Sequential Recovery of Critical Metals from Leached Liquor of Processed Spent Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Leaching Procedure
2.3. Liquid–Liquid Extraction Procedure
3. Results
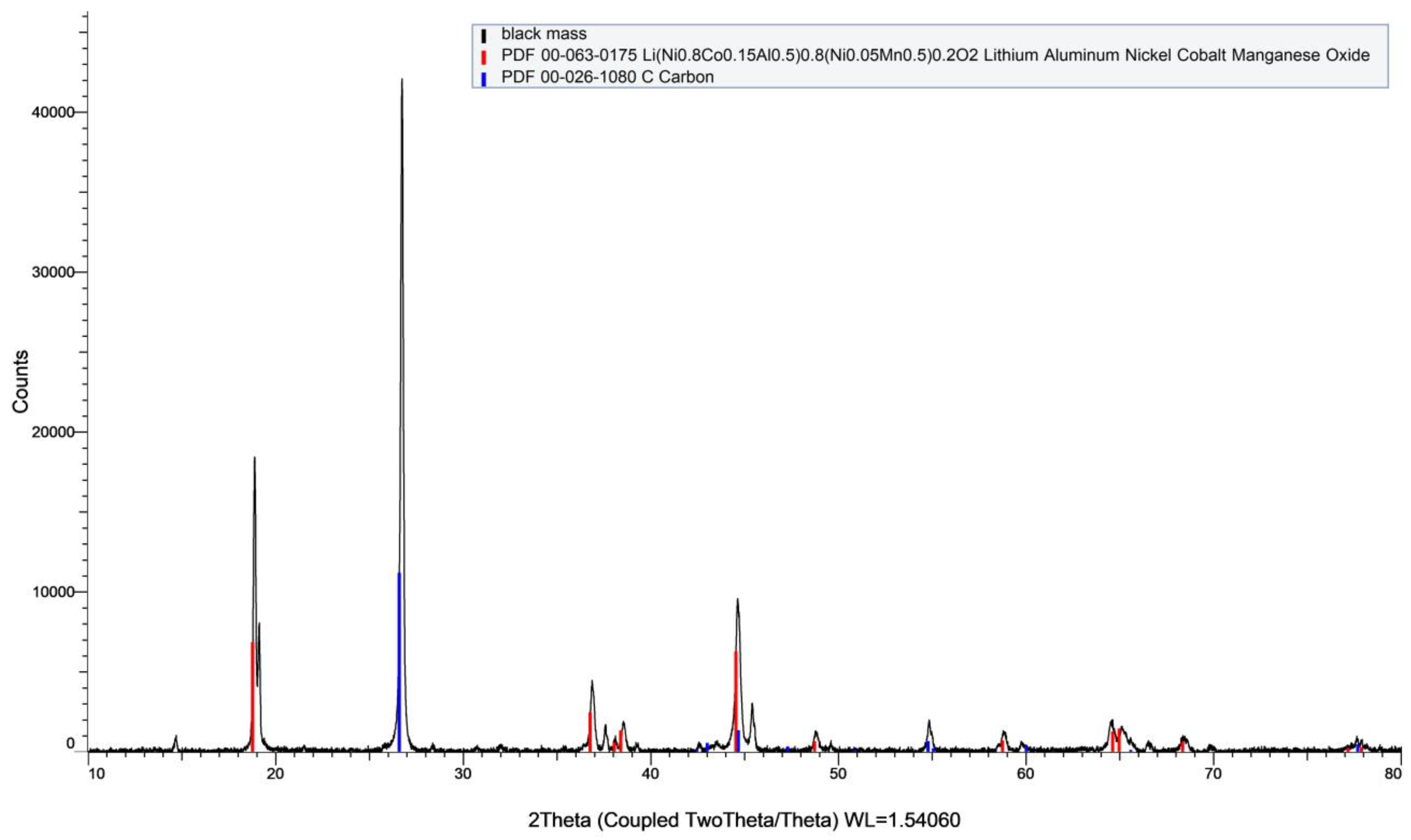
3.1. Leaching
