Developing a Se Quantum Dots@ CoFeOx Composite Nanomaterial as a Highly Active and Stable Cathode Material for Rechargeable Zinc–Air Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
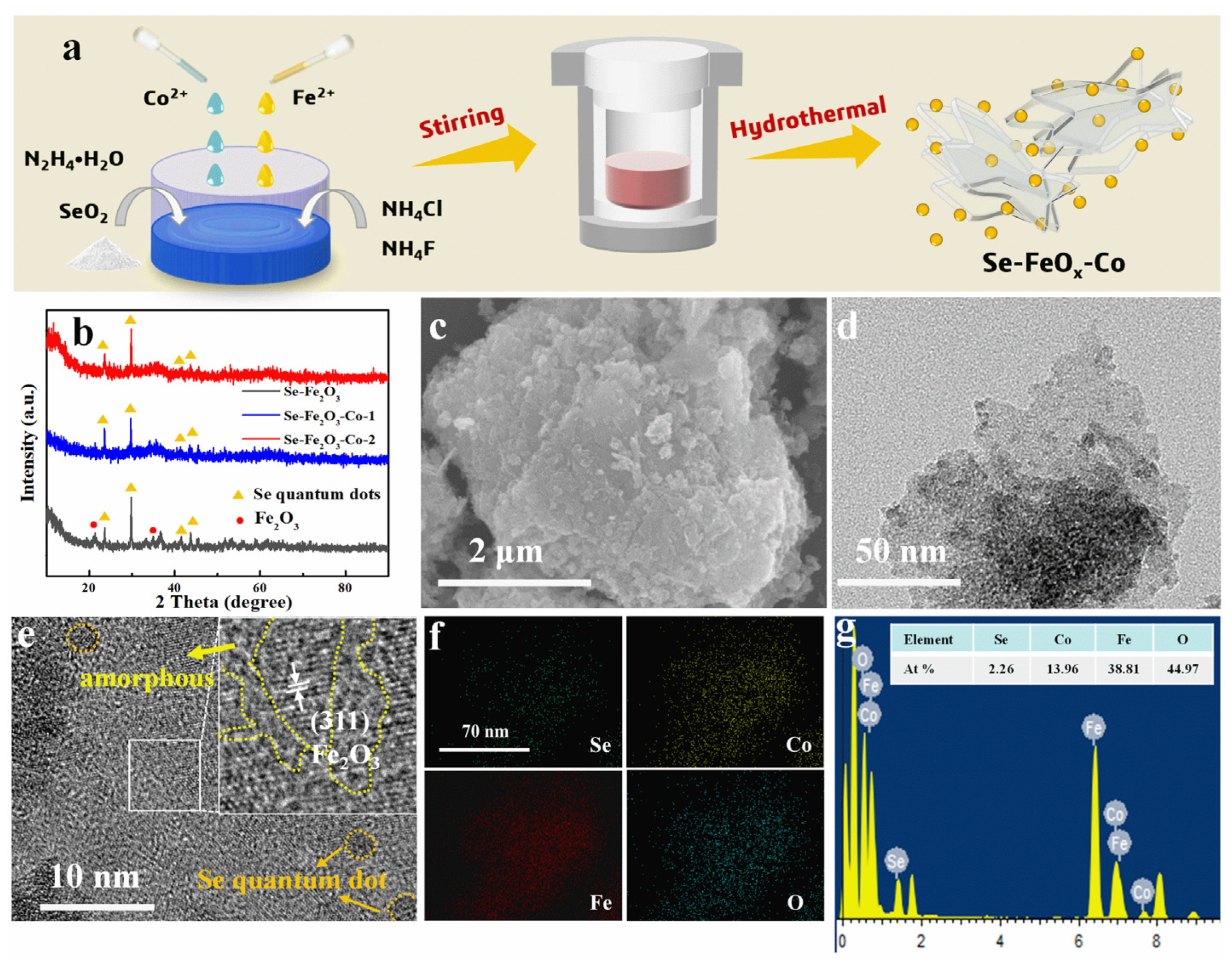
2.2. Synthesis of Se-FeOx-Co Composite Materials
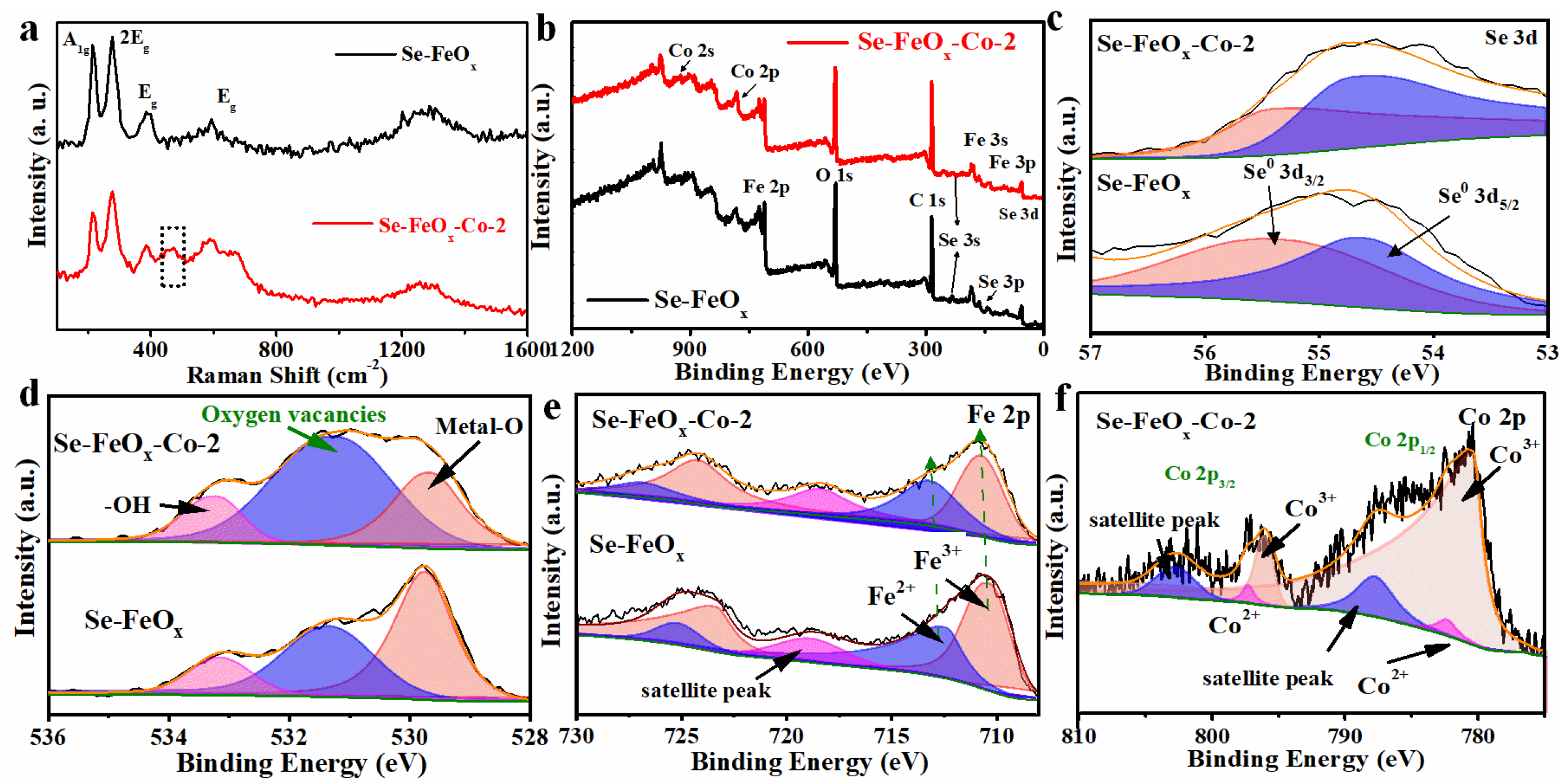
2.3. Materials’ Characterization
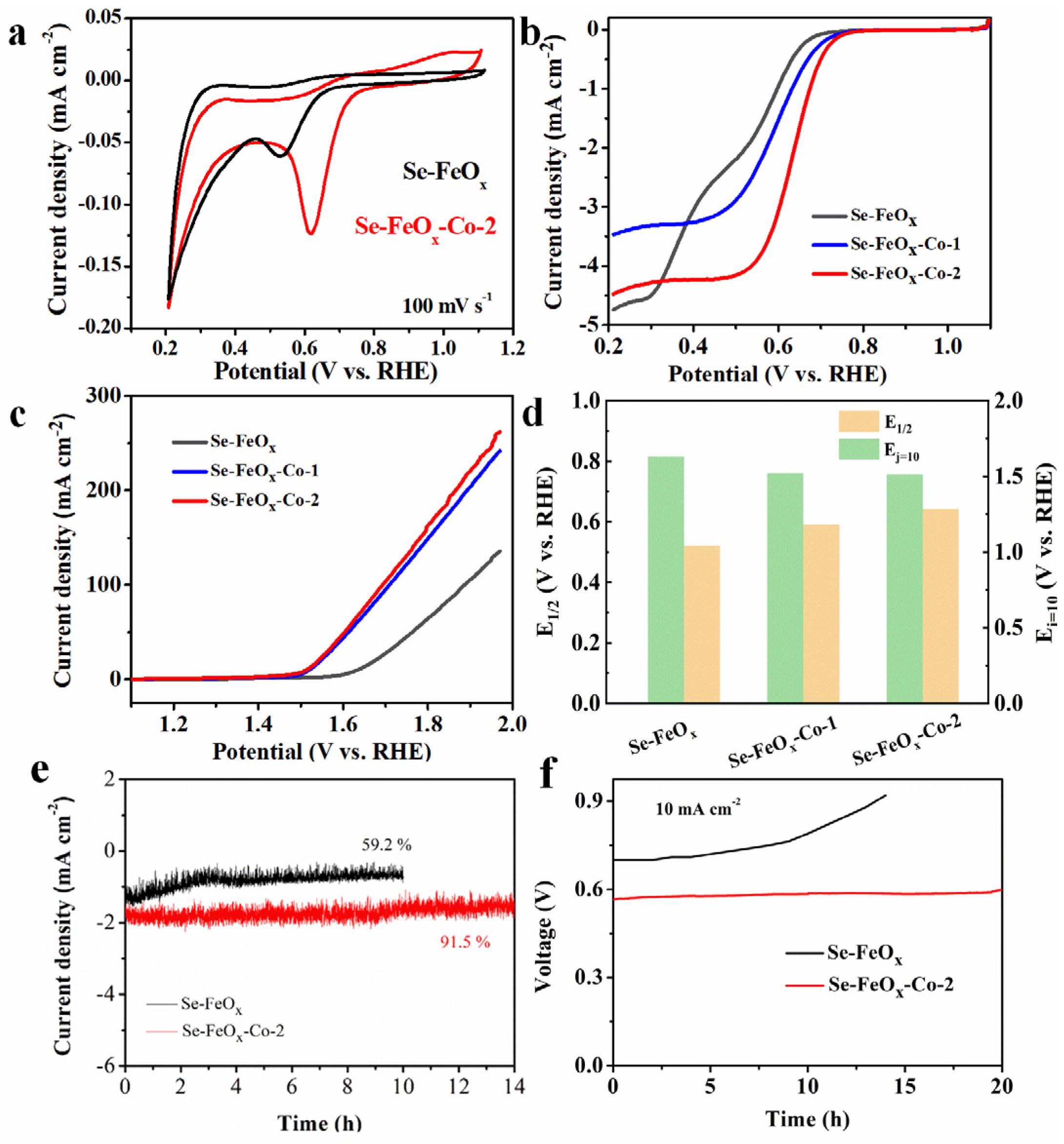
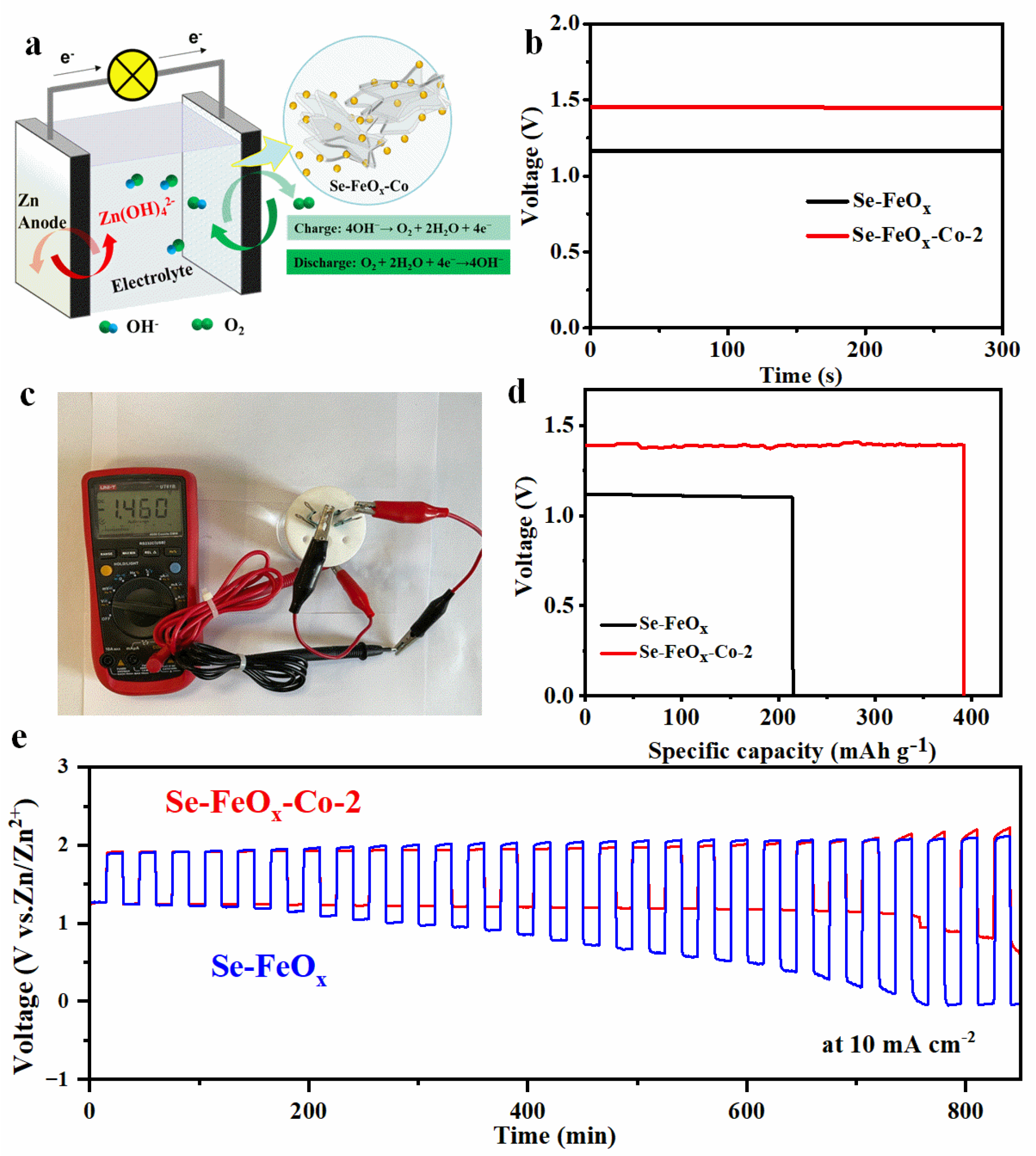
2.4. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Li, S.; Lu, Y. Designing safer lithium-based batteries with nonflammable electrolytes: A review. eScience 2021, 1, 163–177. [Google Scholar] [CrossRef]
- Yuan, M.; Sun, Z.; Yang, H.; Wang, D.; Liu, Q.; Nan, C.; Li, H.; Sun, G.; Chen, S. Self-catalyzed rechargeable lithium-air battery by in situ metal ion doping of discharge products: A combined theoretical and experimental study. Energy Environ. Mater. 2023, 6, e12258. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Niu, Y.; Gong, S.; Liu, X.; Xu, C.; Xu, M.; Sun, S.-G.; Chen, Z. Engineering iron-group bimetallic nanotubes as efficient bifunctional oxygen electrocatalysts for flexible Zn–air batteries. eScinece 2022, 2, 546–556. [Google Scholar] [CrossRef]
- Yu, H.; Fan, F.; He, C.; Zhou, M.; Ma, T.; Wang, Y.; Cheng, C. Sulfur-modulated FeNi nanoalloys as bifunctional oxygen electrode for efficient rechargeable aqueous Zn-air batteries. Sci. China Mater. 2022, 65, 3007–3016. [Google Scholar] [CrossRef]
- Xin, L.; Yang, F.; Rasouli, S.; Qiu, Y.; Li, Z.-F.; Uzunoglu, A.; Sun, C.-J.; Liu, Y.; Ferreira, P.; Li, W.; et al. Understanding Pt nanoparticle anchoring on graphene supports through surface functionalization. ACS Catal. 2016, 6, 2642–2653. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Wang, Y.; Wang, Y.; Chou, S.; Hu, Z.; Zhang, Z. Bifunctional carbon-based cathode catalysts for zinc-air battery: A review. Chin. Chem. Lett. 2022, 33, 683–692. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin- and corrole-based systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of metal catalysts for oxygen reduction reaction: From nanoscale engineering to atomic design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Chen, Z.; Zhong, L.; Zhao, D.; Chi, X.; Zhao, X.; Li, L.; Lu, X.; Leng, K.; et al. Hierarchically porous carbon plates derived from wood as bifunctional ORR/OER electrodes. Adv. Mater. 