Urea-Based Deep Eutectic Solvent with Magnesium/Lithium Dual Ions as an Aqueous Electrolyte for High-Performance Battery-Supercapacitor Hybrid Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of DES Electrolyte
2.2. Physicochemical Characterizations of Electrolytes
2.3. Material and Electrochemical Characterizations of PTCDI/rGO Composite
3. Results and Discussion
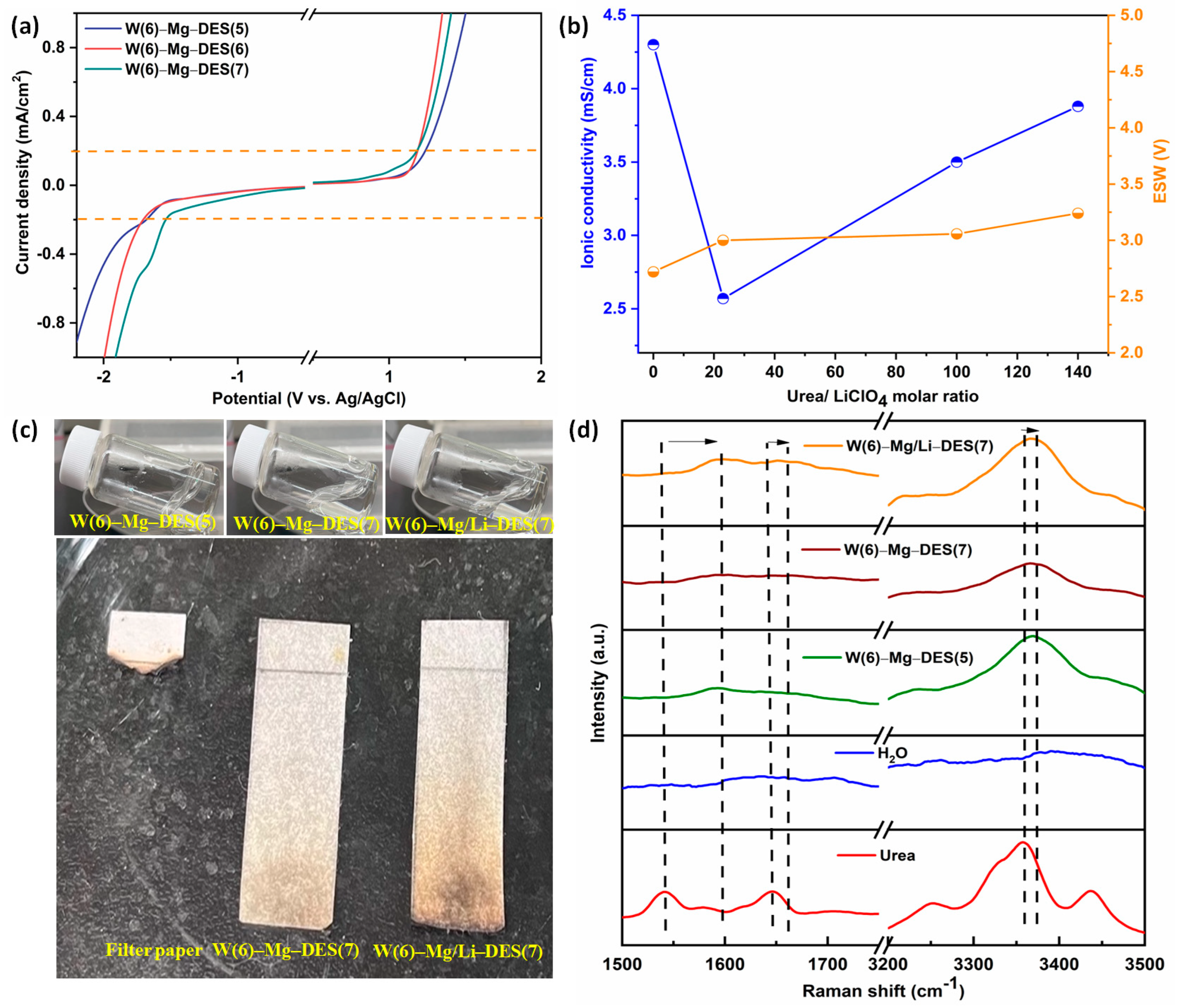
3.1. Characterization of Electrolytes
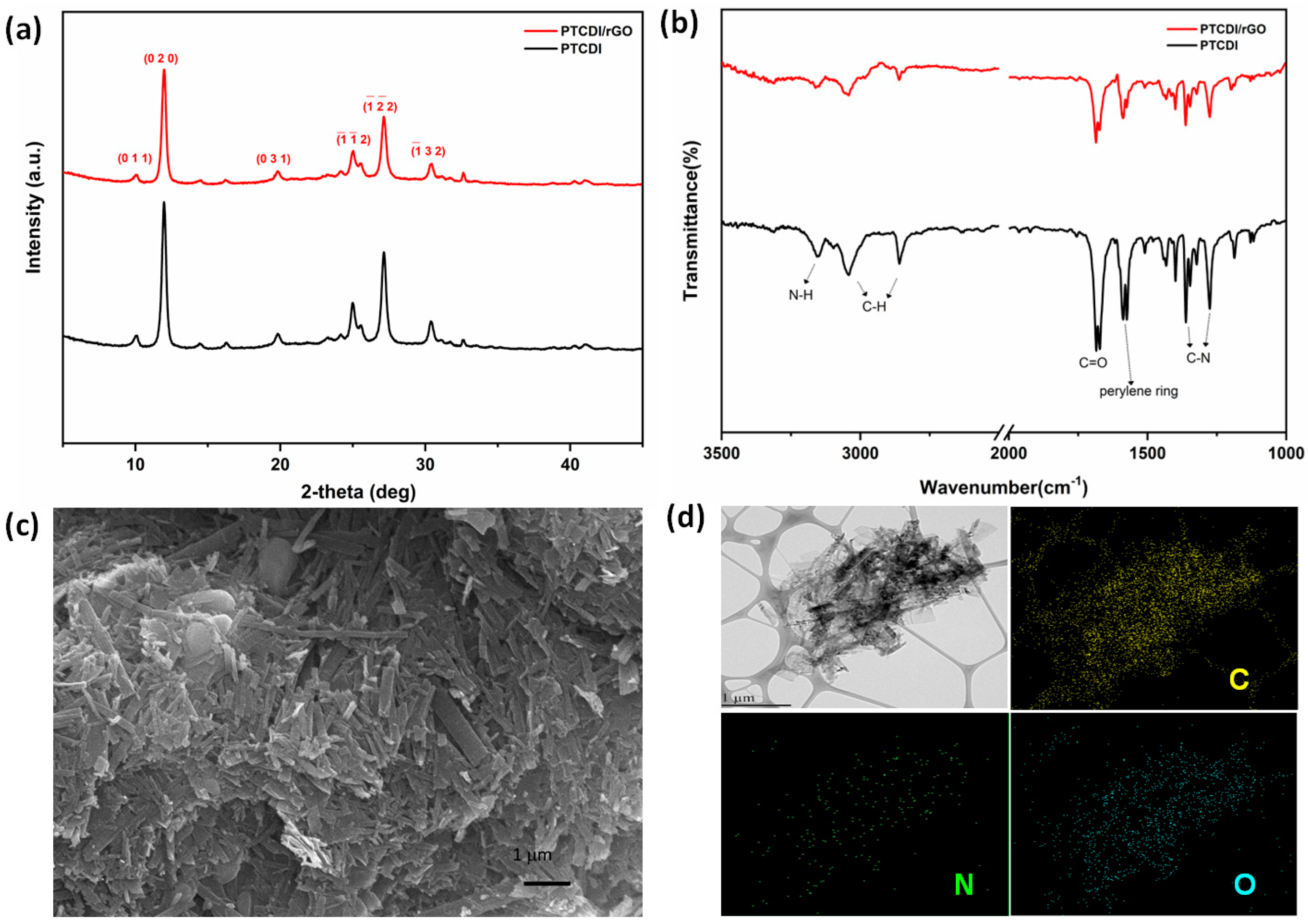
3.2. Material Characterization of the Composite PTCDI/rGO and Pure PTCDI
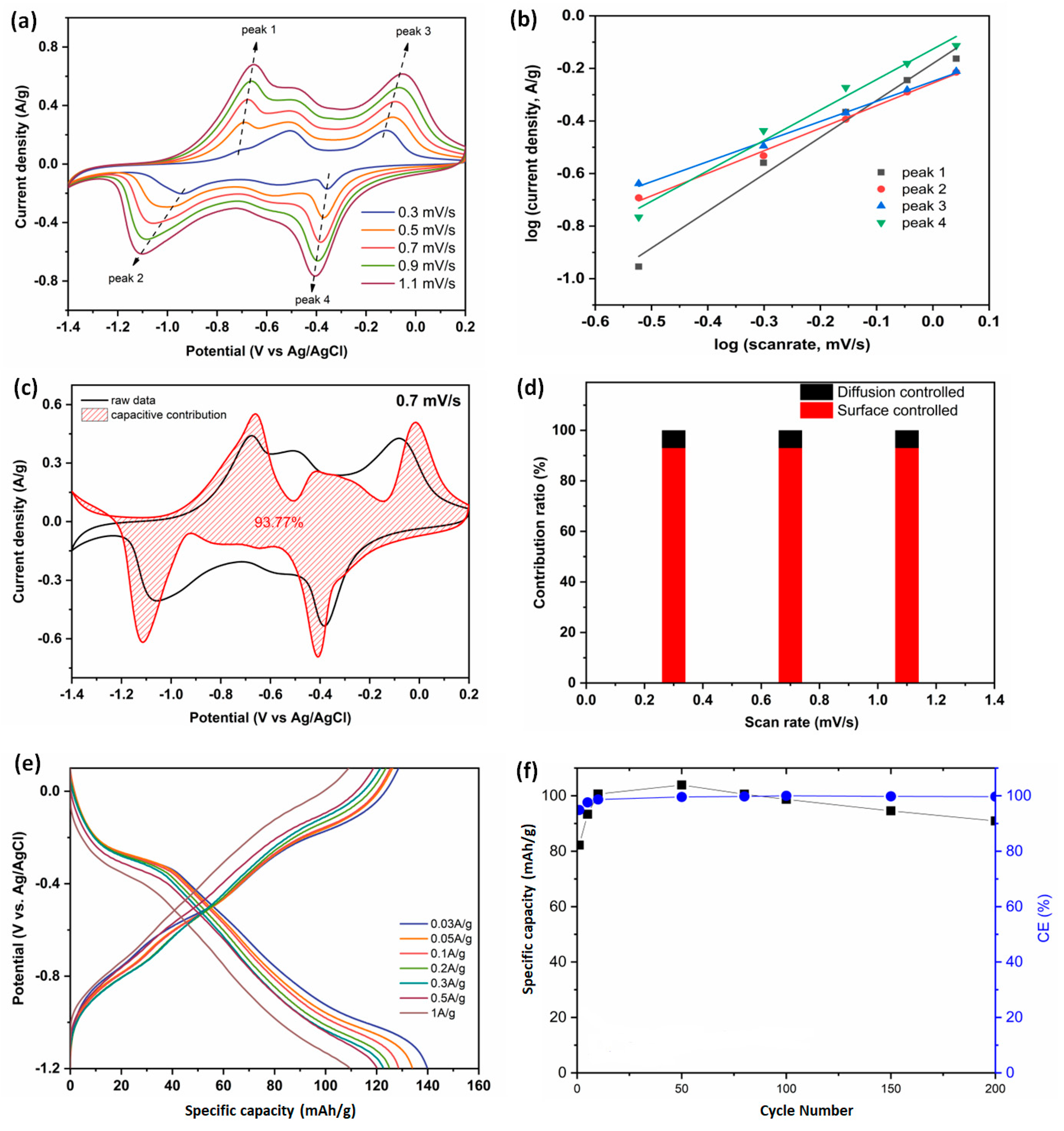
3.3. Electrochemical Performances of the PTCDI/rGO Anode and LMO Cathode in the Electrolyte of W(6)-Mg/Li-DES(7)
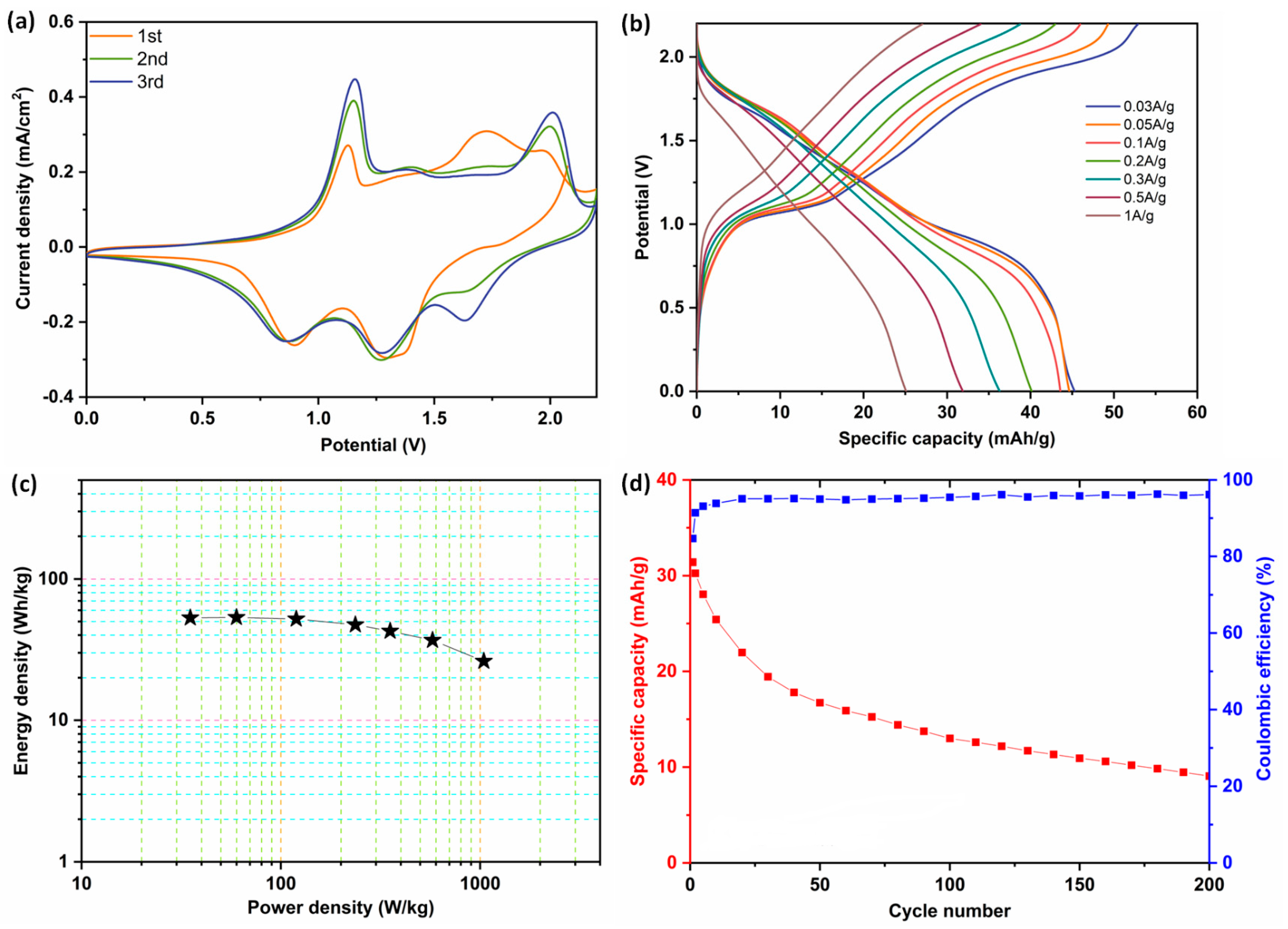
3.4. Two Electrode Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable Lithium Batteries with Aqueous Electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Z.; Chen, B.; Dong, S.; Wang, C.; Du, A.; Wang, L.; Ma, J.; Cui, G. A superior electronic conducting tellurium electrode enabled high-rate capability rechargeable Mg batteries. Mater. Today Energy 2020, 17, 100450. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation-approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Xia, Y. Recent progress in aqueous lithium-ion batteries. Adv. Energy Mater. 2012, 2, 830–840. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Fervent Hype behind Magnesium Batteries: An Open Call to Synthetic Chemists-Electrolytes and Cathodes Needed. Angew. Chem. Int. Ed. 2017, 56, 12064–12084. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Z.; Qiao, L.; Guan, J.; Xu, H.; Wang, X.; Hu, P.; Li, S.; Zhou, X.; Dong, S.; et al. Novel Design Concepts of Efficient Mg-Ion Electrolytes toward High-Performance Magnesium-Selenium and Magnesium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1602055. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Tang, Y. A novel zinc-ion hybrid supercapacitor for long-life and low-cost energy storage applications. Energy Storage Mater. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef]