3.2. Liquid Liquid Extraction
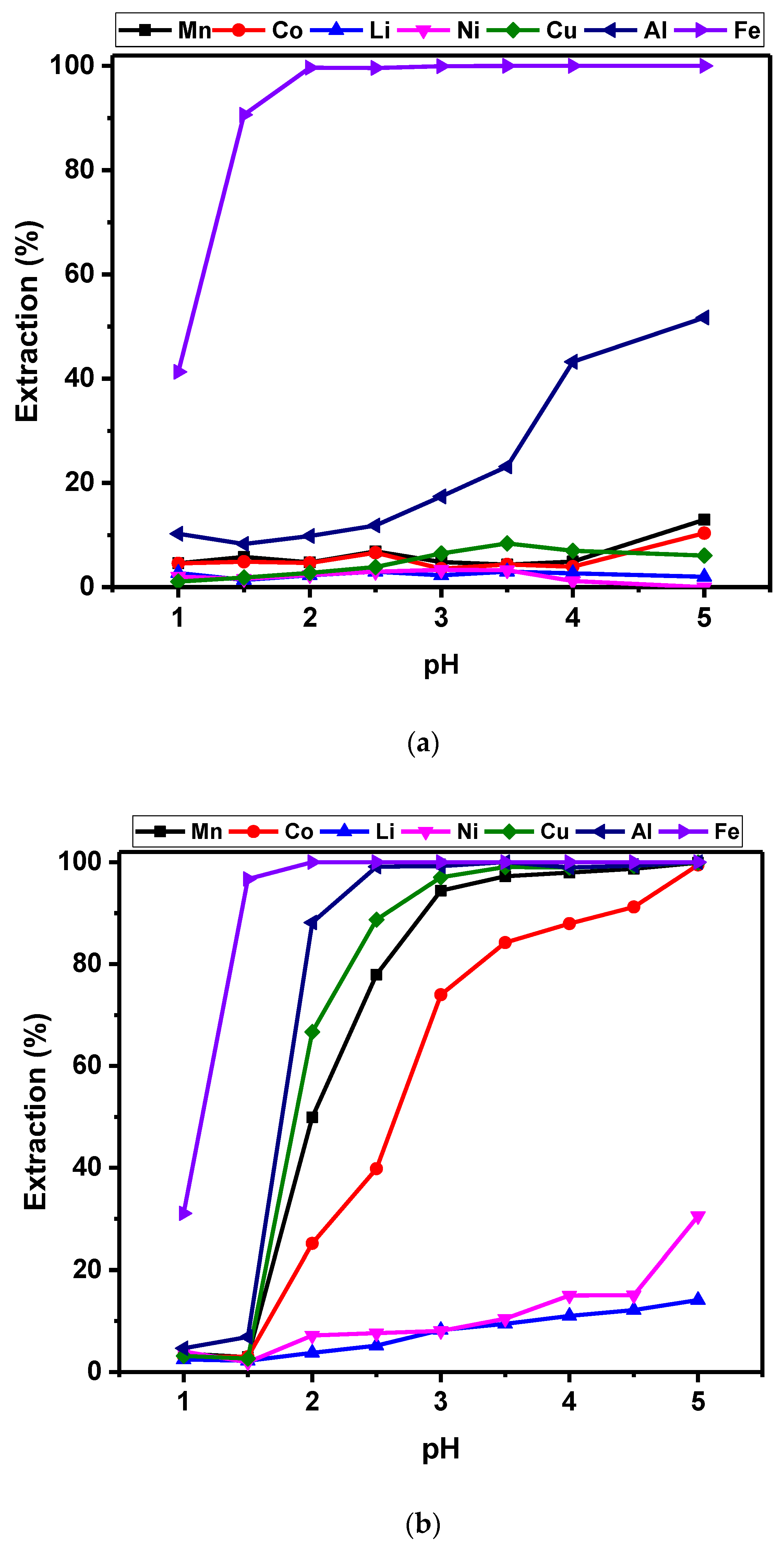
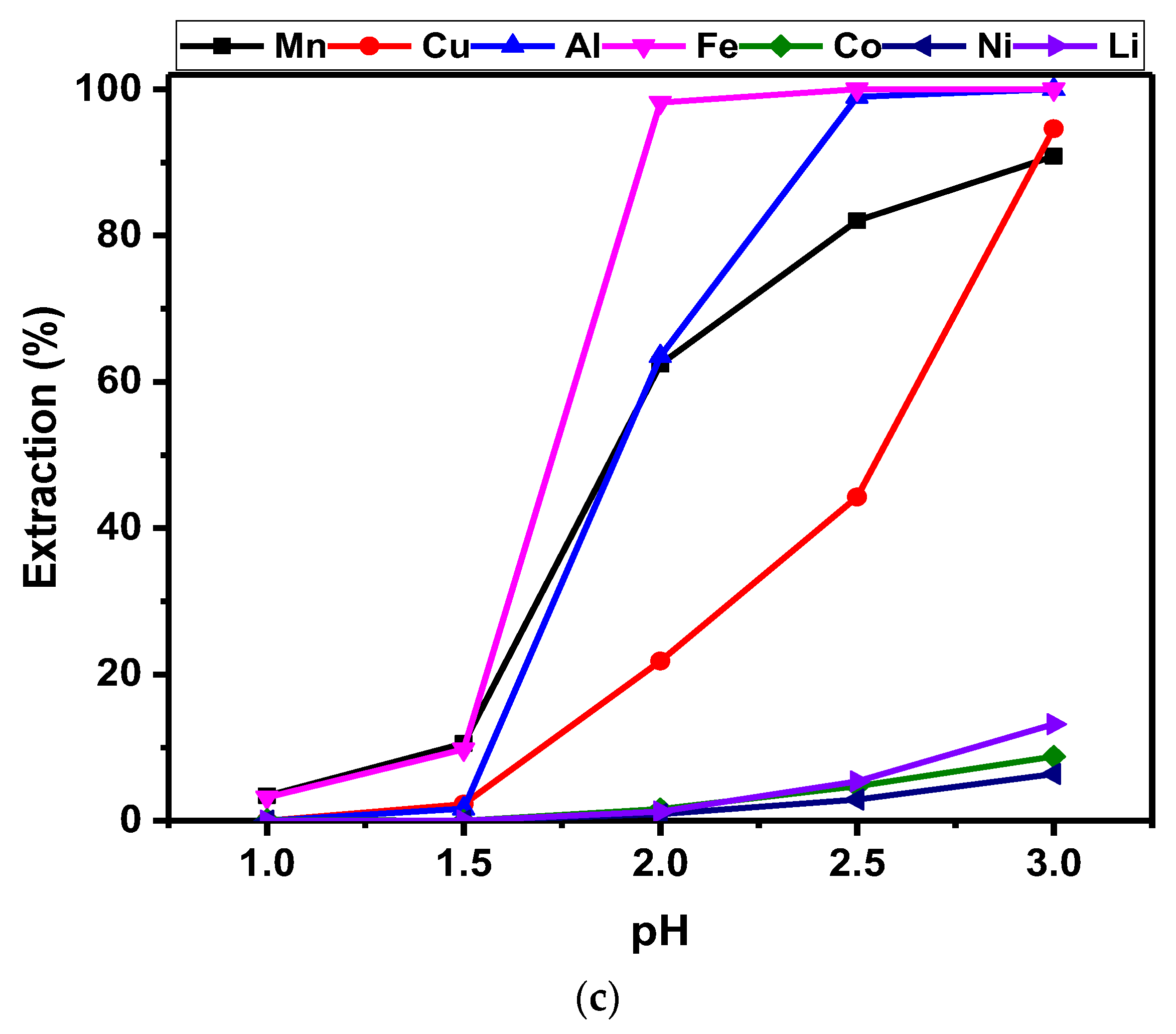
3.2.1. pH Studies
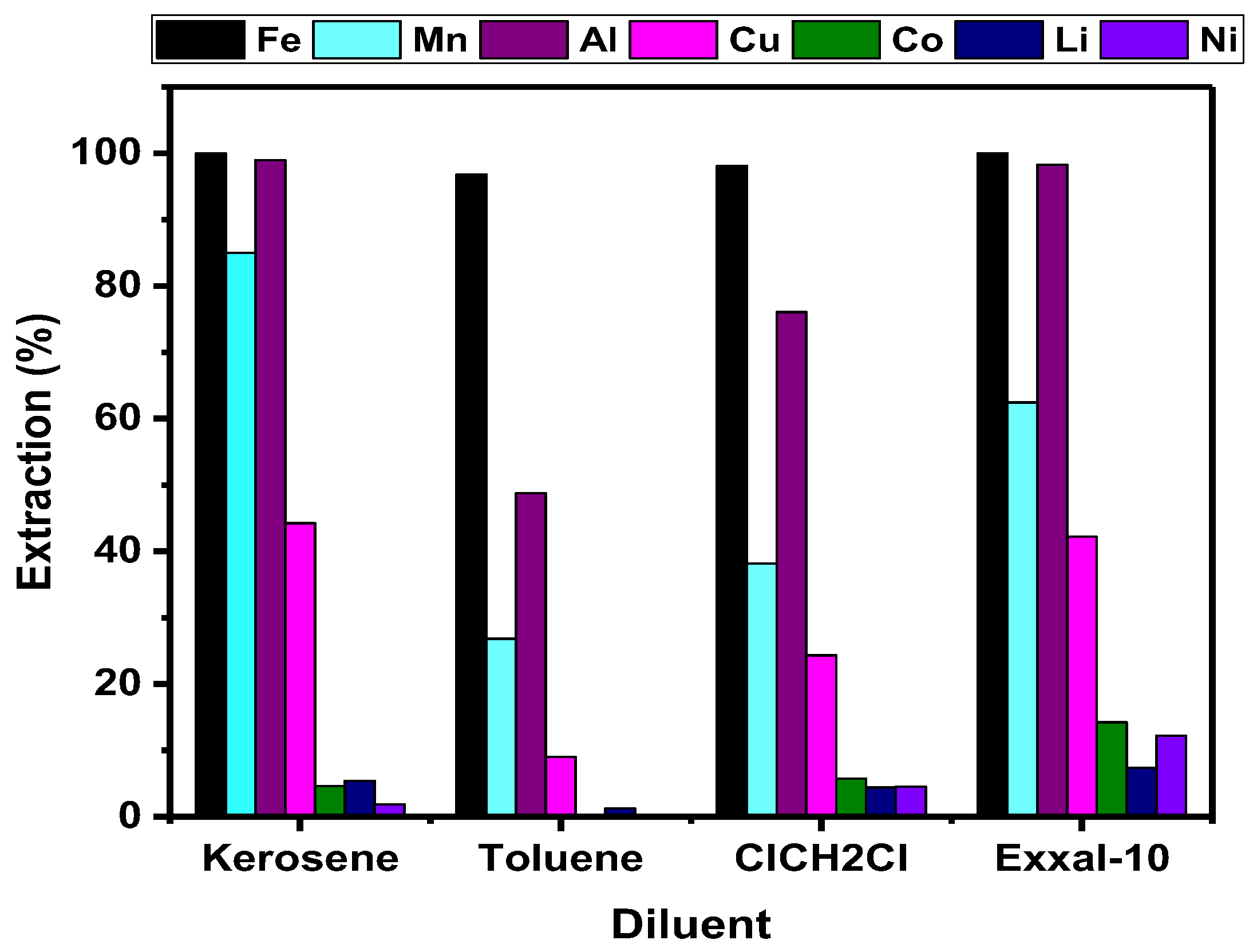
3.2.2. Diluent’s Efficiency
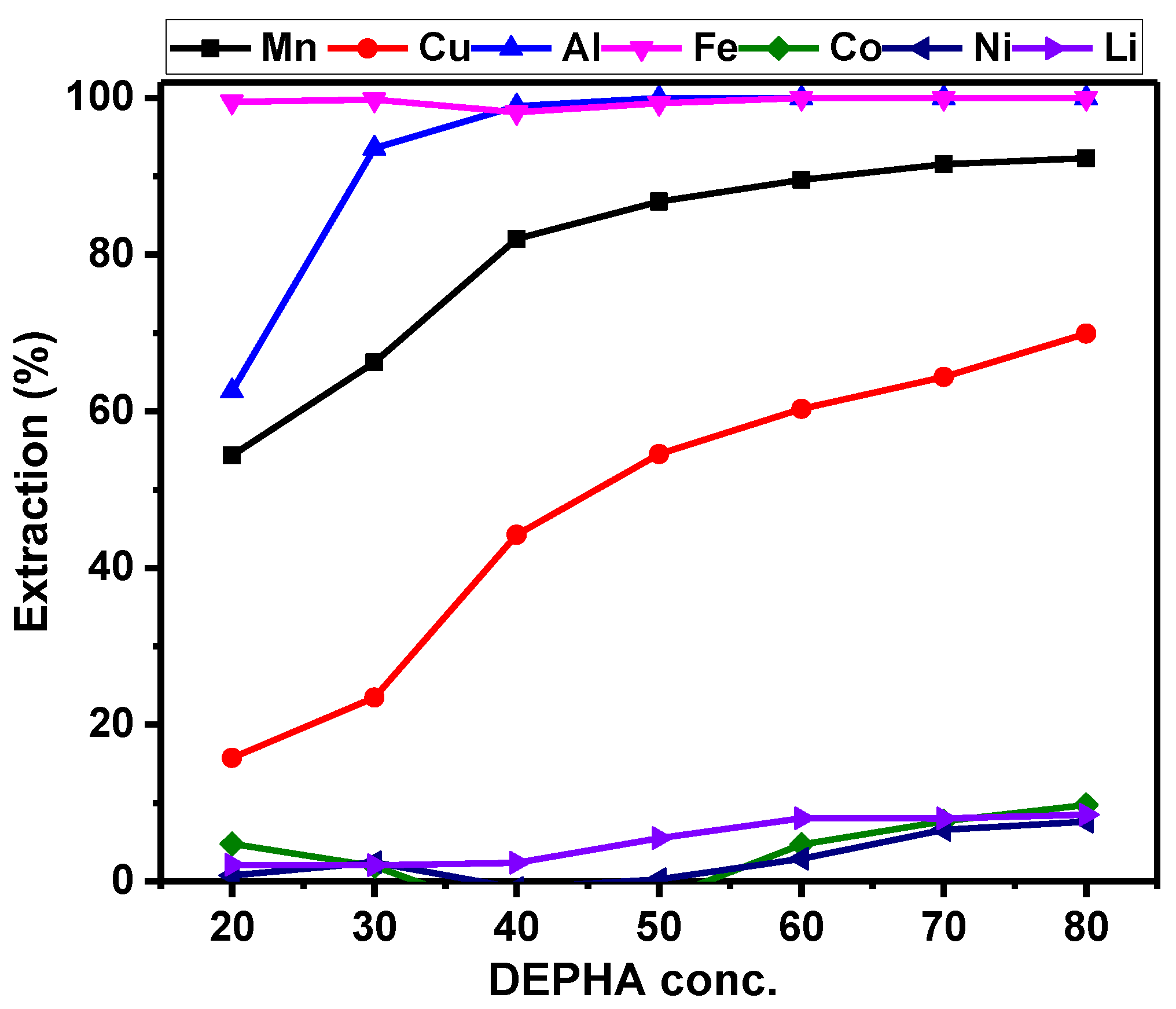
