Abstract
Current Li battery technology employs graphite anode and flammable organic liquid electrolytes. Thus, the current Li battery is always facing the problems of low energy density and safety. Additionally, the sustainable supply of Li due to the scarce abundance of Li sources is another problem. An all-solid-state Mg battery is expected to solve the problems owing to non-flammable solid-state electrolytes, high capacity/safety of divalent Mg metal anode and high abundance of Mg sources; therefore, solid-state electrolytes and all-solid-state Mg batteries have been researched intensively last two decades. However, the realization of all-solid-state Mg batteries is still far off. In this article, we review the recent research progress on all-solid-state Mg batteries so that researchers can pursue recent research trends of an all-solid-state Mg battery. At first, the solid-state electrolyte research is described briefly in the categories of inorganic, organic and inorganic/organic composite electrolytes. After that, the recent research progress of all-solid-state Mg batteries is summarized and analyzed. To help readers, we tabulate electrode materials, experimental conditions and performances of an all-solid-state Mg battery so that the readers can find the necessary information at a glance. In the last, challenges to realize the all-solid-state Mg batteries are visited.
1. Introduction
Since the Li-ion battery (LIB) was commercialized in 1991, its application in portable electronic devices, such as laptop computers and mobile phones, has been widely achieved, significantly affecting our daily lives [1,2]. As the most successful battery technology, LIBs possess several advantages, including high energy density, no memory effect, good capacity retention, etc., overcoming the last generation lead-acid and nickel–hydrogen batteries [3,4]. Current LIBs rely on the intercalation mechanism. The energy density of LIBs has reached 240 Wh kg−1 and 670 Wh L−1 at the cell level due to the innovation and development of materials and cell design in these two decades [5,6]. However, the inherent limitation in the theoretical capacity of current graphite-based anodes makes it almost impossible for LIBs to meet the increasing demand for energy density [7]. Li metal anode is an ideal anode material due to its ultimate high theoretical capacity (Table 1), which can improve the energy density of the batteries. Typically, Li-LMO cells (LMO means Li transition metal oxides) have revealed a high energy density of ~440 Wh kg−1 [8]. However, the dendric growth of Li metal and the scarce abundance of Li sources have hindered the commercialization of the Li metal anode.
Solid-state electrolytes, which are solid-state ion conductors, can suppress dendrite growth due to their high mechanical strength [9]. In addition, their inflammable nature and wide electrochemical window can improve the safety and energy density of LIBs. Therefore, solid-state electrolytes and all-solid-state Li batteries have been researched intensively, especially in the last decade [10]. Regarding the low abundance of Li, it is considered that high-abundance elements, such as Na, K, Mg, Ca, Zn and Al, are employed as charge carriers instead of Li. Particularly, metal anodes of multivalent ions (Mg2+, Ca2+, Zn2+, Al3+) possess higher volumetric capacities than a monovalent Li metal anode. Table 1 summarizes the representative properties of metal anodes. As anode materials, a low redox potential and high specific capacity are desired to achieve the high energy density of batteries. Additionally, a small ionic radius ensures fast ion migration, leading to a high-power density. Mg is in high abundance in the earth’s crust and has a high volumetric capacity and a relatively low redox potential. Mg metal was believed to not form the dendrite. Even though a Mg dendrite formation was found in 2017 [11], Mg metal is less prone to the dendrite formation compared to Li and other metals, which has been verified in both experimental and theoretical studies [12,13]. The self-diffusion barrier of Mg is lower than that of Li, which leads to a uniform Mg metal deposition and tends to low dendrite formation. These features make Mg metal an ideal anode material. Therefore, research on an all-solid-state Mg battery and Mg2+-ion conductive solid-state electrolytes has been intensively carried out recently [14,15]. Usually, the research on solid electrolytes has been conducted by testing their chemical (crystal structure, crystallinity, glass transition temperature, etc.) and electrochemical properties (ionic conductivity, electrochemical window, transference number, etc.). The electrochemical properties are examined using blocking electrodes (Pt, Au, etc.) or metal anodes (Mg metal). Additionally, their compatibility with electrodes is characterized in all-solid-state batteries.
Solid-state electrolytes are categorized into the following three groups: organic, inorganic and organic–inorganic composite [16]. Organic solid-state electrolytes are typically flexible and easy to process for large-scale production. On the contrary, inorganic solid-state electrolytes commonly possess a high ionic conductivity, a high transference number and a wide electrochemical window. The organic–inorganic solid-state electrolytes are proposed and developed to address the defects of organic and inorganic solid-state electrolytes, which can combine the advantages of the flexibility and easy processing of organic solid-state electrolytes and the high conductivity of inorganic solid-state electrolytes [17]. Some good review articles focusing on solid-state electrolytes have been published recently [18,19], while research on all-solid-state Mg batteries has yet to be reviewed.
Therefore, in this review article, we focus on the recent research on all-solid-state Mg batteries, especially in the five years (2018~). At first, solid-state electrolyte research is described briefly in the categories of inorganic, organic and inorganic–organic composite electrolytes. After that, the recent research progress of an all-solid-state Mg battery is summarized and analyzed. To help readers, we tabulate electrode materials, experimental conditions (electrolyte and temperature) and performances (initial capacity and cyclability) of all-solid-state Mg batteries so that the readers can find the necessary information at a glance. Lastly, challenges to realize the all-solid-state Mg batteries are visited.

Table 1.
Properties of various metal anodes [20].
Table 1.
Properties of various metal anodes [20].
| Li | Na | K | Mg | Ca | Zn | Al | |
|---|---|---|---|---|---|---|---|
| Standard redox potential (E vs. SHE) | −3.04 | −2.71 | −2.93 | −2.37 | −2.87 | −0.76 | −1.66 |
| Volumetric capacity (mAh/cm3) | 2062 | 1128 | 591 | 3883 | 2073 | 5851 | 8046 |
| Specific capacity (mAh/g) | 3861 | 1166 | 685 | 2205 | 1337 | 820 | 2980 |
| Abundance (%) | 0.002 | 2.7 | 2.4 | 2.08 | 5 | 0.008 | 8.2 |
| Ionic radius (Å) | 0.76 | 1.02 | 1.38 | 0.72 | 1.00 | 0.74 | 0.535 |
| Relative atomic mass | 6.94 | 22.98 | 39.1 | 24.31 | 40.08 | 65.39 | 26.98 |
| Mass to charge | 6.94 | 22.98 | 39.1 | 12.16 | 20.04 | 32.7 | 8.99 |
2. Solid Electrolytes for Mg Battery
2.1. Inorganic Electrolyte
2.1.1. Oxides
Na+ super ion conductor (NASICON), Na1+xZr2P3-xSixO12, is well-known for permitting the fast migration of the Na+ ion due to the well-ordered three-dimensional network structure [21]. By the successful application of the NASICON structure to a Li+ ion conductive ceramic electrolyte, such as LiZr2(PO4)3 [22,23], Li1+xAlxTi2−x(PO4)3 [24,25] or Li1+xAlxGe2−x(PO4)3 [26,27], it has been highly interested in developing NASICON-type multivalent ion conductors [28,29]. The oxide-based solid electrolytes are usually prepared via calcination of raw materials followed by sintering.
The first report on NASICON-type Mg2+ ion conductors was MgZr4(PO4)6 (MZP) in 1987 [30]. The ionic conductivity was 2.9 × 10−5 and 6.1 × 10−3 S cm−1 at 400 and 800 °C, respectively. MZP was assigned as the rhombohedral structure first, but later it was ascribed to the monoclinic structure, which is like β-Fe2SO4. Nakayama et al. simulated Mg2+ migration energy of 0.63 and 0.71 eV in the rhombohedral and monoclinic structures, respectively [31]. Thus, heteroatom doping into the Zr4+ site to stabilize the rhombohedral structure has been carried out [32,33]. Contrary, another strategy, the introduction of Hf4+ into the Mg2+ site has also been attempted [34]. However, all results exhibited too low Mg2+ ion conductivity, ~10−5 S cm−1, even at 500 °C. Those cannot be applied for an all-solid-state Mg battery operated at ambient temperature. It is noted that some promising results were reported by Mohamed’s group. They reported σ = 3.97 × 10−4 S cm−1 at room temperature in Mg1.05Zn0.4Al0.3Zr1.3(PO4)3. However, this material demonstrated almost the same conductivity, 5.82 × 10−4 S cm−1 at 500 °C [35]. The extremely low activation energy of 0.039 eV was about one order of magnitude lower than that of typical Li+ ion conductive ceramics. They also estimated the Mg2+ ion transference number by the Bruce method to 0.84 [33]. This implies that 16% of electric charge is carried by an anion, i.e., oxygen ion. In addition, they measured the electrochemical window of Mg0.5Si2(PO4)3 to 3.21 V; however, as shown in Figure 1, it seems the decomposition of electrolyte commences below 2 V [36]. We need to re-check their results.
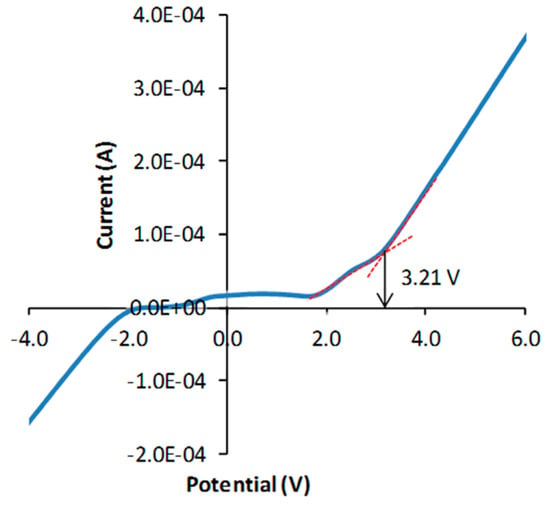
Figure 1.
Linear sweep voltammogram of Mg0.5Si2(PO4)3. Reproduced with permission [36]. Copyright 2016, Elsevier.
In other research, although magnesium phosphate (Mg2.4P2O5.4) [37], magnesium silicate (Mg0.6Al1.2Si1.8O6) [38] and magnesium tungstate (MgHf(WO4)3) [39] are researched, a significant improvement of conductivity is still needed. Indeed, all-solid-state Mg batteries using oxide-based solid electrolytes have not been reported yet.
2.1.2. Chalcogenides
Although chalcogenide-based Li+ ion conductive ceramics like sulfides have succeeded greatly [40], chalcogenide-based Mg2+ ion conductive ceramics were not researched intensively. Only one paper was published about the MgS-P2S5 system in 2014 [41]. However, since Canepa et al. reported trinary spinel chalcogenides with a high Mg2+ ion mobility in 2017 [42], research on the chalcogenide-based Mg2+ ion conductive ceramics has been activated. They predicted the least migration energy of the Mg2+ ion that appeared in MgY2S4, MgY2Se4 and MgSc2Se4, and the values were 360, 361 and 375 meV, respectively (Figure 2); however, only MgSc2Se4 has been successfully synthesized so far. The Mg2+ ion conductivity of MgSc2Se4 was estimated to be ~1 × 10−4 S cm−1, comparable to Li+ ion conductive ceramic electrolytes (garnet-type and NASICON-type) [43]. Unfortunately, electronic conductivity was also relatively high, about 4 × 10−8 S cm−1. Thus, Fichtner et al. synthesized Se-excess, Ti4+, Ce4+-doped MgSc2Se4 to reduce the electronic conductivity, but the electronic conductivity was not drastically lowered [44]. Based on this, they used MgSc2Se4 as a cathode material for a Mg battery using liquid electrolytes. Kundo et al. studied the electronic conduction mechanism of MgSc2Se4 and found that the electronic conductive layer was formed on the surface of particles during the ball milling process [45]. In fact, the electronic conductivity was reduced by avoiding the ball milling process. In addition, the ionic and electronic conductivities of MgSc2Se4 were largely influenced by the sintering process, particularly the cooling process. Indeed, the field-assisted synthesis could sinter MgSc2Se4 in a very short time, leading to a low electronic conductivity of ≈10−11 S cm−1 [46]. Lowering the electronic conductivity of MgSc2Se4 is a critical issue to apply for all-solid-state Mg batteries. Advanced sintering techniques, which can provide rapid heating/cooling rates and short heat treatments, such as spark plasma sintering (SPS) [23], flash sintering [47], microwave sintering [48] and ultrafast high-temperature sintering [49], should be applied for sintering MgSc2Se4.