- Ponrouch, A.; Frontera, C.; Bardé, F.; Palacín, M.R. Towards a calcium-based rechargeable battery. Nat. Mater. 2016, 15, 169–172. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.M.; Li, F. The Rechargeable Aluminum Battery: Opportunities and Challenges. Angew. Chem. Int. Ed. 2019, 58, 11978–11996. [Google Scholar] [CrossRef]
- Lin, M.C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.Y.; Guan, M.; Dai, H. An ultrafast rechargeable Aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef]
- Liang, J.; Li, F.; Cheng, H.M. Metal ion storage: A route to versatile energy storage. Energy Storage Mater. 2018, 11, A1–A3. [Google Scholar] [CrossRef]
- Ponrouch, A.; Bitenc, J.; Dominko, R.; Lindahl, N.; Johansson, P.; Palacin, M.R. Multivalent rechargeable batteries. Energy Storage Mater. 2019, 20, 253–262. [Google Scholar] [CrossRef]
- Yoo, H.D.; Liang, Y.; Dong, H.; Lin, J.; Wang, H.; Liu, Y.; Ru, Q.; Jing, Y.; An, Q.; Zhou, W.; et al. Fast kinetics of magnesium monochloride cations in interlayer-expanded titanium disulfide for magnesium rechargeable batteries. Nat. Commun. 2017, 8, 339. [Google Scholar] [CrossRef]
- Koketsu, T.; Giannini, M.; Ma, J.; Morgan, B.J.; Body, M.; Legein, C.; Dachraoui, W.; Demorterie, A.; Salanne, M.; Dardoize, F.; et al. Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2. Nat. Mater. 2017, 16, 1142–1148. [Google Scholar] [CrossRef]
- Bonnick, P.; Muldoon, J. A Trip to Oz and a Peak Behind the Curtain of Magnesium Batteries. Adv. Funct. Mater. 2020, 30, 1910510. [Google Scholar] [CrossRef]