3.2.3. Extractant Concentration
3.2.4. Extractant Ratio
3.2.5. Separation Factor
3.3. Extraction Using Real Leached Liquor
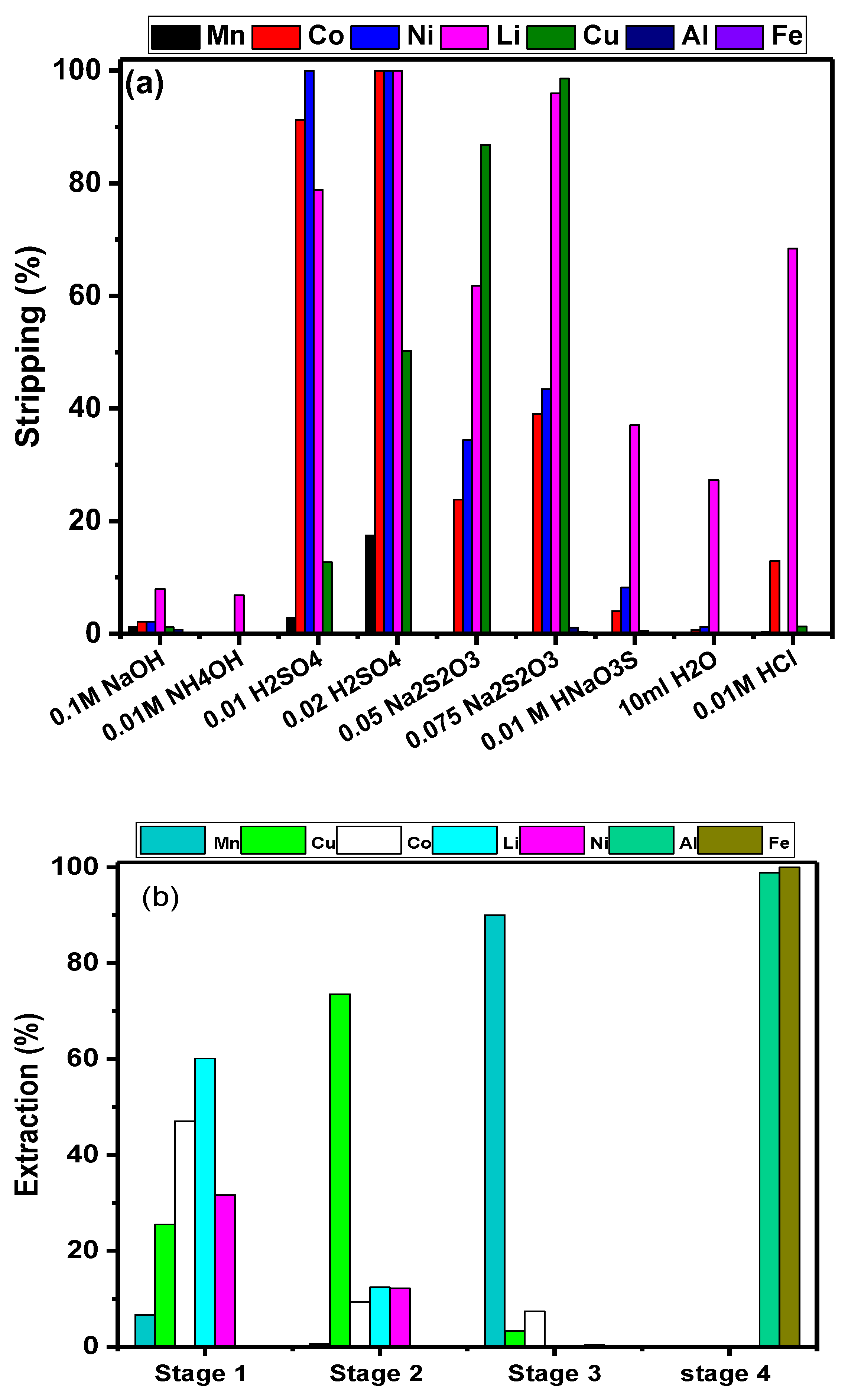
3.4. Stripping
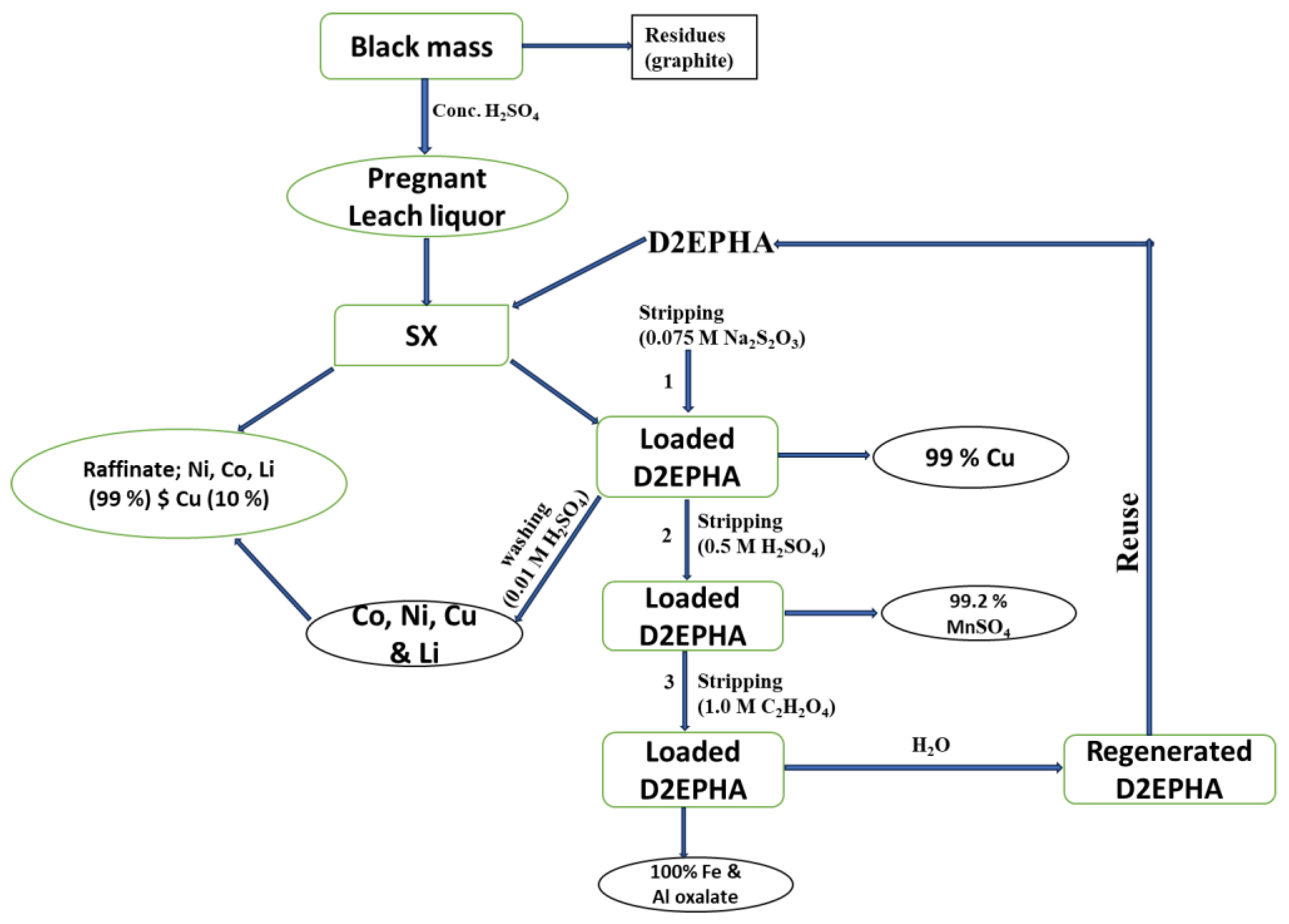
3.5. Conceptual Flow Sheet
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saldaña, G.; San-Martin, J.I.; Zamora, I.; Asensio, F.J.; Oñederra, O. Electric Vehicle into the Grid: Charging Methodologies Aimed at Providing Ancillary Services Considering Battery Degradation. Energies 2019, 12, 2443. [Google Scholar] [CrossRef]
- U.S. Department of Energy Critical Materials Assessment. 2023. Available online: https://www.energy.gov/sites/default/files/2023-07/doe-critical-material-assessment_07312023.pdf (accessed on 2 October 2023).