2019, 31, 1900341. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology control and electronic tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Wang, R.; Zhang, G.; Xu, X.; Liu, J.; Sun, Z.; Liu, M.; Wang, C.; Meng, X.; et al. Activating and stabilizing Co sites in CoP for triggering oxygen electrocatalysis in zinc-air battery. Chem. Eng. J. 2023, 475, 146154. [Google Scholar] [CrossRef]
- Qi, P.; Chen, M.; Luo, T.; Zhao, C.; Lin, C.; Luo, H.; Zhang, D. Solid-state self-catalyzed growth of N-doped carbon tentacles on an M(Fe, Co)Se surface for rechargeable Zn–air batteries. Chem. Commun. 2023, 59, 5898–5901. [Google Scholar] [CrossRef]
- Gui, F.; Jin, Q.; Xiao, D.; Jin, Z.; Zhang, Y.; Cao, Y.; Yang, M.; Tan, Q.; Zhang, C.; Siahrostami, S.; et al. High-performance zinc–air batteries enabled by hybridizing atomically dispersed FeN2 with Co3O4 nanoparticles. J. Mater. Chem. A 2023, 11, 1312–1323. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Shi, C.; Li, Y.; Mi, H.; Deng, L.; Zhang, Q.; He, C.; Ren, X. Rational design of the FeS2/NiS2 heterojunction interface structure to enhance the oxygen electrocatalytic performance for zinc–air batteries. J. Mater. Chem. A 2022, 10, 16627–16638. [Google Scholar] [CrossRef]
- Wang, J.-J.; Li, X.-P.; Cui, B.-F.; Zhang, Z.; Hu, X.-F.; Ding, J.; Deng, Y.-D.; Han, X.-P.; Hu, W.-B. A review of non-noble metal-based electrocatalysts for CO2 electroreduction. Rare Met. 2021, 40, 3019–3037. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Jiang, X.; Wu, X.; Tang, Y.; Sun, D.; Fu, G. Citrulline-induced mesoporous CoS/CoO heterojunction nanorods triggering high-efficiency oxygen electrocatalysis in solid-state Zn-air batteries. Chem. Eng. J. 2022, 434, 134744. [Google Scholar] [CrossRef]
- Weng, C.; Lv, X.; Ren, J.; Wang, Y.; Tian, W.; Gao, L.; Wang, H.; Yuan, Z. Self-promoted electrocatalysts derived from surface reconstruction for rechargeable Zinc–air batteries. ACS Sustain. Chem. Eng. 2022, 10, 6456–6465. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Li, J.; Qian, J.; Liu, Y.; Yang, S.; Wang, Y.; Gao, D.; Xue, D. Interfacial engineering of NiO/NiCo2O4 porous nanofibers as efficient bifunctional catalysts for rechargeable Zinc–air batteries. ACS Appl. Mater. Interfaces 2020, 12, 21661–21669. [Google Scholar] [CrossRef]
