1. Introduction
Commercial lithium-ion batteries have been utilized broadly in the fields of plug-in hybrid/electric vehicles, smartphones, laptop computers, and other portable electronic devices for the past several decades. They significantly reduce environmental pollution and greenhouse gas emissions. The energy densities of lithium-ion batteries are close to their limitation because of the low theoretical capacities of oxide cathodes (i.e., LiFePO
4, LiCoO
2, LiMn
2O
4, and LiNi
xMn
yCo
zO
2) and graphite anode materials [
1]. Lithium–sulfur batteries that use lithium metal anodes instead of graphite can theoretically reach high specific capacity values [
2]. However, there are safety concerns, such as leakage and risk of explosion, associated with organic liquid electrolyte-based lithium–sulfur batteries, and they exhibit poor rate capability and cycling stability due to the huge changes in the volume of sulfur that occur during the charge/discharge process. All-solid-state lithium batteries with nonflammable inorganic solid-state electrolytes have attracted considerable attention in the field of energy storage [
3,
4,
5]. Among the various solid electrolytes, sulfide solid electrolytes have broad application potential because of their high ionic conductivity and low elastic modulus. For example, the Li
10GeP
2S
12 sulfide solid electrolyte [
6] possesses a high ionic conductivity of around 10
−2 S cm
−1 and surpasses the liquid electrolytes used in traditional lithium-ion batteries. The electrochemical stability of the Li
6PS
5Cl electrolyte was also investigated, and it displayed excellent stability when in contact with lithium metal [
7].
Not only is the selection of the sulfide solid electrolyte essential, but the selection of an appropriate cathode material is also key to achieving high performance all-solid-state batteries. The classical oxide cathode materials and transition metal sulfides display different reaction mechanisms during the charge/discharge process. For classical lithium intercalation process in oxide cathode materials, because the 3d metal cationic band is much higher than the p band of oxygen, the ion–electron transfer reactions occur from lithium to the lowest unoccupied energy level of the transition metal d band in ionic oxides. The discharge/charge (lithium ion intercalation/de-intercalation) process relies on the d metal level to host/release the associated electrons; this is called the cation-driven redox process. For transition metal sulfide materials, the p band of sulfur is located in a higher position and is therefore closer or even penetrate the d band of transition metal. Due to the charge transfer of these two bands, the anionic redox process is triggered, and this can improve the reversible capacity of the battery [
8]. The use of transition metal sulfides such as a-TiS
4 [
9], FeS
2 [
10], NiS
2 [
11,
12], and TiS
3 [
12] in all-solid-state batteries has been widely studied due to the low cost of raw materials and their abundant yields. Meanwhile, their relatively high transport kinetics can promote conductivity and reduce charge transfer resistance. Furthermore, sulfide electrolytes show superior compatibility with transition metal sulfide cathodes due to their similar chemical potential, thus realizing high energy density [
13,
14]. However, all-solid-state lithium batteries with sulfide electrolytes and transition metal sulfide cathodes still suffer from inferior interfacial contact within the cathode layer.
Traditional sulfide cathode materials based on insertion reactions can accommodate lithium ions in their lattices without serious structural changes occuring during the intercalation/de-intercalation reaction of
, in which M represents the transition metal [
15]. Although insertion reaction-based cathode materials exhibit stability and long life cycles when used in all-solid-state lithium batteries, they still have intrinsic problems related to their limited space for lithium ions result in to a low specific capacity [
8,
16]. Nevertheless, in transition metal sulfide cathode materials with anionic redox driven chemistry (a-TiS
4 [
9], MoS
3 [
17], and FeS
2 [
10]), sulfur fully or partially exists in the state of
pairs, and this has a strong influence on specific capacity. For example, MoS
2 only has
pairs and has a theoretical specific capacity of 670 mAh g
−1 [
18,
19]. MoS
3 has both
and
pairs and has a higher theoretical specific capacity of 837 mAh g
−1 [
17]. The electronic structure of the
group allows them to donate or receive electrons. Their ability as donors is attributed to the π*
g orbital, which can release electrons that convert
to
. Because of the reversible reaction of
, multi-electron reactions proceed during the charge/discharge process, resulting in a high specific capacity [
8,
19]. In addition, transition metal sulfides with anionic redox driven chemistry display a high voltage plateau of about 2 V, which is close to that of a lithium–sulfur battery.
Even though MoS
2 and MoS
3 demonstrate high theoretical specific capacities, the actual reversible specific capacities of MoS
2 and MoS
3 in all-solid-state lithium batteries are only 592 mAh g
−1 (0.1 C, 0.1–3.0 V) and 747 mAh g
−1 (0.05 A g
−1, 0.5–3.0 V), respectively [
19,
20]. Ionic transport kinetics and interfacial contact within the cathode layer become crucial challenges. Coating a thin electrolyte layer onto the surfaces of active materials is an effectively strategy to enhance ionic transport kinetics and the contact between the solid electrolyte and the active material [
17,
21]. Compared with MoS
2 and MoS
3, MoS
6 is considered one of the most promising cathode materials for all-solid-state lithium batteries because of its ultra-high theoretical specific capacity of 1117 mAh g
−1, which is a result of its high
content as well as its amorphous nature, according to which possess open and random transmission paths to achieve cycling stability [
22]. It therefore has potential applications in high energy all-solid-state batteries.
In this work, a micro-sized cathode material composite, MoS6@15%Li7P3S11, is designed for use in all-solid-state lithium batteries. First, MoS6 was synthesized using a wet chemical method. After the in situ coating of the Li7P3S11 solid electrolyte onto the MoS6, the ionic diffusivity enhanced from 10−12–10−11 cm2 s−1 to 10−11–10−10 cm2 s−1. The Li/Li6PS5Cl/MoS6@15%Li7P3S11 all-solid-state lithium batteries exhibited high initial and reversible discharge capacities of 1083.8 mAh g−1 and 851.5 mAh g−1, respectively, at 0.1 A g−1 and 25 °C within 1.0–3.0 V. Because of the remarkable intimate interfacial contact between the active material MoS6 and the solid electrolyte, these all-solid-state batteries can realize a long cycle life of 1000 cycles with a high reversible specific capacity of 400 mAh g−1 at 1 A g−1 and 25 °C.
