Carbon-Coated Si Nanoparticles Anchored on Three-Dimensional Carbon Nanotube Matrix for High-Energy Stable Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Material Preparation
2.2.1. Pretreatment of Si NPs and CNTs
2.2.2. Preparation of Si/CNT@RF Resin Composites
2.2.3. Preparation of Si/CNT@C Composites
2.3. Material Characterization
2.4. Electrochemical Characterization
3. Results and Discussions
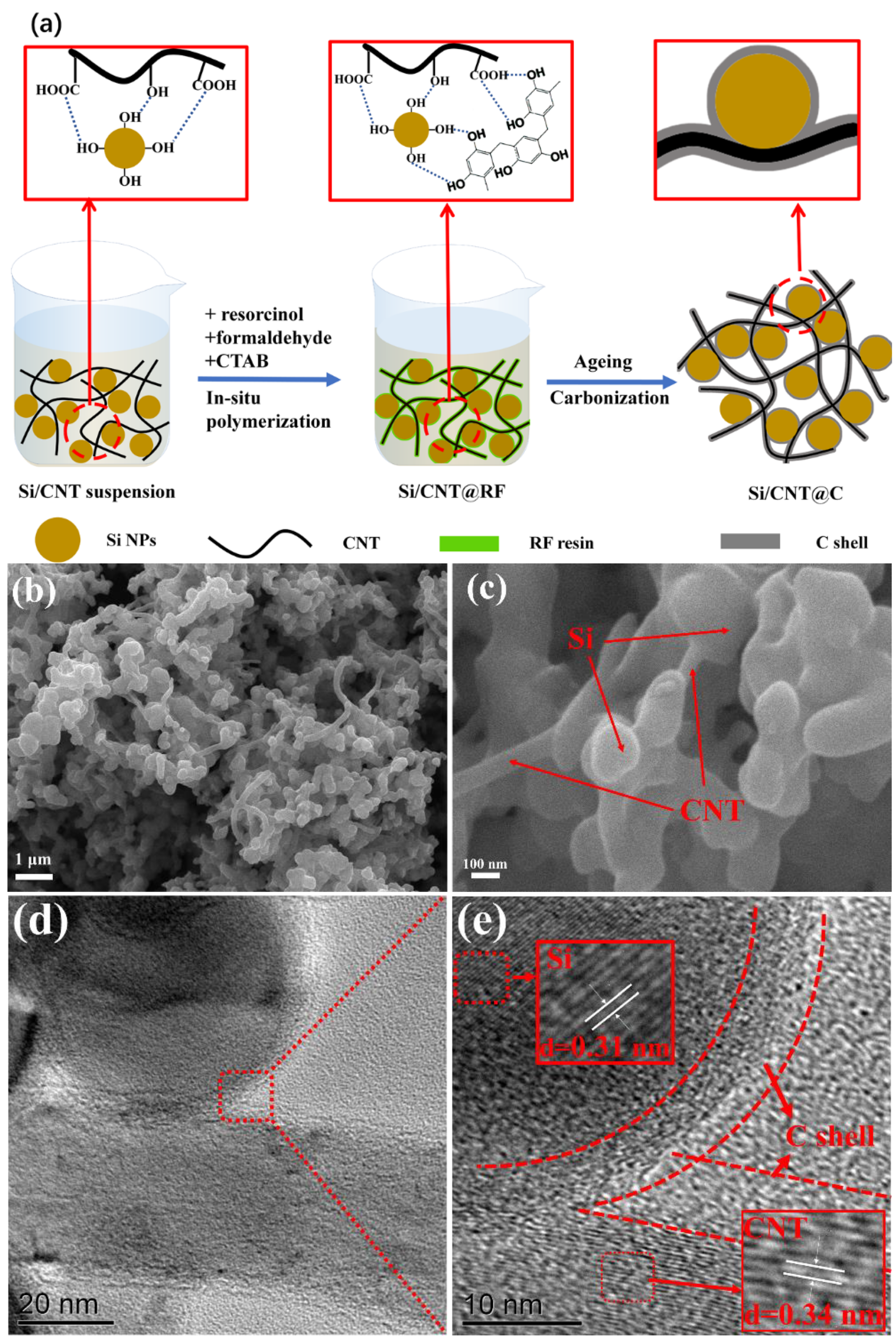
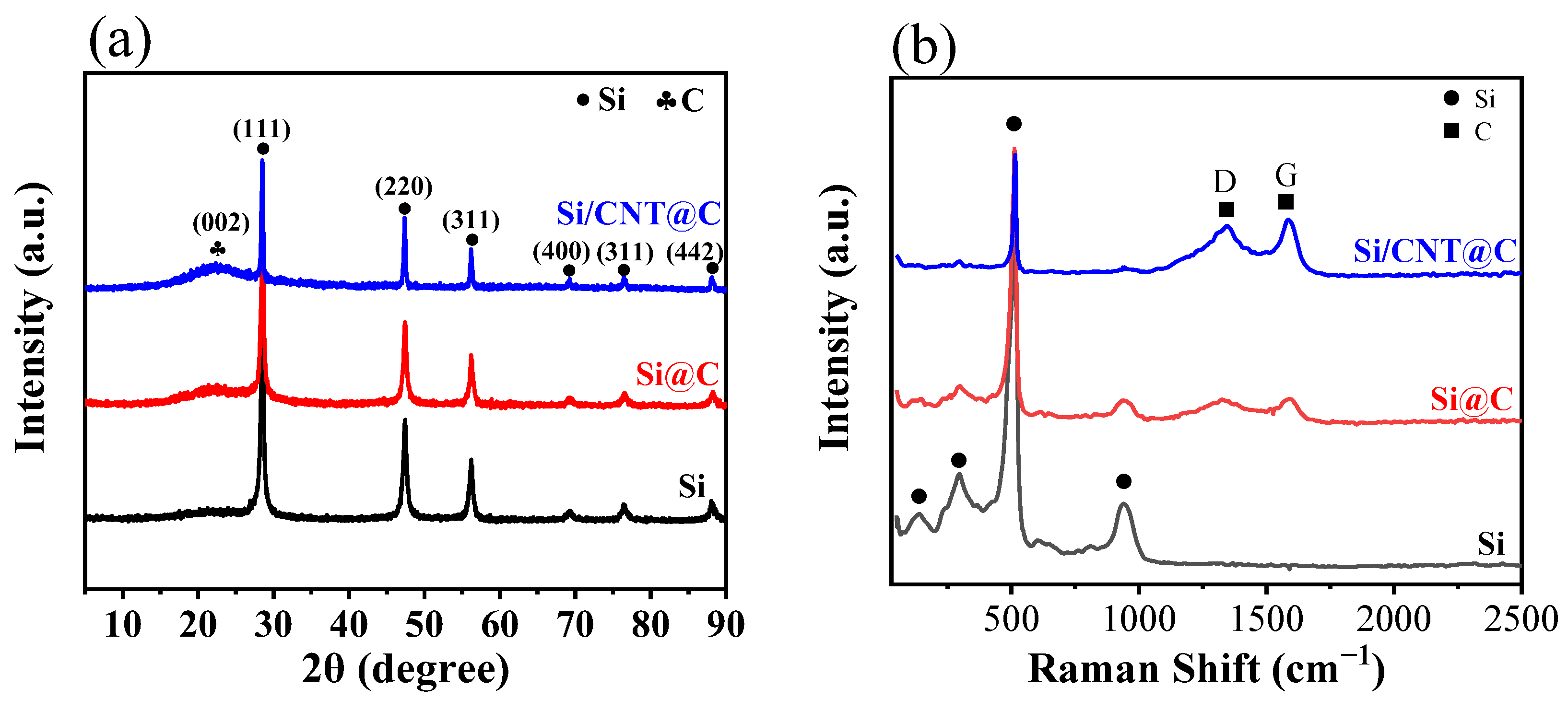
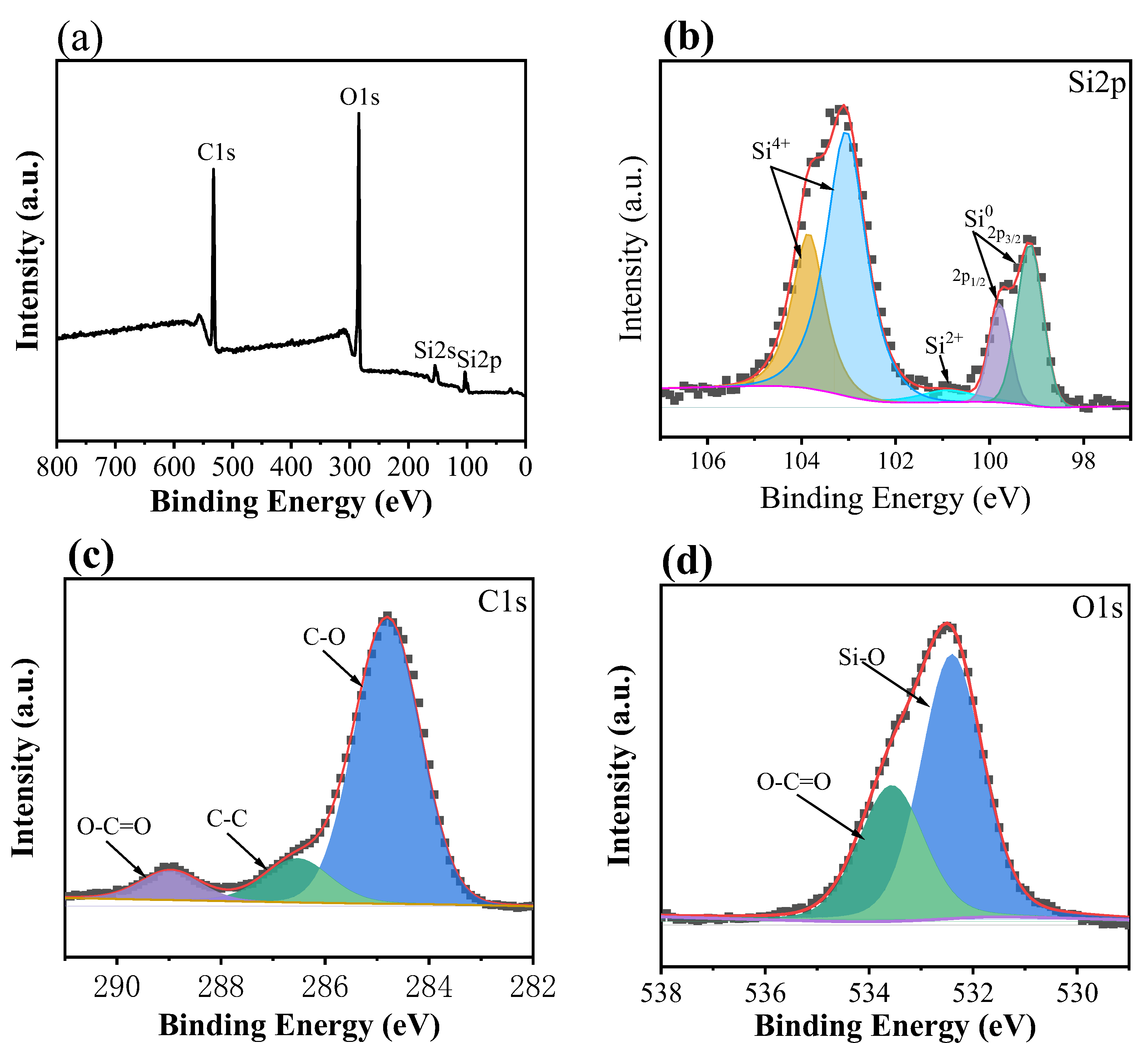
3.1. Morphology, Microstructure and Crystalline Phase Studies
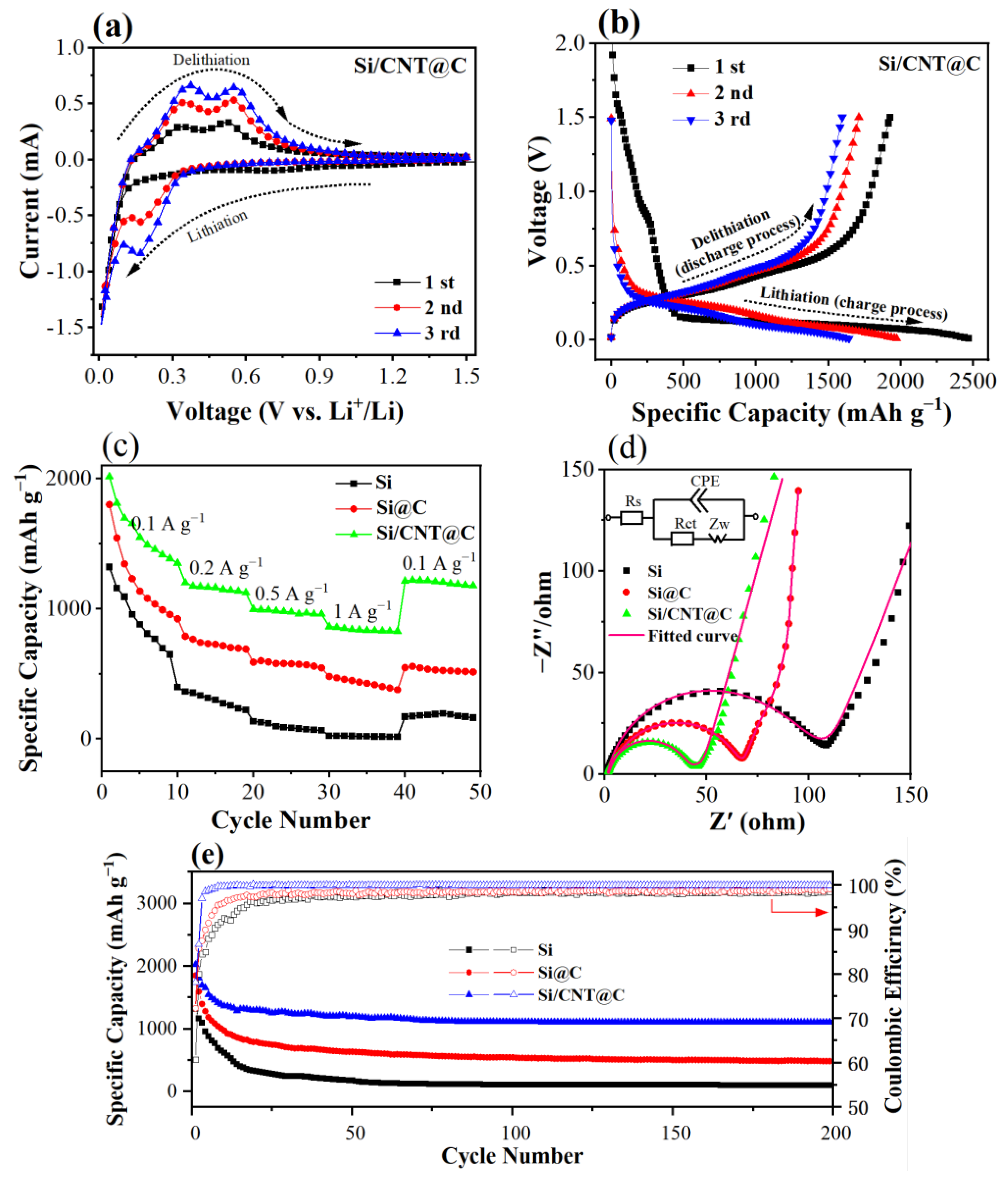
3.2. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, T.; Xu, H.; Yu, X.; Li, H.; Zhang, W.; Cheng, X.; Zhu, W.; Qui, X. Lithiation Behavior of Coaxial Hollow Nanocables of Carbon-Silicon Composite. ACS Nano 2019, 13, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Murphy, P.; Hall, C.; Francis, C.; Kerr, R.; Fabretto, M. Pure Silicon Thin-Film Anodes for Lithium-Ion Batteries: A Review. J. Power Sources 2019, 414, 48–67. [Google Scholar] [CrossRef]
- Wang, F.; Lin, S.; Lu, X.; Hong, R.; Liu, H. Poly-Dopamine Carbon-Coated Stable Silicon/Graphene/CNT Composite as Anode for Lithium Ion Batteries. Electrochim. Acta 2022, 404, 139708. [Google Scholar] [CrossRef]
- Wang, H.W.; Fu, J.Z.; Wang, C.; Wang, J.Y.; Yang, A.K.; Li, C.C.; Sun, Q.F.; Cui, Y.; Li, H.Q. A Binder-Free High Silicon Content Flexible Anode for Li-Ion Batteries. Energy Environ. Sci. 2020, 13, 848–858. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Ma, J.; Kim, N.; Lee, H.-W.; Cui, Y.; Cho, J. Scalable Synthesis of Silicon-Nanolayer-Embedded Graphite for High-Energy Lithium-Ion Batteries. Nat. Energy 2016, 1, 16113. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Huang, B.; Dai, Y. Achieving High Energy Density for Lithium-Ion Battery Anodes by Si/C Nanostructure Design. J. Mater. Chem. A 2019, 7, 2165–2171. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Dou, Y.; Cheng, N.; Cui, D.; Du, Y.; Liu, P.; Al-Mamun, M.; Zhang, S.; Zhao, H. A Yolk-Shell Structured Silicon Anode with Superior Conductivity and High Tap Density for Full Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 8824–8828. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Wang, Z.; Jing, L.; Zheng, X.; Xu, Z.; Yuan, Y.; Liu, X.; Fu, A.; Guo, Y.-G.; Li, H. Microspheres of Si@Carbon-CNTs Composites with a Stable 3D Interpenetrating Structure Applied in High-Performance Lithium-Ion Battery. J. Colloid Interface Sci. 2022, 629, 511–521. [Google Scholar] [CrossRef]
- Wei, R.; Liu, X.Q.; Tian, Y.; Huang, F.F.; Xu, S.Q.; Zhang, J.J. Sn-Based Glass-Graphite-Composite as a High Capacity Anode for Lithium-Ion Batteries. J. Am. Ceram. Soc. 2023, 106, 330–338. [Google Scholar] [CrossRef]
