Experimental Investigation of Thermal Runaway Propagation in a Lithium-Ion Battery Pack: Effects of State of Charge and Coolant Flow Rate
Abstract
:1. Introduction
2. Experimental Setup
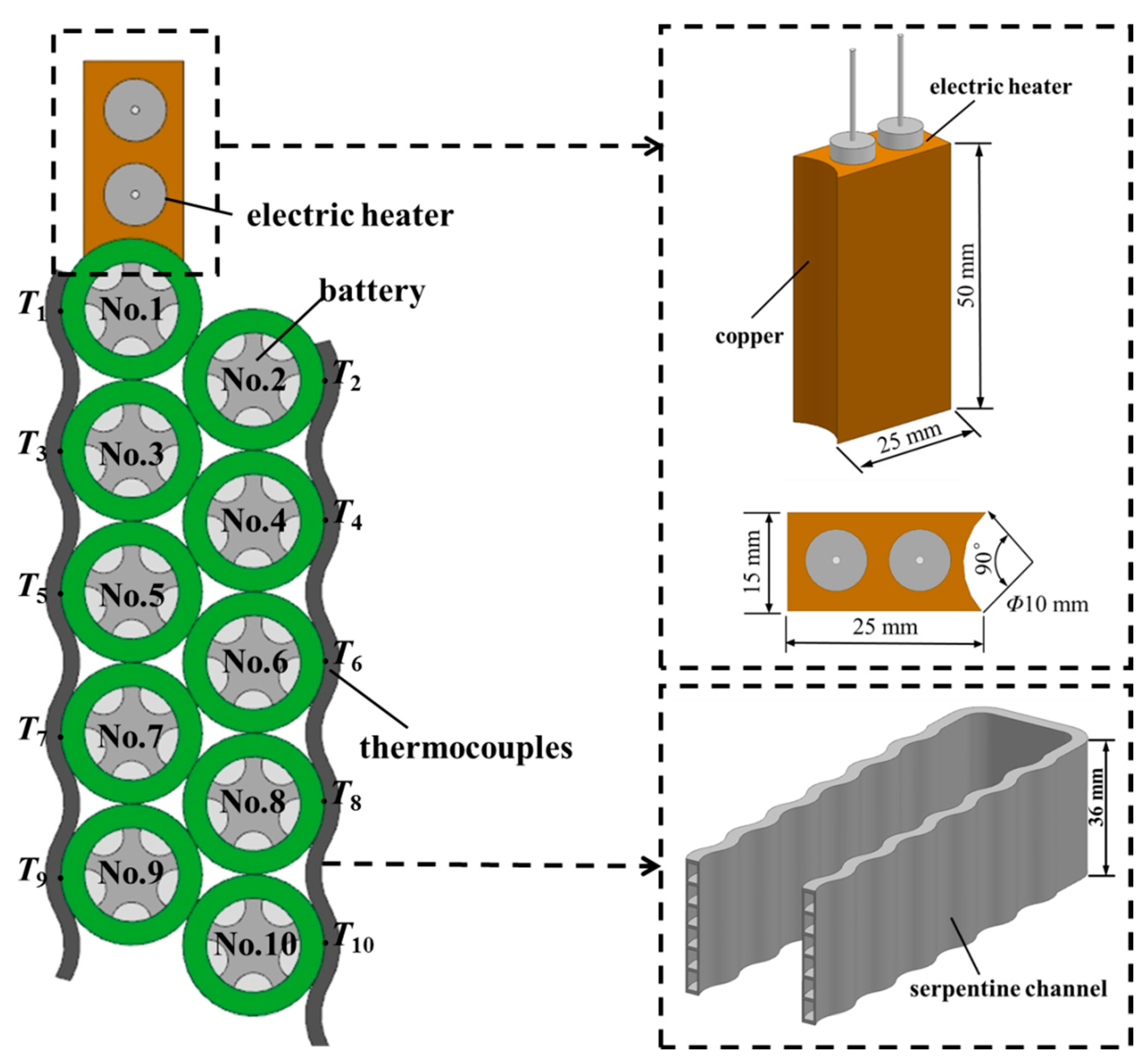