- Deivanayagam, R.; Ingram, B.J.; Shahbazian-Yassar, R.S. Progress in development of electrolytes for magnesium batteries. Energy Storage Mater. 2019, 21, 136–153. [Google Scholar] [CrossRef]
- Jung, K.H.; Jeong, G.S.; Go, C.Y.; Kim, K.C. Conjugacy of organic cathode materials for high-potential lithium-ion batteries: Carbonitriles versus quinones. Energy Storage Mater. 2020, 24, 237–246. [Google Scholar] [CrossRef]
- Lu, D.; Liu, H.; Huang, T.; Xu, Z.; Ma, L.; Yang, P.; Qiang, P.; Zhang, F.; Wu, D. Magnesium ion based organic secondary batteries. J. Mater. Chem. A Mater. 2018, 6, 17297–17302. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, L.; Zhang, K.; Xiong, F.; Tan, S.; Li, M.; An, Q.; Kang, Y.M.; Mai, L. Salt-controlled dissolution in pigment cathode for high-capacity and long-life magnesium organic batteries. Nano Energy 2019, 65, 103902. [Google Scholar] [CrossRef]
- Hu, P.; Wang, H.; Yang, Y.; Yang, J.; Lin, J.; Guo, L. Renewable-Biomolecule-Based Full Lithium-Ion Batteries. Adv. Mater. 2016, 28, 3486–3492. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.; Jung, S.-K.; Ku, K.; Kwon, G.; Seong, W.M.; Kim, H.; Yoon, G.; Kang, I.; Hong, K.; et al. Charge-transfer complexes for high-power organic rechargeable batteries. Energy Storage Mater. 2019, 20, 462–469. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, I.A.; Yuan, Y.; Bommier, C.; Wang, X.; Ma, L.; Leonard, D.P.; Lerner, M.M.; Carter, R.G.; Wu, T.; Greaney, A.P.; et al. Mg-Ion Battery Electrode: An Organic Solid’s Herringbone Structure Squeezed upon Mg-Ion Insertion. J. Am. Chem. Soc. 2017, 139, 13031–13037. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, H. Towards sustainable and versatile energy storage devices: An overview of organic electrode materials. Energy Environ. Sci. 2013, 6, 2280. [Google Scholar] [CrossRef]