- Nurpeissova, A.; Adi, A.; Aishova, A.; Mukanova, A.; Kim, S.S.; Bakenov, Z. Synergistic Effect of 3D Current Collector Structure and Ni Inactive Matrix on the Electrochemical Performances of Sn-Based Anodes for Lithium-Ion Batteries. Mater. Today Energy 2020, 16, 100397. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, Y.M.; Shen, X.P.; Qiu, J.X.; Lian, J.B.; Pu, J.R.; Li, S.; Du, F.H.; Li, S.Q.; Ji, Z.Y.; et al. Ge Nanoparticles Uniformly Immobilized on 3D Interconnected Porous Graphene Frameworks as Anodes for High-Performance Lithium-Ion Batteries. J. Energy Chem. 2022, 69, 161–173. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhuang, Q.F.; Li, Y.; Hu, Y.L.; Liu, Y.Y.; Zhang, Q.B.; Shi, L.; He, C.X.; Zheng, X.; Yu, S.H. Bio-Inspired Synthesis of Transition-Metal Oxide Hybrid Ultrathin Nanosheets for Enhancing the Cycling Stability in Lithium-Ion Batteries. Nano Res. 2022, 15, 5064–5071. [Google Scholar] [CrossRef]
- Hou, T.; Liu, B.; Sun, X.; Fan, A.; Xu, Z.; Cai, S.; Zheng, C.; Yu, G.; Tricoli, A. Covalent Coupling-Stabilized Transition-Metal Sulfide/Carbon Nanotube Composites for Lithium/Sodium-Ion Batteries. ACS Nano 2021, 15, 6735–6746. [Google Scholar] [CrossRef]
- Shen, M.; Ma, H. Metal-Organic Frameworks (MOFs) and Their Derivative as Electrode Materials for Lithium-Ion Batteries. Coord. Chem. Rev. 2022, 470, 214715. [Google Scholar] [CrossRef]
- Li, P.; Hwang, J.-Y.; Sun, Y.-K. Nano/Microstructured Silicon-Graphite Composite Anode for High-Energy-Density Li-Ion Battery. ACS Nano 2019, 13, 2624–2633. [Google Scholar] [CrossRef]
- Fang, S.; Shen, L.; Li, S.; Kim, G.-T.; Bresser, D.; Zhang, H.; Zhang, X.; Maier, J.; Passerini, S. Alloying Reaction Confinement Enables High-Capacity and Stable Anodes for Lithium-Ion Batteries. ACS Nano 2019, 13, 9511–9519. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Tang, B.; Yao, Z.; Zhang, X.; Liu, Z.; Gong, R.; Zhao, P. Mechanical Constraining Double-Shell Protected Si-Based Anode Material for Lithium-Ion Batteries with Long-Term Cycling Stability. J. Alloys Compd. 2020, 846, 156437. [Google Scholar] [CrossRef]
- Zhao, Z.; Cai, M.; Zhao, H.; Ma, Q.; Xie, H.; Xing, P.; Zhuang, Y.X.; Yin, H. Harvesting Si Nanostructures and C-Si Composites by Paired Electrolysis in Molten Salt: Implications for Lithium Storage. ACS Appl. Nano Mater. 2022, 5, 3781–3789. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.-H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, W.; Xu, N.; Wang, X.; Chang, L.; Wang, L.; Fang, L.; Le, Z.; Nie, P. Dealloying Synthesis of Silicon Nanotubes for High-Performance Lithium Ion Batteries. ChemPhysChem 2022, 23, e202100832. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, Y.; Li, P.; Peng, C.; Yin, H.; Li, W.; Wang, D. Electrochemical Conversion of Silica Nanoparticles to Silicon Nanotubes in Molten Salts: Implications for High-Performance Lithium-Ion Battery Anode. ACS Appl. Nano Mater. 2021, 4, 7028–7036. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, J.; Zhang, Y.; Yang, D.; Du, N. Scalable and Low-Cost Synthesis of Porous Silicon Nanoparticles as High-Performance Lithium-Ion Battery Anode. Mater. Today Nano 2022, 18, 100175. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.F.; Xiong, J.; Yang, T.Z.; Qin, Y.; Yan, C.L. Porous Si Nanowires from Cheap Metallurgical Silicon Stabilized by a Surface Oxide Layer for Lithium Ion Batteries. Adv. Funct. Mater. 2015, 25, 6701–6709. [Google Scholar] [CrossRef]
- Pathak, A.D.; Chanda, U.K.; Samanta, K.; Mandal, A.; Sahu, K.K.; Pati, S. Selective Leaching of Al from Hypereutectic Al-Si Alloy to Produce Nano-Porous Silicon (NPs) Anodes for Lithium Ion Batteries. Electrochim. Acta 2019, 317, 654–662. [Google Scholar] [CrossRef]
- Huang, X.; Ding, Y.C.; Li, K.L.; Guo, X.Y.; Zhu, Y.; Zhang, Y.X.; Bao, Z.H. Spontaneous Formation of the Conformal Carbon Nanolayer Coated Si Nanostructures as the Stable Anode for Lithium-Ion Batteries from Silica Nanomaterials. J. Power Sources 2021, 496, 229833. [Google Scholar] [CrossRef]
- Hailu, A.G.; Ramar, A.; Wang, F.M.; Wu, N.L.; Yeh, N.H.; Hsu, C.C.; Chang, Y.J.; Tiong, P.W.L.; Yuwono, R.A.; Khotimah, C.; et al. Investigations of Intramolecular Hydrogen Bonding Effect of a Polymer Brush Modified Silicon in Lithium-Ion Batteries. Adv. Mater. Interfaces 2022, 9, 2102007. [Google Scholar] [CrossRef]
- Yu, C.H.; Chen, X.; Xiao, Z.X.; Lei, C.; Zhang, C.X.; Lin, X.Q.; Shen, B.Y.; Zhang, R.F.; Wei, F. Silicon Carbide as a Protective Layer to Stabilize Si-Based Anodes by Inhibiting Chemical Reactions. Nano Lett. 2019, 19, 5124–5132. [Google Scholar] [CrossRef]
- Gao, X.; Lu, W.Q.; Xu, J. Unlocking Multiphysics Design Guidelines on Si/C Composite Nanostructures for High-Energy-Density and Robust Lithium-Ion Battery Anode. Nano Energy 2021, 81, 105591. [Google Scholar] [CrossRef]
- Bitew, Z.; Tesemma, M.; Beyene, Y.; Amare, M. Nano-Structured Silicon and Silicon Based Composites as Anode Materials for Lithium Ion Batteries: Recent Progress and Perspectives. Sustain. Energy Fuels 2022, 6, 1014–1050. [Google Scholar] [CrossRef]