- Zuo, Z.; Cheng, J.; Guo, H.; Li, Y. Knowledge mapping of research on strategic minerals resources security: A visual analysis using CiteSpace. Resour. Policy 2021, 74, 102372. [Google Scholar] [CrossRef]
- International Energy Agency. Committed Mine Production and Primary Demand for Lithium, 2020–2030. Available online: https://www.iea.org/data--and-statistics/charts/committed-mine-production-and-primary-demand-for-lithium-2020 (accessed on 2 October 2023).
- Garside. Demand for Nickel Worldwide. Statistia. 2023. Available online: https://www.statista.com/statistics/273653/global-nickel-demand/ (accessed on 23 August 2023).
- Cobalt Institute. Electric Vehicle (EV) Demand Growth Offsets Sluggish Portable Electronics Market; Cobalt Market Report; Cobalt Institute: Guildford, UK, 2022. [Google Scholar]
- Steve, L.; Arumugam, M. Can cobalt be eliminated from lithium-ion batteries? ACS Energy Lett. 2022, 7, 3058–3063. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium-ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Zhu, J.; Mathews, I.; Ren, D.; Li, W.; Cogswell, D.; Xing, B.; Sedlatschek, T.; Kantareddy, S.N.R.; Gao, M.Y.T.; Xia, Y.; et al. End-of-life or second-life options for retired electric vehicle batteries. Cell Rep. Phys. Sci. 2021, 2, 100537. [Google Scholar] [CrossRef]
- Jie, Y.; Yang, S.; Li, Y.; Zhao, D.; Lai, Y.; Chen, Y. Oxidizing roasting behavior and leaching performance for the recovery of spent LiFePO4 batteries. Minerals 2020, 10, 949. [Google Scholar] [CrossRef]
- Pindar, S.; Dhawan, N. Recycling of mixed discarded lithium-ion batteries via microwave processing route. Sustain. Mater. Technol. 2020, 25, 2214–9937. [Google Scholar] [CrossRef]
- Gaines, L.; Dai, Q.; Vaughey, J.T.; Gillard, S. Direct Recycling R&D at the ReCell Center. Recycling 2021, 6, 31. [Google Scholar] [CrossRef]
- Rodrigues, B.V.M.; Bukowska, A.; Opitz, S.; Spiewak, M.; Budnyk, S.; Kuśtrowski, P.; Rokicińska, A.; Slabon, A.; Piątek, J. Selective electrochemical recoveries of Cu and Mn from end-of-life Li-ion batteries. Resour. Conserv. Recycl. 2023, 197, 107115. [Google Scholar] [CrossRef]
- Vanderburgt, S.; Santos, R.M.; Chiang, Y.W. Is it worthwhile to recover lithium-ion battery electrolyte during lithium-ion battery recycling? Resour. Conserv. Recycl. 2023, 189, 106733. [Google Scholar] [CrossRef]
- Jiang, S.; Nie, C.; Li, X.; Shi, S.; Gao, Q.; Wang, Y.; Zhu, X.; Wang, Z. Review on comprehensive recycling of spent lithium-ion batteries: A full component utilization process for green and sustainable production. Sep. Purif. Technol. 2023, 315, 123684. [Google Scholar] [CrossRef]
- Yang, C.; Ma, H.; Yuan, R.; Wang, K.; Liu, K.; Long, Y.; Xu, F.; Li, L.; Zhang, H.; Zhang, Y.; et al. Roll-to-roll prelithiation of lithium-ion battery anodes by transfer printing. Nat. Energy 2023, 8, 703–713. [Google Scholar] [CrossRef]
- Dolotko, O.; Gehrke, N.; Malliaridou, T.; Sieweck, R.; Herrmann, L.; Hunzinger, B.; Ehrenberg, H. Universal and efficient extraction of lithium for lithium-ion battery recycling using mechanochemistry. Commun. Chem. 2023, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.G.; Wang, Z.H.; Cao, H.B.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Anthony, T.; Yang, M.Z.; Dahl, U.E.; Pupek, K.Z.; Polzin, B.; Dunlop, A.; Vaughey, J.T. Direct Recycling of Lithium-Ion Battery Cathodes: A Multi-Stage Annealing Process to Recover the Pristine Structure and Performance. ACS Sustain. Chem. Eng. 2022, 10, 13319–13324. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Yosephine, G.; Khaleque, H.N.; Christina, M.; Tsing, B.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterization. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Aziz, A.A.; Monir, M.U.; Teo, S.H.; Hasan, M. 2020. Advances in sustainable approaches to recover metals from e-waste-A review. J. Clean. Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Joseph, J.R.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Zhou, L.F.; Yang, D.; Du, T.; Gong, H.; Luo, W.B. The Current Process for the Recycling of Spent Lithium-Ion Batteries. Front. Chem 2020, 8, 578044. [Google Scholar] [CrossRef]
