Descriptor-Based Graded Electrode Microstructures Design Strategies of Lithium-Ion Batteries for Enhanced Rate Performance
Abstract
1. Introduction
2. Experimental and Numerical Methods
2.1. Materials and Electrochemical Tests
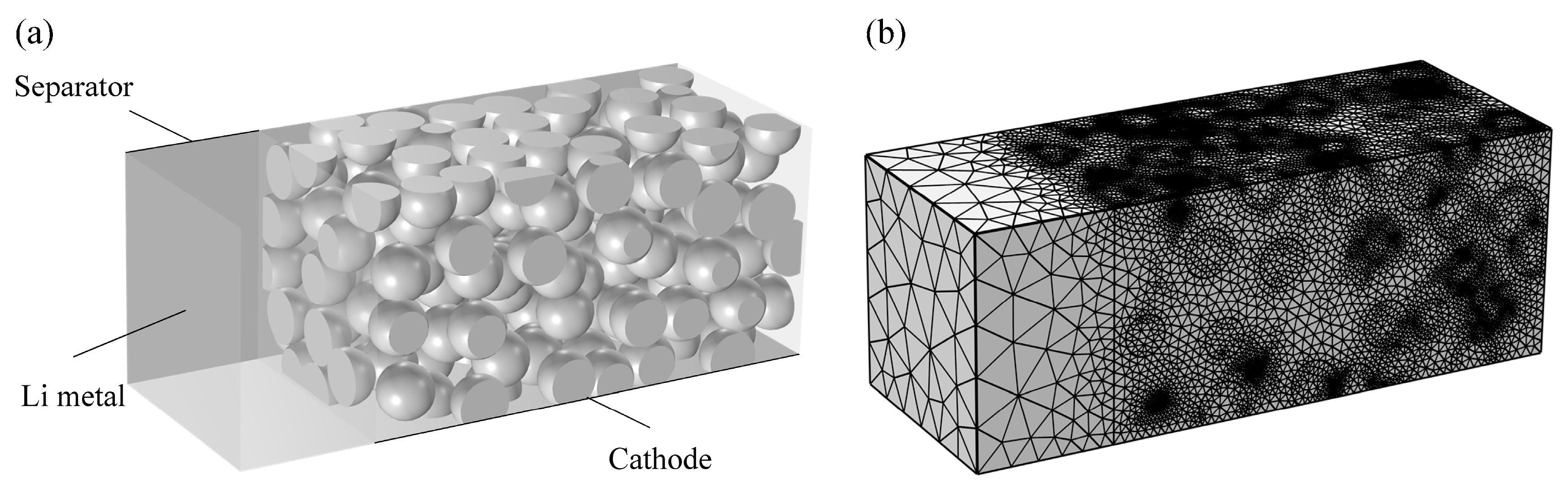
2.2. Electrode Microstructures
2.3. Microstructure-Based Electrochemical Model
2.4. Parameters and Simulation Details
3. Results and Discussion
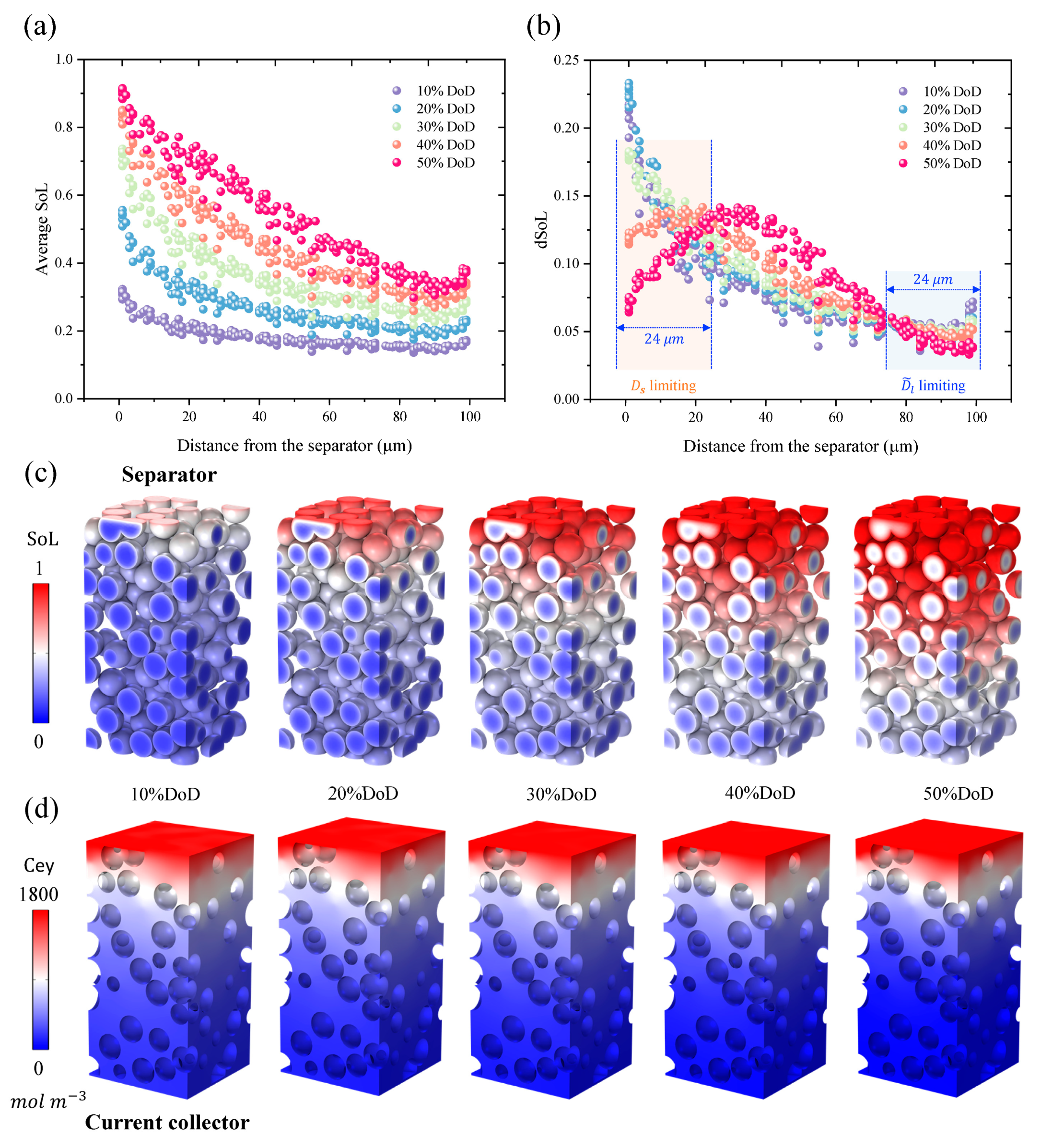
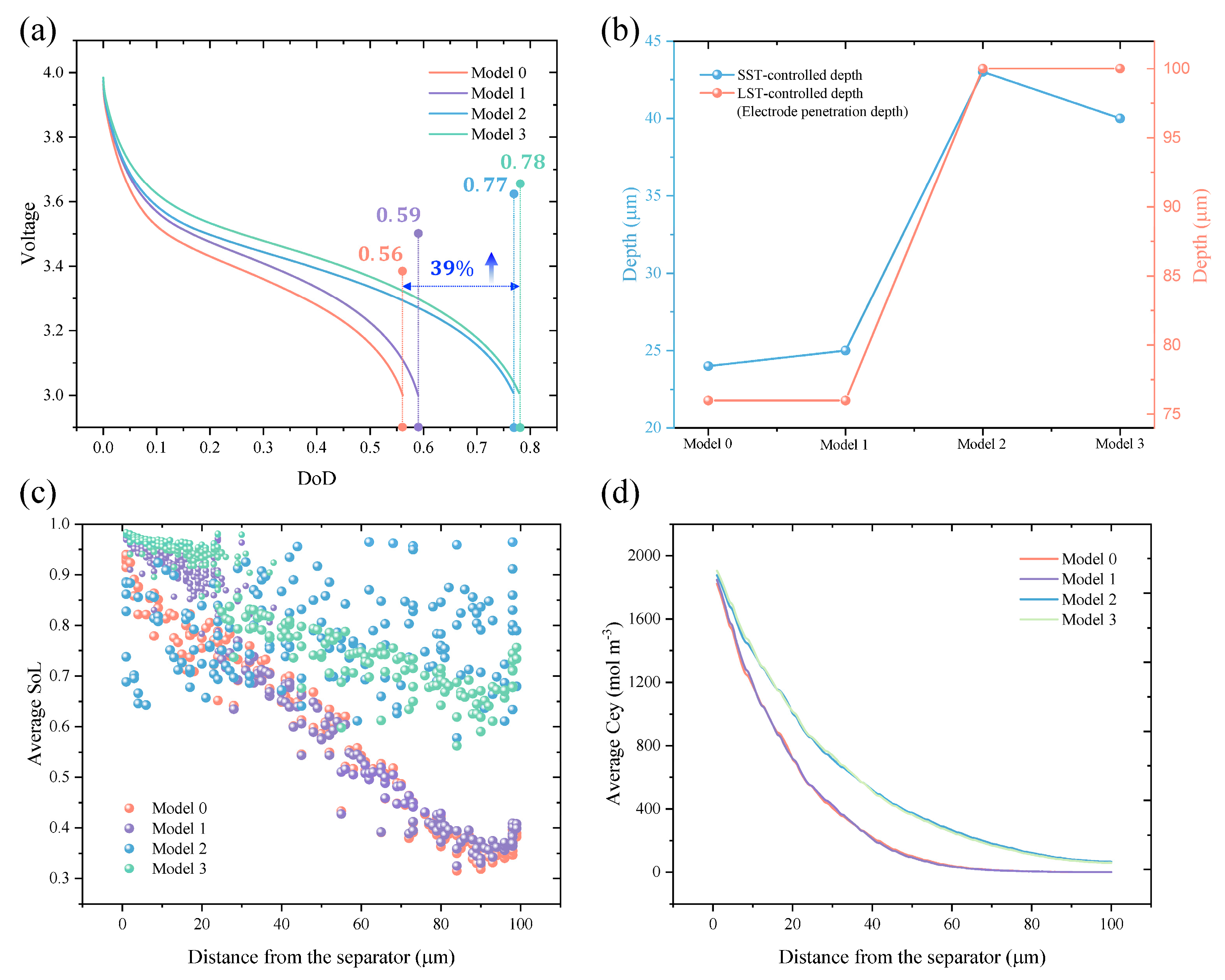
3.1. Quantification of the Electrode Penetration Depth
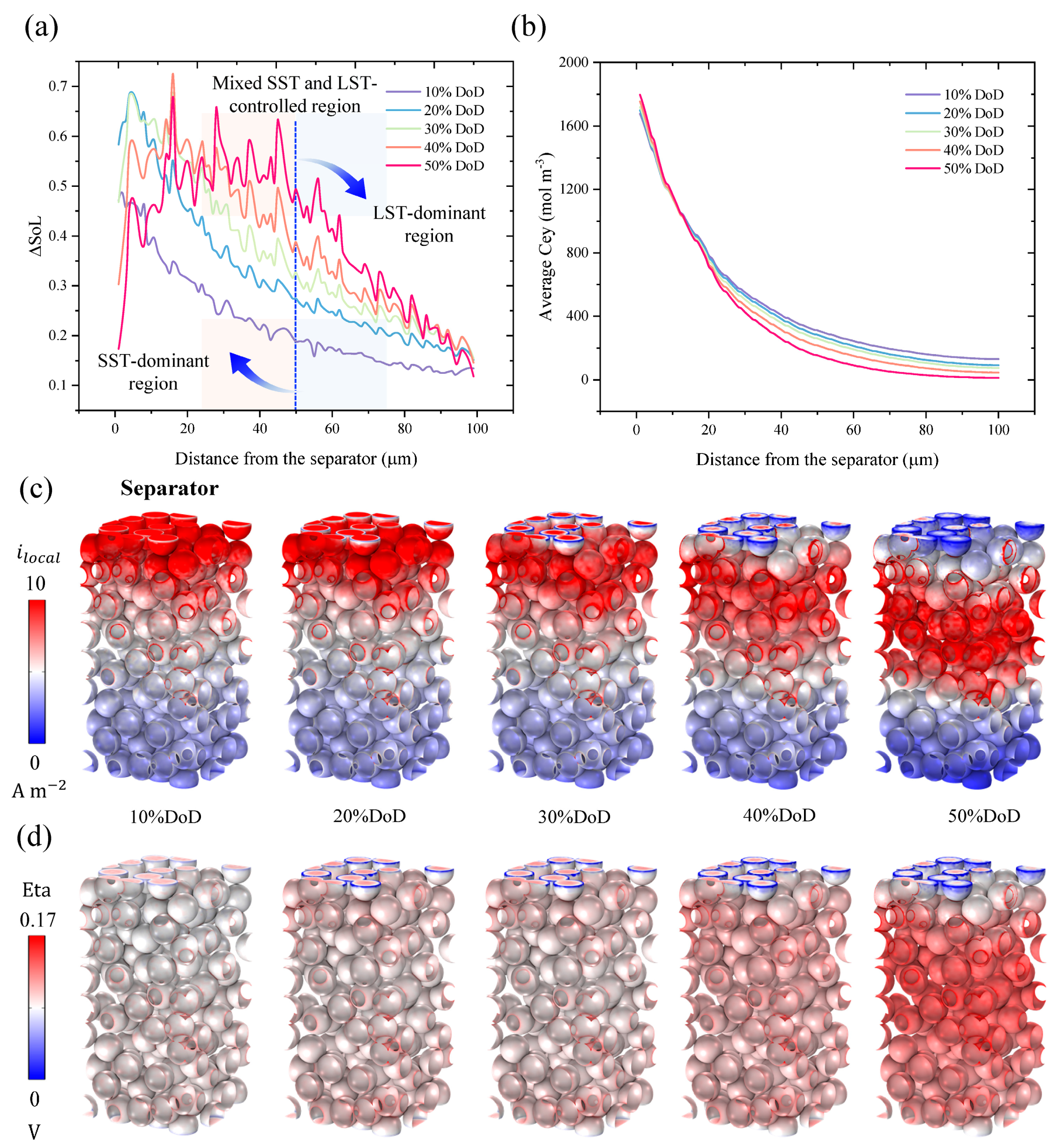
3.2. Analysis of Mixed SST and LST-Controlled Region
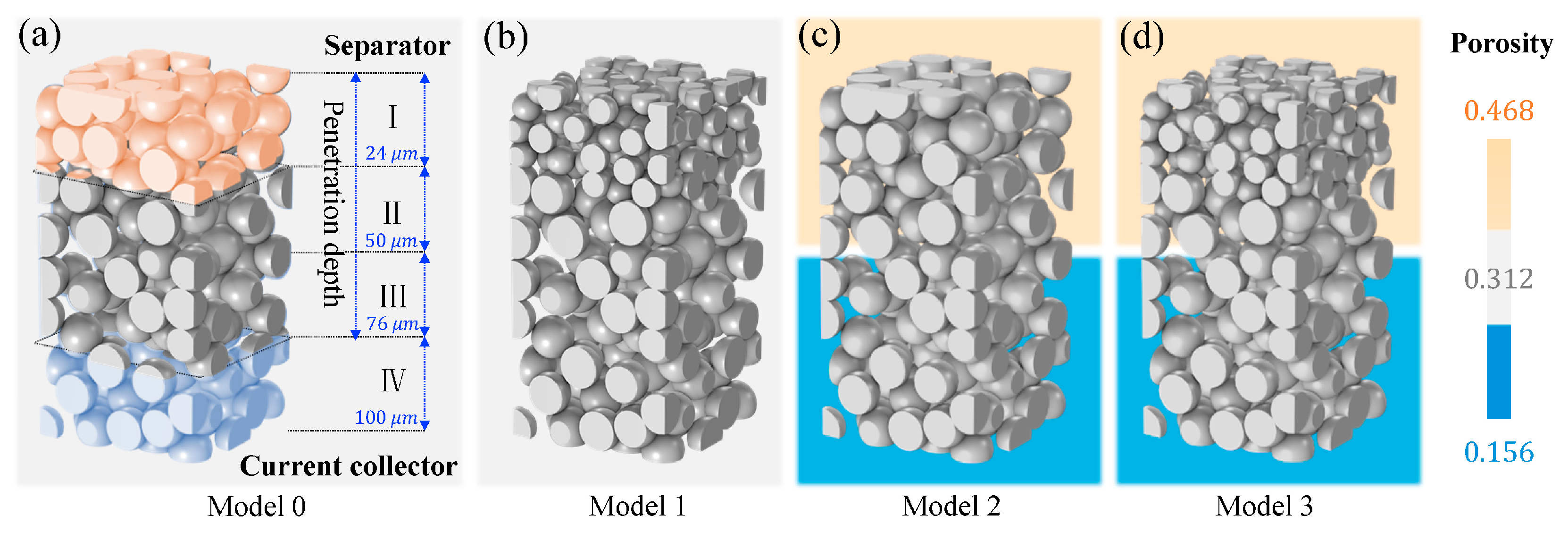
3.3. Regional Graded Electrode Design Strategies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium Ion Batteries: Recent Progress and A Promising Future in Applications. Energy Environ. Mater. 2022, e12450. [Google Scholar] [CrossRef]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M. Lithium-ion batteries–Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Harris, S.J.; Lu, P. Effects of Inhomogeneities—Nanoscale to Mesoscale—On the Durability of Li-Ion Batteries. J. Phys. Chem. C 2013, 117, 6481–6492. [Google Scholar] [CrossRef]