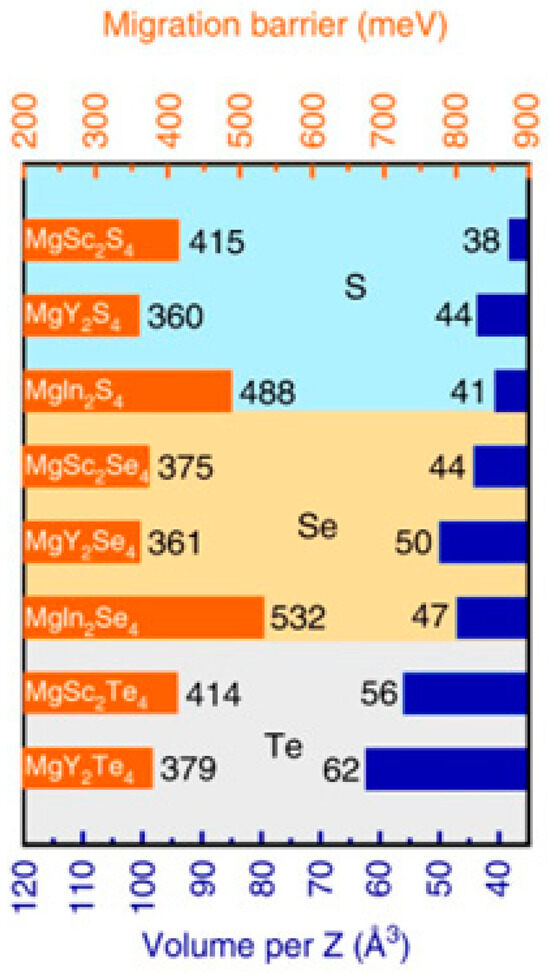
Figure 2.
Computed Mg2+ ion migration barrier in trinary spinel chalcogenides. Reproduced with permission [42]. Copyright 2017, Nature Communications.
The electrochemical window of MgSc2Se4 has not been studied yet, but Au/MgSc2Se4/Au cell was stable during 3 V of applied voltage [46]. Therefore, MgSc2Se4 would possess a reasonable electrochemical window for all-solid-state Mg battery application.
The development of MgSc2Se4 has the following two directions: electrolytes and electrodes. The electronic conductivity of MgSc2Se4 must be lowered for electrolytes while it is maintained for electrodes. The all-solid-state Mg battery composed of MgSc2Se4-based electrodes and MgSc2Se4-based electrolytes should have an intimate electrode/electrolyte interface due to the similar chemical composition and structure, resulting in high and stable performances.
Properties of oxide- and chalcogenide-based electrolytes are summarized in Table 2.

Table 2.
Properties of various oxide- and chalcogenide-based solid electrolytes.
2.1.3. Hydrides
In 2012, Matsuo et al. reported possible Mg conduction in Mg(BH4)2 based on an FPMD simulation [61]. Later, Higashi et al. experimentally proved Mg ion conduction of Mg(BH4)2 and Mg(BH4)(NH2) [62]. Since then, Mg(BH4)2-based electrolytes have been researched the most intensively among the inorganic solid electrolytes. The Mg(BH4)2-based solid electrolytes are usually prepared by mechanical milling without sintering. Also, an all-solid-state Mg battery with inorganic electrolytes has been fabricated by using only Mg(BH4)2-based electrolytes (Chapter 3.1). Although the ionic conductivity of Mg(BH4)(NH2) is 1 × 10−6 S cm−1 at 150 °C [62], it is influenced by the synthetic parameter, for example, the ionic conductivity of 3 × 10−6 S cm−1 at 100 °C was obtained in a glass-ceramic like Mg(NH4)(NH2) [63]. As other strategies, the modification of BH4 ligands [64,65], partial oxidation [66], compositing with ceramic oxides such as MgO, YSZ, TiO2 and Al2O3 [67,68,69,70] have been attempted, and all achieved an improvement of Mg ion conductivity to 10−5~10−6 S cm−1 at ambient temperature, which is slightly lower than those of Li and Na ion conductive inorganic solid electrolytes [10]. Additionally, they exhibited a stable Mg plating/stripping behavior (Figure 3). However, the modification narrowed the electrochemical window to about 1.2~1.4 V. This restricts the choice of cathode materials and decreases the energy density of all-solid-state Mg batteries. Although the moderate ionic conductivity and stability against the Mg metal anode of Mg(BH4)(NH2)-based inorganic solid electrolytes are desirable, the improvement of anodic stability must be considered. Properties of hydride-based electrolytes are summarized in Table 3.
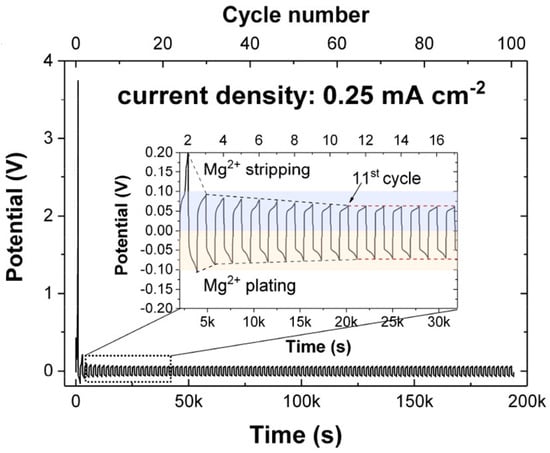
Figure 3.
Galvanostatic cycling of a symmetric Mg/Mg(BH4)2 1.6NH3-MgO cell at 60 °C with a constant current density of 0.25 mA cm−2. Reproduced with permission [69]. Copyright 2020, American Chemical Society.

Table 3.
Properties of hydride-based solid electrolytes.
2.1.4. MOF (Metal–Organic Framework)
MOFs are crystalline solids composed of metal ions coordinated by multifunctional organic molecules with a three-dimensional porous structure. The composition and structure of MOFs can be easily adjusted via the rational selection of the metal ion and organic molecules [73]. Due to the porous structure, diffusivities of guest ions in the pores would be similar to those in a molten salt state [74]; therefore, MOFs have been studied as ionic conductors. To introduce guest ions, pores of MOFs are filled with liquid electrolytes. Thus, MOF-based solid electrolytes would be categorized into liquid–solid composite electrolytes.
Compared to MOF-based Li+ ion conductive electrolytes, the studies on Mg2+ ion conductors are still few, only eight papers have been published so far. The conductivity of MOF-based Mg ion conductive electrolytes ranges from 10−4 to 10−6 S cm−1. Since MOF-based solid electrolytes contain liquid electrolytes, the transference number of Mg2+ ions should be studied, as well as their electrochemical window. Only three papers reported those, 0.25~0.49 of transference number and about 3 V vs. Mg/Mg2+ of oxidative stability [75,76,77]. On the contrary, stable Mg plating/stripping behavior was observed in four papers (Figure 4) [75,76,77,78]; therefore, a Mg metal anode can be applied for the MOF-based electrolytes. Typically, MOF-based Mg ion conductive electrolytes contain around 45~55 wt.% of solvent, which is comparable to gel-polymer electrolytes [79]; however, their conductivities and mechanical properties are not inversely proportional. Hassen et al. reduced the liquid content in MOFs to around 20 wt.% and reported that ionic conductivity was not primarily affected [75]. Also, the same group found conductivity enhancement by treatment of MOF at 150 °C for 24 h, probably due to the removal of coordinated water.
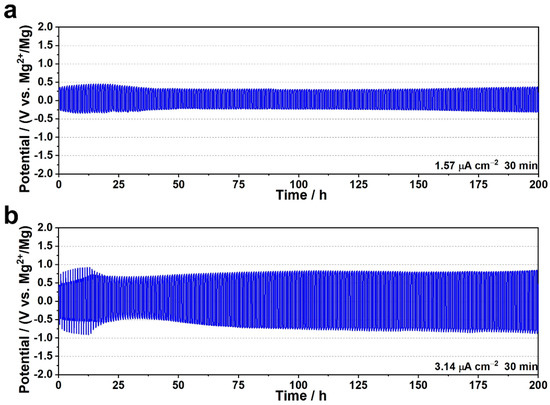
Figure 4.
Mg plating/stripping test of UiO-66-Mg(TFSI)2/[EMIM][TFSI] electrolyte at current density of (a) 1.57 μA cm−2 and (b) 3.14 μA cm−2 at 60 °C. Reproduced with permission [76]. Copyright 2022, Wiley-VCH GmbH.
The MOF-based electrolytes are likely to be stable for a Mg metal anode, a relatively high anodic stability, ~3 V, and a high conductivity, which makes them a good candidate for all-solid-state Mg batteries; however, there are still a lot of unknowns. Particularly, the correlation of the pore structure and the composition of a liquid electrolyte (salt, solvent, and salt concentration) with chemical/electrochemical properties must be clarified. Properties of MOF-based solid electrolytes are summarized in Table 4.

Table 4.
Properties of MOF-based solid electrolytes.
2.2. Organic Electrolyte
Organic electrolytes, namely, polymer electrolytes, are composed of polymer hosts and Mg salts (solid polymer electrolytes, SPEs). In some cases, fillers and plasticizers are added to improve the properties. SPEs have been reported the most, while SPEs with plasticizers are most widely employed for all-solid-state Mg batteries. Herein, recent research on organic electrolytes is briefly summarized. Recently, novel organic electrolytes, i.e., organic crystal electrolytes, have been developed. Organic crystal electrolytes are introduced at the end of this section. For Mg2+ ion conductive electrolytes, only inorganic fillers are employed. Therefore, filler-added polymer electrolytes are reviewed in the next Section 2.3 “Organic-inorganic composite electrolytes”.
2.2.1. Solid Polymer Electrolytes
SPEs are composed of host polymers and Mg salts. The Lewis-base moieties of host polymers allow the dissociation of Mg salts, resulting in an emerging Mg2+ ion conduction. Accordingly, the host polymers contain atoms with lone-pair electrons, such as oxygen, fluorine and nitrogen atoms. Figure 5 depicts the structures of various host polymers. SPEs are prepared by the solution-casting method. Polymer hosts and Mg salts are dissolved into a solvent and then cast onto a substrate to obtain SPE films.
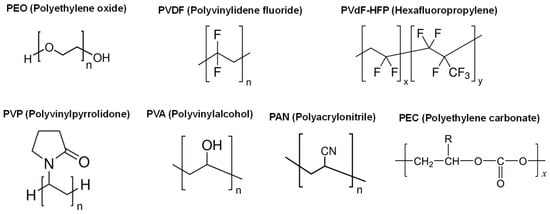
Figure 5.
Structures of various host polymers used in Mg2+ ion conductive polymer electrolytes.
Common host polymers, such as PEO, have been used in Mg2+-ion conductive SPEs [83,84]. Different from Li+ ion conductive SPEs, water-soluble polymers like PVP and PVA are also used [85,86,87,88]. Such polymers allow the use of water as a solvent, facilitating preparation processes and reducing production costs. The ionic conductivities of SPEs using the water-soluble polymer hosts are comparable (10−5~10−6 S cm−1) to the conventional polymer hosts; however, other properties like the electrochemical window are seldom studied. Studies on the other properties must be carried out to clarify the applicability of the water-soluble polymer hosts.
Solvents used for polymer casting would influence the properties of SPEs. Unfortunately, direct research on the influence of solvents has not been performed. Organic and inorganic solvents have been usually employed for synthetic and natural polymers, respectively. A large performance difference by solvents was not confirmed among organic and inorganic solvents.
Natural polymers are also used as host polymers for Mg2+ ion conductive SPEs [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. Natural polymers are attractive in terms of environmental friendliness and resource abundance. Natural polymer-based SPEs exhibit better conductivity, around one order of magnitude higher than synthetic polymer-based SPEs. Notably, SPEs composed of potato starch and Gellan gum reveal a high ionic conductivity of ~10−2 S cm−1 [92], which is comparable to Li10GeP2S12 and even liquid electrolytes [105]. In addition, these SPEs possess high flexibility and are promising for all-solid-state Mg batteries. However, their application to all-solid-state Mg batteries has yet to be reported. The environmental friendliness of natural polymers means that natural polymers will decompose naturally in the long term. Thus, the long-term stability of natural polymer-based SPEs must be tested.
To improve the properties of SPEs, a polymer blend, namely, a mixture of two host polymers, was also studied [106,107,108,109,110,111,112,113,114,115,116]. By the blending, the ionic conductivity increases by one order of magnitude, 10−3~10−4 S cm−1, which is applicable to all-solid-state Mg batteries. Recently, the blend of natural and synthetic polymers emerged as a new research trend in SPEs [117,118,119,120]. For example, in the blend of methyl cellulose (MC) and PVA, the hydrogen bond forms between MC and PVA, stabilizing the polymer blend [119]. In addition, rich-oxygen atoms in MC facilitate the dissociation of Mg salts. As a result, the ionic conductivity increased to ~10−4 S cm−1, which is applicable to all-solid-state Mg batteries [121].
Regarding the Mg salts, in addition to commonly used metal salts in LIBs like TFSI, ClO4-salts, more cost-effective MgSO4, Mg(NO3)2, MgCl2, etc., are used. Thus, water-soluble polymers, such as PVA and PVP, are used for these salts. The ionic conductivity was not influenced by the Mg salts. Aziz et al. added LiFSI into PEC-Mg(TFSI)2 SPE [122]. The ionic conductivity was improved by one order of magnitude by the addition. Also, the Li-contained SPE demonstrated stable Mg stripping and plating. The usage of mixing salt is a new concept for the SPEs. In this system, the contribution of Li-ion conduction must be considered to estimate the ionic conductivity. However, characterization techniques to extract only Mg2+ ion conduction have not been developed yet; therefore, the characterization of the system should be given extra consideration.