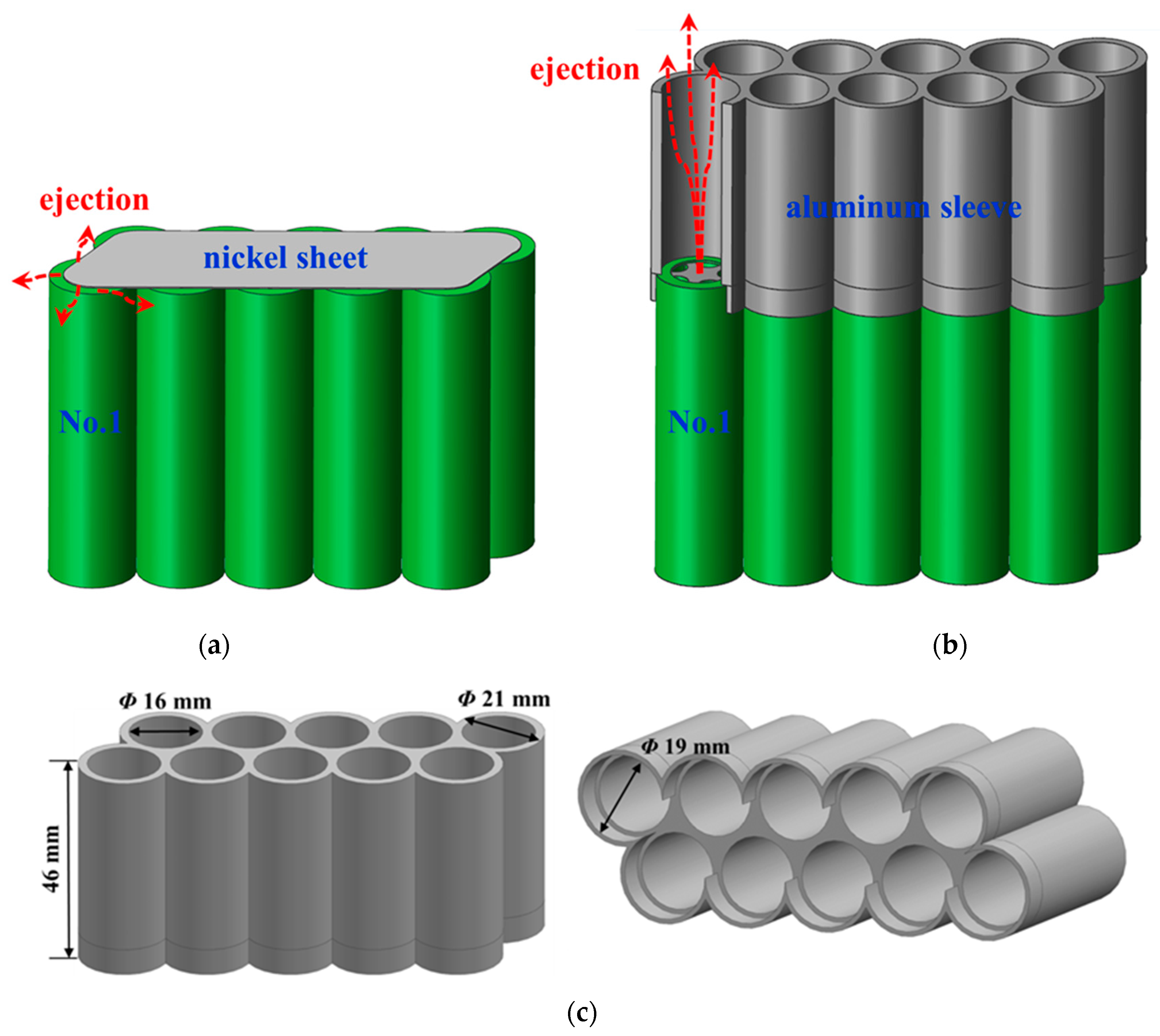
2.1. Battery Module
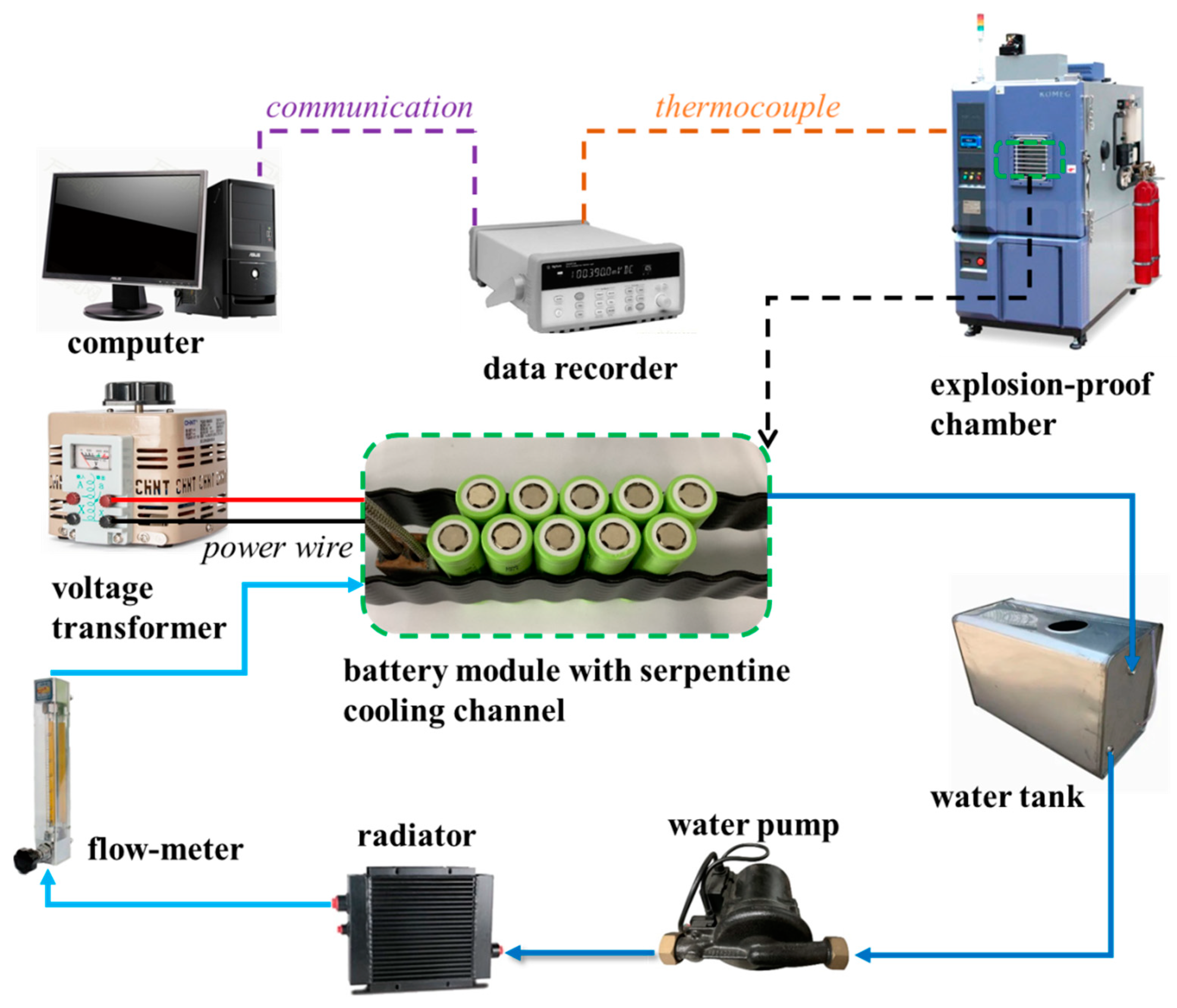
2.2. Experimental System
2.3. Experimental Procedure
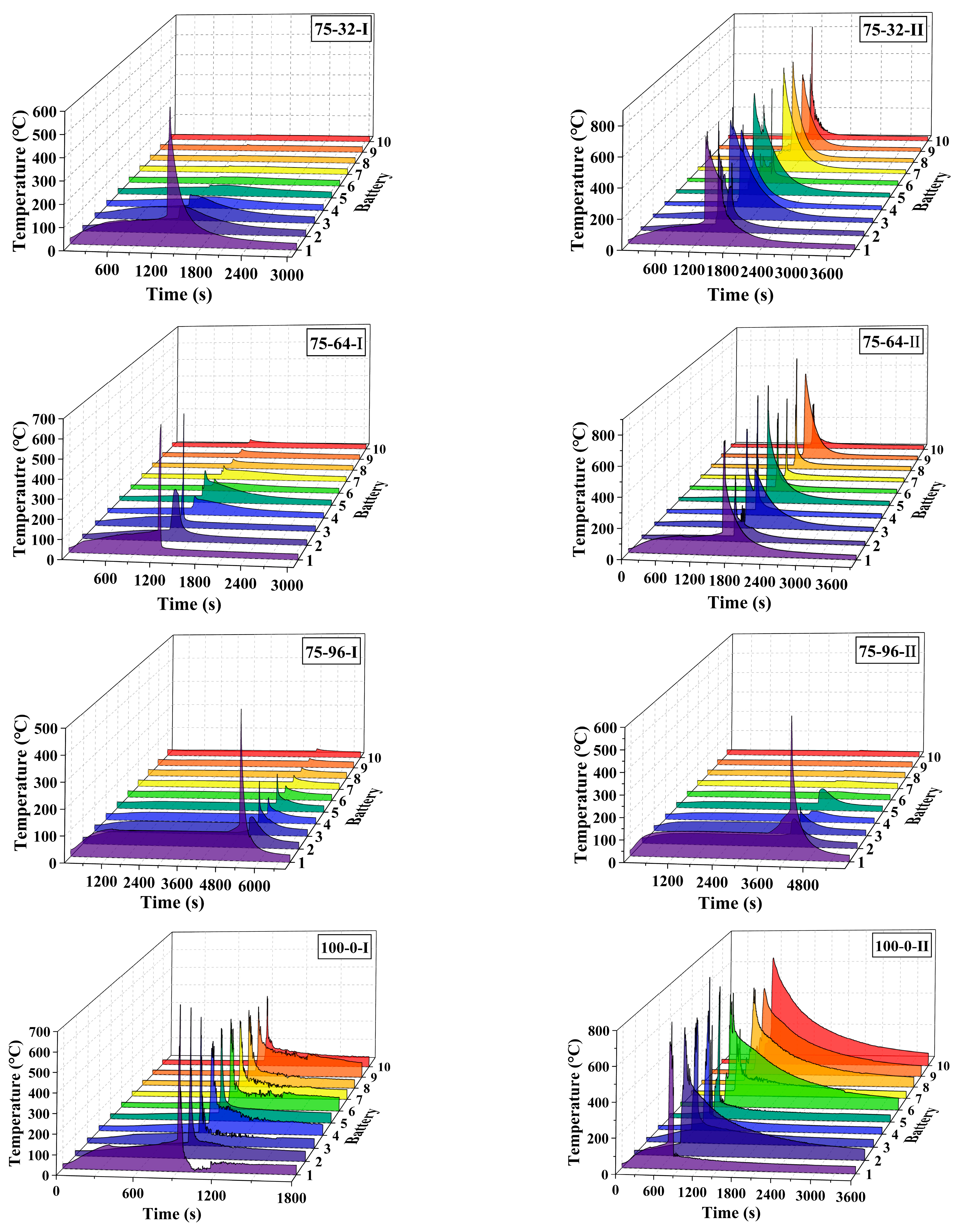
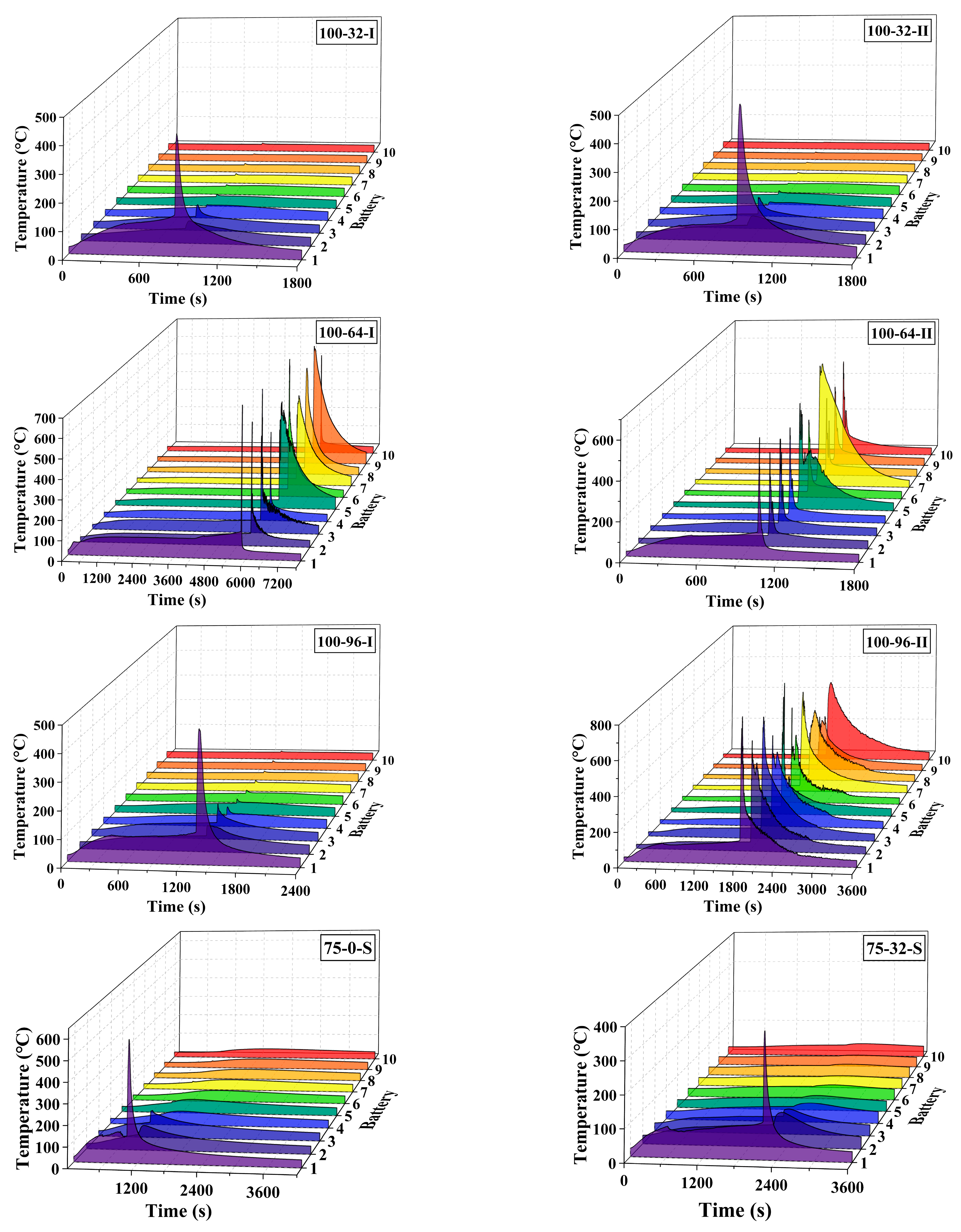
