Effect of Si Content on Extreme Fast Charging Behavior in Silicon–Graphite Composite Anodes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
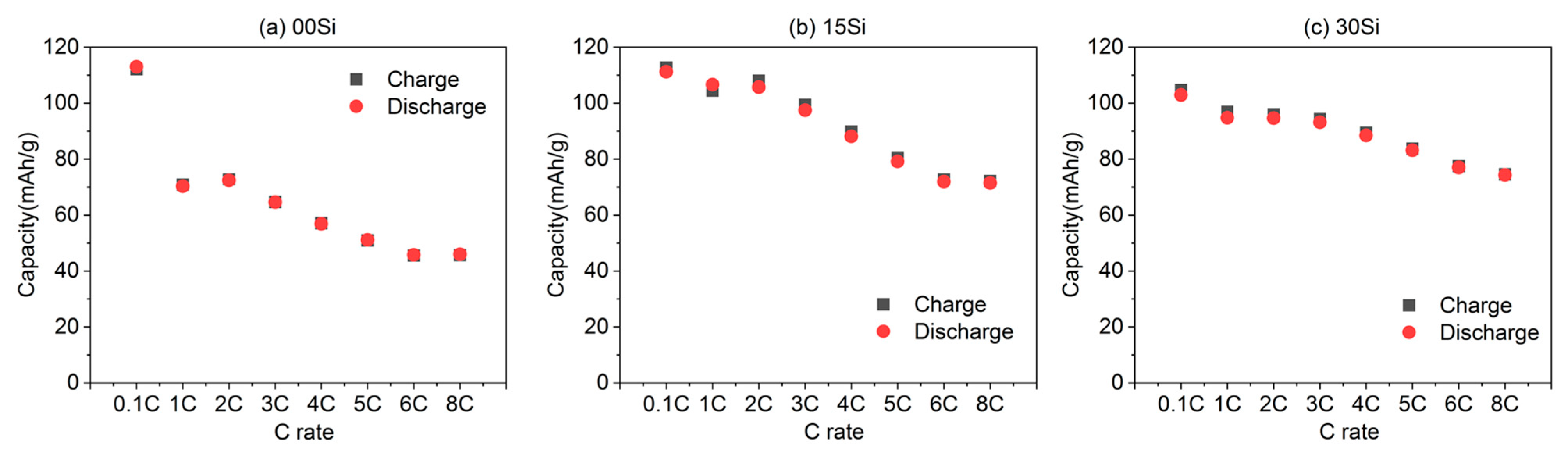
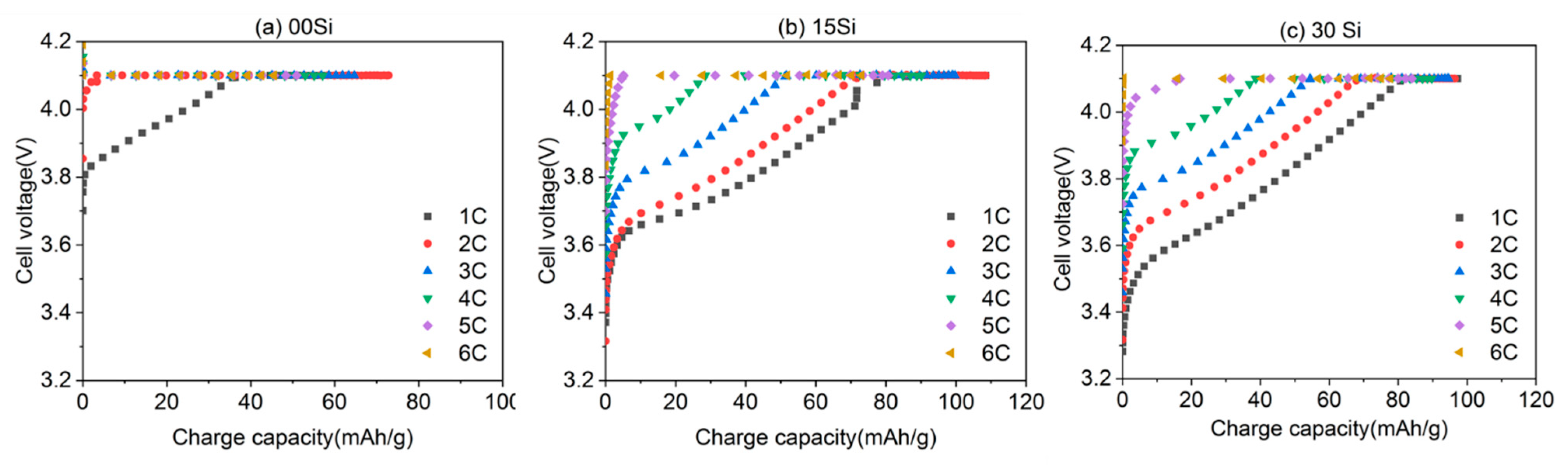
3.1. Electrochemical Performance of Initial Rate Capability Characterization
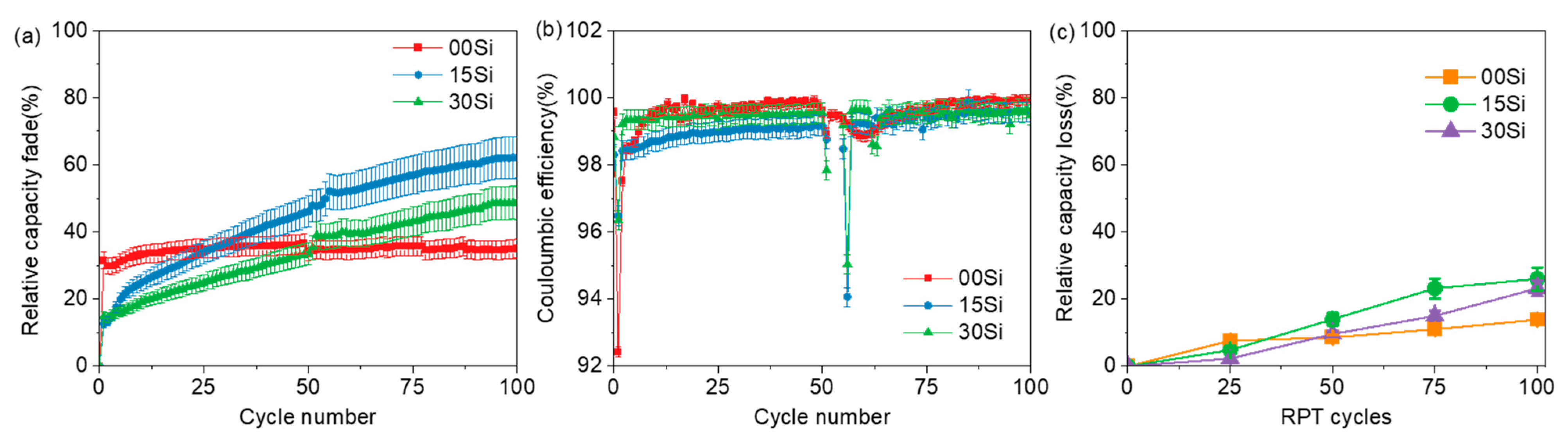
3.2. Electrochemical Performance of Long-Term XFC Aging
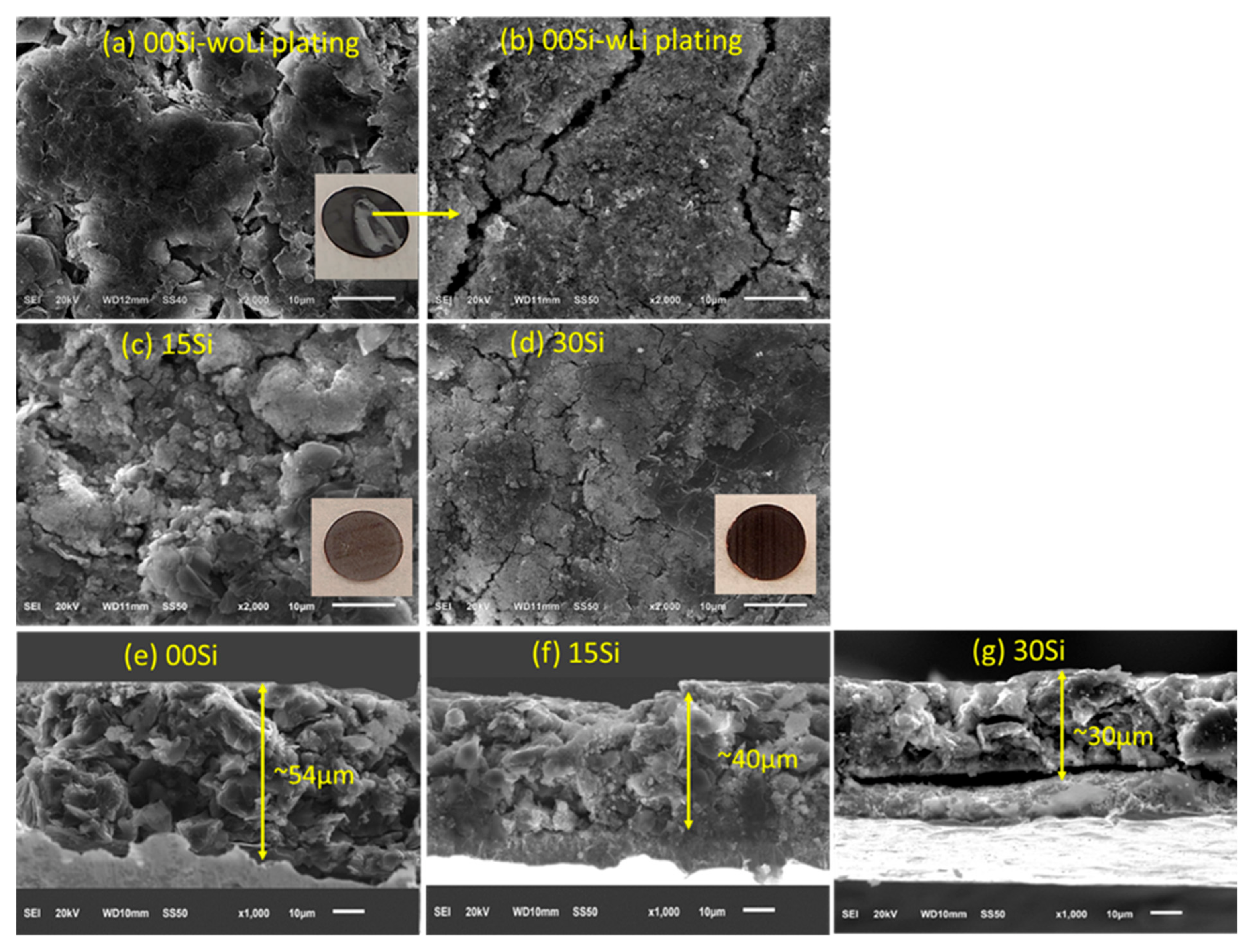
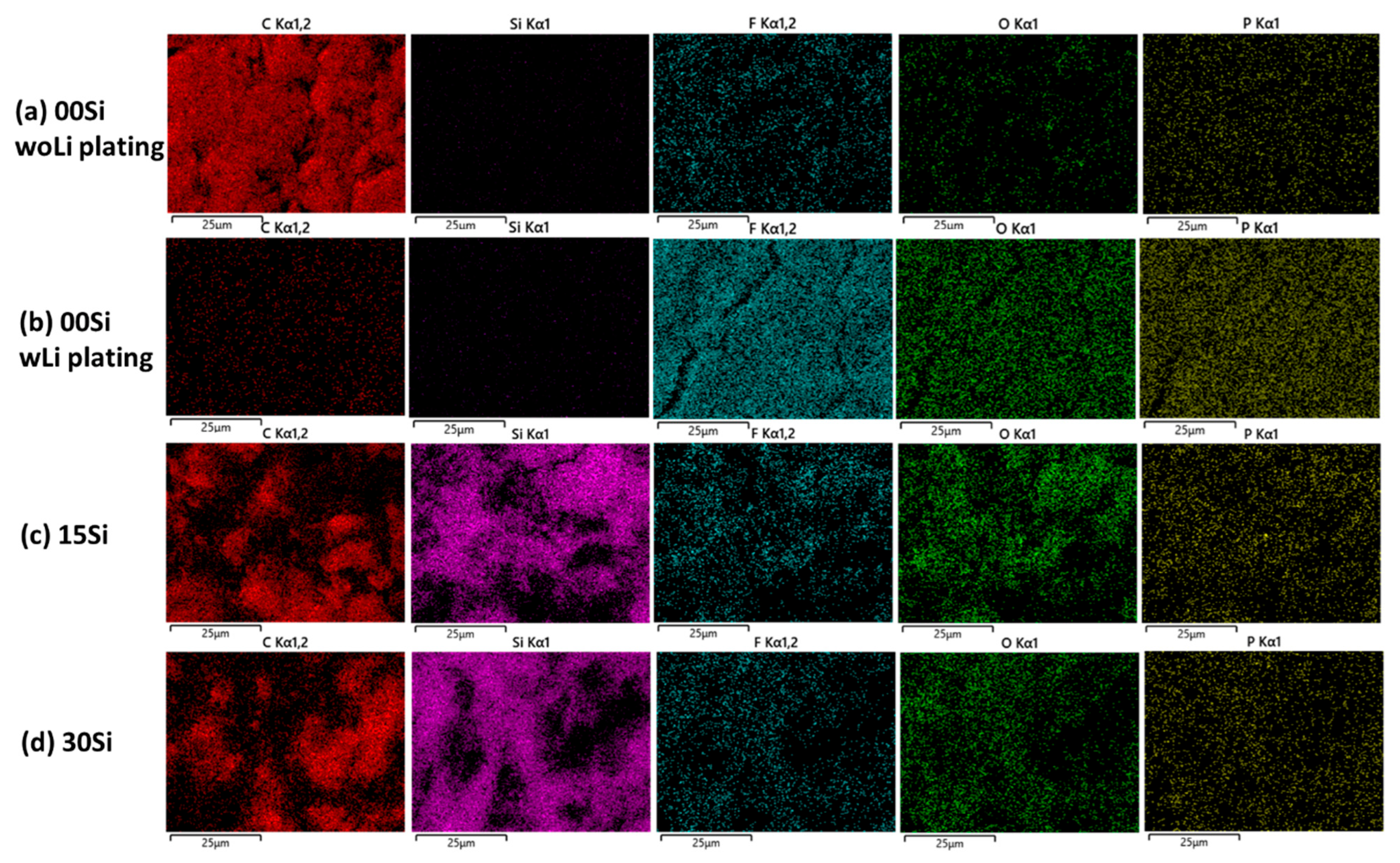
3.3. Morphology and Composition of Aged Anodes
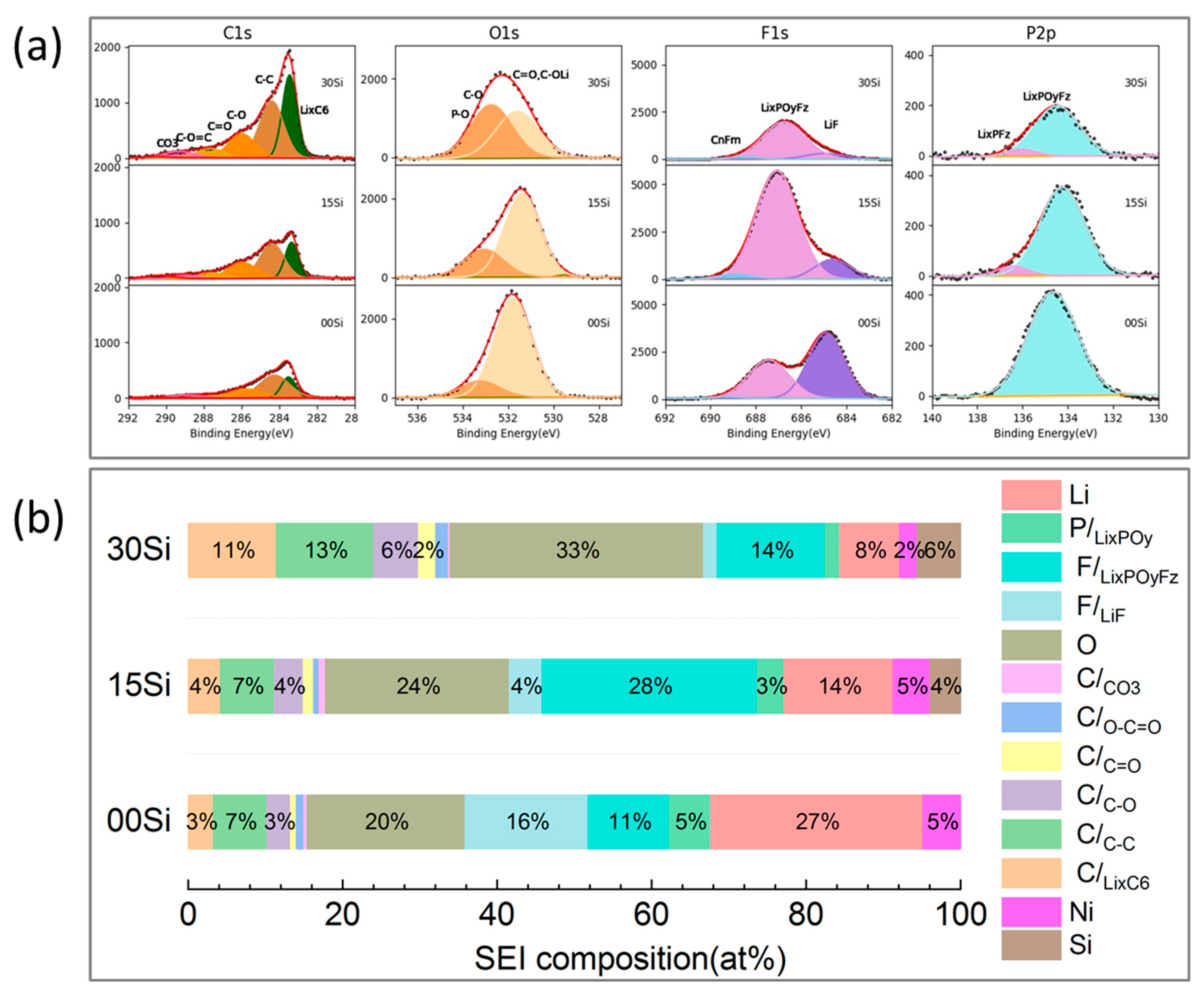
3.4. SEI Composition of the Aged Anodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paul, P.P.; Thampy, V.; Cao, C.; Steinrück, H.-G.; Tanim, T.R.; Dunlop, A.R.; Dufek, E.J.; Trask, S.E.; Jansen, A.N.; Toney, M.F.; et al. Quantification of heterogeneous, irreversible lithium plating in extreme fast charging of lithium-ion batteries. Energy Environ. Sci. 2021, 14, 4979–4988. [Google Scholar] [CrossRef]
- Yang, Z.; Kim, M.; Tsai, Y.; Zapol, P.; Trask, S.E.; Bloom, I. Extreme Fast Charging: Effect of Positive Electrode Material on Crosstalk. J. Electrochem. Soc. 2022, 169, 110505. [Google Scholar] [CrossRef]
- Yang, Z.; Charalambous, H.; Trask, S.E.; Montoya, A.; Jansen, A.; Wiaderek, K.M.; Bloom, I. Extreme fast charge aging: Effect of electrode loading and NMC composition on inhomogeneous degradation in graphite bulk and electrode/electrolyte interface. J. Power Sources 2022, 549, 232119. [Google Scholar] [CrossRef]