Many types of polymers and Mg salts are studied for Mg2+-ion conductive SPEs. Most studies focus on the ionic conductivity, however, other properties, such as electrochemical window, transference number and compatibility with electrodes, are also important for application of all-solid-state batteries. Thus, studies on SPEs should be performed more comprehensively. Properties of SPEs are summarized in Table 5.

Table 5.
Properties of SPEs.
2.2.2. Polymer Electrolytes with Plasticizers (Gel-Polymer Electrolytes)
In general, plasticizers are used to soften a material, to increase its plasticity and to decrease its viscosity. In polymer electrolytes, plasticizers lower Tg (glass-transition temperature) and activate the segmental motion of polymer chains, enhancing ionic conductivity. Studies on all-solid-state Mg batteries have been performed using GPEs the most.
As Li+-ion conductive polymer electrolytes, low molecular weight solvents and ionic liquids have been used as plasticizers (Figure 6). Because plasticizers soften SPEs, the optimum amount of plasticizers must be found to balance the ionic conductivity and mechanical properties of GPEs. The optimum amount of plasticizers varies significantly by the plasticizers used. In the GPE using PYR14TFSI ionic liquid, the highest ionic conductivity was obtained at 10 wt.% of plasticizers [126]. Contrarily, the plasticizer amount of 200 wt.% was reported in the TEGDME system [127]. In such high plasticizer content, it is questioned whether the ionic conduction is mainly caused by the segmental motion of the polymer or dissolved Mg salt in the liquid part.
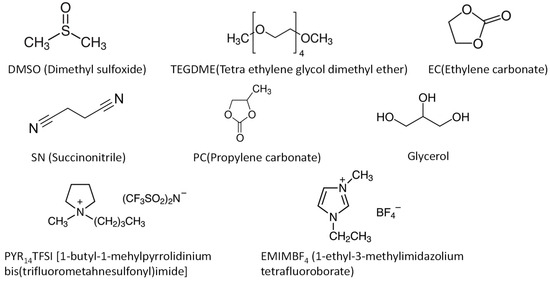
Figure 6.
Structures of various plasticizers used in Mg2+ ion conductive polymer electrolytes.
In GPEs, synthetic polymers are mostly studied. Their conductivity ranges from 10−4 to 10−3 S cm−1, which can be applied for all-solid-state batteries. Gupta et.al. reported a high ionic conductivity of 2 × 10−2 S cm−1 in [PVdF-HFP(30 wt.%)-EMIMBr(70 wt.%)] (30 wt.%)-[PC-Mg(ClO4)2 (0.3 M)] (70 wt.%) system [128]. However, cell data were not reported although the high ionic conductivity is promising. Mixed plasticizers like EC-SN [129] and EC-DEC [130] have also been studied, but they did not significantly improve properties compared with single plasticizers.
The inorganic magnesium aluminum chloride complex (MgCl2-AlCl3, MACC) has been studied in liquid electrolytes [131]. With the addition of AlCl3, the dissociation of MgCl2 is promoted, increasing the solubility of the MgCl2 and Mg2+-ion concentrations. As a result, Mg stripping/plating over potential can be drastically decreased [132]. Wang et al. applied this concept to GPEs for the first time [133]. The ionic conductivity of their PVDF-HFP-based GPEs containing a MgCl2-AlCl3 salt and a TEGDME plasticizer was 4.7 × 10−4 S cm−1. Although this value was comparable to other GPEs, the reversibility of Mg stripping/plating was drastically improved. In polymer electrolyte research, the effect of Mg salt has not been studied intensively. Their results clearly show the importance of Mg salt on the performance of all-solid-state Mg batteries.
In another important study, a single-ion conductive polymer electrolyte was reported by Schaefer et al. [134]. In the single-ion conductive polymer electrolyte, the anion part of Mg salt was polymerized with host polymers. Thus, the mobility of the anion is zero; in other words, the cation transference number is 1. Therefore, undesired side reactions caused by anions can be avoided completely. The authors prepared a P(PEGDMA)-P(TFSI) [poly (ethylenglycol) dimethacrylate- poly styrensulfonyl (trifluoromethylsulfonyl)] network (Figure 7). In this structure, TFSI moiety is involved in the polymer chain, resulting in the immobilization of anion. This type of polymer generally shows a low ionic conductivity due to the low segmental motion of the polymer chain. Thus, the DMSO plasticizer was added, and the Mg2+-ion conductivity was increased to 8.8 × 10−4 S cm−1. It is noted that the high conductivity was achieved by only Mg2+-ion transportation. Some other studies reported higher conductivities; however, the conductivities contain anion transportation. Therefore, the high Mg2+-ion conductivity of single-ion conductive polymer is very attractive. Unfortunately, cell data were not reported.
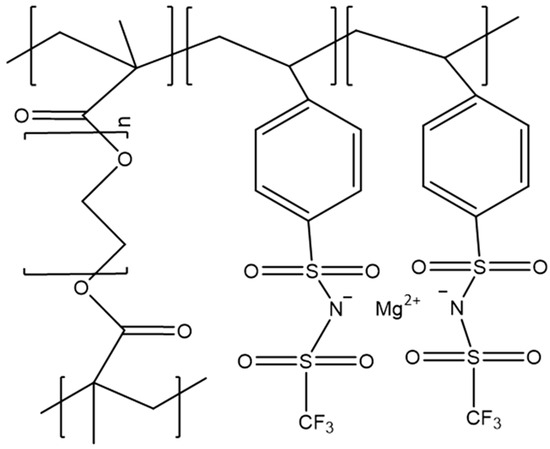
Figure 7.
Structures of magnesiated P(PEGDMA)-P(STFSI) network.
In GPEs, mixed salts like MACC and the single-ion conductive polymer are studied. Such studies have been carried out only in GPEs. Their superior properties are promising to apply for all-solid-state Mg batteries. This concept should be investigated intensively and used in other polymer electrolytes, such as organic–inorganic composite electrolytes. Properties of GPEs are summarized in Table 6.

Table 6.
Properties of GPEs.
2.2.3. Organic Crystal Electrolytes
Recently, Moriya’s group reported a new type of organic electrolyte, organic crystal electrolytes. They are composed of organic molecules and Mg salts and possess ion conduction paths in the crystal lattice (Figure 8). The paths are precisely controlled by organic molecules and Mg salts. Only two Mg ion conductive organic crystal electrolytes have been reported so far [154,155]. In both cases, their room temperature conductivities were higher or comparable to Mg(BH4)2-based inorganic electrolytes, and the cation transference number was higher than that of polymer-based electrolytes. These properties would be improved by adjusting the ion conduction paths. Unfortunately, cell data using the organic crystal electrolytes are not available at the moment. There is still a lot of room to develop organic crystal electrolytes. Properties of organic crystals are summarized in Table 7.
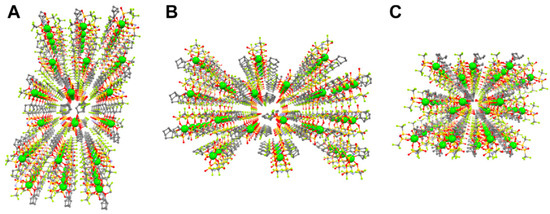
Figure 8.
Packing view with ball and stick model of Mg(TFSA)2(CPME)2. Mg-ions are emphasized as a large sphere. (A) along the a-axis. (B) along the b-axis, and (C) along the c-axis (Mg: green, C: gray, N: pale blue, O: red, F: pale green, S: dark yellow. H atoms are omitted for clarity). Reproduced with permission [154]. Copyright 2021, Frontiers.

Table 7.
Properties of organic crystals.
2.3. Organic–Inorganic Composite Electrolytes
2.3.1. Solid Polymer Electrolytes with Fillers
In the Li+ ion conductive polymer electrolytes, various types of fillers, such as organic/inorganic fillers and active/passive (Li+ ion conductive/non- Li+ ion conductive) fillers are researched. Contrarily, in the Mg2+ ion conductive polymer electrolytes, only inorganic passive fillers, especially metal oxides, are studied. In addition, only nano-particle morphology, not nano-wire, nano-sheet, etc., is employed. Among the studies, the highest conductivity was obtained in filler contents of 3~7 wt.% regardless of the fillers. An interesting study was carried out by Jayanthi et al., in which the ferroelectric material, BaTiO3, was added to PVDF-HFP/MgTf polymer electrolyte as a filler [156]. The presence of ferroelectric domains in the polymer electrolyte facilitates salt dissociation and helps the amorphization of the polymer, enhancing ionic conductivity. A similar study was reported in Na+ ion conductive polymer electrolyte [157]. In this case, K0.5Na0.5NbO3 (KNN) was used as a ferroelectric filler, and it decreased the ionic conductivity of polymer electrolytes, while the stability against the Na metal anode was improved. As a result, a better performance of an all-solid-state Na battery was obtained. This is a good result that electrolyte performance is determined by conductivity and interface properties between electrodes and electrolytes. Thus, electrolyte study must include the construction and evaluation of an all-solid-state battery. Unfortunately, studies on only solid electrolytes have been reported much more than all-solid-state batteries. Properties of SPEs with fillers are summarized in Table 8.

Table 8.
Properties of SPEs with fillers.
2.3.2. Solid Polymer Electrolytes including Plasticizers and Fillers
In this system, polymer electrolytes contain both inorganic fillers and plasticizers. As mentioned, low molecular weight solvents and ionic liquids are employed as plasticizers. Contrarily, metal oxides are used as fillers. Aziz et al. used Ni metal nanoparticles as fillers [172]. Metal fillers have not been applied to Mg2+-ion conductive polymer electrolytes except in this paper. To clarify the effect of metal filler, more research is needed. Sharma et al. added EC-PC and MgAl2O4 into PVDF-HFP/Mg(Tf)2 polymer electrolytes [173]. This system revealed a high transference number of 0.66, which is one of the highest transference numbers in Mg2+-ion conductive polymer electrolytes.
The ionic conductivity of filler/plasticizer-containing polymer electrolytes ranges from 10−5 to 10−3 S cm−1. These values are comparable to other types of polymer electrolytes. Thus, the benefits of using both plasticizers and fillers cannot be emphasized. Currently, the individual effect of plasticizers and fillers on the properties of polymer electrolytes has yet to be fully understood. Thus, the individual effects of plasticizers and fillers must be clarified first. Then, more a complicated system, i.e., polymer electrolytes containing both plasticizers and fillers, should be developed based on the individual effect. Properties of SPEs with plasticizers and fillers are summarized in Table 9.

Table 9.
Properties of SPEs with plasticizers and fillers.
3. All-Solid-State Mg Battery
3.1. Inorganic Electrolyte
Research on all-solid-state Mg batteries using oxide-based electrolytes is not reported. The Mg2+-ion conductivity of the oxide electrolytes is 10−6~10−7 S cm−1 at room temperature. This is too low to support ion conduction in all-solid-state batteries operated at room temperature. Thus, an improvement of room temperature ionic conductivity to at least 10−4 S cm−1 level is needed first. In the case of MgSc2Se4-related materials, although they possess a high Mg2+-ionic conductivity, their electronic conductivities are also high. Thus, these materials are not studied for electrolytes for all-solid-state batteries.
All-solid-state Mg batteries using inorganic electrolytes are reported only in borohydride electrolytes, i.e., Mg(BH4)2-related materials. The materials are usually prepared by mechanical milling, and all-solid-state cells are constructed by pressing Mg metal anodes and cathodes onto the solid electrolyte pellets. While these materials demonstrate good Mg stripping/plating behaviors [62,64,65,67,69], only two papers reported that with respect to full cell configurations. In both cases, TiS2 was used as a cathode, and electrochemical tests were carried out above room temperature (55 and 75 °C) to obtain reasonable capacity. The obtained discharge capacity was about 100 mAh/g, although the C rate was low (141 mAh/g at 0.05 C at 75 °C). Also, capacity decay occurs rapidly (31% capacity retention at the 25th cycle). TiS2 cathode exhibits large (>100 mAh/g) and stable (>100 cycles) capacities at room temperature and a reasonable C rate (1~2 C) in liquid electrolytes [131]. Thus, the low performance of an all-solid-state battery would be caused by a low conductivity of solid electrolytes and a high impedance/low stability of the cathode/electrolyte interface. Indeed, the interface impedance increased with the cycle number [72].
As mentioned, an improvement of room temperature ionic conductivity is needed to realize all-solid-state Mg batteries using inorganic electrolytes. Additionally, various cathode materials and properties of the electrode/electrolyte interface must be tested and characterized, respectively. In summary, all-solid-state Mg batteries using inorganic electrolytes are still far from realization.
3.2. MOF
In the all-solid-state Mg batteries using MOF-based electrolytes, since MOFs are used to support liquid electrolytes, they can be said “Quasi-solid electrolytes”. Although stable Mg stripping/plating is observed, only one paper reported it in the full cell configuration. The full cell using PTCDA (Perylenetetracarboxilic dianhydride) cathode demonstrates a small discharge capacity of 36 mAh/g at 1 mAh/g at 60 °C. The capacity fade was also large; only 61% of capacity remained in the 3rd cycle. The PTCDA cathode is employed for Na and K batteries [179,180] and reveals a good performance. However, it has not been applied for Mg batteries, even including liquid electrolytes. Therefore, the poor performance of all-solid-state Mg batteries using MOF-based quasi-solid electrolyte has been attributed to some factors, such as the cathode itself, the cathode/electrolyte interface, the electrolyte itself and so on. The usage of common cathode materials like MoS6 can reduce the factors, facilitating the evaluation of all-solid-state Mg batteries. Consequently, the common cathode materials should be used for the MOF-based quasi-solid electrolytes at this moment.
