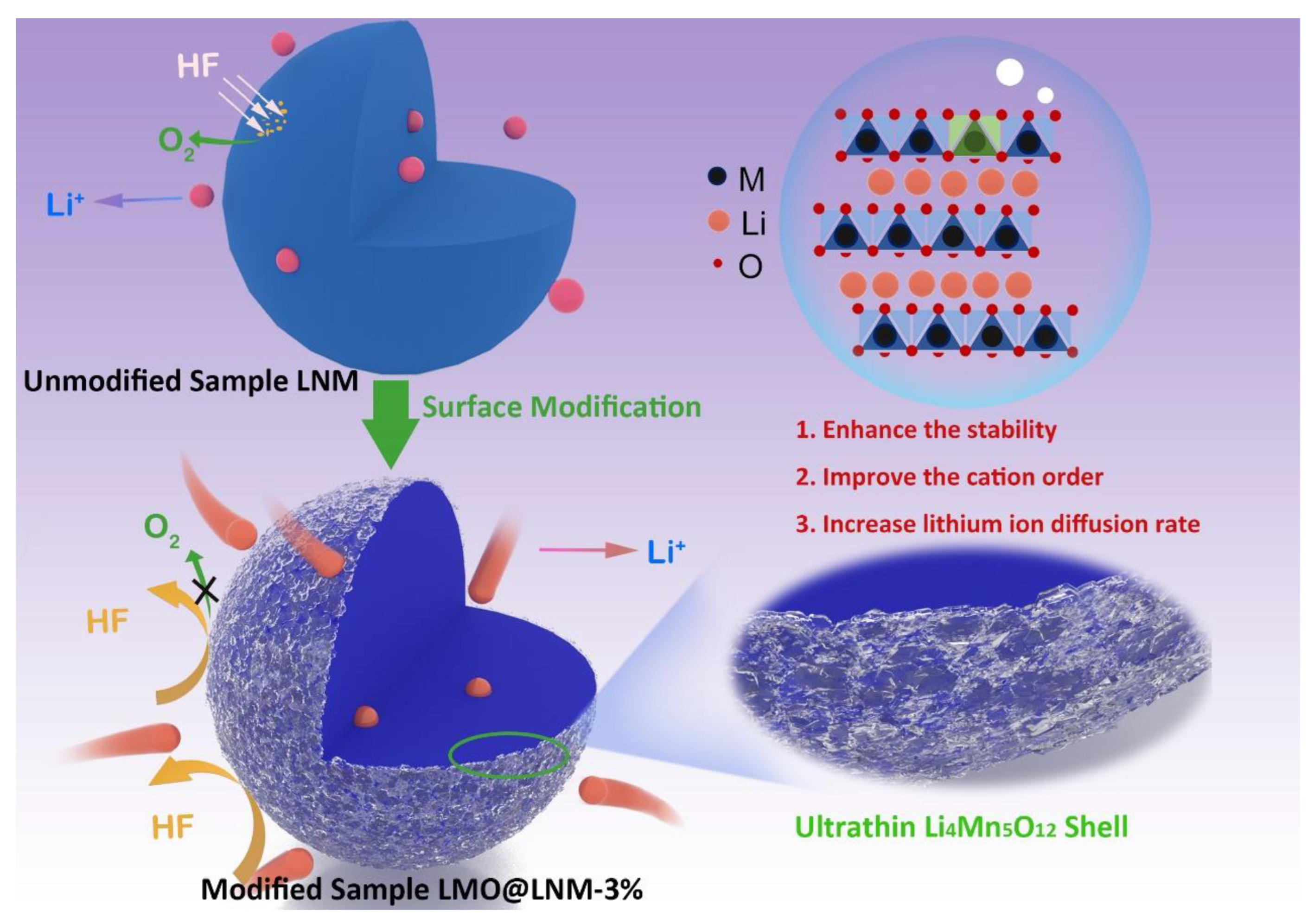
Building Ultrathin Li4Mn5O12 Shell for Enhancing the Stability of Cobalt-Free Lithium-Rich Manganese Cathode Materials
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Li1.2Mn0.6Ni0.2O2
2.2. Preparation of Li4Mn5O12/Li1.2Mn0.6Ni0.2O2 Materials
2.3. Preparation and Assembly of Electrodes
2.4. Characterization and Testing of Materials
3. Results and Discussion
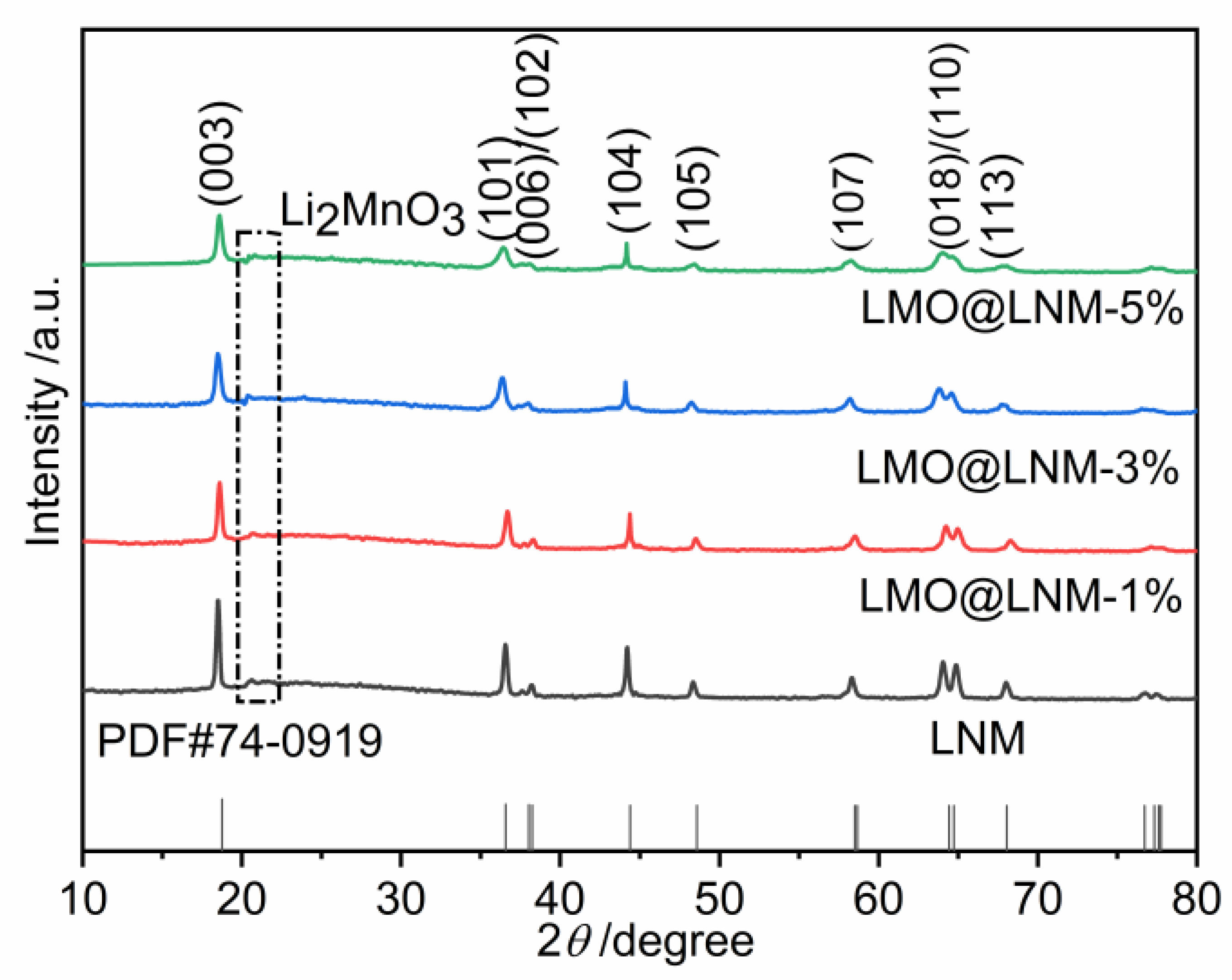
3.1. X-ray Diffraction Analysis
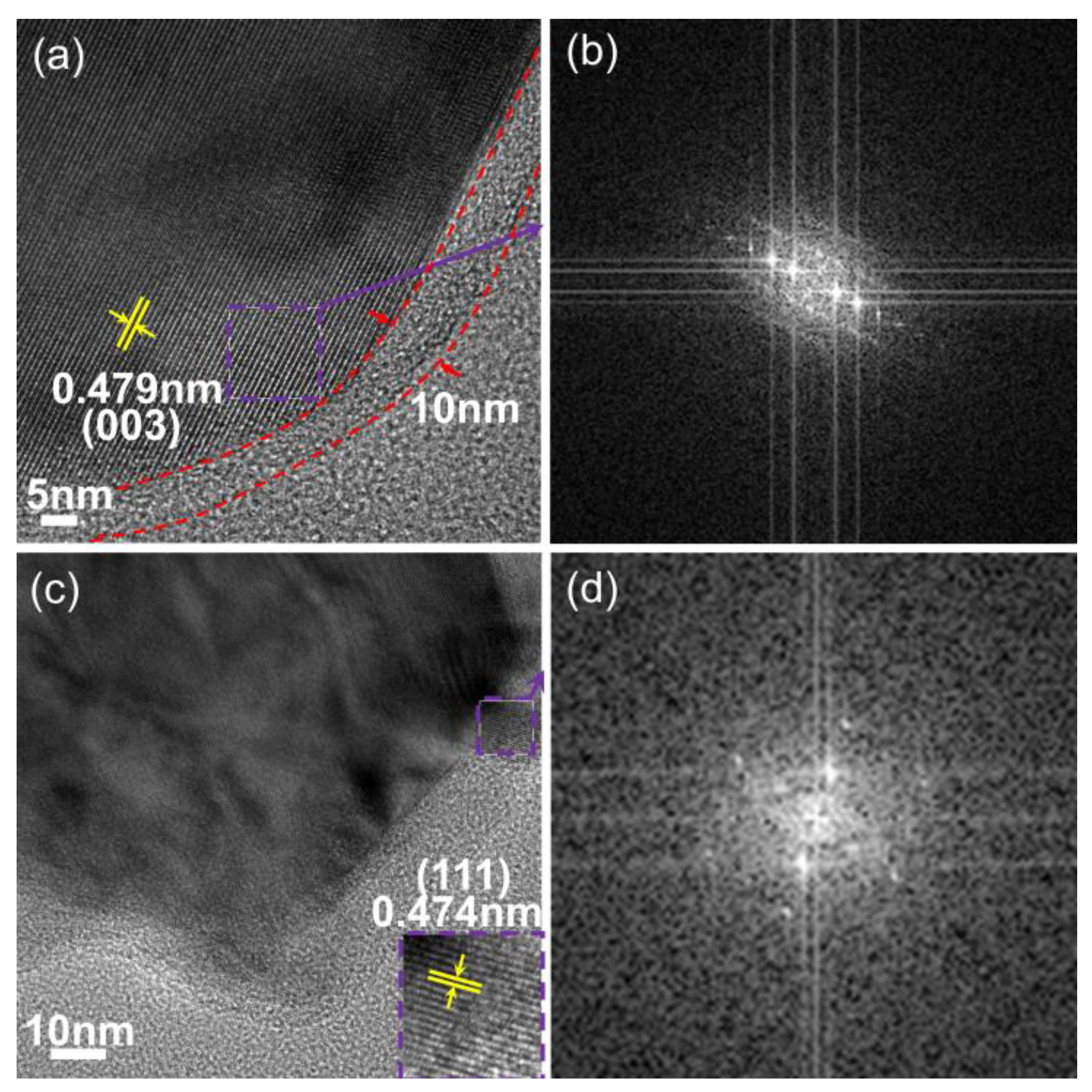
3.2. Material Morphology and Composition Analysis
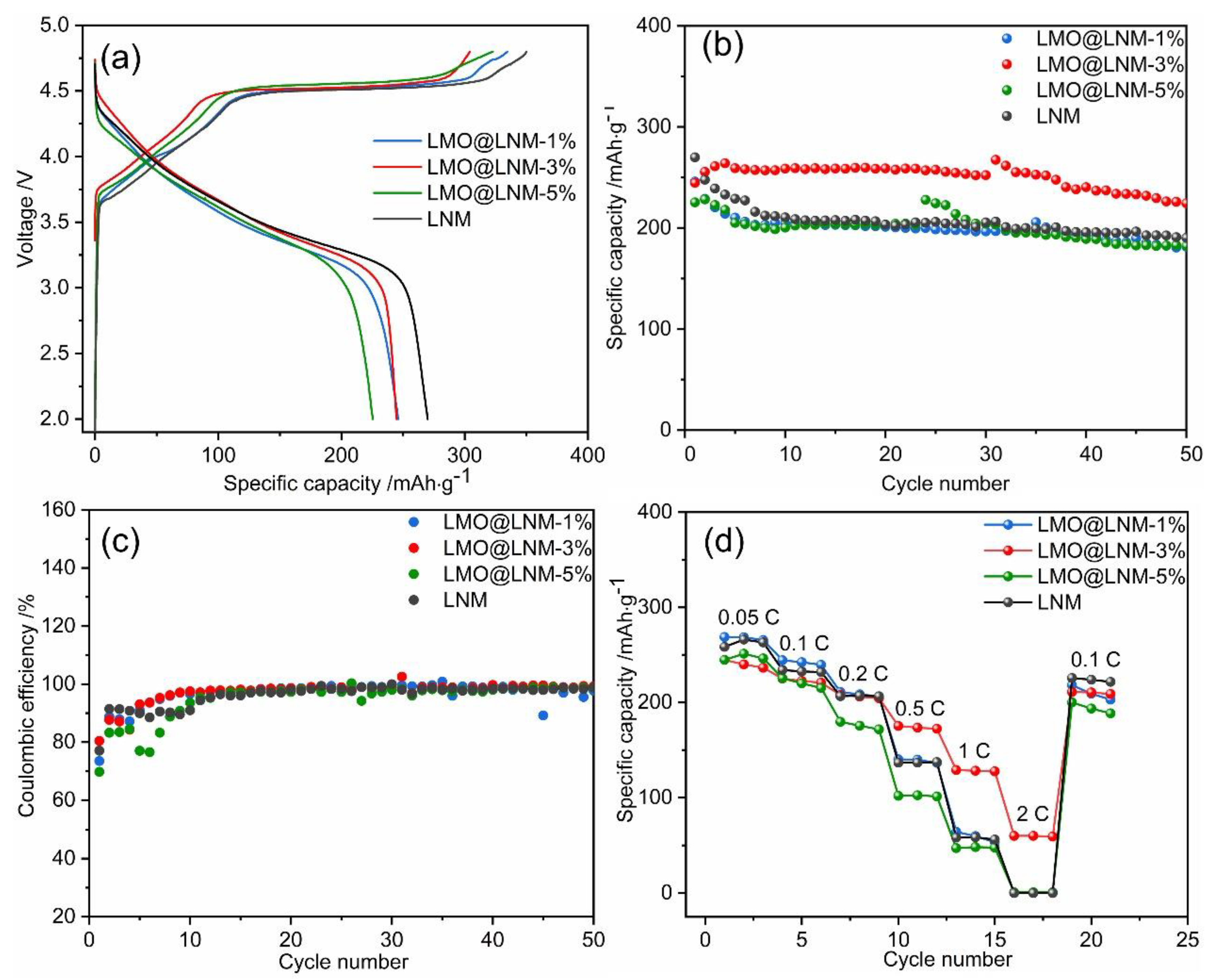
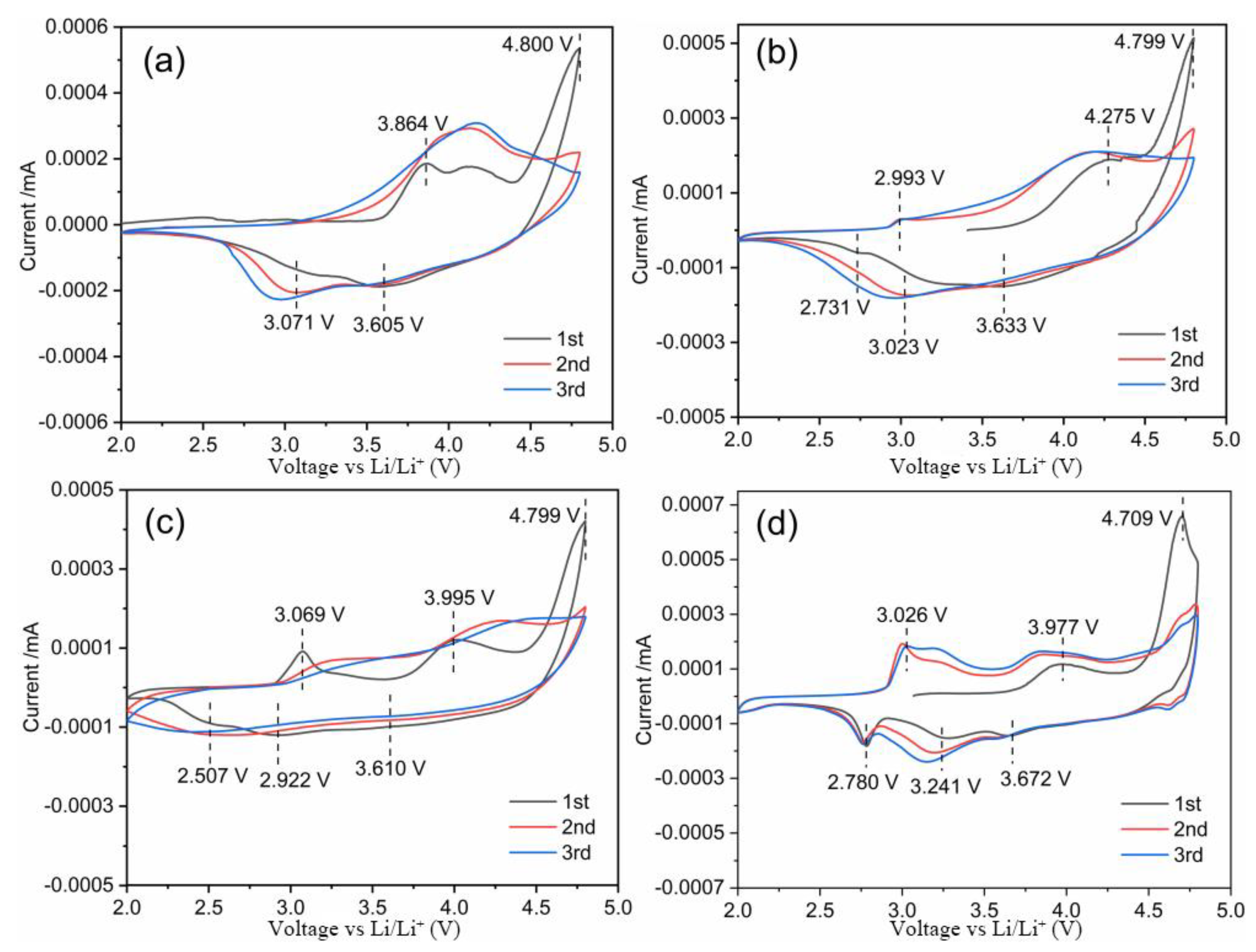
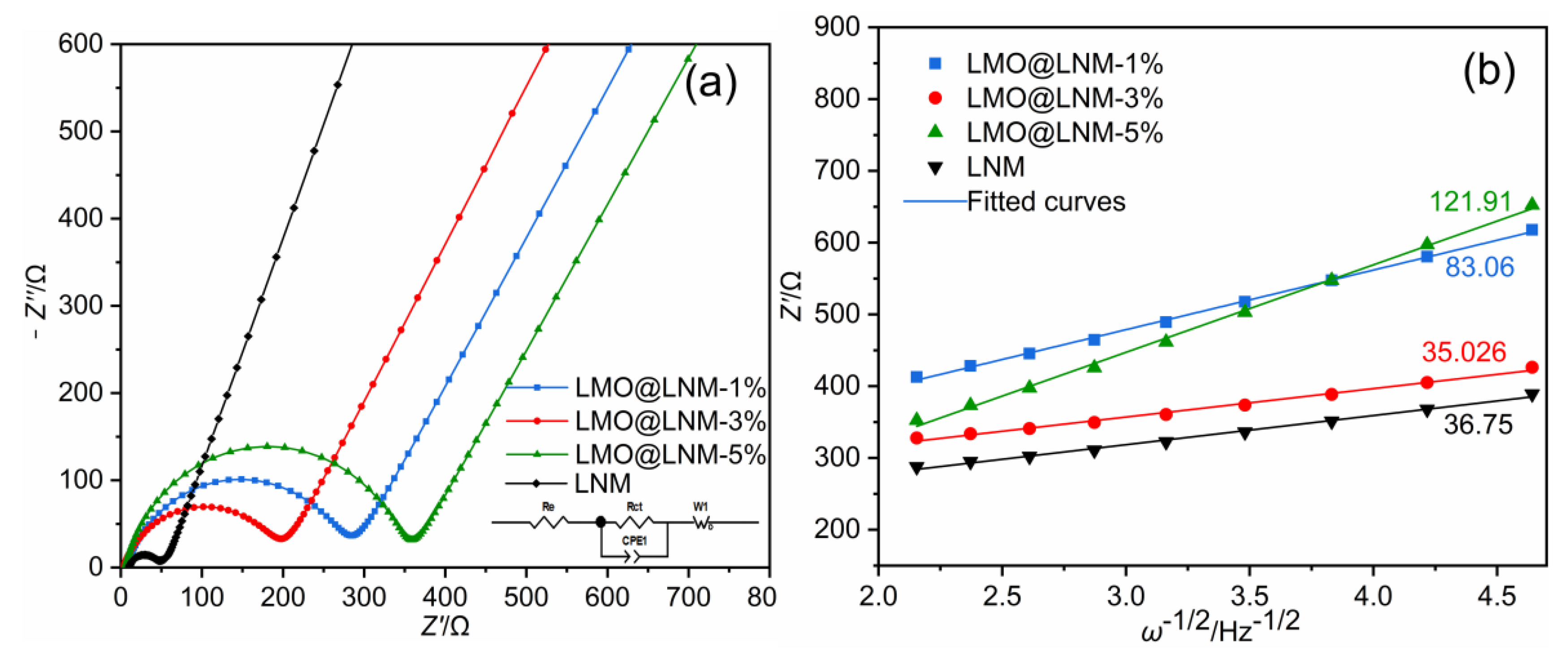
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Wang, Y.; Song, Y.; Jia, X.; Yang, J.; Li, Y.; Liao, J.; Song, H. Immobilizing Polysulfide via Multiple Active Sites in W18O49 for Li-S batteries by Oxygen Vacancy Engineering. Energy Storage Mater. 2021, 43, 422–429. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Wu, F.; Li, Q.; Chen, L.; Zhang, Q.; Wang, Z.; Lu, Y.; Bao, L.; Chen, S.; Su, Y. Improving the Structure Stability of LiNi0.8Co0.1Mn0.1O2 by Surface Perovskite-like La2Ni0.5Li0.5O4 Self-Assembling and Subsurface La3+ Doping. ACS Appl. Mater. Interfaces 2019, 11, 36751–36762. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, H.; Yuan, X.; Ma, H.; Ma, Z.; Zhao, Y.; Zhao, J.; Wang, X.; Chen, D.; Dong, Y. Direct ink writing preparation of LiFePO4/MWCNTs electrodes with high-areal Li-ion capacity. Ceram. Int. 2021, 47, 21161–21166. [Google Scholar] [CrossRef]