- Mao, C.; Ruther, R.E.; Li, J.; Du, Z.; Belharouak, I. Identifying the limiting electrode in lithium ion batteries for extreme fast charging. Electrochem. Commun. 2018, 97, 37–41. [Google Scholar] [CrossRef]
- Yang, Z.; Trask, S.E.; Gilbert, J.A.; Li, X.; Tsai, Y.; Jansen, A.N.; Ingram, B.J.; Bloom, I. Exploring the Promise of Multifunctional “Zintl-Phase-Forming” Electrolytes for Si-Based Full Cells. ACS Appl. Mater. Interfaces 2022, 14, 53860–53871. [Google Scholar] [CrossRef]
- Kim, N.; Chae, S.; Ma, J.; Ko, M.; Cho, J. Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nat. Commun. 2017, 8, 812. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting Issues of the Practical Implementation of Si Anode in High-Energy Lithium-Ion Batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nature Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Key, B.; Trask, S.E.; Yang, Z.; Lu, W. Silicon Nanoparticles: Stability in Aqueous Slurries and the Optimization of the Oxide Layer Thickness for Optimal Electrochemical Performance. ACS Appl. Mater. Interfaces 2017, 9, 32727–32736. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Andersen, H.F.; Foss, C.E.L.; Voje, J.; Tronstad, R.; Mokkelbost, T.; Vullum, P.E.; Ulvestad, A.; Kirkengen, M.; Mæhlen, J.P. Silicon-Carbon composite anodes from industrial battery grade silicon. Sci. Rep. 2019, 9, 14814. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, X.; Tian, Z.; Yang, S.; Cao, G. A high-performance silicon/carbon composite as anode material for lithium ion batteries. Nano Express 2021, 2, 010021. [Google Scholar] [CrossRef]
- Landa-Medrano, I.; Eguia-Barrio, A.; Sananes-Israel, S.; Porcher, W.; Trad, K.; Moretti, A.; Carvalho, D.V.; Passerini, S.; de Meatza, I. Insights into the Electrochemical Performance of 1.8 Ah Pouch and 18650 Cylindrical NMC:LFP|Si:C Blend Li-ion Cells. Batteries 2022, 8, 97. [Google Scholar] [CrossRef]
- Ruther, R.E.; Hays, K.A.; An, S.J.; Li, J.; Wood, D.L.; Nanda, J. Chemical Evolution in Silicon–Graphite Composite Anodes Investigated by Vibrational Spectroscopy. ACS Appl. Mater. Interfaces 2018, 10, 18641–18649. [Google Scholar] [CrossRef]
- Kirkaldy, N.; Samieian, M.A.; Offer, G.J.; Marinescu, M.; Patel, Y. Lithium-Ion Battery Degradation: Measuring Rapid Loss of Active Silicon in Silicon–Graphite Composite Electrodes. ACS Appl. Energy Mater. 2022, 5, 13367–13376. [Google Scholar] [CrossRef]
- Lee, B.S.; Oh, S.-H.; Choi, Y.J.; Yi, M.-J.; Kim, S.H.; Kim, S.-Y.; Sung, Y.-E.; Shin, S.Y.; Lee, Y.; Yu, S.-H. SiO-induced thermal instability and interplay between graphite and SiO in graphite/SiO composite anode. Nat. Commun. 2023, 14, 150. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, D.; Seifert, H.J.; Pfleging, W. Investigation of Fast-Charging and Degradation Processes in 3D Silicon-Graphite Anodes. Nanomaterials 2021, 12, 140. [Google Scholar] [CrossRef]