Since stable Mg stripping/plating was achieved at room temperature in MOF-based solid electrolytes, they would be attractive for all-solid-state Mg battery applications; therefore, studies on cathode side must be carried out intensively.
3.3. Organic Electrolyte
Although studies on SPEs (without fillers and plasticizers) are reported by many groups, SPEs are not applied for all-solid-state Mg batteries. Some groups studied primary Mg batteries using SPEs [90,94,101,103,104,108,115,117,119,123]. Since this review article focuses on rechargeable all-solid-state Mg batteries, the studies on primary batteries are not introduced here. The main reason for the lack of research on SPEs for rechargeable all-solid-state Mg batteries is their relatively low ionic conductivity. The conductivity of most SPEs ranges 10−5~10−7 S cm−1 at RT. An improvement of the conductivity to 10−3~10−4 S cm−1 is needed to achieve reasonable performance of all-solid-state Mg batteries. Potato starch [89] and gellan gum [92]-based SPEs exhibit an extremely high ionic conductivity of ~10−2 S cm−1. These are two orders of magnitude higher than other SPEs. A close investigation on these SPEs, such as reproducibility and characterization procedures, should be performed since such a very high conductivity of these SPEs cannot be easily accepted.
Gel Polymer Electrolyte
Gel polymer electrolytes (GPEs) composed of post polymers, Mg salts and plasticizers possess a higher conductivity, 10−3~10−4 S cm−1, than SPEs. Additionally, their high flexibility makes battery construction easy. Thus, all-solid-state Mg batteries using GPEs are reported the most. The all-solid-state Mg batteries using GPEs can be operated at room temperature [127,133,140,148]; however, in all cases, initial and steady-state capacities are low. Because stable Mg stripping/plating is observed in GPEs, the cathode itself and the cathode/electrolyte interface would be the reason for the low performance. Detail characterization and post-mortem analysis of the interface and cathode should be carried out.
Ge et al. fabricates pouch cell-type all-solid-state Mg batteries for the first time [127]. Only this study reports the performance of a pouch cell-type all-solid-state Mg battery with GPE. The pouch cell can reduce the weight of battery cases, resulting in a high energy density. Additionally, the authors performed safety tests, such as cutting the pouch cell and flammability tests of GPE and pouch cell. The study is meaningful in verifying the possible application of pouch cell configuration for all-solid-state Mg batteries, although an improvement in the performance of the pouch cells is needed.
Sheha et al. studied dual polymer/liquid electrolytes (Figure 9) [135]. The electrolyte is composed of the following two layers: liquid electrolyte and GPE. Both electrolytes are separated by a glass fiber membrane. The liquid electrolyte (APC, all phenyl complex) and GPE are faced on the cathode and anode (Mg metal) sides, respectively. In the all-solid-state battery, poor contact between a porous electrode and a solid electrolyte increases the impedance of the battery and causes low performance. At the moment, an effective solution for the contact issue has yet to be found. Their concept would be helpful in solving the contact issue. Therefore, the dual electrolyte configuration is likely to be applied for first-generation all-solid-state Mg batteries. The authors reported a high initial capacity using the BaTiO3 cathode (557 mAh/g at 20 mA/g at 55 °C), but the capacity was rapidly decayed within 15 cycles. The reason for a low cyclability is unclear since the cyclability of the BaTiO3 cathode has not been tested in liquid electrolytes. The dual electrolytes should be studied using common cathode materials at first. For safety, the usage of the flammable liquid electrolytes must be minimized. The influence of the liquid electrolyte on the safety of the batteries, the formation of CEI (cathode-electrolyte interphase) and the properties of the new interface, i.e., the GPE/liquid electrolyte should be studied for successful application of the dual electrolyte system (In fact, the battery is not pure all-solid-state Mg batteries since the batteries contain a small amount of liquid electrolyte).
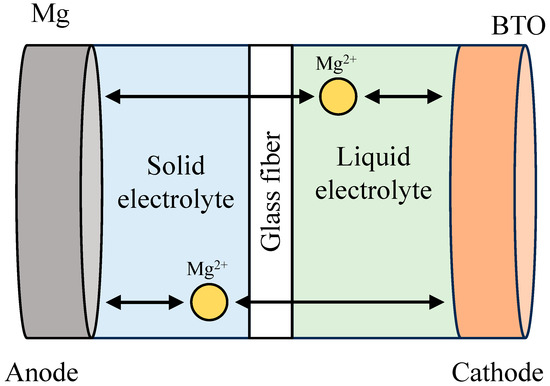
Figure 9.
Configuration of Mg battery using the dual GPE/liquid electrolyte (Mg/GPE/APC/BaTiO3 cathode). Reproduced with permission [135]. Copyright 2020, American Chemical Society.
In the GPE research, Mo6S8, which is the most commonly used cathode material, is applied for the all-solid-state battery [127,148]. This facilitates the evaluation of the all-solid-state batteries since the performance of the cathode has been studied in liquid electrolytes for a long time. Mo6S8 cathode provides a high capacity (130 mAh g−1 at 0.1 C and 98 mAh g−1 at 0.5 C) in liquid electrolytes [181,182]. Contrarily, the all-solid-state batteries using GPEs demonstrate about 70 mAh g−1 (68 mAh g−1 at 0.1 C [148] and 73 mAh g−1 at 0.3 C [127]) even though the same cathode materials are used. Because the ionic conductivity of electrolytes would not largely influence battery performance at such a low C rate, the high impedance at the cathode/electrolyte interface could be a reason for the low performance. Common electrode materials should be used for all-solid-state batteries more intensively. It is interesting to note that better battery performance was obtained in the PECH-OH-based GPE compared to the PTHF-based GPE. As shown in Table 6, the PTHF-based GPE revealed about two orders of magnitude higher Mg2+-ion conductivity than the PECH-OH-based GPE. This is a good example that battery performance is determined by not only the properties of electrolytes. GPE is the most promising for all-solid-state Mg batteries at this moment. The ionic conductivity of GPEs is comparable to Li+-ion conductive polymer electrolytes. Thus, compatibility with electrodes and properties of the electrode/electrolyte interface would determine the performance of all-solid-state Mg batteries. Many studies on GPEs are reported, while their application to all-solid-state Mg batteries is not researched intensively. Such study is strongly required, not only simple studies on properties of GPEs, to realize all-solid-state Mg batteries.
3.4. Organic–Inorganic Composite Electrolytes
Only three papers have been reported in terms of all-solid-state Mg batteries using the organic–inorganic composite electrolytes recently [168,177,178]. Compared with GPEs, all-solid-state Mg batteries with composite electrolytes demonstrate a better initial performance and cyclability. Particularly, when SPEs contain both fillers and plasticizers, a very stable cyclability was achieved, although only two papers were reported. Both papers exhibited 99% capacity retention at the 100th cycle [177] and 98% capacity retention at the 70th cycle [178]. The compatibility of polymer electrolytes with electrodes, especially cathodes, is likely to be improved by adding fillers. Wang et al. successfully prepared the pouch cell-type all-solid-state Mg batteries [178]. The cell demonstrated excellent performance. However, the solid electrolyte contained two salts, Mg(BH4)2 and LiBH4. The ratio of Mg/Li is 0.1/1.5. Thus, it is unclear the influence of Li intercalation on observed capacity.
Despite fewer examples, fillers would improve the stability of all-solid-state Mg batteries. The application of the organic–inorganic composite electrolytes for all-solid-state Mg batteries must be studied more intensively. Although pouch cell-type all-solid-state battery was reported, common coin cell configuration and cathode materials should be adopted at this moment because the application of organic–inorganic composite electrolytes for all-solid-state Mg battery is still infant stage. Properties of all-solid-state Mg batteries are summarized in Table 10.

Table 10.
Properties of all-solid-state Mg batteries.
4. Challenges
The all-solid-state Mg battery is a good option to replace LIBs due to high safety, energy density and resource abundance. Thanks to the many efforts of researchers, technologies for the all-solid-state Mg battery have progressed significantly. Despite the significant progress, further research is still required to realize the all-solid-state Mg battery. Herein, the challenges are summarized.
- (1)
- Inorganic electrolytes
The Mg2+-ion conductivity of current ceramic electrolytes is 10−6~10−7 S cm−1 at room temperature (except [33,35,59]). Even with hydride-based inorganic electrolytes, the conductivity is ~10−5 S cm−1. For all-solid-state Mg batteries, the required conductivity is >~10−4 S cm−1 at room temperature to ensure room temperature operation. Thus, an improvement in ionic conductivity must be investigated in inorganic electrolytes. Some groups have studied the Mg2+-ion conductivity of ceramic electrolytes intensively; however, an effective solution to enhance the conductivity is yet to be found at this moment. Thus, thin film ceramic electrolytes would effectively compensate for the low conductivity. Currently, only one paper is reported with respect to thin film ceramic electrolytes [58]. The thin film ceramic electrolytes should be studied more intensively. Adding inorganic fillers will likely improve the ionic conductivity in the hydride-based electrolytes [67,68]. The effect of fillers should be a good research topic for further study.
- (2)
- Study on Mg salts
Compared to inorganic electrolytes, organic polymer electrolytes are more promising in the application of all-solid-state Mg batteries at this moment. Various polymer hosts have been studied. On the other hand, a systematic study on Mg salts is not carried out. Fichtner et al. reported the corrosion of the battery case and current collectors by Cl-contained salt [148]. Although various types of Mg salts, such as MgSO4, MgCl2, Mg(ClO4)2, Mg(TFSI)2, Mg(NO3)2 and Mg(Tf)2, have been employed so far, a suitable Mg salt for the all-solid-state Mg battery is still under investigation. Therefore, the effect of Mg salts on the properties of SPEs (and GPEs) and the performances of all-solid-state Mg batteries should be studied systematically.
- (3)
- Mechanical properties of solid electrolytes
High flexibility is one of the benefits of SPEs and GPEs, which facilitates the construction of the all-solid-state Mg battery. However, quantitative evaluation of the flexibility, i.e., mechanical properties, has not been carried out yet. The mechanical properties of solid electrolytes influence cell pressure, contact with electrodes, suppression of Mg dendrite formation, etc., significantly. Consequently, both the electrochemical and mechanical properties of SPEs and GPEs must be characterized precisely.
- (4)
- Construction of all-solid-state Mg battery
As mentioned in a previous section, most research focuses on solid electrolytes and only some papers try to fabricate all-solid-state Mg batteries. The compatibility of solid electrolytes with an all-solid-state Mg battery cannot be evaluated by only ionic conductivity, electrochemical window, transference number, etc. Therefore, solid electrolytes must be evaluated in all-solid-state batteries in addition to the above-mentioned properties. Particularly, the properties of the electrode/solid electrolyte interface that largely affect the performance of all-solid-state Mg batteries can be characterized only in the all-solid-state battery configuration.
- (5)
- Energy density
At the moment, the energy density of an all-solid-state Mg battery cannot be discussed since the development is still in the infant stage. To improve the energy density, one good strategy is a decrease in electrolyte thickness. A thin electrolyte can reduce the volume and weight of the all-solid-state battery, resulting in an enhancement of the energy density. Therefore, the decrease in the thickness of electrolytes must be studied.
- (6)
- Cathode materials
Some groups employ novel cathode materials for the all-solid-state Mg batteries. In this case, the performance of the cathode material is unknown. Thus, it is impossible to clarify the reason for the poor performance of the all-solid-state Mg battery, i.e., the cathode itself or other components. Common cathode materials such as Mo6S8 and TiS2, which are well characterized in liquid electrolytes, should be used to characterize and evaluate the all-solid-state Mg batteries. After that, the development of cathode materials for all-solid-state batteries would be carried out.
An all-solid-state Mg battery would replace current LIBs owing to high safety, energy density and resource abundance, although the research is still infant stage. To realize the all-solid-state Mg battery, above mentioned studies must be carried out intensively. Additionally, research on an Mg battery using liquid electrolytes should be referred to, especially for the cathode selection. The following possible strategy is suggested: (1) Development of polymer-based solid electrolytes (with/without plasticizers and fillers), (2) Investigation of compatibility with cathode materials in all-solid-state batteries (common cathodes will be used at first, then novel cathodes for all-solid-state batteries will be studied), (3) Decreasing thickness of electrolytes and applying a pouch cell configuration to improve energy density. The first-generation all-solid-state Mg battery would employ polymer-based solid electrolytes because it would need more time to develop inorganic electrolytes. Most probably, dual polymer/liquid electrolytes, as shown in Figure 9, would be employed to solve poor contact between porous cathodes and solid electrolytes. Then, full all-solid-state Mg batteries could be developed based on knowledge and experiences obtained in dual polymer/liquid electrolytes. Ceramic electrolytes could be applied after the realization of SPE and GPE-based all-solid-state Mg batteries.