- Wang, S.; Feng, S.; Liang, J.; Su, Q.; Zhao, F.; Song, H.; Zheng, M.; Sun, Q.; Song, Z.; Jia, X.; et al. Insight into MoS 2 –MoN Heterostructure to Accelerate Polysulfide Conversion toward High-Energy-Density Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2003314. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, S.; Jia, X.; Yang, J.; Li, Y.; Liao, J.; Song, H. Freestanding sandwich-like hierarchically TiS2–TiO2/Mxene bi-functional interlayer for stable Li–S batteries. Carbon 2022, 188, 533–542. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Zhang, X.; Li, L.; Wu, F. Advanced cathode materials for lithium-ion batteries using nanoarchitectonics. Nanoscale Horiz. 2016, 1, 423–444. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Wang, S.; Liao, J.; Yang, X.; Liang, J.; Sun, Q.; Liang, J.; Zhao, F.; Koo, A.; Kong, F.; Yao, Y.; et al. Designing a highly efficient polysulfide conversion catalyst with paramontroseite for high-performance and long-life lithium-sulfur batteries. Nano Energy 2018, 57, 230–240. [Google Scholar] [CrossRef]
- Bi, L.; Wei, X.; Qiu, Y.; Song, Y.; Long, X.; Chen, Z.; Wang, S.; Liao, J. A highly ionic transference number eutectogel hybrid electrolytes based on spontaneous coupling inhibitor for solid-state lithium metal batteries. Nano Res. 2022, 16, 1717–1725. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Antolini, E. LiCoO2: Formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties. Solid State Ionics 2004, 170, 159–171. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Tarascon, J.-M. Optimization of Insertion Compounds Such as LiMn[sub 2]O[sub 4] for Li-Ion Batteries. J. Electrochem. Soc. 2002, 149, K31–K46. [Google Scholar] [CrossRef]