- Kumar, M.S.; Das, P.; Yasoda, K.Y.; Kothurkar, N.K.; Malik, S.; Batabyal, S.K. Fabrication of organic nanocomposite of polyaniline for enhanced electrochemical performance. J. Energy Storage 2020, 31, 101700. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.Q.; Li, B. Organic quinones towards advanced electrochemical energy storage: Recent advances and challenges. J. Mater. Chem. A Mater. 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Nevers, D.R.; Brushett, F.R.; Wheeler, D.R. Engineering radical polymer electrodes for electrochemical energy storage. J. Power Sources 2017, 352, 226–244. [Google Scholar] [CrossRef]
- Senoh, H.; Yao, M.; Sakaebe, H.; Yasuda, K.; Siroma, Z. A two-compartment cell for using soluble benzoquinone derivatives as active materials in lithium secondary batteries. Electrochim. Acta 2011, 56, 10145–10150. [Google Scholar] [CrossRef]
- Williams, D.L.; Byrne, J.; Driscoll, J.S. A High Energy Density Lithium/Dichloroisocyanuric Acid Battery System. J. Electrochem. Soc. 1969, 116, 2. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Huang, T.H.; Lin, T.W. Fabrication of 3D hierarchically structured carbon electrode for supercapacitors by carbonization of polyaniline/carbon nanotube/graphene composites. Inorg. Chim. Acta 2019, 489, 217–223. [Google Scholar] [CrossRef]
- Kumar, M.S.; Yasoda, K.Y.; Batabyal, S.K.; Kothurkar, N.K. Carbon-polyaniline nanocomposites as supercapacitor materials. Mater. Res. Express 2018, 5, 045505. [Google Scholar] [CrossRef]
- Lu, M.N.; Dai, C.S.; Tai, S.Y.; Lin, T.W.; Lin, J.Y. Hierarchical nickel sulfide/carbon nanotube nanocomposite as a catalytic material toward triiodine reduction in dye-sensitized solar cells. J. Power Sources 2014, 270, 499–505. [Google Scholar] [CrossRef]
- Yasoda, K.Y.; Kumar, S.; Kumar, M.S.; Ghosh, K.; Batabyal, S.K. Fabrication of MnS/GO/PANI nanocomposites on a highly conducting graphite electrode for supercapacitor application. Mater. Today Chem. 2021, 19, 100394. [Google Scholar] [CrossRef]