Author Contributions
J.P. and G.J.; analysis, writing—original draft preparation, K.Z.W. and M.K.; writing—review and editing, M.K.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Winter, M.; Barnett, N.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.W.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, D.; Tan, H.; Zhang, X.; Rui, X.; Yu, Y. 3D porous V2O5 architectures for high-rate lithium storage. J. Energy Chem. 2020, 40, 15–21. [Google Scholar] [CrossRef]
- Zeng, J.; Peng, C.; Wang, R.; Cao, C.; Wang, X.; Liu, J. Micro-sized FeS2@FeSO4 core-shell composite for advanced lithium storage. J. Alloys Compd. 2020, 814, 151922. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Whittingham, M.S. Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 2014, 114, 11414–11443. [Google Scholar] [CrossRef]
- Xu, W.; Wnag, J.L.; Ding, F.; Chen, X.L.; Nasybutin, E.; Zhang, Y.H.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Song, X.; Jin, Z. Recent progress in constructing halogenated interfaces for highly stable lithium metal anodes. Energy Storage Mater. 2023, 54, 732–775. [Google Scholar] [CrossRef]
- Sarkar, S.; Thangadurai, V. Critical current densities for high-performance all-solid-state Li-metal batteries: Fundamentals mechanisms, interfaces materials and applications. ACS Energy Lett. 2022, 7, 1492–1527. [Google Scholar] [CrossRef]
- Feng, Z.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Hebie, S.; Mgo, H.P.K.; Lepretre, J.-C.; Iojoiu, C.; Cointeaux, L.; Berthelot, R.; Alloin, F. Electrolyte based on easily synthesized, low cost triphenolate-borohydride salt for high performance Mg(TFSI)2-glyme rechargeable magnesium batteries. ACS Appl. Mater. Interfaces 2017, 9, 28377–28385. [Google Scholar] [CrossRef]
- Wei, C.; Tan, L.; Zhang, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Highly reversible Mg metal anodes enabled by interfacial liquid metal engineering for high-energy Mg-S batteries. Energy Storage Mater. 2022, 48, 447–457. [Google Scholar] [CrossRef]
- Jackle, M.; Helmbrecht, K.; Smits, M.; Stottmeister, D.; Gross, A. Self-diffusion barriers: Possible descriptors for dendrite growth in batteries. Energy Environ. Sci. 2018, 11, 3400–3407. [Google Scholar] [CrossRef]
- Kotobuki, M. Recent progress of ceramic electrolytes for post Li and Na batteries. Funct. Mater. Lett. 2021, 14, 2130003. [Google Scholar] [CrossRef]
- Shuai, H.; Xu, J.; Huang, K. Progress in retrospect of electrolytes for secondary magnesium batteries. Coord. Chem. Rev. 2020, 422, 213478. [Google Scholar] [CrossRef]
- Chen, C.; Wang, K.; He, H.; Hanc, E.; Kotobuki, M.; Lu, L. Processing and properties of garnet-type Li7La3Zr2O12 ceramic electrolytes. Small 2022, 19, 2205550. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hits, G.T.; Nolan, A.M.; Wachman, E.D.; Mo, Y.; et al. Garnet-type solid-state electrolytes: Materials, interfaces and batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; CHongyang, Y.; Tengfei, Z.; Xuebin, Y. Solid state electrolytes for rechargeable magnesium-ion batteries: From structure to mechanism. Small 2022, 18, 2106981. [Google Scholar]
- Stefania, F.; Marisa, F.; Belen, B.G.A.; Matteo, B.; Segio, B.; Michele, P.; Claudio, G. Solid-state post Li metal ion batteries: A sustainable forthcoming reality? Adv. Energy Mater. 2021, 11, 2100785. [Google Scholar]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on Sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Yan, B.; Wang, Z.; Ren, H.; Lu, X.; Qu, Y.; Liu, W.; Jiang, K.; Kotobuki, M. Interfacial modification of Na3Zr2Si2PO12 solid electrolyte by femtosecond laser etching. Ionics 2023, 29, 865–870. [Google Scholar] [CrossRef]
- Nakayama, M.; Nakano, K.; Harada, M.; Tanibata, N.; Takeda, H.; Noda, Y.; Kobatashi, R.; Karasuyama, M.; Takeuchi, I.; Kotobuki, M. Na superionic conductor-type LiZr2(PO 4)3 as a promising solid electrolyte for use in all-solid-state Li metal batteries. Chem. Comm. 2022, 58, 9328. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, M.; Yanagiya, S. Li-ion conductivity of NASICON-type Li1+2xZr2-xCax(PO4)3 solid electrolyte prepared by spark plasma sintering. J. Alloys Compd. 2021, 862, 158641. [Google Scholar] [CrossRef]
- Kotobuki, M.; Koishi, M. Preparation of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte via a sol-gel method using various Ti Sources. J. Asian Ceram. Soc. 2020, 8, 891–897. [Google Scholar] [CrossRef]
- Kotobuki, M.; Koishi, M. Preparation of Li1.5Al0.5Ti1.5(PO4)3 solid electrolyte via a sol-gel route using various Al sources. Ceram. Int. 2013, 39, 4645–4649. [Google Scholar] [CrossRef]
- Hayamizu, K.; Haishi, T. Ceramic-glass pellet thickness and Li diffusion in NASICON-type LAGP (Li1.5Al0.5Ge1.5(PO4)3) studied by pulsed field gradient NMR spectroscopy. Solid State Ion. 2022, 380, 115924. [Google Scholar] [CrossRef]
- Yan, B.; QU, Y.; Ren, H.; Lu, X.; Wang, Z.; Liu, W.; Wang, Y.; Kotobuki, M.; Jiang, K. A solid-liquid composite electrolyte with a vertical microporous Li1.5Al0.5Ge1.5(PO4)3 skeleton that prepared by femtosecond laser structuring and filled with ionic liquid. Mater. Chem. Phys. 2022, 287, 126265. [Google Scholar] [CrossRef]
- Lee, W.; Tamura, S.; Imanaka, N. Synthesis and characterization of divalent ion conductors with NASICON-type structures. J. Asian Ceram. Soc. 2019, 7, 221–227. [Google Scholar] [CrossRef]
- Shao, Y.J.; Zhong, G.M.; Lu, Y.X.; Liu, L.L.; Zhao, C.L.; Zhang, Q.Q. A novel NASICON-based glass-ceramic composite electrolyte with enhanced Na-ion conductivity. Energy Storage Mater. 2019, 23, 514–521. [Google Scholar] [CrossRef]
- Ikeda, S.; Takahashi, M.; Ishikawa, J.; Ito, K. Solid electrolytes with multivalent cation conduction. 1. Conducting species in MgZrPO4 system. Solid State Ion. 1987, 23, 125–129. [Google Scholar] [CrossRef]
- Nakano, K.; Noda, Y.; Tanibata, N.; Nakayama, M.; Kajihara, K.; Kanamura, K. Computational investigation of the Mg-ion conductivity and phase stability of MgZr4(PO4)6. RSC Adv. 2019, 9, 12590–12595. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, J.; Morota, K.; Kuwata, N.; Nakamura, Y.; Maekawa, H.; Hattori, T.; Imanaka, N.; Okazaki, Y.; Adachi, G.-Y. High temperature 31P NMR study on Mg2+ ion conductors. Solid State Comm. 2001, 120, 295–298. [Google Scholar] [CrossRef]
- Anuar, N.K.; Adnan, S.B.R.S.; Jaafar, M.H.; Mohamed, N.S. Studies on structural and electrical properties of Mg0.5+y(Zr2-yFey)2(PO4)3 ceramic electrolytes. Ionics 2016, 22, 1125–1133. [Google Scholar] [CrossRef]
- Tamura, S.; Yamane, M.; Hoshino, Y.; Imanaka, N. Highly conducting divalent Mg2+ cation solid electrolytes with well-ordered three-dimensional network structure. J. Solid State Chem. 2016, 235, 7–11. [Google Scholar] [CrossRef]
- Anuar, N.K.; Mohamed, N.S. Structural and electrical properties of novel Mg0.9+0.5yZn0.4AlyZr1.6-y(PO4)3 ceramic electrolytes synthesized via nitrate sol-gel method. J. Sol-Gel Sci. Technol. 2016, 80, 249–258. [Google Scholar] [CrossRef]
- Halim, Z.A.; Adnan, S.B.R.S.; Mohamed, N.S. Effect of sintering temperature on the structural, electrical and electrochemical properties of novel Mg0.5Si2(PO4)3 ceramic electrolytes. Ceram. Int. 2016, 42, 4452–4461. [Google Scholar] [CrossRef]
- Su, J.; Tsuruoka, T.; Tsujita, T.; Nishitani, Y.; Nakura, K.; Terabe, K. Aromic layer deposition of a magnesium phosphate solid electrolyte. Chem. Mater. 2019, 31, 5566–5575. [Google Scholar] [CrossRef]
- Takeda, H.; Nakano, K.; Tanibata, N.; Nakayama, M. Novel Mg-ion conductive oxide of μ-cordierite Mg0.6Al1.2Si1.8O6. Sci. Technol. Adv. Mater. 2020, 21, 131–138. [Google Scholar] [CrossRef]
- Omote, A.; Yotsuhashi, S.; Zenitani, Y.; Yamada, Y. High ion conductivity in MgHf(WO4)3 solids with ordered structure: 1-D alignments of Mg2+ and Hf4+ ions. J. Am. Ceram. Soc. 2011, 94, 2285–2288. [Google Scholar] [CrossRef]
- Fujii, Y.; Kobayashi, M.; Miura, A.; Rosero-Navarro, N.C.; Li, M.; Sun, J.; Kotobuki, M.; Tadanaga, K. Fe-P-S electrodes for all-solid-state lithium secondary batteries using sulfide-based solid electrolytes. J. Power Sources 2020, 449, 227576. [Google Scholar] [CrossRef]
- Yamanaka, T.; Hayashi, A.; Yamauchi, A.; Tatsumisago, M. Preparation of magnesium ion conducting MgS-P2S5-MgI2 glasses by a mechanochemical technique. Solid State Ion. 2014, 262, 601–603. [Google Scholar] [CrossRef]
- Canepa, P.; Bo, S.-H.; Gautam, G.S.; Key, B.; Richards, W.D.; Shi, T.; Tian, Y.; Wang, Y.; Li, J.; Ceder, G. High magnesium mobility in ternary spinel chalcogenides. Nat. Comm. 2017, 8, 1759. [Google Scholar] [CrossRef] [PubMed]
- Koishi, M.; Kotobuki, M. Preparation of Y-doped Li7La3Zr2O12 by co-precipitation method. Ionics 2022, 28, 2065–2072. [Google Scholar] [CrossRef]
- Wang, L.-P.; Zhao-Karger, Z.; Klein, F.; Chable, J.; Braun, T.; Schuer, A.R.; Wang, C.-R.; Guo, Y.-G.; Fichtner, M. MgSc2Se4-magnesium solid ionic conductor for all-solid-state Mg batteries? ChemSusChem 2019, 12, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Solomatin, N.; Kauffmann, Y.; Kraytsberg, A.; Ein-Eli, Y. Revealing and excluding the root cause of the electronic conductivity in Mg-ion MgSc2Se4 solid electrolyte. Appl. Mater. Today 2021, 23, 100998. [Google Scholar] [CrossRef]
- Kundu, S.; Solomatin, N.; Kraytsberg, A.; Ein-Eli, Y. MgSc2Se4 solid electrolyte for rechargeable Mg batteries: An electric field-assisted all-solid-state synthesis. Energy Technol. 2022, 10, 2200896. [Google Scholar] [CrossRef]
- Clemenceau, T.; Andriamady, N.; Kumar, M.K.; Bardran, A.; Avila, V.; Dhal, K.; Hopkins, M.; Vendrell, X.; Marshall, D.; Raj, R. Flash sintering of Li-ion conducting ceramic in a few seconds at 850 °C. Scr. Mater. 2019, 172, 1–5. [Google Scholar] [CrossRef]
- Yan, B.; Kang, L.; Kotobuki, M.; He, L.; Liu, B.; Jiang, K. Boron group element doping of Li1.5Al0.5Ge1.5(PO4)3 based on microwave sintering. J. Solid State Electrochem. 2021, 25, 527–534. [Google Scholar] [CrossRef]
- Wang, C.; Ping, W.; Bai, Q.; Cui, H.; Hensleigh, R.; Wang, R.; Brozena, A.H.; Xu, Z.; Dai, J.; Pei, Y.; et al. A general method to synthesize and sinter bulk ceramics in seconds. Science 2020, 368, 521–526. [Google Scholar] [CrossRef]
- Anuar, N.K.; Adnan, S.B.R.S.; Mohamed, N.S. Characterization of Mg0.5Zr2(PO4)3 for potential use as electrolyte in solid state magnesium batteries. Ceram. Int. 2014, 40, 13719–13727. [Google Scholar] [CrossRef]
- Mohammed, A.; Kale, G.M. Novel sol-gel synthesis of MgZr4P6O24 composite solid electrolyte and newer insight into the Mg2+-ion conducting properties using impedance spectroscopy. J. Phys. Chem. C 2016, 120, 17909–17915. [Google Scholar]