- Karunawan, J.; Abdillah, O.B.; Floweri, O.; Aji, M.P.; Santosa, S.P.; Sumboja, A.; Iskandar, F. Improving the Structural Ordering and Particle-Size Homogeneity of Li-Rich Layered Li1.2Ni0.13Co0.13Mn0.54O2 Cathode Materials through Microwave Irradiation Solid-State Synthesis. Batteries 2022, 9, 31. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, H. High-Energy Cathode Materials (Li2MnO3–LiMO2) for Lithium-Ion Batteries. J. Phys. Chem. Lett. 2013, 4, 1268–1280. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Song, Y.; Zhang, J.; Jia, X.; Yang, J.; Shao, D.; Li, Y.; Liao, J.; Song, H. Synergistic regulating of dynamic trajectory and lithiophilic nucleation by Heusler alloy for dendrite-free Li deposition. Energy Storage Mater. 2022, 50, 505–513. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Liao, J.; Sun, Q.; Zhao, F.; Luo, J.; Lin, X.; Niu, X.; Wu, M.; Li, R.; et al. Efficient Trapping and Catalytic Conversion of Polysulfides by VS4 Nanosites for Li–S Batteries. ACS Energy Lett. 2019, 4, 755–762. [Google Scholar] [CrossRef]
- Ates, M.N.; Mukerjee, S.; Abraham, K.M. A Search for the Optimum Lithium Rich Layered Metal Oxide Cathode Material for Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A1236–A1245. [Google Scholar] [CrossRef]
- Boulineau, A.; Simonin, L.; Colin, J.-F.; Bourbon, C.; Patoux, S. First Evidence of Manganese–Nickel Segregation and Densification upon Cycling in Li-Rich Layered Oxides for Lithium Batteries. Nano Lett. 2013, 13, 3857–3863. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2014, 44, 699–728. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Guo, S.; Xu, Z.; Zhu, H.; Zhang, X.; Yuan, Y.; He, P.; Ishida, M.; Zhou, H. Direct Visualization of the Reversible O2−/O− Redox Process in Li-Rich Cathode Materials. Adv. Mater. 