Advances in Cathodes for High-Performance Magnesium-Sulfur Batteries: A Critical Review
Abstract
1. Introduction
2. Reaction Mechanisms and Challenges in Mg-S Batteries
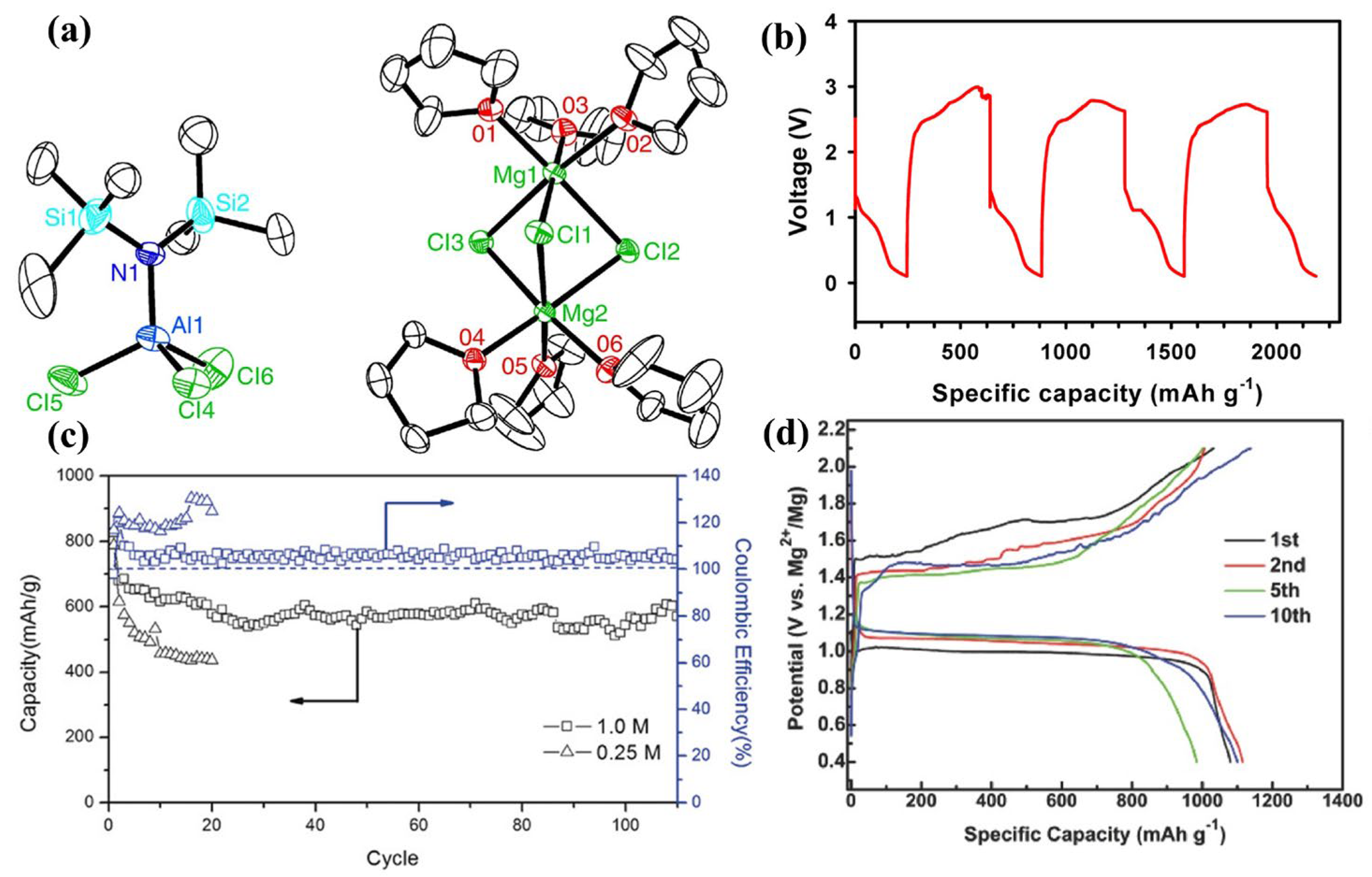
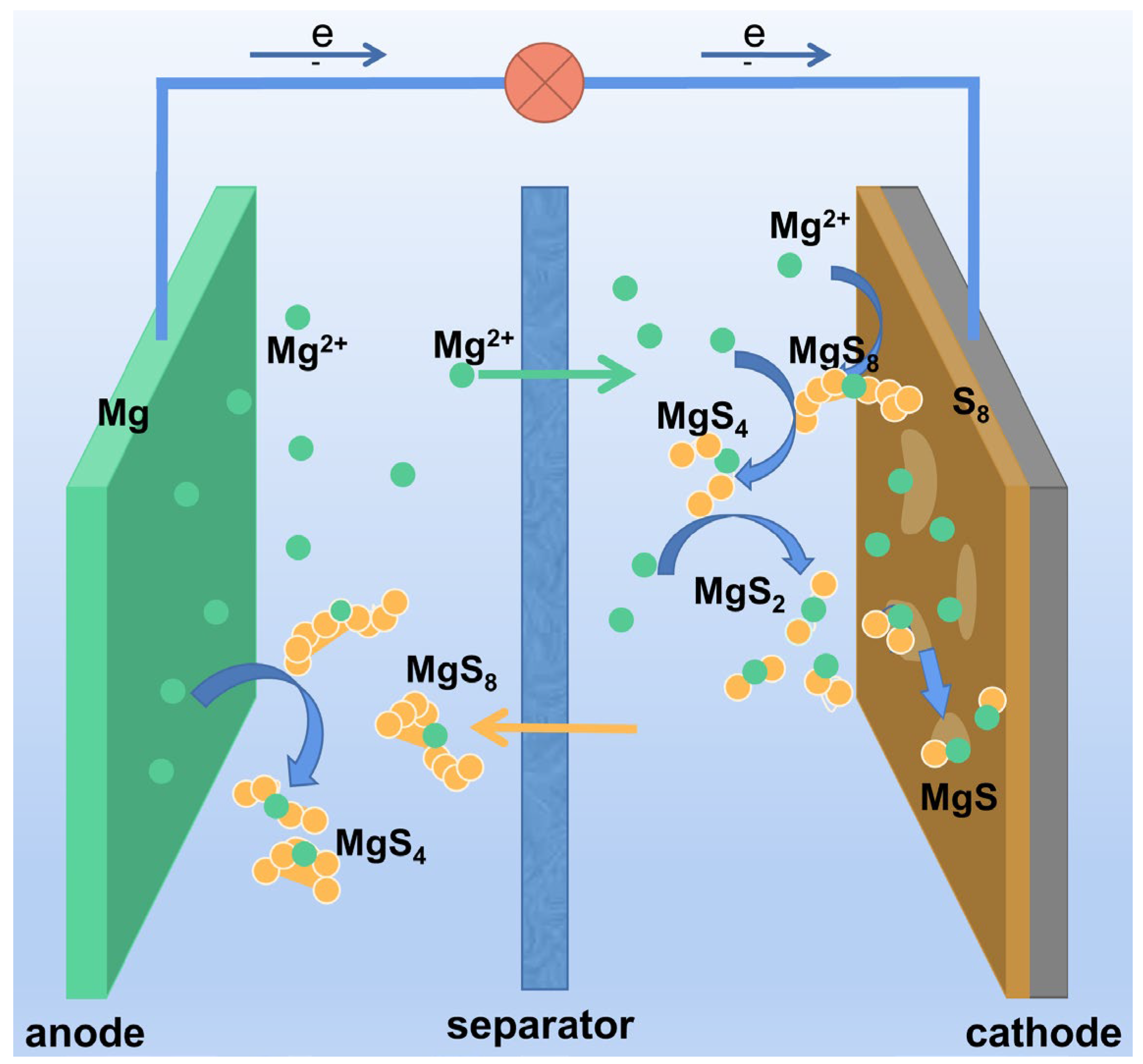
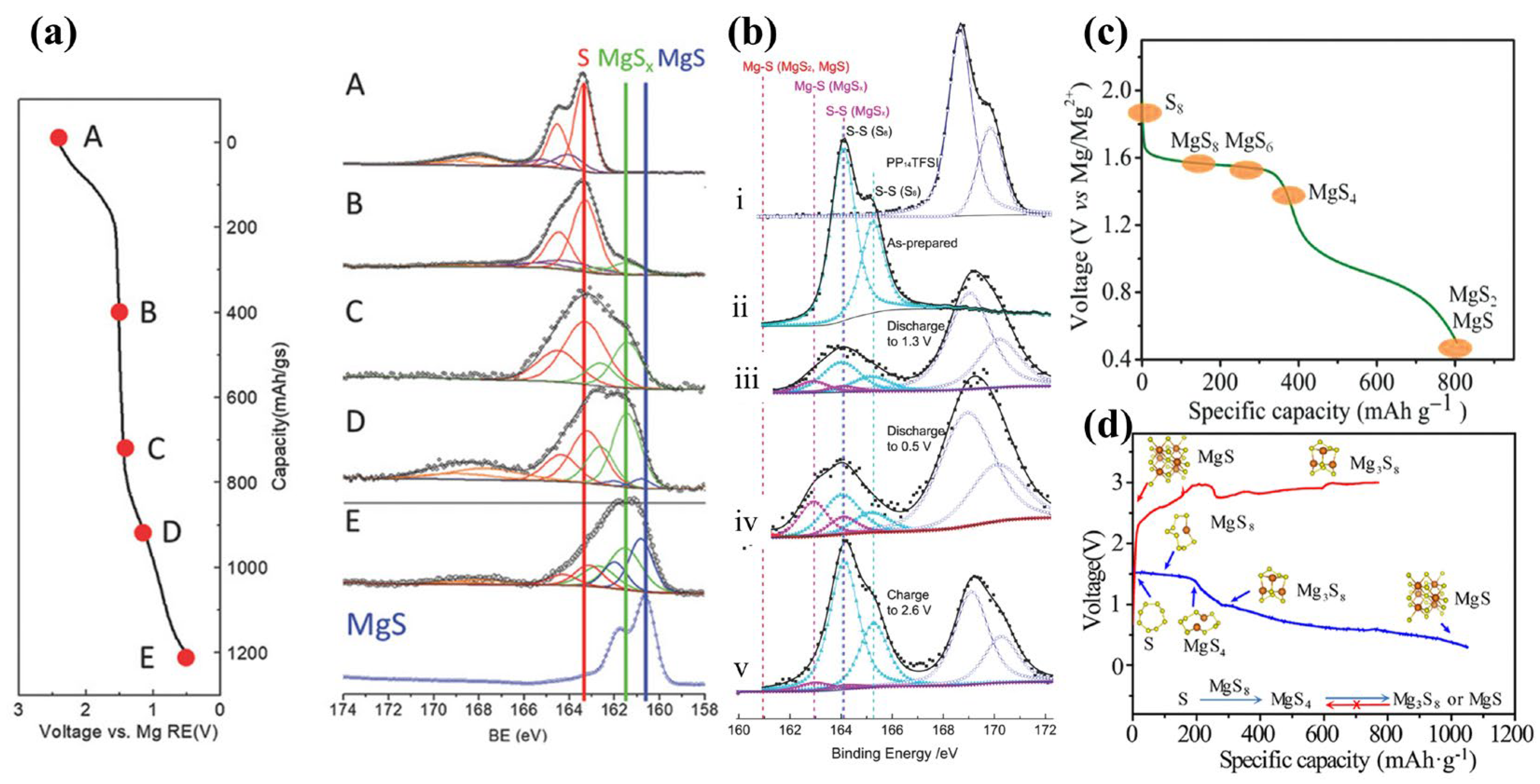
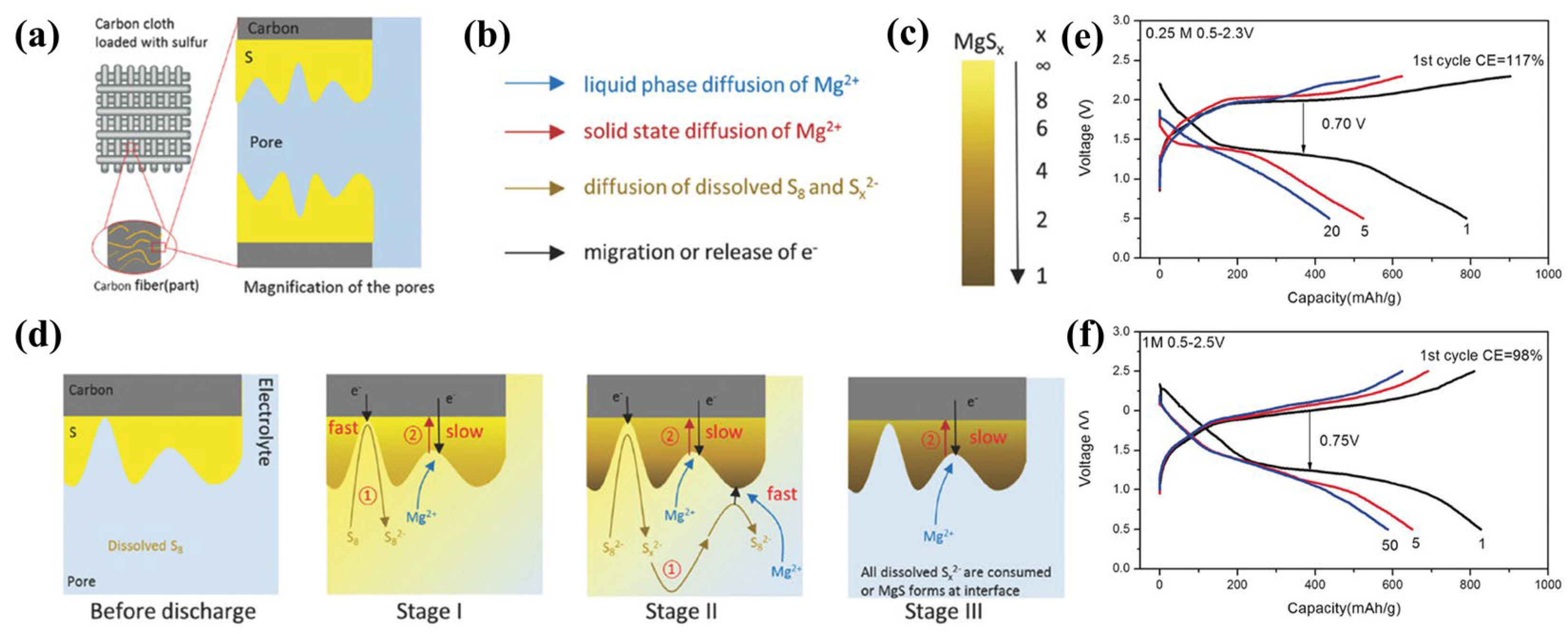
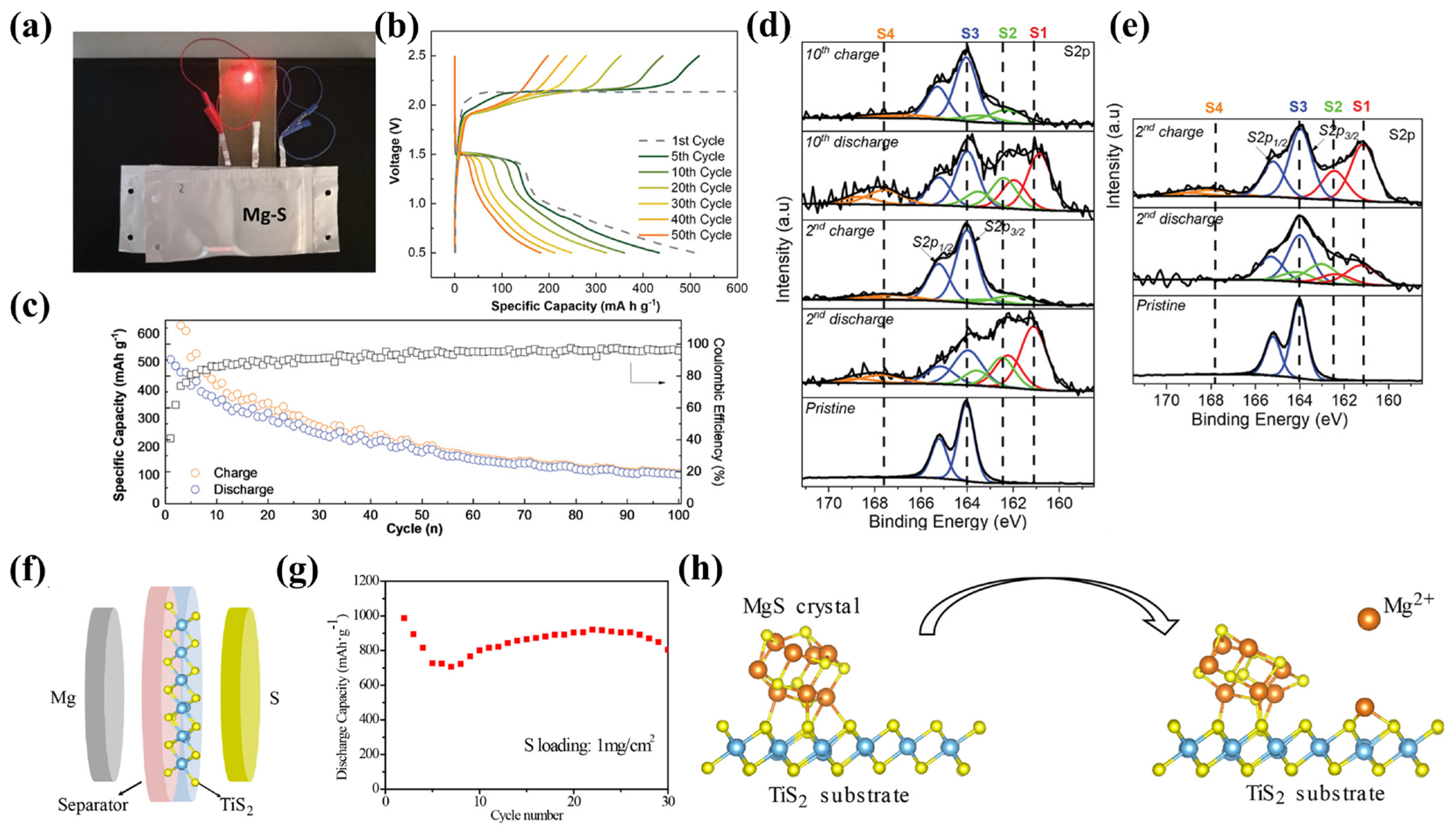
2.1. Reaction Mechanisms in Mg-S Batteries
- Scheme I:
- Scheme II:
- Scheme II:
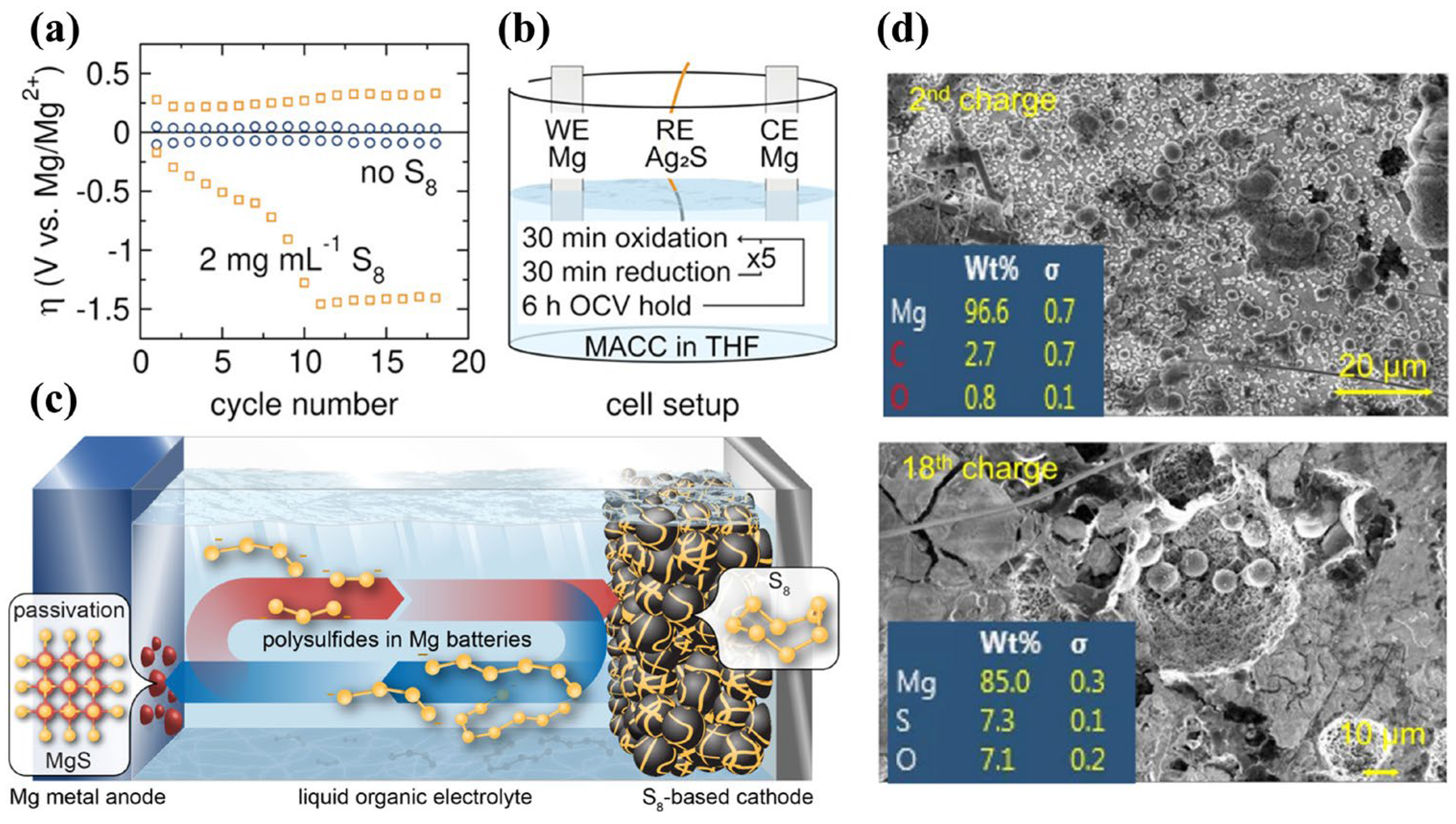
2.2. Challenges in Cathode Materials
3. Research Progresses on Sulfur Cathodes
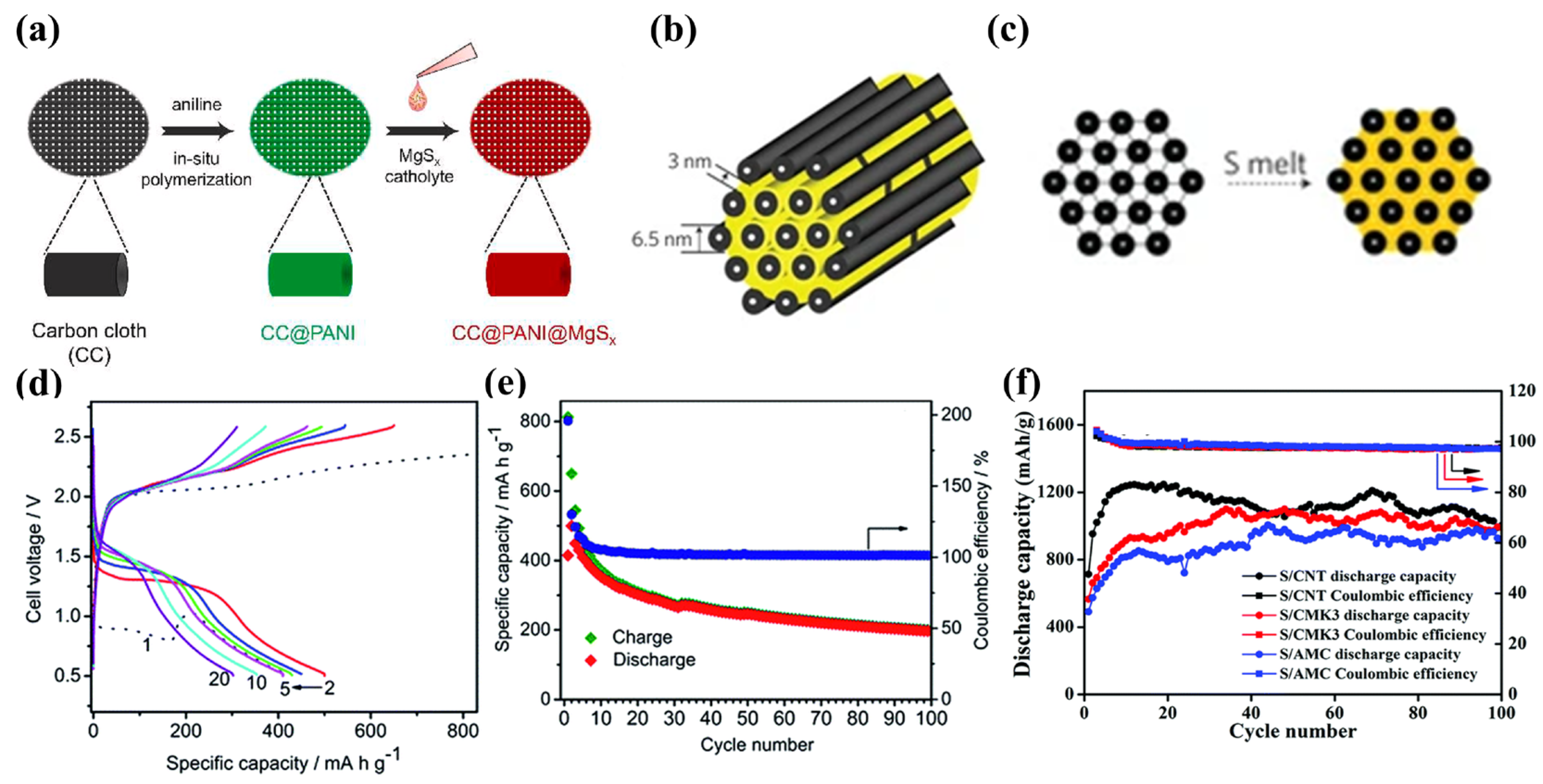
3.1. Physical Adsorption Cathode
3.2. Chemisorbed Cathode
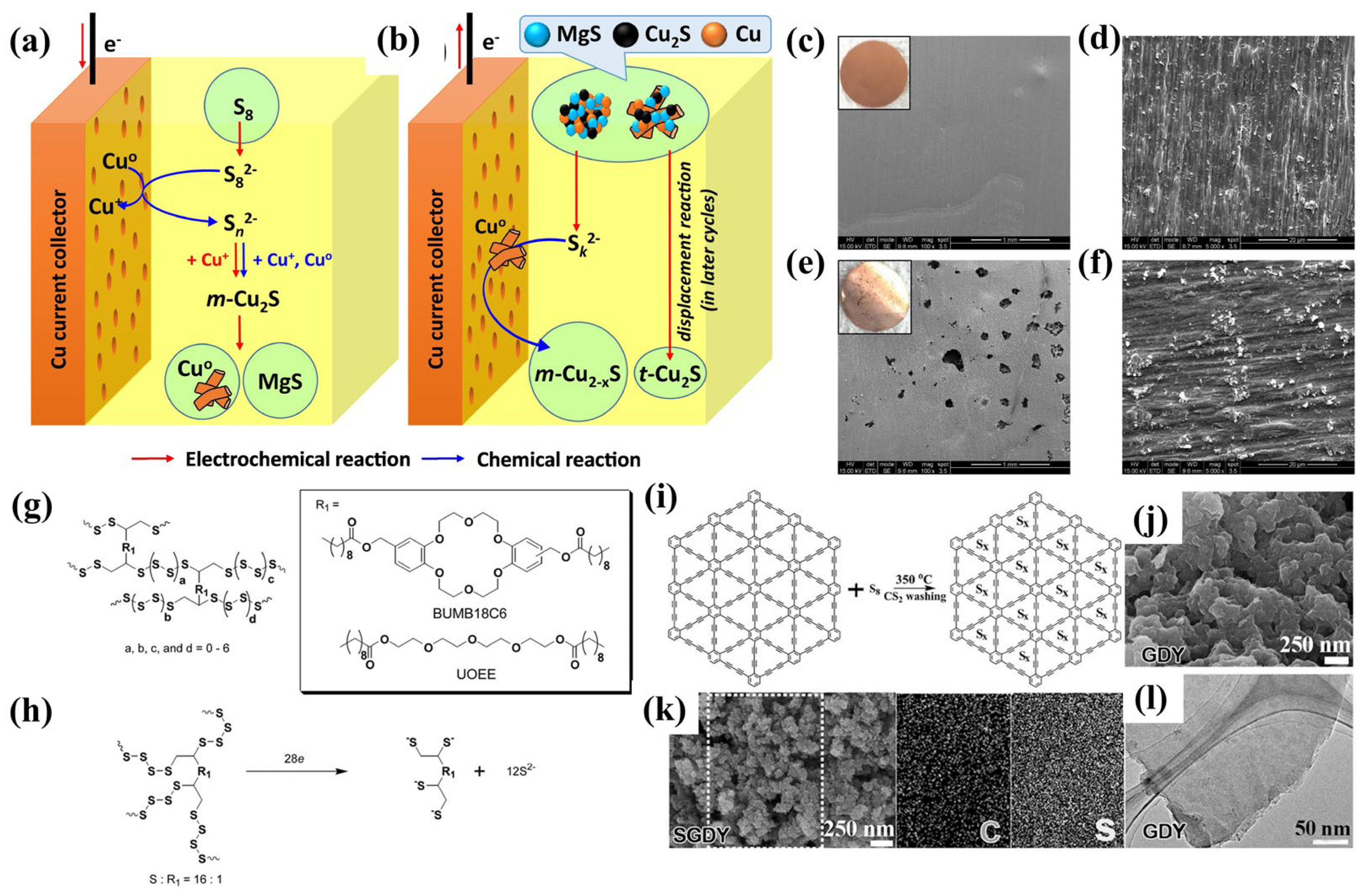
3.3. Covalently Bonded Sulfur Cathode
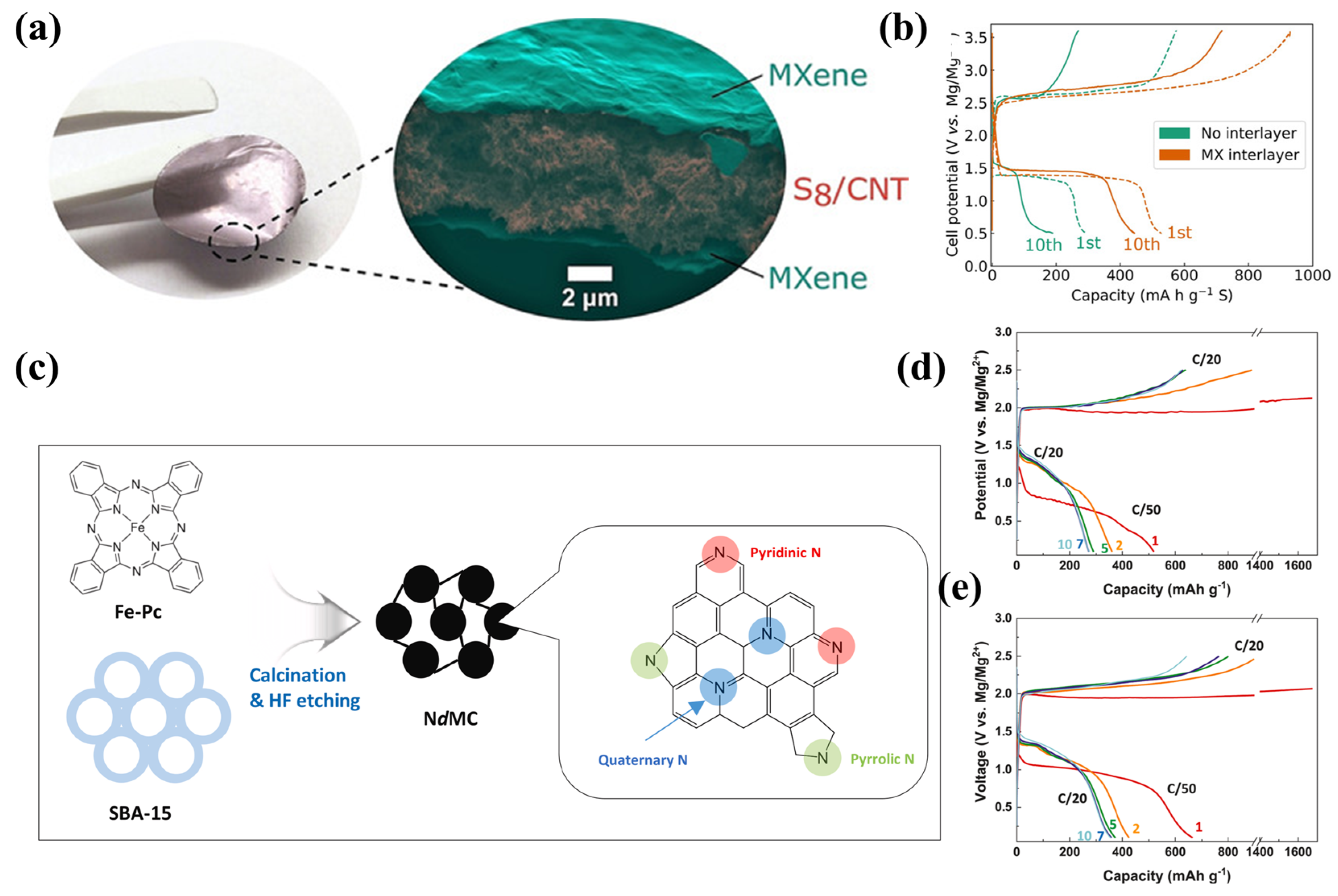
3.4. Interlayer Modification on Cathode Side
4. Research Progresses on Non-Nucleophilic Electrolytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franke, K.; Sensfuß, F.; Bernath, C.; Lux, B. Carbon-neutral energy systems and the importance of flexibility options: A case study in China. Comput. Ind. Eng. 2021, 162, 107712. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; He, Y.-B.; Kang, F.; Chen, L.; Li, H.; Yang, Q.-H. Solid-state lithium batteries: Safety and prospects. eScience 2022, 2, 138–163. [Google Scholar] [CrossRef]
- Mohtadi, R.; Tutusaus, O.; Arthur, T.S.; Zhao-Karger, Z.; Fichtner, M. The metamorphosis of rechargeable magnesium batteries. Joule 2021, 5, 581–617. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Tian, H.; Gao, T.; Li, X.; Wang, X.; Luo, C.; Fan, X.; Yang, C.; Suo, L.; Ma, Z.; Han, W.; et al. High power rechargeable magnesium/iodine battery chemistry. Nat. Commun. 2017, 8, 14083. [Google Scholar] [CrossRef] [PubMed]
- Blake, I.C. Fiftieth Anniversary: The Anniversary Issue on Primary Cell. J. Electrochem. Soc. 1952, 99, 202C. [Google Scholar] [CrossRef]
- Gregory, T.D.; Hoffman, R.J.; Winterton, R.C. Nonaqueous Electrochemistry of Magnesium: Applications to Energy Storage. J. Electrochem. Soc. 1990, 137, 775–780. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef]
- Mizrahi, O.; Amir, N.; Pollak, E.; Chusid, O.; Marks, V.; Gottlieb, H.; Larush, L.; Zinigrad, E.; Aurbach, D. Electrolyte Solutions with a Wide Electrochemical Window for Rechargeable Magnesium Batteries. J. Electrochem. Soc. 2008, 155, A103. [Google Scholar] [CrossRef]
- Kim, H.S.; Arthur, T.S.; Allred, G.D.; Zajicek, J.; Newman, J.G.; Rodnyansky, A.E.; Oliver, A.G.; Boggess, W.C.; Muldoon, J. Structure and compatibility of a magnesium electrolyte with a sulphur cathode. Nat. Commun. 2011, 2, 427. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, S.; Li, T.; Su, D.; Guo, S.; Wang, G. Recent Advances in Rechargeable Magnesium-Based Batteries for High-Efficiency Energy Storage. Adv. Energy Mater. 2020, 10, 1903591. [Google Scholar] [CrossRef]