- Wang, S.; Ren, P.; Takyi-Aninakwa, P.; Jin, S.; Fernandez, C. A Critical Review of Improved Deep Convolutional Neural Network for Multi-Timescale State Prediction of Lithium-Ion Batteries. Energies 2022, 15, 5053. [Google Scholar] [CrossRef]
- Wang, S.; Takyi-Aninakwa, P.; Jin, S.; Yu, C.; Fernandez, C.; Stroe, D.-I. An improved feedforward-long short-term memory modeling method for the whole-life-cycle state of charge prediction of lithium-ion batteries considering current-voltage-temperature variation. Energy 2022, 254, 124224. [Google Scholar] [CrossRef]
- Browning, K.L.; Browning, J.F.; Doucet, M.; Yamada, N.L.; Liu, G.; Veith, G.M. Role of conductive binder to direct solid–electrolyte interphase formation over silicon anodes. Phys. Chem. Chem. Phys. 2019, 21, 17356–17365. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Morrissette, J.W.; Meisner, Q.; Son, S.-B.; Trask, S.E.; Tsai, Y.; Lopykinski, S.; Naik, S.; Bloom, I. Extreme Fast-Charging of Lithium-Ion Cells: Effect on Anode and Electrolyte. Energy Technol. 2021, 9, 2000696. [Google Scholar] [CrossRef]
- Hamzelui, N.; Eshetu, G.G.; Figgemeier, E. Customizing Active Materials and Polymeric Binders: Stern Requirements to Realize Silicon-Graphite Anode Based Lithium-Ion Batteries. J. Energy Storage 2021, 35, 102098. [Google Scholar] [CrossRef]