2018, 30, e1705197. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered Li[Ni[sub x]Co[sub 1−2x]Mn[sub x]]O[sub 2] Cathode Materials for Lithium-Ion Batteries. Electrochem. Solid-state Lett. 2001, 4, A200–A203. [Google Scholar] [CrossRef]
- Ji, X.; Xia, Q.; Xu, Y.; Feng, H.; Wang, P.; Tan, Q. A review on progress of lithium-rich manganese-based cathodes for lithium ion batteries. J. Power Sources 2020, 487, 229362. [Google Scholar] [CrossRef]
- He, W.; Yuan, D.; Qian, J.; Ai, X.; Yang, H.; Cao, Y. Enhanced high-rate capability and cycling stability of Na-stabilized layered Li1.2[Co0.13Ni0.13Mn0.54]O2 cathode material. J. Mater. Chem. A 2013, 1, 11397–11403. [Google Scholar] [CrossRef]
- Zhu, W.; Tai, Z.; Shu, C.; Chong, S.; Guo, S.; Ji, L.; Chen, Y.; Liu, Y. The superior electrochemical performance of a Li-rich layered cathode material with Li-rich spinel Li4Mn5O12 and MgF2 double surface modifications. J. Mater. Chem. A 2020, 8, 7991–8001. [Google Scholar] [CrossRef]
- Ding, G.; Yan, F.; Zhu, Z.; Chen, J.; Hu, Z.; Li, G.; Liu, J.; Gao, L.; Jiang, W.; Sun, F. Mussel-inspired polydopamine-assisted uniform coating of Li+ conductive LiAlO2 on nickel-rich LiNi0.8Co0.1Mn0.1O2 for high-performance Li-ion batteries. Ceram. Int. 2021, 48, 5714–5723. [Google Scholar] [CrossRef]
- Akkinepally, B.; Reddy, I.N.; Ko, T.J.; Yoo, K.; Shim, J. Dopant effect on Li+ ion transport in NASICON-type solid electrolyte: Insights from molecular dynamics simulations and experiments. Ceram. Int. 2022, 48, 12142–12151. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.; Fu, C.; Luo, D.; Fan, J.; Li, L. K+-Doped Li1.2Mn0.54Co0.13Ni0.13O2: A Novel Cathode Material with an Enhanced Cycling Stability for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 10330–10341. [Google Scholar] [CrossRef]
- Wu, Y.; Manthiram, A. Effect of surface modifications on the layered solid solution cathodes (1−z) Li[Li1/3Mn2/3]O2−(z) Li[Mn0.5−yNi0.5−yCo2y]O2. Solid State Ionics 2009, 180, 50–56. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Xu, H.; Li, J.; Qiu, X. Stabilized cobalt-free lithium-rich cathode materials with an artificial lithium fluoride coating. Int. J. Miner. Met. Mater. 2022, 29, 917–924. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Chen, W.; Chen, J.; Yang, W.; Zou, H.; Lin, Z. Enhanced Electrochemical Performance of Li-Rich Cathode Materials by Organic Fluorine Doping and Spinel Li1+xNiyMn2–yO4 Coating. ACS Sustain. Chem. Eng. 2019, 8, 121–128. [Google Scholar] [CrossRef]
- Zhao, H.; Li, W.; Li, J.; Xu, H.; Zhang, C.; Li, J.; Han, C.; Li, Z.; Chu, M.; Qiu, X. Enhance performances of Co-free Li-rich cathode by eutesctic melting salt treatment. Nano Energy 2021, 92, 106760. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Shi, J.-L.; Liang, J.-Y.; Yin, Y.-X.; Zhang, J.-N.; Yu, X.-Q.; Guo, Y.-G. Suppressing Surface Lattice Oxygen Release of Li-Rich Cathode Materials via Heterostructured Spinel Li4 Mn5 O12 Coating. Adv. Mater. 2018, 30, e1801751. [Google Scholar] [CrossRef]