- Bitenc, J.; Dominko, R. Opportunities and Challenges in the Development of Cathode Materials for Rechargeable Mg Batteries. Front. Chem. 2018, 6, 634. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Zhu, D.; Li, Z.; Sun, F.; Zhu, W.; Chen, Y.; Zhang, J.; Ren, L.; Zhang, S.; et al. Synchrotron Radiation Spectroscopic Studies of Mg2+ Storage Mechanisms in High-Performance Rechargeable Magnesium Batteries with Co-Doped FeS2 Cathodes. Adv. Energy Mater. 2022, 12, 2201608. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Y.; Li, B.; Wang, L.; Xu, H.; Chong, L.; Zou, J. Rechargeable magnesium batteries: Development, opportunities and challenges. Chin. J. Nonferrous Met. 2021, 31, 3192–3216. [Google Scholar]
- Zhao-Karger, Z.; Zhao, X.; Wang, D.; Diemant, T.; Behm, R.J.; Fichtner, M. Performance Improvement of Magnesium Sulfur Batteries with Modified Non-Nucleophilic Electrolytes. Adv. Energy Mater. 2015, 5, 1401155. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Liu, R.; Dai, W.; Li, Z.; Diemant, T.; Vinayan, B.P.; Bonatto Minella, C.; Yu, X.; Manthiram, A.; Behm, R.J.; et al. Toward Highly Reversible Magnesium-Sulfur Batteries with Efficient and Practical Mg[B(hfip)4]2 Electrolyte. ACS Energy Lett. 2018, 3, 2005–2013. [Google Scholar] [CrossRef]
- Gao, T.; Ji, X.; Hou, S.; Fan, X.; Li, X.; Yang, C.; Han, F.; Wang, F.; Jiang, J.; Xu, K.; et al. Thermodynamics and Kinetics of Sulfur Cathode during Discharge in MgTFSI2-DME Electrolyte. Adv. Mater. 2018, 30, 1704313. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, Y.; Zhao, S.; Feng, J.; Li, J.; Chen, H.; Yang, A.; Shi, F.; Jia, L.; Wu, Y.; et al. In Situ X-ray Absorption Spectroscopic Investigation of the Capacity Degradation Mechanism in Mg/S Batteries. Nano Lett. 2019, 19, 2928–2934. [Google Scholar] [CrossRef]
- Du, W.; Hao, Z.; Iacoviello, F.; Sheng, L.; Guan, S.; Zhang, Z.; Brett, D.J.L.; Wang, F.R.; Shearing, P.R. A Multiscale X-Ray Tomography Study of the Cycled-Induced Degradation in Magnesium-Sulfur Batteries. Small Methods 2021, 5, 2001193. [Google Scholar] [CrossRef]
- Robba, A.; Vizintin, A.; Bitenc, J.; Mali, G.; Arčon, I.; Kavčič, M.; Žitnik, M.; Bučar, K.; Aquilanti, G.; Martineau-Corcos, C.; et al. Mechanistic Study of Magnesium-Sulfur Batteries. Chem. Mater. 2017, 29, 9555–9564. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Ambient-Temperature Energy Storage with Polyvalent Metal-Sulfur Chemistry. Small Methods 2017, 1, 1700217. [Google Scholar] [CrossRef]
- Haecker, J.; Duc Hien, N.; Rommel, T.; Zhao-Karger, Z.; Wagner, N.; Friedrich, K.A. Operando UV/vis Spectroscopy Providing Insights into the Sulfur and Polysulfide Dissolution in Magnesium-Sulfur Batteries. ACS Energy Lett. 2022, 7, 1–9. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Gomes, R.; Bhattacharyya, A.J. Probing the Polysulfide Confinement in Two Different Sulfur Hosts for a Mg|S Battery Employing Operando Raman and Ex-Situ UV-Visible Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 1159–1164. [Google Scholar] [CrossRef]
- Laskowski, F.A.L.; Stradley, S.H.; Qian, M.D.; See, K.A. Mg Anode Passivation Caused by the Reaction of Dissolved Sulfur in Mg-S Batteries. ACS Appl. Mater. Inter. 2021, 13, 29461–29470. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, C.; Xiao, R.; Li, R.; Mu, T.; Huo, H.; Ma, Y.; Yin, G.; Zuo, P. Interface regulation of Mg anode and redox couple conversion in cathode by copper for high-performance Mg-S battery. Chem. Eng. J. 2023, 451, 138663. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Zhao-Karger, Z.; Diemant, T.; Chakravadhanula, V.S.K.; Schwarzburger, N.I.; Cambaz, M.A.; Behm, R.J.; Kuebel, C.; Fichtner, M. Performance study of magnesium-sulfur battery using a graphene based sulfur composite cathode electrode and a non-nucleophilic Mg electrolyte. Nanoscale 2016, 8, 3296–3306. [Google Scholar] [CrossRef]