- Heubner, C.; Liebmann, T.; Lohrberg, O.; Cangaz, S.; Maletti, S.; Michaelis, A. Understanding Component-Specific Contributions and Internal Dynamics in Silicon/Graphite Blended Electrodes for High-Energy Lithium-Ion Batteries. Batter. Supercaps 2022, 5, e202100182. [Google Scholar] [CrossRef]
- Yao, K.P.C.; Okasinski, J.S.; Kalaga, K.; Almer, J.D.; Abraham, D.P. Operando Quantification of (De)Lithiation Behavior of Silicon–Graphite Blended Electrodes for Lithium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803380. [Google Scholar] [CrossRef]
- Tanim, T.R.; Paul, P.P.; Thampy, V.; Cao, C.; Steinrück, H.-G.; Nelson Weker, J.; Toney, M.F.; Dufek, E.J.; Evans, M.C.; Jansen, A.N.; et al. Heterogeneous Behavior of Lithium Plating during Extreme Fast Charging. Cell Rep. Phys. Sci. 2020, 1, 100114. [Google Scholar] [CrossRef]
- Moon, J.; Lee, H.C.; Jung, H.; Wakita, S.; Cho, S.; Yoon, J.; Lee, J.; Ueda, A.; Choi, B.; Lee, S.; et al. Interplay between electrochemical reactions and mechanical responses in silicon–graphite anodes and its impact on degradation. Nat. Commun. 2021, 12, 2714. [Google Scholar] [CrossRef]
- Usseglio-Viretta, F.L.E.; Mai, W.; Colclasure, A.M.; Doeff, M.; Yi, E.; Smith, K. Enabling fast charging of lithium-ion batteries through secondary- /dual- pore network: Part I—Analytical diffusion model. Electrochim. Acta 2020, 342, 136034. [Google Scholar] [CrossRef]