3. Results and Discussions
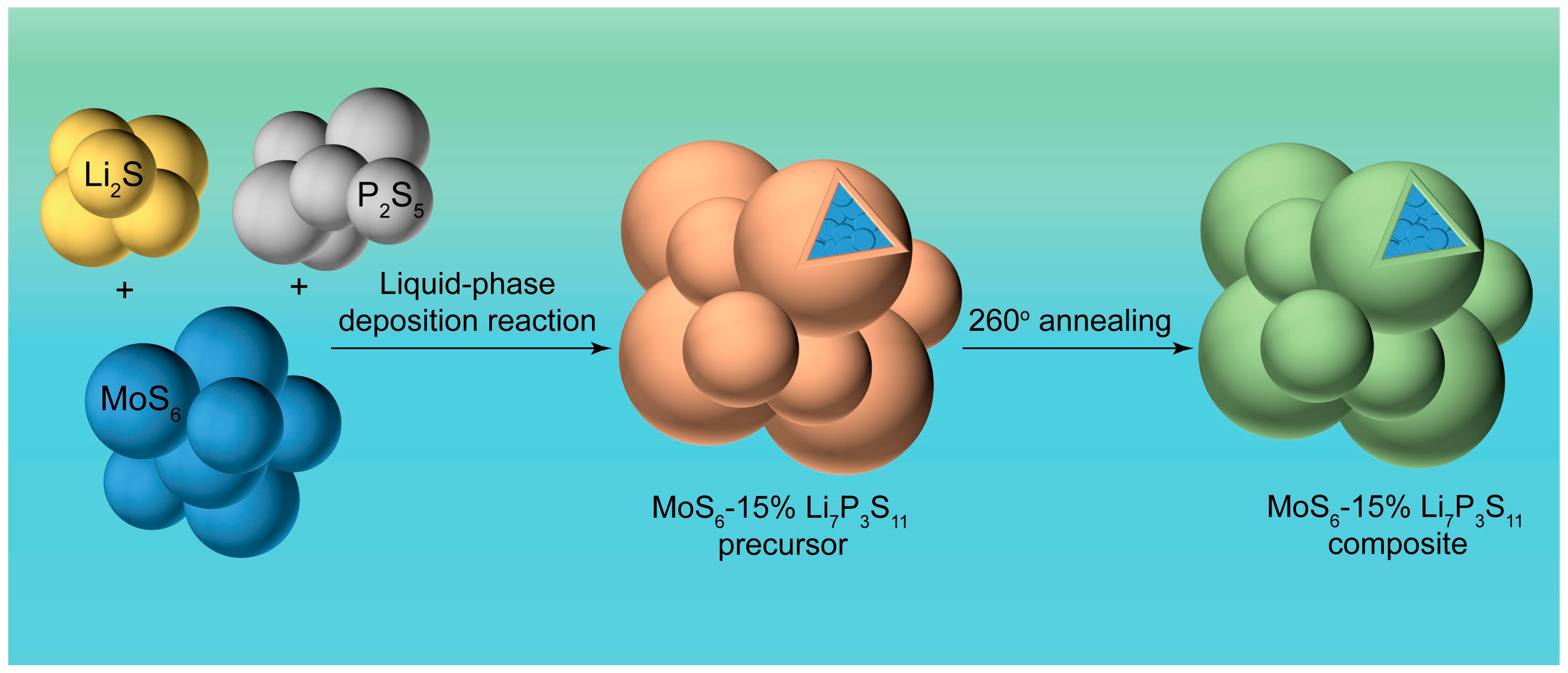
To better demonstrate the properties of the prepared sample, the Mo/S atom ratio was measured using an inductively coupled plasma emission spectrometer (ICP) (
Table S1). The actual remaining weight ratios of the Mo and S were 31.5 wt% and 63.5 wt%, respectively, indicating an Mo/S ratio of 6.036, which is in agreement with the theoretical value of 6. The procedure for synthesizing the MoS
6@15%Li
7P
3S
11 composite is schematically illustrated in
Figure 1. A Li
7P
3S
11 solid electrolyte precursor was in situ coated onto the MoS
6 surface during facile liquid phase deposition. After a 260 °C annealing treatment, the MoS
6@15%Li
7P
3S
11 composite was successfully prepared.