- Yamada, Y.; Wang, J.; Ko, S.; Watanabe, E.; Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 2019, 4, 269–280. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Sun, J.; Liu, Y.; Hou, L. Recent Progress in ‘Water-in-Salt’ Electrolytes Toward Non-lithium Based Rechargeable Batteries. Front. Chem. 2020, 8, 595. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kim, W. Water-in-Salt Electrolyte Enables Ultrafast Supercapacitors for AC Line Filtering. ACS Energy Lett. 2021, 6, 769–777. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, L.; Li, Z.; Dou, H.; Zhang, X. Design strategies and research progress for Water-in-Salt electrolytes. Energy Storage Mater. 2022, 44, 10–28. [Google Scholar] [CrossRef]
- Cao, J.; Su, E. Hydrophobic deep eutectic solvents: The new generation of green solvents for diversified and colorful applications in green chemistry. J. Clean. Prod. 2021, 314, 127965. [Google Scholar] [CrossRef]
- Cruz, H.; Jordão, N.; Branco, L.C. Deep eutectic solvents (DESs) as low-cost and green electrolytes for electrochromic devices. Green Chem. 2017, 19, 1653–1658. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, L.; Yu, G. Eutectic Electrolytes as a Promising Platform for Next-Generation Electrochemical Energy Storage. Acc Chem. Res. 2020, 18, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Lin, T.J.; Vedhanarayanan, B.; Tsai, C.C.; Chen, T.Y.; Lin, T.W. A 1.9-V all-organic battery-supercapacitor hybrid device with high-rate capability and wide temperature tolerance in a metal-free water-in-salt electrolyte. J. Colloid. Interface Sci. 2022, 612, 76–87. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Chen, X.B.; Birbilis, N.; Yang, H. Effect of water presence on choline chloride-2urea ionic liquid and coating platings from the hydrated ionic liquid. Sci. Rep. 2016, 6, 29225. [Google Scholar] [CrossRef]
