally, B.; Reddy, I.N.; Ko, T.J.; Yoo, K.; Shim, J. Dopant effect on Li+ ion transport in NASICON-type solid electrolyte: Insights from molecular dynamics simulations and experiments. Ceram. Int. 2022, 48, 12142–12151. [Google Scholar] [CrossRef]
- Fu, S.; Arinicheva, Y.; Hüter, C.; Finsterbusch, M.; Spatschek, R. Grain boundary characterization and potential percolation of the solid electrolyte LLZO. Batteries 2023, 9, 222. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, P.; Wang, N.; Huang, F.; Dou, S. Research progress in stable interfacial constructions between composite polymer electrolytes and electrodes. Energy Environ. Sci. 2022, 15, 2753–2775. [Google Scholar] [CrossRef]
- Park, K.; Yu, B.-C.; Jung, J.-W.; Li, Y.; Zhou, W.; Gao, H.; Son, S.; Goodenough, J.B. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: Interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem. Mater. 2016, 28, 8051–8059. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Pei, N.; Chen, Z.; Li, R.; Fu, L.; Zhang, P.; Zhao, J. The critical role of fillers in composite polymer electrolytes for lithium battery. Nanomicro Lett. 2023, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Anothumakkool, B.; Kurungot, S.; Winter, M.; Nair, J.R. In situ polymerization process: An essential design tool for lithium polymer batteries. Energy Environ. Sci. 2021, 14, 2708–2788. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, J.; Chen, R.; Fang, F.; Nie, K.; Qi, W.; Zhang, J.-N.; Yang, R.; Yu, X.; Li, H.; et al. 4.2V poly(ethylene oxide)-based all-solid-state lithium batteries with superior cycle and safety performance. Adv. Energy Mater. 2020, 32, 191–198. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, J.; Shen, X.; Li, R.; Chen, Z.; Huang, X.; Zhang, P.; Zhao, J. New UV-initiated lithiated-interpenetrating network gel-polymer electrolytes for lithium-metal batteries. J. Power Sources. 2022, 541, 231681. [Google Scholar] [CrossRef]
- Liu, M.; Xie, W.; Li, B.; Wang, Y.; Li, G.; Zhang, S.; Wen, Y.; Qiu, J.; Chen, J.; Zhao, P. Garnet Li7LaZr2O12-based solid-state lithium batteries achieved by in situ thermally polymerized gel polymer electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 43116–43126. [Google Scholar] [CrossRef]
- D’Angelo, A.J.; Panzer, M.J. Enhanced lithium ion transport in poly(ethylene glycol) diacrylate-supported solvate ionogel electrolytes via chemically cross-linked ethylene oxide pathways. J. Phys. Chem. B. 2017, 121, 890–895. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, Y.M.; Cho, K.Y.; Han, G.-B.; Lee, J.-N.; Lee, D.J.; Choi, J.W.; Park, J.-K. A gel polymer electrolyte based on initiator-free photopolymerization for lithium secondary batteries. Electrochim. Acta 2012, 60, 23–30. [Google Scholar] [CrossRef]
- Suk, J.; Lee, Y.H.; Kim, D.Y.; Kim, D.W.; Cho, S.Y.; Kim, J.M.; Kang, Y. Semi-interpenetrating solid polymer electrolyte based on thiol-ene cross-linker for all-solid-state lithium batteries. J. Power Sources 2016, 334, 154–161. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, K.-H.; Cho, S.-J.; Park, J.-S.; Cho, K.Y.; Lee, C.K.; Lee, S.B.; Shim, J.K.; Lee, S.-Y. A shape-deformable and thermally stable solid-state electrolyte based on a plastic crystal composite polymer electrolyte for flexible/safer lithium-ion batteries. J. Mater. Chem. A 2014, 2, 10854–10861. [Google Scholar] [CrossRef]
- Nair, J.R.; Destro, M.; Bella, F.; Appetecchi, G.B.; Gerbaldi, C. Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J. Power Sources 2016, 306, 258–267. [Google Scholar] [CrossRef]
- Lee, K.-P.; Gopalan, A.I.; Santhosh, P.; Lee, S.H.; Nho, Y.C. Gamma radiation induced distribution of gold nanoparticles into carbon nanotube-polyaniline composite. Compos. Sci. Technol. 2007, 67, 811–816. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Shan, M.; Guo, Q.; Wang, Z.; Qian, X. Nano-structure and property transformations of carbon systems under γ-ray irradiation: A review. RSC Adv. 2013, 3, 154g. [Google Scholar] [CrossRef]