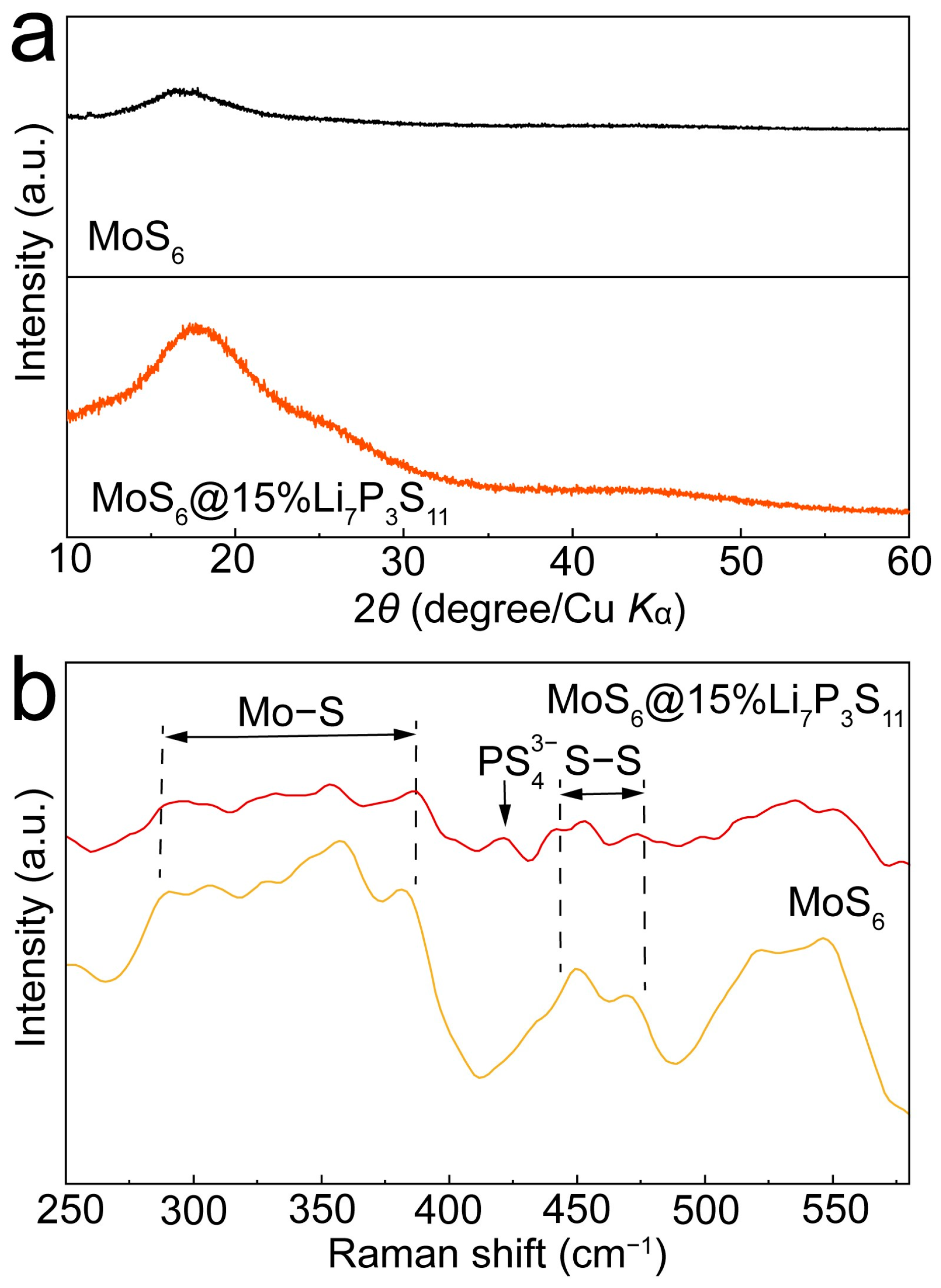
The XRD patterns of the MoS
6 and MoS
6@15%Li
7P
3S
11 composite are shown in
Figure 2a, and they confirm the amorphous nature of MoS
6. No characteristic peaks were detected for the Li
7P
3S
11 sulfide electrolytes, indicating the low amount of Li
7P
3S
11 sulfide electrolytes in the coating layer. In order to confirm the existence of the Li
7P
3S
11 sulfide solid electrolytes in the MoS
6@15%Li
7P
3S
11 composite, Raman spectroscopy was performed (
Figure 2b). The peaks located in the ranges of 286–385 cm
−1 and 518–550 cm
−1 correspond to vibrations of molybdenum sulfide bonds and bridging disulfide/terminal disulfide [
26,
27]. Furthermore, the peak located at 421cm
−1 can be attributed to the
in the Li
7P
3S
11 sulfide solid electrolyte [
21].
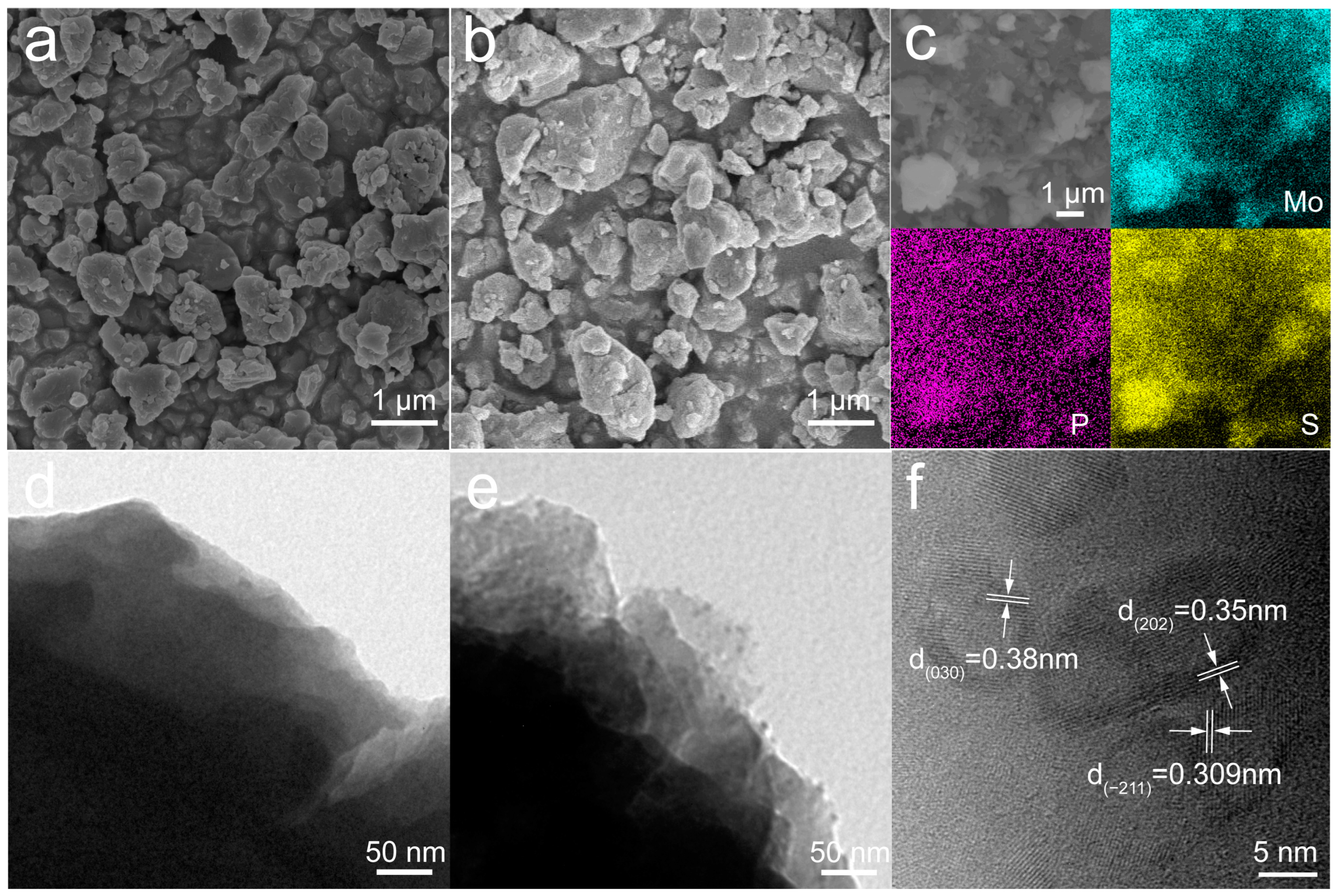
The morphology and microstructure of the MoS
6 and MoS
6@15%Li
7P
3S
11 composite were observed using SEM and HRTEM (
Figure 3). The particle sizes of the MoS
6 and MoS
6@15%Li
7P
3S
11 composite were in the range of 1–4 μm (
Figure 3a,b). EDS mapping of the MoS
6@15%Li
7P
3S
11 composite clearly illustrates the good distribution of molybdenum (blue), phosphorus (purple), and sulfur (yellow) (
Figure 3c). The HRTEM images further confirmed that Li
7P
3S
11 solid electrolytes were uniformly growing on the surface of the MoS
6 (
Figure 3d,e), and this facilitates improvements in ionic transport kinetics and the formation of intimate interfacial contact. The
d-spacings of 0.38, 0.35, and 0.309 nm correspond to the
d030,
d202, and
d−211 spacing of the Li
7P
3S
11, respectively (
Figure 3f) [
21].
The electrochemical performance of the MoS
6 was tested in all-solid-state lithium batteries. Two types of battery configuration were employed at 25 °C and 0.2 A g
−1, i.e., Li/Li
6PS
5Cl/MoS
6 and Li/75%Li
2S-24%P
2S
5-1%P
2O
5/Li
10GeP
2S
12/MoS
6 all-solid-state batteries. As
Figure S2 shows, the Li/Li
6PS
5Cl/MoS
6 all-solid-state battery exhibited a reversible specific capacity of 361.8 mAh g
−1 after 200 cycles, which was higher than the value exhibited by the Li/Li
10GeP
2S
12/75%Li
2S-24%P
2S
5-1%P
2O
5/MoS
6 all-solid-state battery (78.3 mAh g