- Bo, Z.; Zhou, M.; Zhou, S.; Song, Y.; Liu, Z.; Liao, H.; Yang, H.; Yan, J.; Cen, K.; Fan, X.; et al. Controlling Interfacial Structural Evolution in Aqueous Electrolyte via Anti-Electrolytic Zwitterionic Waterproofing. Adv. Funct. Mater. 2022, 32, 202207140. [Google Scholar] [CrossRef]
- Khan, M.; Khan, K.; Hemant, S.; Kumar, M.; Gupta, K. Detection of Urea Adulteration in Milk Using Near-Infrared Raman Spectroscopy. Food Anal. Methods 2014, 8, 93–102. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Huang, X.; Chen, L. Physical and electrochemical properties of new binary room-temperature molten salt electrolyte based on LiBETI and acetamide. Solid State Ion. 2004, 175, 277–280. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Li, H.; Huang, X.; Chen, L. Spectroscopic studies on the mechanism of liquid formation and ionic conductivity in the LiCF3SO3/acetamide complex system. Vib. Spectrosc. 2005, 37, 1–10. [Google Scholar] [CrossRef]
- Wu, X.; Qi, Y.; Hong, J.J.; Li, Z.; Hernandez, A.S.; Ji, X. Rocking-Chair Ammonium-Ion Battery: A Highly Reversible Aqueous Energy Storage System. Angew. Chem. 2017, 129, 13206–13210. [Google Scholar] [CrossRef]
- Lei, X.; Zheng, Y.; Zhang, F.; Wang, Y.; Tang, Y. Highly stable magnesium-ion-based dual-ion batteries based on insoluble small-molecule organic anode material. Energy Storage Mater. 2020, 30, 34–41. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, L.; Wang, Y.G.; Wang, C.; Xia, Y. Aqueous Lithium-Ion Batteries Using Polyimide-Activated Carbon Composites Anode and Spinel LiMn2O4 Cathode. ACS Sustain. Chem. Eng. 2017, 5, 1503–1508. [Google Scholar] [CrossRef]