- Amanze, C.; Zheng, X.; Man, M.; Yu, Z.; Ai, C.; Wu, X.; Xiao, S.; Xia, M.; Yu, Y.; Wu, X.; et al. Recovery of heavy metals from industrial wastewater using bioelectrochemical system inoculated with novel Castellaniella species. Environ. Res. 2022, 205, 112467. [Google Scholar] [CrossRef]
- Sun, L.; Liu, B.; Wu, T.; Wang, G.; Huang, Q.; Su, Y.; Wu, F. Hydrometallurgical recycling of valuable metals from spent lithium-ion batteries by reductive leaching with stannous chloride. Int. J. Min. Metall. Mater. 2021, 28, 991–1000. [Google Scholar] [CrossRef]
- Partinen, J.; Halli, P.; Wilson, B.P.; Lundström. The impact of chlorides on NMC leaching in hydrometallurgical battery recycling. Miner. Eng. 2023, 202, 108244. [Google Scholar] [CrossRef]
- Tang, Y.C.; Wang, J.Z.; Shen, Y.H. Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction. Minerals 2023, 13, 285. [Google Scholar] [CrossRef]
- Sahu, S.; Devi, N. Two-step leaching of spent lithium-ion batteries and effective regeneration of critical metals and graphitic. R. Soc. Chem. Adv. 2023, 13, 7193–7205. [Google Scholar] [CrossRef]
- Peng, C.; Liu, F.; Wang, Z.; Wilson, B.P.; Lundström, M. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 2019, 415, 179–188. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 2, S3632–S3639. [Google Scholar] [CrossRef]
- Fan, X.; Song, C.; Lu, X.; Shi, Y.; Yang, S.; Zheng, F.; Huang, Y.; Liu, K.; Wang, H.; Li, Q. Separation and recovery of valuable metals from spent lithium-ion batteries via concentrated sulfuric acid leaching and regeneration of LiNi1/3Co1/3Mn1/3O2. J. Alloys Compd. 2021, 863, 158775. [Google Scholar] [CrossRef]
- Guimarães, L.F.; Botelho Junior, A.B.; Espinosa, D.C.R. Sulfuric acid leaching of metals from waste Li-ion batteries without using reducing agent. Miner. Eng. 2022, 183, 107597. [Google Scholar] [CrossRef]
- Urias, P.M.; Dos, R.; Menêzes, L.H.; Cardoso, V.L.; de Resende, M.M.; de Souza, F.J. Leaching with mixed organic acids and sulfuric acid to recover cobalt and lithium from lithium-ion batteries. Environ. Technol. 2021, 42, 4027–4037. [Google Scholar] [CrossRef]
- Yunze, Z.; Liu, B.; Zhang, L.; Guo, S. Microwave Pyrolysis of Macadamia Shells for Efficiently Recycling Lithium from Spent Lithium-ion Batteries. J. Hazard. Mater. 2020, 396, 122740. [Google Scholar] [CrossRef]
- Liu, C.; Ji, H.; Liu, J.; Liu, P.; Zeng, G.; Luo, X.; Guan, Q.; Mi, X.; Li, Y.; Zhang, J.; et al. An emission-free controlled potassium pyrosulfate roasting-assisted leaching process for selective lithium recycling from spent Li-ion batteries. Waste Manag. 2022, 153, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, W.; Jia, K.; Li, S.; Jia, P.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Chen, S. Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite. Processes 2023, 11, 1451. [Google Scholar] [CrossRef]
- Kihlbom, C. Separation of Cobalt and Nickel Using CYANEX 272 for Solvent Extraction [Dissertation] (TRITA-CBH-GRU). 2021. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-293919 (accessed on 12 October 2023).
- Doreen, K.; Sandra, P.; Martin, B. Recovery of Al, Co, Cu, Fe, Mn, and Ni from Spent LIBs after Li Selective Separation by the COOL-Process. Part 1: Leaching of Solid Residue from COOL-Process. Chem. Ing. Tech. 2021, 93, 1833–1839. [Google Scholar] [CrossRef]
- Viet, N.H.N.; Man, S.L. Separation of Co (II), Cu (II), Ni (II), and Mn (II) from synthetic sulfuric acid leaching solution of spent lithium-ion batteries by solvent extraction. Physicochem. Probl. Miner. Process 2020, 56, 599–610. [Google Scholar]
- Viet, N.H.N.; Man, S.L. Separation of Co (II), Ni (II), Mn (II) and Li(I) from synthetic sulfuric acid leaching solution of spent lithium-ion batteries by solvent extraction. J. Chem. Technol. Biotechnol. 2021, 96, 1205–1217. [Google Scholar]
- Ajiboye, E.A.; Panda, P.K.; Adebayo, A.O.; Ajayi, O.O.; Tripathy, B.C.; Ghosh, M.K.; Basu, S. Leaching kinetics of Cu, Ni and Zn from waste silica rich integrated circuits using mild nitric acid. Hydrometallurgy 2019, 188, 161–168. [Google Scholar] [CrossRef]
- Benjamasutin, P.; Promphan, R. Determination of Optimal Parameters for the Application of Hydrogen Peroxide as Reducing Agent in the Leaching Process. Master’s Thesis, Chalmer University of Technology, Gothenburg, Sweden, 2020. Available online: https://hdl.handle.net/20.500.12380/300808 (accessed on 12 October 2023).