- Imanaka, N.; Okazaki, Y.; Adachi, G.-Y. Divalent magnesium ion conducting characteristics in phosphate based solid electrolyte composites. J. Mater. Chem. C 2000, 10, 1431–1435. [Google Scholar] [CrossRef]
- Imanaka, N.; Okazaki, Y.; Adachi, G. Optimization of divalent magnesium ion conduction in phosphate based polycrystalline solid electrolytes. Ionics 2001, 7, 440–446. [Google Scholar] [CrossRef]
- Liang, B.; Kreshishian, V.; Liu, S.; Yi, E.; Jia, D.; Zhou, Y.; Kieffer, J.; Ye, B.; Kaine, R.M. Processing liquid-feed flame spray pyrolysis synthesized Mg0.5Ce0.2Zr1.8(PO4)3 nanopowders to free standing thin films and pellets as potential electrolytes in all-solid-state Mg batteries. Electrochim. Acta 2018, 272, 144–153. [Google Scholar] [CrossRef]
- Kajihara, K.; Nagano, H.; Tsujita, T.; Munakata, H.; Kanamura, K. High-temperature conductivity measurements of magnesium-ion-conducting solid oxide Mg0.5-x(Zr1-xNbx)2(PO4)3 (x = 0.15) using Mg metal electrodes. J. Electrochem. Soc. 2017, 164, A2183–A2185. [Google Scholar] [CrossRef]
- Mustafa, M.; Rani, M.S.A.; Adnan, S.B.R.S.; Salleh, F.M.; Mohamed, N.S. Characteristics of new Mg0.5(Zr1-xSnx)2(PO4)3 NASICON structured compound as solid electrolytes. Ceram. Int. 2020, 46, 28145–28155. [Google Scholar] [CrossRef]
- Imanaka, N.; Okazaki, Y.; Adachi, G. Divalent magnesium ionic conduction in Mg1-2x(Zr1-xNbx)4P6O24 (x = 0-0.4) solid solutions. Electrochem. Solid State Lett. 2000, 3, 327–329. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, C.; Wang, Y.; Yi, E.; Wang, W.; Kieffer, J.; Laine, R.M. Processing combustion synthesized Mg0.5Zr2(PO4)3 nanopowders to thin films as potential solid electrolytes. Electrochem. Comm. 2020, 116, 106753. [Google Scholar] [CrossRef]
- Halim, Z.A.; Adnan, S.B.R.S.; Salleh, F.M.; Mohamed, N.S. Effects of Mg2+ interstitial ion on the properties of Mg0.5+x/2Si2-xAlx(PO4)3 ceramic electrolytes. J. Magnes. Alloy 2017, 5, 439–447. [Google Scholar] [CrossRef]
- Sulaiman, M.; Su, N.C.; Mohamed, N.S. Sol-gel synthesis and characterization of β-MgSO4: Mg(NO3)2-MgO composite solid electrolyte. Ionics 2017, 23, 443–452. [Google Scholar] [CrossRef]
- Matsuo, M.; Oguchi, H.; Sato, T.; Takamura, H.; Tsuchida, E.; Ikeshoji, T.; Orimo, S.-I. Sodium and magnesium ionic conduction in comlex hydrides. J. Alloys Compd. 2013, 580, S98–S101. [Google Scholar] [CrossRef]
- Higashi, S.; Miwa, K.; Aoki, M.; Takechi, K. A novel inorganic solid state ion conductor for rechargeable Mg batteries. Chem. Comm. 2014, 50, 1320. [Google Scholar] [CrossRef] [PubMed]
- Ruyet, R.L.; Berthelot, R.; Salager, E.; Florian, P.; Fleutot, B.; Janot, R. Investigation of Mg(BH4)(NH2)-based composite materials with enhanced Mg2+ ionic conductivity. J. Phys. Chem. C 2019, 123, 10756–10763. [Google Scholar] [CrossRef]
- Roedern, E.; Kuhnel, R.-S.; Remhof, A.; Battaglia, C. Magnesium ethylenediamine borohydride as solid-state electrolyte for magnesium batteries. Sci. Rep. 2017, 7, 46189. [Google Scholar] [CrossRef] [PubMed]
- Kisu, K.; Kim, S.; Inukai, M.; Oguchi, H.; Takagi, S.; Orimo, S.-I. Magnesium borohydride ammonia borane as a magnesium ionic conductor. ACS Appl. Energy Mater. 2020, 3, 3174–3179. [Google Scholar] [CrossRef]
- Luo, X.; Rawal, A.; Cazorla, C.; Aguey-Zinsou, K.F. Facile self-forming superionic conductors based on complex borohydride surface oxidation. Adv. Sus. Syst. 2020, 4, 1900113. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Zhang, R.; Liu, Z.; Deng, H.; Cen, W.; Yan, Y.; Chen, Y. Oxygen vacancies boosted fast Mg2+ migration in solids at room temperature. Energy Storage Mater. 2022, 51, 630–637. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Burankova, T.; Wei, S.; Embs, J.P.; Skibsted, J.; Jensen, T.R. Fast room-temperature Mg2+ conductivity in Mg(BH4)2∙1.6NH3-Al2O3 nanocomposites. J. Phys. Chem. Lett. 2022, 13, 2211–2216. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Jorgensen, M.; Skov, L.N.; Skibsted, J.; Jensen, T.R. Ammine magnesium borohydride nanocomposites for all-solid-state magnesium batteries. ACS Appl. Energy Mater. 2020, 3, 9264–9270. [Google Scholar] [CrossRef]
- Skov, L.N.; Grinderslev, J.B.; Rosenkranz, A.; Lee, Y.-S.; Jensen, T.R. Towards solid-state magnesium batteries: Ligand-assisted superionic conductivity. Batter. Supercaps 2022, 5, e202200163. [Google Scholar] [CrossRef]
- Ruyet, R.L.; Fleutot, B.; Berthelot, R.; Benabed, Y.; Hatier, G.; Filinchuk, Y.; Janot, R. Mg3(BH4)4(NH2)2 as inorganic solid electrolyte with high Mg2+ ionic conductivity. ACS Appl. Energy Mater. 2020, 3, 6093–6097. [Google Scholar] [CrossRef]
- Pang, Y.; Nie, Z.; Xu, F.; Sun, L.; Yang, J.; Sun, D.; Fang, F.; Zheng, S. Borohydride ammoniate solid electrolyte design for all-solid-state Mg batteries. Energy Environ. Mater. 2022, e12527. [Google Scholar] [CrossRef]
- Rouhani, F.; Rafizadeh-Masuleh, F.; Morsali, A. Highly electroconductive metal-organic framework: Tunable by metal ion sorption quantity. J. Am. Chem. Soc. 2019, 141, 11173–11182. [Google Scholar] [CrossRef]
- Yanai, N.; Uemura, T.; Horike, S.; Shimomura, S.; Kiragawa, S. Inclusion and dynamics of a polymer-Li salt complex in coordination nanochannels. Chem. Comm. 2011, 47, 1722–1724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassan, H.K.; Farkas, A.; Varzi, A.; Jacob, T. Mixed metal-organic frameworks as efficient semi-solid electrolytes for magnesium-ion batteries. Batter. Supecaps 2022, 5, e202200260. [Google Scholar] [CrossRef]
- Wei, Z.; Maile, R.; Riegger, L.M.; Rohnke, M.; Muller-Buschbaum, K.; Janek, J. Ionic liquid-incorporated metal-organic framework with high magnesium ion conductivity for quasi-solid-state magnesium batteries. Batter. Supecaps 2022, 5, e202200318. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, J.; Ning, D.; Hunag, Y.; Lei, W.; Li, J.; Li, J.; Schuck, G.; Shen, J.; Guo, Y.; et al. Design of metal-organic frameworks for improving pseudo-solid-state magnesium-ion electrolytes: Open metal sites, isoreticular expansion and framework topology. J. Mater. Sci. Technol. 2023, 144, 15–27. [Google Scholar] [CrossRef]
- Luo, J.; Li, Y.; Zhang, H.; Wang, A.; Lo, W.-S.; Dong, Q.; Wong, N.; Povinelli, C.; Shao, Y.; Chereddy, S.; et al. A metal-organic framework thin film for selective Mg2+ transport. Angew. Chem. Int. Ed. 2019, 58, 15313–15317. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, M.L.; Ameloot, R.; Wiers, B.M.; Long, J.R. Metal-organic frameworks as solid magnesium electrolytes. Energy Environ. Sci. 2014, 7, 667–671. [Google Scholar] [CrossRef]
- Park, S.S.; Tukchinsky, Y.; Dinca, M. Single-ion Li+, Na+ and Mg2+ solid electrolytes supported by a mesoporous anionic Cu-azolate metal-organic framework. J. Am. Chem. Soc. 2017, 139, 13260–13263. [Google Scholar] [CrossRef]
- Miner, E.M.; Park, S.S.; Dinca, M. High Li+ and Mg2+ conductivity in a Cu-azolate metal-organic framework. J. Am. Chem. Soc. 2019, 141, 4422–4427. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yamada, T.; Jing, Y.; Toyao, T.; Shimizu, K.-I.; Sadakiyo, M. Super Mg2+ conductivity around 10-3 S cm-1 observed in a porous metal-organic framework. J. Am. Chem. Soc. 2022, 144, 8669–8675. [Google Scholar]
- Walke, P.; Venturini, J.; Spranger, R.j.; Wullen, L.; Nilges, T. Fast Magnesium Conductiong Electrospun Solid Polymer Electrolyte. Battery Supercap. 2022, 5, e202200285. [Google Scholar] [CrossRef]
- Reddy, M.J.; Chu, P.P. Ion pair formation and its effect in PEO:Mg solid polymer electrolyte system. J. Power Sources 2002, 109, 340–346. [Google Scholar] [CrossRef]
- Basha, S.K.S.; Rao, M.C. Spectroscopic and electrochemical properties of PVP based polymer electrolyte films. Polym. Bull. 2018, 75, 3641–3666. [Google Scholar] [CrossRef]
- Basha, S.K.S.; Sundari, G.S.; Kumar, K.V.; Rao, M.C. Preparation and dielectric properties of PVP-based polymer electrolyte films for solid-state battery application. Polym. Bull. 2018, 75, 925–945. [Google Scholar] [CrossRef]
- Rathore, M.; Dalvi, A. Electrical characterization of PVA-MgSO4 and PVA-Li2SO4 polymer salt composite electrolytes. Mater. Today Proc. 2019, 10, 106–111. [Google Scholar] [CrossRef]
- Kalagi, S.S. Activation energy dependence on doping concentration in PVA-MgCl2 composites. Mater. Today Proc. 2023, 72, 2691–2696. [Google Scholar]
- Komal, B.; Yadav, M.; Kumar, M.; Tiwari, T.; Srivastava, N. Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system. e-Polymers 2019, 19, 453–461. [Google Scholar] [CrossRef]
- Tamilosai, R.; Palanisamy, P.N.; Selvasekarapandian, S.; Maheshwari, T. Solium alginate incorporated with magnesium nitrate as a novel solid biopolymer electrolyte for magnesium-ion batteries. J. Mater. Sci. Mater. Electron. 2021, 32, 22270–22285. [Google Scholar] [CrossRef]
- Ismayl, J.K.; Hegde, S.; Vasachar, R.; Sanjeev, G. Novel solid biopolymer electrolyte based on methyl cellulose with enhanced ion transport properties. J. Appl. Polym. Sci. 2022, 139, 51826. [Google Scholar]
- Buvaneshwari, P.; Mathavan, T.; Selvasekarapandian, S.; Krishna, M.V.; Naachiyar, R.M. Preparation and characterization of biopolymer electrolyte based on gellan gum with magnesium perchlorate for magnesium battery. Ionics 2022, 28, 3843–3854. [Google Scholar] [CrossRef]
- Rajapaksha, H.G.N.; Perera, K.S.; Vidanapathirana, K.P. Characterization of a natural rubber based solid polymer electrolyte to be used for a magnesium rechargeable cell. Polym. Bull. 2022, 79, 4879–4890. [Google Scholar] [CrossRef]
- Priya, S.S.; Karthika, M.; Selvasekarapandian, S.; Manjuladevi, R. Preparation and characterization of polymer electrolyte based on biopolymer I-Carrageenen with magnesium nitrate. Solid State Ion. 2018, 327, 136–149. [Google Scholar] [CrossRef]
- Ali, N.I.; Abidin, S.Z.Z.; Majid, S.R.; Jaafar, N.K. Role of Mg(NO3)2 as defective agent in ameliorating the electrical conductivity, structural and electrochemical properties of agarose-based polymer electrolytes. Polymers 2021, 13, 3357. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, M.; Selvanayagam, S.; Selvasekarapandian, S.; Chandra, M.V.L.; Sangeetha, P.; Manjuladevi, R. Magnesium ion-conducting solid polymer electrolyte based on cellulose acetate with magnesium nitrate (Mg(NO3)2•6H2O) for electrochemical studies. Ionics 2020, 26, 4553–4565. [Google Scholar] [CrossRef]
- Sangeetha, P.; Selvakumari, T.M.; Selvasekarapandian, S.; Srikumar, S.R.; Manjukadevi, R.; Mahalakshmi, M. Preparation and characterization of biopolymer K-carrageenan with MgCl2 and its application to electrochemical devices. Ionics 2020, 26, 233–244. [Google Scholar] [CrossRef]