- Gavilán-Arriazu, E.M.; Mercer, M.P.; Pinto, O.A.; Oviedo, O.A.; Barraco, D.E.; Hoster, H.E.; Leiva, E.P.M. Numerical simulations of cyclic voltammetry for lithium-ion intercalation in nanosized systems: Finiteness of diffusion versus electrode kinetics. J. Solid State Electrochem. 2020, 24, 3279–3287. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhao, G.; Zhu, L.; Sun, Y.; Besanbacher, F.; Yu, M. Rechargeable Mg-Ion Full Battery System with High Capacity and High Rate. ACS Appl. Mater. Interfaces 2021, 13, 40451–40459. [Google Scholar] [CrossRef]
- Wen, Y.; Ma, C.; Chen, H.; Zhang, H.; Li, M.; Zhao, P.; Qiu, J.; Ming, H.; Cao, G.; Tang, G. Stabilizing LiMn2O4 cathode in aqueous electrolyte with optimal concentration and components. Electrochim. Acta 2020, 362, 137079. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Wang, Y.; Xia, Y. Cycling Stability of Spinel LiMn2O4 with Different Particle Sizes in Aqueous Electrolyte. Electrochim. Acta 2015, 173, 178–183. [Google Scholar] [CrossRef]
- Wang, F.; Fan, X.; Gao, T.; Sun, W.; Ma, Z.; Yang, C.; Han, F.; Xu, K.; Wang, C. High-Voltage Aqueous Magnesium Ion Batteries. ACS Cent. Sci. 2017, 3, 1121–1128. [Google Scholar] [CrossRef]
- Chen, L.; Bao, J.L.; Dong, X.; Truhlar, D.G.; Wang, Y.; Wang, C.; Xia, Y. Aqueous Mg-ion battery based on polyimide anode and prussian blue cathode. ACS Energy Lett. 2017, 2, 1115–1121. [Google Scholar] [CrossRef]
- Mizuno, Y.; Okubo, M.; Hosona, E.; Kudo, T.; Oh-Ishi, K.; Okazawa, A.; Kojima, N.; Kurona, R.; Nishimura, N.I.; Yamada, A. Electrochemical Mg2+ intercalation into a bimetallic CuFe Prussian blue analog in aqueous electrolytes. J. Mater. Chem. A Mater. 2013, 1, 13055–13059. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, K.; Zhu, K.; Cang, R.; Yan, J.; Cheng, K.; Wang, G.; Cao, D. Aqueous Magnesium-Ion Battery Based on a Carbon-Coated FeVO4 Anode and a Mg-OMS-1 Cathode. Chem.