- Dan-Thien, N.; Horia, R.; Eng, A.Y.S.; Song, S.-W.; Seh, Z.W. Material design strategies to improve the performance of rechargeable magnesium-sulfur batteries. Mater. Horiz. 2021, 8, 830–853. [Google Scholar] [CrossRef]
- Schmidt, A.; Koger, H.; Barthelemy, A.; Studer, G.; Esser, B.; Krossing, I. Is One of the Least Coordinating Anions Suitable to Serve as Electrolyte Salt for Magnesium-Ion Batteries? Batter. Supercaps 2022, 5, e202200340. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, Y.; Xiao, J.; Zhang, J.; Wang, M.; Zhang, Y. A non-nucleophilic gel polymer magnesium electrolyte compatible with sulfur cathode. Nano Res. 2020, 13, 2749–2754. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Meng, Z.; Xiu, Y.; Dasari, B.; Zhao-Karger, Z.; Fichtner, M. Designing gel polymer electrolyte with synergetic properties for rechargeable magnesium batteries. Energy Storage Mater. 2022, 48, 155–163. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Fan, H.; Zhao, Y.; Su, Y.; Liu, H.; Li, X.; Su, Y.; Yuan, H.; Pan, T.; et al. Stable Solid Electrolyte Interphase In Situ Formed on Magnesium-Metal Anode by using a Perfluorinated Alkoxide-Based All-Magnesium Salt Electrolyte. Adv. Mater. 2022, 34, 2203783. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, D.; Ghosh, A.; Kumar, A.; Mitra, S. Nitrogen and Sulfur Doped Carbon Cloth as Current Collector and Polysulfide Immobilizer for Magnesium-Sulfur Batteries. Chemelectrochem 2019, 6, 684–689. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, H.; NuLi, Y.; Zhou, J.; Yang, J.; Wang, J. Sulfur@microporous Carbon Cathode with a High Sulfur Content for Magnesium-Sulfur Batteries with Nucleophilic Electrolytes. J. Phys. Chem. C 2018, 122, 26764–26776. [Google Scholar] [CrossRef]
- Gao, T.; Hou, S.; Wang, F.; Ma, Z.; Li, X.; Xu, K.; Wang, C. Reversible S0/MgSx Redox Chemistry in a MgTFSI2/MgCl2/DME Electrolyte for Rechargeable Mg/S Batteries. Angew. Chem. 2017, 129, 13711–13715. [Google Scholar] [CrossRef]
- Muthuraj, D.; Pandey, M.; Krishna, M.; Ghosh, A.; Sen, R.; Johari, P.; Mitra, S. Magnesium polysulfide catholyte (MgSx): Synthesis, electrochemical and computational study for magnesium-sulfur battery application. J. Power Sources 2021, 486, 229326. [Google Scholar] [CrossRef]
- Lee, M.; Jeong, M.; Nam, Y.S.; Moon, J.; Lee, M.; Lim, H.D.; Byun, D.; Yim, T.; Oh, S.H. Nitrogen-doped graphitic mesoporous carbon materials as effective sulfur imbibition hosts for magnesium-sulfur batteries. J. Power Sources 2022, 535, 231471. [Google Scholar] [CrossRef]
- Kaland, H.; Haskjold Fagerli, F.; Hadler-Jacobsen, J.; Zhao-Karger, Z.; Fichtner, M.; Wiik, K.; Wagner, N.P. Performance Study of MXene/Carbon Nanotube Composites for Current Collector- and Binder-Free Mg-S Batteries. Chemsuschem 2021, 14, 1864–1873. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Euchner, H.; Zhao-Karger, Z.; Cambaz, M.A.; Li, Z.; Diemant, T.; Behm, R.J.; Gross, A.; Fichtner, M. Insights into the electrochemical processes of rechargeable magnesium-sulfur batteries with a new cathode design. J. Mater. Chem. A 2019, 7, 25490–25502. [Google Scholar] [CrossRef]