- Baek, M.; Kim, J.; Jin, J.; Choi, J.W. Photochemically driven solid electrolyte interphase for extremely fast-charging lithium-ion batteries. Nat. Commun. 2021, 12, 6807. [Google Scholar] [CrossRef]
- Hasanain, F.; Guenther, K.; Mullett, W.M.; Craven, E. Gamma sterilization of pharmaceuticals-a review of the irradiation of excipients, active pharmaceutical ingredients, and final drug product formulations. PDA J. Pharm. Sci. Technol. 2014, 68, 113–137. [Google Scholar] [CrossRef]
- Rouif, S. Radiation cross-linked polymers: Recent developments and new applications. Nucl. Instrum. Methods Phys. Res. B 2005, 236, 68–72. [Google Scholar] [CrossRef]
- Shen, X.; Zeng, Y.Z.; Li, R.; Zhang, P.; Zhao, J. In situ solidification of flame-retardant lithium-ion batteries by γ-ray irradition. Energy Storage Sci. Technol. 2022, 11, 1816–1821. [Google Scholar] [CrossRef]
- Murray, K.A.; Kennedy, J.E.; McEvoy, B.; Vrain, O.; Ryan, D.; Cowman, R.; Higginbotham, C.L. Effects of gamma ray and electron beam irradiation on the mechanical, thermal, structural and physicochemical properties of poly (ether-block-amide) thermoplastic elastomers. J. Mech. Behav. Biomed. Mater. 2013, 17, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Imperiyka, M.; Ahmad, A.; Hanifah, S.A.; Rahman, M.Y.A. Role of salt concentration lithium perchlorate on ionic conductivity and structural of (glycidyl methacrylate -co-ethyl methacrylate) (70/30) based on a solid polymer electrolyte. Adv. Mater. Res. 2012, 626, 454–458. [Google Scholar] [CrossRef]
- Lazauskaite, R.; Grazulevicius, J.V. Synthesis and cationic photopolymerization of electroactive monomers containing functional groups. Polym. Adv. Technol. 2005, 16, 571–581. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Tominaga, Y.; Yamazaki, K.; Nanthana, V. Effect of anions on lithium ion conduction in poly(ethylene carbonate)-based polymer electrolytes. J. Electrochem. Soc. 2015, 162, A3133–A3136. [Google Scholar] [CrossRef]
- Chai, J.; Liu, Z.; Ma, J.; Wang, J.; Liu, X.; Liu, H.; Zhang, J.; Cui, G.; Chen, L. In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv. Sci. 2017, 4, 1600377. [Google Scholar] [CrossRef]
- Ju, J.; Wang, Y.; Chen, B.; Ma, J.; Dong, S.; Chai, J.; Qu, H.; Cui, L.; Wu, X.; Cui, G. Integrated interface strategy toward room temperaturesolid-state lithium batteries. ACS Appl. Mater. Interfaces 2018, 10, 13588–13597. [Google Scholar] [CrossRef]
- Lameiras, P.; Nuzillard, J.M. Highly viscous binary solvents: DMSO-d6/glycerol and DMSO-d6/glycerol-d8 forpolar and apolar mixture analysis by NMR. Anal. Chem. 2016, 88, 4508–4515. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yang, X.; Pei, N.; Li, R.; Zeng, Y.; Zhang, P.; Zhao, J. In Situ Solidification by γ−ray Irradiation Process for Integrated Solid−State Lithium Battery. Batteries 2023, 9, 255. https://doi.org/10.3390/batteries9050255
Chen Z, Yang X, Pei N, Li R, Zeng Y, Zhang P, Zhao J. In Situ Solidification by γ−ray Irradiation Process for Integrated Solid−State Lithium Battery. Batteries. 2023; 9(5):255. https://doi.org/10.3390/batteries9050255
Chicago/Turabian StyleChen, Zhiqiang, Xueying Yang, Nanbiao Pei, Ruiyang Li, Yuejin Zeng, Peng Zhang, and Jinbao Zhao. 2023. "In Situ Solidification by γ−ray Irradiation Process for Integrated Solid−State Lithium Battery" Batteries 9, no. 5: 255. https://doi.org/10.3390/batteries9050255
APA StyleChen, Z., Yang, X., Pei, N., Li, R., Zeng, Y., Zhang, P., & Zhao, J. (2023). In Situ Solidification by γ−ray Irradiation Process for Integrated Solid−State Lithium Battery. Batteries, 9(5), 255. https://doi.org/10.3390/batteries9050255