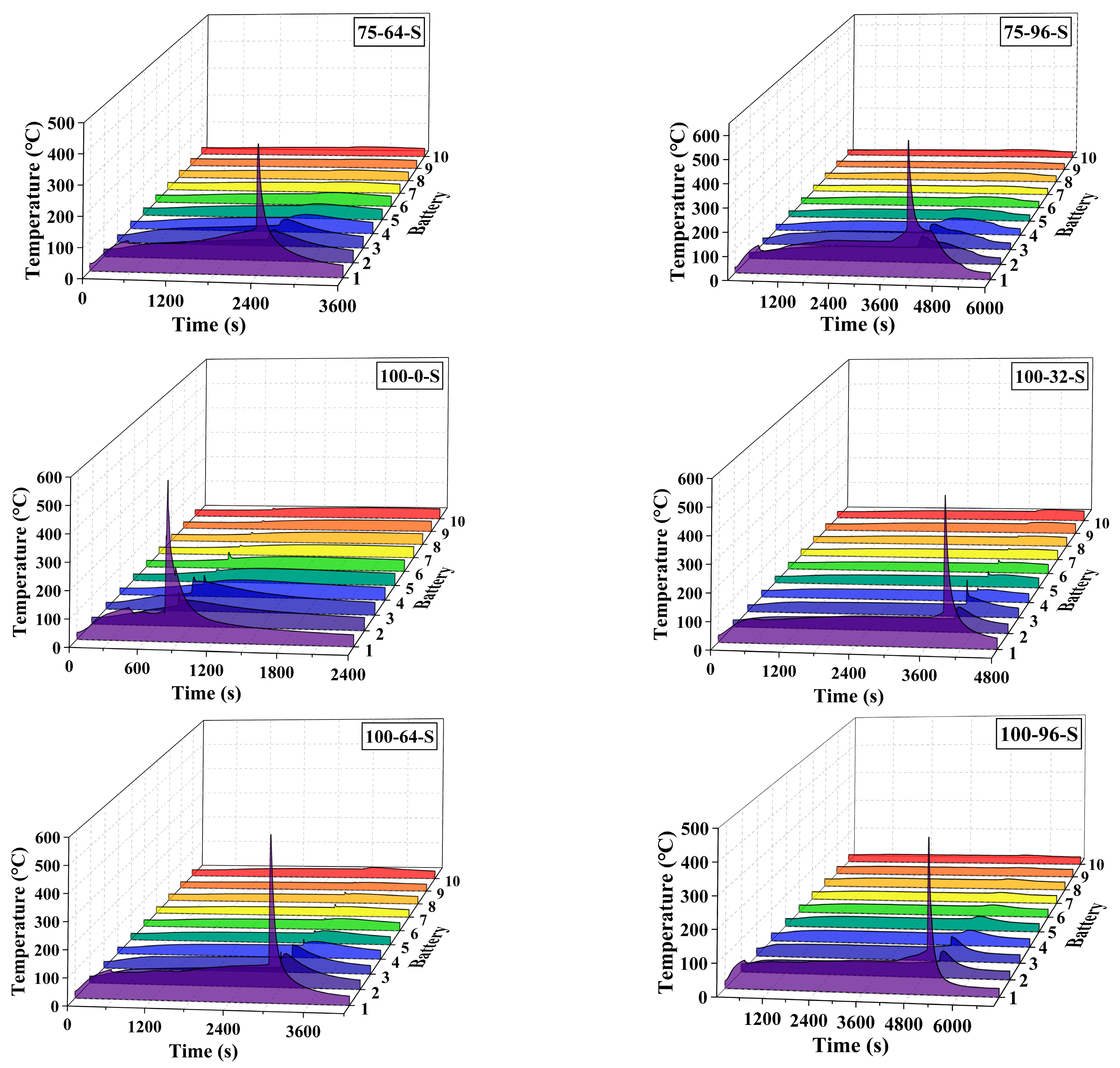
3. Results and Discussion
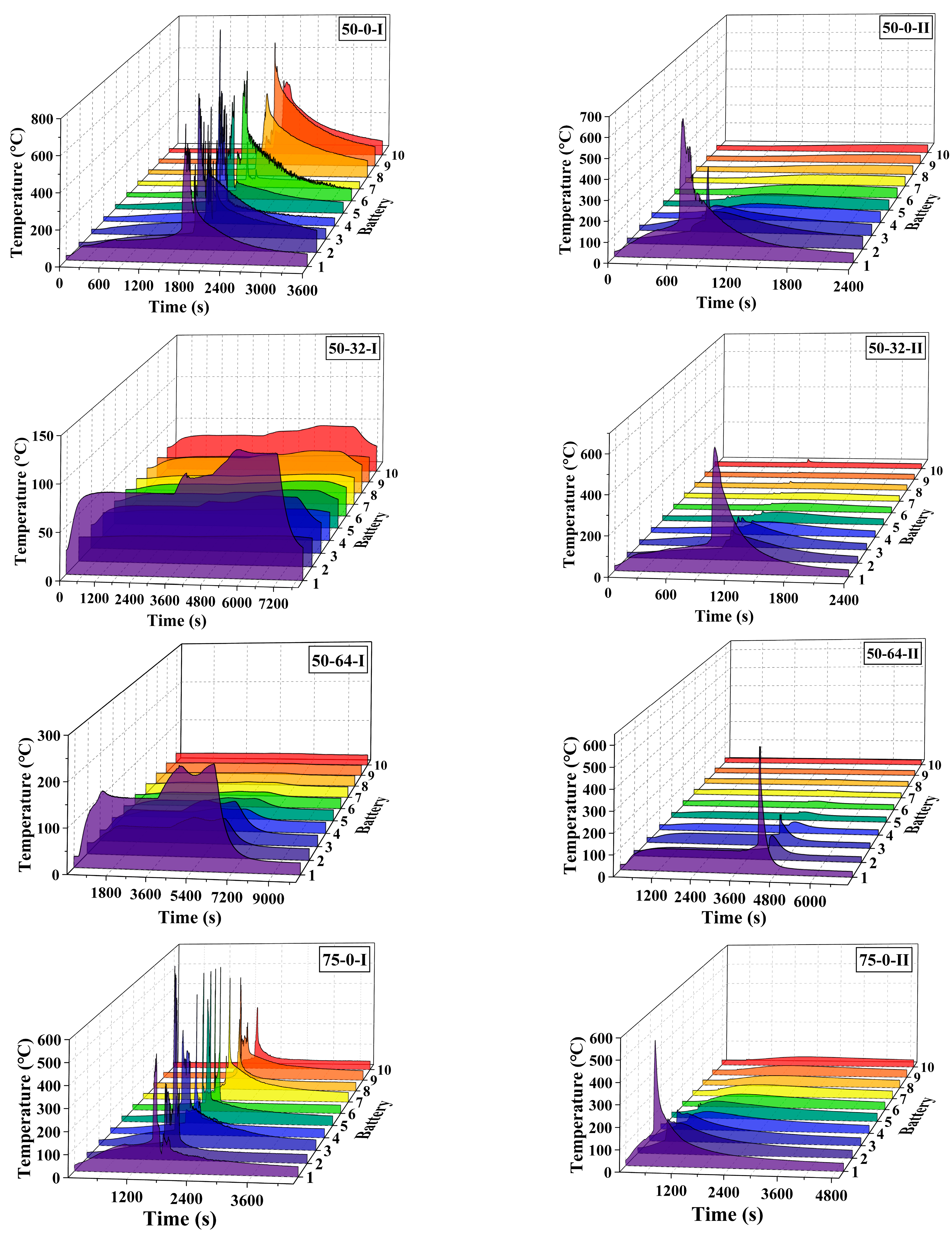
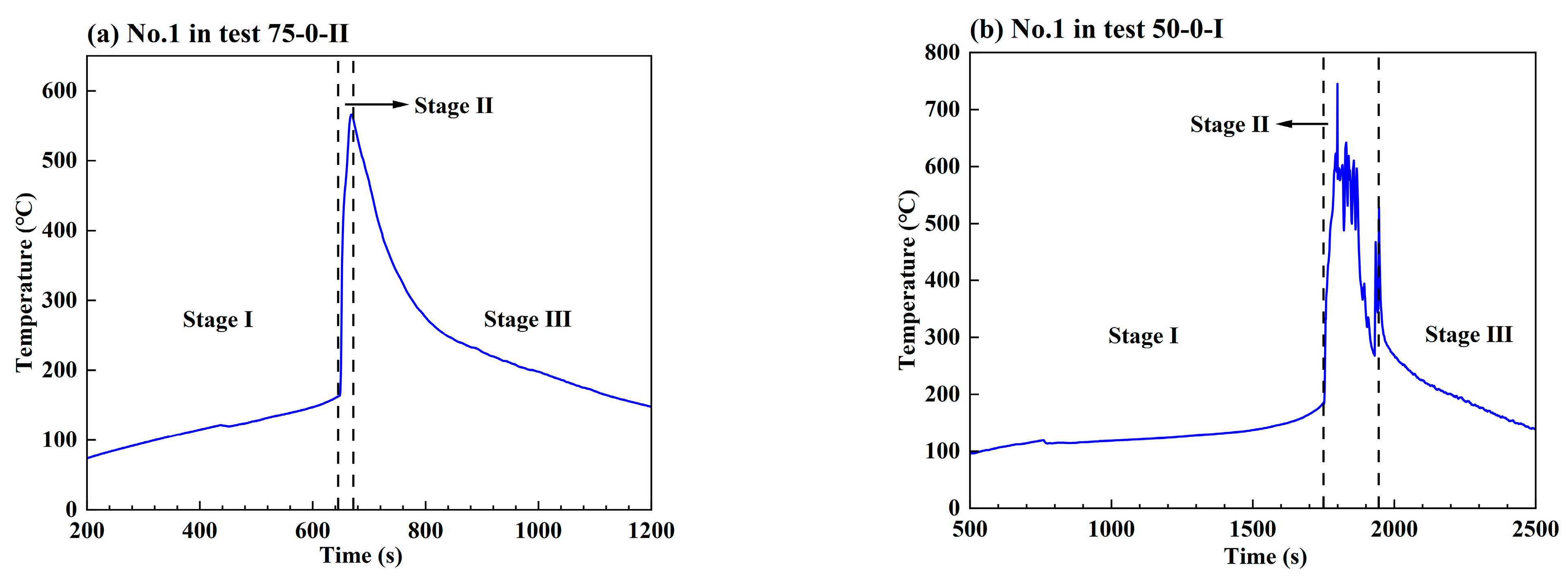
3.1. General Results
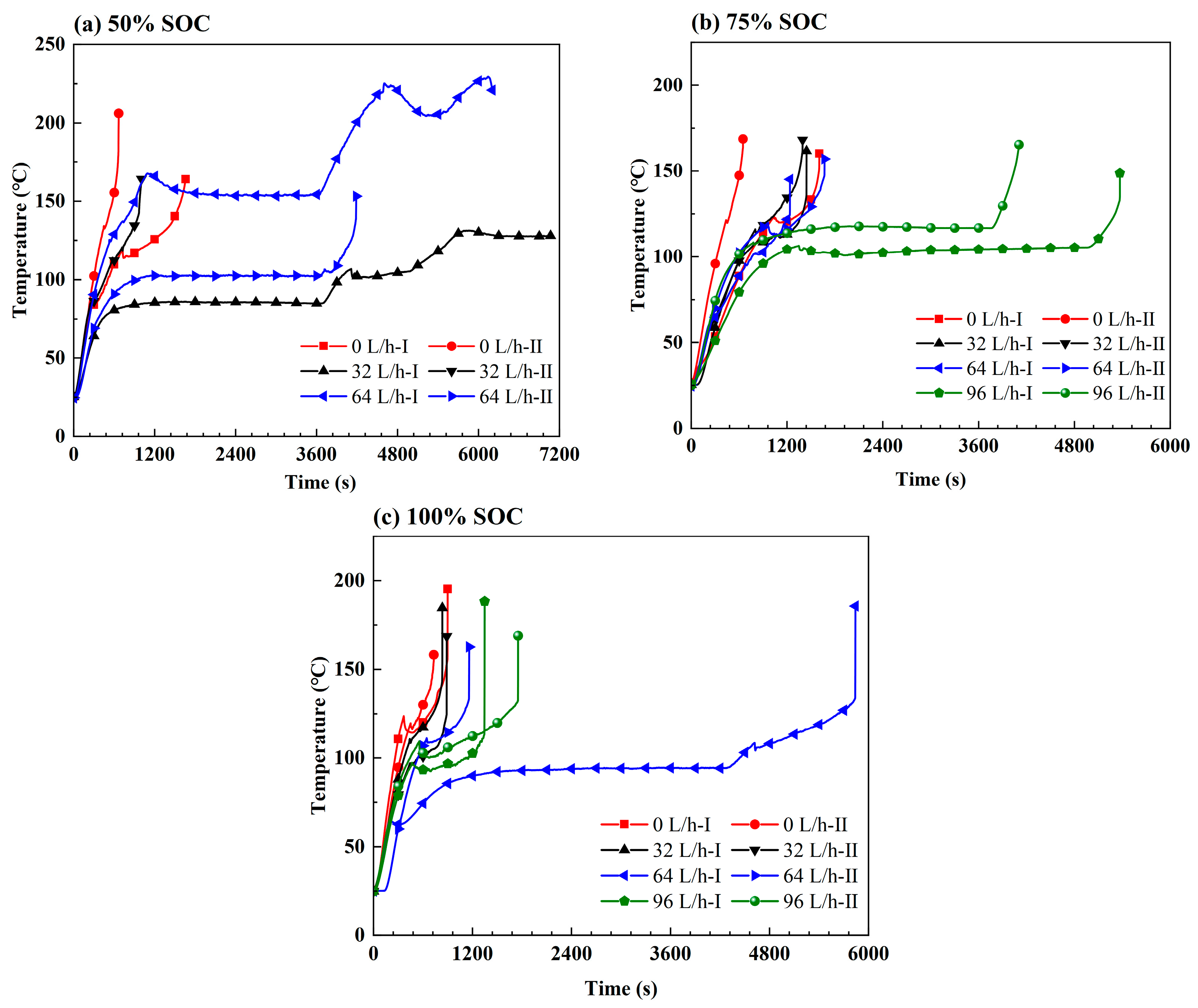
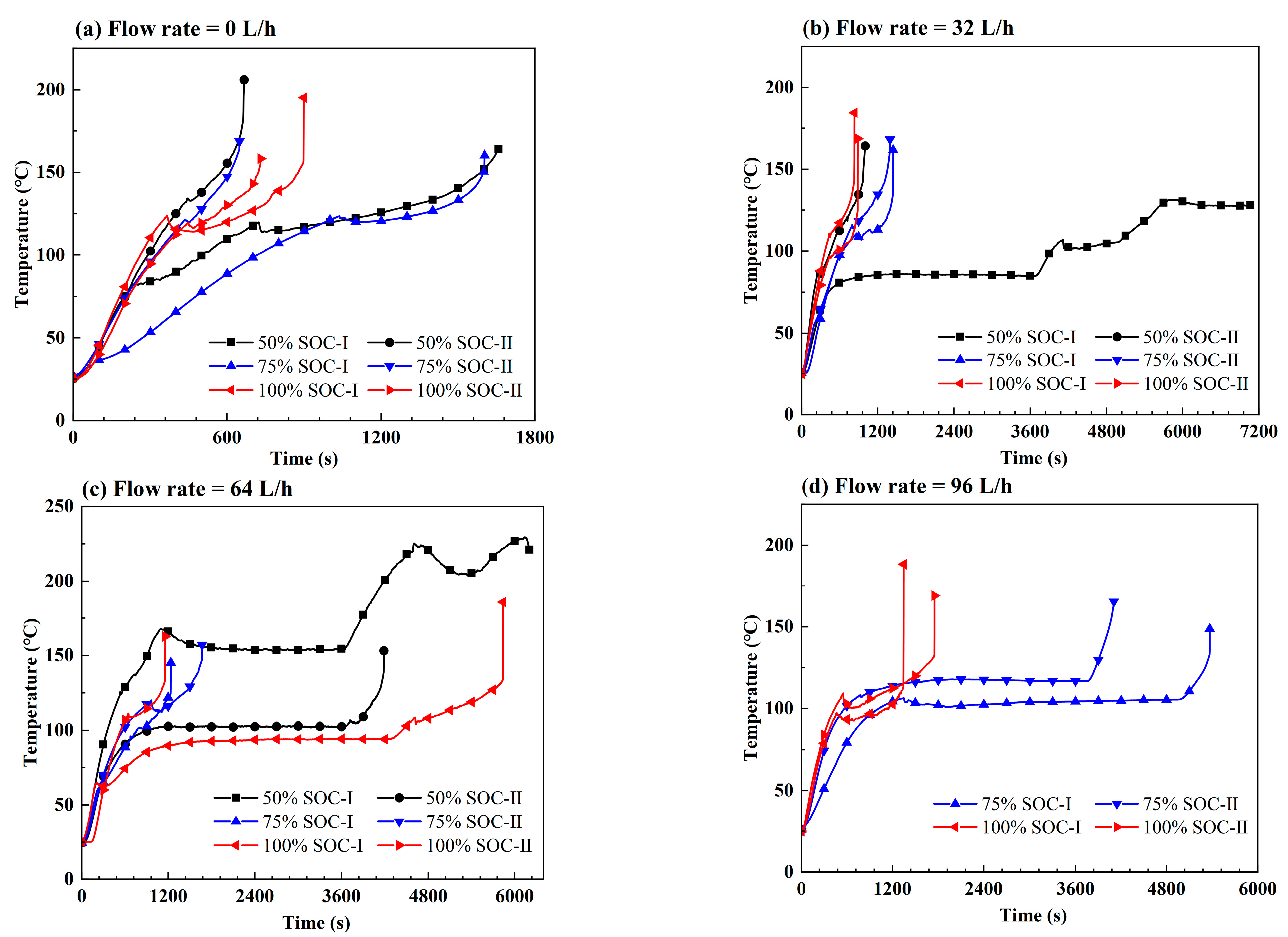