- Ford, H.O.; Doyle, E.S.; He, P.; Boggess, W.C.; Oliver, A.G.; Wu, T.; Sterbinsky, G.E.; Schaefer, J.L. Self-discharge of magnesium-sulfur batteries leads to active material loss and poor shelf life. Energy Environ. Sci. 2021, 14, 890–899. [Google Scholar] [CrossRef]
- Richter, R.; Hacker, J.; Zhao-Karger, Z.; Danner, T.; Wagner, N.; Fichtner, M.; Friedrich, K.A.; Latz, A. Insights into Self-Discharge of Lithium- and Magnesium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 8457–8474. [Google Scholar] [CrossRef]
- Wang, P.; Kuester, K.; Starke, U.; Liang, C.; Niewa, R.; Buchmeiser, M.R. Performance enhancement of rechargeable magnesium-sulfur batteries based on a sulfurized poly(acrylonitrile) composite and a lithium salt. J. Power Sources 2021, 515, 230604. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, W.; NuLi, Y.; Wang, B.; Yang, J.; Wang, J. Sulfurized-Pyrolyzed Polyacrylonitrile Cathode for Magnesium-Sulfur Batteries Containing Mg2+/Li+ Hybrid Electrolytes. Chem. Eng. J. 2022, 427, 130902. [Google Scholar] [CrossRef]
- Wang, L.; Jankowski, P.; Njel, C.; Bauer, W.; Li, Z.; Meng, Z.; Dasari, B.; Vegge, T.; Lastra, J.M.G.; Zhao-Karger, Z.; et al. Dual Role of Mo6S8 in Polysulfide Conversion and Shuttle for Mg-S Batteries. Adv. Sci. 2022, 9, 2104605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, W.; Zhang, D.; Ren, W.; Zhang, S.; Yan, Y.; Zhang, Y.; Lee, S.J.; Lee, J.S.; Ma, Z.F.; et al. Toward High-Performance Mg-S Batteries via a Copper Phosphide Modified Separator. ACS Nano 2022, 17, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zhao, Y.; Zhang, Z.; Dong, S.; Cui, Z.; Tang, K.; Lu, C.; Han, P.; Zhou, X.; Cui, G. Selenium sulfide cathode with copper foam interlayer for promising magnesium electrochemistry. Energy Storage Mater. 2020, 26, 23–31. [Google Scholar] [CrossRef]
- Nakayama, Y.; Matsumoto, R.; Kumagae, K.; Mori, D.; Mizuno, Y.; Hosoi, S.; Kamiguchi, K.; Koshitani, N.; Inaba, Y.; Kudo, Y.; et al. Zinc Blende Magnesium Sulfide in Rechargeable Magnesium-Sulfur Batteries. Chem. Mater. 2018, 30, 6318–6324. [Google Scholar] [CrossRef]
- Zou, Q.; Sun, Y.; Liang, Z.; Wang, W.; Lu, Y.-C. Achieving Efficient Magnesium-Sulfur Battery Chemistry via Polysulfide Mediation. Adv. Energy Mater. 2021, 11, 2101552. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, J.; Hu, J.; Li, C. High Rate Magnesium-Sulfur Battery with Improved Cyclability Based on Metal-Organic Framework Derivative Carbon Host. Adv. Mater. 2018, 30, 1704166. [Google Scholar] [CrossRef] [PubMed]
- Sungjemmenla; Soni, C.B.; Vineeth, S.K.; Kumar, V. Exploration of the Unique Structural Chemistry of Sulfur Cathode for High-Energy Rechargeable Beyond-Li Batteries. Adv. Energy Sustain. Res. 2022, 3, 2100157. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zhang, Z.; Qu, H.; Cui, Z.; Qiao, L.; Wang, L.; Chai, J.; Lu, T.; Dong, S.; Dong, T.; et al. An efficient organic magnesium borate-based electrolyte with non-nucleophilic characteristics for magnesium-sulfur battery. Energy Environ. Sci. 2017, 10, 2616–2625. [Google Scholar] [CrossRef]