- She, Z.M.; Uceda, M.; Pope, M.A. Encapsulating a Responsive Hydrogel Core for Void Space Modulation in High-Stability Graphene-Wrapped Silicon Anodes. ACS Appl. Mater. Interfaces 2022, 14, 10363–10372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Du, Y.; Yang, Y.J.; Jin, H.C.; Shi, H.B.; Bai, L.Y.; Ouyang, Y.G.; Ding, F.; Hou, G.L.; Yuan, F.L. Large-Scale Production of Highly Stable Silicon Monoxide Nanowires by Radio-Frequency Thermal Plasma as Anodes for High-Performance Li-Ion Batteries. J. Power Sources 2021, 497, 229906. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xu, Y.N.; Du, H.B. One-Step Synthesis of Uniformly Distributed SiOx-C Composites as Stable Anodes for Lithium-Ion Batteries. Dalton Trans. 2022, 51, 11909–11915. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.T.; Wang, Y.; Zeng, Y.; Yu, M.; Liu, T.; Chen, J.; Wang, K.; Xie, J.Y.; Li, L.S. Boosting Cyclability and Rate Capability of SiOx via Dopamine Polymerization-Assisted Hybrid Graphene Coating for Advanced Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 17388–17395. [Google Scholar] [CrossRef] [PubMed]
- Di, S.H.; Zhang, D.X.; Weng, Z.; Chen, L.; Zhang, Y.; Zhang, N.; Ma, R.Z.; Chen, G.; Liu, X.H. Crosslinked Polymer Binder via Phthalic Acid for Stabilizing SiOx Anodes. Macromol. Chem. Phys. 2022, 223, 2200068. [Google Scholar] [CrossRef]
- Zhao, J.K.; Wei, D.A.; Wang, J.J.; Yang, K.M.; Wang, Z.L.; Chen, Z.J.; Zhang, S.G.; Zhang, C.; Yang, X.J. Inorganic Crosslinked Supramolecular Binder with Fast Self-Healing for High Performance Silicon Based Anodes in Lithium-Ion Batteries. J. Colloid Interface Sci. 2022, 625, 373–382. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.K.; Kim, H.; Jung, I.H. Ambidextrous Polymeric Binder for Silicon Anodes in Lithium-Ion Batteries. Chem. Mater. 2022, 34, 5791–5798. [Google Scholar] [CrossRef]
- Rajeev, K.K.; Jang, W.; Kim, S.; Kim, T.H. Chitosan-Grafted-Gallic Acid as a Nature-Inspired Multifunctional Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 3166–3178. [Google Scholar]
- Deng, L.; Zheng, Y.; Zheng, X.M.; Or, T.; Ma, Q.Y.; Qian, L.T.; Deng, Y.P.; Yu, A.P.; Li, J.T.; Chen, Z.W. Design Criteria for Silicon-Based Anode Binders in Half and Full Cells. Adv. Energy Mater. 2022, 12, 2200850. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Lai, Y.; Yang, Z.; Yang, Q.; Liu, Y.; Zheng, Z.; Liu, Y.; Sun, Y.; Zhong, B.; et al. Revisiting the Preparation Progress of Nano-Structured Si Anodes toward Industrial Application from the Perspective of Cost and Scalability. Adv. Energy Mater. 2022, 12, 2102181. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, D. Comparative Life Cycle Assessment of Lithium-Ion Batteries with Lithium Metal, Silicon Nanowire, and Graphite Anodes. Clean Technol. Environ. Policy 2018, 20, 1233–1244. [Google Scholar] [CrossRef]
- Maxwell, T.L.; Balk, T.J. The Fabrication and Characterization of Bimodal Nanoporous Si with Retained Mg through Dealloying. Adv. Eng. Mater. 2018, 20, 1700519. [Google Scholar] [CrossRef]