- Sauerteig, D.; Ivanov, S.; Reinshagen, H.; Bund, A. Reversible and irreversible dilation of lithium-ion battery electrodes investigated by in-situ dilatometry. J. Power Sources 2017, 342, 939–946. [Google Scholar] [CrossRef]
- Li, X.; Colclasure, A.M.; Finegan, D.P.; Ren, D.; Shi, Y.; Feng, X.; Cao, L.; Yang, Y.; Smith, K. Degradation mechanisms of high capacity 18650 cells containing Si-graphite anode and nickel-rich NMC cathode. Electrochim. Acta 2019, 297, 1109–1120. [Google Scholar] [CrossRef]
- Frankenberger, M.; Trunk, M.; Seidlmayer, S.; Dinter, A.; Dittloff, J.; Werner, L.; Gernhäuser, R.; Revay, Z.; Märkisch, B.; Gilles, R.; et al. SEI Growth Impacts of Lamination, Formation and Cycling in Lithium Ion Batteries. Batteries 2020, 6, 21. [Google Scholar] [CrossRef]
- Yang, Z.; Kim, M.; Yu, L.; Trask, S.E.; Bloom, I. Chemical Interplay of Silicon and Graphite in a Composite Electrode in SEI Formation. ACS Appl. Mater. Interfaces 2021, 13, 56073–56084. [Google Scholar] [CrossRef]
| Cathode | Si-Gr Composite Anode | |||
|---|---|---|---|---|
| Composition | NMC811 | 00Si | 15Si | 30Si |
| active material | 90 wt% NMC811 (Targray) | 0 wt% Paraclete Energy Si, 88 wt% Hitachi MagE3 graphite | 15 wt% Paraclete Energy Si, 73 wt% Hitachi MagE3 graphite | 30 wt% Paraclete Energy Si, 58 wt% Hitachi MagE3 graphite |