- Ha, S.-Y.; Lee, Y.-W.; Woo, S.W.; Koo, B.; Kim, J.-S.; Cho, J.; Lee, K.T.; Choi, N.-S. Magnesium(II) Bis(trifluoromethane sulfonyl) Imide-Based Electrolytes with Wide Electrochemical Windows for Rechargeable Magnesium Batteries. ACS Appl. Mater. Inter. 2014, 6, 4063–4073. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Gil Bardaji, M.E.; Fuhr, O.; Fichtner, M. A new class of non-corrosive, highly efficient electrolytes for rechargeable magnesium batteries. J. Mater. Chem. A 2017, 5, 10815–10820. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Performance Enhancement and Mechanistic Studies of Magnesium-Sulfur Cells with an Advanced Cathode Structure. ACS Energy Lett. 2016, 1, 431–437. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Hou, Y. MXenes: Synthesis strategies and lithium-sulfur battery applications. eScience 2022, 2, 164–182. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, D.; Zhu, W.; Sun, F.; Zou, J.; Laine, R.M.; Ding, W. Rational design of high concentration electrolytes and MXene-based sulfur host materials toward high-performance magnesium sulfur batteries. Chem. Eng. J. 2022, 428, 131031. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, R.; Zhang, Y.; Huang, G.; Jiang, B.; Xu, C.; Pan, F. The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries. J. Magnes. Alloy. 2021, 9, 78–89. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Y.; Wang, B.; Wang, M.; Zhang, Y.; Wang, Q.; Wu, H. Construction of Electrocatalytic and Heat-Resistant Self-Supporting Electrodes for High-Performance Lithium-Sulfur Batteries. Nano-Micro Lett. 2019, 11, 1–17. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Wu, H.B.; Lou, X.W.D. Metal-Organic Framework-Assisted Synthesis of Compact Fe2O3 Nanotubes in Co3O4 Host with Enhanced Lithium Storage Properties. Nano-Micro Lett. 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Choi, J.; Na, S.; Yoo, D.-J.; Kim, J.H.; Cho, B.W.; Kim, Y.-T.; Yim, T.; Choi, J.W.; Oh, S.H. Critical role of elemental copper for enhancing conversion kinetics of sulphur cathodes in rechargeable magnesium batteries. Appl. Surf. Sci. 2019, 484, 933–940. [Google Scholar] [CrossRef]
- Sun, J.; Deng, C.; Bi, Y.; Wu, K.-H.; Zhu, S.; Xie, Z.; Li, C.; Amal, R.; Luo, J.; Liu, T.; et al. In Situ Sulfurized Carbon-Confined Cobalt for Long-Life Mg/S Batteries. ACS Appl. Energy Mater. 2020, 3, 2516–2525. [Google Scholar] [CrossRef]
- Shimokawa, K.; Furuhashi, T.; Kawaguchi, T.; Park, W.-Y.; Wada, T.; Matsumoto, H.; Kato, H.; Ichitsubo, T. Electrochemically synthesized liquid-sulfur/sulfide composite materials for high-rate magnesium battery cathodes. J. Mater. Chem. A 2021, 9, 16585–16593. [Google Scholar] [CrossRef]
- Robba, A.; Meznar, M.; Vizintin, A.; Bitenc, J.; Bobnar, J.; Arcon, I.; Randon-Vitanova, A.; Dominko, R. Role of Cu current collector on electrochemical mechanism of Mg-S battery. J. Power Sources 2020, 450, 227672. [Google Scholar] [CrossRef]