- Vieceli, N.; Casasola, R.; Lombardo, G.; Ebin, B.; Petranikova, M. Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Manag. 2021, 125, 192–203. [Google Scholar] [CrossRef]
- Choubey, P.K.; Dinkar, O.M.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective extraction and separation of Li, Co, and Mn from leach liquor of discarded lithium-ion batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef]
- Punt, T.; Luckay, R.C.; Akdogan, G.; Bradshaw, S.M.; Van Wyk, A.P. Comparison of phosphorus-based extractants on manganese separation from citrate leach solutions for recycling of lithium-ion batteries. S. Afr. J. Sci. 2023, 119. [Google Scholar] [CrossRef]
- Pardon, K.K.; Michael, A.H. Diluent effect on the solvent extraction rate of copper. Sep. Sci. Technol. 2002, 37, 1135–1152. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Engdahl, E.L.; Aneheim, E.; Ekberg, C.; Foreman, M.; Skarnemark, G. Diluent effects in solvent extraction. In Proceedings of the First ACSEPT International Workshop, Lisbon, Portugal, 7–10 September 2010. [Google Scholar]
- Ritcey, G.M.; Lucas, G.H. Proceedings of International Solvent Extraction Conference; Society of Chemical Industry: London, UK, 1974; Volume 3, p. 2899. [Google Scholar]
- Nilsson, M.; Andersson, S.; Ekberg, C.; Foreman, M.R.S.; Hudson, M.J.; Liljenzin, J.; Magnusson, D.; Skarnemark, G. Extraction properties of 6,6’-bis-(5,6-dipentyl- [1,2,4] triazin-3-yl)- [2,2] bipyridinyl (C5-BTBP). Solvent Extr. Ion Exch. 2005, 24, 299–318. [Google Scholar] [CrossRef]
- Khorfan, S.; Koudsi, Y. Effect of organic diluents on the extraction of uranium from phosphoric acid. J. Radioanal. Nucl. Chem. Lett. 1995, 199, 339–345. [Google Scholar] [CrossRef]
- Ramazan, D.; Emrah, T. Extraction of Th (IV) metal ions with trioctylphosphine oxide dissolved in kerosene using multi-dropped liquid membrane technique. Heliyon 2022, 8, e09258. [Google Scholar] [CrossRef]
- Reuna, S.; Väisänen, A. Purification of recovered phosphoric acid by extracting aluminum with di-2-ethylhexyl phosphoric acid. Chem. Pap. 2022, 76, 417–425. [Google Scholar] [CrossRef]
- Zhou, L.; Yongqing, Z.; Lijin, Z.; Xuefeng, W.; Ran, J.; Lu, W. High-Value Recovery of the Iron via Solvent Extraction from Waste Nickel-Cadmium Battery Sulfuric Acid Leachate Using Saponified D2EHPA. Separations 2023, 10, 251. [Google Scholar] [CrossRef]



| Metal | Ni | Co | Li | Mn | Cu | Al | Fe |
|---|---|---|---|---|---|---|---|
| Conc. (g/L × 100) | 1.21 | 0.89 | 0.31 | 0.57 | 0.101 | 0.102 | 0.14 |
| pH | 2.0 | 2.5 | 3.0 |
|---|---|---|---|
| Mn/Co | 24 | 95 | 52 |
| Mn/Ni | 31 | 252 | 146 |
| Mn/Li | 80 | 389 | 65 |
| Mn/Cu | 6 | 6 | 6 |
| Al/Mn | 1 | 21 | 452 |
| Fe/Mn | 1376 | 252 | 1074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajiboye, A.E.; Dzwiniel, T.L. Sequential Recovery of Critical Metals from Leached Liquor of Processed Spent Lithium-Ion Batteries. Batteries 2023, 9, 549. https://doi.org/10.3390/batteries9110549
Ajiboye AE, Dzwiniel TL. Sequential Recovery of Critical Metals from Leached Liquor of Processed Spent Lithium-Ion Batteries. Batteries. 2023; 9(11):549. https://doi.org/10.3390/batteries9110549
Chicago/Turabian StyleAjiboye, Ayorinde Emmanuel, and Trevor L. Dzwiniel. 2023. "Sequential Recovery of Critical Metals from Leached Liquor of Processed Spent Lithium-Ion Batteries" Batteries 9, no. 11: 549. https://doi.org/10.3390/batteries9110549
APA StyleAjiboye, A. E., & Dzwiniel, T. L. (2023). Sequential Recovery of Critical Metals from Leached Liquor of Processed Spent Lithium-Ion Batteries. Batteries, 9(11), 549. https://doi.org/10.3390/batteries9110549