−1), indicating a better capacity retention. For the purpose of comparison, cycles of 50MoS
6:50Li
10GeP
2S
12, 40MoS
6:50Li
10GeP
2S
12:10Super P, and 40MoS
6:40Li
10GeP
2S
12:20Super P were performed at 0.1 A g
−1 and 25 °C to determine the appropriate mixing ratio, as is shown in
Figure S3. The capacity retention of the 40MoS
6:50Li
10GeP
2S
12:10Super P was 544.8 mAh g
−1 after 10 cycles; this was higher than the 394.69 mAh g
−1 and 438.29 mAh g
−1 values observed for the 50MoS
6:50Li
10GeP
2S
12 and 40MoS
6:40Li
10GeP
2S
12:20Super P, respectively, and indicated a high reversible discharge capacity.
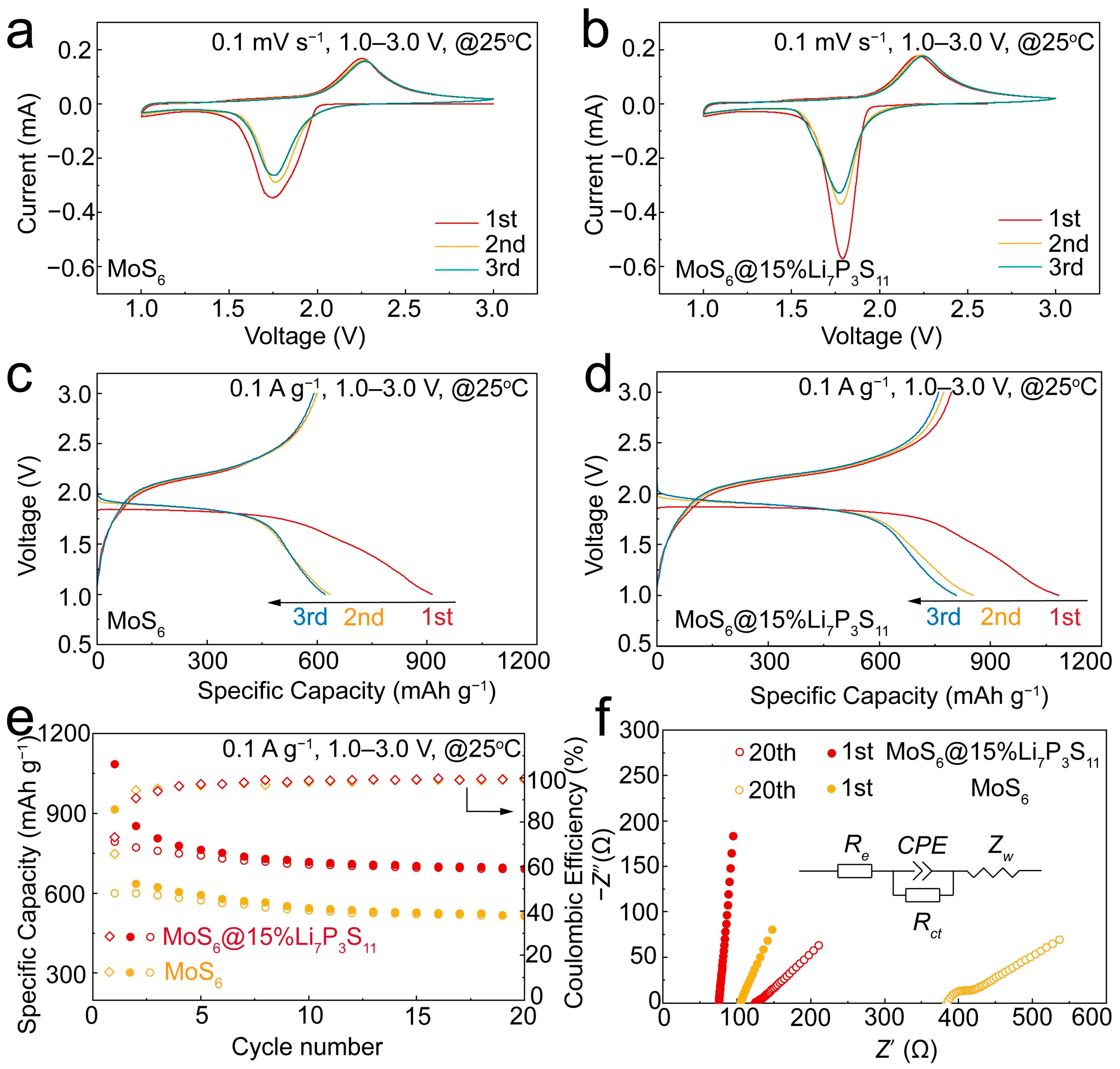
To reveal the electrochemical reaction mechanisms of the MoS
6 and MoS
6@15% Li
7P
3S
11 composite, CV curves were generated for the Li/Li
6PS
5Cl/MoS
6 and Li/Li
6PS
5Cl/MoS
6@15%Li
7P
3S
11 all-solid-state batteries for the first three cycles, and these are shown in
Figure 4a,b. During the first cathodic scan, there was a reduction peak at around 1.75 V which can be attributed to the lithiation process of the MoS
6 and a further conversion process. During the initial anodic scanning period, the oxidation peak which occurred at 2.25 V corresponds to the de-lithiation processes and the forming of molybdenum sulfides. The CV curves of the MoS
6@15%Li
7P
3S
11 composite are similar to those of the MoS
6; they show the same electrochemical reaction process [
21,
28]. Even so, the redox peaks of the MoS
6@15%Li
7P
3S
11 composite are narrower and stronger than those of the MoS
6, illustrating enhancements in the reversibility and electrochemical reaction kinetics resulting from favorable ionic diffusion. In the second cycle, the CV curves display an apparent tendency to overlap, which indicates that the MoS
6@15%Li
7P
3S
11 composite has excellent cycling stability. Clearly, the redox reactions of the MoS
6 and MoS
6@15%Li
7P
3S
11 composite occur within the potential window of 1.0–3.0 V, which was selected to further evaluate electrochemical performances.
Figure 4c,d present the galvanostatic discharge–charge curves of the first three cycles of the MoS
6 and MoS
6@15% Li
7P
3S
11 composite in the all-solid-state lithium batteries at 0.1 A g
−1 and 25 °C. The all-solid-sate lithium battery with the MoS