—A Eur. J. 2017, 23, 17118–17126. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.D.; Masese, T.; Orikasa, Y.; Mori, T.; Minato, T.; Tassel, C.; Kobayashi, Y.; Kageyama, H.; Uchimoto, Y. MgFePO4F as a feasible cathode material for magnesium batteries. J. Mater. Chem. A Mater. 2014, 2, 11578–11582. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, K.; Zhu, K.; Cang, R.; Wang, X.; Wang, G.; Cao, D. Assembly of Aqueous Rechargeable Magnesium Ions Battery Capacitor: The Nanowire Mg-OMS-2/Graphene as Cathode and Activated Carbon as Anode. ACS Sustain. Chem. Eng. 2017, 5, 6727–6735. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-Y.; Kumar, M.S.; Vedhanarayanan, B.; Shen, H.-H.; Lin, T.-W. Urea-Based Deep Eutectic Solvent with Magnesium/Lithium Dual Ions as an Aqueous Electrolyte for High-Performance Battery-Supercapacitor Hybrid Devices. Batteries 2023, 9, 69. https://doi.org/10.3390/batteries9020069
Tsai H-Y, Kumar MS, Vedhanarayanan B, Shen H-H, Lin T-W. Urea-Based Deep Eutectic Solvent with Magnesium/Lithium Dual Ions as an Aqueous Electrolyte for High-Performance Battery-Supercapacitor Hybrid Devices. Batteries. 2023; 9(2):69. https://doi.org/10.3390/batteries9020069
Chicago/Turabian StyleTsai, Hsin-Yen, Munusamy Sathish Kumar, Balaraman Vedhanarayanan, Hsin-Hui Shen, and Tsung-Wu Lin. 2023. "Urea-Based Deep Eutectic Solvent with Magnesium/Lithium Dual Ions as an Aqueous Electrolyte for High-Performance Battery-Supercapacitor Hybrid Devices" Batteries 9, no. 2: 69. https://doi.org/10.3390/batteries9020069
APA StyleTsai, H.-Y., Kumar, M. S., Vedhanarayanan, B., Shen, H.-H., & Lin, T.-W. (2023). Urea-Based Deep Eutectic Solvent with Magnesium/Lithium Dual Ions as an Aqueous Electrolyte for High-Performance Battery-Supercapacitor Hybrid Devices. Batteries, 9(2), 69. https://doi.org/10.3390/batteries9020069