- He, P.; Ford, H.O.; Merrill, L.C.; Schaefer, J.L. Investigation of the Effects of Copper Nanoparticles on Magnesium-Sulfur Battery Performance: How Practical Is Metallic Copper Addition? ACS Appl. Energy Mater. 2019, 2, 6800–6807. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, B.; Xu, H.; Cui, Z.; Dong, S.; Du, A.; Ma, J.; Wang, Q.; Zhou, X.; Cui, G. Self-Established Rapid Magnesiation/De-Magnesiation Pathways in Binary Selenium-Copper Mixtures with Significantly Enhanced Mg-Ion Storage Reversibility. Adv. Funct. Mater. 2018, 28, 1701718. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, Z.; Zhang, Z.; Du, A.; Dong, S.; Li, Z.; Li, G.; Cui, G. Current Design Strategies for Rechargeable Magnesium-Based Batteries. ACS Nano 2021, 15, 15594–15624. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Z.; Kong, Q.; Zhang, Q.; Wang, T.; Dong, S.; Gu, L.; Wang, X.; Ma, J.; Han, P.; et al. Highly Reversible Cuprous Mediated Cathode Chemistry for Magnesium Batteries. Angew. Chem. 2020, 132, 11574–11579. [Google Scholar] [CrossRef]
- Thiele, P.; Neumann, J.; Westphal, A.; Ludwig, R.; Bonsa, A.-M.; Appelhagen, A.; Malcher, P.; Koeckerling, M. Electrical Energy Storage by a Magnesium-Copper-Sulfide Rechargeable Battery. J. Electrochem. Soc. 2017, 164, A770–A774. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, N.; Yang, J.; Wang, J.; Nuli, Y. Application of a Sulfur Cathode in Nucleophilic Electrolytes for Magnesium/Sulfur Batteries. J. Electrochem. Soc. 2017, 164, A2504–A2512. [Google Scholar] [CrossRef]
- Xiao, R.; Yu, T.; Yang, S.; Chen, K.; Li, Z.; Liu, Z.; Hu, T.; Hu, G.; Li, J.; Cheng, H.-M.; et al. Electronic structure adjustment of lithium sulfide by a single-atom copper catalyst toward high-rate lithium-sulfur batteries. Energy Storage Mater. 2022, 51, 890–899. [Google Scholar] [CrossRef]
- He, D.; Xue, P.; Song, D.; Qu, J.; Lai, C. Tri-Functional Copper Sulfide as Sulfur Carrier for High-Performance Lithium-Sulfur Batteries. J. Electrochem. Soc. 2017, 164, A1499–A1502. [Google Scholar] [CrossRef]
- Yang, D.; Li, M.; Zheng, X.; Han, X.; Zhang, C.; Jacas Biendicho, J.; Llorca, J.; Wang, J.; Hao, H.; Li, J.; et al. Phase Engineering of Defective Copper Selenide toward Robust Lithium-Sulfur Batteries. ACS Nano 2022, 16, 11102–11114. [Google Scholar] [CrossRef] [PubMed]
- Itaoka, K.; Kim, I.-T.; Yamabuki, K.; Yoshimoto, N.; Tsutsumi, H. Room temperature rechargeable magnesium batteries with sulfur-containing composite cathodes prepared from elemental sulfur and bis(alkenyl) compound having a cyclic or linear ether unit. J. Power Sources 2015, 297, 323–328. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Z.; He, J.; Cui, Z.; Chai, J.; Ma, J.; Yang, Z.; Huang, C.; Cui, G. A Delicately Designed Sulfide Graphdiyne Compatible Cathode for High-Performance Lithium/Magnesium-Sulfur Batteries. Small 2017, 13, 1702277. [Google Scholar] [CrossRef]
- Zheng, N.; Jiang, G.; Chen, X.; Mao, J.; Jiang, N.; Li, Y. Battery Separators Functionalized with Edge-Rich MoS2/C Hollow Microspheres for the Uniform Deposition of Li2S in High-Performance Lithium-Sulfur Batteries. Nano-Micro Lett. 2019, 11, 43. [Google Scholar] [CrossRef]
- Hencz, L.; Chen, H.; Ling, H.Y.; Wang, Y.; Lai, C.; Zhao, H.; Zhang, S. Housing Sulfur in Polymer Composite Frameworks for Li-S Batteries. Nano-Micro Lett. 2019, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-Z.; Nuli, Y.-N.; Yang, J. Conductive sulfur-containing material/polyaniline composite for cathode material of rechargeable magnesium batteries. Acta Phys. -Chim. Sin. 2007, 23, 327–331. [Google Scholar] [CrossRef]