- Wang, X.; Wen, K.; Chen, T.; Chen, S.; Zhang, S. Supercritical Fluid-Assisted Preparation of Si/CNTs@FG Composites with Hierarchical Conductive Networks as a High-Performance Anode Material. Appl. Surf. Sci. 2020, 522, 146507. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Kang, W.; Ye, Y.; Yuan, Y.; Qiu, Z.; Wang, C.; Zhang, X.; Ke, Y.; Tang, Y. Silicon-Nanoparticle-Based Composites for Advanced Lithium-Ion Battery Anodes. Nanoscale 2020, 12, 7461–7484. [Google Scholar] [CrossRef]
- Liu, L.; Lyu, J.; Li, T.; Zhao, T. Well-Constructed Silicon-Based Materials as High-Performance Lithium-Ion Battery Anodes. Nanoscale 2016, 8, 701–722. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, J.; Yub, H.; Zhang, J. Carbon-Coated Si/N-Doped Porous Carbon Nanofibre Derived from Metal-Organic Frameworks for Li-Ion Battery Anodes. J. Alloys Compd. 2022, 902, 163635. [Google Scholar] [CrossRef]
- Zeng, Y.; Huang, Y.; Liu, N.; Wang, X.; Zhang, Y.; Guo, Y.; Wu, H.-H.; Chen, H.; Tang, X.; Zhang, Q. N-Doped Porous Carbon Nanofibers Sheathed Pumpkin-Like Si/C Composites as Free-Standing Anodes for Lithium-Ion Batteries. J. Energy Chem. 2021, 54, 727–735. [Google Scholar] [CrossRef]
- Park, B.H.; Lee, G.-W.; Choi, S.B.; Kim, Y.-H.; Kim, K.B. Triethoxysilane-Derived SiOx-Assisted Structural Reinforcement of Si/Carbon Nanotube Composite for Lithium-Ion Battery. Nanoscale 2020, 12, 22140–22149. [Google Scholar] [CrossRef]
- Yi, Z.; Lin, N.; Zhao, Y.; Wang, W.; Qian, Y.; Zhu, Y.; Qian, Y. A Flexible Micro/Nanostructured Si Microsphere Cross-Linked by Highly-Elastic Carbon Nanotubes toward Enhanced Lithium Ion Battery Anodes. Energy Storage Mater. 2019, 17, 93–100. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Shi, X.; Song, D.; Zhang, H.; Li, C.; Wang, K.-Y.; Xiao, C.; Liu, X.; Zhang, L. Low-Temperature and High-Performance Si/Graphite Composite Anodes Enabled by Sulfite Additive. Chem. Eng. J. 2021, 421, 127782. [Google Scholar] [CrossRef]
- Zhao, E.; Luo, S.; Gu, Y.; Yang, L.; Hirano, S.-I. Preactivation Strategy for a Wide Temperature Range in Situ Gel Electrolyte-Based LiNi0.5Co0.2Mn0.3O2 Parallel to Si-Graphite Battery. ACS Appl. Mater. Interfaces 2021, 13, 59843–59854. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sun, X.; Xu, Y.; Wei, C.; Liang, G. By Self-Assembly of Electrostatic Attraction to Encapsulate Protective Carbon-Coated Nano-Si into Graphene for Lithium-Ion Batteries. Ionics 2022, 28, 1099–1108. [Google Scholar] [CrossRef]
- Ren, Y.; Xiang, L.; Yin, X.; Xiao, R.; Zuo, P.; Gao, Y.; Yin, G.; Du, C. Ultrathin Si Nanosheets Dispersed in Graphene Matrix Enable Stable Interface and High Rate Capability of Anode for Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2110046. [Google Scholar] [CrossRef]