- Agyeman, D.; Zheng, Y.; Lee, T.; Park, M.; Tamakloe, W.; Lee, G.; Jang, H.; Cho, K.; Kang, Y. Synergistic catalysis of the lattice oxygen and transition metal facilitating ORR and OER in perovskite catalysts for Li–O2 batteries. ACS Catal. 2021, 11, 424–434. [Google Scholar] [CrossRef]
- Huang, H.; Huang, A.; Liu, D.; Han, W.; Kuo, C.; Chen, H.; Li, L.; Pan, H.; Peng, S. Tailoring oxygen reduction reaction kinetics on perovskite oxides via oxygen vacancies for low-temperature and knittable zinc–air batteries. Adv. Mater. 2023, 35, 2303109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Prabhakaran, S.; Ramakrishnan, S.; Karthikeyan, S.C.; Kim, A.R.; Kim, D.H.; Yoo, D.J. Developing outstanding bifunctional electrocatalysts for rechargeable Zn-air batteries using high-purity spinel-type ZnCo2Se4 nanoparticles. Small 2023, 19, 2207096. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Li, H.; Askari, S.; Wang, J.; Wolff, N.; Behrens, M.; Kienle, L.; Benedikt, J. Nitrogen-doped NiCo2O4 nanowires on carbon paper as a self-supported air cathode for rechargeable Zn-air batteries. Int. J. Hydrog. Energy 2023, 48, 26107–26118. [Google Scholar] [CrossRef]
- Bo, L.; Nian, F.; Pu, L.; Li, P.; Hu, Y.; Tong, J. Simply embedding α-Fe2O3/Fe3O4 nanoparticles in N-doped graphitic carbon polyhedron layered arrays as excellent electrocatalyst for rechargeable Zn-air battery. Sep. Purif. Technol. 2023, 312, 123413. [Google Scholar] [CrossRef]
- Samanta, A.; Das, S.; Jana, S. Doping of Ni in α-Fe2O3 nanoclews to boost oxygen evolution electrocatalysis. ACS Sustain. Chem. Eng. 2019, 7, 12117–12124. [Google Scholar] [CrossRef]
- Niu, Y.; Yuan, Y.; Zhang, Q.; Chang, F.; Yang, L.; Chen, Z.; Bai, Z. Morphology-controlled synthesis of metal-organic frameworks derived lattice plane-altered iron oxide for efficient trifunctional electrocatalysts. Nano Energy 2021, 82, 105699. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, H.; Wang, N.; Chen, B.; Mai, Y.; Feng, X. Patterning graphene surfaces with iron-oxide-embedded mesoporous polypyrrole and derived N-doped carbon of tunable pore size. Small 2018, 14, 1702755. [Google Scholar] [CrossRef]
- Hof, F.; Liu, M.; Valenti, G.; Picheau, E.; Paolucci, F.; Pénicaud, A. Size control of nanographene supported iron oxide nanoparticles enhances their electrocatalytic performance for the oxygen reduction and oxygen evolution reactions. J. Phys. Chem. C 2019, 123, 20774–20780. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Z.; Ye, X.; Zhang, L.; Feng, J.; Wang, A. Caffeine derived graphene-wrapped Fe3C nanoparticles entrapped in hierarchically porous FeNC nanosheets for boosting oxygen reduction reaction. J. Colloid Interface Sci. 2023, 637, 216–224. [Google Scholar] [CrossRef]