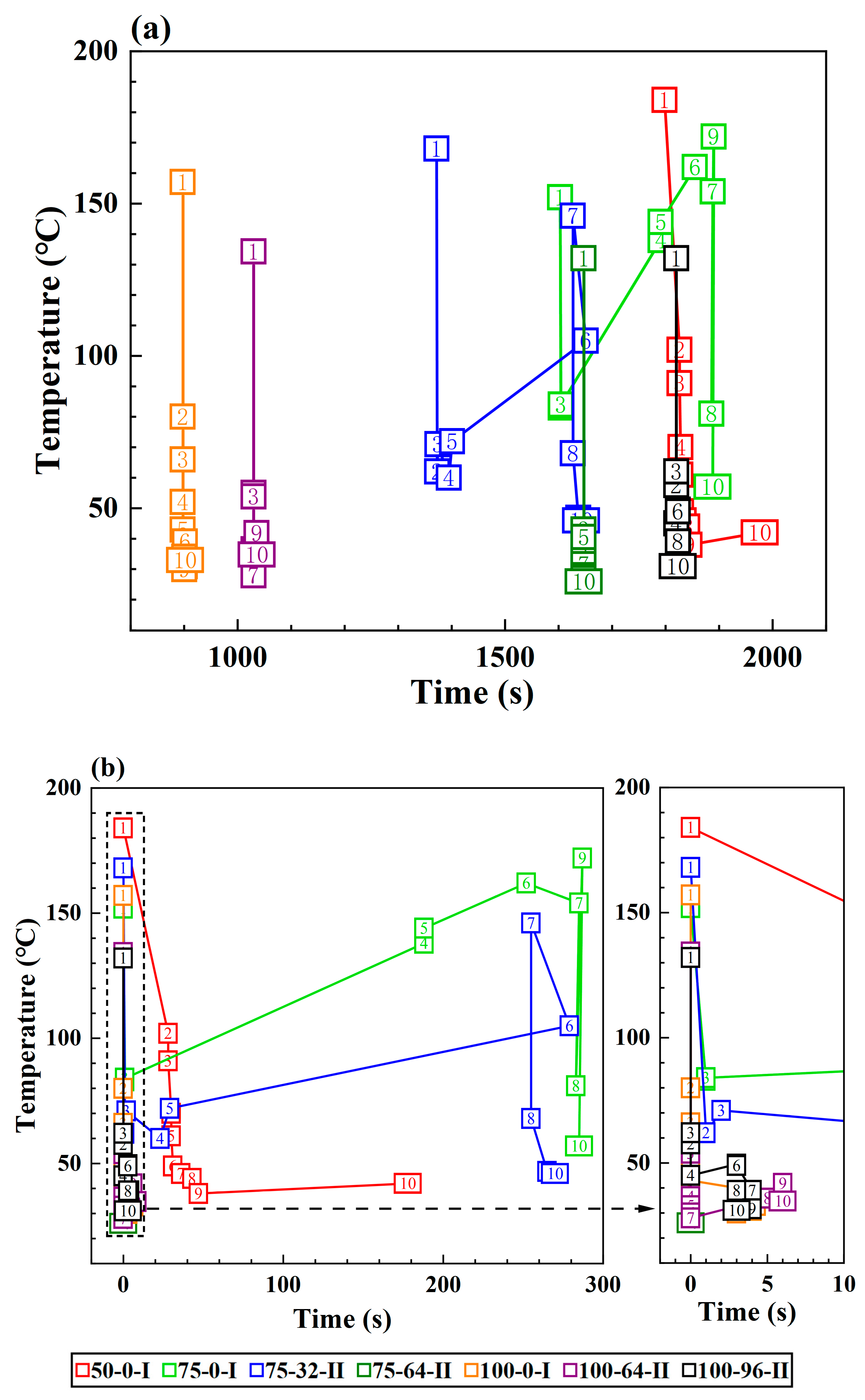
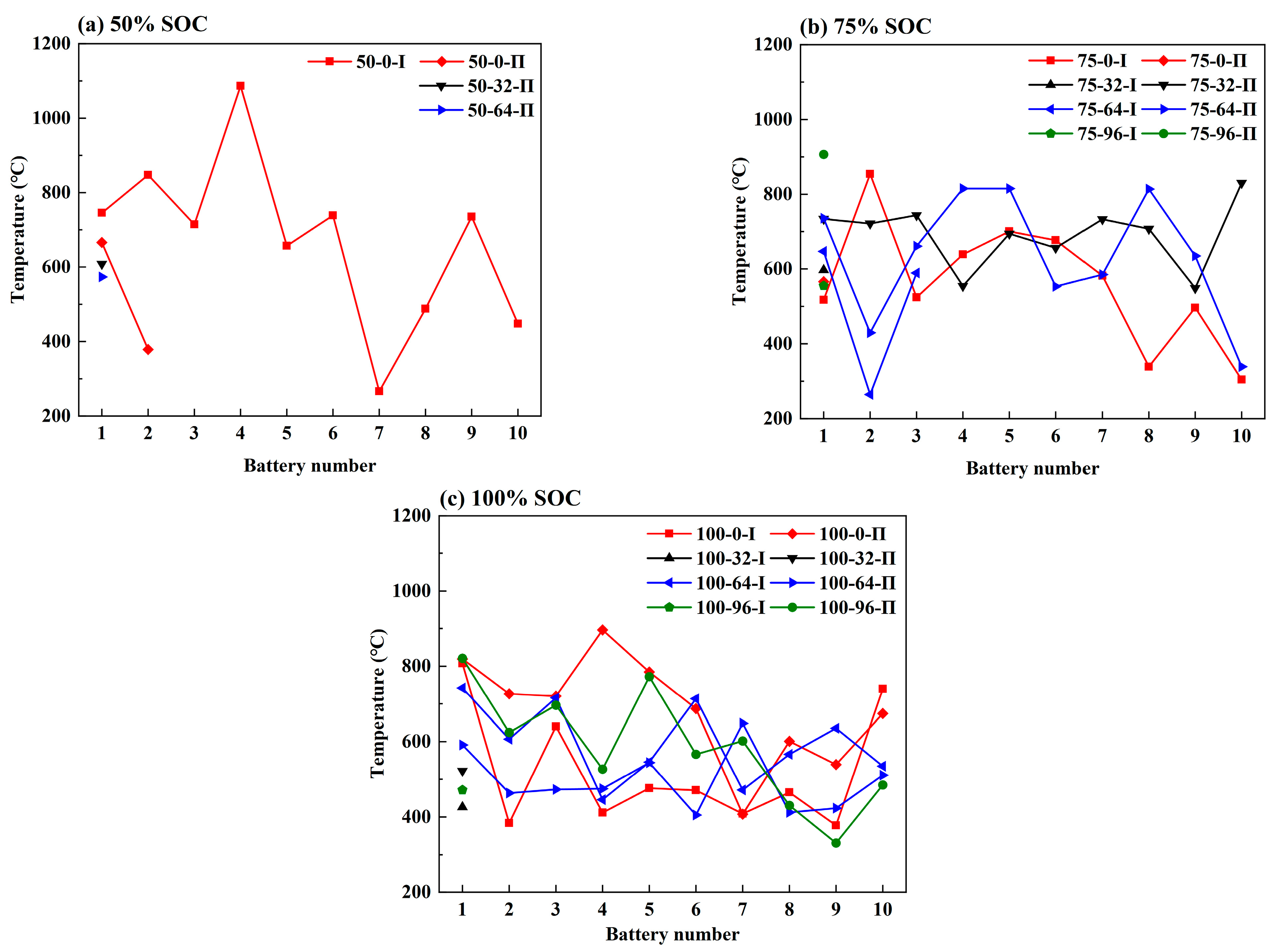
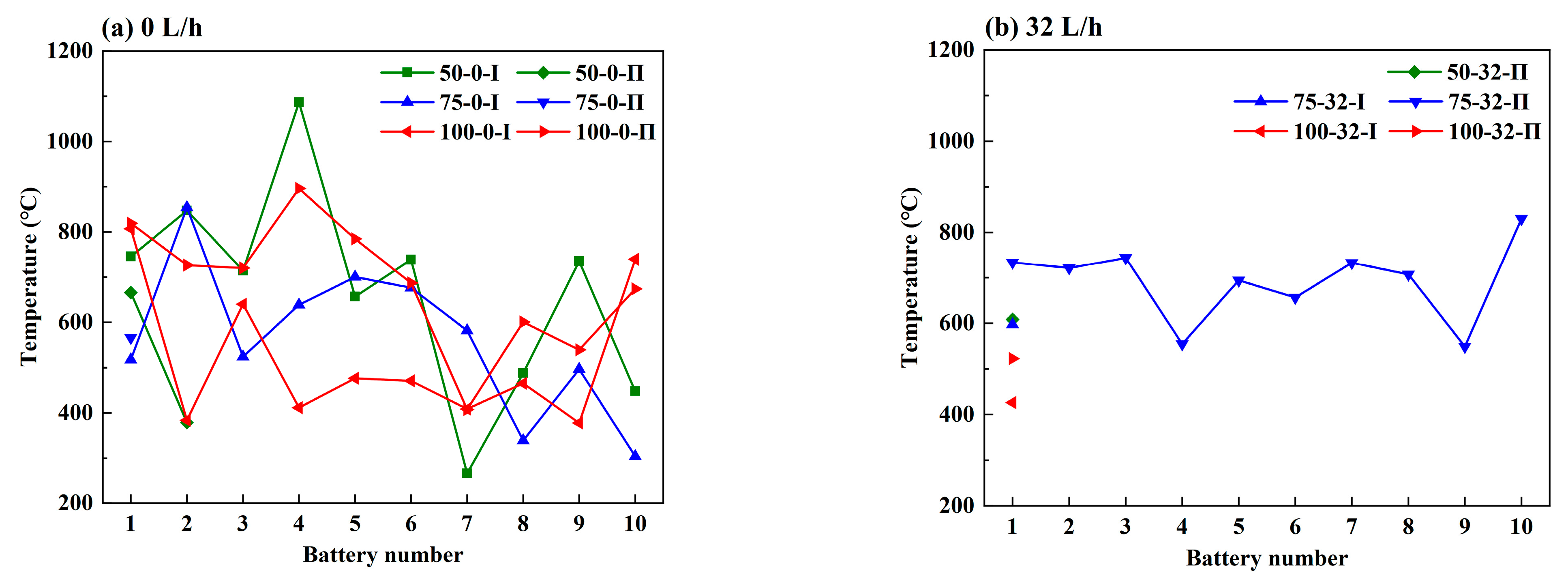
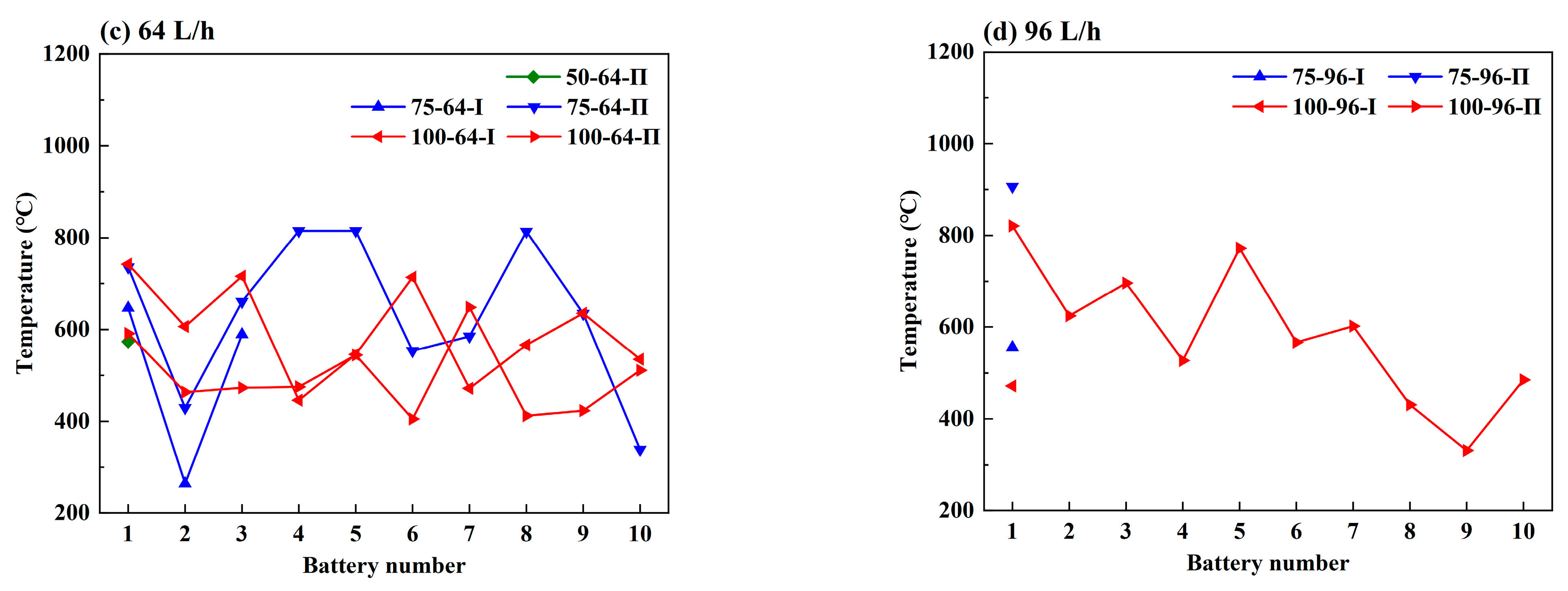
3.2. Effect of the Coolant Flow Rate and SOC
4. Conclusions
- (1)
- The retarding effect of BTMS on TR propagation in LIBs depends on the coolant flow rate as well as the battery SOC value; higher SOC increases the difficulty of TR propagation prevention by BTMS. Lower SOC values and higher coolant flow rates generally result in delayed TR occurrence in No.1 batteries and fewer TR batteries in the pack during the tests.
- (2)
- The TR propagation rate, indicated by the time intervals in TR occurrence between adjacent batteries is associated with the battery SOC value; higher SOC values lead to more rapid TR propagation in the pack. The batteries with 100% SOC show extremely rapid TR propagation, which is completed in only a few seconds compared to several minutes for batteries with 50% SOC and 75% SOC.