- Qiu, S.; Fang, T.; Zhu, Y.; Hua, J.; Chu, H.; Zou, Y.; Zeng, J.-L.; Xu, F.; Sun, L. Li1.2Mn0.6Ni0.2O2 with 3D porous rod-like hierarchical micro/nanostructure for high-performance cathode material. J. Alloys Compd. 2019, 790, 863–870. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, M.; Deng, Y.; Zheng, Q.; Xu, C.; Lin, D. La2O3-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with enhanced specific capacity and cycling stability for lithium-ion batteries. Ceram. Int. 2016, 42, 15623–15633. [Google Scholar] [CrossRef]
- Gao, L.; Xu, Z.; Zhang, S. The co-doping effects of Zr and Co on structure and electrochemical properties of LiFePO4 cathode materials. J. Alloys Compd. 2018, 739, 529–535. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Shi, Q.; Zhong, C.; Gong, D.; Lu, L.; Wang, X.; Liu, G.; Zhan, C. Enhanced high-voltage robustness of ultra-high nickel cathodes by constructing lithium-ion conductor buffer layer for highly stable lithium-ion batteries. Appl. Surf. Sci. 2022, 605, 154684. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Wang, Y.-Y.; Lai, Q.-Y.; Zhao, Y.; Chen, L.-M.; Ji, X.-Y. Study of capacitive properties for LT-Li4Mn5O12 in hybrid supercapacitor. J. Solid State Electrochem. 2008, 13, 905–912. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, X.; Yang, Y. A comparison of preparation method on the electrochemical performance of cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium ion battery. Electrochim. Acta 2011, 56, 3071–3078. [Google Scholar] [CrossRef]
- Wen, L. Improved Electrochemical Performance of LaF3-coated Layered Oxide Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material for Lithium- Ion Batteries Prepared by Sol-Gel Method. Int. J. Electrochem. Sci. 2021, 16, 210344. [Google Scholar] [CrossRef]