- Wang, M.; Ji, S.; Wang, H.; Wang, X.; Linkov, V.; Wang, R. Foamed carbon-supported nickel-iron oxides interspersed with bamboo-like carbon nanotubes for high-performance rechargeable Zinc-air batteries. Small 2022, 18, 2204474. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, J.; Yang, W.; Fu, Q.; Sun, K.; Song, Y.; Wei, Z.; Liao, Q.; Zhu, X. Green and facile synthesis of iron oxide nanoparticle-embedded N-doped biocarbon as an efficient oxygen reduction electrocatalyst for microbial fuel cells. Chem. Eng. J. 2020, 385, 123393. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, C.; Wang, W.; Pan, L.; Zou, J.; Wang, L.; Zhang, X.; Li, G. Tailoring the hetero-structure of iron oxides in the framework of nitrogen doped carbon for the oxygen reduction reaction and zinc–air batteries. J. Mater. Chem. A 2020, 8, 25791–25804. [Google Scholar] [CrossRef]
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.Y. Carbon quantum dots for energy applications: A review. ACS Appl. Nano Mater. 2021, 4, 6515–6541. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Meng, D.; Wei, L.; Shi, J.; Jiang, Q.; Tang, J. A review of enhanced electrocatalytic composites hydrogen/oxygen evolution based on quantum dot. J. Ind. Eng. Chem. 2023, 121, 27–39. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, T.; Huang, H. Semiconducting quantum dots for energy conversion and storage. Adv. Funct. Mater. 2023, 33, 2213770. [Google Scholar] [CrossRef]
- Qian, J.; Guo, X.; Wang, T.; Liu, P.; Zhang, H.; Gao, D. Bifunctional porous Co-doped NiO nanoflowers electrocatalysts for rechargeable zinc-air batteries. Appl. Catal. B Environ. 2019, 250, 71–77. [Google Scholar] [CrossRef]
- Lian, Y.; Yang, W.; Zhang, C.; Sun, H.; Deng, Z.; Xu, W.; Song, L.; Ouyang, Z.; Wang, Z.; Guo, J.; et al. Unpaired 3d electrons on atomically dispersed cobalt centres in coordination polymers regulate both oxygen reduction reaction (ORR) activity and selectivity for use in zinc–air batteries. Angew. Chem. Int. Ed. 2020, 59, 286–294. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Velusamy, D.B.; Sengodan, S.; Nagaraju, G.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Rational design of multifunctional electrocatalyst: An approach towards efficient overall water splitting and rechargeable flexible solid-state zinc–air battery. Appl. Catal. B Environ. 2022, 300, 120752. [Google Scholar] [CrossRef]