- Dillon, S.J.; Sun, K. Microstructural design considerations for Li-ion battery systems. Curr. Opin. Solid State Mater. Sci. 2012, 16, 153–162. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Tan, C.; Heenan, T.M.; Lagnoni, M.; O’Regan, K.; Daemi, S.; Bertei, A.; Jones, H.G.; Hinds, G. Multi-length scale microstructural design of lithium-ion battery electrodes for improved discharge rate performance. Energy Environ. Sci. 2021, 14, 5929–5946. [Google Scholar] [CrossRef]
- Lu, X.; Bertei, A.; Finegan, D.P.; Tan, C.; Daemi, S.R.; Weaving, J.S.; O’Regan, K.B.; Heenan, T.M.; Hinds, G.; Kendrick, E. 3D microstructure design of lithium-ion battery electrodes assisted by X-ray nano-computed tomography and modelling. Nat. Commun. 2020, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.E.; Walker, B.C.; Skelton, C.B.; Gorzkowski, E.P.; Rowenhorst, D.J.; Wheeler, D.R. Modeling 3D microstructure and ion transport in porous Li-ion battery electrodes. J. Electrochem. Soc. 2011, 158, A781. [Google Scholar] [CrossRef]
- Shearing, P.R.; Howard, L.E.; Jørgensen, P.S.; Brandon, N.P.; Harris, S.J. Characterization of the 3-dimensional microstructure of a graphite negative electrode from a Li-ion battery. Electrochem. Commun. 2010, 12, 374–377. [Google Scholar]
- Chowdhury, R.; Banerjee, A.; Zhao, Y.; Liu, X.; Brandon, N. Simulation of bi-layer cathode materials with experimentally validated parameters to improve ion diffusion and discharge capacity. Sustain. Energy Fuels 2021, 5, 1103–1119. [Google Scholar] [CrossRef]