- Shao, F.; Li, H.; Yao, L.; Xu, S.; Li, G.; Li, B.; Zou, C.; Yang, Z.; Su, Y.; Hu, N.; et al. Binder-Free, Flexible, and Self-Standing Non-Woven Fabric Anodes Based on Graphene/Si Hybrid Fibers for High-Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 27270–27277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Jin, H.; Zong, P.; Bai, Y.; Lian, K.; Xu, H.; Ma, F. A Robust Hierarchical 3D Si/CNTs Composite with Void and Carbon Shell as Li-Ion Battery Anodes. Chem. Eng. J. 2019, 360, 974–981. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Chen, D.; Fu, T.; Sun, D.; Zhao, J. Binder-Free Carbon-Coated Silicon–Reduced Graphene Oxide Nanocomposite Electrode Prepared by Electrophoretic Deposition as a High-Performance Anode for Lithium-Ion Batteries. ChemElectroChem 2016, 3, 757–763. [Google Scholar] [CrossRef]
- An, W.; Xiang, B.; Fu, J.; Mei, S.; Guo, S.; Huo, K.; Zhang, X.; Gao, B.; Chu, P.K. Three-Dimensional Carbon-Coating Silicon Nanoparticles Welded on Carbon Nanotubes Composites for High-Stability Lithium-Ion Battery Anodes. Appl. Surf. Sci. 2019, 479, 896–902. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Chou, S.; Xu, Y.; Li, W.; Kong, B.; Dou, S.X.; Liu, H.K.; Yang, J. Critical Thickness of Phenolic Resin-Based Carbon Interfacial Layer for Improving Long Cycling Stability of Silicon Nanoparticle Anodes. Nano Energy 2016, 27, 255–264. [Google Scholar] [CrossRef]
- Fang, H.; Zou, W.; Yan, J.; Xing, Y.; Zhang, S. Facile Fabrication of Fe2O3 Nanoparticles Anchored on Carbon Nanotubes as High-Performance Anode for Lithium-Ion Batteries. ChemElectroChem 2018, 5, 2458–2463. [Google Scholar] [CrossRef]
- Guan, B.; Wang, X.; Xiao, Y.; Liu, Y.; Huo, Q. A Versatile Cooperative Template-Directed Coating Method to Construct Uniform Microporous Carbon Shells for Multifunctional Core-Shell Nanocomposites. Nanoscale 2013, 5, 2469–2475. [Google Scholar] [CrossRef]
- Ma, X.Y.; Yin, Z.L.; Tong, H.; Yu, S.; Li, Y.; Ding, Z.Y. 3D Graphene-Like Nanosheets/Silicon Wrapped by Catalytic Graphite as a Superior Lithium Storage Anode. J. Electroanal. Chem. 2020, 873, 114350. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, Z.-Q.; Jia, R.; Yang, Y.; Zhu, F.-Y.; Yang, Z.-L.; Huang, Y.; Li, X.; Xu, W. Facile Electrostatic Self-Assembly of Silicon/Reduced Graphene Oxide Porous Composite by Silica Assist as High Performance Anode for Li-Ion Battery. Appl. Surf. Sci. 2018, 456, 379–389. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.L.; Zhang, X.F.; Luo, B.; Jin, M.H.; Liang, M.H.; Dayeh, S.A.; Picraux, S.T.; Zhi, L.J. Adaptable Silicon-Carbon Nanocables Sandwiched between Reduced Graphene Oxide Sheets as Lithium Ion Battery Anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef]