- Li, Y.-S.; Church, J.S.; Woodhead, A.L. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- El Mendili, Y.; Bardeau, J.F.; Randrianantoandro, N.; Gourbil, A.; Greneche, J.M.; Mercier, A.M.; Grasset, F. New evidences of in situ laser irradiation effects on γ-Fe2O3 nanoparticles: A Raman spectroscopic study. J. Raman Spectrosc. 2011, 42, 239–242. [Google Scholar] [CrossRef]
- Zheng, H.; Bao, J.; Huang, Y.; Xiang, L.; Faheem; Ren, B.; Du, J.; Nadagouda, M.N.; Dionysiou, D.D. Efficient degradation of atrazine with porous sulfurized Fe2O3 as catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2019, 259, 118056. [Google Scholar] [CrossRef]
- Karuppasamy, P.; Karthika, A.; Senthilkumar, S.; Rajapandian, V. An efficient and highly sensitive amperometric quercetin sensor based on a lotus flower like SeO2-decorated rGO nanocomposite modified glassy carbon electrode. Electrocatalysis 2022, 13, 269–282. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Zhang, J.; Chu, X.; Xu, J.; Li, J.; Liu, Y.; Yu, H.; Yan, L.; Zhang, L.; et al. Encapsulating selenium into mesoporous carbon for high-performance aqueous Cu-Se battery. Chem. Eng. J. 2023, 454, 140433. [Google Scholar] [CrossRef]
- Liu, K.; Niu, J.; Liu, L.; Tian, F.; Nie, H.; Liu, X.; Chen, K.; Zhao, R.; Sun, S.; Jiao, M.; et al. LUMO-mediated Se and HOMO-mediated Te nanozymes for selective redox biocatalysis. Nano Lett. 2023, 23, 5131–5140. [Google Scholar] [CrossRef]
- Sapna; Budhiraja, N.; Kumar, V.; Singh, S.K. Synergistic effect in structural and supercapacitor performance of well dispersed CoFe2O4/Co3O4 nano-hetrostructures. Ceram. Int. 2018, 44, 13806–13814. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Qin, Z. ZnO/ZnFe2O4 nanocomposite as a broad-spectrum photo-Fenton-like photocatalyst with near-infrared activity. Catal. Sci. Technol. 2017, 7, 2236–2244. [Google Scholar] [CrossRef]