- Wu, F.; Lu, H.; Su, Y.; Li, N.; Bao, L.; Chen, S. Preparation and electrochemical performance of Li-rich layered cathode material, Li[Ni0.2Li0.2Mn0.6]O2, for lithium-ion batteries. J. Appl. Electrochem. 2009, 40, 783–789. [Google Scholar] [CrossRef]
- Lu, C.; Wu, H.; Zhang, Y.; Liu, H.; Chen, B.; Wu, N.; Wang, S. Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J. Power Sources 2014, 267, 682–691. [Google Scholar] [CrossRef]
- Yu, T.; Li, J.; Xu, G.; Li, J.; Ding, F.; Kang, F. Improved cycle performance of Li[Li0.2Mn0.54Co0.13Ni0.13]O2 by Ga doping for lithium ion battery cathode material. Solid State Ionics 2017, 301, 64–71. [Google Scholar] [CrossRef]
- Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries. Nano Energy 2019, 58, 786–796. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, P.; Xie, Q.; Zhang, G.; Zheng, H.; Cai, Y.; Li, Z.; Wang, L.; Zhu, Z.-Z.; Mai, L.; et al. Double-shell Li-rich layered oxide hollow microspheres with sandwich-like carbon@spinel@layered@spinel@carbon shells as high-rate lithium ion battery cathode. Nano Energy 2019, 59, 184–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, T.; Lin, L.; Yuan, M.; Li, H.; Sun, G.; Ma, S. Hierarchical Li1.2Mn0.54Ni0.13Co0.13O2 hollow spherical as cathode material for Li-ion battery. J. Nanoparticle Res. 2017, 19, 373. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, S.; Huang, C.; Xia, J.; Wang, X.; Xiang, K.; Zeng, P.; Zhang, Y.; Jamil, S. Synergetic Effects of Multifunctional Composites with More Efficient Polysulfide Immobilization and Ultrahigh Sulfur Content in Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 13562–13572. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Long, N.; Liu, T.; Cheng, X.; Zheng, R.; Zhu, H.; Ye, W.; Shu, J. Effect of calcination temperature on morphology and electrochemical performance of PbLi2Ti6O14. Ceram. Int. 2018, 44, 9506–9513. [Google Scholar] [CrossRef]