6 cathodes delivered a high initial discharge capacity of 913.9 mAh g
−1. After coupling with the Li
7P
3S
11 solid-electrolyte thin layer, the all-solid-state lithium battery with the MoS
6@15%Li
7P
3S
11 composite cathodes delivered initial and reversible discharge capacities of 1083.8 mAh g
−1 and 851.5 mAh g
−1, respectively (
Figure 4d), which were the one of highest values observed among the many sulfide-based cathode materials, i.e., rGO-MoS
3, cubic FeS
2, Co
9S
8@Li
7P
3S
11, and MoS
2@Li
7P
3S
11 [
10,
17,
19,
21]. As is shown in
Table S2, the initial discharge capacities of the rGO-MoS
3, FeS
2, Co
9S
8@Li
7P
3S
11, and MoS
2@Li
7P
3S
11 were 1241.4 mAh g
−1, 750 mAh g
−1, 633 mAh g
−1, and 868.4 mAh g
−1, respectively, while their respective reversible capacities were around 760 mAh g
−1, 730 mAh g
−1, 574 mAh g
−1, and 669.2 mAh g
−1. The increased reversible discharge capacity compared with that of the MoS
6 could be attributed to the better interface compatibility between the active material and the solid electrolyte. In addition, the initial Coulombic efficiency of the MoS
6@15%Li
7P
3S
11 composite was 73.2%, which was higher than that of the MoS
6 (65.6%). These values were calculated using the equation
, where
C is the specific capacity of the charge or discharge process. As is shown in
Figure 4e, the MoS
6@15%Li
7P
3S
11 composite exhibited a remarkably stable cyclic performance with an impressive reversible specific capacity of 693.2 mAh g
−1 after 20 cycles, while the MoS
6 only showed a reversible specific capacity of 517.7 mAh g
−1. The excellent electrochemical performance of the MoS
6@15%Li
7P
3S
11 composite can be attributed to the Li
7P
3S
11 thin layer, which can improve the cathode–electrolyte compatibility. In fact, the coating ratio of the Li
7P
3S
11 solid electrolyte also affected the electrochemical performance of the MoS
6@Li
7P
3S
11 composite (
Figure S4). The Li/Li
6PS
5Cl/MoS
6@10%Li
7P
3S
11 and Li/Li
6PS
5Cl/MoS
6@20%Li
7P
3S
11 delivered initial discharges of 967.8 mAh g
−1 and 991.4 mAh g
−1, respectively. After 20 cycles, the capacity retention values of the 10%Li
7P
3S
11- and 20%Li
7P
3S
11-coated electrodes were 476.1 mAh g
−1 and 496.3 mAh g