- Li, C.; Lin, Y.; Li, X.; Li, Z.; Luo, P.; Jin, Y.; Li, Z. Effect of Co-doping concentration on α-Fe2O3/Graphene as anode materials for lithium ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130681. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Fu, J.; Jiang, G.; Lui, G.; Luo, D.; Deng, Y.P.; Zhang, J.; Cano, Z.P.; Yu, A.; et al. An oxygen-vacancy-rich semiconductor-supported bifunctional catalyst for efficient and stable zinc–air batteries. Adv. Mater. 2019, 31, 1806761. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Wu, D.; Yang, J. Optimizing the electronic structure of cobalt via synergized oxygen vacancy and Co-N-C to boost reversible oxygen electrocatalysis for rechargeable Zn-air batteries. Appl. Catal. B Environ. 2020, 278, 119300. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.; Tang, Y.; Meng, Q.; Zhou, W.; Chen, S.; Deng, S.; Wang, J. Unveiling the role of cobalt doping in optimizing ammonia electrosynthesis on iron–cobalt oxyhydroxide hollow nanocages. J. Mater. Chem. A 2023, 11, 14424–14431. [Google Scholar] [CrossRef]
- Li, M.; Tang, Y.; Shi, W.; Chen, F.; Shi, Y.; Gu, H. Design of visible-light-response core–shell Fe2O3/CuBi2O4 heterojunctions with enhanced photocatalytic activity towards the degradation of tetracycline: Z-scheme photocatalytic mechanism insight. Inorg. Chem. Front. 2018, 5, 3148–3154. [Google Scholar] [CrossRef]
- Zhu, H.; Dong, S.; Du, X.; Du, H.; Xia, J.; Liu, Q.; Luo, Y.; Guo, H.; Li, T. Defective CuO-rich CuFe2O4 nanofibers enable the efficient synergistic electrochemical reduction of nitrate to ammonia. Catal. Sci. Technol. 2022, 12, 4998–5002. [Google Scholar] [CrossRef]
- Luo, J.; Han, X.; Ge, J.; Wang, Y.; Zhao, X.; Zhang, F.; Lei, X. Cu9S5/Fe2O3 nanospheres as advanced negative electrode materials for high performance battery-like hybrid capacitors. ACS Appl. Energy Mater. 2022, 5, 7016–7025. [Google Scholar] [CrossRef]
- Yang, H.; Wang, B.; Li, H.; Ni, B.; Wang, K.; Zhang, Q.; Wang, X. Trimetallic sulfide mesoporous nanospheres as superior electrocatalysts for rechargeable Zn–air batteries. Adv. Energy Mater. 2018, 8, 1801839. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Yang, Q.; Li, X.; Li, H.; Wang, Y.; Jiao, T.; Huang, Z.; Dong, B.; Zhang, W.; et al. Highly efficient electrochemical reduction of nitrogen to ammonia on surface termination modified Ti3C2Tx MXene nanosheets. ACS Nano 2020, 14, 9089–9097. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, S.; Zeng, X.; Li, X.; Xiao, L.; Chen, K.; Lu, Q.; Xu, Q.; Weng, J.; Xu, J. Holey amorphous FeCoO-coated black phosphorus for robust polysulfide adsorption and catalytic conversion in lithium–sulfur batteries. J. Mater. Chem. A 2022, 10, 11676–11683. [Google Scholar] [CrossRef]