- Aziz, S.B.; Al-Zangana, S.; Woo, H.J.; Kadir, M.F.Z.; Abdullah, O.G. The compatibility of chitosan with divalent salts over monovalent salts for the preparation of solid polymer. Results Phys. 2018, 11, 826–836. [Google Scholar] [CrossRef]
- Sangeetha, P.; Selvakumari, T.M.; Selvasekarapandian, S.; Mahalakshmi, M. Characterization of solid biopolymer electrolytes based on kappa-carrageenan with magnesium nitrate hexahydrate and its application to electrochemical devices. Polym. Plast. Technol. Mater. 2021, 60, 1317–1330. [Google Scholar]
- Ismayil, J.K.; Hegde, S.; Sanjeev, G.; Murari, M.S. An insight into the suitability of magnesium ion-conducting biodegradable methyl cellulose solid polymer electrolyte film in energy storage devices. J. Mater. Sci. 2023, 58, 5389–5412. [Google Scholar]
- Priya, S.S.; Karthika, M.; Selvasekarapandian, S.; Manjuladevi, R.; Monisha, S. Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics 2018, 24, 3861–3875. [Google Scholar] [CrossRef]
- Helen, P.A.; Perumal, P.; Sivaraj, P.; Diana, M.I.; Selvin, P.C. Mg-ion conducting electrolytes based on chitosan biopolymer host for the rechargeable Mg batteries. Mater. Today Proc. 2022, 50, 2668–2670. [Google Scholar] [CrossRef]
- Kiruthika, S.; Malathi, M.; Selvasekarapandian, S.; Tamilarasan, K.; Moniha, V.; Manjuladevi, R. Eco-friendly biopolymer electrolyte, pectin with magnesium nitrate salt, for application in electrochemical devices. J. Solid State Electrochem. 2019, 23, 2181–2193. [Google Scholar] [CrossRef]
- Kiruthika, S.; Malathi, M.; Selvasekarapandian, S.; Tamilarasan, K.; Maheshwari, T. Conducting biopolymer electrolyte based on pectin with magnesium chloride salt for magnesium battery application. Polym. Bull. 2020, 77, 6299–6317. [Google Scholar] [CrossRef]
- Kayama, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yoneyama, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Rathika, R.; Suthanthiraraj, S.A. Ionic interactions and dielectric relaxation of PEO/PVDF-Mg[(CF3SO2)2N2] blend electrolytes for magnesium ion rechargeable batteries. Macromol. Res. 2016, 24, 422–428. [Google Scholar] [CrossRef]
- Shenbagavalli, S.; Muthuvinayagam, M.; Revathy, M.S. Electrical properties of Mg2+ ion-conductive PEO:P(PVdF-HFP) based solid blend polymer electrolytes. Polymer 2022, 256, 125242. [Google Scholar] [CrossRef]
- Manjuladevi, R.; Thamilselvan, M.; Selvasekarapandian, S.; Mangalam, R.; Premalatha, M.; Monisha, S. Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium perchlorate. Solid State Ion. 2017, 308, 90–100. [Google Scholar] [CrossRef]
- Ponmani, S.; Prabhu, M.R. Development and study of solid polymer electrolytes based on PVdF-HFP/PVAc:Mg(ClO4)2 for Mg ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 15086–15096. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R.; Rhee, H.-W. Magnesium ion conducting solid polymer blend electrolyte based on biodegradable polymers and application in solid-state batteries. Ionics 2015, 21, 125–132. [Google Scholar] [CrossRef]
- Manjuladevi, R.; Thamilselvan, M.; Selavasekarapandian, S.; Selvin, P.C.; Mangalam, R.; Monisha, S. Preparation and characterization of blend polymer electrolyte film based on poly(vinyl alcohol)-poly(aceylonitrile)/MgCl2 for energy storage devices. Ionics 2018, 24, 1083–1095. [Google Scholar] [CrossRef]
- Ponraj, T.; Ramalingam, A.; Selvasekarapandian, S.; Srikumar, S.R.; Manjuladevi, R. Mg-ion conducting triblock copolymer electrolyte based on poly(VdCl-co-AN-co-MMA) with magnesium nitrate. Ionics 2020, 26, 789–800. [Google Scholar] [CrossRef]
- Hiraoka, K.; Inoue, M.; Takahashi, K.; Hayamizu, K.; Watanabe, M.; Seki, S. Analysis of ionic transport and electrode interfacial reaction, and NMR one-dimensional imaging of ther-based polymer electrolytes. J. Electrochem. Soc. 2021, 168, 060501. [Google Scholar] [CrossRef]
- Park, B.; Andersson, R.; Pate, S.G.; Liu, J.; O’brien, C.P.; Hernandez, G.; Mindemark, J.; Schaefer, J.L. Ion coordination and transport in magnesium polymer electrolytes based on polyester-co-polycarbonate. Energy Mater. Adv. 2021, 2021, 9895403. [Google Scholar] [CrossRef]
- Manjuladevi, R.; Selvasekarapandian, S.; Thamilselvan, M.; Mangalam, R.; Monisha, S.; Selvin, P.C. A study on blend polymwe electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium nitrate for magnesium battery. Ionics 2018, 24, 3493–3506. [Google Scholar] [CrossRef]
- Ponmani, S.; Kalaiselvimary, J.; Prabhu, M.R. Structural, electrical, and electrochemical properties of poly(vinylidene fluoride-co-hexaflouropropylene)/poly(vinyl acetate)-based polymer blend electrolytes for rechargeable magnesium ion batteries. J. Solid State Electrochem. 2018, 22, 2605–2615. [Google Scholar] [CrossRef]
- Nayak, P.; Ismayil; Cyriac, V.; Hegde, S.; Sanjeev, G.; Murari, M.S.; Sudhakar, Y.N. Magnesium ion conducting free-standing biopolymer blend electrolyte films for electrochemical device application. J. Non-Cryst. Solids 2022, 592, 121741. [Google Scholar] [CrossRef]
- Suvarnna, K.; Kirubavathy, S.J.; Selvasekarapandian, S.; Krishna, M.V.; Ramaswamy, M. Corn silk extract-based solid-state biopolymer electrolyte and its application to electrochemical storage devices. Ionics 2022, 28, 1767–1782. [Google Scholar] [CrossRef]
- Kanakaraj, T.M.; Bhajantri, R.F.; Chavan, C.; Cyriac, V.; Bulla, S.S.; Ismayil. Investigation on the structural and ion transport properties of magnesium salt doped HPMC-PVA based polymer blend for energy storage applications. J. Non-Cryst. Solids 2023, 603, 122276. [Google Scholar]
- Koduru, H.K.; Marinov, Y.G.; Kaleemulla, S.; Rafailov, P.M.; Hadjichristov, G.B.; Scaramuzza, N. Fabrication and characterization of magnesium-ion-conducting flexible polymer electrolyte membranes based on a nanocomposite of poly(ethylene oxide) and potate starch nanocrystals. J. Solid State Electrochem. 2021, 25, 2409–2428. [Google Scholar] [CrossRef]
- Kotobuki, M.; Kanamura, K. Fabrication of all-solid-state battery using Li5La3Ta2O12 Ceramic electrolyte. Ceram. Int. 2013, 39, 6481–6487. [Google Scholar] [CrossRef]
- Aziz, A.A.; Tominaga, Y. Effect of Li salt addition on electrochemical properties of poly(ethylene carbonate)-Mg salt electrolytes. Polym. J. 2019, 51, 61–67. [Google Scholar] [CrossRef]
- Perumal, P.; Abhilash, K.P.; Sivaraj, P.; Selvin, P.C. Study on Mg-ion conducting solid biopolymer electrlytes based on tamarind seed polysaccharide for magnesium ion batteries. Mater. Res. Bull. 2019, 118, 110490. [Google Scholar] [CrossRef]
- Aziz, A.A.; Yominaga, Y. Magnesium ion-conductive polye(ethylene carbonate) electrolytes. Ionics 2018, 24, 3475–3481. [Google Scholar] [CrossRef]
- Viviani, M.; Meereboer, N.L.; Saeaswati, N.L.P.A.; Loos, K.; Portale, G. Lithium and magnesium polymeric electrolytes prepared using poly(glycidyl ether)-based polymers with short grafted chains. Polym. Chem. 2020, 11, 2070–2079. [Google Scholar] [CrossRef]
- Sarangika, H.N.M.; Dissanayake, M.A.K.L.; Senadeera, G.K.R.; Rathnayake, R.R.D.V.; Pitawala, H.M.J.C. Polyethylene oxide and ionic liquid-based solid polymer electrolyte for rechargeable magnesium batteries. Ionics 2017, 23, 2829–2835. [Google Scholar] [CrossRef]
- Ge, X.; Song, F.; Du, A.; Zhang, Y.; Xie, B.; Huang, L.; Zhao, J.; Dong, S.; Zhou, X.; Cui, G. Robust self-standing single-ion polymer electrolytes enabling high-safety magnesium batteries at elevated temperature. Adv. Energy Mater. 2022, 12, 2201464. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, A.; Tripathi, S.K. Structural and electrochemical studies of bromide derived ionic liquid-based gel polymer electrolyte for energy storage. J. Energy Storage 2020, 32, 101723. [Google Scholar] [CrossRef]
- Hambali, D.; Zainol, N.H.; Othman, L.; Isa, K.B.M.; Osman, Z. Magnesium ion-conducting gel polymer electrolytes based on poly(vinylidene chloride-co-acrylonitrile) (PVdC-co-AN): A comparative study between magnesium trifluoromethanesulfonate (MgTf2) and magnesium bis(trifluoromethanesulfonimide) (Mg(TFSI)2). Ionics 2019, 25, 1187–1198. [Google Scholar] [CrossRef]
- Maheshwaran, C.; Mishra, K.; Kanchan, D.K.; Kumar, D. Mg2+ conducting polymer gel electrolytes: Physical and electrochemical investigations. Ionics 2020, 26, 2969–2980. [Google Scholar] [CrossRef]
- Kotobuki, M.; Yan, B.; Lu, L. Recent progress on cathode materials for rechargeable magnesium batteries. Energy Storage Mater. 2023, 54, 227–253. [Google Scholar] [CrossRef]
- Doe, R.E.; Han, R.; Hwang, J.; Gmitter, A.J.; Shterenberg, I.; Yoo, H.D.; Pour, N.; Aurbach, D. Novel electrolyte solutions comprisingly fully inorganic salts with high anodic stability for rechargeable magnesium batteries. Chem. Comm. 2014, 50, 243–245. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, X.; Liu, F.; Fan, L.-Z. Porous polymer electrolytes for long-cycle stable quasi-solid-state magnesium batteries. J. Energy Chem. 2021, 59, 608–614. [Google Scholar] [CrossRef]
- Merrill, L.C.; Ford, H.O.; Scaefer, J.L. Application of single-ion conducting gel polymer electrolytes in magnesium batteries. ACS Appl. Energy Mater. 2019, 2, 6355–6363. [Google Scholar] [CrossRef]
- Sheha, E.; Liu, F.; Wang, T.; Farrag, M.; Liu, J.; Yacout, N.; Kebebe, M.A.; Sharma, N.; Fan, L.-Z. Dual polymer/liquid electrolyte with BaTiO3 electrode for magnesium batteries. ACS Appl. Energy Mater. 2020, 3, 5882–5892. [Google Scholar] [CrossRef]
- Maheshwaran, C.; Kanchan, D.K.; Gohel, K.; Mishra, K.; Kumar, D. Effect of Mg(CF3SO3)2 concentration on structural and electrochemical properties of ionic liquid incorporated polymer electrolyte membranes. J. Solid State Electrochem. 2020, 24, 655–665. [Google Scholar] [CrossRef]
- Hambali, D.; Osman, Z.; Othman, L.; Isa, K.B.M.; Harudin, N. Magnesium (II) bis(trifluoromethanesulfonimide) doped PVdC-co-AN gel polymer electrolytes for rechargeable batteries. J. Polym. Res. 2020, 27, 159. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, A.; Tripathi, S.K. Structural, electrical and electrochemical studies of ionic liquid-based polymer gel electrolyte using magnesium salt for supercapacitor application. J. Polym. Res. 2021, 28, 235. [Google Scholar] [CrossRef]
- Aziz, A.; Yoshimoto, N.; Yamabuki, K.; Tominaga, Y. Ion-conductive, thermal and electrochemical properties of poly(ethylene carbonate)-Mg electrolytes with glyme solution. Chem. Lett. 2018, 47, 1258–1261. [Google Scholar] [CrossRef]
- Sharma, J.; Hashmi, S.A. Plastic crystal-incorporated magnesium ion conducting gel polymer electrolyte for battery application. Bull. Mater. Sci. 2018, 41, 147. [Google Scholar] [CrossRef]