−1, respectively. Obviously, the Li/Li
6PS
5Cl/MoS
6@15%Li
7P
3S
11 displayed the highest reversible capacity. To explore the principles of cathode kinetics and capacity variation for the Li/Li
6PS
5Cl/MoS
6 and Li/Li
6PS
5Cl/MoS
6@15%Li
7P
3S
11 all-solid-state lithium batteries, EIS was conducted with an amplitude of 15 mV from 10
6 to 0.1 Hz. The corresponding equivalent circuit model is shown in
Figure 4f, in which
Re is the resistance of the electrolyte, the semicircle shows the emergence of the interfacial charge transfer resistance (
Rct), the constant phase angle element (
CPE) is used to indicate the behavior of the non-ideal capacitance of the double-layer, and
Zw represents the Warburg resistance, which indicates that the lithium ions diffuse into the bulk electrodes [
21,
28]. The fitted
Re and
Rct results are listed in
Table S3. After the first cycle, the EIS plots of the MoS
6 and MoS
6@15%Li
7P
3S
11 composite-based all-solid-state lithium batteries were straight lines. The MoS
6@15%Li
7P
3S
11 composite exhibited an
Re value of 75.89 Ω, which was lower than that of the MoS
6 (105.42 Ω). After 20 cycles, the MoS
6 exhibited higher
Re (384.11 Ω) and
Rct (52.96 Ω) values. In contrast, the MoS
6@15%Li
7P
3S
11 composite exhibited respective
Re and
Rct values of 124.79 Ω and 10.78 Ω due to the intimate interfacial contact.
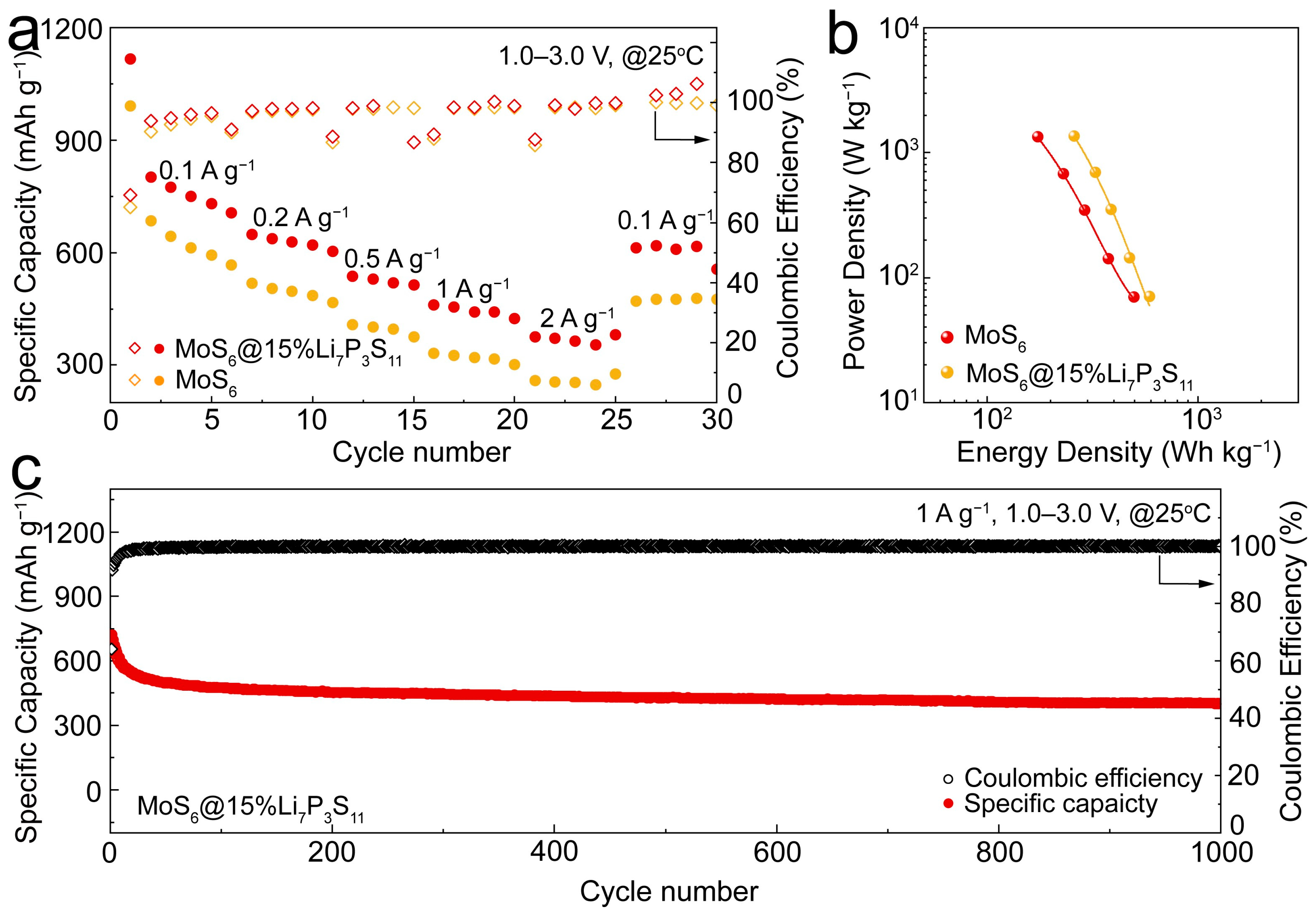
The rate capabilities of the MoS
6 and MoS
6@15%Li
7P
3S
11 composite cathodes were measured under various current densities ranging from 0.1 to 2 A g
−1 (
Figure 5a). The MoS
6@15%Li
7P
3S
11 composite cathodes exhibited superior reversible discharge capacities of 801.5, 648.1, 536.3, 454.4, and 370.8 mAh g
−1 under current densities of 0.1, 0.2, 0.5, 1, and 2 A g
−1, respectively, while the MoS
6 only exhibited reversible discharge capacities 683.9, 516.8, 407.7, 326.2, and 255.8 mAh g
−1. The excellent rate capability of the MoS
6@15%Li
7P
3S
11 composite can be attributed to its enhanced ionic diffusivity, which led to improved electrochemical reaction kinetics. The Ragone plot shown in
Figure 5b gives the relationship between the average power density and energy density. The power density of the MoS
6 and MoS
6@15%Li
7P
3S