- Lee, K.; Zhang, L.; Lui, H.; Hui, R.; Shi, Z.; Zhang, J. Oxygen reduction reaction (ORR) catalyzed by carbon-supported cobalt polypyrrole (Co-PPy/C) electrocatalysts. Electrochim. Acta 2009, 54, 4704–4711. [Google Scholar] [CrossRef]
- Xu, C.; Guo, C.; Liu, J.; Hu, B.; Dai, J.; Wang, M.; Jin, R.; Luo, Z.; Li, H.; Chen, C. Accelerating the oxygen adsorption kinetics to regulate the oxygen reduction catalysis via Fe3C nanoparticles coupled with single Fe-N4 sites. Energy Storage Mater. 2022, 51, 149–158. [Google Scholar] [CrossRef]
- Gao, S.; Fan, B.; Feng, R.; Ye, C.; Wei, X.; Liu, J.; Bu, X. N-doped-carbon-coated Fe3O4 from metal-organic framework as efficient electrocatalyst for ORR. Nano Energy 2017, 40, 462–470. [Google Scholar] [CrossRef]
- Mao, S.; Wen, Z.; Huang, T.; Hou, Y.; Chen, J. High-performance bi-functional electrocatalysts of 3D crumpled graphene–cobalt oxide nanohybrids for oxygen reduction and evolution reactions. Energy Environ. Sci. 2014, 7, 609–616. [Google Scholar] [CrossRef]
- Du, S.; Ren, Z.; Qu, Y.; Wu, J.; Xi, W.; Zhu, J.; Fu, H. Co3O4 nanosheets as a high-performance catalyst for oxygen evolution proceeding via a double two-electron process. Chem. Commun. 2016, 52, 6705–6708. [Google Scholar] [CrossRef]
- Tian, G.; Zhao, M.; Yu, D.; Kong, X.; Huang, J.; Zhang, Q.; Wei, F. Nitrogen-doped graphene/carbon nanotube hybrids: In situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Rasaki, S.A.; Chen, Z.; Shen, H.; Guo, H.; Thomas, T.; Yang, M. Cobalt nanoparticles modified single-walled titanium carbonitride nanotube derived from solid-solid separation for oxygen reduction reaction in alkaline solution. Electrocatalysis 2020, 11, 579–592. [Google Scholar] [CrossRef]
- Tang, K.; Hu, H.; Xiong, Y.; Chen, L.; Zhang, J.; Yuan, C.; Wu, M. Hydrophobization engineering of the air–cathode catalyst for improved oxygen diffusion towards efficient zinc–air batteries. Angew. Chem. Int. Ed. 2022, 61, e202202671. [Google Scholar] [CrossRef]
- Faisal, S.N.; Haque, E.; Noorbehesht, N.; Liu, H.; Islam, M.M.; Shabnam, L.; Roy, A.K.; Pourazadi, E.; Islam, M.S.; Harris, A.T.; et al. A quadrafunctional electrocatalyst of nickel/nickel oxide embedded N-graphene for oxygen reduction, oxygen evolution, hydrogen evolution and hydrogen peroxide oxidation reactions. Sustain. Energy Fuels 2018, 2, 2081–2089. [Google Scholar] [CrossRef]