- Vujković, M.; Stojković, I.; Cvjetićanin, N.; Mentus, S. Gel-combustion synthesis of LiFePO4/C composite with improved capacity retention in aerated aqueous electrolyte solution. Electrochim. Acta 2013, 92, 248–256. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Peng, X.; Zhou, L.; Yan, J.; Song, Y.; Bi, L.; Long, X.; He, L.; Xie, Q.; Wang, S.; et al. Building Ultrathin Li4Mn5O12 Shell for Enhancing the Stability of Cobalt-Free Lithium-Rich Manganese Cathode Materials. Batteries 2023, 9, 123. https://doi.org/10.3390/batteries9020123
Qiu Y, Peng X, Zhou L, Yan J, Song Y, Bi L, Long X, He L, Xie Q, Wang S, et al. Building Ultrathin Li4Mn5O12 Shell for Enhancing the Stability of Cobalt-Free Lithium-Rich Manganese Cathode Materials. Batteries. 2023; 9(2):123. https://doi.org/10.3390/batteries9020123
Chicago/Turabian StyleQiu, Yuhong, Xuefeng Peng, Lichun Zhou, Jie Yan, Yaochen Song, Linnan Bi, Xin Long, Liang He, Qingyu Xie, Sizhe Wang, and et al. 2023. "Building Ultrathin Li4Mn5O12 Shell for Enhancing the Stability of Cobalt-Free Lithium-Rich Manganese Cathode Materials" Batteries 9, no. 2: 123. https://doi.org/10.3390/batteries9020123
APA StyleQiu, Y., Peng, X., Zhou, L., Yan, J., Song, Y., Bi, L., Long, X., He, L., Xie, Q., Wang, S., & Liao, J. (2023). Building Ultrathin Li4Mn5O12 Shell for Enhancing the Stability of Cobalt-Free Lithium-Rich Manganese Cathode Materials. Batteries, 9(2), 123. https://doi.org/10.3390/batteries9020123