- Guo, C.Z.; Liao, W.L.; Li, Z.B.; Sun, L.T.; Chen, C.G. Easy Conversion of Protein-Rich Enoki Mushroom Biomass to a Nitrogen-Doped Carbon Nanomaterial as a Promising Metal-Free Catalyst for Oxygen Reduction Reaction. Nanoscale 2015, 7, 15990–15998. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Zhao, Y.; Li, Y.; Shi, J. A Novel Raspberry-Like Yolk-Shell Structured Si/C Micro/Nano-Spheres as High-Performance Anode Materials for Lithium-Ion Batteries. J. Alloys Compd. 2020, 844, 156201. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Peng, J.; Wu, Z.; Li, X.; Wang, X. Structure Design and Performance of the Graphite/Silicon/Carbon Nanotubes/Carbon (GSCC) Composite as the Anode of a Li-Ion Battery. Energy Fuels 2021, 35, 13491–13498. [Google Scholar] [CrossRef]
- Wang, S.; Liao, J.; Wu, M.; Xu, Z.; Gong, F.; Chen, C.; Wang, Y.; Yan, X. High Rate and Long Cycle Life of a CNT/RGO/Si Nanoparticle Composite Anode for Lithium-Ion Batteries. Part. Part. Syst. Charact. 2017, 34, 1700141. [Google Scholar] [CrossRef]
- Ramachandran, A.; Sarojiniamma, S.; Varatharajan, P.; Appusamy, I.S.; Yesodha, S.K. Nano Graphene Shell for Silicon Nanoparticles: A Novel Strategy for a High Stability Rechargeable Battery Anode. ChemistrySelect 2018, 3, 11190–11199. [Google Scholar] [CrossRef]
- Zhu, X.; Choi, S.H.; Tao, R.; Jia, X.; Lu, Y. Building High-Rate Silicon Anodes Based on Hierarchical Si@C@CNT Nanocomposite. J. Alloys Compd. 2019, 791, 1105–1113. [Google Scholar] [CrossRef]
- Xu, R.; Wei, R.; Hu, X.; Li, Y.; Wang, L.; Zhang, K.; Wang, Y.; Zhang, H.; Liang, F.; Yao, Y. A Strategy and Detailed Explanations to the Composites of Si/MWCNTs for Lithium Storage. Carbon 2021, 171, 265–275. [Google Scholar] [CrossRef]
- Fu, Z.; Bian, F.; Ma, J.; Zhang, W.; Gan, Y.; Xia, Y.; Zhang, J.; He, X.; Huang, H. In Situ Synthesis of a Si/CNTs/C Composite by Directly Reacting Magnesium Silicide with Lithium Carbonate for Enhanced Lithium Storage Capability. Energy Fuels 2021, 35, 20386–20393. [Google Scholar] [CrossRef]
- Kong, X.; Luo, S.; Rong, L.; Xie, X.; Zhou, S.; Chen, Z.; Pan, A. Enveloping a Si/N-Doped Carbon Composite in a CNT-Reinforced Fibrous Network as Flexible Anodes for High Performance Lithium-Ion Batteries. Inorg. Chem. Front. 2021, 8, 4386–4394. [Google Scholar] [CrossRef]
- Han, N.; Li, J.; Wang, X.; Zhang, C.; Liu, G.; Li, X.; Qu, J.; Peng, Z.; Zhu, X.; Zhang, L. Flexible Carbon Nanotubes Confined Yolk-Shelled Silicon-Based Anode with Superior Conductivity for Lithium Storage. Nanomaterials 2021, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, Y.; Qiao, Y.; Jiang, J.; Huang, M.; Qu, M.; Peng, G.; Xie, Z. 1-Aminopyrene-Modified Functionalized Carbon Nanotubes Wrapped with Silicon as a High-Performance Lithium-Ion Battery Anode. Solid State Ion. 2021, 369, 115724. [Google Scholar] [CrossRef]