- Shudo, Y.; Fukuda, M.; Islam, M.S.; Kuroiwa, K.; Sekine, Y.; Karim, M.R.; Hayami, S. 3D porous Ni/NiOx as a bifunctional oxygen electrocatalyst derived from freeze-dried Ni(OH)2. Nanoscale 2021, 13, 5530–5535. [Google Scholar] [CrossRef]
- Xiong, P.; Sun, B.; Sakai, N.; Ma, R.; Sasaki, T.; Wang, S.; Zhang, J.; Wang, G. 2D superlattices for efficient energy storage and conversion. Adv. Mater. 2020, 32, 1902654. [Google Scholar] [CrossRef]
- Cheng, Y.; Pang, K.; Wu, X.; Zhang, Z.; Xu, X.; Ren, J.; Huang, W.; Song, R. In situ hydrothermal synthesis MoS2/guar gum carbon nanoflowers as advanced electrocatalysts for electrocatalytic hydrogen evolution. ACS Sustain. Chem. Eng. 2018, 6, 8688–8696. [Google Scholar] [CrossRef]
- Kong, H.; Lv, C.; Yan, C.; Chen, G. Engineering mesoporous single crystals Co-doped Fe2O3 for high-performance lithium ion batteries. Inorg. Chem. 2017, 56, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. A three-dimensional branched cobalt-doped α-Fe2O3 Nanorod/MgFe2O4 heterojunction array as a flexible photoanode for efficient photoelectrochemical water oxidation. Angew. Chem. Int. Ed. 2013, 52, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Wang, Y.; Han, X.; Hu, W. Developing a Se Quantum Dots@ CoFeOx Composite Nanomaterial as a Highly Active and Stable Cathode Material for Rechargeable Zinc–Air Batteries. Batteries 2023, 9, 561. https://doi.org/10.3390/batteries9110561
Zhang D, Wang Y, Han X, Hu W. Developing a Se Quantum Dots@ CoFeOx Composite Nanomaterial as a Highly Active and Stable Cathode Material for Rechargeable Zinc–Air Batteries. Batteries. 2023; 9(11):561. https://doi.org/10.3390/batteries9110561
Chicago/Turabian StyleZhang, Donghao, Yang Wang, Xiaopeng Han, and Wenbin Hu. 2023. "Developing a Se Quantum Dots@ CoFeOx Composite Nanomaterial as a Highly Active and Stable Cathode Material for Rechargeable Zinc–Air Batteries" Batteries 9, no. 11: 561. https://doi.org/10.3390/batteries9110561
APA StyleZhang, D., Wang, Y., Han, X., & Hu, W. (2023). Developing a Se Quantum Dots@ CoFeOx Composite Nanomaterial as a Highly Active and Stable Cathode Material for Rechargeable Zinc–Air Batteries. Batteries, 9(11), 561. https://doi.org/10.3390/batteries9110561