11 composite were calculated using the equation
, where
ED is the energy density of the active materials,
m is the loading weight of the active materials or the cathode layer, and
t is the duration of the discharge process. The MoS
6 and MoS
6@15%Li
7P
3S
11 composite numbers that were used in the energy density and power density calculations are listed in
Tables S4 and S5. At current densities of 0.1 and 2.0 A g
−1, the MoS
6@15%Li
7P
3S
11 composite cathodes delivered energy and power densities of 588 Wh kg
−1 and 1358 W kg
−1, respectively, based on the total cathode layer which is composed of the MoS
6@15%Li
7P
3S
11 composite, Li
10GeP
2S
12, and super P. These values were significantly higher than the energy and power densities of 495.8 Wh kg
−1 and 1332.2 W kg
−1 exhibited by the MoS
6 at the same respective current densities. The long-term cycling stability of the MoS
6@15%Li
7P
3S
11 composite cathodes at 1 A g
−1 is further shown in
Figure 5c, which shows that the cathodes exhibited a high reversible capacity of 400 mAh g
−1 after 1000 cycles.
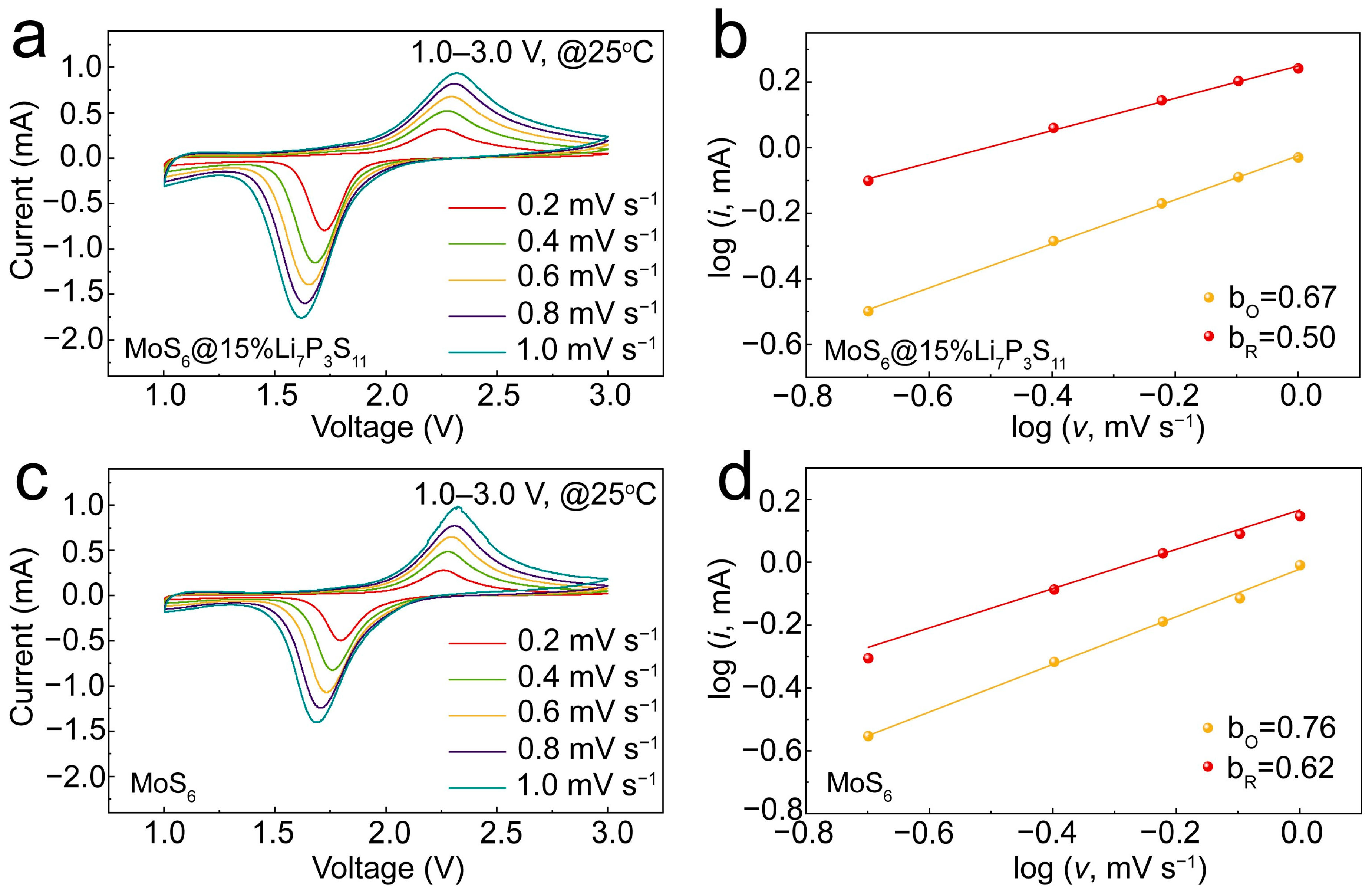
CV measurements were conducted to illustrate the electrochemical reaction kinetics. The relationship between the peak current (
i) and the scan rate obeys the power law:
. The
b-value is fitted using a log(
v)-log(
i) plot. A
b-value of 1.0 indicates a surface-mediated mode, while a
b-value of 0.5 indicates a diffusion-controlled mode. The CV curves shown in
Figure 6a,c show similar shapes and gradually broadened redox peaks. At the same scan rate, the curve intensity of the MoS
6@15%Li
7P
3S
11 composite was higher than that of the MoS
6, indicating that the MoS
6@15%Li
7P
3S
11 composite can maintain fast electrochemical kinetics with an increased scan rate. As shown in
Figure 6b, the fitted
b-values of the reduction peak and the oxidation peak were 0.50 and 0.67, respectively, for the MoS
6@15%Li
7P
3S
11 composite; these values are lower than the fitted
b-values of 0.62 and 0.76 recorded for the MoS
6 (
Figure 6d), indicating that the electrochemical reaction kinetics are dominated by diffusion-controlled processes. This condition allows lithium ions to become fully intercalated and thereby realize high reversible capacity [
17,
29].