- NuLi, Y.; Guo, Z.; Liu, H.; Yang, J. A new class of cathode materials for rechargeable magnesium batteries: Organosulfur compounds based on sulfur-sulfur bonds. Electrochem. Commun. 2007, 9, 1913–1917. [Google Scholar] [CrossRef]
- Wang, P.; Kappler, J.; Sievert, B.; Haecker, J.; Kuester, K.; Starke, U.; Ziegler, F.; Buchmeiser, M.R. Characteristics of magnesium-sulfur batteries based on a sulfurized poly(acrylonitrile) composite and a fluorinated electrolyte. Electrochim. Acta 2020, 361, 137024. [Google Scholar] [CrossRef]
- Wu, D.; Ren, W.; Yang, Y.; Wang, J.; NuLi, Y. A Se-Doped S@CMK3 Composite as a High-Performance Cathode for Magnesium-Sulfur Batteries with Mg2+/Li+ Hybrid Electrolytes. J. Phys. Chem. C 2021, 125, 25959–25967. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.-Y.; Chen, X.; Li, B.-Q.; Kaskel, S.; Zhang, Q.; Huang, J.-Q. Promoting the sulfur redox kinetics by mixed organodiselenides in high-energy-density lithium-sulfur batteries. eScience 2021, 1, 44–52. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Lin, X.-M.; Minella, C.B.; Wang, D.; Diemant, T.; Behm, R.J.; Fichtner, M. Selenium and selenium-sulfur cathode materials for high-energy rechargeable magnesium batteries. J. Power Sources 2016, 323, 213–219. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, B.; Fang, T.; Li, Y.; Zhou, Z.; Wang, Q.; Zhang, J.; Zhao, Y. A Multifunctional Separator Enables Safe and Durable Lithium/Magnesium-Sulfur Batteries under Elevated Temperature. Adv. Energy Mater. 2020, 10, 1902023. [Google Scholar] [CrossRef]
- Bosubabu, D.; Li, Z.; Meng, Z.; Wang, L.-P.; Fichtner, M.; Zhao-Karger, Z. Mitigating self-discharge and improving the performance of Mg-S battery in Mg[B(hfip)4]2 electrolyte with a protective interlayer. J. Mater. Chem. A 2021, 9, 25150–25159. [Google Scholar] [CrossRef]
- Wang, P.; Buchmeiser, M.R. Rechargeable Magnesium-Sulfur Battery Technology: State of the Art and Key Challenges. Adv. Funct. Mater. 2019, 29, 1905248. [Google Scholar] [CrossRef]
- Bucur, C.B. Challenges of a Rechargeable Magnesium Battery: A Guide to the Viability of this Post Lithium-Ion Battery; Springer International Publishing: Cham, Switzerland, 2018; pp. 11–38. [Google Scholar]
- Connell, J.G.; Genorio, B.; Lopes, P.P.; Strmcnik, D.; Stamenkovic, V.R.; Markovic, N.M. Tuning the Reversibility of Mg Anodes via Controlled Surface Passivation by H2O/Cl– in Organic Electrolytes. Chem. Mater. 2016, 28, 8268–8277. [Google Scholar] [CrossRef]
- Cheng, Y.; Stolley, R.M.; Han, K.S.; Shao, Y.; Arey, B.W.; Washton, N.M.; Mueller, K.T.; Helm, M.L.; Sprenkle, V.L.; Liu, J.; et al. Highly active electrolytes for rechargeable Mg batteries based on a [Mg2(mu-Cl)2](2+) cation complex in dimethoxyethane. Phys. Chem. Chem. Phys. 2015, 17, 13307–13314. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Oliver, A.G.; Zajicek, J.; Allred, G.D.; Boggess, W.C. Corrosion of magnesium electrolytes: Chlorides—The culprit. Energy Environ. Sci. 2013, 6, 482–487. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Z.; Qiao, L.; Guan, J.; Xu, H.; Wang, X.; Hu, P.; Du, H.; Li, S.; Zhou, X.; et al. Novel Design Concepts of Efficient Mg-Ion Electrolytes toward High-Performance Magnesium-Selenium and Magnesium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1602055. [Google Scholar] [CrossRef]