| binder | 5 wt% poly(vinylidene fluoride) (PVDF, Solvay 5130) | 10 wt% lithium polyacrylate (LiPAA) | 10 wt% lithium polyacrylate (LiPAA) | 10 wt% lithium polyacrylate (LiPAA) |
| conducting agent | 5 wt% Timcal C45 carbon | 2 wt% Timcal C45 carbon | 2 wt% Timcal C45 carbon | 2 wt% Timcal C45 carbon |
| loading density (mg/cm2) | 9.08 | 6.4 | 3.0 | 2.32 |
| coating thickness (μm) | 33 | 48 | 27 | 22 |
| N/P ratio | ~1.33 | ~1.31 | ~1.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Trask, S.E.; Wu, X.; Ingram, B.J. Effect of Si Content on Extreme Fast Charging Behavior in Silicon–Graphite Composite Anodes. Batteries 2023, 9, 138. https://doi.org/10.3390/batteries9020138
Yang Z, Trask SE, Wu X, Ingram BJ. Effect of Si Content on Extreme Fast Charging Behavior in Silicon–Graphite Composite Anodes. Batteries. 2023; 9(2):138. https://doi.org/10.3390/batteries9020138
Chicago/Turabian StyleYang, Zhenzhen, Stephen E. Trask, Xianyang Wu, and Brian J. Ingram. 2023. "Effect of Si Content on Extreme Fast Charging Behavior in Silicon–Graphite Composite Anodes" Batteries 9, no. 2: 138. https://doi.org/10.3390/batteries9020138
APA StyleYang, Z., Trask, S. E., Wu, X., & Ingram, B. J. (2023). Effect of Si Content on Extreme Fast Charging Behavior in Silicon–Graphite Composite Anodes. Batteries, 9(2), 138. https://doi.org/10.3390/batteries9020138