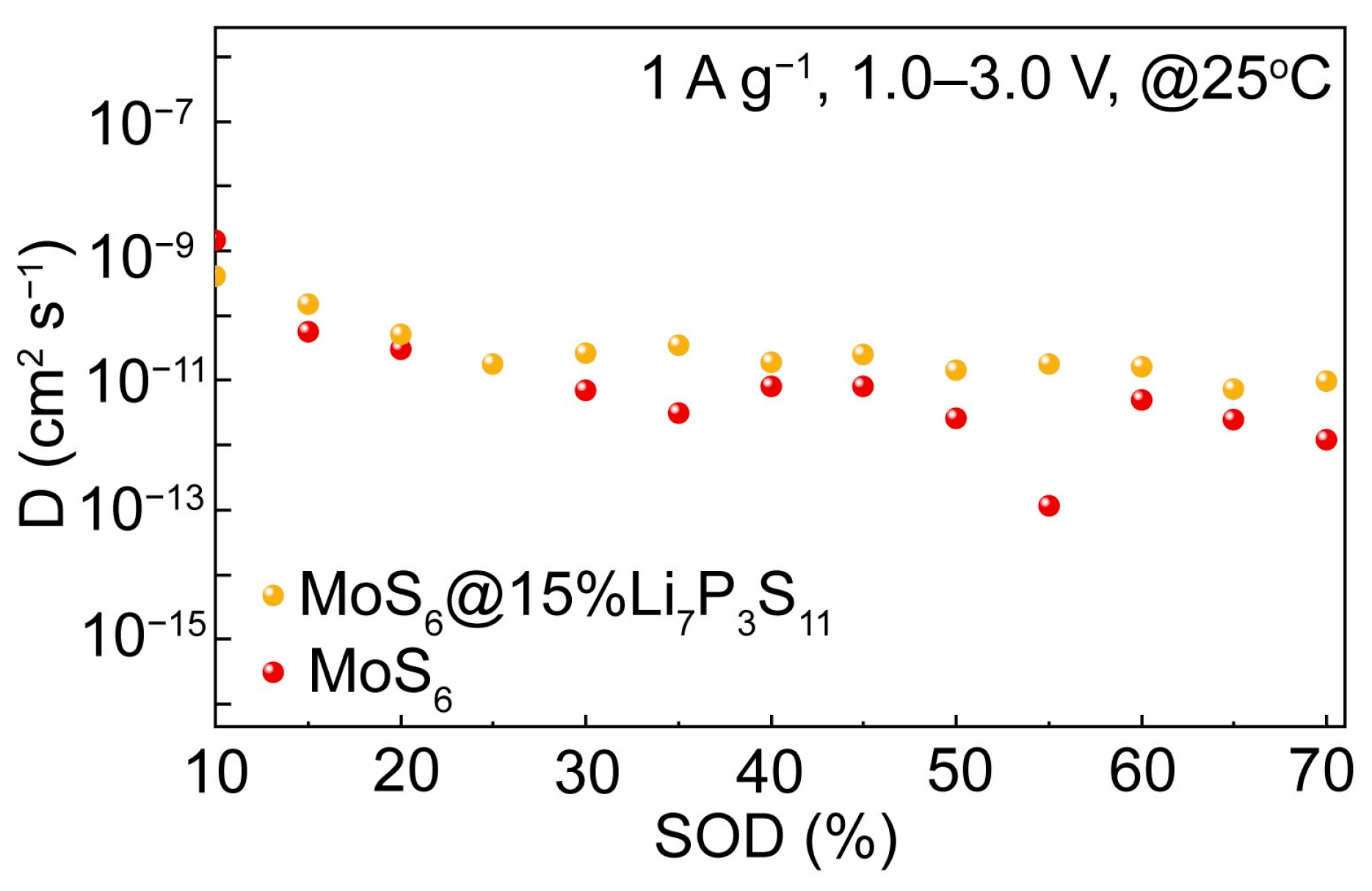
To further quantify the ionic transport kinetics of the Li/Li
6PS
5Cl/MoS
6@15%Li
7P
3S
11 and Li/Li
6PS
5Cl/MoS
6 all-solid-state batteries, the GITT was used and calculated were made to determine the lithium ion diffusion coefficients at 1 A g
−1 and 1.0–3.0 V. As is shown in
Figure 7, the ion diffusion coefficient range of the MoS
6@15%Li
7P
3S
11 composite was calculated to be 10
−11–10
−10 cm
2 s
−1, which is higher than that of the MoS
6 (10
−12–10
−11 cm
2 s
−1). Obviously, the ionic diffusivity of the MoS
6@15%Li
7P
3S
11 composite was enhanced significantly; this is beneficial for the rapid transportation of lithium ions and thus significantly improved the electrochemical performances related to high discharge capacity and rate capability.