- Huang, C.; Leung, C.L.A.; Leung, P.; Grant, P.S. A solid-state battery cathode with a polymer composite electrolyte and low tortuosity microstructure by directional freezing and polymerization. Adv. Energy Mater. 2021, 11, 2002387. [Google Scholar] [CrossRef]
- Lv, Z.; Yue, M.; Ling, M.; Zhang, H.; Yan, J.; Zheng, Q.; Li, X. Controllable Design Coupled with Finite Element Analysis of Low-Tortuosity Electrode Architecture for Advanced Sodium-Ion Batteries with Ultra-High Mass Loading. Adv. Energy Mater. 2021, 11, 2003725. [Google Scholar] [CrossRef]
- Zhang, X.; Hui, Z.; King, S.T.; Wu, J.; Ju, Z.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; Wang, L. Gradient Architecture Design in Scalable Porous Battery Electrodes. Nano Lett. 2022, 22, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guan, P.; Liu, C. Experimental and simulation investigations of porosity graded cathodes in mitigating battery degradation of high voltage lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A3163. [Google Scholar] [CrossRef]
- Wu, J.; Ju, Z.; Zhang, X.; Xu, X.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Low-Tortuosity Thick Electrodes with Active Materials Gradient Design for Enhanced Energy Storage. ACS Nano 2022, 16, 4805–4812. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Pinfield, V.J.; Wu, B.; Wang, H.; Jiao, K.; Leung, D.Y.; Xuan, J. Towards the digitalisation of porous energy materials: Evolution of digital approaches for microstructural design. Energy Environ. Sci. 2021, 14, 2549–2576. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, Z.; Zhu, Y.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; Yu, G. Multiscale understanding and architecture design of high energy/power lithium-ion battery electrodes. Adv. Energy Mater. 2021, 11, 2000808. [Google Scholar] [CrossRef]
- Boyce, A.M.; Lu, X.; Brett, D.J.; Shearing, P.R. Exploring the influence of porosity and thickness on lithium-ion battery electrodes using an image-based model. J. Power Sources 2022, 542, 231779. [Google Scholar] [CrossRef]
- Dai, Y.; Srinivasan, V. On graded electrode porosity as a design tool for improving the energy density of batteries. J. Electrochem. Soc. 2015, 163, A406. [Google Scholar] [CrossRef]
- Hui, Z.; Mayilvahanan, K.S.; Ganko, K.; Yang, Y.; Zhang, X.; Ju, Z.; Takeuchi, K.J.; Marschilok, A.C.; Yu, G.; Takeuchi, E. Optimal electrode-scale design of Li-ion electrodes: A general correlation. Energy Storage Mater. 2021, 39, 176–185. [Google Scholar] [CrossRef]
- Zielke, L.; Hutzenlaub, T.; Wheeler, D.R.; Chao, C.W.; Manke, I.; Hilger, A.; Paust, N.; Zengerle, R.; Thiele, S. Three-phase multiscale modeling of a LiCoO2 cathode: Combining the advantages of FIB–SEM imaging and X-Ray tomography. Adv. Energy Mater. 2015, 5, 1401612. [Google Scholar]