- (3)
- The maximum temperature of the TR battery partially depends on the battery position in the pack, rather than the battery SOC value and coolant flow rate. The upstream batteries generally show a slightly higher maximum temperature during the TR process compared to the downstream ones. Therefore, a weak correlation between the maximum temperature during the TR process and the external heating duration as well as the pre-TR stage temperature of the battery can be speculated.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hannan, M.A.; Lipu, M.S.H.; Hussain, A.; Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations. Renew. Sustain. Energy Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Chen, Z. Low temperature preheating techniques for Lithium-ion batteries: Recent advances and future challenges. Appl. Energy 2022, 313, 118832. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Q.; Zhen, C.; Niu, Y.; Han, Y.; Zeng, G.; Chen, D.; Feng, C.; Chen, N.; Lv, W.; et al. Recent progress in flame-retardant separators for safe lithium-ion batteries. Energy Storage Mater. 2021, 37, 628–647. [Google Scholar] [CrossRef]
- Li, Z.; Kong, Q.; Tian, H.; Cai, Y.; Su, Z. Interaction of superionic conductor and heterostructure LiVOPO4-Li2.72Ti2(PO4)3 cathode materials for lithium-ion batteries. Mater. Lett. 2023, 335, 133824. [Google Scholar] [CrossRef]
- Chen, L.; Fan, X.; Hu, E.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Li, J.; Su, D.; Yang, X.; et al. Achieving High Energy Density through Increasing the Output Voltage: A Highly Reversible 5.3 V Battery. Chem-Us 2019, 5, 896–912. [Google Scholar] [CrossRef]
- Yan, P.; Zhu, Y.; Pan, X.; Ji, H. A novel flame-retardant electrolyte additive for safer lithium-ion batteries. Int. J. Energy Res. 2021, 45, 2776–2784. [Google Scholar] [CrossRef]
- Xu, J.; Duan, Q.; Zhang, L.; Liu, Y.; Sun, J.; Wang, Q. The enhanced cooling effect of water mist with additives on inhibiting lithium ion battery thermal runaway. J. Loss Prevent. Proc. 2022, 77, 104784. [Google Scholar] [CrossRef]
- Hu, J.; Liu, T.; Wang, X.; Wang, Z.; Wu, L. Investigation on thermal runaway of 18,650 lithium ion battery under thermal abuse coupled with charging. J. Energy Storage 2022, 51, 104482. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Lu, L.; Wu, P.; Kulp, C.; Prasser, S. Thermal runaway propagation model for designing a safer battery pack with 25 Ah LiNixCoyMnzO2 large format lithium ion battery. Appl. Energy 2015, 154, 74–91. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A Review on Lithium-Ion Batteries Safety Issues: Existing Problems and Possible Solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- We Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Kong, D.; Wang, G.; Ping, P.; Wen, J. Numerical investigation of thermal runaway behavior of lithium-ion batteries with different battery materials and heating conditions. Appl. Therm. Eng. 2021, 189, 116661. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, F. A review on passive and active strategies of enhancing the safety of lithium-ion batteries. Int. J. Heat Mass Transf. 2022, 184, 122288. [Google Scholar] [CrossRef]
- Jiang, Z.; Qu, Z. Lithium-ion battery thermal management using heat pipe and phase change material during discharge-charge cycle: A comprehensive numerical study. Appl. Energy 2019, 242, 378–392. [Google Scholar] [CrossRef]
- Rao, Z.; Qian, Z.; Kuang, Y.; Li, Y. Thermal performance of liquid cooling based thermal management system for cylindrical lithium-ion battery module with variable contact surface. Appl. Therm. Eng. 2017, 123, 1514–1522. [Google Scholar] [CrossRef]
- Greco, A.; Jiang, X.; Cao, D. An investigation of lithium-ion battery thermal management using paraffin/porous-graphite-matrix composite. J. Power Sources 2015, 278, 50–68. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A critical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Z.; He, W.; Zhao, J. Thermal issues about Li-ion batteries and recent progress in battery thermal management systems: A review. Energy Convers. Manag. 2017, 150, 304–330. [Google Scholar] [CrossRef]
- Ke, Q.; Li, X.; Guo, J.; Cao, W.; Wang, Y.; Jiang, F. The retarding effect of liquid-cooling thermal management on thermal runaway propagation in lithium-ion batteries. J. Energy Storage 2022, 48, 104063. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Liu, J.; Huang, X. Layer-to-layer thermal runaway propagation of open-circuit cylindrical li-ion batteries: Effect of ambient pressure. J. Energy Storage 2022, 55, 105709. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Bian, H.; Jiang, F.; Yang, Y. Characteristics of and factors influencing thermal runaway propagation in lithium-ion battery packs. J. Energy Storage 2021, 41, 102956. [Google Scholar] [CrossRef]
- Fang, J.; Cai, J.; He, X. Experimental study on the vertical thermal runaway propagation in cylindrical Lithium-ion batteries: Effects of spacing and state of charge. Appl. Therm. Eng. 2021, 197, 117399. [Google Scholar] [CrossRef]
- Li, H.; Duan, Q.; Zhao, C.; Huang, Z.; Wang, Q. Experimental investigation on the thermal runaway and its propagation in the large format battery module with Li(Ni1/3Co1/3Mn1/3)O-2 as cathode. J. Hazard Mater. 2019, 375, 241–254. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, S.; Chen, Y.; Zhao, L.; Chen, M. Experimental and analytical investigation on the thermal runaway propagation characteristics of lithium-ion battery module with NCM pouch cells under various state of charge and spacing. J. Energy Storage 2023, 72, 108380. [Google Scholar] [CrossRef]
- Cao, W.; Zhao, C.; Wang, Y.; Dong, T.; Jiang, F. Thermal modeling of full-size-scale cylindrical battery pack cooled by channeled liquid flow. Int. J. Heat Mass Transf. 2019, 138, 1178–1187. [Google Scholar] [CrossRef]
- US DOE. FreedomCAR Battery Test Manual for Power-Assist Hybrid Electric Vehicles: DOE/ID-11069; US Department of Energy: Washington, DC, USA, 2013.


| Parameters | Values |
|---|---|
| Type | 18650 |
| Electrode material | Anode: graphite; Cathode: NCA |
| Voltage range | 3.0–4.2 V |
| Rated capacity | 2.75 Ah |
| Nominal voltage | 3.6 V |
| Energy density | 170 Wh/L |
| Mass | 49 g |
| Specific heat | 1098.2 J/(kg·K) |
| Thermal conductivity | 2.586 W/(m·K) |
| SOC | Flow Rate (L/h) | Without Sleeve | With Sleeve | |
|---|---|---|---|---|
| 50% | 0 | (50-0-I) *: 10 ** | (50-0-II): 2 | |
| 32 | (50-32-I): 0 | (50-32-II): 1 | ||
| 64 | (50-64-I): 0 | (50-64-II): 1 | ||
| 75% | 0 | (75-0-I): 10 | (75-0-II): 1 | (75-0-S): 1 |
| 32 | (75-32-I): 1 | (75-32-II): 10 | (75-32-S): 1 | |
| 64 | (75-64-I): 3 | (75-64-II): 10 | (75-64-S): 1 | |
| 96 | (75-96-I): 1 | (75-96-II): 1 | (75-96-S): 1 | |
| 100% | 0 | (100-0-I): 10 | (100-0-II): 10 | (100-0-S): 1 |
| 32 | (100-32-I): 1 | (100-32-II): 1 | (100-32-S): 1 | |
| 64 | (100-64-I): 10 | (100-64-II): 10 | (100-64-S): 1 | |
| 96 | (100-96-I): 1 | (100-96-II): 10 | (100-96-S): 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Ke, Q.; Guo, J.; Wang, Y.; Qiu, Y.; Cen, J.; Jiang, F. Experimental Investigation of Thermal Runaway Propagation in a Lithium-Ion Battery Pack: Effects of State of Charge and Coolant Flow Rate. Batteries 2023, 9, 552. https://doi.org/10.3390/batteries9110552
Wu W, Ke Q, Guo J, Wang Y, Qiu Y, Cen J, Jiang F. Experimental Investigation of Thermal Runaway Propagation in a Lithium-Ion Battery Pack: Effects of State of Charge and Coolant Flow Rate. Batteries. 2023; 9(11):552. https://doi.org/10.3390/batteries9110552
Chicago/Turabian StyleWu, Wanyi, Qiaomin Ke, Jian Guo, Yiwei Wang, Yishu Qiu, Jiwen Cen, and Fangming Jiang. 2023. "Experimental Investigation of Thermal Runaway Propagation in a Lithium-Ion Battery Pack: Effects of State of Charge and Coolant Flow Rate" Batteries 9, no. 11: 552. https://doi.org/10.3390/batteries9110552
APA StyleWu, W., Ke, Q., Guo, J., Wang, Y., Qiu, Y., Cen, J., & Jiang, F. (2023). Experimental Investigation of Thermal Runaway Propagation in a Lithium-Ion Battery Pack: Effects of State of Charge and Coolant Flow Rate. Batteries, 9(11), 552. https://doi.org/10.3390/batteries9110552