- Mohtadi, R.; Matsui, M.; Arthur, T.S.; Hwang, S.J. Magnesium borohydride: From hydrogen storage to magnesium battery. Angew. Chem. Int. Ed. Engl. 2012, 51, 9780–9783. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Z.; Cui, Z.; Du, A.; Lu, C.; Dong, S.; Ma, J.; Zhou, X.; Cui, G. Strong anion receptor-assisted boron-based Mg electrolyte with wide electrochemical window and non-nucleophilic characteristic. Electrochem. Commun. 2017, 83, 72–76. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; NuLi, Y.; Li, D.; Wang, Y.; Xiang, X. A new class of electrolytes based on magnesium bis(diisopropyl)amide for magnesium-sulfur batteries. Chem. Commun. 2019, 55, 6086–6089. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zheng, Z.; Zhao, L.; Li, W.; Wang, J.; Dai, M.; Zhao, Y.; Xiao, J.; Wang, G.; Ding, X.; et al. Extending Cycle Life of Mg/S Battery by Activation of Mg Anode/Electrolyte Interface through an LiCl-Assisted MgCl2 Solubilization Mechanism. Adv. Funct. Mater. 2020, 30, 1909370. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, G.; Zhao, S.; Li, W.; Shi, F.; Li, J.; Feng, J.; Zhao, Y.; Wu, Y.; Guo, J.; et al. Improving a Mg/S Battery with YCl3 Additive and Magnesium Polysulfide. Adv. Sci. 2019, 6, 1800981. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Hu, A.; Zhou, M.; Chen, W.; Lei, T.; Yan, Y.; Huang, J.; Yang, C.; Wang, X.; et al. In Situ/Operando Raman Techniques in Lithium–Sulfur Batteries. Small Struct. 2022, 3, 2100170. [Google Scholar] [CrossRef]
- Cuan, J.; Zhou, Y.; Zhou, T.; Ling, S.; Rui, K.; Guo, Z.; Liu, H.; Yu, X. Borohydride-Scaffolded Li/Na/Mg Fast Ionic Conductors for Promising Solid-State Electrolytes. Adv. Mater. 2019, 31, 2100170. [Google Scholar] [CrossRef]
- Gao, T.; Hou, S.; Khue, H.; Wang, F.; Eidson, N.; Fan, X.; Han, F.; Luo, C.; Mao, M.; Li, X.; et al. Existence of Solid Electrolyte Interphase in Mg Batteries: Mg/S Chemistry as an Example. ACS Appl. Mater. Inter. 2018, 10, 14767–14776. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.Y.; Zhan, Y.; Sun, X.Y.; Li, Z.; Xu, H.; Laine, R.M.; Zou, J.X. Advances in Cathodes for High-Performance Magnesium-Sulfur Batteries: A Critical Review. Batteries 2023, 9, 203. https://doi.org/10.3390/batteries9040203
Yao YY, Zhan Y, Sun XY, Li Z, Xu H, Laine RM, Zou JX. Advances in Cathodes for High-Performance Magnesium-Sulfur Batteries: A Critical Review. Batteries. 2023; 9(4):203. https://doi.org/10.3390/batteries9040203
Chicago/Turabian StyleYao, Ying Ying, Yang Zhan, Xin Yu Sun, Zhao Li, Hao Xu, Richard M. Laine, and Jian Xin Zou. 2023. "Advances in Cathodes for High-Performance Magnesium-Sulfur Batteries: A Critical Review" Batteries 9, no. 4: 203. https://doi.org/10.3390/batteries9040203
APA StyleYao, Y. Y., Zhan, Y., Sun, X. Y., Li, Z., Xu, H., Laine, R. M., & Zou, J. X. (2023). Advances in Cathodes for High-Performance Magnesium-Sulfur Batteries: A Critical Review. Batteries, 9(4), 203. https://doi.org/10.3390/batteries9040203