- Wiedemann, A.H.; Goldin, G.M.; Barnett, S.A.; Zhu, H.; Kee, R.J. Effects of three-dimensional cathode microstructure on the performance of lithium-ion battery cathodes. Electrochim. Acta 2013, 88, 580–588. [Google Scholar] [CrossRef]
- Ebner, M.; Geldmacher, F.; Marone, F.; Stampanoni, M.; Wood, V. X-ray tomography of porous, transition metal oxide based lithium ion battery electrodes. Adv. Energy Mater. 2013, 3, 845–850. [Google Scholar] [CrossRef]
- Nyman, A.; Behm, M.; Lindbergh, G. Electrochemical characterisation and modelling of the mass transport phenomena in LiPF6-EC-EMC. Electrochim. Acta 2008, 53, 6356. [Google Scholar] [CrossRef]
- Zavalis, T.G.; Behm, M.; Lindbergh, G. Investigations of Short-Circuit Scenarios in a Lithium-Ion Battery Cell. J. Electrochem. Soc. 2012, 159, A848. [Google Scholar] [CrossRef]
- Cai, L.; White, R.E. Mathematical modeling of a lithium ion battery with thermal effects in COMSOL Inc. Multiphysics (MP) software. J. Power Sources 2011, 196, 5985–5989. [Google Scholar] [CrossRef]
- Zheng, W.; Shui, M.; Shu JI, E.; Gao, S.; Xu, D.; Chen, L.; Feng, L.; Ren, Y. GITT studies on oxide cathode LiNi1/3Co1/3Mn1/3O2 synthesized by citric acid assisted high-energy ball milling. Bull. Mater. Sci. 2013, 36, 495–498. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Danner, T.; Singh, M.; Hein, S.; Kaiser, J.; Hahn, H.; Latz, A. Thick electrodes for Li-ion batteries: A model based analysis. J. Power Sources 2016, 334, 191–201. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Kim, S.; Deng, Y.; Minor, A.M.; Song, X.; Battaglia, V.S. Effects of various conductive additive and polymeric binder contents on the performance of a lithium-ion composite cathode. J. Electrochem. Soc. 2008, 155, A887. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Q.; Liu, Y.; Chen, S. Descriptor-Based Graded Electrode Microstructures Design Strategies of Lithium-Ion Batteries for Enhanced Rate Performance. Batteries 2023, 9, 227. https://doi.org/10.3390/batteries9040227
Shan Q, Liu Y, Chen S. Descriptor-Based Graded Electrode Microstructures Design Strategies of Lithium-Ion Batteries for Enhanced Rate Performance. Batteries. 2023; 9(4):227. https://doi.org/10.3390/batteries9040227
Chicago/Turabian StyleShan, Qiang, Yuwen Liu, and Shengli Chen. 2023. "Descriptor-Based Graded Electrode Microstructures Design Strategies of Lithium-Ion Batteries for Enhanced Rate Performance" Batteries 9, no. 4: 227. https://doi.org/10.3390/batteries9040227
APA StyleShan, Q., Liu, Y., & Chen, S. (2023). Descriptor-Based Graded Electrode Microstructures Design Strategies of Lithium-Ion Batteries for Enhanced Rate Performance. Batteries, 9(4), 227. https://doi.org/10.3390/batteries9040227


.png)