- Park, G.D.; Choi, J.H.; Jung, D.S.; Park, J.-S.; Kang, Y.C. Three-Dimensional Porous Pitch-Derived Carbon Coated Si Nanoparticles-CNT Composite Microsphere with Superior Electrochemical Performance for Lithium Ion Batteries. J. Alloys Compd. 2020, 821, 153224. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Hou, Z.; Zhang, W.; Zhu, Y.; Qian, Y. A Deep Reduction and Partial Oxidation Strategy for Fabrication of Mesoporous Si Anode for Lithium Ion Batteries. ACS Nano 2016, 10, 2295–2304. [Google Scholar] [CrossRef]
- Luo, Z.; Xiao, Q.; Lei, G.; Li, Z.; Tang, C. Si Nanoparticles/Graphene Composite Membrane for High Performance Silicon Anode in Lithium Ion Batteries. Carbon 2016, 98, 373–380. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, X.; Ban, B.; Li, J.; Chen, J. Carbon Nanotubes-Enhanced Lithium Storage Capacity of Recovered Silicon/Carbon Anodes Produced from Solar-Grade Silicon Kerf Scrap. Electrochim. Acta 2021, 381, 138269. [Google Scholar] [CrossRef]
- Liang, A.-H.; Xu, T.-H.; Liou, S.; Li, Y.-Y. Silicon Single Walled Carbon Nanotube-Embedded Pitch-Based Carbon Spheres Prepared by a Spray Process with Modified Antisolvent Precipitation for Lithium Ion Batteries. Energy Fuels 2021, 35, 9705–9713. [Google Scholar] [CrossRef]
- Saha, S.; Jana, M.; Khanra, P.; Samanta, P.; Koo, H.; Chandra Murmu, N.; Kuila, T. Band gap modified boron doped NiO/Fe3O4 nanostructure as the positive electrode for high energy asymmetric supercapacitors. RSC Adv. 2016, 6, 1380–1387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, H.; Liu, Q.; Feng, X.; Yan, J.; Wang, L.; He, L.; Zhang, L.; Wang, G. Carbon-Coated Si Nanoparticles Anchored on Three-Dimensional Carbon Nanotube Matrix for High-Energy Stable Lithium-Ion Batteries. Batteries 2023, 9, 118. https://doi.org/10.3390/batteries9020118
Fang H, Liu Q, Feng X, Yan J, Wang L, He L, Zhang L, Wang G. Carbon-Coated Si Nanoparticles Anchored on Three-Dimensional Carbon Nanotube Matrix for High-Energy Stable Lithium-Ion Batteries. Batteries. 2023; 9(2):118. https://doi.org/10.3390/batteries9020118
Chicago/Turabian StyleFang, Hua, Qingsong Liu, Xiaohua Feng, Ji Yan, Lixia Wang, Linghao He, Linsen Zhang, and Guoqing Wang. 2023. "Carbon-Coated Si Nanoparticles Anchored on Three-Dimensional Carbon Nanotube Matrix for High-Energy Stable Lithium-Ion Batteries" Batteries 9, no. 2: 118. https://doi.org/10.3390/batteries9020118
APA StyleFang, H., Liu, Q., Feng, X., Yan, J., Wang, L., He, L., Zhang, L., & Wang, G. (2023). Carbon-Coated Si Nanoparticles Anchored on Three-Dimensional Carbon Nanotube Matrix for High-Energy Stable Lithium-Ion Batteries. Batteries, 9(2), 118. https://doi.org/10.3390/batteries9020118