- Ponraj, T.; Tamalingam, A.; Selvasekarapandian, S.; Srikumar, S.R.; Manjuladevi, R. Plasticized solid polymer electrolyte based on triblock copolymer polyvinylidene chloride-co-acrylonitrile-co-methyl methacrylate for magnesium ion batteries. Polym. Bull. 2021, 78, 35–57. [Google Scholar] [CrossRef]
- Tominaga, Y.; Kato, S.; Nishimura, N. Preparation and electrochemical characterization of magnesium gel electrolytes based on crosslinked poly(tetrahydrofuran). Polymer 2021, 224, 123743. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Kadir, M.F.Z.; Brza, M.A.; Karim, W.O. The study of EDLC device fabricated from plasticized magnesium ion conducting chitosan based polymer electrolyte. Polym. Test. 2020, 90, 106714. [Google Scholar] [CrossRef]
- Ponmami, S.; Prabhu, M.R. Sulfonate based ionic liquid incorporated polymer electrolytes for Magnesium secondary battery. Polym. Plast. Technol. Eng. 2019, 58, 978–991. [Google Scholar]
- Nishino, H.; Liu, C.; Kanehashi, S.; Mayumi, K.; Tominaga, Y.; Shimomura, T.; Ito, K. Ionics transport and mechanical properties of slide-ring gel swollen with Mg-ion electrolytes. Ionics 2020, 26, 255–261. [Google Scholar] [CrossRef]
- Singh, R.; Janakiraman, S.; Khalifa, M.; Anandhan, S.; Ghosh, S.; Venimadhav, A.; Biswas, K. An electroactive β-phase polyvinylidene fluoride as gel polymer electrolyte for magnesium-ion battery application. J. Electroanal. Chem. 2019, 851, 113417. [Google Scholar] [CrossRef]
- Bhatt, P.J.; Pathak, N.; Mishra, K.; Kanchan, D.K.; Kumar, D. Effect of different cations on ion-transport behavior in polymer gel electrolytes intended for application in flexible electrochemical devices. J. Electron. Mater. 2022, 51, 1371–1384. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Meng, Z.; Xiu, Y.; Dasari, B.; Zhao-Karger, Z.; Fichtner, M. Designing gel polymer electrolytes with synergetic properties for rechargeable magnesium batteries. Energy Storage Mater. 2022, 48, 155–163. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Muchakayala, R.; Song, S. High-performance Mg-ion conducting poly(vinyl alcohol) membranes: Preparation, characterization and application in supercapacitors. J. Membr. Sci. 2018, 555, 280–289. [Google Scholar] [CrossRef]
- Singh, R.; Janakiraman, S.; Agrawal, A.; Ghosh, S.; Venimadhav, A.; Biswas, K. An amorphous poly(vinylidene fluoride-co-hexafluoropropylene) based gel polymer electrolyte for magnesium ion battery. J. Electroanal. Chem. 2020, 858, 113788. [Google Scholar] [CrossRef]
- Singh, R.; Janakiraman, S.; Khalifa, M.; Anandhan, S.; Ghosh, S.; Venimadhav, A.; Biswas, K. A high thermally stable polyacrylonitrile (PAN)-based gel polymer electrolyte for rechargeable Mg-ion battery. J. Mater. Sci. Mater. Electron. 2020, 31, 22912–22925. [Google Scholar] [CrossRef]
- Maheshwaran, C.; Kanchan, D.K.; Mishra, K.; Kumar, D.; Gohel, K. Flexible, magnesium-ion conducting polymer electrolyte membrane: Mechanical, structural, thermal, and electrochemical impedance spectroscopic properties. J. Mater. Sci. Mater. Electron. 2020, 31, 15013–15027. [Google Scholar] [CrossRef]
- Abdulwahid, R.T.; Aziz, S.B.; Brza, M.A.; Kadir, M.F.Z.; Karim, W.O.; Hamsan, H.M.; Asnawi, A.S.F.M.; Abdullah, R.M.; Nofal, M.M.; Dannoun, E.M.A. Electrochemical performance of polymer blend electrolytes based on chitosan: Dextran: Impedance, dielectric properties, and energy storage study. J. Mater. Sci. Mater. Electron. 2021, 32, 14846–14862. [Google Scholar] [CrossRef]
- Ota, T.; Uchiyama, S.; Tsukada, K.; Moriya, M. Room-temperature Mg-ion conduction through molecular crystal Mg{N(SO2CF3)2}2(CH3OC5H9)2. Front. Energy Res. 2021, 9, 640777. [Google Scholar] [CrossRef]
- Mori, S.; Obora, T.; Namaki, M.; Kondo, M.; Moriya, M. Organic crystalline solid electrolytes with high Mg-ion conductivity composed of nonflammable ionic liquid analogs and Mg(TFSA)2. Inorg. Chem. 2022, 61, 7358–7364. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Kalapriya, K. Structural, transport, morphological, and thermal studies of nano barium titanate-incorporated magnesium ion conducting solid polymer electrolytes. Polym. Polym. Compos. 2021, 29, S1158–S1167. [Google Scholar]
- Wang, Y.; Wang, Z.; Zheng, F.; Sun, J.; Oh, J.A.S.; WU, T.; Chen, G.; Huang, Q.; Kotobuki, M.; Lu, L. Ferroelectric engineered electrode-composite polymer electrolyte interfaces for all-solid-state sodium metal battery. Adv. Sci. 2022, 9, 2105849. [Google Scholar] [CrossRef] [PubMed]
- Jeyabanu, K.; Sundaramahalingam, K.; Devendran, P.; Manikandan, A.; Nallamuthu, N. Effect of electrical conductivity studied for CuS nanofillers mixed magnesium ion based PVA-PVP blend polymer solid electrolytes. Phys. B Condenced Matter. 2019, 572, 129–138. [Google Scholar] [CrossRef]
- Jayalakshmi, K.; Ismayil; Hedge, S.; Ravindrachary, V.; Sanjeev, G.; Mazumdar, N.; Sindhoora, K.M.; Masti, K.; Jayalakshmi, S.P.; Murari, M.S. Methyl cellulose-based solid polymer electrolytes with dispersed zinc oxide nanoparticles: A promising candidate for battery applications. J. Phys. Chem. Solids 2023, 173, 111119. [Google Scholar] [CrossRef]
- Nidhi, S.P.; Kumar, R. Synthesis and characterization of magnesium ion conductive in PVDF based nanocomposite polymer electrolytes disperse with MgO. J. Alloys Compd. 2019, 789, 6–14. [Google Scholar] [CrossRef]
- Polu, A.; Kumar, R. Preparation and characterization of PEG-Mg(CH3COO)2-CeO2 composite polymer electrolytes for battery application. Bull. Mater. Sci. 2014, 37, 309–314. [Google Scholar] [CrossRef]
- Sarojini, S.; Padmapriya, L. Effect of size of the filler on the electrical conductivity of magnesium ion conducting polymer electrolyte. Mater. Today Proc. 2022, 68, 454–462. [Google Scholar] [CrossRef]
- Helen, P.A.; Selvin, P.C.; Lashmi, D.; Diana, M.I. Amelioration of ionic conductivity (303K) with the supplement of MnO2 filler in the chitosan biopolymer electrolyte for magnesium batteries. Polym. Bull. 2023, 80, 7715–7740. [Google Scholar] [CrossRef]
- Maheshwaran, C.; Kanchan, D.K.; Mishra, K.; Kumar, D.; Gohel, K. Effect of active MgO nano-particles dispersion in small amount within magnesium-ion conducting polymer electrolyte matrix. Nano-Struct. Nano-Objects 2020, 24, 100587. [Google Scholar] [CrossRef]
- Nidhi, S.P.; Kumar, R. Effect of Al2O3 on electrical properties of polymer electrolyte for electrochemical device application. Mater. Today Proc. 2021, 46, 2175–2178. [Google Scholar] [CrossRef]
- Nidhi, S.P.; Kumar, R. Effect of nanoparticles on electrical properties of PVDF-based Mg2+ ion conducting polymer electrolytes. Bull. Mater. Sci. 2021, 44, 140. [Google Scholar] [CrossRef]
- Sundar, M.; Selladurai, S. Effect of fillers on magnesium-poly(ethylene oxide) solid polymer electrolyte. Ionics 2006, 12, 281–286. [Google Scholar] [CrossRef]
- Ponmani, S.; Selvakumar, K.; Prabhu, M.R. The effect of the geikeilite (MgTiO3) nanofiller concentration in PVdF-HFP/PVAc-based polymer blend electrolytes for magnesium ion battery. Ionics 2020, 26, 2353–2369. [Google Scholar] [CrossRef]
- Helen, P.A.; Ajith, K.; Diana, M.I.; Lakshmi, D.; Selvin, P.C. Chitosan based biopolymer electrolyte reinforced with V2O5 filler for magnesium batteries: An inclusive investigation. J. Mater. Sci. Mater. Electron. 2022, 33, 3925–3937. [Google Scholar] [CrossRef]
- Mallikarjun, A.; Sangeetha, M.; Mettu, M.R.; Reddy, J.M.; Kumar, S.J.; Sreekanth, T.; Rao, V.S. Impedance spectroscopy and electrochemical cell studies of Mg2+ ion conducting with dispersed ZrO2 nano filler in PVDF-HFP based nano composite solid polymer electrolytes. Mater. Today Proc. 2022, 62, 5204–5208. [Google Scholar] [CrossRef]
- Patel, N.S.; Kumar, R. PVDF-HFP based nanocomposite polymer electrolytes for energy storage devices dispersed with various nano-fillers. AIP Conf. Proc. 2020, 2220, 080044. [Google Scholar]
- Dannoun, E.M.A.; Aziz, S.B.; Brza, M.A.; Nofal, M.M.; Asnawi, A.S.F.M.; Yusof, Y.M.; Al-Zangana, S.; Hamsan, M.H.; Kadir, M.F.Z.; Woo, H.J. The study of plasticized solid polymer blend electrolytes based on natural polymers and their application for energy storage EDLC devices. Polymers 2020, 12, 2531. [Google Scholar] [CrossRef]
- Sharma, J.; Hashmi, S. Magnesium ion-conducting gel polymer electrolyte nanocomposites: Effect of active and passive nanofillers. Polym. Compos. 2019, 40, 1295–1306. [Google Scholar] [CrossRef]
- Song, S.; Kotobuki, M.; Zheng, F.; Li, Q.; Xu, C.; Wang, Y.; Li, W.D.Z.; Hu, N.; Lu, L. Communication-A composite polymer electrolyte for safer Mg batteries. J. Electrochem. Soc. 2017, 164, A741–A743. [Google Scholar] [CrossRef]
- Aziz, S.B.; Dannoun, E.M.A.; Hamsan, M.H.; Abdulwahid, R.T.; Mishra, K.; Nofal, M.M.; Kadir, M.F.Z. Improving EDLC device performance constructed from plasticized magnesium ion conducting chitosan based polymer electrolytes via metal complex dispersion. Membranes 2021, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Deivanayagam, R.; Cheng, M.; Wang, M.; Vasudevan, V.; Foroozan, T.; Medhekar, N.V.; Shahbazian-Yassar, R. Composite polymer electrolyte for highly cyclable room-temperature solid-state magnesium batteries. ACS Appl. Energy Mater. 2019, 2, 7980–7990. [Google Scholar] [CrossRef]
- Sun, J.; Zou, Y.; Gao, S.; Shao, L.; Chen, C. Robust strategy of quasi-solid-state electrolytes to boost the stability and compatibility of Mg ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 54711–54719. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Truck, J.; Hacker, J.; Schlosser, A.; Kuster, K.; Starke, U.; Reinders, L.; Buchmeiser, M.R. A design concept for halogen-free Mg2+/Li+-dual salt-containing gel-polymer-electrolytes for rechargeable magnesium batteries. Energy Storage Mater. 2022, 49, 509–517. [Google Scholar] [CrossRef]
- Luo, W.; Allen, M.; Raji, V.; Ji, X. An organic pigment as a high-performance cathode for sodium-ion batteries. Adv. Energy Mater. 2014, 4, 1400554. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, W.; Cater, M.; Zhou, L.; Dai, J.; Fu, K.; Lacey, S.; Li, T.; Wan, J.; Han, J.; et al. Organic electrode for non-aqueous potassium-ion batteries. Nano Energy 2015, 18, 205–211. [Google Scholar] [CrossRef]
- Chen, Y.; Parent, L.R.; Shao, Y.; Wang, C.; Sprenkle, V.L.; Li, G.; Liu, J. Facile synthesis of chevrel phase nanocubes and their applications for multivalent energy storage. Chem. Mater. 2014, 26, 4904–4907. [Google Scholar] [CrossRef]
- Mao, M.; Lin, Z.; Tong, Y.; Yue, J.; Zhao, C.; Lu, J.; Zhang, Q.; Gu, L.; Suo, L.; Hu, Y.S.; et al. Iodine vapor transport-triggered preferential growth of chevrel Mo6S8 nanosheets for advanced multivalent batteries. ACS Nano 2020, 14, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).